Abstract
We conducted a prospective study of adult allogeneic hematopoietic cell transplantation (HCT) recipients to assess pre- and post-HCT physical function. Baseline measurements included a wrist actigraphy, a 6 min walk test (6MWT), an international physical activity questionnaire (IPAQ), and a Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) as well as serial post-HCT assessments of 6MWT, IPAQ, and FACT-BMT. Forty-seven patients were evaluable for functionality assessments, with a median follow-up of 54.5 months for surviving recipients. No patients demonstrated vigorous or very vigorous activity at any time during monitoring by wrist actigraphy; patients spent a median of 6 h daily sedentary. Self-reported activity via the IPAQ showed 36%, 43%, and 21% of subjects reporting light, moderate, and vigorous activity prior to HCT, respectively. Post-HCT 6MWTs on day +30 demonstrated the greatest association with subsequent survival and non-relapse mortality. A decline in 6MWT distance over time also demonstrated worsened overall survival. This study shows the feasibility of fitness assessments and the ability to risk stratify for subsequent mortality, particularly using the 6MWT on the day +30 single time point assessment and change scores from baseline to day +30 post HCT. These pilot findings suggest important targets for future study.
INTRODUCTION
Candidate selection for hematopoietic stem cell transplant (HCT) entails determination of patient tolerability to ascertain risk of treatment-related complications, including comorbidity burden, performance status, and chronological age [1–4]. However, current paradigms can miss an underlying frailties, such as decreased functionality [5]. Poor functionality has also been correlated with worse outcomes in cancer patients, particularly HCT recipients with decreased exercise tolerance and physical function [6, 7].
While functionality assessments for patient risk stratification are customary in other medical disciplines [8, 9], parameters that predict for transplant morbidity and mortality remain unclear [10, 11]. Self-reported physical function declines early after transplantation [12–14], with full physical recovery often protracted [13, 15, 16] and is associated with increased medical risk [17]. Although a multicenter exercise training intervention during transplant had no impact on survival, the impact of patienttailored interventions based on pre-HCT baseline activity may impact patient functionality and outcomes [18]. Metrics to identify patients with diminished functional capacity may better prognosticate post-HCT outcomes and inform physician interventions to mitigate excess risk.
Given the limited data evaluating direct and indirect functional assessments in HCT patients, we conducted a prospective pilot study to assess pre- and post-HCT physical function using wrist actigraphy, a 6 min walk test (6MWT), and self-reported activity and quality-of-life (QOL) surveys. The primary objective of the trial was to assess the relationship between pre-HCT physical function measures and post-HCT overall survival (OS). Secondary aims included an assessment of physical function trajectory over time post-HCT and the relationship of this change with post-HCT survival.
MATERIALS/SUBJECTS AND METHODS
Population cohort
Eligible patients included independently mobile adults ≥18 years who received allogeneic HCT at Moffitt Cancer Center (Tampa, FL, USA). Potential patients were identified by treating physicians, who also determined suitability for HCT as per institutional eligibility criteria. No additional restrictions were imposed by this protocol regarding patient age, disease remission status, performance status, comorbidities, or physician-perceived frailty. All HCT donor types, graft sources, malignancy types, and planned conditioning intensity were permitted. Patients having received intravenous chemotherapy or radiotherapy within 2 weeks prior to entering the study were deemed ineligible and thus no patients were on active treatment at time of baseline study testing. All patients underwent transplantation as inpatients and received routine standard of care post HCT, including inpatient physical therapy and post-discharge physical therapy as required; patients did not receive any additional exercise intervention regimen. IRB (PRO 00014631) approval was obtained to conduct this study.
Research procedures
After providing informed consent, enrolled subjects completed baseline research procedures within 30 days before the HCT hospital admission date. This included assessments of fitness (wrist actigraphy, 6MWT), the International Physical Activity Questionnaire (IPAQ) [19], and patient-reported QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplantation (FACT-BMT) [20]. Post-HCT study measures included 6MWT, IPAQ, and FACT-BMT assessed at days 30, 90, and 180, respectively. Analyses were based on all available data for each measure/time point.
Wrist actigraphy
Activity was monitored using the triaxial accelerometer ActiGraph GT3X unit (ActiGraph, Pensacola, FL, USA). Monitors were placed on the patients’ nondominant wrist and worn continuously for 7 days (completed by the time of HCT admission). Using Actigraph-associated software, metabolic equivalent of task units (METs) per hour based on individual energy expenditure were calculated stratifying patients into previously validated sedentary/light (<3.0 METs), moderate (≥3.0 METs and <6.0 METs), and vigorous physical activity intensities (≥6.0 METs) [21–23].
6 min walk test (6MWT)
The 6MWT was used to objectively measure aerobic fitness [24, 25], given the accuracy of the test to estimate peak oxygen uptake [26] among HCT candidates [10]. The 6MWT was administered in the outpatient setting [27]. Predicted normative values for 6MWT were calculated using validated formulas after normalization by height and weight [28].
IPAQ questionnaire
The IPAQ short form has been validated in cancer patients and can be used to assess their perceived activity over a 7-day period [19, 29, 30]. Estimation of self-reported METs was categorized into low, moderate, and high activity groups corresponding to sedentary/light intensity (<3.3 METs), moderate intensity (≥3.3 METs and <8.0 METs), and vigorous intensity (≥8.0 METs) [19].
FACT-BMT questionnaire
FACT-BMT version 4.0 is a 37-item self-report questionnaire, which includes a 10-item bone marrow transplant subscale. The QOL instrument is used to score overall QOL and evaluate individual domains, including physical, functional, social/family, emotional well-being, and a BMT-specific domain score [20].
Statistical methods
The planned enrollment in this pilot study was based on feasibility and intent to generate early signals for subsequent adequately powered trials. Feasibility was defined as completion of protocol testing at baseline and serially after HCT. Descriptive statistics were used to summarize the patient population. Physical activity parameters were evaluated both as a continuous and discrete variable when applicable. The correlation of baseline functional measures was studied (Spearman correlation coefficient). OS was estimated from the time of HCT and from the individual studied time points to assess the effect of post-HCT function on survival; surviving patients were censored at last follow-up using the Kaplan–Meier method. The log-rank test compared the OS distribution of ≥2 groups in each variable. Non-relapse mortality (NRM) was plotted using malignancy relapse as a competing risk event per the Fine and Gray method. The Gray method was used to compare NRM between ≥2 groups. The analysis of variance test, or its nonparametric alternatives, was used for secondary analyses of the physical fitness variables, with a composite endpoint that measured the trend of QOL scores over time.
RESULTS
Patient characteristics
Between November 2013 and April 2015, a total of 50 patients were enrolled. Three were excluded from the study and subsequent analyses due to incomplete baseline testing (n = 1) or ineligibility for HCT after signing consent (n = 2). Baseline demographics of the 47 evaluable subjects are detailed in Table 1 with median time between baseline assessment and admission for HCT being 16 days. The median age of the cohort was 60 years (range, 24–75 years), with the majority ≥40 years (n = 38 [81%]). The cohort contained more male patients (n = 31 [66%]), the majority of participants had a good performance status with Karnofsky performance score (KPS) > 80 (n = 42 [89%]), and normal lung function based on FEV1 > 80% (n = 37 [79%]). However, some patients (n = 19, 40%) had a high comorbidity burden based on HCT-CI scores ≥3. Conditioning regimens were evenly distributed among myeloablative and nonmyeloablative, with 40% of patients having a high or very-high HCT-specific Disease Risk Index (DRI) [31], and 85% of patients (n = 40) having received an 8/8 HLA-matched HCT. The median follow-up for surviving patients was 54.5 months (range, 26.3–59.7 months) and 25.7 months for all patients (range, 0.6, 59.7 months).
Table 1.
Baseline pre-HCT characteristics of evaluable subjects.
| Variables | N = 47 | % |
|---|---|---|
| Age, y | ||
| <40 | 9 | 19.1 |
| 40–64 | 28 | 59.6 |
| ≥65 | 10 | 21.3 |
| Sex | ||
| Female | 16 | 34 |
| Male | 31 | 66 |
| Primary diagnosis | ||
| Acute lymphoblastic leukemia | 5 | 10.6 |
| Acute myeloid leukemia | 11 | 23.4 |
| Chronic lymphocytic leukemia | 3 | 6.4 |
| Chronic myelogenous leukemia | 2 | 4.3 |
| Hodgkin’s disease | 3 | 6.4 |
| Myelodysplastic syndrome | 9 | 19.1 |
| Multiple myeloma | 3 | 6.4 |
| Myeloproliferative syndromes | 2 | 4.3 |
| Non-Hodgkin lymphoma | 9 | 19.1 |
| Disease Risk Index (DRI) | ||
| Low | 3 | 6.4 |
| Intermediate | 25 | 53.2 |
| High/very high | 19 | 40.4 |
| Conditioning intensity | ||
| Myeloablative | 23 | 48.9 |
| Reduced intensity/nonmyeloablative | 24 | 51.1 |
| KPS | ||
| ≤80 | 5 | 10.6 |
| >80 | 42 | 89.4 |
| FEV1 | ||
| ≤80 | 10 | 21.3 |
| >80 | 37 | 78.7 |
| DLCO | ||
| >80% | 6 | 12.8 |
| 66%−80% | 27 | 57.4 |
| ≤65% | 14 | 29.8 |
| Hematopoietic Cell Transplantation-Specific Comorbidity Index (HCT-CI) | ||
| 0 | 3 | 6.4 |
| 1–2 | 25 | 53.2 |
| ≥3 | 19 | 40.4 |
| Match grade | ||
| 8/8 | 40 | 85.1 |
| Other | 7 | 14.9 |
| Baseline Body Mass Index | ||
| <30 | 38 | 80.9 |
| ≥30 | 9 | 19.1 |
Pre- and post-HCT assessments of physical function
Actigraphy.
Two patients wore actigraph monitors for <24 h and were excluded from the actigraph analyses. Remainder of the patients (n = 45, 96%) tolerated ActiGraph monitoring, with a median wear time of 165.4 h (range, 104.6–168 h). None of the patients demonstrated vigorous or very vigorous activity at any time during monitoring. The majority of awake hours per day were spent in light activity (median 82% [range, 69–92%]), and the remainder of awake hours were spent in moderate activity (median 18% [range, 8–31%]). The median MET rate for all patients was 2.3 (range, 1.7–3.2), which is equivalent to physical activities such as mild stretching, dusting, or polishing furniture, and food shopping [32]. In addition, the median daily awake hours spent sedentary was 6 h (range, 3.4–12 h).
IPAQ.
All evaluable patients (n = 47) completed the baseline IPAQ questionnaire, and 28 patients (60%) completed the IPAQ questionnaire at all study time points. At baseline, 36% (n = 17) of all patients self-reported being the following activity groups: light activity, 43% (n = 20) moderate activity, and 21% (n = 10) vigorous activity, with a median time spent sitting (sedentary) of 6 h (range, 2–15 h). At follow-up of evaluable patients on day 30, only one subject self-reported vigorous activity; the majority self-reported light (n = 23 [59%]) and moderate activity (n = 15 [38%]). Activity levels improved at days 90 and 180 posttransplant as a whole. At day 90, light, moderate, and vigorous activities were self-reported by 31% (n = 11), 50% (n = 18), and 19% (n = 7) of patients, respectively. At day 180, 26% (n = 8), 35% (n = 11), and 39% (n = 12) self-reported light, moderate, and vigorous activity, respectively.
6 min walk test (6MWT).
At baseline, all 47 evaluable patients completed the baseline 6MWT. Only 21 (45%) completed the 6MWT at all study time points with the lowest participation rate at the day 30 assessment (57%, N = 27). The mean distance walked was 431, 400, 408, and 442 m at baseline and on days 30, 90, and 180, respectively. At all-time points, the mean distance walked during testing was lower than predicted normative values for healthy adults (Fig. 1) [28].
Fig. 1. Observed and Predicted 6-min walk test.

Baseline and serial post-HCT 6-min walk test results for the study population stratified by gender are shown below. At each time point, HCT recipients had a lower observed 6-min walk test results than what would have been predicted for each gender.
Association of physical function measures and mortality
Pre-HCT physical function.
OS and NRM for the cohort at 2 years were 53% (95% CI, 41%–70%) and 32% (95% CI, 19%–45%), respectively. Cumulative incidence of relapse at 2 years was 28% (95% CI, 16–41%). The cumulative incidence of acute graft-versushost disease (aGVHD) by days 30 and 100 for the cohort was 42.6% and 57.4%, respectively. Actigraphy-based measures were not associated with OS or NRM, when examined by METs (continuous and categorical) or daily average sedentary bouts (P = not significant [NS] for all; Supplementary Figs. 1a, b and 2a, b).Pre-HCT activity by IPAQ did not show a significant association with post-HCT OS or NRM (P = NS for all; Supplementary Fig. 3a,b). Baseline pre-HCT 6MWT levels demonstrated no difference in survival, however, there was a trend for inferior survival for those with baseline 6MWT levels below the median value (Fig. 2).
Fig. 2. Overall survival according to 6 min walk test values at baseline and serial time points post-hematopoietic stem cell transplant.

The starting point for survival estimation for each plot is the time point of the 6 min walk test (6MWT) assessment (i.e., survival estimation starts at baseline, days 30, 90, and 180 reflecting the respective post-hematopoietic stem cell transplant (HCT) 6MWT assessment time point). At-risk population includes patients who completed the testing at the individual time point (i.e., 47 patients completed the 6MWT at baseline, 27 subjects completed the 6MWT at day 30 post HCT).
Post-HCT physical function.
To better understand the impact of change in functionality after transplantation, we evaluated change from baseline function at the different study time points. Among serial post-HCT measures of physical function by the 6MWT, day 30 had the greatest association with subsequent survival (Fig. 2) and NRM (Fig. 3). Furthermore, larger declines in 6MWT distances over time, in particular on day 30, demonstrated an association with worsened OS (Fig. 4).
Fig. 3. None-relapse mortality (NRM) according to distance walked on 6MWT at baseline and serial post-HCT assessments.
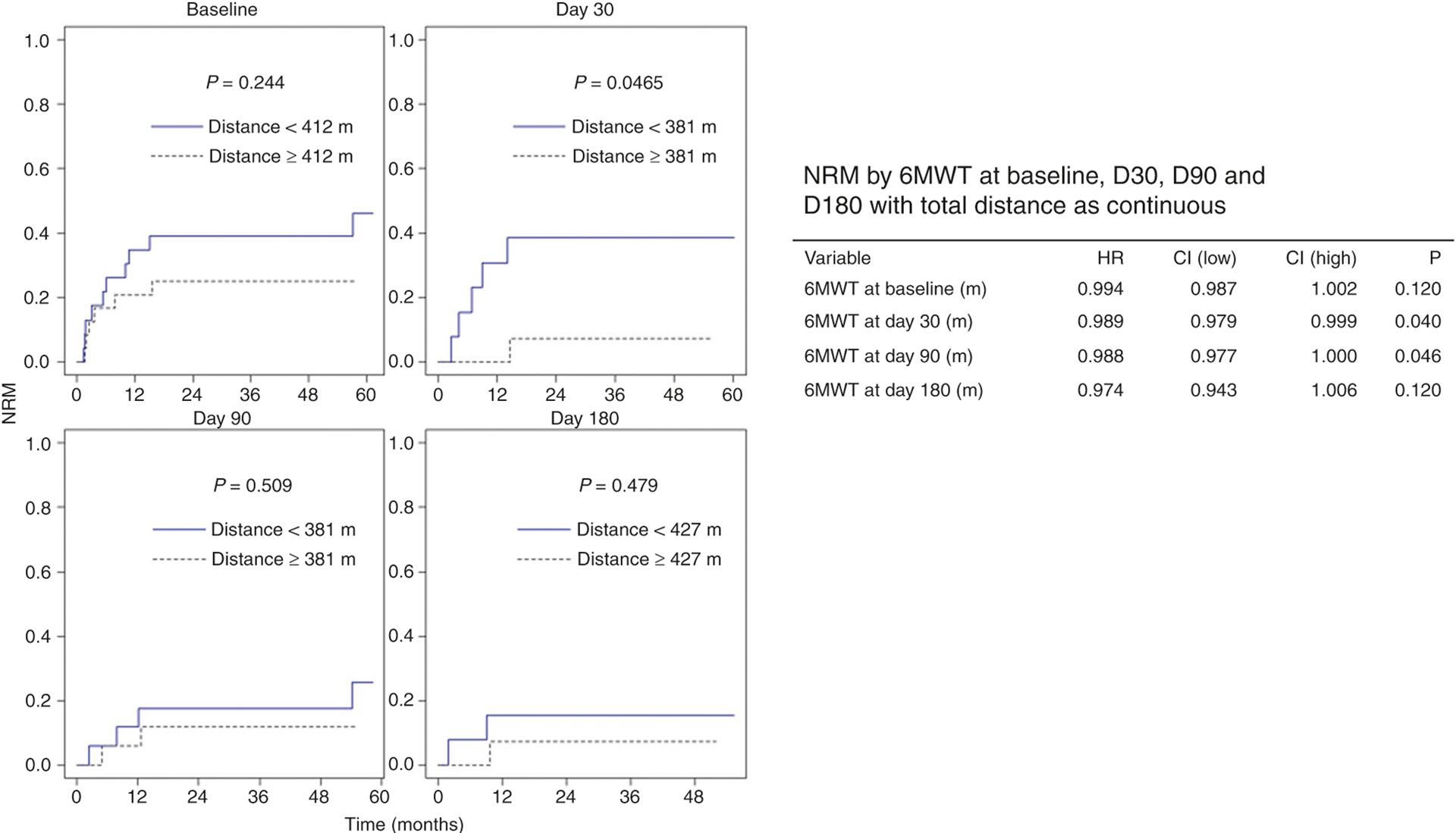
The starting point for survival estimation for each plot is the time point of the 6 min walk test (6MWT) assessment (i.e., survival estimation starts at baseline, days 30, 90, and 180 reflecting the respective post-hematopoietic stem cell transplant (HCT) 6MWT assessment time point). At-risk population includes patients who completed the testing at the individual time point. NRM based on dichotomized medians at each study time point for the 6MWT as well as a continuous value of total distance walked is shown.
Fig. 4. Overall survival according to change in 6 min walk test from baseline to serial post-hematopoietic stem cell transplant time points.

Median values were used to dichotomize the score change. The median of change in the 6 min walk test (6MWT) distance from baseline to day 30 was 51.2 m and from baseline to day 90 was 33.8 m. The at-risk population included patients who completed the testing at the time points shown. Twenty-seven patients completed the 6MWT at baseline and day 30 post-hematopoietic stem cell transplant. Seventeen subjects completed the 6MWT at baseline and at day 90 post HCT).
Exploratory studies: hospital stay, self-reported post-HCT function (IPAQ), and QOL
Pretransplant activity, as measured by actigraphy, the 6MWT, and IPAQ at baseline, was not associated with duration of transplant hospital stay (P = NS). Objective activity measurements and sedentary time, captured by actigraphy, were not correlated with activity as indicated by the self-reported IPAQ questionnaire at baseline or at any follow-up time points. At baseline, 21% and 43% of patients underwent vigorous or moderate activity, respectively, as indicated by the IPAQ questionnaire. By contrast, when comparing physical activity by objective actigraphy monitoring, no patients demonstrated participation in vigorous activity, and only three subjects demonstrated moderate activity by actigraphy monitoring. Actigraphy was also not correlated with the 6MWT (r = 0.19; P = NS). METs and sedentary time, measured by actigraphy, were not associated with QOL (FACT-BMT score) at baseline or at follow-up QOL assessment (P = NS). Similarly, the 6MWT and IPAQ were not associated with QOL (FACT-BMT score) at baseline or at serial time points (P = NS).
DISCUSSION
Herein we describe the physical functionality in a pilot a cohort of HCT recipients prior to and after HCT by both subjective and objective measurements. This study illustrates several important findings: (1) physical function assessment in this population is feasible pre-HCT but more challenging after HCT; (2) HCT recipients demonstrate limited vigorous activity pre-HCT; (3) objective and self-reported physical function assessments have limited correlation; (4) HCT recipients overestimate their physical functionality when compared to objective measurements; (5) METs or sedentary time pre-HCT may not adequately predict risk for subsequent mortality, morbidity, or QOL, and that (6) serial real-time objective measures of physical function by 6MWT appear to stratify risk for subsequent mortality, in particular both day 30 single time point assessments and change scores from baseline to day 30 post HCT. These pilot findings bring new insight to the field and suggest important targets for future studies.
The time period for baseline testing was selected to optimize recovery from prior chemotherapy treatment and reflect functional capacity immediately before initiating conditioning chemotherapy for HCT. Causes for screen failures for potentially eligible patients (n = 112) included patient preference (n = 18, 16%), inability to complete testing prior to HCT admission (n = 27, 24%), and disease concerns or donor unavailability (n = 17, 15%). Patients enrolled were in general assessed to have high KPS by their HCT physician. The majority of evaluable patients completed both pre- and post-HCT assessments. However, serial assessments were not completed by all evaluable patients, with surveys being more readily completed in comparison to 6MWTs (Supplementary Fig. S4). We sought to identify whether pre-HCT factors were indicative of completing the assessment, and we found no difference in age, gender, diagnosis, DRI, HCT-CI, KPS, FEV1, or match grade among patients who completed each testing modality evaluated. Completing direct functionality measurements may be a surrogate for survival and for identifying patients who require intensive rehabilitation to improve outcomes. OS and NRM differed among patients who completed the 6MWT and those who did not, with patients who completed testing having better survival (P = 0.0093 and P = 0.0045, respectively). We found similar findings among patients who completed the IPAQ and FACT-BMT. To understand limiting factors for completion, we also evaluated aGVHD as a potential complication that would result in decreased functionality and death. We found no difference in cumulative incidence of aGVHD in patients who completed the 6MWT, IPAQ, or FACT compared to those who did not complete the test (P = NS) (Supplementary Fig. S5a–c).
Our data highlight the diminished functional capacity of HCT recipients at baseline. The median MET rate reflected activities equivalent to mild stretching, dusting, or polishing furniture, and food shopping [32]. In addition, median hours spent in sedentary behavior was 6 h daily but ranged up to 12 h. In keeping with these actigraphy data, both the baseline and subsequent post-HCT 6MWT data showed impairments in objective physical function compared to predicted values [28]. The actual 6MWT performances among the study subjects were less than the average walking distance of healthy 70- to 80-year-old individuals [33]. Rather, the performance was in line with those with serious medical conditions, such as chronic obstructive pulmonary disease or congestive heart failure [34]. When comparing to other cancer patients both on and off cancer directed treatment, 6MWT performance in HCT recipients in our cohort was substantially lower [35].
We also found differences between actual and perceived measurements of activity. On self-report, 64% of patients indicated participating in moderate and vigorous activities, but this did not correlate with objective measurement by actigraphy and 6MWT. When considering the slight variations of activity categorization of METs by actigraphy and IPAQ, actigraphy showed one patient to have an MET value of 3.1 (moderate activity), a value which would have been categorized as light activity by IPAQ. This patient self-reported light activity by IPAQ, whereas most patients reported higher than objectively measured activity levels. Overestimation of physical activity by IPAQ and inconsistency with accelerometer-measured physical activity have been reported [36, 37]. However, pre-HCT self-reported physical activity has also been reported to be predictive of survival after allograft [38, 39], suggesting that actigraphy and self-reported physical activity may measure different aspects of functionality. Although activity levels differed between the two measurements, a median of 6 h was reported for both self-reported and objective sedentary behaviors.
The primary objective of this study was to evaluate the association between pre-HCT physical functionality measures and subsequent mortality, with the goal of identifying predictive measures that could supplement currently available tools. Although no single baseline measure had significant association with survival, our data showed that both the single time point 6MWT (day 30) and changes in the 6MWT (in particular, changes from baseline to day 30) post-HCT had significant association with subsequent OS and NRM. In our cohort, patients with a decrease in 6MWT capabilities had worse OS over time. This finding is in line with prior reports of HCT recipients [40] and patients with chronic medical disease such as chronic obstructive pulmonary disease, in which similar changes in walk test distance can result in clinically noticeable differences in walking ability [34]. Interestingly, low usual gait speed, as seen in serial measurements of 6MWT in our cohort, has recently been incorporated into definitions of sarcopenia by expert consensus, highlighting the impact of such findings on clinical outcomes [41]. Similar to results from prior reports, these data demonstrate that detectable improvements in physical activity do not translate into an improved perception of QOL or correlate with self-reported physical activity [42]. However, our group previously demonstrated the predictive value of actigraphy-based sleep and activity measurements for post-transplantation recovery of QOL [43]. Collectively, the data speak to the different nuanced aspects of activity each of these individual tests measure.
We note several potential considerations for our study. First, the tests used in this pilot study may have been under-powered to fully detect the prognostic ability of measures for HCT mortality. Second, on the basis of cohort size, the tests may have been unable to adequately assess the effect of other patient, disease, and HCT variables on outcomes. Third, the ability to distinguish change in outcomes according to these factors may have been impacted by the limited variation in the observed physical function at baseline pre-HCT. Fourth, the limited functional reserve at the time of HCT, as demonstrated by our data, may in part explain prior large, multicenter efforts to improve patient outcomes with a self-directed exercise program after HCT [18]. Last, it may be that the degree of decline in function early post-HCT is indicative of mortality risk (as supported by our data). Although activity after HCT did return to baseline functionality for survivors in our cohort, modest decreases in activity were associated with worse survival. This association suggests that early interventions may be needed to minimize this important early post-HCT decline.
In summary, both self-reported and objective fitness activity measurements in HCT recipients are feasible before HCT and can be serially monitored. Although actigraphy monitoring was not evaluated serially in this pilot, we continue to believe, given the objective measurement over a prolonged time period, that it is useful for identifying at-risk patients. Our data suggest that the 6MWT can predict for mortality after HCT and for risk stratifying patients beyond currently known paradigms. We aim to validate these results in a larger group of patients to evaluate the impact of functionality, accounting for transplant-specific factors.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank all the patients and their families as well as all physicians, nurses, medical providers, and data managers for their contribution to this study. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Bone Marrow Transplantation. This work has been supported in part by the Biostatistics and Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). Editorial assistance was provided by the Moffitt Cancer Center’s Office of Scientific Writing by Dr Paul Fletcher & Daley Drucker. No compensation was given beyond their regular salaries.
FUNDING
We acknowledge the following funding support: H. Lee Moffitt Cancer Center BMT-CI Foundation Grant (to AM), Moffitt Cancer Center Support Grant P30-CA076292.
COMPETING INTERESTS
AM receives grant funding from Novartis. BCB is a co-inventor for provisional patents related to the use of the CD83 CAR T cells (WO2019165156), JAK inhibitors (WO2017058950A1), and STAT3 inhibitors (WO2015120436A2) in GVHD prevention and treatment; BCB nor his institution(s) have received payment related to claims described in the patents. HF is on the advisory boards for Incyte and Jazz and speakers bureau for Sanofi. FLL is a scientific advisor for Kite/Gilead, Novartis, BMS/Celgene, Allogene, Wugen, Calibr, and GammaDelta Therapeutics, is a consultant for Cellular BioMedicine Group Inc., and has institutional patents for Survivin Dendritic Cell Vaccine; improving CAR T Cell Therapy. TN receives research support to the institution (not to the individual) from Novartis and Karyopharm. HJ consulted for RedHill BioPharma, Janssen Scientific Affairs, and Merck. The other authors declare no competing interests.
Footnotes
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41409-021-01428-1.
REFERENCES
- 1.Artz AS. From biology to clinical practice: aging and hematopoietic cell transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2012;18 1 Suppl:S40–5. 10.1016/j.bbmt.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–52. 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorror M, Storer B, Sandmaier BM, Maloney DG, Chauncey TR, Langston A, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 5.Muffly LS, Boulukos M, Swanson K, Kocherginsky M, Cerro PD, Schroeder L, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2013;19:429–34. 10.1016/j.bbmt.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31:876–85. 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 7.Wood WA, Le-Rademacher J, Syrjala KL, Jim H, Jacobsen PB, Knight JM, et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902). Cancer. 2016;122:91–98. 10.1002/cncr.29717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–500. [DOI] [PubMed] [Google Scholar]
- 9.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2009;120: e169–276. 10.1161/CIRCULATIONAHA.109.192690. [DOI] [PubMed] [Google Scholar]
- 10.Wood WA, Deal AM, Reeve BB, Abernethy AP, Basch E, Mitchell SA, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone Marrow Transplant. 2013;48:1342–9. 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 11.Wingard JR, Wood WA, Martens M, Le-Rademacher J, Logan B, Knight JM, et al. Pretransplantation exercise and hematopoietic cell transplantation survival: a secondary analysis of Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0902). Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2017;23:161–4. 10.1016/j.bbmt.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danaher EH, Ferrans C, Verlen E, Ravandi F, van Besien K, Gelms J, et al. Fatigue and physical activity in patients undergoing hematopoietic stem cell transplant. Oncol Nurs Forum. 2006;33:614–24. 10.1188/06.ONF.614-624. [DOI] [PubMed] [Google Scholar]
- 13.Bevans MF, Marden S, Leidy NK, Soeken K, Cusack G, Rivera P, et al. Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:101–9. 10.1038/sj.bmt.1705406. [DOI] [PubMed] [Google Scholar]
- 14.Altmaier EM, Ewell M, McQuellon R, Geller N, Carter SL, Henslee-Downey J, et al. The effect of unrelated donor marrow transplantation on health-related quality of life: a report of the unrelated donor marrow transplantation trial (T-cell depletion trial). Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2006;12:648–55. 10.1016/j.bbmt.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol: Off J Am Soc Clin Oncol. 2005;23:599–608. 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 16.Bush NE, Donaldson GW, Haberman MH, Dacanay R, Sullivan KM. Conditional and unconditional estimation of multidimensional quality of life after hematopoietic stem cell transplantation: a longitudinal follow-up of 415 patients. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2000;6:576–91. [DOI] [PubMed] [Google Scholar]
- 17.Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers ME, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–43. 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen PB, Le-Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2014;20:1530–6. 10.1016/j.bbmt.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. 10.1249/01.Mss.0000078924.61453.Fb. [DOI] [PubMed] [Google Scholar]
- 20.McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357–68. 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 21.Crouter SE, Kuffel E, Haas JD, Frongillo EA, Bassett DR Jr. Refined two-regression model for the ActiGraph accelerometer. Med Sci Sports Exerc. 2010;42:1029–37. 10.1249/MSS.0b013e3181c37458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. 2011;14:411–6. 10.1016/j.jsams.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81. [DOI] [PubMed] [Google Scholar]
- 24.Burr JF, Bredin SS, Faktor MD, Warburton DE. The 6-minute walk test as a predictor of objectively measured aerobic fitness in healthy working-aged adults. Phys Sportsmed. 2011;39:133–9. [DOI] [PubMed] [Google Scholar]
- 25.Solway SBD, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–70. [DOI] [PubMed] [Google Scholar]
- 26.Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31. 10.1186/1471-2466-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 28.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7. 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 29.Booth M Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71 2 Suppl:S114–20. [PubMed] [Google Scholar]
- 30.Johnson-Kozlow MSJ, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr., Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 33.Casanova C, Celli BR, Barria P, Casas A, Cote C, de Torres JP, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37:150–6. 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 34.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–82. 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631–6. 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 36.Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7. 10.1186/1479-5868-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerin E, Cain KL, Oyeyemi AL, Owen N, Conway TL, Cochrane T, et al. Correlates of agreement between accelerometry and self-reported physical activity. Med Sci Sports Exerc. 2016;48:1075–84. 10.1249/MSS.0000000000000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra A, Yue B, Kim J, Anasetti C, Pidala JA, Riches ML. et al. Physical activity as a predictor of outcomes in hematopoietic stem cell transplantation (HSCT) recipients. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33 15_Suppl:7027. 10.1200/jco.2015.33.15_suppl.7027. [DOI] [Google Scholar]
- 39.Jayani R, Pidala J, Jim H, Whiting J, Mo Q, MIshra A. Association of patient-reported physical activity on allogeneic hematopoietic cell transplant outcomes. Clin Hematol Int. 2021;3:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones LW, Devlin SM, Maloy MA, Wood WA, Tuohy S, Espiritu N, et al. Prognostic importance of pretransplant functional capacity after allogeneic hematopoietic cell transplantation. Oncologist. 2015;20:1290–7. 10.1634/theoncologist.2015-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriat Soc. 2020. 10.1111/jgs.16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knols RH, de Bruin ED, Uebelhart D, Aufdemkampe G, Schanz U, Stenner-Liewen F, et al. Effects of an outpatient physical exercise program on hematopoietic stem-cell transplantation recipients: a randomized clinical trial. Bone Marrow Transplant. 2011;46:1245–55. 10.1038/bmt.2010.288. [DOI] [PubMed] [Google Scholar]
- 43.Hoogland AI, Bulls HW, Gonzalez BD, Small BJ, Liu L, Pidala J, et al. Circadian rhythmicity as a predictor of quality of life in allogeneic hematopoietic cell transplant patients. J Pain Symptom Manag. 2019;57:952–60.e1. 10.1016/j.jpainsymman.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


