Abstract
Background:
Diagnoses of HIV in the United States decreased by 17% in 2020 due to COVID-related disruptions. The extent to which this decrease is attributable to changes in HIV testing versus HIV transmission is unclear. We seek to better understand this issue by analyzing the discrepancy in expected versus observed HIV diagnoses in 2020 among persons who acquired HIV between 2010 and 2019 because changes in diagnosis patterns in this cohort cannot be attributed to changes in transmission.
Methods:
We developed 3 methods based on the CD4-depletion model to estimate excess missed diagnoses in 2020 among persons with HIV (PWH) infected from 2010 to 2019. We stratified the results by transmission group, sex assigned at birth, race/ethnicity, and region to examine differences by group and confirm the reliability of our estimates. We performed similar analyses projecting diagnoses in 2019 among PWH infected from 2010 to 2018 to evaluate the accuracy of our methods against surveillance data.
Results:
There were approximately 3100–3300 (approximately 18%) fewer diagnoses than expected in 2020 among PWH infected from 2010 to 2019. Females (at birth), heterosexuals, persons who inject drugs, and Hispanic/Latino PWH missed diagnoses at higher levels than the overall population. Validation and stratification analyses confirmed the accuracy and reliability of our estimates.
Conclusions:
The substantial drop in number of previously infected PWH diagnosed in 2020 suggests that changes in testing played a substantial role in the observed decrease. Levels of missed diagnoses differed substantially across population subgroups. Increasing testing efforts and innovative strategies to reach undiagnosed PWH are needed to offset this diagnosis gap. These analyses may be used to inform future estimates of HIV transmission during the COVID-19 pandemic.
Keywords: missed diagnoses, impact of COVID-19, CD4 depletion model, HIV testing
INTRODUCTION
The outbreak of COVID-19 in 2020 has significantly disrupted the HIV continuum of care in the United States. Recent work has shown that COVID-19 and the associated responses led to reductions in pre-exposure prophylaxis uptake and adherence,1 antiretroviral therapy uptake and adherence,2 and HIV testing and care provision.3,4 It is therefore not surprising, given the reduction in testing, that HIV diagnoses in the United States decreased substantially in 2020, with official data suggesting a drop of approximately 17% as compared to 2019.5,6
However, it is unclear to what extent the drop in HIV diagnoses is attributable to disruptions in testing versus pandemic-related changes in transmission behaviors (for instance, fewer sexual partners) leading to reductions in transmission (incidence), resulting in fewer persons requiring testing and, in turn, diagnosis. Both effects have likely contributed; however, estimating their relative magnitudes is not straightforward. This lack of clarity has caused problems in HIV surveillance because most models used to estimate incidence are derived from diagnosis data.7–9 Uncertainty regarding the interpretation of 2020 diagnosis data has called many of the underlying assumptions in such models into question. For this reason, the Centers for Disease Control and Prevention (CDC) has not published official incidence estimates for 2020. Although the diagnosis data have been published, CDC has urged caution in the interpretation of these data.5,6
In the absence of an incidence estimate, modeling studies have attempted to quantify both present and future effects of COVID-19 on trends in HIV by running multiple simulations under varying assumptions on changes in transmission behaviors, testing, and care provision.10–12 Although such studies have value, projected long-term effects may vary substantially depending on which particular assumptions are used, limiting their utility. It is therefore critical to better understand the proximate causes of this drop in diagnoses to better understand its future implications.
In the last decade, CD4 measurements taken at diagnosis have been widely used to estimate year of HIV infection. Such measurements form the basis of modern incidence estimation methods.7–9,13,14 Research has shown show that, historically, 60%–70% of HIV infections are not diagnosed within the same calendar year of infection.8 In addition, each year, only 30%–35% of new HIV diagnoses result from infections within that same year.8 Thus, our goal is to estimate the discrepancy between expected and observed diagnoses in 2020 (referred to as excess missed diagnoses) among PWH infected in or before 2019. We use the terminology excess missed diagnoses because each year there are already many missed diagnoses, and hence, we are referring to additional missed diagnoses above the expected level.
METHODS
In this section, we provide a brief description of the 4 different methods we developed and the data used for estimating excess missed HIV diagnoses in 2020. The full mathematical details of all methods are provided in Appendix A (Supplemental Digital Content, http://links.lww.com/QAI/C4). We also describe our procedures for assessing the methods’ consistency and robustness to stratification, as well as their validation, with full details on these topics given in Appendices B and C, respectively.
Data from CDC’s National HIV Surveillance System (NHSS) were used for all analyses. Collection of these data, complete since 2009 and which are used to construct annual incidence estimates, provide the annual number of new HIV diagnoses for each infection year (as estimated by the CD4-depletion model).8,9,15–17
A minority, ranging from 10% to 15%, of new diagnoses do not have an associated CD4 measurement. To account for this limitation, we assumed that the infection-year distribution of new diagnoses without a CD4 measurement is identical to that with a CD4 measurement. This assumption is also used in incidence estimation.7–9
Some of the proposed methods require incidence data as an input. In such cases, we used incidence estimates published by CDC for years 2009–2019.15–17
The analyses in this article are restricted to the cohort of persons infected before 2020, as any observed drop in diagnoses in this group cannot be attributed to changes in incidence in 2020 because such persons were infected before the beginning of the COVID-19 pandemic. By removing uncertainty around incidence in 2020, we can provide a reliable estimate of the potentially large portion of PWH unaware of their status due to COVID-19. In addition, focusing on this population allows us to better quantify the effect of COVID-19-related testing disruption on HIV diagnosis in 2020.
Diagnosis-Based Method
For the diagnosis-based method, we used a 4-year log-normal linear regression model to project the number of HIV diagnoses in 2020 based on diagnosis trends in 2016–2019.18 The difference between the projected number and the observed number of diagnoses in 2020 is the number of excess missed diagnoses. We distributed these diagnoses by year of infection based on the distribution among the observed diagnoses in 2020.
To derive the infection-year distribution, each HIV case diagnosed in 2020 was weighed to account for those excess missed diagnoses in 2020. We calculated this weight as the projected number of diagnoses divided by the observed number of HIV diagnoses in 2020. We stratified HIV cases diagnosed in 2020 by year of HIV infection based on their first CD4 test after diagnosis. We derived the excess missed diagnoses by year of infection from the increased case counts based on weighted and unweighted numbers, where the unweighted case counts sum to the total observed number of HIV diagnoses in 2020 and the weighted case counts sum to the total projected number of HIV diagnoses in 2020.
We reduced the estimated number of excess missed diagnoses with infection in 2020 by 50%, and we redistributed them proportionally to those with infection years before 2020. This adjustment was motivated by the fact that infections that occurred before 2020 may conceivably be diagnosed at any point during 2020. However, infections that occurred during 2020 can only be diagnosed postinfection, reducing the available time to be diagnosed by 6 months on average.
Incidence-Based Methods
Our second approach for estimating excess missed diagnoses, the incidence-based methods, incorporates incidence data in addition to diagnosis data. Within this approach, we use 2 related, but distinct, methods for estimating the probability of diagnosis after k years and will refer to these 2 methods as the incidence-based, linear regression method and incidence-based, recent-year average method. Because of the similarity in the methods, we first describe the general framework common to each formulation below.
Incidence-based methods work by projecting diagnoses in a year y among PWH infected in a year x as follows:
Projected diagnoses in year y among PWH infected in year x = Number of PWH infected year x× Probability of diagnosis after (y–x) yrs.
The number of excess missed diagnoses in year y among those infected in x is as follows:
Excess missed diagnoses = projected diagnoses − observed diagnoses
For the total number of PWH infected in year x, we used CDC US HIV incidence estimates for year x from CDC’s NHSS.15–17
To estimate the probability of diagnosis y–x years after infection, we used both incidence and diagnosis data. For example, define the estimate of the probability of diagnosis in 2020 of PWH infected in 2017, or 3 years after infection, as
We first computed the probability of diagnosis after 3 years for PWH infected in the years preceding 2017 with NHSS data. For example,
From our estimates p3(2016), p3(2015), p3(2014), ..., we can fit a function , which allows us to extrapolate the expected probability of diagnosis in 2020 for PWH infected in 2017. Then
Expected diag. 2020, PWH inf. in 2017 = 2017 incidence estimate .
The difference between the expected and observed diagnoses among PWH infected in 2017 gave us the excess missed diagnoses in 2020 for 2017 infections. We repeated this process for infection years for which we had reliable data (beginning in 2010). Summing these results, we obtained our estimate for total excess missed diagnoses in 2020.
As with any function fitted to measured data, may be defined in different ways. In the current analysis, we considered 2 ways of defining , as described below. These 2 definitions result in distinct methods.
Incidence-Based Method: Linear Regression
In the linear regression formulation of the incidence-based method, we defined by applying least-squares linear fitting with surveillance data for , the probability of diagnosis after k years in a diagnosis year x. We note, however, that recent-year averages were used for , k ≥ 8 because of data limitations.
Incidence-Based Method: Recent-Year Average
For the recent-year average formulation of the incidence-based method, we defined as a constant function, as the average (arithmetic mean) probability over the past 3 years of diagnosis after k years, except when fewer than 3 years were available (in which case we used the number of available years).
Note that because of averages being used in the linear-regression approach for , k ≥ 8 (see above), the 2 incidence-based methods are identical in these cases.
Diagnosis Delay-Based Method
This method is structurally similar to the incidence-based methods because it also estimates the number of diagnoses in a year y among PWH infected in a year x as follows:
Projected diagnoses in year y among PWH infected in year x = Number of PWH infected year x× Probability of diagnosis after (y–x) yrs.
However, the diagnosis delay-based method is distinct in 2 key respects. First, instead of assuming known HIV incidence in years before 2020, we estimated the annual number of infections based on the observed CD4 data, but we limited the analysis to HIV cases diagnosed up to 2019 and we used a simplified estimation procedure (with case counts by calendar year). This method is the same as the one used to estimate HIV incidence at the national level.8,19
Second, the method of estimating the probability of diagnosis is different from the incidence-based methods. In the diagnosis delay-based method, we estimated this probability through a conditional probability analysis. We write the cumulative probability that an infection acquired in year 0 is diagnosed by year k as . We estimated for a given year N (in this case, N = 12, the longest possible period for which our data are complete) and then obtained through conditional probability:
Repeating the procedure
similarly for N–3, N–4, and so forth.
The are cumulative probabilities, which give the probability of diagnosis within k years of infection. However, we need the annual diagnosis probability—the probability of diagnosis exactly k years after infection, . This is obtained as follows:
Robustness to Stratification
To ensure confidence in our estimates on population subgroups, we performed a stratification robustness analysis. We refer to the sum of the different population stratifications as SeparateSum and the analysis performed on the entire population as TotalPopulation. For example, for sex assigned at birth, we have
We then evaluated robustness to stratification by comparing SeparateSum and TotalPopulation. If a method is consistent and robust to stratification, the 2 quantities should be similar. We quantified stratification robustness with 2 metrics: % total difference and % year-by-year difference, and provide their detailed mathematical definitions in Appendix B (Supplemental Digital Content, http://links.lww.com/QAI/C5).
2019 Validation Analysis
To validate of our developed methods, we performed an analysis against 2019 diagnosis data. The same NHSS diagnosis data and published incidence data as used in the excess missed diagnosis analyses were used; however, to derive our estimates, we used only diagnosis data of persons older than 13 years from the years 2010–2018. We then applied the diagnosis delay-based and both incidence-based methods to project expected diagnoses in 2019 for the infection years 2010–2018 (note that, for reasons described in Appendix C (Supplemental Digital Content, http://links.lww.com/QAI/C6), the diagnosis-based method was not considered in the validation analysis). We evaluated the quality of the estimates by comparing the projected and observed diagnoses in 2019 for each infection year.
We evaluated our results both for accuracy compared with observed data and robustness with stratification. We used several quantitative metrics in our analysis: %Error (total), %Error (year-by-year), %Difference (summed vs. total), and %Difference, summed vs. full population (year-by-year). Full definitions of these metrics are provided in Appendix C (Supplemental Digital Content, http://links.lww.com/QAI/C6).
RESULTS
Total Missed HIV Diagnoses in 2020
Our estimates of the total number of excess missed diagnoses in 2020 for PWH (aged 13 or older) infected from 2010 to 2019 ranged from 3154 to 3315 for our different estimation methods (Fig. 1), indicating that approximately 18% of the expected number of ~18,000 HIV diagnoses from previous infections (as our projection methods estimate) were missed in 2020. These estimates were consistent across all methods, with the differences between them 5% or less. In addition, the different methods showed similar results on infection-year distribution, with the correlation coefficients between the different methods 0.92 or higher.
FIGURE 1.
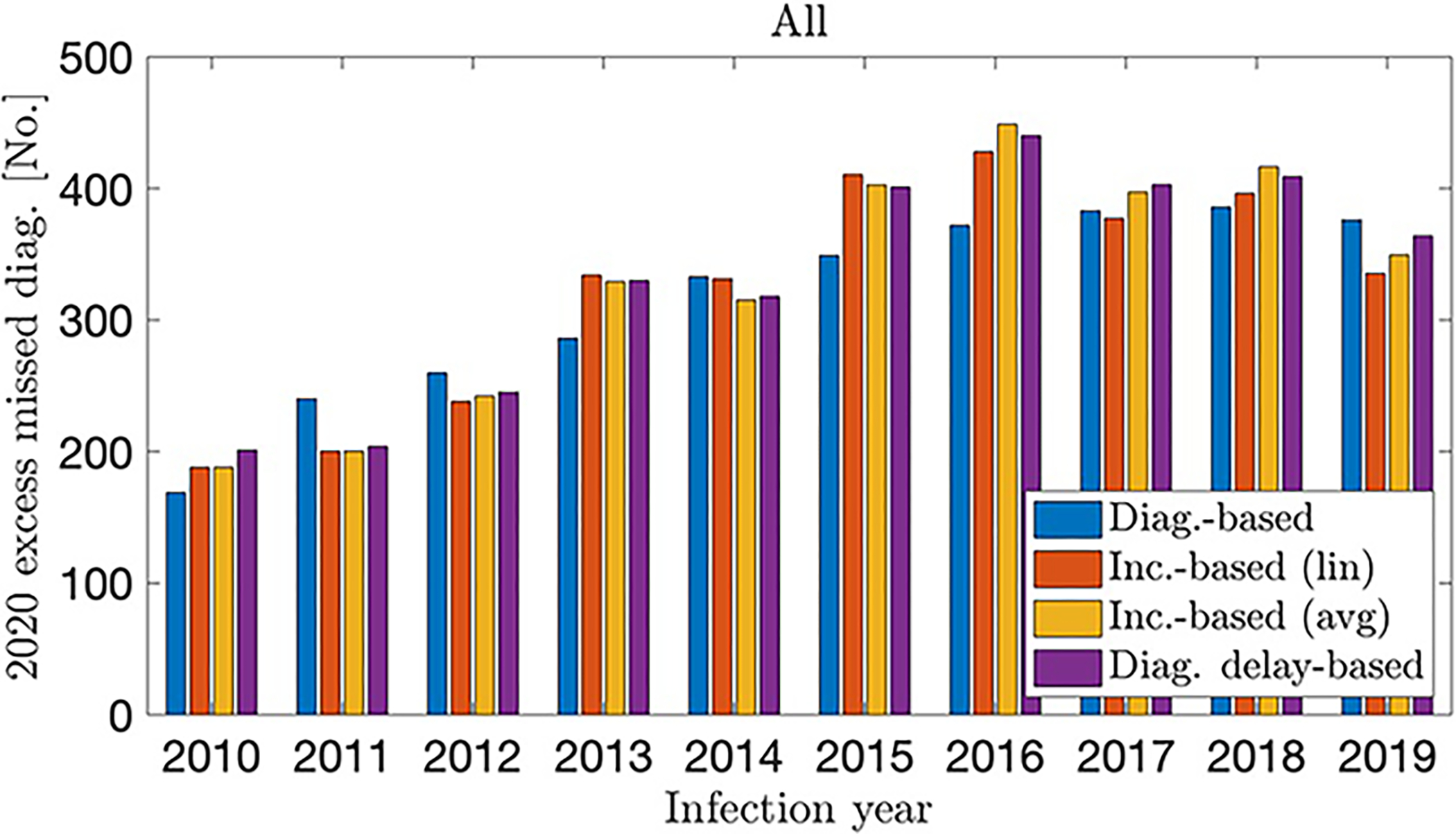
Excess missed HIV diagnoses in 2020, by infection year for each method, United States
Qualitatively, the methods showed some small differences. The diagnosis-based and incidence-based (linear fit) methods showed the highest concentrations of excess undiagnosed infections occurring in the years 2016–2019. The incidence-based (averaged) and diagnosis delay-based methods showed a comparatively less even infection-year distribution and estimated that the highest concentration of excess missed diagnoses in 2020 occurred among infection years 2015–2018.
Stratification by Population Subgroups
In Figure 2, we show the estimated excess missed diagnoses stratified by transmission group: men who have sex with men (MSM), heterosexuals (HET), persons who inject drugs (PWID), and men who have sex with men who also inject drugs (MSM/PWID). We estimated 1726–2034 excess missed diagnoses among MSM, 741–867 among heterosexuals, 263–446 among PWID, and 207–216 among MSM/PWID.
FIGURE 2.
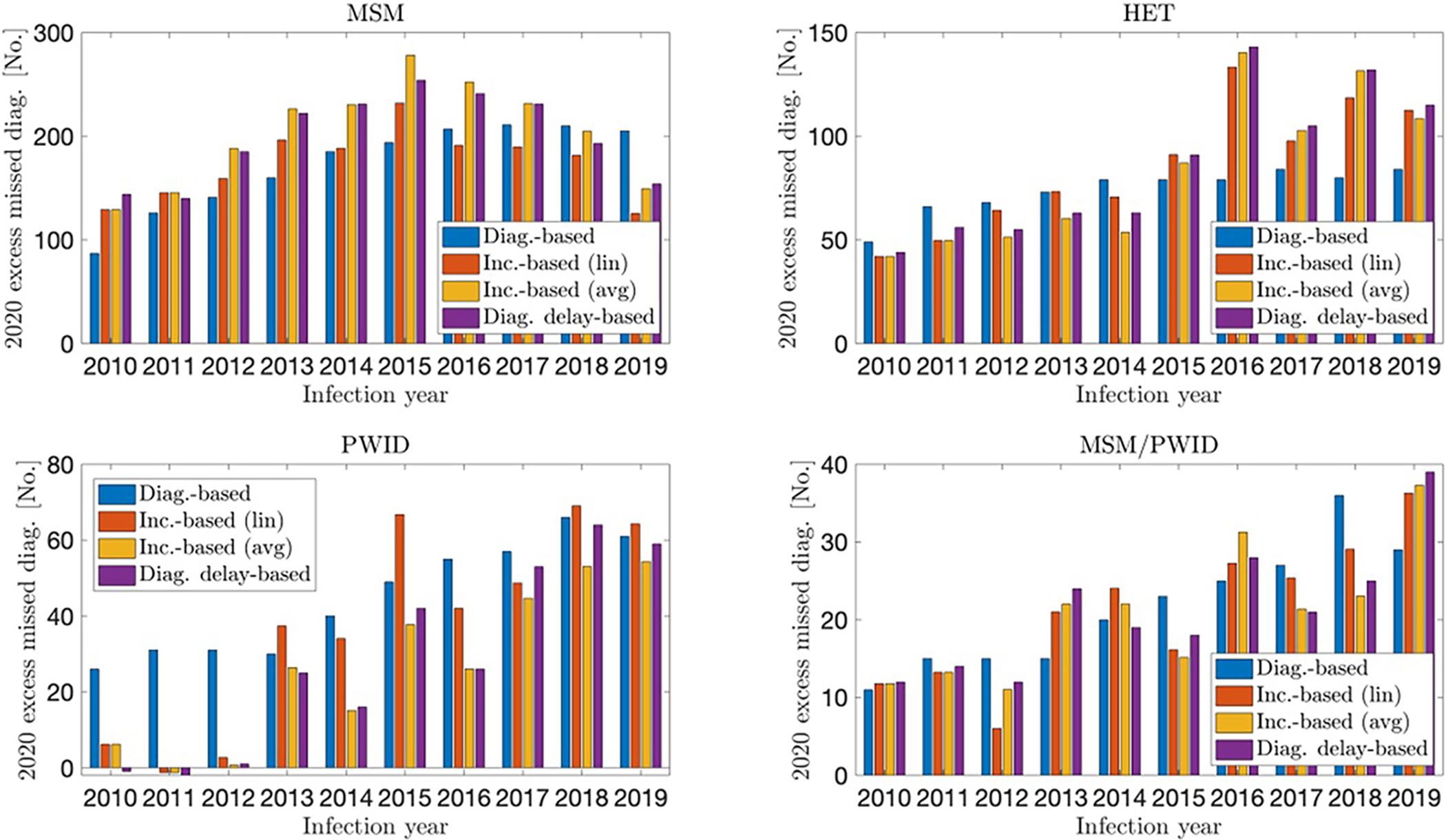
Excess missed HIV diagnoses in 2020, by infection year and transmission group, United States. Comparison between methods.
Missed diagnoses among MSM showed a regular distribution by infection year. Somewhat higher levels of missed diagnoses were observed among infections from 2014 to 2016 and somewhat lower levels among older and more recent infections. Among MSM/PWID, we observed an asymmetric trend, with more recent infections missing diagnoses at substantially higher levels compared with older infections (~60% of estimated missed diagnoses from infection years 2016–2019).
The incidence-based and diagnosis delay-based methods showed virtually no diagnoses missed among PWID infected from 2010 to 2012. Among heterosexuals, the incidence-based and diagnosis delay-based methods showed a marked infection-year trend, with missed diagnoses occurring at a notably higher level among PWH infected more recently (~60% of estimated missed diagnoses from infection years 2016–2019).
Figure 3 (a) displays the number of expected HIV diagnoses in 2020, broken down by observed versus missed, overall, and by each transmission group (left axis), as well as the percent of expected HIV diagnoses that were missed within each transmission group (right axis). For all PWH infected from 2010 to 2019, 18% (~3288) of the approximately 18,000 expected HIV diagnoses in 2020 were missed (note the method used in this plot is the averaged incidence-based method, similar qualitatively and quantitatively to the diagnosis delay-based method in this analysis. The other methods gave similar results, and the complete analyses are shown in Appendix B [Supplemental Digital Content, http://links.lww.com/QAI/C5]). This percentage was slightly lower for MSM (16%), somewhat higher for PWID (19%), and substantially higher for heterosexuals (24%) and MSM/PWID (31%). Figure 3 (b) shows the percentage of total excess missed diagnoses in 2020 attributed to each transmission group. MSM accounted for 61% of the 3288 excess missed HIV diagnoses in 2020, followed by heterosexuals (25%), PWID (8%), and MSM/PWID (6%).
FIGURE 3.
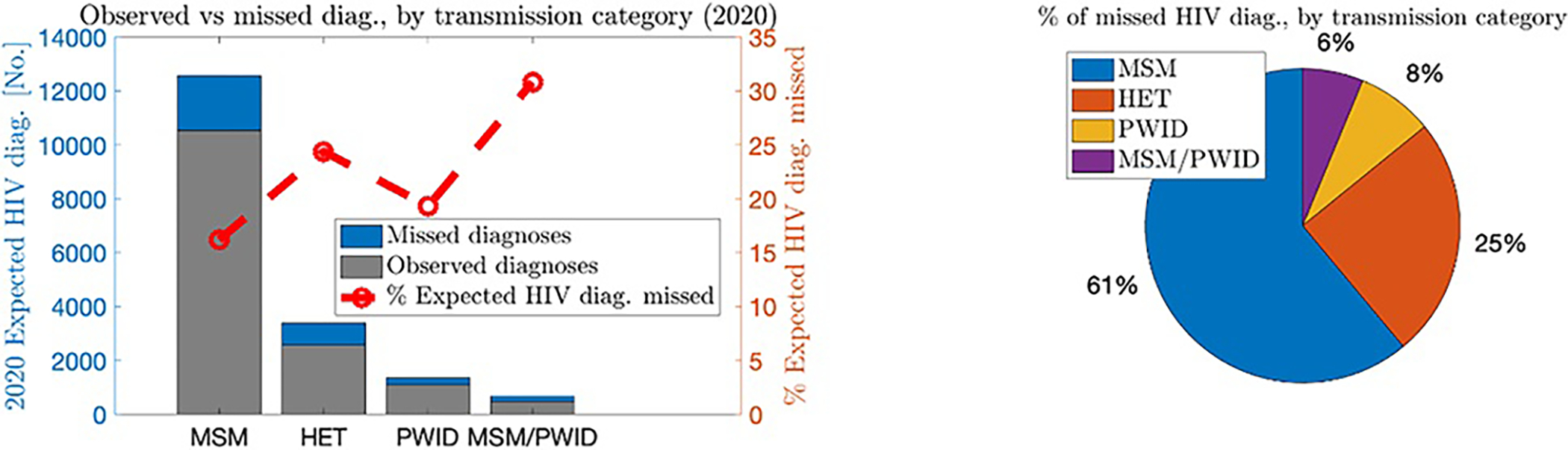
Level of excess missed HIV diagnoses in 2020 among PWH acquiring infection 2010–2019, by transmission group, United States. Results shown were computed with the incidence-based (averaged) method.
Table 1 reports the total excess missed diagnoses, as measured by each method, overall, and by sex-at-birth, race/ethnicity, transmission group, and region. Table 2 reports the corresponding percentage of diagnoses missed, as compared to expected 2020 diagnoses. Elevated levels of missed diagnoses were found among female sex assigned at birth (~23%–25%) and Hispanic/Latino PWH (~22%–25%). Among racial/ethnic groups, the largest number of missed HIV diagnoses was among black PWH (1267–1366), representing roughly 40% of all total excess missed diagnoses. Missed diagnoses seem slightly elevated in the Northeast (~19%–20%) and slightly lower in the Midwest (~15%). The South showed the most overall excess missed diagnoses among geographic regions (1709–1855, around 55% of total excess missed diagnoses). Although MSM, men (at birth), and Black/White PWH had larger raw numbers of missed diagnosis, they showed lower levels of missed diagnosis as compared to other population groups. Further details are provided in Appendix B (Supplemental Digital Content, http://links.lww.com/QAI/C5).
TABLE 1. -.
Total Excess Missed HIV Diagnoses in 2020 Among PWH Acquiring Infection 2010–2019 for Each Examined Group and Method. United States
| Total Missed, Group | Diag.-Based | Inc.-Based (Linear Fit) | Inc.-Based (Averaged) | Diag. Delay-Based |
|---|---|---|---|---|
|
| ||||
| Total | 3154 | 3238 | 3288 | 3315 |
| Sex at birth | ||||
| Male | 2407 | 2424 | 2631 | 2624 |
| Female | 722 | 790 | 669 | 707 |
| Race/Ethnicity | ||||
| Black or African American | 1357 | 1366 | 1267 | 1328 |
| Hispanic/Latino | 1109 | 1054 | 1119 | 1165 |
| White | 580 | 610 | 688 | 654 |
| Transmission group | ||||
| MSM | 1726 | 1737 | 2034 | 1995 |
| HET | 741 | 854 | 828 | 867 |
| PWID | 446 | 370 | 263 | 283 |
| MSM/PWID | 216 | 209 | 207 | 212 |
| Region | ||||
| Northeast | 451 | 512 | 547 | 518 |
| West | 537 | 649 | 674 | 698 |
| South | 1855 | 1762 | 1709 | 1748 |
| Midwest | 313 | 351 | 368 | 374 |
TABLE 2. -.
Percentage of Expected HIV Diagnoses Missed in 2020 Among PWH Acquiring Infection 2010–2019 for Each Examined Group and Method, United States
| % Missed, Group | Diag.-Based | Inc.-Based (Linear Fit) | Inc.-Based (Averaged) | Diag. Delay-Based |
|---|---|---|---|---|
|
| ||||
| Total | 17.6% | 18.1% | 18.3% | 18.4% |
| Sex at birth | ||||
| Male | 16.3% | 16.3% | 17.5% | 17.4% |
| Female | 23.8% | 25.6% | 22.6% | 23.5% |
| Race/Ethnicity | ||||
| Black or African American | 17.4% | 17.5% | 16.4% | 17.1% |
| Hispanic/Latino | 21.6% | 20.8% | 21.8% | 22.5% |
| White | 14.5% | 15.2% | 16.8% | 16.1% |
| Transmission group | ||||
| MSM | 14.1% | 14.2% | 16.2% | 15.9% |
| HET | 22.4% | 24.9% | 24.3% | 25.2% |
| PWID | 28.8% | 25.1% | 19.3% | 20.5% |
| MSM/PWID | 31.6% | 31.0% | 30.8% | 31.2% |
| Region | ||||
| Northeast | 18.1% | 20.1% | 21.2% | 20.3% |
| West | 14.9% | 17.5% | 18.1% | 18.6% |
| South | 19.6% | 18.8% | 18.4% | 18.7% |
| Midwest | 13.5% | 14.8% | 15.4% | 15.7% |
Stratification robustness analyses confirmed the consistency and reliability of our results, and we provide these details in Appendix B (Supplemental Digital Content, http://links.lww.com/QAI/C5).
The validation study on 2019 data confirmed the accuracy of the developed methods, with the examined methods showing good agreement with NHSS data. Over the entire population, the %Error (total) was less than 1% and %Error (year-by-year) less than 5% for all methods. Further analyses on population subgroups showed similar accuracy. Full details are given in Appendix C (Supplemental Digital Content, http://links.lww.com/QAI/C6).
DISCUSSION
We developed 4 methods to estimate excess missed HIV diagnoses in 2020 among PWH infected from 2010 to 2019. All methods estimate that there were approximately 3100–3300 (~18%) fewer diagnoses than roughly 18,000 expected among this population in 2020. Our estimation techniques, despite their differing formulations, are consistently in agreement, providing evidence toward their reliability. These missed diagnoses cannot be attributed to changes in incidence in 2020 and firmly establish that disruptions in testing during the COVID-19 pandemic is responsible for a large portion of the observed drop in HIV diagnoses in the United States in 2020. They further indicate that COVID-19 and associated disruptions have led to a decrease in awareness of infection status.
Our results indicate that different subpopulations were not equally affected by COVID-19-related disruptions. We found that heterosexuals, PWID, and particularly MSM/PWID, missed diagnoses at elevated levels as compared to MSM. Despite MSM having a lower proportion of missed diagnoses than other transmission groups, MSM comprise most (61%, n~2000) of all missed diagnoses (n = 3,288, using the averaged incidence-based method). We also found that women (assigned at birth) and Hispanic/Latino PWH also showed disproportionately high levels of missed diagnosis.
Although the different methods generally agree, we note that the diagnosis-based method at times gave qualitatively different infection-year trends as compared to the other methods. This is unsurprising because the other 3 methods are closely related in contrast to the diagnosis-based method, which is distinct. The diagnosis-based method postulates that undiagnosed infections follow an infection-year distribution based on that of PWH with diagnosed infection. By contrast, the other methods make no assumptions on infection-year distribution and hence may be more suitable for identifying qualitative trends. This was further supported by our validation study (Appendix C, Supplemental Digital Content, http://links.lww.com/QAI/C6), in which the incidence-based and diagnosis delay-based methods accurately predicted the infection-year distributions of our examined subpopulations.
We note that the true number of excess missed diagnoses of PWH infected prepandemic is almost certainly higher than that reported here; because of limitations on completeness of data, our estimates only go back to infections dating from 2010 to later. Considering the decreasing trend in excess missed diagnoses for older infections and the increased probability of a stage 3 (AIDS) disease classification in 11+ years after infection,20 we believe the numbers of excess missed diagnoses from 2009 and earlier are likely small compared with those in the years considered. Nonetheless, they are almost certainly non-zero. The numbers shown here should therefore be interpreted as low-end estimates for total excess missed diagnoses among PWH infected before 2020.
The public health ramifications of these excess missed diagnoses are potentially severe because reduced infection awareness is associated with longer delays in linking to care and viral suppression and increased HIV transmission.21–23 Increasing testing efforts and innovative strategies toward correcting this diagnosis gap in the coming years may be necessary to avoid putting the goals of the Ending the HIV Epidemic in the United States (EHE) initiative at risk.24 We showed that our methods may also be applied to population stratifications to identify groups who missed diagnoses at high levels.
Given the potential consequences of this diagnosis gap, future analyses in this area are important. Applying these methods to identify additional disproportionately affected subpopulations may help identify where more targeted or tailored approaches are needed to reach those undiagnosed PWH. Such analyses will help inform efforts toward achieving health equity, eliminating disparities and improving the health of all population groups. Although only one-way stratifications were considered in the present, analyses on further stratification may also be useful in identifying priority populations. Similar projections may also be performed at different geographic scales, including, for instance, individual EHE jurisdictions, to help prioritize intervention strategies. Quantifying the costs and benefits of such interventions, including future increased incidence due to a persisting diagnosis gap, is also critical.
Although the overall effect of COVID-19 on incidence in 2020 remains unclear at the time of writing, the results shown here strongly suggest that factors unrelated to changes in incidence, including disruptions in testing, played a significant role in the observed reduction in diagnosis levels. As such, this analysis may allow for a more precise quantification of such incidence-independent effects, enabling us to better understand and ultimately estimate the other presumed major contributor to the diagnosis drop in 2020: the effect of the COVID-19 pandemic on HIV incidence.
Supplementary Material
Acknowledgments
This work was supported by the Centers for Disease Control and Prevention.
Footnotes
The authors have no conflicts of interest to disclose.
The data used for this analysis were collected as part of the public health program activity called PS18–1802 Integrated HIV Surveillance and Prevention Programs for Health Departments (Component A), which is a routine disease surveillance activity across 60 jurisdictions throughout the United States. A project determination made by the National Center for HIV, Hepatitis, STD, and TB Prevention (NCHHSTP) on behalf of the Centers for Disease Control and Prevention (CDC) on January 22, 2018, determined this project to be exempt from needing IRB review as it is not human subjects research. The National Center for HIV, Hepatitis, STD, and TB Prevention (NCHHSTP) on behalf of the Centers for Disease Control and Prevention (CDC) approved the use of these data in the current document on August 25, 2022.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
REFERENCES
- 1.Huang Y-LA, Zhu W, Wiener J, et al. Impact of coronavirus disease 2019 (COVID-19) on human immunodeficiency virus (HIV) pre-exposure prophylaxis prescriptions in the United States—a time-series analysis. Clin Infect Dis. 2022. doi: 10.1093/cid/ciac038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu W, et al. Impact of the COVID–19 pandemic on prescriptions for antiretroviral drugs for HIV treatment in the United States, 2019–2021. AIDS. 2022. doi: 10.1097/QAD.0000000000003315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moitra E, Tao J, Olsen J, et al. Impact of the COVID-19 pandemic on HIV testing rates across four geographically diverse urban centres in the United States: an observational study. Lancet Reg Health Am. 2022;7:100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rick F, Odoke W, Hombergh J, et al. Impact of coronavirus disease(COVID-19) on HIV testing and care provision across four continents. HIV Med. 2022;23:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. HIV Surveillance Report, 2020. Vol 33; 2022. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. accessed Jun. 01, 2022. [Google Scholar]
- 6.DiNenno EA, Delaney KP, Pitasi MA, et al. HIV testing before and during the COVID-19 pandemic — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2022;71:820–824. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Song R, Johnson AS, et al. HIV incidence, prevalence, and undiagnosed infections in U.S. Men who have sex with men. Ann Intern Med. 2018;168:685. [DOI] [PubMed] [Google Scholar]
- 8.Song R, Hall HI, Green TA, et al. Using CD4 data to estimate HIV incidence, prevalence, and percent of undiagnosed infections in the United States. JAIDS J Acquired Immune Deficiency Syndromes. 2017;74:3–9. [DOI] [PubMed] [Google Scholar]
- 9.Hall HI, Song R, Tang T, et al. HIV trends in the United States: diagnoses and estimated incidence. JMIR Public Health Surveill. 2017;3:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fojo A, Wallengren E, Schnure M, et al. Potential effects of the coronavirus disease 2019 (COVID-19) pandemic on human immunodeficiency virus (HIV) transmission: a modeling study in 32 US cities. Clin Infect Dis. 2022. doi: 10.1093/cid/ciab1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zang X, Krebs E, Chen S, et al. The potential epidemiological impact of coronavirus disease 2019 (COVID-19) on the human immunodeficiency virus (HIV) epidemic and the cost-effectiveness of linked, opt-out HIV testing: a modeling study in 6 us cities. Clin Infect Dis. 2021;72:e828–e834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenness SM, Le Guillou A, Chandra C, et al. Projected HIV and bacterial sexually transmitted infection incidence following COVID-19–related sexual distancing and clinical service interruption. J Infect Dis. ;223:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satcher Johnson A, Song R, Hall HI. Estimated HIV incidence, prevalence, and undiagnosed infections in US States and Washington, DC, 2010–2014. JAIDS J Acquired Immune Deficiency Syndromes. 2017;76:116–122. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AS, Song R. Incident and prevalent HIV infections attributed to sexual transmission in the United States, 2018. Sex Transm Dis. 2021;48: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2015. HIV Surveill Supplemental Rep. 20182018;23. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed Jun 01, 2022. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveill Supplemental Rep. 2021;26:2021. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed Jun. 01, 2022. [Google Scholar]
- 17.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveill Supplemental Rep.20122012;17. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-17-4.pdf. Accessed Jun 01, 2022. [Google Scholar]
- 18.Satcher-Johnson A, Song R, Siddigi A, et al. Impact of the COVID-19 Pandemic on HIV Diagnosis in the United States, 2020. IAS; 2022. [Google Scholar]
- 19.Song R, Green T. An improved approach to accounting for reporting delay in case surveillance systems. JP J Biostat. 2012;7:1–14. [Google Scholar]
- 20.Crepaz N, Song R, Lyss SB, et al. Estimated time from HIV infection to diagnosis and diagnosis to first viral suppression during 2014–2018. AIDS. 2021;35:2181–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Q, Lim S, Wu B, et al. Estimating the probability of diagnosis within1 year of HIV acquisition. AIDS. 2020;34:1075–1080, Jun. [DOI] [PubMed] [Google Scholar]
- 22.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175:588. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Purcell DW, Sansom SL, et al. Vital signs: HIV transmission along the continuum of care — United States, 2016. MMWR Morb Mortal Wkly Rep. 2019;68:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HHS Office of Infectious Disease and HIV/AIDS Policy, “What Is Ending the HIV Epidemic in the U.S.?, 2021. https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview. Accessed Aug 01, 2022.
- 25.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6. doi: 10.1214/aos/1176344136 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


