Abstract
BACKGROUND
Patients with ≥2 ventricular arrhythmia (VA) events within 3 months (clustered VA) have increased risk for mortality.
OBJECTIVES
The aim of this study was to examine the association of risk factors including scar characteristics on cardiovascular magnetic resonance imaging with clustered VA and VA cycle length in nonischemic cardiomyopathy (NICM) and ischemic cardiomyopathy (ICM).
METHODS
Data from 329 primary prevention implantable cardioverter-defibrillator recipients (mean age 57 years, 26% women) were analyzed from the Left Ventricular Structural Predictors of Sudden Cardiac Death study.
RESULTS
Twenty-one patients developed clustered VA (median time 2.7 years after implantable cardioverter-defibrillator placement). Men had the greatest risk for recurrent VA. Patients with NICM and scar had the highest incidence rate of clustered VA. In patients with NICM, each 1-g increase in core scar correlated with greater clustered VA risk (HR: 1.19; 95% CI: 1.07–1.32). Gray scar was similar among subgroups. Patients with NICM with clustered VA had the longest mean VA cycle length (297 ± 40 milliseconds). Higher core scar burden correlated with longer VA cycle length in patients with NICM (P = 0.002), and higher body mass index correlated with shorter VA cycle length in those with ICM (P = 0.02). Type of VA was similar between cardiomyopathy subgroups, and no scar pattern predominated.
CONCLUSIONS
Clustered VA was most common in patients with NICM and scar, with greatest risk among those with larger core scar. Core scar correlated with slower VA in patients with NICM, and higher body mass index correlated with faster VA in those with ICM. Type of VA was similar by cardiomyopathy etiology, and no dominant scar pattern was associated with clustered VA. (J Am Coll Cardiol EP 2022;8:957–966) © 2022 by the American College of Cardiology Foundation.
Keywords: cardiovascular magnetic resonance, risk stratification, sudden cardiac death, ventricular arrhythmia
Three or more episodes of ventricular arrhythmias (VAs) within 24 hours constitute electrical storm (ES). This commonly accepted definition has evolved from its first description by Fries et al1 in 1997. Since the initial description, a variety of ES definitions have been published.2
Despite the significant heterogeneity in defining ES, multiple studies report a uniform association between increased mortality risk and ES.3 The incremental risk for mortality related to repetitive VAs at intervals longer than 24 hours or the frequency of events within a constant period (clustered arrhythmias) was previously unknown. Elsokkari et al2 investigated clustered arrhythmias and reported an increased risk for mortality in patients with as few as 2 VA events within 3 months compared with those without clustered arrhythmias. The increasing frequency of VA events was associated with an incremental risk for mortality.
Although clustered arrhythmias have been linked to increased mortality risk, the associated risk factors and predictors remain unclear. Prior studies have reported a link between myocardial scar and increased risk for the first occurrence of VA in non-ischemic cardiomyopathy (NICM) and ischemic cardiomyopathy (ICM).4–8 The objectives of this study were, in patients with NICM and those with ICM: 1) to assess clinical characteristics associated with increased risk for clustered VA; 2) to study the association between extent and pattern of myocardial scar and clustered VA; 3) to identify the type of VA, such as monomorphic or polymorphic ventricular tachycardia (VT) or ventricular fibrillation (VF), in relation to scar burden; and 4) to quantify VA cycle length in relation to scar burden.
METHODS
STUDY POPULATION.
The Left Ventricular Structural Predictors of Sudden Cardiac Death study (NCT01076660) is a prospective, observational, multicenter cohort study of patients with ICM and NICM eligible for primary prevention implantable cardioverter-defibrillator (ICD) placement according to clinical guidelines. The goal of the study was to identify predictors of sudden cardiac death in patients with ICDs using electrophysiological and structural phenotyping. The Institutional Review Board at each participating center approved the study.
The study enrolled 382 patients between November 2003 and December 2010 at The Johns Hopkins Hospital (Baltimore, Maryland) and Christiana Hospital (Newark, Delaware). Preimplantation cardiovascular magnetic resonance (CMR) images were acquired close to the time of ICD implantation (median 3 days). Details of the study methodology have been published previously.7,9,10
All patients provided comprehensive baseline medical histories and underwent medical examinations, resting 12-lead electrocardiography, fasting blood collection, and clinical echocardiography. Patients were evaluated twice per year, and all stored electrograms were collected and adjudicated at these scheduled follow-up visits or after patient-perceived ICD therapy. Two clinical cardiac electrophysiologists blinded to patient demographics adjudicated arrhythmic events, with adjudication by a third electrophysiologist in cases of disagreement. We performed a retrospective analysis and excluded patients who did not receive gadolinium (n = 49) and those with incomplete ICD therapy data available (n = 4), for a final study size of 329 patients.
CMR IMAGE ACQUISITION AND SCAR ANALYSIS.
Details of the CMR and late gadolinium enhancement (LGE) image acquisition and analysis protocols have been published previously.7,9,10 In brief, CMR images were obtained using a 1.5-T whole-body scanner (Signa CV/I, GE Healthcare, or Avanto, Siemens). Per protocol, short- and long-axis cine and 2-dimensional LGE images were obtained. Images were then analyzed for the presence of LGE by 2 readers blinded to outcome measures. Core scar was defined as myocardium with signal intensity >50% of maximal signal intensity. Gray scar was defined as signal intensity <50% of maximal signal intensity but greater than that of peak remote healthy myocardium. Scar patterns were categorized as: 1) subendocardial based (with or without transmural involvement); 2) midwall striae; 3) midwall patchy; 4) subepicardial; and 5) involving the right ventricular insertion point(s).11
EXPOSURES.
The primary exposures were the amount of core and gray zone scar as determined by CMR.
OUTCOMES.
The primary outcome was the occurrence of clustered VA. VA was defined as an adjudicated appropriate ICD firing for VT or VF above the programmed rate cutoff. Clustered VA was defined as ≥2 VA events more than 24 hours apart within 90 days. Self-terminating VAs or those terminated by antitachycardia pacing (ATP) were excluded. Each VA event was classified as monomorphic VT, polymorphic VT, VF, or ventricular flutter. Ventricular flutter was defined as a wide complex rhythm with a single predominant QRS configuration and rates in excess of 250 beats/min. Polymorphic VT comprised ventricular rhythm with rates in excess of 100 beats/min but generally <300 beats/min, without a single predominant QRS configuration. VF was defined as a rapid, chaotic rhythm without definable QRS waves or T waves. VT rates were determined from ICD device interrogation, and the beat-to-beat cycle was reported. The mean cycle length during the detection window was used to determine the tachycardia cycle length.
COVARIATES.
Baseline medical history and examination at the time of study enrollment were used to determine demographics (age, sex, ethnicity), body mass index (BMI), New York Heart Association functional class, duration of cardiomyopathy, and presence of comorbidities such as hypertension, diabetes, hypercholesterolemia, atrial fibrillation, and medication use. Left ventricular ejection fraction at enrollment was assessed using echocardiography, nuclear scintigraphy, or ventriculography. Patients were defined as having NICM if they had no history of myocardial infarction, revascularization, or coronary artery stenosis of >50% of ≥2 major epicardial vessels or involving the left main or proximal left anterior descending coronary artery.
STATISTICAL ANALYSIS.
Categorical variables are presented as percentages, and continuous variables are presented as either mean ± SD or median (IQR). Categorical variables were compared using chi-square tests, and continuous variables were compared using Student’s t-tests. Values of P < 0.05 were considered to indicate statistical significance.
Two regression models were constructed: model 1 used core and gray scar as covariates, and model 2 extended model 1 by adjusting for age, sex, and ethnicity. Multinomial logistic regression using models 1 and 2 was used to study the association between scar burden and risk for clustered VA. To account for multiple individual VA events per patient, we constructed a zero-inflated, negative binomial regression model. The absence of scar was used to account for excess zero counts of VA events. Last, we performed Fine and Gray competing risk regression analysis using models 1 and 2 to account for the competing risk for death over time while assessing the risk for clustered VA.
We performed stratified stepwise backward regression assessing for variables significantly associated with VT cycle length. We tested the following variables: age, sex, ethnicity, BMI, left ventricular ejection fraction, beta-blocker use, antiarrhythmic medication use, presence of atrial fibrillation, QRS duration, amount of gray scar, and amount of core scar. We created 2 additional models using variables of significance: model 3 included log(core scar) in patients with NICM and BMI in those with ICM, and model 4 extended model 3 by adjusting for age, sex, and ethnicity. Using stratified multivariate regression analysis, we tested the association of core scar burden and VA cycle length in patients with NICM and the association of BMI with VA cycle length in those with ICM. Last, we quantified the VA subtype frequency and assessed for statistically significant differences using pairwise comparison of means. Accounting for a small sample size, we assessed scar pattern frequency after stratifying by type of cardiomyopathy on a descriptive basis. All statistical analysis was performed using Stata version 16 (StataCorp).
RESULTS
STUDY POPULATION.
Table 1 shows the baseline characteristics of the 329 patients included in the study. The mean age of patients was 57 ± 13 years. Thirty-three percent of patients were non-White, and 26% were women. Forty-seven percent of patients had NICM. The median follow-up time was 6.8 years (IQR: 4.4–9.9 years). The median time to clustered VA was 2.7 years (IQR: 1.1–5.4 years). In the ICM group, 166 of 174 participants (95%) had scar on CMR. In the NICM group, 64 of 155 participants (41%) had scar on CMR. Comparison of baseline characteristics demonstrated that the only significant differences (P < 0.05) among the 3 groups of no VA, unclustered VA, and clustered VA were scar characteristics and diuretic medication use. Ninety-five percent of the clustered VA group had scar on CMR. Those without VA (no VA) had the least amount of core scar, and the clustered VA group had the most amount of gray scar. Diuretic medication use was more common in the unclustered VA group. At baseline, <10% of the patients were on antiarrhythmic agents. Antiarrhythmic medications consisted of amiodarone for atrial fibrillation, and no patients were on sotalol. Absolute scar amount and mean VA cycle length within subgroups of cardiomyopathy and clustered VA are shown in Table 2.
TABLE 1.
Baseline Patient Characteristics of the Study Population Stratified by Clustered VA
| No VA (n = 260) | Unclustered VA (n = 48) | Clustered VA (n = 21) | P Value | |
|---|---|---|---|---|
|
| ||||
| Age, y | 56.7 ± 13.3 | 57.6 ± 12.0 | 53.7 ± 10.2 | 0.51 |
| Female | 28.8 | 14.6 | 19.0 | 0.09 |
| Non-White | 32.3 | 35.4 | 28.6 | 0.84 |
| BMI, kg/m2 | 28.8 ± 5.9 | 28.8 ± 5.0 | 29.7 ± 5.1 | 0.79 |
| Nonischemic | 48.5 | 37.5 | 52.4 | 0.33 |
| LVEF, % | 28.0 ± 10.2 | 24.3 ± 9.0 | 26.1 ± 8.6 | 0.06 |
| NYHA functional class | 0.70 | |||
| I | 23.1 | 31.3 | 28.6 | |
| II | 45.0 | 35.4 | 42.9 | |
| III | 31.9 | 33.3 | 28.6 | |
| Duration of CM, y | 3.8 ± 5.2 | 5.3 ± 6.0 | 5.7 ± 5.6 | 0.07 |
| Hypertension | 56.9 | 62.5 | 52.4 | 0.69 |
| Diabetes | 27.3 | 20.8 | 28.6 | 0.63 |
| Hypercholesterolemia | 58.1 | 58.3 | 57.1 | 1.00 |
| Smoker | 46.9 | 64.6 | 47.6 | 0.08 |
| Aspirin | 70.4 | 79.2 | 57.1 | 0.17 |
| Statin | 66.2 | 79.2 | 57.1 | 0.12 |
| ACE inhibitor/ARB | 90.4 | 89.6 | 85.7 | 0.79 |
| Beta-blocker | 93.8 | 89.6 | 90.5 | 0.51 |
| Spironolactone | 25.8 | 33.3 | 14.3 | 0.24 |
| Diuretic | 55.0 | 75.0 | 57.1 | 0.04 |
| Antiarrhythmic | 6.2 | 10.4 | 14.3 | 0.26 |
| Digoxin | 16.9 | 18.8 | 28.6 | 0.40 |
| Atrial fibriUation | 17.4 | 16.7 | 28.6 | 0.42 |
| Ventricular rate, beats/min | 72.8 ± 13.9 | 71.5 ± 12.5 | 65.9 ± 12.4 | 0.08 |
| QRS duration, ms | 118.2 ± 31.3 | 120.0 ± 26.0 | 122.6 ± 25.6 | 0.78 |
| LBBB | 24.6 | 18.8 | 14.3 | 0.41 |
| CRT-D | 28.1 | 14.6 | 23.8 | 0.14 |
| Hematocrit, % | 41.7 ± 26.2 | 41.7 ± 4.7 | 39.8 ± 4.3 | 0.94 |
| Sodium, mEq/L | 139.0 ± 3.0 | 139.3 ± 3.1 | 138.4 ± 3.3 | 0.53 |
| Potassium, mEq/L | 4.3 ± 0.4 | 4.2 ± 0.4 | 4.3 ± 0.4 | 0.71 |
| Blood urea nitrogen, mg/dL | 18.9 ± 8.1 | 19.9 ± 7.1 | 19.5 ± 7.9 | 0.70 |
| Creatinine, mg/dL | 1.0 ± 0.5 | 1.0 ± 0.3 | 1.0 ± 0.2 | 0.99 |
| eGFR, mL/min/1.73 m2 | 82.7 ± 22.9 | 81.6 ± 19.9 | 87.3 ± 21.3 | 0.62 |
| NT-proBNP, pg/mL | 2,745.6 ± 7,198.3 | 2,476.9 ± 1,641.5 | 2,692.1 ± 2,430.3 | 0.97 |
| hsCRP, mg/L | 6.6 ± 12.0 | 9.6 ± 17.6 | 6.5 ± 7.5 | 0.33 |
| IL-6, pg/mL | 3.7 ± 11.0 | 5.0 ± 7.2 | 2.8 ± 3.1 | 0.67 |
| IL-10, pg/mL | 65.1 ± 757.6 | 8.7 ± 41.4 | 21.8 ± 63.4 | 0.87 |
| cTnT, ng/nL | 0.158 ± 1.254 | 0.016 ± 0.038 | 0.026 ± 0.067 | 0.70 |
| cTnI, ng/mL | 0.180 ± 1.103 | 0.091 ± 0.227 | 0.116 ± 0.309 | 0.86 |
| CK-MB, ng/mL | 3.9 ± 5.9 | 3.6 ± 2.3 | 4.4 ± 6.7 | 0.90 |
| Myoglobin, ng/mL | 39.5 ± 108.0 | 37.4 ± 39.3 | 63.9 ± 143.9 | 0.69 |
| Scar on CMR, % | 65.4 | 83.3 | 95.2 | 0.001 |
| Gray scar, g | 8.7 ± 11.6 | 12.9 ± 11.5 | 15.4 ± 13.2 | 0.006 |
| Core scar, g | 11.9 ± 14.3 | 19.0 ± 18.5 | 18.7 ± 11.8 | 0.002 |
| Total scar, g | 20.7 ± 24.9 | 31.9 ± 28.9 | 34.2 ± 23.0 | 0.003 |
Values are mean ± SD or %.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; CK-MB = creatine kinase-myocardial band; CM = cardiomyopathy; CMR = cardiovascular magnetic resonance; CRT-D = cardiac resynchronization therapy and defibrillator; cTnI = cardiac troponin I; cTnT = cardiac troponin T; eGFR = estimated glomerular filtration rate; hsCRP = high-sensitivity C-reactive protein; IL = interleukin; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; VA = ventricular arrhythmia.
TABLE 2.
Mean Scar Amount and Cycle Length Within Subgroups of Cardiomyopathy and Clustered VA
| Core Scar, g | Gray Scar, g | Cycle Length, ms | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| NICM | ICM | NICM | ICM | NICM | ICM | |
|
| ||||||
| No VA | 2.58 ± 6.31 | 20.70 ± 14.12 | 2.42 ± 6.88 | 14.69 ± 11.98 | NA | NA |
| Unclustered VA | 2.75 ± 3.90 | 28.78 ± 16.84 | 2.56 ± 3.55 | 19.08 ± 9.97 | 247.9 ± 45.4 | 255.6 ± 44.5 |
| Clustered VA | 14.10 ± 12.16 | 23.86 ± 9.53 | 12.50 ± 16.65 | 18.64 ± 7.70 | 296.9 ± 39.0 | 258.3 ± 48.7 |
| Overall | 3.42 ± 7.24 | 22.37 ± 14.66 | 3.15 ± 8.03 | 15.67 ± 11.55 | 267.5 ± 49.0 | 256.5 ± 45.2 |
Values are mean ± SD.
ICM = ischemic cardiomyopathy; NA = not applicable; NICM = nonischemic cardiomyopathy; VA = ventricular arrhythmia.
VA EVENTS.
Two hundred VA events occurred among the 69 patients with VA. Forty-eight patients had unclustered VA and 21 had clustered VA. Four patients had recurrent VA within 24 hours, and of those, 2 had additional VA at later dates that met criteria for clustering. In sensitivity analysis, the inclusion of 2 patients who did not proceed to develop clustered VA did not significantly change the results of the regression analysis or competing risk analysis. Table 3 summarizes the crude event numbers as incidence rates for clustered VA. The incidence rate of clustered VA was 9 per 1,000 person-years of follow-up. Patients with scar on CMR had a significantly greater incidence rate of clustered VA (13 per 1,000 person-years) than those with no scar (1 per 1,000 person-years). The incidence rate of clustered VA did not vary when stratifying only by type of cardiomyopathy but not scar presence. In subgroup analysis, the greatest incidence rate of clustered VA was among patients with NICM with scar (29 per 1,000 person-years).
TABLE 3.
Crude Event Numbers and Incidence Rate for Clustered Ventricular Arrhythmia Stratified by Presence of Scar and Type of Cardiomyopathy
| Group | Events | Follow-Up, PY | Rate, per 1,000 PY |
|---|---|---|---|
|
| |||
| NICM, no scar | 1 | 673.4 | 1.5 (0.2–10.5) |
| NICM, scar | 10 | 346.7 | 28.8 (15.5–53.6) |
| ICM, no scar | 0 | 59.7 | 0 |
| ICM, scar | 10 | 1,234.3 | 8.1 (4.4–15.1) |
| Total | 21 | 2,318.7 | 9.1 (5.9–13.1) |
PY = person-years; other abbreviations as in Table 2.
REGRESSION ANALYSIS.
Table 4 shows a summary of results from regression analysis. Multinomial logistic regression analysis comparing patients with clustered VA with the reference group who had no VA showed that the presence of core scar in patients with NICM was significantly associated with risk for clustered VA (OR: 1.26; 95% CI: 1.08–1.46). Core scar was not associated with increased risk for clustered VA in patients with ICM, and gray scar was not associated with clustered VA in those with NICM or ICM. Multiple event analysis showed that the number of expected individual VA events increased by a factor of 1.32 (95% CI: 1.16–1.51) in patients with NICM with every 1-g increase in core scar burden. Gray scar burden was not associated with risk for increasing number of VA events in patients with NICM or ICM. Model 2 within the multiple event analysis also identified that among patients with NICM, men were more likely to have a higher incidence of VA events than women (rate ratio: 6.86; 95% CI: 1.92–24.54).
TABLE 4.
Results of Regression and Competing Risk Analysis Stratified by Type of Cardiomyopathy
| Nonischemic | Ischemic | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Model 1 | Model 2 | Model 1 | Model 2 | ||
|
| |||||
| Fine and Gray analysisa | Core scar, g | 1.19 (1.09–1.30) | 1.19 (1.07–1.32) | 0.99 (0.94–1.05) | 0.98 (0.93–1.04) |
| Gray scar, g | 0.93 (0.85–1.02) | 0.93 (0.83–1.04) | 1.02 (0.96–1.09) | 1.04 (0.96–1.12) | |
| Multinomial logistic regressionb | Core scar, g | 1.24 (1.07–1.44) | 1.26 (1.08–1.46) | 1.00 (0.94–1.06) | 0.99 (0.93–1.06) |
| Gray scar, g | 0.91 (0.80–1.03) | 0.89 (0.78–1.01) | 1.03 (0.96–1.10) | 1.04 (0.96–1.12) | |
| Múltiple event analysisc | Core scar, g | 1.30 (1.09–1.55) | 1.32 (1.16–1.51) | 0.99 (0.95–1.04) | 0.99 (0.95–1.04) |
| Gray scar, g | 1.01 (0.85–1.21) | 0.96 (0.85–1.09) | 1.06 (1.00–1.13) | 1.05 (0.99–1.12) | |
Model 1 was adjusted for core scar and gray scar; model 2 was adjusted for core scar, gray scar, age, sex, and ethnicity.
HR (95% CI) for clustered ventricular arrhythmia.
Odds (95% CI) of clustered ventricular arrhythmia compared with no ventricular arrhythmia.
Event rate ratio (95% CI) from zero-inflated, negative binomial regression for ventricular arrhythmia events.
FINE AND GRAY COMPETING RISK ANALYSIS.
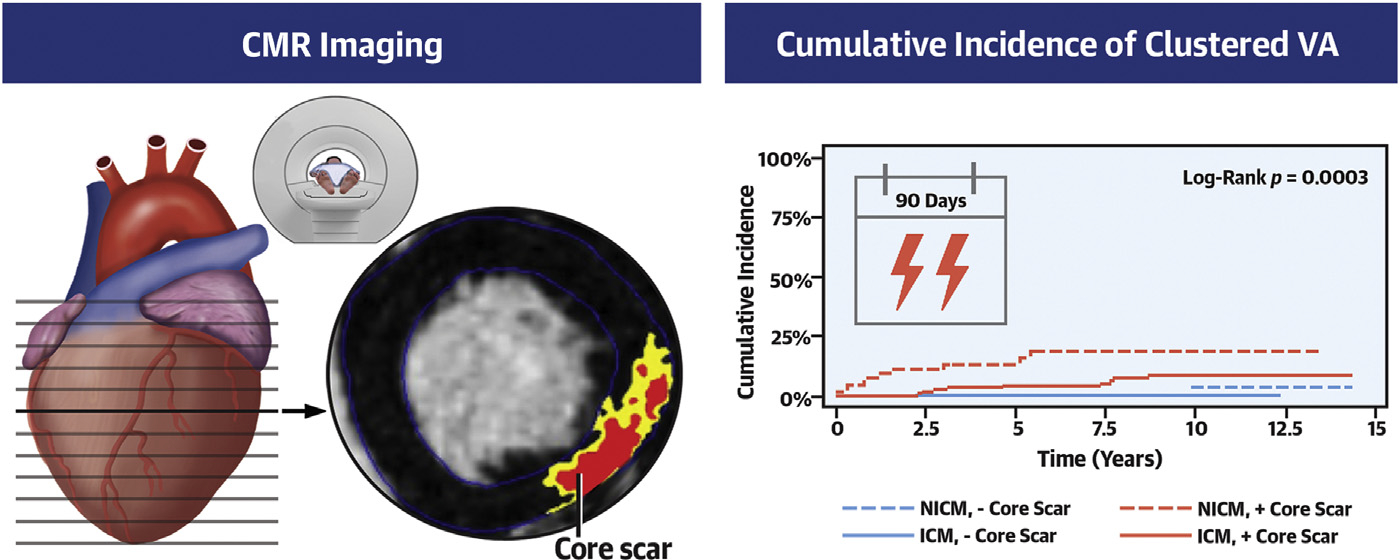
In concordance with findings from regression analyses, Fine and Gray competing risk analysis confirmed the association between core scar and clustered VA risk among patients with NICM (HR: 1.19; 95% CI: 1.07–1.32) and confirmed the lack of association in those with ICM (Figure 1). Also, in agreement with regression analyses, no association between gray scar and clustered VA was observed in patients with either type of cardiomyopathy.
FIGURE 1. Cumulative Incidence of Clustered VA Stratified by Type of Cardiomyopathy.
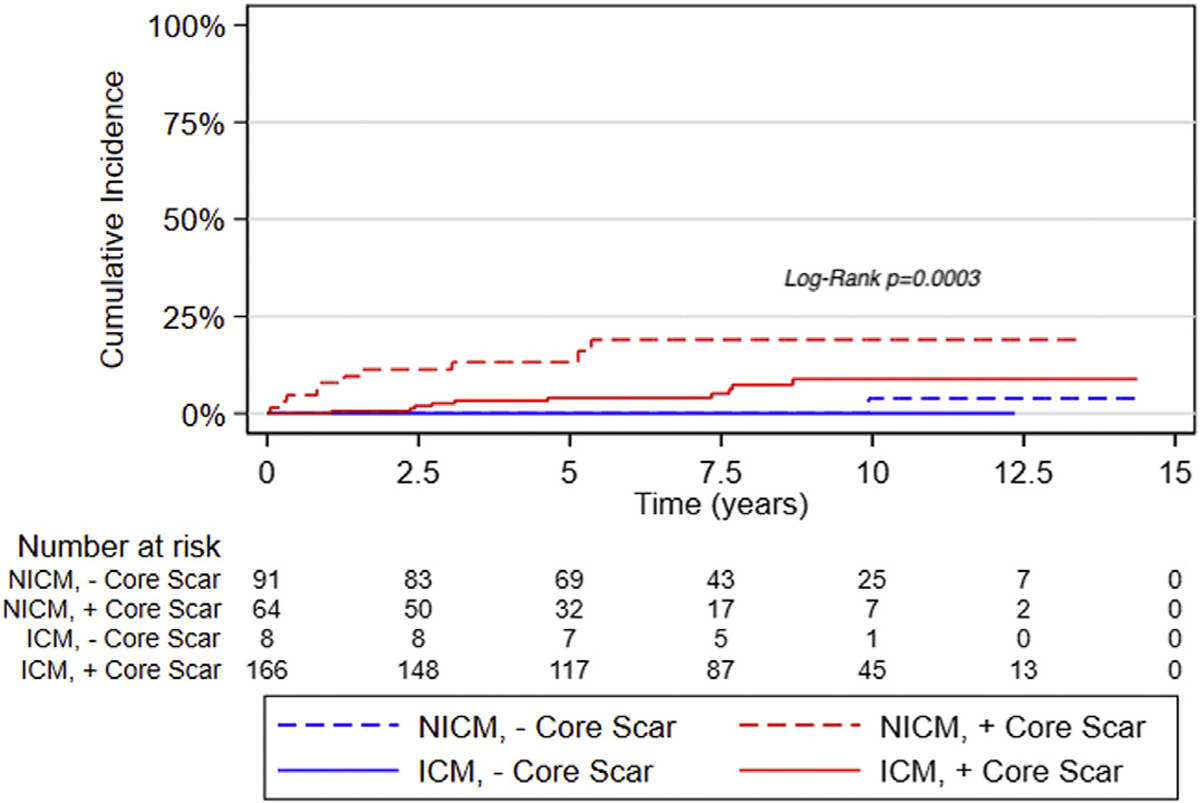
Patients with nonischemic cardiomyopathy (NICM) and core scar have the highest cumulative incidence of clustered ventricular arrhythmia (VA). ICM = ischemic cardiomyopathy.
VA CYCLE LENGTH.
In VA cycle length analysis, core scar amount was the only variable of significance in patients with NICM, and BMI was the only variable of significance in those with ICM. Stratified multivariable regression analysis revealed that higher core scar burden was associated with longer VA cycle length in patients with NICM, and higher BMI was associated with shorter VA cycle length in those with ICM (Table 5).
TABLE 5.
Association of Cycle Length With Core Scar and BMI
| Nonischemic, log (Core Scar) | Ischemic, BMI | |||
|---|---|---|---|---|
|
|
|
|||
| Model 1 | Model 2 | Model 1 | Model 2 | |
|
| ||||
| Cycle | 26.06 | 28.42 | −3.85 | −3.75 |
| length, ms | (11.60 to 40.52) | (10.93 to 45.91) | (−6.71 to −0.99) | (−6.91 to −0.60) |
BMI = body mass index.
TYPE OF VA AND SCAR PATTERN.
The most common type of VA was monomorphic VT (97 of 200 events). When stratified by the presence of clustered VA and type of cardiomyopathy, monomorphic VT was relatively more common within the clustered VA subgroups (Figure 2). The NICM and clustered VA subgroup had the most amount of polymorphic VT. Subepicardial and subendocardial scars were more prevalent than other patterns (Figure 3), and the septal, inferior, and lateral walls were more commonly affected (Figure 4), but there was no predominant pattern or location. Furthermore, patients with NICM and unclustered VA did not have any subepicardial scar, but scar was relatively more common in the insertion point and septal regions. Patients with ICM had predominantly subendocardial-based scar with or without transmural involvement or no scar.
FIGURE 2. Proportional Frequency of VA Type Stratified by Cardiomyopathy and Clustered VA.
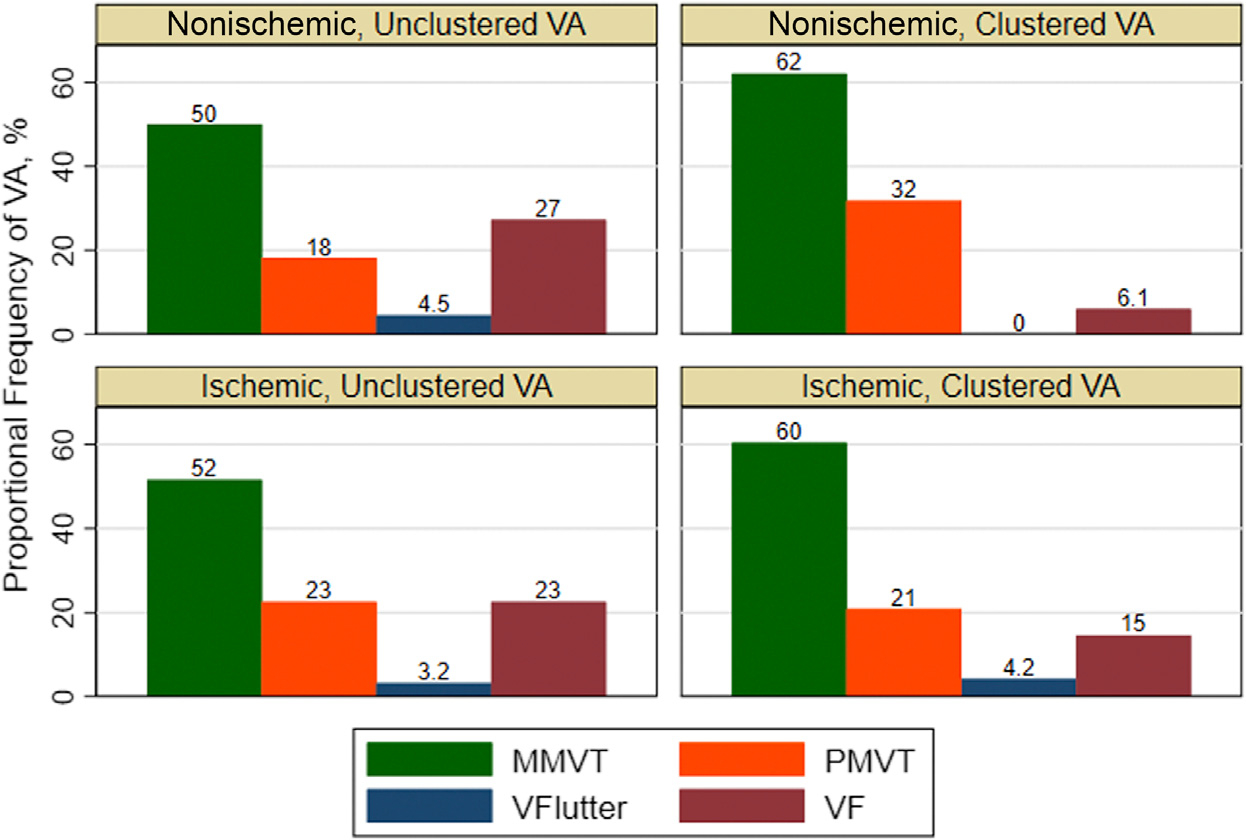
Monomorphic ventricular tachycardia (MMVT) was the most common type of ventricular arrhythmia (VA) event among the 4 subgroups of clustered VA and cardiomyopathy. The nonischemic cardiomyopathy with clustered VA subgroup had the most amount of polymorphic ventricular tachycardia (PMVT). VF = ventricular fibrillation; VFlutter = ventricular flutter.
FIGURE 3. Proportional Frequency of Scar Pattern in Patients With Nonischemic Cardiomyopathy Stratified by Clustered VA.
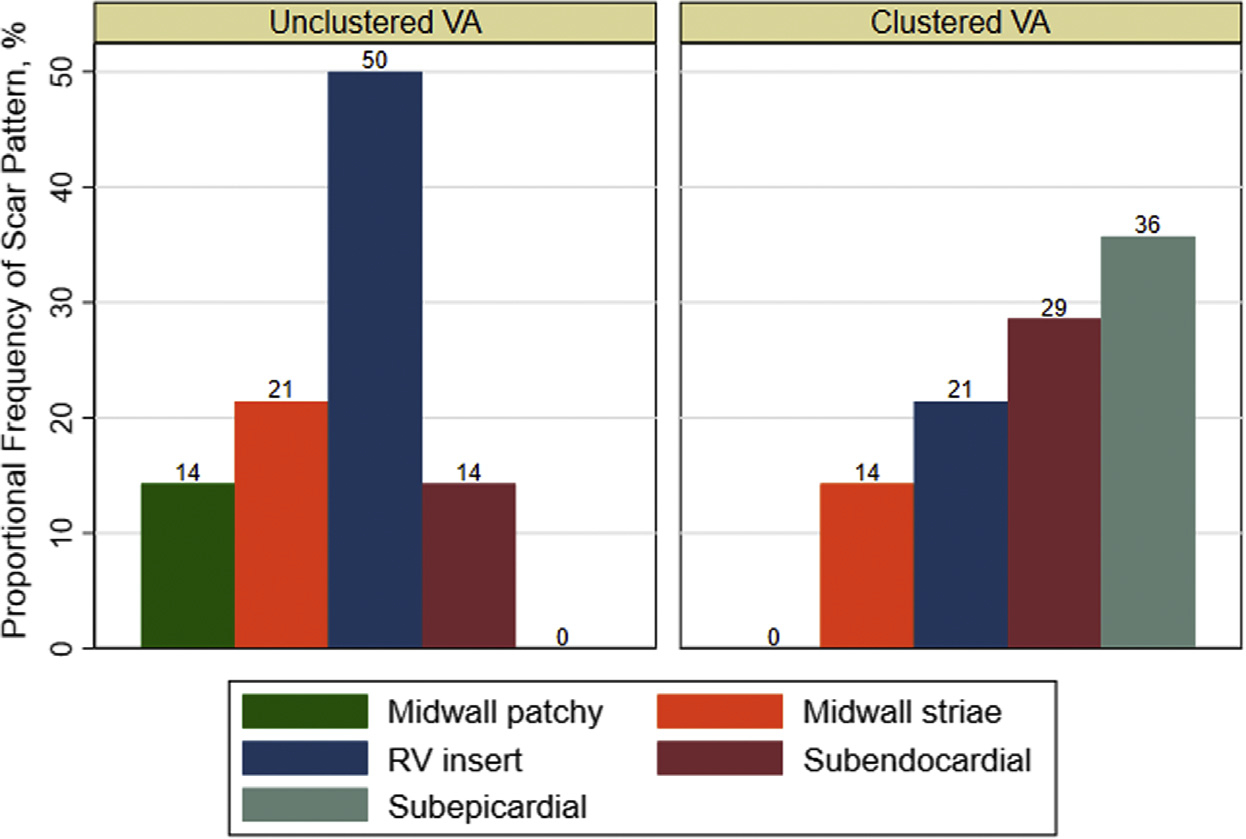
Subepicardial and subendocardial scars were more prevalent than other patterns in the nonischemic cardiomyopathy with clustered ventricular arrhythmia (VA) subgroup, but no single scar pattern predominated. RV insert = right ventricular insertion point.
FIGURE 4. Proportional Frequency of Scar Location in Patients With Nonischemic Cardiomyopathy Stratified by Clustered VA.
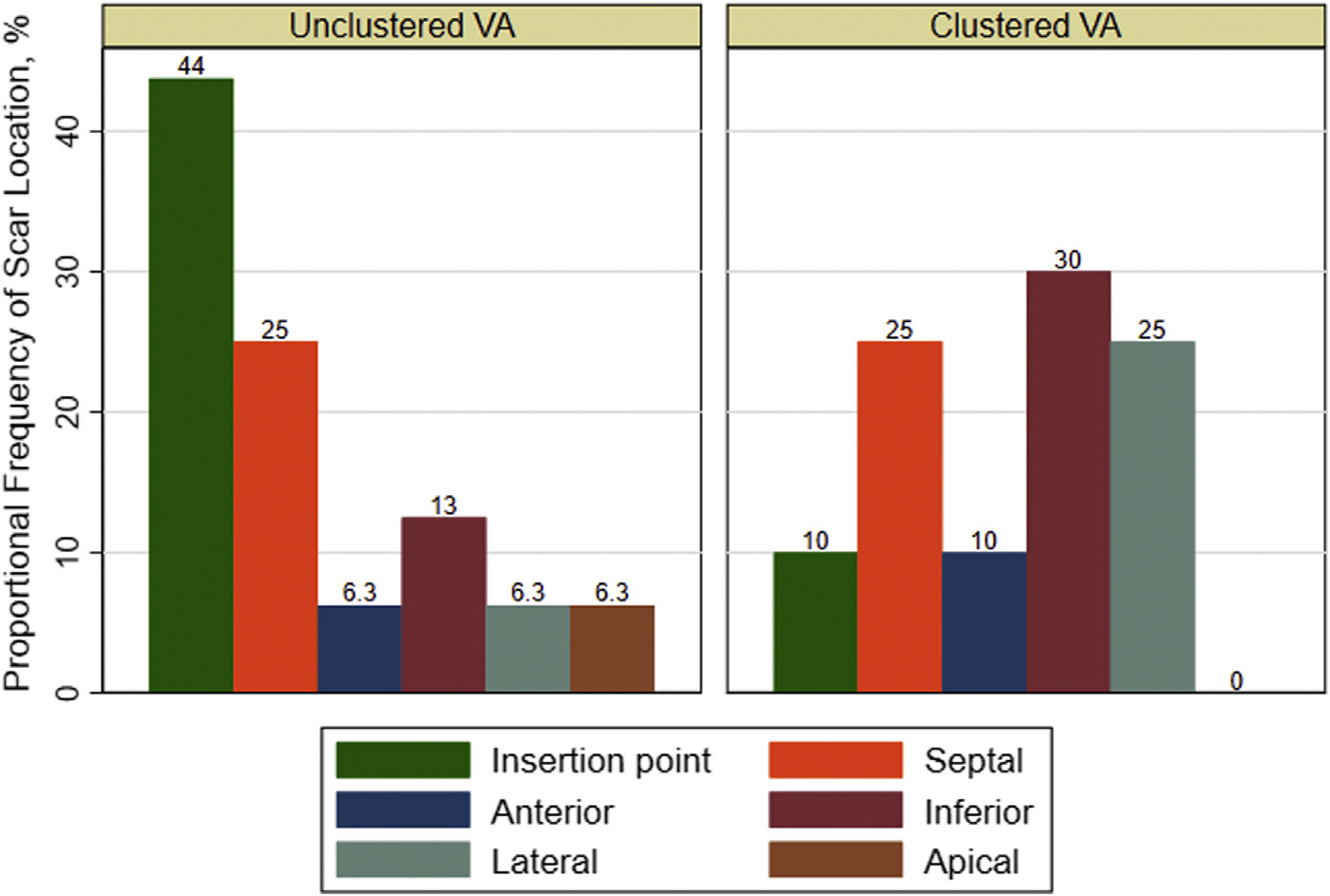
The septal, inferior, and lateral walls were more commonly affected by scar in the nonischemic cardiomyopathy with clustered ventricular arrhythmia (VA) subgroup, but no single scar location was most affected.
DISCUSSION
STUDY FINDINGS.
We examined risk factors for the development of clustered VA, including clinical characteristics and CMR metrics. We also described characteristics of the VAs seen in the clustered vs nonclustered cohorts. Our main findings are as follows: 1) men were more likely to have recurrent VA; 2) clustered VA was most common in those with NICM and scar (Central Illustration); 3) the amount of core scar in patients with NICM predicted risk for clustered VA; 4) no predominant scar pattern or location was associated with presence of clustered VA; 5) a greater amount of core scar predicted the occurrence of slower VA in patients with NICM; 6) higher BMI is related to faster VA in patients with ICM; and 7) VA subtype frequencies were similar across the type of cardiomyopathy.
CENTRAL ILLUSTRATION. Core Scar on Cardiovascular Magnetic Resonance Imaging Is Associated With Clustered Ventricular Arrhythmia in Patients With Nonischemic Cardiomyopathy.
CMR = cardiovascular magnetic resonance; ICM = ischemic cardiomyopathy; NICM = nonischemic cardiomyopathy; VA = ventricular arrhythmia.
COMPARISON WITH PRIOR RESEARCH AND MECHANISM.
Prior studies have highlighted the use of LGE on CMR to identify areas of scar in patients with NICM and ICM as well as to risk-stratify patients at risk for sudden death.4–8,12 Although clustered VAs have been associated with increased mortality risk, little is known about risk factors for clustering. Specifically, scar burden as a predictor has not been previously studied and is a novel finding of this study.2 Despite a similar incidence of clustered VA and VA subtypes in both types of cardiomyopathies, we noted that scar burden and risk for clustered VA depend on the type of cardiomyopathy.
The etiology of VA in ICM is thought to be re-entry related to myocardial scar from prior myocardial infarction, usually in a coronary distribution.13,14 Prior research has also shown that substrate spatial complexity analysis of LGE may help refine the risk for VA in those with ICM, especially as most patients with ICM and LGE on CMR do not experience VA.15 In contrast, the pattern of distribution of myocardial scar in patients with NICM is typically nonconfluent and often involves the midwall or subepicardial regions.16–18 Extensive work has been conducted to identify the association of scar patterns and arrhythmic risk in patients with ICM and NICM and has yielded a mixture of findings because of the heterogeneity not only of patient characteristics but of the underlying substrate.5,6,11,19 The results of our study align with those of prior studies showing a variety of scar patterns present in patients with NICM and subendocardial-based scar pattern predominating in patients with ICM.
We also identified a sex difference in recurrent VA risk in patients with NICM, whereby men were more likely to have recurrent VA than women. Elsokkari et al2 also showed that men were more likely to have clustered VA. However, on the basis of prior work, the etiology of sex differences in the incidence of VA and associated mortality in patients with NICM is unclear.20 It is posited that the incidence of VA is lower in women; however, the mortality associated with VA may be higher.
The relationship between BMI and VA has been previously reported. Pietrasik et al21 identified higher BMI as an independent risk factor for VA in patients with ICM by conducting a subanalysis of the MADIT II (Multicenter Automatic Defibrillator Implantation Trial II) study. The proposed mechanism of higher incidence of VA in this population was increased sympathetic activity caused by leptin, as the level of leptin positively correlates with BMI.22 Analysis of conduction velocity maps in a series of patients with ICM showed that conduction velocity was slower in areas of dense scar.23 Nishimura et al24 recently reported on the circuit determinants of VA cycle length. They noted that the principal determinant of VA cycle length was outer loop conduction velocity, which correlated with the extent of dense scar present in the outer loop. They found that in patients with NICM, there was no correlation between dense scar area and cycle length. However, the sample size of the study was only 49 patients. Overall, findings from our analysis support the notion that though scar burden plays a role, there may be multiple characteristics responsible for differences in VA cycle length.
In terms of the frequency of VA subtypes, there was a greater frequency of monomorphic VT in the clustered VA group compared with the unclustered VA group. This was an expected finding, as monomorphic VT was the most frequent type of VA, and those with clustered VA had more overall VA events than those with unclustered VA. There was a signal toward a higher occurrence of polymorphic VT in the NICM with clustered VA subgroup. Various patterns of LGE have been described in NICM (midwall scar25; anteroseptal and inferolateral location26; epicardial, transmural, combined septal and free wall LGE27) that are associated with composite VA and sudden cardiac death outcomes depending on the cohort studied. There have been very limited prior studies investigating scar predictors of type of VA. In a prior study of 87 patients with NICM, larger core extent, particularly transmural involvement of the basal wall, was associated with monomorphic VT, while the presence vs absence of scar was not associated with polymorphic VT or VF.28
STUDY LIMITATIONS.
First, the inherent limitations of a retrospective analysis of a cohort study apply, and baseline characteristics may have changed over time during the study. Patients were recruited on the basis of availability of the CMR scanner, which may have introduced bias. ICD programming parameters were not prescribed but determined by implanting electrophysiologists, as is the case for other contemporary ICD trials. Although device programming parameters evolved over the course of the study because of changes in clinical practice, we observed only a slight decline over time in appropriate ICD therapies, as we previously reported.29 Although clustered ATP for VA has also been associated with increased mortality,2 ATP therapy rates are most influenced by ICD programming parameters30 and may not reflect sustained VA. We thus chose to focus on clinically relevant, life-threatening arrhythmias terminated by ICD shocks, which are associated with the highest mortality.2 Including ATP therapy in future studies may enhance mechanistic insights.
We were not able to account for changes in antiarrhythmic drug therapy over time, which could have influenced VA characteristics over time, such as clustering pattern and cycle length. A limited number of clustered VA events precluded further stratification or analysis with extended models. Specifically, constructing more complex regression models using variables associated with heart failure mortality identified in the MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) study was not feasible.31 Similarly, analysis for the scar pattern and location was completed on a descriptive basis because of the relatively small sample size in subgroups.
FUTURE RESEARCH.
If our findings are confirmed in other cohorts, future research should explore whether earlier identification and treatment of patients with NICM with higher core scar burden reduces recurrence of VA and improves outcome.
CONCLUSIONS
We found that patients with NICM and scar were at the highest risk for clustered VA. Men were more likely to have recurrent VA than women. Among the NICM subgroup, core scar burden, as identified by CMR, was an important predictor of clustered VA. In contrast, neither core nor gray scar burden was associated with risk for clustered VA in patients with ICM. We additionally found that greater core scar burden was associated with slower VAs in patients with NICM, and higher BMI was associated with faster VAs in those with ICM. The type of VA present did not significantly differ between types of cardiomyopathy, and no distinct scar pattern predominated in those with NICM and clustered VA, though subepicardial and subendocardial scars with septal, inferior, or lateral locations were more frequent.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Patients with clustered VA have an increased risk for mortality, but risk factors for clustered VA are unknown. In this cohort consisting of primary prevention ICD recipients, an increasing burden of core scar on CMR was correlated with a greater risk for clustered VA and slower VA in patients with NICM, without a predominant scar pattern or location.
TRANSLATIONAL OUTLOOK:
The data presented in this study are expected to guide studies in using CMR for risk stratification for sudden cardiac death in patients with NICM.
ACKNOWLEDGMENT
The authors thank Lisa Yanek, MPH, for her expertise and review of statistical methodology.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This work was supported by National Heart, Lung, and Blood Institute grant R01HL103812 (to Dr Wu) and National Heart, Lung, and Blood Institute grant R01HL132181 (to Drs Wu and Tomaselli). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ATP
antitachycardia pacing
- BMI
body mass index
- CMR
cardiovascular magnetic resonance
- ES
electrical storm
- ICD
implantable cardioverter-defibrillator
- ICM
ischemic cardiomyopathy
- LGE
late gadolinium enhancement
- NICM
nonischemic cardiomyopathy
- VA
ventricular arrhythmia
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Fries R, Heisel A, Huwer H, et al. Incidence and clinical significance of short-term recurrent ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillator. Int J Cardiol. 1997;59:281–284. [DOI] [PubMed] [Google Scholar]
- 2.Elsokkari I, Parkash R, Tang A, et al. Mortality risk increases with clustered ventricular arrhythmias in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol EP. 2020;6:327–337. [DOI] [PubMed] [Google Scholar]
- 3.Guerra F, Shkoza M, Scappini L, Flori M, Capucci A. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Europace. 2014;16:347–353. [DOI] [PubMed] [Google Scholar]
- 4.Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. Eur J Heart Fail. 2013;15:1019–1027. [DOI] [PubMed] [Google Scholar]
- 5.Disertori M, Rigoni M, Pace N, et al. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. J Am Coll Cardiol Img. 2016;9:1046–1055. [DOI] [PubMed] [Google Scholar]
- 6.Di Marco A, Anguera I, Schmitt M, et al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. J Am Coll Cardiol HF. 2017;5:28–38. [DOI] [PubMed] [Google Scholar]
- 7.Wu KC, Gerstenblith G, Guallar E, et al. Combined cardiac magnetic resonance imaging and C-reactive protein levels identify a cohort at low risk for defibrillator firings and death. Circ Cardiovasc Imaging. 2012;5:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu KC. Sudden cardiac death substrate imaged by magnetic resonance imaging: from investigational tool to clinical applications. Circ Cardiovasc Imaging. 2017;10:e005461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almehmadi F, Joncas SX, Nevis I, et al. Prevalence of myocardial fibrosis patterns in patients with systolic dysfunction: prognostic significance for the prediction of sudden cardiac arrest or appropriate implantable cardiac defibrillator therapy. Circ Cardiovasc Imaging. 2014;7:593–600. [DOI] [PubMed] [Google Scholar]
- 12.Klem I, Klein M, Khan M, et al. Relationship of LVEF and myocardial scar to long-term mortality risk and mode of death in patients with non-ischemic cardiomyopathy. Circulation. 2021;143:1343–1358. [DOI] [PubMed] [Google Scholar]
- 13.Wijnmaalen AP, Schalij MJ, von der Thüsen JH, Klautz RJ, Zeppenfeld K. Early reperfusion during acute myocardial infarction affects ventricular tachycardia characteristics and the chronic electroanatomic and histological substrate. Circulation. 2010;121:1887–1895. [DOI] [PubMed] [Google Scholar]
- 14.Lo R, Chia KK, Hsia HH. Ventricular tachycardia in ischemic heart disease. Card Electrophysiol Clin. 2017;9:25–46. [DOI] [PubMed] [Google Scholar]
- 15.Okada DR, Miller J, Chrispin J, et al. Substrate spatial complexity analysis for the prediction of ventricular arrhythmias in patients with ischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2020;13:e007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrohon J, Moon J, Prasad S, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. [DOI] [PubMed] [Google Scholar]
- 17.Becker MA, Cornel JH, Van de Ven PM, van Rossum AC, Allaart CP, Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. J Am Coll Cardiol Img. 2018;11:1274–1284. [DOI] [PubMed] [Google Scholar]
- 18.Nakahara S, Tung R, Ramirez RJ, et al. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy: implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55:2355–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin DG, Lee H-J, Park J, et al. Pattern of late gadolinium enhancement predicts arrhythmic events in patients with non-ischemic cardiomyopathy. Int J Cardiol. 2016;222:9–15. [DOI] [PubMed] [Google Scholar]
- 20.Kim SK, Bennett R, Ingles J, Kumar S, Zaman S. Arrhythmia in cardiomyopathy: sex and gender differences. Curr Heart Fail Rep. 2021;18(5):274–283. [DOI] [PubMed] [Google Scholar]
- 21.Pietrasik G, Goldenberg I, McNitt S, Moss AJ, Zareba W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol. 2007;18:181–184. [DOI] [PubMed] [Google Scholar]
- 22.Maffei á, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. [DOI] [PubMed] [Google Scholar]
- 23.Aronis KN, Ali RL, Prakosa A, et al. Accurate conduction velocity maps and their association with scar distribution on magnetic resonance imaging in patients with postinfarction ventricular tachycardias. Circ Arrhythm Electrophysiol. 2020;13:e007792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura T, Upadhyay GA, Aziz ZA, et al. Circuit determinants of ventricular tachycardia cycle length: characterization of fast and unstable human ventricular tachycardia. Circulation. 2021;143:212–226. [DOI] [PubMed] [Google Scholar]
- 25.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 26.Piers SR, Tao Q, van Huls van Taxis CF, Schalij MJ, van der Geest RJ, Zeppenfeld K. Contrast-enhanced MRI–derived scar patterns and associated ventricular tachycardias in nonischemic cardiomyopathy: implications for the ablation strategy. Circ Arrhythm Electrophysiol. 2013;6:875–883. [DOI] [PubMed] [Google Scholar]
- 27.Di Marco A, Brown PF, Bradley J, et al. Improved risk stratification for ventricular arrhythmias and sudden death in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2021;77:2890–2905. [DOI] [PubMed] [Google Scholar]
- 28.Piers SR, Everaerts K, van der Geest RJ, et al. Myocardial scar predicts monomorphic ventricular tachycardia but not polymorphic ventricular tachycardia or ventricular fibrillation in non-ischemic dilated cardiomyopathy. Heart Rhythm. 2015;12:2106–2114. [DOI] [PubMed] [Google Scholar]
- 29.Wu KC, Wongvibulsin S, Tao S, et al. Baseline and dynamic risk predictors of appropriate implantable cardioverter defibrillator therapy. J Am Heart Assoc. 2020;9:e017002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 31.Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]


