Abstract
Introduction
The SARS-CoV-2 pandemic remains a threat to public health. Soon after its outbreak, it became apparent that children are less severely affected. Indeed, opposing clinical manifestations between children and adults are observed for other infections. The SARS-CoV-2 outbreak provides the unique opportunity to study the underlying mechanisms. This protocol describes the methods of an observational study that aims to characterise age dependent differences in immune responses to primary respiratory infections using SARS-CoV-2 as a model virus and to assess age differences in clinical outcomes including lung function.
Methods and analysis
The study aims to recruit at least 120 children and 60 adults that are infected with SARS-CoV-2 and collect specimen for a multiomics analysis, including single cell RNA sequencing of nasal epithelial cells and peripheral blood mononuclear cells, mass cytometry of whole blood samples and nasal cells, mass spectrometry-based serum and plasma proteomics, nasal epithelial cultures with functional in vitro analyses, SARS-CoV-2 antibody testing, sequencing of the viral genome and lung function testing. Data obtained from this multiomics approach are correlated with medical history and clinical data. Recruitment started in October 2020 and is ongoing.
Ethics and dissemination
The study was reviewed and approved by the Ethics Committee of Charité – Universitätsmedizin Berlin (EA2/066/20). All collected specimens are stored in the central biobank of Charité – Universitätsmedizin Berlin and are made available to all participating researchers and on request.
Trial registration number
DRKS00025715, pre-results publication.
Keywords: immunology, paediatrics, virology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Sample collection from children and adults with primary SARS-CoV-2 infection at multiple time points, however, samples from severely ill patients are not included.
Mass cytometry, single-cell RNA sequencing and mass spectrometry-based serum and plasma proteomics display the local and systemic immune response.
Air–liquid interface cultures reproduce in vivo conditions and will be used for functional studies.
Analysis of clinical data and lung function testing complement the multiomics approach.
Introduction
In December 2019, the novel coronavirus SARS-CoV-2 emerged as cause of acute pneumonia.1 2 By August 2021, more than 200 million people were infected with SARS-CoV-2.3 Soon after the beginning of the pandemic, it became obvious that children have an increased resilience against the primary infection. The course of disease in children is more likely to be milder and severe or even fatal courses remain extremely rare.4–8 Various hypotheses to explain the reduced susceptibility and mortality of children are currently discussed, including reduced virus entry via ACE-2 in children,9 preactivated components of the immune system, such as cross-reactive T cells10–13 and antibodies,14 or a more accentuated innate immunity in children15 16 (table 1). Most of these findings are complementary in the explanation of the observed phenomenon, however, some findings are in part contradictory and require further investigation. Opposing clinical manifestations between children and adults are also observed for other viral respiratory infections.15–17 This points to major changes in the general immune response pattern during ageing. In the past, comparative immune response analyses to primary infections in various age groups were difficult to perform, as many adults had already been exposed to the pathogens. The SARS-CoV-2 outbreak provides the unique opportunity to study the age-dependent changes in immune responses in a controlled manner.
Table 1.
Hypotheses to explain the resilience of children in SARS-CoV-2 infections
| Hypothesis | Proposed explanation | Scientific findings |
| Reduced susceptibility for SARS-CoV-2 in children81 | Reduced virus entry via ACE-2 in children | SARS-Cov-2 uses ACE-2 in the upper and lower airways for host cell entry82 The age-dependency of ACE-2 expression is controversely discussed.83–85 |
| Even though an increased expression of ACE-2 renders the individual more susceptible to viral infection, ACE-2 also initiates anti-inflammatory signalling and might contribute to a milder immune response.86 | ||
| Age-dependent differential immune activation pattern | Preactivated immune components in children entail a milder immune response | In early childhood, infections of the upper respiratory tract are frequent. It has been proposed that previous infections with coronaviridae might contribute to a cross-reactive immunity.87 |
| Pre-existing T cell reactivity to SARS-CoV-2 could affect the severity of COVID-19.10–13 19 | ||
| Cross-reactive antibodies entail a milder immune response to SARS-CoV-2.14 Of note, uninfected infants do not express cross-reactive antibodies.88 | ||
| The polyclonality and polyreactivity of IgM naturally present in children recognises SARS-CoV-2 particles.89 | ||
| Children possess a stronger innate immunity than adults | Children display a higher basal expression of pattern recognition receptors than adults and a stronger innate antiviral response.76 77 | |
| The nasopharyngeal mucosa of children exhibits a stronger innate immunity and expresses more antiviral cytokines than adults.90 | ||
| Children expose a different cytokine response on SARS-CoV-2 infection than adults | In COVID-19, a cytokine storm leads to acute respiratory distress syndrome.63 64 | |
| Certain cytokine patterns correlate with COVID-19 severity.91 | ||
| Proinflammatory cytokine concentrations might be lower in children infected with SARS-CoV-2 than in adults.92 | ||
| Coinfections lead to a milder immune reaction, for example, because of virus competition or primed immune components | In COVID-19, coinfection with other pathogens is not rare, especially in children.93–95 |
To understand the mechanisms behind the lower susceptibility of children compared with adults to develop severe COVID-19 disease, we have established the observational study RECAST (increased REsilience of Children compared to Adults in SARS-CoV-2 infecTion) focusing on the differences in the clinical presentation, lung function and the immune response to SARS-CoV-2 in children compared with adults.
The complexity of immune responses requires a multilevel approach to display changes on various layers, including local immune cell composition, cytokine signalling and systemic response. It can be assumed that the combination of several mechanisms leads to the largely different phenotypes. At the same time, modern techniques allow to engage on an exploratory approach analysing simultaneously the involvement of canonical and non-canonical immune response patterns. The multiomics approach presented here allows deeply detailed characterisation of the various layers of age dependent specific immune responses. Therefore, we believe that the presented study design will contribute to a further understanding even beyond COVID-19.
To meet these requirements adequately, we chose a multiomics approach, including: (1) single-cell RNA sequencing (scRNAseq) of peripheral blood mononuclear cells (PBMCs) and nasal epithelial cells, (2) mass spectrometry-based serum and plasma proteomics, which has been used to identify prognostic marker signatures for SARS-CoV-2 disease severity and devise risk-adapted treatment strategies,18 (3) mass cytometry (cytometry by time-of-flight, CyTOF) of whole blood samples and nasal cells, which has been used to elucidate the role of T cell cytotoxicity in COVID19 and to identify a dysregulation of the myeloid cell compartment as hallmark of severe COVID,20 (4) highly differentiated nasal epithelial cultures and functional in vitro analyses, which have been used to display age-related differences in the nasal epithelium,21 (5) antibody testing and (6) sequencing of the viral genome. Obtained data are complemented with anamnestic and clinical information, lung function testing, including spirometry and multiple breath washout (MBW), which is a standardised method which allows to assess the ventilation homogeneity of the lungs already in preschool children as well as smell and taste. Longitudinal sampling allows monitoring of the immune response over the course of disease and beyond. Due to the maturation of the immune system during childhood, age-specific immune response pattern against SARS-CoV-2 can be expected.22 23 Thus, participants of all age groups are enrolled.
Participant recruitment began in October 2020 and is ongoing. Of particular interest is the recruitment of children infected with various SARS-CoV-2 virus variants of concern.
Study objectives
This study aims to characterise and compare primary infections with SARS-CoV-2 in children and adults, and to identify age-related determinants of disease course and prognosis. The immune system is not only highly complex, but immune response patterns also vary depending on individual predisposition; moreover, it also matures throughout the ageing of an individual. Furthermore, of pivotal interest for the interpretation of these data is the correlation between immune response pattern and clinical outcome.
Methods and analysis
Study design
RECAST is a prospective observational cohort study at Charité – Universitätsmedizin Berlin in Berlin, Germany. It is a substudy of the Pa-COVID study of the Charité,24 aiming to characterise the disease course of patients suffering from COVID-19.
Data are collected longitudinally from patients with confirmed COVID-19 at three time points, directly after the diagnosis and at follow-up visits after 2 weeks and 4–6 months, and from healthy age-matched controls.
Recruitment started in October 2020 and is planned to end in October 2023.
Study population
Inclusion criteria
Main inclusion criteria for the index person are a primary acute SARS-CoV-2 infection in a minor (<18 years of age) with positive PCR or antigen testing (both will be confirmed by PCR testing).
Exclusion criteria
Subjects with pre-existing conditions affecting the immune response, such as diseases requiring chemotherapy or syndromes with immunodeficiency and subjects with concomitant medication that affects the immune response, such as systemic steroids, biologicals or investigational therapeutics targeting SARS-CoV-2, are excluded.
Study procedures
Patient identification and recruitment
A network of participating paediatric outpatient practices (n=20) has been established as sentinels to provide access to a pool of >25 000 paediatric patients. All children who tested positive for SARS-CoV-2 by PCR or antigen testing as well as their household members are eligible for inclusion.
Healthy controls are recruited from clinical routine diagnostic settings if the diagnostic screening for SARS-CoV-2 was negative.
Medical history, clinical assessment and functional testing
Assessed data include epidemiological and demographic parameters, medical history and potential risk factors, clinical course—including all diagnostic results of the present medical attendance—and household and family constellation. A complete list of all items is attached in online supplemental appendix table E1.
bmjopen-2022-065221supp001.pdf (87.8KB, pdf)
Data are collected at first contact and during the follow-up visits. Symptoms of post-COVID-19/long-COVID25 are documented and symptoms of myalgic encephalomyelitis/chronic fatigue syndrome are assessed with the Canadian consensus criteria,26 27 Chalder Fatigue Scale28 and PedsQL Multidimensional Fatigue Scale.29–37 Loss of smell and taste are assessed with the ‘U-Sniff’ test, a 12-item odour identification, the ‘Sniffin’ Sticks’ olfactory threshold test and taste samples for sweet, sour, salty and bitter tastes in children aged 6 years or older.38 39 For adults, health status and quality of life are assessed with the St George’s Respiratory Questionnaire40 and health status and mental health are evaluated withthe 9-question Patient Health Questionnaire (PHQ-9) 41 and the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5) 42 questionnaires. For children, quality of life is assessed using the Revised questionnaire to assess Health-Related Quality of Life in children and adolescents (KINDL) 43.
Disease severity is classified according to clinical features using the criteria outlined in the WHO COVID-19 clinical management guideline44 as asymptomatic, mild, moderate, severe or critical disease. Also, clinical progression is classified according to the WHO clinical progression scale.45 Applied classification scales are shown in tables 2 and 3.
Table 2.
COVID disease severity
| Patient state | Descriptor | Score |
| Uninfected | Uninfected; no viral RNA detected | 0 |
| Ambulatory: mild disease | Asymptomatic; viral RNA detected | 1 |
| Symptomatic; independent | 2 | |
| Symptomatic; assistance needed | 3 | |
| Hospitalised: moderate disease | Hospitalised; no oxygen therapy | 4 |
| Hospitalised; oxygen by mask or nasal prongs | 5 | |
| Hospitalised: severe disease | Hospitalised; oxygen by non-invasive ventilation (NIV) or high flow | 6 |
| Intubation and mechanical ventilation, pO2/pFiO2≥150 or SpO2/FiO2≥200 | 7 | |
| Mechanical ventilation, pO2/pFiO2<150 (SpO2/FiO2<200) or vasopressors | 8 | |
| Mechanical ventilation, pO2/pFiO2<150 and vasopressors, dialysis or extracorporeal membrane oxygenation | 9 | |
| Dead | Dead | 10 |
Abbreviated criteria for COVID-19 disease severity according to WHO COVID-19 Clinical management guideline.44
FiO2, fractional inspired oxygen; pO2, partial pressure of oxygen.
Table 3.
WHO clinical progression scale
| Disease severity | Definition | Criteria |
| Asymptomatic | ||
| Mild | Symptomatic patients meeting the case definition for COVID-19 without evidence of viral pneumonia or hypoxia. | |
| Moderate | Pneumonia | Clinical signs of (non-severe) pneumonia Adolescent or adult: fever, cough, dyspnoea, fast breathing Child: cough or difficulty breathing+fast breathing and/or chest indrawing Diagnosis can be made on clinical grounds; chest imaging (radiograph, CT scan, ultrasound) may assist in diagnosis and identify or exclude pulmonary complications. |
| Severe | Severe pneumonia | Adolescent or adult: plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress; or peripheral oxygen saturation (SpO2) <90% on room air Child: plus at least one of the following: Central cyanosis or SpO2<90%; severe respiratory distress (eg, fast breathing, grunting, very severe chest indrawing); general danger sign: inability to breastfeed or drink, lethargy or unconsciousness, or convulsions. |
| Critical disease | Acute respiratory distress syndrome or sepsis/septic shock |
Oxygenation impairment, invasive ventilation or bilevel non-invasive ventilation (NIV) or continuous positive airway pressure (CPAP) (≥ 5 cmH2O) required or Infection and ≥2 systemic inflammatory response syndrome criteria |
Modified from WHO working group.45
Functional testing, including lung function testing and MBW, will be conducted at the follow-up visits after 2 weeks and 4–6 months.
Patient and public involvement
We will disseminate all findings in an appropriate and understandable manner to all participants, including children. We welcome the collaboration of participants and public in the interpretation and dissemination of all findings.
Sample collection
Samples will be collected from SARS-CoV-2 positive participants at each of three time points, directly after the diagnosis and at follow-up visits after 2 weeks and 4–6 months, and once from healthy age-matched controls.
Nasal and pharyngeal swab samples are collected for a SARS-CoV-2-PCR, scRNAseq and establishment of air–liquid cell cultures. In addition, PBMCs are collected for single-cell sequencing, whole blood for mass cytometry and plasma and serum for mass-spectrometry-based proteomics, and SARS-CoV-2-specific antibody testing.
Study database
For Pa-COVID-19, a study protocol was established that harmonises clinical, molecular and immunological phenotyping assessment in COVID-19 patients.24 All data are added to an electronic case report form (SecuTrial). Participants included in RECAST are part of Pa-COVID-19. All participants are assigned a pseudonym consisting of a six-digit or seven-digit alphanumerical participant code. A separate log allows to match each participant and their code. Access to SecuTrial requires a username and password. All local data are secured by password.
Sample description
Patients recruited in RECAST are grouped into six age categories (table 4). Due to the nature of observatory studies and the lack of pre-existing data, it is not possible to predict the extent of assumptive differences. Preliminary findings suggest that for most planned analyses a sample size of 15 is sufficient. The outlined sample sizes should suffice even for comparisons between children of different age groups.
Table 4.
Age categories of the RECAST participants
| Age group | Disease state | No | Time points | |
| Children | Nursery (0–3 years) | SARS-CoV-2− | ≥30 | 1 |
| SARS-CoV-2+ | ≥30 | 3 (days 0, 14, 180) | ||
| Kindergarten (3–6 years) | SARS-CoV-2− | ≥30 | 1 | |
| SARS-CoV-2+ | ≥30 | 3 (days 0, 14, 180) | ||
| Primary school (6–12 years) | SARS-CoV-2− | ≥30 | 1 | |
| SARS-CoV-2+ | ≥30 | 3 (days 0, 14, 180) | ||
| Secondary school (13–18 years) |
SARS-CoV-2− | ≥30 | 1 | |
| SARS-CoV-2+ | ≥30 | 3 (days 0, 14, 180) | ||
| Adults | <60 years | SARS-CoV-2− | ≥30 | 1 |
| SARS-CoV-2+ | ≥30 | 3 (days 0, 14, 180) | ||
| >60 years | SARS-CoV-2− | ≥30 | 1 | |
| SARS-CoV-2+ | ≥30 | 3 (days 0, 14, 180) | ||
| Total | ≥360 | ≥ 720 | ||
RECAST, REsilience of Children compared to Adults in SARS-CoV-2 infection.
Planned analyses and outcomes of interest
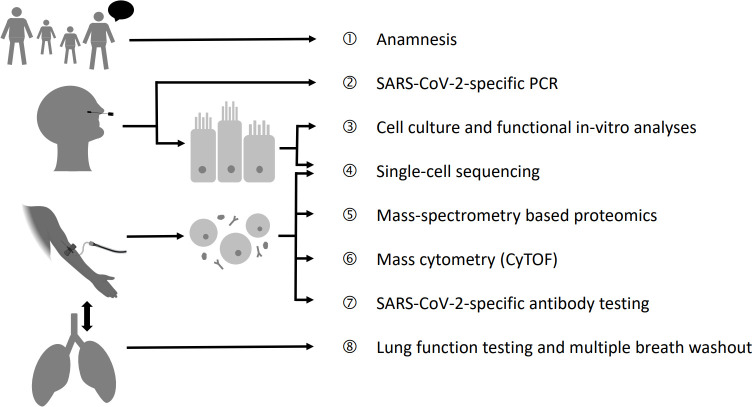
We propose a multiomics workup for all patients. A synopsis of the planned analyses is depicted in figure 1.
Figure 1.
Performed analyses in the RECAST study. The RECAST study consist of eight elements, including the anamnesis and clinical data, a comprehensive multiomics workup of the immune landscape of the blood and the upper airways and lung function testing. CyTOF, cytometry by time-of-flight; RECAST, REsilience of Children compared to Adults in SARS-CoV-2 infection.
SARS-CoV-2-specific PCR and antibody testing
All participants are screened for an active SARS-CoV-2 infection with Real Time Quantitative PCRs (RT-qPCRs) targeting E and N genes.46 Antibody testing is conducted for all serum and saliva samples with SARS-CoV-2-specific IgG-ELISAs and IgA-ELISAs. In case of a reactive screening result, confirmatory testing with a recombinant immunofluorescence assay and a plaque reduction neutralisation test47 are conducted.
Nasal epithelial culture and functional in vitro analyses
Conditional reprogramming allows for the generation of long-term cultures of primary airway epithelial cells.48–52 Without the need of genetic modification or clone selection, conditional reprogramming enables cell expansion, while redifferentiated cultures retain their organ-specific phenotype.53 We establish highly differentiated polarised in vitro air–liquid interface cultures that reproduce and allow for the analysis of physiological in vivo conditions, such as heterogeneous cell composition with preserved lineage54 as well as functional characteristics, including production of airway surface liquid55 56 and mucociliary clearance.57 58 For material collection, FLOQswabs (Copan, Italy) are used. Swabs are transferred into DMEM/F12 medium (Gibco, USA) and transported to our laboratory within 2 hours.
Mass cytometry of whole blood samples
Whole blood is fixed with a proteomic stabiliser for preservation of surface and intracellular markers. Blood samples are stored at −80°C until batch-based analysis. Thawed samples are stained in batches of nine patient and one anchor reference sample. On barcoding of individual samples, they are pooled and stained with metal isotype conjugated anti-human antibodies as described previously.19 20 CyTOF technology allows for the detection of more than 40 different barcodes simultaneously to identify cell populations in a high-throughput setting.19 56
Mass spectrometry-based serum and plasma proteomics
A platform technology with semiautomated sample preparation to allow for ultra-high-throughput liquid chromatography and mass-spectrometry based analyses of the proteome has recently been established by members of our group.59 In a directed approach, we characterise the immune response-related serum and plasma proteome, with focus on the acute phase response and the complement system. However, the plethora of proteome signals that are generated per sample also allows for an undirected approach, delivering predictive proteome signatures. To facilitate the computation of such extensive bulk data, a deep neural network is employed.60
Single-cell sequencing of nasal epithelial cell samples and PBMCs
The nasopharynx is the entry point for an infection with SARS-CoV-261 and as such of distinguished concern in the exploration of the individual immune response pattern. Using scRNAseq of nasal and bronchial samples, we were previously able to identify cell types and states that correlate with a severe disease course of COVID-19.62 Here, scRNAseq will be applied to define the composition and transcriptional activity of immune and epithelial cells in the nasal environment of children and adults throughout the various states of SARS-CoV-2 infection. Nasal swabs (FLOQswabs, Copan, Italy) are used for sample collection. Following sample collection, swabs are directly transferred into cold Gibco Dulbecco's Modified Eagle Medium: Nutrient Mixture F12 (DMEM/F12) (Gibco, USA) and transported to our biosafety laboratory within 1 hour for further processing. Library preparation is performed according to manufacturer’s protocol (10×) and sequencing is performed using the Illumina NextSeq 6000 platform.
In addition to the analysis of cells in the respiratory environment, PBMCs are isolated to study the transcriptional activity of blood cell populations. Cell separation, library preparation and comparative single cell transcriptome analyses are conducted according to the manufacturer’s protocol. Differential transcriptome profiles of immune cells of the blood will help us to characterise the distinctive features of the systemic and localised immune response to SARS-CoV-2 infections in children and adults.
Lung function testing and MBW
SARS-CoV-2 infections cause severe lung damage in adults.63 64 In a large review with 2135 children, 45% (951 children) were classified with a severity of moderate, severe or critical, all with lung involvement per definition.7 There is evidence that children with acute lung injury experience the same lung pathologies as adults.65 To assess the extent of transient and permanent functional lung impairment, we investigate the lung function with spirometry and MBW. MBW measures the lung ventilation homogeneity.66 67 This technique is already feasible without sedation in children from 2 years of age.67 Spirometry depends on the cooperation of the participant and may usually be conducted with children aged 6 years or older. The technical MBW procedures are in accordance with the American Thoracic Society Technical Statement.68 Measurements are conducted by personnel certified according to European Cystic Fibrosis Society-Clinical Trial Network (ECFS-CTN certified) and for study measurements Exhalyzer D (Ecomedics, Dürnden, Switzerland) will be used.69 N2 wash-out is used as tracer gas to determine the lung clearance index (LCI) as an outcome measure. The LCI increases with lung ventilation inhomogeneity.
Biobanking
Collected material is processed and stored at the central biobank of Charité (ZeBanC, https://biobank.charite.de). Material that is not immediately used is subjected to cryopreservation.
Ethics and dissemination
All procedures in this study are in compliance with the principles of the 1964 Declaration of Helsinki and its amendments. We act in adherence to the principles of Good Clinical Practice (International Council for Harmonisation, 1996). The study was reviewed and approved by the Charité Ethics Committee (EA2/066/20). All participants enrolled give written informed consent in person, for participants minor of age the written informed consent of the legal guardian is also required.
Study procedures never interfere with the medical management of participants. Samples required for medical management always have priority. There is no direct benefit for patients participating in the study. Results from the study might improve our understanding of the disease and benefit the public health.
Data are monitored regularly. Informed consent forms are audited by a monitor appointed by the Charité Clinical Trial Management Unit. Data monitoring of collected data is performed in the course of the study.
As established for Pa-COVID,24 we reiterate the fundamental principle in this study that all contributors and researchers who have access to samples commit to unrestricted data sharing. In accordance with FAIR data principles, all data collected shall be findable, accessible, interoperable and reusable.70
Results originating from nasal epithelial culture and functional in vitro analyses, mass cytometry, mass spectrometry-based proteomics, single-cell sequencing and lung function testing and MBW as well as clinical data will be will be disseminated separately or in context in a variety of ways including abstracts, posters and presentations at conferences and published manuscripts in peer-reviewed journals. As soon as all analyses are completed, a comprehensive review will be published to put the findings in context of each other.
Discussion
The SARS-CoV-2 pandemic has accelerated scientific research in the field of virology and related immunobiology for nearly 2 years, yet many crucial questions remain unanswered. Soon after the emergence of the virus it became apparent that, while children are just as likely to be infected with SARS-CoV-2 as adults, they are less severely affected.4–8 71 RECAST is an observational study that aims to elucidate the differences between children and adults in primary SARS-CoV-2 infections using a multiomics approach. Revealing age-dependent differences will help to develop better suited therapeutics and vaccination strategies beyond SARS-CoV-2 infections.
Previous multiomics approaches conducted with specimen from adult donors served to elucidate the immune response in COVID-19,62 72 to identify predictors of severe disease courses73 74 and to isolate possible targets for therapy.62 74 75 Multiomics-based studies focusing on SARS-CoV-2 infections in children remain rare and are limited to small participant numbers and only analyse a limited number of multiomics dimensions: A study including 24 infected children analysed the single-cell transcriptional landscape in the upper airways76; with single-cell multiomic profling of matched nasal, tracheal, bronchial and blood samples of 19 infected children, a study characterised the immune landscape with focus on the upper airways77; the plasma proteomic and metabolomic data of 18 infected children was analysed in another study78; clinical characteristics and serum markers were analysed in a larger group that summarised children and young adults and T cell response in a paediatric subgroup of 11 participants was examined79; and a study with 24 infected children analysed the T cell response and specific antibody response.80 Even though these studies contributed greatly to a better understanding of age-related immune response patterns in COVID-19, there is still a substantial demand for research. Especially studies analysing the immune response over the whole age and severity spectrum applying a multiomics approach are needed. In addition, mechanistic investigations, revealing the causal relationship between the different immune defence layers, are missing. In RECAST, we will conduct a full multiomics workup with at least 120 infected children, a larger number of participants than in previously published multiomics studies. Moreover, we will conduct follow-up visits for 6 months to profile the development of age-specific immune response patterns over the course of time. Biobanking and long-term storage of samples will be used to perform subsequent mechanistic studies on first data collection and hypotheses formulation. The recruitment of family members, both infected and non-infected, allows to assess the effect of genetic relationships. The combination of high resolution multidimensional immunological methods with clinical endpoints in the RECAST study will enable us to contribute to the understanding of the increased resilience of children to SARS-CoV-2 infections.
Supplementary Material
Acknowledgments
We thank participating children and their families for their close collaboration. We also thank all outpatient pediatricians who are involved in the recruitment.
Footnotes
Contributors: BS, JR, MAM, VMC, MR and LES initiated the project and led the BMBF grant proposal. All authors contributed to the design of the study. SS, JR, NZ, MK, TB, HS-B, JM and ER are collecting data biological material and are conducting functional testing. BS, MAM, VMC, MR, LES, IL, RE, JR and VS lead their respective research field and supervise the conduction of experiments and the interpretation of results. CD-H, PMB and AB are conducting experiments. SS and JR produced the first draft of the protocol. All authors provided critical review of the manuscript and have approved the final version.
Funding: This work was supported by the German Federal Ministry of Education and Research (grant number 01KI20337).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Zhou P, Yang X-L, Wang X-G, et al. Addendum: a pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;588:270–3.:E6. 10.1038/s41586-020-2951-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in china, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO COVID-19 dashboard. 2020.
- 4.Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 2020;179:1029–46. 10.1007/s00431-020-03684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-cov-2) infection in children and adolescents. JAMA Pediatr 2020;174:882. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in china. Pediatrics 2020;145:e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 8.Bhopal SS, Bagaria J, Olabi B, et al. Children and young people remain at low risk of COVID-19 mortality. The Lancet Child & Adolescent Health 2021;5:e12–3. 10.1016/S2352-4642(21)00066-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dioguardi M, Cazzolla AP, Arena C, et al. Innate immunity in children and the role of ACE2 expression in SARS-cov-2 infection. Pediatr Rep 2021;13:363–82. 10.3390/pediatric13030045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-cov-2 T cell epitopes in unexposed humans. Science 2020;370:89–94. 10.1126/science.abd3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrock E, Fujimura E, Kula T, et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020;370:eabd4250. 10.1126/science.abd4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loyal L, Braun J, Henze L, et al. Cross-reactive cd4+ t cells enhance sars-cov-2 immune responses upon infection and vaccination. Science 2021;374:eabh1823. 10.1126/science.abh1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun J, Loyal L, Frentsch M, et al. SARS-cov-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020;587:270–4. 10.1038/s41586-020-2598-9 [DOI] [PubMed] [Google Scholar]
- 14.Guo L, Wang Y, Kang L, et al. Cross-Reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg Microbes Infect 2021;10:664–76. 10.1080/22221751.2021.1905488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coates BM, Staricha KL, Wiese KM, et al. Influenza A virus infection, innate immunity, and childhood. JAMA Pediatr 2015;169:956–63. 10.1001/jamapediatrics.2015.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prendergast AJ, Klenerman P, Goulder PJR. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol 2012;12:636–48. 10.1038/nri3277 [DOI] [PubMed] [Google Scholar]
- 17.Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ 2019;366:l5021. 10.1136/bmj.l5021 [DOI] [PubMed] [Google Scholar]
- 18.Demichev V, Tober-Lau P, Lemke O, et al. A time-resolved proteomic and prognostic map of COVID-19. Cell Syst 2021;12:780–94. 10.1016/j.cels.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georg P, Astaburuaga-García R, Bonaguro L, et al. Complement activation induces excessive t cell cytotoxicity in severe covid-19. Cell 2022;185:493–512. 10.1016/j.cell.2021.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulte-Schrepping J, Reusch N, Paclik D, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020;182:1419–1440. 10.1016/j.cell.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balázs A, Millar-Büchner P, Mülleder M, et al. Age-Related differences in structure and function of nasal epithelial cultures from healthy children and elderly people. Front Immunol 2022;13. 10.3389/fimmu.2022.822437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw AC, Goldstein DR, Montgomery RR. Age-Dependent dysregulation of innate immunity. Nat Rev Immunol 2013;13:875–87. 10.1038/nri3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015;282:20143085. 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurth F, Roennefarth M, Thibeault C, et al. Studying the pathophysiology of coronavirus disease 2019: a protocol for the Berlin prospective COVID-19 patient cohort (pa-COVID-19). Infection 2020;48:619–26. 10.1007/s15010-020-01464-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koczulla AR, Ankermann T, Behrends U, et al. S1 guideline post-COVID/long-COVID. Pneumologie 2021;75:869–900. 10.1055/a-1551-9734 [DOI] [PubMed] [Google Scholar]
- 26.Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome. Journal of Chronic Fatigue Syndrome 2003;11:7–115. 10.1300/J092v11n01_02 [DOI] [Google Scholar]
- 27.Kedor C, Freitag H, Meyer-Arndt L, et al. Chronic covid-19 syndrome and chronic fatigue syndrome (me/cfs) following the first pandemic wave in germany – a first analysis of a prospective observational study. Infectious Diseases (except HIV/AIDS) [Preprint] 2021. 10.1101/2021.02.06.21249256 [DOI]
- 28.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res 1993;37:147–53. 10.1016/0022-3999(93)90081-p [DOI] [PubMed] [Google Scholar]
- 29.Varni JW, Burwinkle TM, Katz ER, et al. The pedsql in pediatric cancer: reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer 2002;94:2090–106. 10.1002/cncr.10428 [DOI] [PubMed] [Google Scholar]
- 30.Daniel LC, Brumley LD, Schwartz LA. Fatigue in adolescents with cancer compared to healthy adolescents. Pediatr Blood Cancer 2013;60:1902–7. 10.1002/pbc.24706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varni JW, Burwinkle TM, Szer IS. The pedsql multidimensional fatigue scale in pediatric rheumatology: reliability and validity. J Rheumatol 2004;31:2494–500. [PubMed] [Google Scholar]
- 32.Varni JW, Limbers CA. The pedsql multidimensional fatigue scale in young adults: feasibility, reliability and validity in a university student population. Qual Life Res 2008;17:105–14. 10.1007/s11136-007-9282-5 [DOI] [PubMed] [Google Scholar]
- 33.Varni JW, Limbers CA, Bryant WP, et al. The pedsql multidimensional fatigue scale in type 1 diabetes: feasibility, reliability, and validity. Pediatr Diabetes 2009;10:321–8. 10.1111/j.1399-5448.2008.00482.x [DOI] [PubMed] [Google Scholar]
- 34.Varni JW, Limbers CA, Bryant WP, et al. The pedsql multidimensional fatigue scale in pediatric obesity: feasibility, reliability and validity. Int J Pediatr Obes 2010;5:34–42. 10.3109/17477160903111706 [DOI] [PubMed] [Google Scholar]
- 35.Varni JW, Limbers CA, Bryant WP, et al. Assessment of Fatigue in Pediatric Patients With Short Stature Utilizing the PedsQL™ Multidimensional Fatigue Scale. Children’s Health Care 2012;41:162–81. 10.1080/02739615.2012.657068 [DOI] [Google Scholar]
- 36.Varni JW, Beaujean AA, Limbers CA. Multidimensional fatigue scale. Qual Life Res 2013;22:2581–94. 10.1007/s11136-013-0370-4 [DOI] [PubMed] [Google Scholar]
- 37.Panepinto JA, Torres S, Bendo CB, et al. PedsQL™ Multidimensional fatigue scale in sickle cell disease: feasibility, reliability, and validity. Pediatr Blood Cancer 2014;61:171–7. 10.1002/pbc.24776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schriever VA, Agosin E, Altundag A, et al. Development of an international odor identification test for children: the universal sniff test. J Pediatr 2018;198:265–72. 10.1016/j.jpeds.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 39.Gellrich J, Sparing-Paschke L-M, Thieme T, et al. Normative data for olfactory threshold and odor identification in children and adolescents. Int J Pediatr Otorhinolaryngol 2019;123:5–9. 10.1016/j.ijporl.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 40.Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med 1991;85 Suppl B:25–31; 10.1016/s0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 41.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blevins CA, Weathers FW, Davis MT, et al. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress 2015;28:489–98. 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- 43.Ravens-Sieberer U, Bullinger M. Assessing health-related quality of life in chronically ill children with the german KINDL: first psychometric and content analytical results. Qual Life Res 1998;7:399–407. 10.1023/a:1008853819715 [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization . n.d. COVID-19 clinical management: living guidance. [PubMed]
- 45.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. The Lancet Infectious Diseases 2020;20:e192–7. 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-ncov) by real-time RT-PCR. Euro Surveill 2020;25. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465–9. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Krawczyk E, Suprynowicz FA, et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat Protoc 2017;12:439–51. 10.1038/nprot.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Ory V, Chapman S, et al. Rock inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. The American Journal of Pathology 2012;180:599–607. 10.1016/j.ajpath.2011.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chapman S, Liu X, Meyers C, et al. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest 2010;120:2619–26. 10.1172/JCI42297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gentzsch M, Boyles SE, Cheluvaraju C, et al. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 2017;56:568–74. 10.1165/rcmb.2016-0276MA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salomon JJ, Albrecht T, Graeber SY, et al. Chronic rhinosinusitis with nasal polyps is associated with impaired TMEM16A-mediated epithelial chloride secretion. Journal of Allergy and Clinical Immunology 2021;147:2191–2201. 10.1016/j.jaci.2021.02.008 [DOI] [PubMed] [Google Scholar]
- 53.Schulze J, Mache C, Balázs A, et al. Analysis of severe acute respiratory syndrome 2 replication in explant cultures of the human upper respiratory tract reveals broad tissue tropism of wild-type and B.1.1.7 variant viruses. J Infect Dis 2021;224:2020–4.:jiab523. 10.1093/infdis/jiab523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinovich KM, Iosifidis T, Buckley AG, et al. Conditionally reprogrammed primary airway epithelial cells maintain morphology, lineage and disease specific functional characteristics. Sci Rep 2017;7:17971. 10.1038/s41598-017-17952-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bove PF, Dang H, Cheluvaraju C, et al. Breaking the in vitro alveolar type II cell proliferation barrier while retaining ion transport properties. Am J Respir Cell Mol Biol 2014;50:767–76. 10.1165/rcmb.2013-0071OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mall MA, Button B, Johannesson B, et al. Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with βenac-overexpressing mice. Journal of Biological Chemistry 2010;285:26945–55. 10.1074/jbc.M110.151803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Wu Y, Rong L. Conditionally reprogrammed human normal airway epithelial cells at ali: a physiological model for emerging viruses. Virol Sin 2020;35:280–9. 10.1007/s12250-020-00244-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duerr J, Leitz DHW, Szczygiel M, et al. Conditional deletion of nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat Commun 2020;11:2012. 10.1038/s41467-020-15743-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Messner CB, Demichev V, Wendisch D, et al. Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection. Cell Syst 2020;11:11–24.:S2405-4712(20)30197-6. 10.1016/j.cels.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demichev V, Messner CB, Vernardis SI, et al. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat Methods 2020;17:41–4. 10.1038/s41592-019-0638-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sungnak W, Huang N, Bécavin C, et al. SARS-cov-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26:681–7. 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol 2020;38:970–9. 10.1038/s41587-020-0602-4 [DOI] [PubMed] [Google Scholar]
- 63.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020;383:2255–73. 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kommoss FKF, Schwab C, Tavernar L, et al. The pathology of severe COVID-19-related lung damage. Dtsch Arztebl Int 2020;117:500–6.:arztebl.2020.0500. 10.3238/arztebl.2020.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell WB. Thromboinflammation in COVID-19 acute lung injury. Paediatr Respir Rev 2020;35:20–4. 10.1016/j.prrv.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stahl M, Joachim C, Blessing K, et al. Multiple breath washout is feasible in the clinical setting and detects abnormal lung function in infants and young children with cystic fibrosis. Respiration 2014;87:357–63. 10.1159/000357075 [DOI] [PubMed] [Google Scholar]
- 67.Stahl M, Joachim C, Kirsch I, et al. Multicentre feasibility of multiple-breath washout in preschool children with cystic fibrosis and other lung diseases. ERJ Open Res 2020;6:00408-2020. 10.1183/23120541.00408-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beydon N, Davis SD, Lombardi E, et al. An official american thoracic society/european respiratory society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 2007;175:1304–45. 10.1164/rccm.200605-642ST [DOI] [PubMed] [Google Scholar]
- 69.Saunders C, Jensen R, Robinson PD, et al. Integrating the multiple breath washout test into international multicentre trials. Journal of Cystic Fibrosis 2020;19:602–7. 10.1016/j.jcf.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 70.Wilkinson MD, Dumontier M, Aalbersberg IJJ, et al. The fair guiding principles for scientific data management and stewardship. Sci Data 2016;3:160018. 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 2020;39:355–68. 10.1097/INF.0000000000002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stephenson E, Reynolds G, Botting RA, et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med 2021;27:904–16. 10.1038/s41591-021-01329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernardes JP, Mishra N, Tran F, et al. Longitudinal multi-omics analyses identify responses of megakaryocytes, erythroid cells, and plasmablasts as hallmarks of severe COVID-19. Immunity 2020;53:1296–314. 10.1016/j.immuni.2020.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trump S, Lukassen S, Anker MS, et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol 2021;39:705–16. 10.1038/s41587-020-00796-1 [DOI] [PubMed] [Google Scholar]
- 75.Stukalov A, Girault V, Grass V, et al. Multilevel proteomics reveals host perturbations by SARS-cov-2 and SARS-cov. Nature 2021;594:246–52. 10.1038/s41586-021-03493-4 [DOI] [PubMed] [Google Scholar]
- 76.Loske J, Röhmel J, Lukassen S, et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-cov-2 infection in children. Nat Biotechnol 2022;40:319–24. 10.1038/s41587-021-01037-9 [DOI] [PubMed] [Google Scholar]
- 77.Yoshida M, Worlock KB, Huang N, et al. Local and systemic responses to SARS-cov-2 infection in children and adults. Nature 2022;602:321–7. 10.1038/s41586-021-04345-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang C, Li X, Ning W, et al. Multi-Omic profiling of plasma reveals molecular alterations in children with COVID-19. Theranostics 2021;11:8008–26. 10.7150/thno.61832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pierce CA, Preston-Hurlburt P, Dai Y, et al. Immune responses to SARS-cov-2 infection in hospitalized pediatric and adult patients. Sci Transl Med 2020;12:564.:eabd5487. 10.1126/scitranslmed.abd5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen CA, Li APY, Hachim A, et al. SARS-cov-2 specific t cell responses are lower in children and increase with age and time after infection. Nat Commun 2021;12:4678. 10.1038/s41467-021-24938-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-cov-2 infection among children and adolescents compared with adults: A systematic review and meta-analysis. JAMA Pediatr 2021;175:143–56. 10.1001/jamapediatrics.2020.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-cov-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Z, Guo L, Huang L, et al. Distinct disease severity between children and older adults with coronavirus disease 2019 (COVID-19): impacts of ACE2 expression, distribution, and lung progenitor cells. Clin Infect Dis 2021;73:e4154–65. 10.1093/cid/ciaa1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yonker LM, Neilan AM, Bartsch Y, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-cov-2): clinical presentation, infectivity, and immune responses. The Journal of Pediatrics 2020;227:45–52. 10.1016/j.jpeds.2020.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koch CM, Prigge AD, Anekalla KR, et al. Immune response to SARS-cov-2 in the nasal mucosa in children and adults. Infectious Diseases (except HIV/AIDS) [Preprint]. 10.1101/2021.01.26.21250269 [DOI]
- 86.Felsenstein S, Hedrich CM. SARS-cov-2 infections in children and young people. Clinical Immunology 2020;220:108588. 10.1016/j.clim.2020.108588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee CH, Pinho MP, Buckley PR, et al. Potential CD8+ T cell cross-reactivity against SARS-cov-2 conferred by other coronavirus strains. Front Immunol 2020;11:579480. 10.3389/fimmu.2020.579480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fraley E, LeMaster C, Banerjee D, et al. Cross-Reactive antibody immunity against SARS-cov-2 in children and adults. Cell Mol Immunol 2021;18:1826–8. 10.1038/s41423-021-00700-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Massalska MA, Gober H-J. How children are protected from covid-19? a historical, clinical, and pathophysiological approach to address covid-19 susceptibility. Front Immunol 2021;12:646894. 10.3389/fimmu.2021.646894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pierce CA, Sy S, Galen B, et al. Natural mucosal barriers and COVID-19 in children. JCI Insight 2021;6:e148694. 10.1172/jci.insight.148694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozsurekci Y, Aykac K, Er AG, et al. Predictive value of cytokine/chemokine responses for the disease severity and management in children and adult cases with COVID-19. J Med Virol 2021;93:2828–37. 10.1002/jmv.26683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jia R, Wang X, Liu P, et al. Mild cytokine elevation, moderate CD4+ T cell response and abundant antibody production in children with COVID-19. Virol Sin 2020;35:734–43. 10.1007/s12250-020-00265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneider JG, Relich RF, Datta D, et al. Identifying risk factors that distinguish symptomatic severe acute respiratory syndrome coronavirus 2 infection from common upper respiratory infections in children. Cureus 2021;13:e13266. 10.7759/cureus.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Q, Xing Y, Shi L, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics 2020;146. 10.1542/peds.2020-0961 [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Wang H, Wang F, et al. Co-Infections of SARS-cov-2 with multiple common respiratory pathogens in infected children. Medicine (Baltimore) 2021;100:e24315. 10.1097/MD.0000000000024315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065221supp001.pdf (87.8KB, pdf)