Abstract
Patient: Female, 29-year-old
Final Diagnosis: Plasmodium falciparum-induced autoimmune hemolytic anemia
Symptoms: Anemia • tachycardia
Clinical Procedure:—
Specialty: Hematology • Infectious Diseases
Objective:
Unusual clinical course
Background:
Sickle cell disease (SCD) is an autosomal recessive hereditary condition characterized by chronic hemolytic anemia and painful vaso-occlusive episodes. Homozygous sickle cell patients are at increased risk of morbidity and mortality from malaria. Autoimmune hemolytic anemia (AIHA) secondary to, or in the setting of, malarial infection is rare. In our case, the concurrence of Plasmodium falciparum malarial parasitemia and AIHA led to severe hemolytic anemia with an extensive packed red blood cell transfusion requirement. The patient’s underlying SCD also contributed to the severity of the anemia and persistence of the malarial infection.
Case Report:
We report the case of a 29-year-old woman in the second trimester of pregnancy, with a history of SCD, who presented with severe anemia beyond her typical baseline in the setting of P. falciparum malaria. Hemolysis markers, including lactate dehydrogenase and bilirubin, were elevated. Direct Coombs testing was positive for IgG and C3 antibodies. Treatment with antimalarial agents and steroids led to clinical improvement and eventual clearance of the parasitemia.
Conclusions:
Our patient’s clinical course was most compatible with P. falciparum malaria-induced AIHA. Although she received a short course of steroids, it was treatment and clearance of the parasitemia that led to resolution of the hemolysis and a return to baseline hemoglobin levels. While the exact mechanism of AIHA in malaria is not well characterized, several unique mechanisms have been proposed and should be considered in cases of P. falciparum malaria manifesting with particularly severe hemolytic anemia.
Keywords: Anemia, Hemolytic, Autoimmune; Anemia, Sickle Cell; Malaria
Background
Sickle cell disease (SCD) is an autosomal recessive hereditary condition characterized by chronic hemolytic anemia and painful vaso-occlusive episodes [1]. SCD is most common in sub-Saharan Africa, although it affects approximately 100,000 Americans [2,3]. Plasmodium falciparum malaria can present with a broad spectrum of clinical conditions including severe anemia and cerebral malaria. More than 20 polymorphisms, including sickle cell trait, have been selected in human populations as they offer protection against fatal P. falciparum infections [4]. We present the case of a 29-year-old woman in the second trimester of pregnancy, with a history of SCD, who presented with severe anemia beyond her typical baseline in the setting of P. falciparum malaria. Hemolysis markers including lactate dehydrogenase and bilirubin were raised. Direct Coombs testing was positive for IgG and C3 antibodies. The concurrence of P. falciparum malarial parasitemia and autoimmune hemolytic anemia (AIHA) on a background of SCD led to severe hemolytic anemia with an extensive packed red blood cell transfusion requirement. Treatment with antimalarial agents and steroids led to clinical improvement and eventual clearance of the parasitemia.
Case Report
A 29-year-old woman in the second trimester of pregnancy, with a history of SCD (HbSS genotype), presented with severe anemia. Three months prior to presentation she had traveled to Mali and Senegal, where she was diagnosed with P. falciparum malaria and started on an unknown antimalarial. However, according to her own report, she was not fully compliant with her prescribed course of treatment. The patient did not report any sickle cell vaso-occlusive episodes after she was diagnosed with P. falciparum malaria.
During an outpatient visit at our institution to follow up on her pregnancy, she was found to have severe anemia beyond her typical baseline. She was therefore referred for inpatient admission.
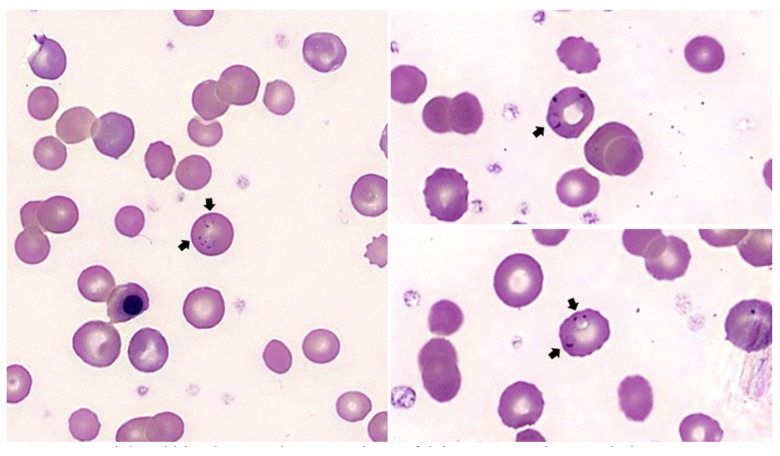
Upon presentation, she was tachycardic, though well-appearing and in no distress. She was anemic to a hemoglobin (Hgb) level of 4.6 g/dL (from her typical baseline of 8–9 g/dL). Reticulocyte percentage was 21.8% (from her typical baseline of 7–8%). Lactate dehydrogenase (LDH) was 734 U/L (from her typical baseline of approximately 300 U/L). Total bilirubin was 4.6 mg/dL (from her typical baseline of 1.5 mg/dL). Haptoglobin was undetectable. Direct Coombs testing was positive for IgG and C3. A peripheral blood smear demonstrated P. falciparum in 1% of her red blood cells (Figure 1) and blood parasite culture was positive for P. falciparum. She was transfused with 4 units of packed red blood cells (PRBCs) and admitted for management of malarial parasitemia and concurrent AIHA.
Figure 1.
Peripheral blood smear demonstrating Plasmodium falciparum parasite morphology. Peripheral blood smear on presentation demonstrated P. falciparum in 1% of red blood cells (arrows).
For treatment of malaria, she was started on intravenous artesunate 2.4 mg/kg every 12 hours. Following 3 doses of intravenous artesunate, 2 consecutive blood parasite cultures and 2 peripheral smears were negative for P. falciparum. She was then transitioned to quinine 648 mg every 8 hours for 24 hours (3 doses total), and clindamycin 450 mg every 8 hours for 7 days. For the management of concurrent AIHA, she was started on intravenous methylprednisolone 500 mg daily for 2 days and thereafter transitioned to prednisone 1 mg/kg/day (equivalent to prednisone 60 mg daily) which she received for 2 days while hospitalized. Laboratory markers of hemolysis (LDH and bilirubin) down-trended and hemoglobin stabilized with no further transfusion requirement. She was thereafter discharged on prednisone 60 mg daily for 7 days (she was also completing her course of clindamycin at the time of discharge). She was scheduled for followup 1 week after discharge.
One week following discharge, repeat thick and thin smears sent in the outpatient setting demonstrated recurrent P. falciparum parasitemia (to <1%). She was readmitted for refractory malaria and started on artemether/lumefantrine 80–480 mg for 6 doses (every 8 hours for the first 2 doses, followed by every 12 hours for the following 4 doses). She thereafter again cleared her parasitemia. During this second hospitalization, her hemoglobin and hemolysis markers remained stable at or near her baseline levels. Prednisone was discontinued with no evidence of recurrent AIHA. Prior to discharge, she had 2 blood parasite cultures and 2 peripheral smears that were negative for P. falciparum. Parasitemia and AIHA did not reoccur during followup after this second hospital discharge.
Discussion
The geographic distribution of SCD mirrors that of malaria [5,6]. This corresponding epidemiological distribution is not coincidental, as there is evidence that heterozygotes for the sickle cell gene (HbAS genotype), also known as sickle cell trait, are protected against mortality from malaria. Sickle cell trait confers a high degree of resistance to complicated and severe malarial infections [7–10]. The invasion and development stages of P. falciparum are reduced secondary to the unique physical and biochemical properties of the red blood cells (RBCs) in patients with sickle cell trait [11,12]. Furthermore, RBCs in patients with sickle cell trait that are parasitized by P. falciparum have a high propensity to sickle and undergo deformation due to low oxygen tension caused by the parasite. These infected cells become vulnerable to phagocytosis and undergo destruction by the spleen [11,13].
In contrast, patients who are homozygous for the sickle cell gene (HbSS genotype) experience increased susceptibility to severe and complicated infections and are at increased risk of morbidity and mortality from malaria [14–16]. P. falciparum-induced destruction of parasitized RBCs precipitates a severe exacerbation of the pre-existing chronic hemolytic anemia in these patients [17,18]. Malarial infection can also trigger painful vaso-occlusive episodes in sickle cell patients and is a leading cause of hospitalizations in endemic regions [14,19]. Furthermore, patients with SCD often have impaired splenic function, frequently to the extent of functional asplenia due to vaso-occlusive disease-related auto-splenectomy, rendering them incapable of clearing the parasitized erythrocytes [20].
There are 5 cases in the literature describing an association between SCD and AIHA [21]. However, AIHA related to SCD is not the likely cause of our patient’s severe anemia at presentation. Anemia is frequently associated with malaria secondary to destruction of RBCs by the Plasmodium parasites, splenic sequestration of RBCs, production of inflammatory cytokines, dyserythropoiesis related to infection, and bone marrow suppression [22]. AIHA secondary to, or in the setting of, malarial infection is rare. While 9 cases in the literature describe an association between malaria and AIHA [23–29], there is only 1 case of P. falciparum malaria mimicking AIHA in pregnancy that resolved following treatment with antimalarial agents alone [29]. Lastly, the risk of delayed hemolytic anemia after antimalarial treatment, specifically intravenous artesunate, is well documented but we do not believe that this was the likely cause for our patient’s presentation since it did not reoccur with treatment at our institution [30–37].
In our case, the concurrence of P. falciparum malarial parasitemia and AIHA led to severe hemolytic anemia with an extensive PRBC transfusion requirement. The patient’s underlying SCD also contributed to the severity of the anemia and persistence of the malarial infection. Although she received a short course of steroids, it was treatment and clearance of the parasitemia that led to resolution of the hemolysis and a return to baseline hemoglobin levels.
Conclusions
While it is difficult to definitively establish that this patient’s AIHA resulted from her malarial infection, this relationship appears likely since treatment of the malaria led to resolution of the AIHA. Several unique mechanisms for AIHA in patients with malaria have been proposed. For example, alterations in the levels of antibody- and complement-binding in the setting of Plasmodium infection may play a role, as may reduction in the threshold for splenic sequestration of RBCs [38–40]. Although the exact mechanism of AIHA in malaria is not well characterized, it should be considered in cases of P. falciparum malaria manifesting with particularly severe hemolytic anemia.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Done
Division of Hematology and Medical Oncology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York City, NY, USA.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet Lond Engl. 2010;376(9757):2018–31. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 2.Wastnedge E, Waters D, Patel S, et al. The global burden of sickle cell disease in children under five years of age: A systematic review and meta-analysis. J Glob Health. 2018;8(2):021103. doi: 10.7189/jogh.08.021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC . Centers for Disease Control and Prevention; 2020. Data & Statistics on Sickle Cell Disease | CDC [Internet] [cited 2022 Feb 7]. Available from: https://www.cdc.gov/ncbddd/sicklecell/data.html. [Google Scholar]
- 4.Maier AG, Matuschewski K, Zhang M, Rug M. Plasmodium falciparum . Trends Parasitol. 2019;35(6):481–82. doi: 10.1016/j.pt.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet Lond Engl. 2013;381(9861):142–51. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiebe A, Longbottom J, Gleave K, et al. Geographical distributions of African malaria vector sibling species and evidence for insecticide resistance. Malar J. 2017;16(1):85. doi: 10.1186/s12936-017-1734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allison AC. Polymorphism and natural selection in human populations. Cold Spring Harb Symp Quant Biol. 1964;29:137–49. doi: 10.1101/sqb.1964.029.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Aidoo M, Terlouw DJ, Kolczak MS, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet Lond Engl. 2002;359(9314):1311–12. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 9.Hill JA, Bell DA, Brintnell W, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med. 2008;205(4):967–79. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willcox M, Björkman A, Brohult J, et al. A case-control study in northern Liberia of Plasmodium falciparum malaria in haemoglobin S and beta-thalassaemia traits. Ann Trop Med Parasitol. 1983;77(3):239–46. doi: 10.1080/00034983.1983.11811704. [DOI] [PubMed] [Google Scholar]
- 11.Friedman MJ. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci USA. 1978;75(4):1994–97. doi: 10.1073/pnas.75.4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274(5672):701–3. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 13.Shear HL, Roth EF, Fabry ME, et al. Transgenic mice expressing human sickle hemoglobin are partially resistant to rodent malaria. Blood. 1993;81(1):222–26. [PubMed] [Google Scholar]
- 14.McAuley CF, Webb C, Makani J, et al. High mortality from Plasmodium falciparum malaria in children living with sickle cell anemia on the coast of Kenya. Blood. 2010;116(10):1663–68. doi: 10.1182/blood-2010-01-265249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makani J, Komba AN, Cox SE, et al. Malaria in patients with sickle cell anemia: Burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115(2):215–20. doi: 10.1182/blood-2009-07-233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luzzatto L. Sickle cell anaemia and malaria. Mediterr J Hematol Infect Dis. 2012;4(1):e2012065. doi: 10.4084/MJHID.2012.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haldar K, Mohandas N. Malaria, erythrocytic infection, and anemia. Hematol Am Soc Hematol Educ Program. 2009:87–93. doi: 10.1182/asheducation-2009.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White NJ. Anaemia and malaria. Malar J. 2018;17(1):371. doi: 10.1186/s12936-018-2509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menendez C, Fleming AF, Alonso PL. Malaria-related anaemia. Parasitol Today Pers Ed. 2000;16(11):469–76. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- 20.Adeloye A, Luzzatto L, Edington GM. Severe malarial infection in a patient with sickle-cell anaemia. Br Med J. 1971;2(5759):445–46. doi: 10.1136/bmj.2.5759.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaplin H, Zarkowsky HS. Combined sickle cell disease and autoimmune hemolytic anemia. Arch Intern Med. 1981;141(8):1091–93. [PubMed] [Google Scholar]
- 22.Roberts DJ, Casals-Pascual C, Weatherall DJ. The clinical and patho-physiological features of malarial anaemia. Curr Top Microbiol Immunol. 2005;295:137–67. doi: 10.1007/3-540-29088-5_6. [DOI] [PubMed] [Google Scholar]
- 23.Sonani R, Bhatnagar N, Maitrey G. Autoimmune hemolytic anemia in a patient with Malaria. Asian J Transfus Sci. 2013;7(2):151–52. doi: 10.4103/0973-6247.115581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A, Sharma S, Choudhury J. Autoimmune hemolytic anemia in Plasmodium vivax malaria. Indian J Pediatr. 2017;84(6):483–84. doi: 10.1007/s12098-017-2327-z. [DOI] [PubMed] [Google Scholar]
- 25.Taneja S, Agarwal N. Autoimmune haemolytic anaemia associated with P. vivax malaria. Trop Doct. 2019;49(1):54–65. doi: 10.1177/0049475518804381. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AS, Delisca G, Booth GS. Warm autoimmune hemolytic anemia secondary to Plasmodium ovale infection: A case report and review of the literature. Transfus Apher Sci. 2013;49(3):571–73. doi: 10.1016/j.transci.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Sharma V, Samant R, Hegde A, Bhaja K. Autoimmune hemolysis in malaria: A report of three cases. J Assoc Physicians India. 2012;60:129–31. [PubMed] [Google Scholar]
- 28.Sitcharungsi R, Anurathapan U, Sirachainan N, Chanthavanich P. Auto-immune haemolytic anaemia concurrent with Plasmodium vivax infection: A case report. Ann Trop Paediatr. 2011;31(1):87–91. doi: 10.1179/1465328110Y.0000000003. [DOI] [PubMed] [Google Scholar]
- 29.Drouin J, Rock G, Jolly EE. Plasmodium falciparum malaria mimicking auto-immune hemolytic anemia during pregnancy. CMAJ. 1985;132(3):265–67. [PMC free article] [PubMed] [Google Scholar]
- 30.Camprubí D, Pereira A, Rodriguez-Valero N, et al. Positive direct antiglobulin test in post-artesunate delayed haemolysis: More than a coincidence? Malar J. 2019;18(1):123. doi: 10.1186/s12936-019-2762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24(4–5):143–50. doi: 10.1016/j.blre.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Raffray L, Receveur MC, Beguet M, et al. Severe delayed autoimmune haemolytic anaemia following artesunate administration in severe malaria: A case report. Malar J. 2014;13:398. doi: 10.1186/1475-2875-13-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jauréguiberry S, Ndour PA, Roussel C, et al. Postartesunate delayed hemolysis is a predictable event related to the lifesaving effect of artemisinins. Blood. 2014;124(2):167–75. doi: 10.1182/blood-2014-02-555953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh S, Singh SK, Tentu AK, et al. Artesunate-induced severe autoimmune hemolytic anemia in complicated malaria. Indian J Crit Care Med. 2018;22(10):753–56. doi: 10.4103/ijccm.IJCCM_298_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ascoli Bartoli T, Lepore L, D’Abramo A, et al. Systematic analysis of direct antiglobulin test results in post-artesunate delayed haemolysis. Malar J. 2021;20(1):206. doi: 10.1186/s12936-021-03735-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Nardo P, Oliva A, Giancola ML, et al. Haemolytic anaemia after oral artemether-lumefantrine treatment in a patient affected by severe imported falciparum malaria. Infection. 2013;41(4):863–65. doi: 10.1007/s15010-013-0451-x. [DOI] [PubMed] [Google Scholar]
- 37.Kurth F, Lingscheid T, Steiner F, et al. Hemolysis after oral artemisinin combination therapy for uncomplicated Plasmodium falciparum malaria. Emerg Infect Dis. 2016;22(8):1381–36. doi: 10.3201/eid2208.151905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Facer CA, Bray RS, Brown J. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. I. Incidence and class specificity. Clin Exp Immunol. 1979;35(1):119–27. [PMC free article] [PubMed] [Google Scholar]
- 39.Facer CA. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. II. Specificity of erythrocyte-bound IgG. Clin Exp Immunol. 1980;39(2):279–88. [PMC free article] [PubMed] [Google Scholar]
- 40.Merry AH, Looareesuwan S, Phillips RE, et al. Evidence against immune haemolysis in falciparum malaria in Thailand. Br J Haematol. 1986;64(1):187–94. doi: 10.1111/j.1365-2141.1986.tb07586.x. [DOI] [PubMed] [Google Scholar]