Abstract
D-Dopachrome tautomerase (D-DT; or MIF-2) is a multifunctional protein with immunomodulatory properties and a documented pathogenic role in inflammation and cancer that is associated with activation of the cell surface receptor CD74. Alongside D-DT, macrophage migration inhibitory factor (MIF) is also known to activate CD74, promoting pathogenesis. While the role of the MIF/CD74 axis has been extensively studied in various disease models, the late discovery of the D-DT/CD74 axis has led to a poor investigation into the D-DT-induced activation mechanism of CD74. A previous study has identified 4-(3-carboxyphenyl)-2,5-pyridinedicarboxylic acid (4-CPPC) as the first selective and reversible inhibitor of D-DT and reported its potency to block the D-DT-induced activation of CD74 in a cell-based model. In this study, we employ molecular dynamics simulations and nuclear magnetic resonance experiments to study 4-CPPC-induced changes to the dynamic profile of D-DT. We found that binding of the inhibitor remarkably promotes the conformational flexibility of C-terminal without impacting the structural stability of the biological assembly. Consequently, long-range intrasubunit (>11 Å) and intersubunit (>30 Å) communications are enabled between distal regions. Communication across the three subunits is accomplished via 4-CPPC, which serves as a communication bridge after Val113 is displaced from its hydrophobic pocket. This previously unrecognized structural property of D-DT is not shared with its human homolog, MIF, which exhibits an impressive C-terminal rigidity even in the presence of an inhibitor. Considering the previously reported role of MIF’s C-terminal in the activation of CD74, our results break new ground for understanding the functionality of D-DT in health and disease.
Significance
D-Dopachrome tautomerase (D-DT) is an important immunostimulatory protein with a poorly understood structure/function relationship. Utilizing a protein dynamic approach, we demonstrate that interactions between the C-terminal and 4-CPPC enable novel intra- and intersubunit communications across the three monomers of D-DT. This structural property of D-DT provides new insights into the migration inhibitory factor superfamily and promotes understanding of D-DT functionality.
Introduction
Ligand binding often prompts conformational changes that inform the biological activity of a protein. Depending on the ligand, these structural events may have either a salutary or deleterious functional effect, providing, at the same time, mechanistic insight into the development of novel modulators (1,2). For transmembrane proteins, binding of an agonist triggers conformational changes that are essential for signaling cascades inside the cell (3). On the other hand, binding of an antagonist promotes structural changes that induce an inactive receptor state (4). Ligand-induced conformational changes have also been observed in soluble proteins, such as enzymes, and are coupled to their activities (5). These protein motions have been studied over a broad timescale range by a variety of methods, including NMR, cryoelectron microscopy, protein crystallography, spectrophotometry, and molecular dynamics (MD) simulations (6,7).
While several proteins undergo loop and large domain conformational changes to perform biological activities, others accomplish their tasks with minimal flexibility. A representative example of the latter is migration inhibitory factor (MIF). MIF is a well-known immunomodulatory protein that was first described in the 1960s (8,9). The MIF trimer possesses several nonoverlapping biological activities, including cytokine (10), chemokine (11), endonuclease (12), and catalytic (13). Considering the homotrimeric biological assembly is only ∼37 kDa, it is of great interest to understand how a small protein like MIF coordinates multiple biological activities. Recent studies exploring the dynamic features of MIF have shown that its central β-sheet is responsible for regulating dynamic signal transmission between distal functional sites (14,15). While no major conformational changes have been observed, dynamic regulation of these activities occur through correlated backbone and side-chain motions on the fast timescale.
D-Dopachrome tautomerase (D-DT) is a homolog of MIF and the second human member of the MIF superfamily. D-DT was originally isolated from rat liver and named after its ability to catalyze tautomerization of D-dopachrome (16). Despite the 34% sequence identity, MIF and D-DT share endonuclease, tautomerase, and certain cytokine activities. The cytokine activity is the most notable, as it is related to activation of the CD74/CD44 coreceptor complex (10,17). Activation of the receptor complex is associated with a cascade of MAP kinase signaling events resulting in various downstream effects, such as cell proliferation and inhibition of apoptosis (18).
The shared keto/enol tautomerase activity is of great interest as it is responsible for the identification of all reported small-molecule MIF and D-DT modulators (19,20,21,22,23,24,25). The enzymatic pocket is formed between two subunits and is controlled by the preserved N-terminus proline. In the absence of a defined biological substrate, various naturally occurring (4-hydroxyphenylpyruvate [4-HPP] and phenylpyruvate [PP]) and synthetic (D-dopachrome) model substrates have been used. The MIF and D-DT active sites share several key residues important for the binding and tautomerization of these substrates, though overall, the catalytic site of MIF is appreciably more hydrophobic than that of D-DT and contains several aromatic residues that D-DT lacks (25). These differences have culminated in numerous selective inhibitors for the better-studied MIF and a shared covalent inhibitor with D-DT known as 4-iodo-6-phenylpyrimidine. Meanwhile, only two reversible D-DT inhibitors have been described thus far, with the first, 4-(3-Carboxyphenyl)-2,5-pyridinedicarboxylic acid (4-CPPC), being reported recently (23,26,27). Crystallographic analysis of the D-DT/4-CPPC structure shows that the ligand binds via an induced fit mechanism, forcing the C-terminal to adopt an open conformation. Consequently, the C-terminal becomes highly flexible, which explains the lack of electron density for its last four residues, Met114-Leu117 (26). Considering the previously reported rigidity of the MIF trimer (28), the role of C-terminal residues in the MIF-induced activation of CD74 (19), and the similar structural topology between MIF and D-DT, it is of great importance to understand the potential biological significance of D-DT’s enhanced C-terminal flexibility.
In this study, we investigate the effect of 4-CPPC binding on the dynamic profile of D-DT. MD simulations confirmed the previously reported crystallographic findings and exposed mechanistic insights into the role of C-terminal flexibility. After we experimentally excluded the scenario of structural instability, we showed the effect on the C-terminal to be clearly dynamic, enabling long-range intersubunit communications across the D-DT trimer. These exciting findings offer a new avenue for understanding the vastly unexplored biological functionality of D-DT with a focus on the CD74 activation mechanism. At the same time, our analysis provides key information, which aids the efforts made thus far to explain the divergent functional profiles of MIF and D-DT.
Materials and methods
Materials and general methods
All chemicals used for buffers and synthesis were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Thermo Fisher Scientific (Waltham, MA, USA) and used without further purification. 1H NMR and 13C NMR spectra of 4-CPPC were collected on a JEOL (Peabody, MA, USA) ECA 600 MHz Fourier transform-NMR spectrometer. Protein NMR experiments were performed on a Bruker (Billerica, MA, USA) Avance NEO 600 MHz spectrometer at 30°C. Mass spectrometry (MS) spectra for small molecules were recorded on a JEOL Accu TOF LC time-of-flight mass spectrometer equipped with a Direct Analysis in Real Time (DART) ion source. Isothermal microcalorimetric measurements were performed on an affinity isothermal titration calorimetry (ITC) instrument (TA Instruments, New Castle, DE, USA).
Synthesis of 2,5-dimethyl-4-(3-methylphenyl)pyridine (1)
In a 25 mL round-bottomed flask, m-Tolylboronic acid (219 mg, 1.61 mmol) and 4-bromo-2,5-dimethylpyridine (200 mg, 1.08 mmol) were dissolved in a mixture of 1:1 iPrOH:water (4 mL). After adding Na3PO4, 12H2O (1.43 g, 3.76 mmol), and 10% Pd/C (40 mg, 3.5 mol %) to the flask, the reaction mixture was stirred for 12 h at 80°C under argon. The reaction mixture was diluted with water (10 mL) and Et2O (10 mL) and filtered through Celite. The filtrate was separated into two layers, and the aqueous layer was extracted with Et2O (2 × 10 mL). The combined organic layers were washed with brine (10 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. Gradient chromatography of the residue (silica gel) with 0%–2% MeOH in dichloromethane yielded compound 1 (115 mg, 54%). 1H NMR (600 MHz, CDCl3): δ 8.39 (s, 1H), 7.31 (m, 1H), 7.18–7.21p (m, 1H), 7.08–7.12 (m, 2H), 7.01 (s, 1H). 2.55 (s, 3H), 2.40 (s, 3H), 2.22 (s, 3H). 13C NMR (150 MHz, CDCl3): δ 155.61, 150.24, 149.96, 139.27, 139.10, 129.21, 128.64, 128.29,127.63, 123.77, 125.6, 23.79, 21.50, 16.90. High-resolution MS (DART) calc'd = calculated 198.1277 for C14H16N [M + H]+, found 198.1266.
Synthesis of 4-CPPC
In a 25 mL round-bottomed flask, compound 1 (115 mg, 0.583 mmol) and KMnO4 (507 mg, 3.21 mmol) were added to water (5 mL). After the reaction mixture was refluxed for 4 h, the excess of KMnO4 was reduced with Na2SO3 (74.0 mg, 0.587 mmol), filtered through Celite, and washed with hot water (95°C). The volume of the filtrate was reduced to 7 mL under vacuum. The filtrate was then acidified with 6 M HCl in an ice-water bath to yield white precipitates. The precipitates were collected under vacuum filtration, washed with ice-cold water (25 mL), and dried under vacuum overnight to yield 4-CPPC (70 mg, 42%). 1H NMR (600 MHz, deuterated dimethyl sulfoxide [DMSO-d6]): δ 9.10 (s, 1H), 8.07 (dt, J = 6.0 Hz, 1.8, 1H), 8.05 (s, 1H), 8.01 (t, J = 1.8 Hz, 1H), 7.77 (m, 1H), 7.67 (t, J = 6 Hz, 1H). 13C NMR (150 MHz, DMSO-d6): δ 167.27, 166.91, 165.48, 150.19, 149.96, 148.61, 137.87, 132.59, 131.04, 129.97, 129.61, 129.03, 128.93, 125.51. High-resolution MS (DART) calcd 288.0503 for C14H10NO6 [M + H]+, found 288.0486.
Protein expression and purification
Wild-type D-DT for kinetic experiments, ITC, and NMR studies was expressed and purified as previously described (26,29). For kinetic assays and ITC, a pET-22b plasmid encoding D-DT was transformed into BL21-Gold (DE3) E. coli cells and grown in Luria-Bertani medium at 37°C to an OD600 0.6–0.8. The 15N-labeled D-DT was expressed in a similar way, but instead of Luria-Bertani, the cells were grown in M9 minimal medium with 15NH4Cl to an OD600 0.8–1. Protein expression was induced with 1 mM IPTG and incubated at 37°C for 4 h (kinetics and ITC) or 20°C for 18 h (NMR). The cell culture was harvested by centrifugation and resuspended in 20 mM Tris and 20 mM NaCl (pH 8.5) supplemented with 1 mM phenylmethylsulfonyl fluoride. Cells were lysed on ice by sonication and then centrifuged to remove cell debris. The supernatant was filtered through a 0.22 μm filter, loaded onto a Q-Sepharose (anion-exchange) column, washed with 20 mM Tris and 20 mM NaCl (pH 8.5), and eluted with 5% of 20 mM Tris, and 1 M NaCl (pH 8.5). Additional contaminants were removed by size-exclusion chromatography (HiLoad 16/600 Superdex 75 pg). Protein purity was assessed by sodium dodecyl-sulfate polyacrylamide gel electrophoresis. The concentration of D-DT was determined by ultraviolet-visible spectroscopy utilizing ε280 = 5500 M−1cm−1 and Pierce Bicinchoninic Acid Protein Assay Kit (Thermo Fisher Scientific).
ITC
Before the ITC experiment, all samples were degassed for 5 min at room temperature. The titration experiment was carried out with the ligand (4-CPPC) in a 264 μL rotating syringe and wild-type D-DT in the isothermal sample cell. The final concentrations of ligand and protein were 500 and 100 μM, respectively. 4-CPPC was injected into the sample cell as 50 aliquots of 3.5 μL. The control run was carried out under identical conditions with the D-DT/4-CPPC titration experiment, but instead of protein, the sample cell contained buffer (20 mM Tris, 20 mM NaCl [pH 8.50]). The syringe rotational speed was 125 Rpm, each injection lasted 4 s, and the delay between injections was 300 s. Before the first injection, the instrument equilibrated for 1800 s. Data generated for ligand-to-buffer and ligand-to-protein experiments were processed in the NanoAnalyze software (v.3.11.0). The corrected heat plot of D-DT/4-CPPC was fitted in the independent model.
Tautomerase inhibition assay
Tautomerase inhibition assay was carried out in a similar manner as previously described (26). A 30 mM stock solution of 4-HPP was prepared in 0.5 M ammonium acetate (pH 6.20) and incubated overnight, under rocking, to favor the formation of the keto isoform. Serial dilutions of 4-HPP were then made, yielding a final concentration range between 0 and 2 mM. Following 4-HPP, borate was added at a final concentration of 0.420 M. The inhibitor (4-CPPC) was dissolved in 100% DMSO and added to the mixture providing a final concentration range of 0–75 μM. In all wells, the final concentration of DMSO was 1%. D-DT was then added at a final concentration of 250 nM. The reaction was measured in a Tecan Infinite M-Plex microplate reader at 306 nm for 300 s using 10 s intervals (ε306 = 11,400 M−1 cm−1). The kinetic experiments were performed in triplicate.
NMR titrations
All NMR data were processed using NMRPipe (30) and analyzed in Sparky (31). The DMSO tolerance of the D-DT protein was tested in a buffer of 20 mM Tris and 20 mM NaCl (pH 7.4). DMSO-d6 (Sigma-Aldrich) was titrated into the D-DT sample to final (v/v) percentages of 3.2%, 10%, 18.9%, and 26.8%, after which the integrity of the structure was monitored through 1H 1D and 2D experiments. Based on this analysis, 1H15N transverse relaxation optimized spectroscopy- heteronuclear single quantum coherence spectra of D-DT were subsequently collected in a buffer of 20 mM Tris, 20 mM NaCl, and 15% DMSO-d6 (pH 7.4). 4-CPPC was dissolved in 100% DMSO-d6 to create a stock solution at a concentration of 50 mM. Titrations were performed by adding small aliquots of 4-CPPC to the sample with gentle mixing by pipette before the acquisition of spectra.
MD simulations and analysis
The starting models for our 1 μs simulations were obtained from the crystal structures of apo D-DT (PDB: 1DPT) and D-DT/4-CPPC (PDB: 6C5F). The biological assembly of D-DT was generated in PyMOL (32), and the missing residues were added with Chimera (33). Protein structure files (PSFs) with hydrogens were generated using the Visual Molecular Dynamics plugin psfgen (34). The PSF for D-DT was produced using the CHARMM36 topology (35); meanwhile, a separate topology for 4-CPPC was generated using the CGENFF webserver. The resulting PSF and PDB pair were then used to generate a new set of PSF/PDB pairs that placed the protein in a TIP3P water box. TIP3P waters and the system’s net charge were set to 0 by the addition of sodium and chloride ions. Minimization, heating to 300 K, and equilibration processes were carried out with a 2 fs timestep, analogous to what has been previously described (15). Simulations were then run using NAMD 2.12 (36). Generalized cross correlation analysis was performed for the α-carbons using g_correlation (37), a GROMACS (38) plugin. Root-mean-square fluctuation (RMSF) analysis was performed in GROMACS. Analysis of the simulations was carried out in PyMOL. All simulations and subsequent analyses were completed in triplicate.
Results and discussion
Apo and 4-CPPC-bound D-DT demonstrate distinct dynamic profiles at the C-terminal
In a previous study, we reported the cocrystal structure of D-DT in complex with 4-CPPC and showed that ligand binding is associated with conformational changes of the C-terminal (26). The ligand-induced displacement of the C-terminal drastically increased the flexibility of the last four amino acids, Met114-Leu117, which, in protein crystallography, is expressed as lack of electron density. It is of great interest to note that increased flexibility of the C-terminal is an uncommon feature of the MIF superfamily members. Besides D-DT, only Plasmodium falciparum MIF has previously revealed a flexible C-terminal upon ligand binding (24).
To explore the significance of this finding, we performed 1 μs MD simulations of the apo D-DT- and 4-CPPC-bound structures. The length of these trajectories was within the timescale of loop motion and thus appropriate to examine the dynamic profile of the C-terminal (7). In agreement with the crystallographic observations, RMSF analyses of the apo D-DT and D-DT/4-CPPC structures showed noticeable differences, which are associated with 4-CPPC binding. Upon ligand binding, the C-terminal residues Lys109-Val113 are moderately affected, while Met114-Leu117 demonstrate a sharp increase of their dynamic activity (Fig. 1). A careful examination of the RMSF plots reveals that 4-CPPC has only a local dynamic effect, perturbing the C-terminal and, to a lesser extent, the α2/β4 loop (Val66-Gly68). Both regions are proximal to the 4-CPPC-binding site. The average RMSF values of 0.70 ± 0.3 and 0.76 ± 0.6 Å for the corresponding structures of apo D-DT (black dash line) and D-DT/4-CPPC further support that 4-CPPC does not alter the global dynamic profile of D-DT (Fig. 1). Interestingly, the previously reported RMSF value of apo MIF, which was determined at 0.90 Å by 1 μs MD simulations, is similar to the RMSF value of apo D-DT reported herein (28).
Figure 1.
Root-mean-square fluctuation of apo D-DT and D-DT/4-CPPC. The average root-mean-square fluctuation values of apo D-DT (0.70 ± 0.3 Å) and D-DT/4-CPPC (0.76 ± 0.6 Å) are shown as black and red dashed lines, respectively. This analysis was carried out in triplicate (n = 3) and the error values are shown as standard deviations (SD). Snapshots of the D-DT/4-CPPC structure, at 100 ns intervals, demonstrate the intense dynamic activity (black dashed circles) of the C-terminal. The C-terminal residues Met114-Leu117 are highlighted in red. To see this figure in color, go online.
4-CPPC binding enables novel correlations across the D-DT trimer
Correlation analysis is a tool employed to study physical motions of atoms within a protein under different stimuli such as mutations, protein-protein interactions, or ligand binding. Through this method, the dynamic characteristics of a protein are analyzed, and information about correlated motions are extracted (39). In the case of D-DT, the cross-correlation analyses of Cα fluctuations for apo D-DT and D-DT/4-CPPC were plotted and compared (Fig. 2, A and B). Correlation values range between 0 and 1, with 0 and 1 indicating no correlation and absolute correlation, respectively. Absolute correlation is only observed when comparing a residue with itself. Large correlation values are expected when analyzing adjacent residues. However, such values are not useful for understanding protein dynamics because fluctuations of residue “X” are expected to influence the adjacent residues (X−1 and X+1). In contrast, the large correlation values between distal D-DT residues provide important structural and functional insights.
Figure 2.
Correlation analyses of apo D-DT and D-DT/4-CPPC structures. (A and B) Correlation of Cα atoms was derived from 1 μs MD simulations using the apo (PDB: 1DPT) and 4-CPPC-bound (PDB: 6C5F) crystal structures of D-DT. Each black box (A–C) represents the three monomers of D-DT. Correlation values vary between 0 and 1, indicating no correlation and absolute correlation, respectively. The Y and X axes show the 351 amino acids of the D-DT trimer (Pro1-Leu117). The correlation plots of (A) apo D-DT and (B) D-DT/4-CPPC demonstrate noticeable differences, which are associated with binding of 4-CPPC. Correlations within a single monomer (intrasubunit) or between monomers (intersubunit) are marked in ellipses and boxes, respectively. The apo D-DT correlation plot shows the intra- and intersubunit correlations found in both structures. These correlations are marked in yellow (A) and called “independent” because they are not influenced by 4-CPPC binding. The intra- and intersubunit correlations associated with 4-CPPC binding (B) are shown in red. (C) The independent correlations (yellow) are illustrated on the D-DT monomer. (D) The ligand-induced correlations (red) are presented on the D-DT trimer. To see this figure in color, go online.
The D-DT correlations within a single monomer (intrasubunit) or between monomers (intersubunit) are marked in ellipses and boxes, respectively (Fig. 2, A and B). For clarity, independent and ligand-induced correlations are shown on different plots. The independent intra- and intersubunit correlations are marked in yellow on the apo D-DT plot (Fig. 2 A). Such correlations are called “independent” because they are formed in both apo D-DT and D-DT/4-CPPC structures and thus are not influenced by 4-CPPC binding. On the other hand, the ligand-induced intra- and intersubunit correlations are enabled by 4-CPPC binding. These correlations are presented on the D-DT/4-CPPC plot (Fig. 2 B).
Analysis of the independent correlations highlights the importance of the core β-sheet in coordinating communications across the D-DT trimer (Fig. 2 C). The N-terminal residues 1–3 form strong correlations with residues 37–39 and 61–63. At the same time, residues 61–63 are correlated with residues 99–101. A similar pattern of correlated motion was previously reported for MIF (28). While the two proteins also possess similar global flexibility, D-DTRMSF = 0.70 Å (reported herein) and MIFRMSF = 0.90 Å (28), our findings demonstrate common dynamic features that can be utilized for further elucidating their functionalities. Additional correlations within the D-DT monomer involve the following: residues 6–8 with 56–57, 7–10 with 43–45, 55–57 with 93–95, and 63–64 with 101–103. All reported intrasubunit correlations are illustrated on the D-DT monomer (Fig. 2 C). As expected, the intersubunit correlations, which are marked in yellow boxes (Fig. 2 A), are located at the three dimeric interfaces and are responsible for subunit-subunit communications.
Binding of 4-CPPC in the active site of D-DT enables new intra- and intersubunit communications, which are highlighted on the D-DT/4-CPPC plot (Fig. 2 B). The ligand-induced intrasubunit correlation, marked in the ellipse, associates residues 64–68 with 115–117. Residues 64–68 are located on the α2/β4 loop, while residues 115–117 are found at the C-terminal (Fig. 2 D). Intersubunit correlations between these two regions were noted across the D-DT trimer. At the same time, the C-terminal residues 115–117 of subunit A form strong correlations with the C-terminal residues 115–117 from subunits B and C. These intra- and intersubunit communications are all shown in the biological assembly of D-DT (Fig. 2 D).
Interactions between the C-terminal and 4-CPPC trigger the formation of new intra- and intersubunit communications
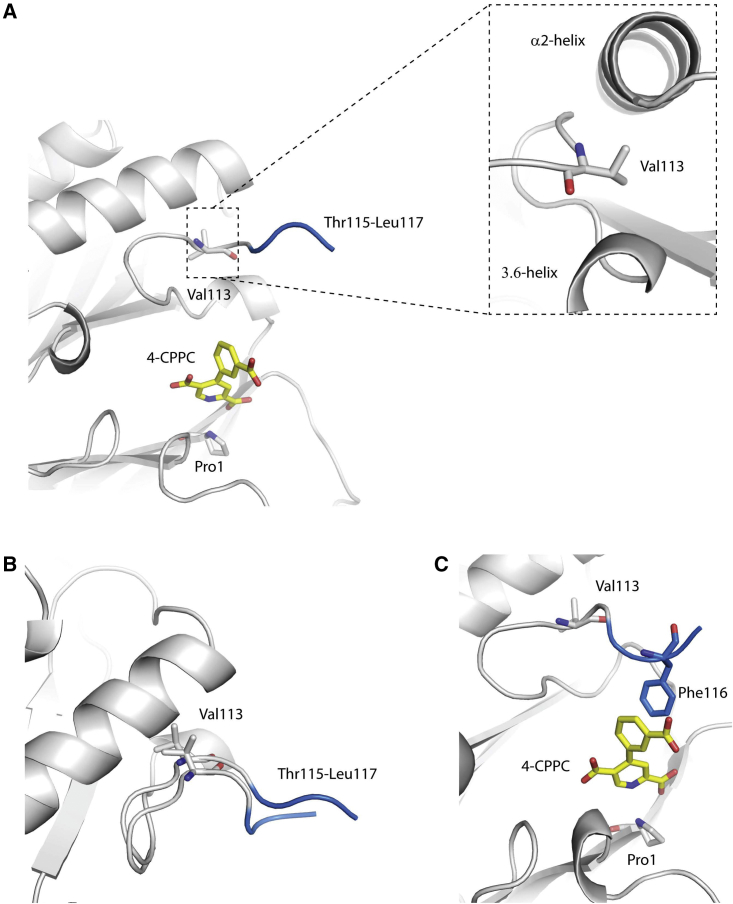
Structural analysis of D-DT demonstrates that the ligand-induced correlated regions are found at distances beyond the physical limits of direct communication. For example, the C-terminal residues 115–117 of chain A are found >30 Å away from the corresponding C-terminal segments of chains B and C. Similarly, residues 64–68 and 115–117 of subunit A are located >11 Å apart. To explain these findings, we closely examined the MD simulations of D-DT/4-CPPC. At the beginning of the simulations, the C-terminal is largely flexible, and Thr115-Leu117 are extended out into the solvent unable to form interactions with either the protein or 4-CPPC (Fig. 3 A). At the same time, Val113 is hidden in a hydrophobic pocket formed between the α2-helix and β5/C-terminal 3.6 helix. In that pocket, Val113 possesses the same orientation as seen in the crystal structures of apo D-DT and D-DT/4-CPPC (Fig. 3 A). As the simulations progress and the C-terminal retains its high flexibility, Val113 is displaced from the hydrophobic pocket (Fig. 3 B). This leads to a C-terminal conformational change that favors the formation of an aromatic-aromatic interaction between Phe116 and the benzoic moiety of 4-CPPC (Fig. 3 C). This interaction enables communication between the C-terminal and α2/β4 loop of the same monomer. In turn, intersubunit communications between the three C-termini are enabled via the adjacent α2-helix. Residues 64–68 of the α2/β4 loop, as well as the α2-helix, play a key role in the intersubunit correlations. Via this pathway, the ligand induces novel intra- and intersubunit communications across the biological assembly of D-DT.
Figure 3.
Intra- and intersubunit communication are triggered via interactions between the C-terminal and 4-CPPC. (A) Upon binding of 4-CPPC (yellow sticks), the C-terminal adopts a conformation that exposes The115-Leu117 (blue) into the solvent. Val113 (gray sticks) is hidden in a hydrophobic pocket, formed between the α2-helix and β5/C-terminal 3.6 helix. (B) As the simulations progress, Val113 is displaced from the hydrophobic pocket, allowing C-terminal residues Thr115-Leu117 to adopt a new conformation. (C) Due to the new conformation of the C-terminal, 4-CPPC forms aromatic-aromatic interactions with Phe116 (blue sticks) and enables several long-range communications across the D-DT trimer. To see this figure in color, go online.
Experimental interrogation of the D-DT/4-CPPC complex confirms the computational findings
To experimentally verify the computational findings, we employed solution NMR, a method that is regularly paired with MD simulations to examine protein dynamics under various stimuli. As part of this effort, we first synthesized 4-CPPC via a two-step pathway (Scheme S1). The structures o 4-CPPC and its biaryl precursor 1 were confirmed by 1H and 13C NMR (Fig. S1).
We performed ITC and kinetic experiments to confirm that the newly synthesized molecule binds D-DT as a competitive inhibitor. For the ITC experiments, the heat emitted from the ligand-to-buffer titration was used to correct our calorimetric data. The corrected data points of 4-CPPC-to-protein titration were fitted in the independent model, providing a KD of 51 μM (Fig. S2). The binding mode of 4-CPPC was then examined using the keto/enol enzymatic assay of D-DT. Our kinetic findings were in agreement with the previously published data, providing a competitive type of inhibition with an inhibition constant (Ki) of 38 μM (Fig. S3) (26).
To assist solubility of 4-CPPC and minimize NMR spectral artifacts during titration of D-DT with 4-CPPC, a background of 15% (v/v) DMSO-d6 was used without significantly altering the D-DT structure (Fig. S4). 1H 15N transverse relaxation optimized spectroscopy-heteronuclear single quantum coherence spectral overlays of apo D-DT with 0% vs. 15% DMSO-d6 showed only modest chemical shift perturbations in a consistent spectral pattern and direction, localized mainly to flexible loop regions. At first glance, the structural stability of D-DT in 15% DMSO-d6 may be unexpected. However, precipitation of a macromolecule in various organic solvents is highly influenced by the protein’s amino acid composition, pH, and polarity (40). In the case of D-DT, at low concentrations of DMSO, the protein has a higher affinity for water than DMSO, supporting its retention of natural structure. Protein precipitation and denaturation analyses of ribonuclease, lysozyme, β-lactoglobulin, and chymotrypsinogen have shown that these proteins retain their natural structures at DMSO concentrations higher than 15% (40).
According to the KD value of 4-CPPC for D-DT (Fig. S2), we designed two titration experiments. The first experiment was performed with the ligand and protein at a 1:1 molar ratio (Fig. 4 A) and the second with the ligand in a fivefold excess (Fig. S5 A). For the first titration experiment, we noticed that 4-CPPC induced many changes in the C-terminal face of the protein, including moderate chemical shift perturbations and a large number of line-broadened peaks, which is a qualitative signature of millisecond dynamics (Fig. 4 B and C). Chemical shift perturbations map to both α-helices and loop regions, while line broadening localizes mostly around the α2 helix, residues spanning the opening of the solvent cavity, and the C-terminal residues 111 and 112, which immediately precede the site involved in the induced fit of 4-CPPC (26). Residues known to be involved in the D-DT/4-CPPC interaction, or that become flexible upon binding of D-DT to 4-CPPC, are structurally or dynamically affected in the NMR experiments. Most notably, residues surrounding the 4-CPPC-binding pocket become highly flexible, consistent with the MD simulations.
Figure 4.
Solution interactions of 4-CPPC with D-DT. (A) 1H-15N heteronuclear single quantum coherence NMR spectral overlay of apo D-DT (black) with 4-CPPC-bound D-DT (red). Sample conditions were 0.5 mM D-DT in 20 mM Tris, 20 mM NaCl, and 15% (v/v) DMSO-d6 (pH 7.4). (B) NMR chemical shift perturbations caused by 4-CPCC. Gray vertical bars highlight sites of NMR line broadening. Blue and red dashed lines denote the 10% trimmed mean + 1.5σ of all shifts, respectively. Green triangles correspond to the 4-CPPC-interacting residues, while blue triangles correspond to residues that lack electron density in the 4-CPPC-bound x-ray crystal structure (26). (C) Chemical shifts greater than 1.5σ of the 10% trimmed mean (green spheres) are mapped onto the D-DT trimer and monomer structures (PDB: 7MSE), while blue spheres indicate sites of line broadening. The 4-CPPC ligand is also shown with the D-DT monomer, using PDB: 6C5F as the reference for alignment. To see this figure in color, go online.
The second titration experiment was performed having the ligand in a high excess. Under these conditions, 4-CPPC has a drastic impact on the global flexibility of D-DT (Fig. S5, A and B). Regions located distant from the binding site of 4-CPPC demonstrate elevated dynamic activity, which is consistent with the formation of intersubunit communications. While it is difficult to distinguish intra- and intersubunit communication in a symmetric molecule by NMR, the locations and types of the NMR-detectable perturbations provide some clues. NMR revealed chemical shift perturbations and extensive line broadening around the α2 helix and β4 sheet at the monomer-monomer interface, suggestive of intersubunit crosstalk. Likewise, most of the line-broadened resonances surround the D-DT solvent channel, which prior studies of both MIF and D-DT demonstrated to facilitate intermonomer communication (14,29). Other portions of D-DT, namely the α1 helix and loops decorating the periphery of the structure, display minimal evidence of line broadening and local chemical shift perturbations. These sites are also distal to the subunit interfaces, suggesting only intrasubunit effects. Given the low binding affinity of 4-CPPC, the chemical shift perturbation differences between the two titration experiments are attributed to the saturation level of D-DT. At stoichiometric quantities (1:1 molar ratio), the three active sites are not fully occupied, and thus the ligand has a moderate impact on the dynamics of the trimer. On the other hand, at a fivefold excess, all active sites are occupied to enable displacement of all three C-termini, which in turn enables the global communication between the three subunits. The limited solubility of 4-CPPC at NMR-level concentrations in >80% aqueous buffer precluded longer spin relaxation experiments to quantitate rates of conformational exchange and altered structural populations. Future work will focus on optimizing 4-CPPC solubility, perhaps through chemical modification, to facilitate further NMR studies.
Conclusions
In the absence of large-amplitude collective displacements, such as domain movements, proteins mediate fundamental biochemical processes via correlated motions of residues. Correlated motion is a fundamental property of the MIF β-sheet (41) that is used to finely tune at least two of the protein’s biological activities (14,15,28). Computational and experimental evidence demonstrate that MIF can execute a fascinating variety of functions without undergoing any major conformational changes. Instead, local flexibility is sufficient to modulate its functionality (14,28).
In contrast to MIF, D-DT reveals an unexpected mobility of its C-terminal. Conformational changes of the C-terminal resulting from phosphorylation (42), ion (43), or ligand (44) binding, or protein-protein interactions (45), are known to regulate a variety of biological tasks, the most notable being channel gating (46), signaling (47), and catalysis (44). For D-DT, the biological significance of C-terminal flexibility is enigmatic. A previous study comparing the D-DT/CD74 and MIF/CD74 interaction features showed that D-DT has a higher binding rate to CD74 but also a faster dissociation compared with MIF (17). These findings suggest that the D-DT-induced activation mechanism of CD74 may be noticeably different from the corresponding mechanism of MIF, involving distinct binding surfaces and/or conformations. Further studies on D-DT’s C-terminal are required to elucidate the potential functionality of this site. Such studies may include deletion(s) and/or single point mutation(s) of the residues (Met114-Leu117) previously shown to have enhanced flexibility upon 4-CPPC binding. The structural alterations to the C-terminal as a consequence of these modifications may increase or reduce the affinity to a substrate/inhibitor or even point the importance of C-terminal for activation of CD74. Collectively, the findings presented herein offer a fresh perspective for exploring and understanding the D-DT-induced activation mechanism of CD74.
Author contributions
A.P. expressed and purified D-DT, carried out the enzymatic assays, performed the MD simulations, prepared samples for ITC, analyzed data, and wrote and edited the manuscript. E.C. expressed and purified D-DT for NMR experiments, conducted the NMR experiments, analyzed data, and wrote and edited the manuscript. V.M.R. performed the ITC experiment. M.S. synthesized the precursor compound 1 and 4-CPPC. L.X. supervised organic synthesis and ITC experiments, analyzed data, and wrote and edited the manuscript. G.P.L. supervised the NMR experiments, analyzed data, and wrote and edited the manuscript, and G.P. conceived of supervised the project, analyzed data, and wrote and edited the manuscript. All authors have given approval to the final version of the manuscript.
Acknowledgments
This work was supported by start-up funds from Brown University, Rhode Island Foundation Medical Research grant GR5290658, and NIH R01 GM144451 (G.P.L.) and start-up funds from the University of the Pacific and Scholarly/Artistic Activities grant (G.P.). We are grateful for financial support for purchasing an isothermal titration calorimeter from the National Science Foundation (MRI-1828179).
Declaration of interests
The authors declare no competing interests.
Editor: Alexandr Kornev.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2023.02.019.
Supporting material
References
- 1.Pan M., Yu Y., et al. Zhao M. Mechanistic insight into substrate processing and allosteric inhibition of human p97. Nat. Struct. Mol. Biol. 2021;28:614–625. doi: 10.1038/s41594-021-00617-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen C.Y., Chang Y.C., et al. Tsai M.D. Temperature-resolved cryo-EM uncovers structural bases of temperature-dependent enzyme functions. J. Am. Chem. Soc. 2019;141:19983–19987. doi: 10.1021/jacs.9b10687. [DOI] [PubMed] [Google Scholar]
- 3.Zhao P., Truong T.T., et al. Wootten D. Implications of ligand-receptor binding kinetics on GLP-1R signalling. Biochem. Pharmacol. 2022;199:114985. doi: 10.1016/j.bcp.2022.114985. [DOI] [PubMed] [Google Scholar]
- 4.Wu B., Chien E.Y.T., et al. Stevens R.C. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karthikeyan S., Zhou Q., et al. Zhang H. Ligand binding-induced conformational changes in riboflavin kinase: structural basis for the ordered mechanism. Biochemistry. 2003;42:12532–12538. doi: 10.1021/bi035450t. [DOI] [PubMed] [Google Scholar]
- 6.Khurana L., ElGindi M., et al. Pantouris G. Elucidating the role of an immunomodulatory protein in cancer: from protein expression to functional characterization. Methods Enzymol. 2019;629:307–360. doi: 10.1016/bs.mie.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 7.Henzler-Wildman K., Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]
- 8.Bloom B.R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 9.David J.R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng L., Metz C.N., et al. Bucala R. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernhagen J., Krohn R., et al. Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., An R., et al. Dawson T.M. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science. 2016;354:aad6872. doi: 10.1126/science.aad6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubetsky J.B., Swope M., et al. Lolis E. Pro-1 of macrophage migration inhibitory factor functions as a catalytic base in the phenylpyruvate tautomerase activity. Biochemistry. 1999;38:7346–7354. doi: 10.1021/bi990306m. [DOI] [PubMed] [Google Scholar]
- 14.Pantouris G., Khurana L., et al. Lolis E.J. Regulation of MIF enzymatic activity by an allosteric site at the central solvent channel. Cell Chem. Biol. 2020;27:740–750.e5. doi: 10.1016/j.chembiol.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkins A., Skeens E., et al. Pantouris G. The N-terminus of MIF regulates the dynamic profile of residues involved in CD74 activation. Biophys. J. 2021;120:3893–3900. doi: 10.1016/j.bpj.2021.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odh G., Hindemith A., et al. Rorsman H. Isolation of a new tautomerase monitored by the conversion of D-dopachrome to 5,6-dihydroxyindole. Biochem. Biophys. Res. Commun. 1993;197:619–624. doi: 10.1006/bbrc.1993.2524. [DOI] [PubMed] [Google Scholar]
- 17.Merk M., Zierow S., et al. Bucala R. The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF) Proc. Natl. Acad. Sci. USA. 2011;108:E577–E585. doi: 10.1073/pnas.1102941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi X., Leng L., et al. Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pantouris G., Syed M.A., et al. Lolis E.J. An analysis of MIF structural features that control functional activation of CD74. Chem. Biol. 2015;22:1197–1205. doi: 10.1016/j.chembiol.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A.K., Pantouris G., et al. Cho T.Y. Structural basis for decreased induction of class IB PI3-kinases expression by MIF inhibitors. J. Cell Mol. Med. 2017;21:142–153. doi: 10.1111/jcmm.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho Y., Crichlow G.V., et al. Lolis E.J. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc. Natl. Acad. Sci. USA. 2010;107:11313–11318. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cournia Z., Leng L., et al. Jorgensen W.L. Discovery of human macrophage migration inhibitory factor (MIF)-CD74 antagonists via virtual screening. J. Med. Chem. 2009;52:416–424. doi: 10.1021/jm801100v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilstam P.V., Pantouris G., et al. Bucala R. A selective small-molecule inhibitor of macrophage migration inhibitory factor-2 (MIF-2), a MIF cytokine superfamily member, inhibits MIF-2 biological activity. J. Biol. Chem. 2019;294:18522–18531. doi: 10.1074/jbc.RA119.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pantouris G., Rajasekaran D., et al. Lolis E.J. Crystallographic and receptor binding characterization of Plasmodium falciparum macrophage migration inhibitory factor complexed to two potent inhibitors. J. Med. Chem. 2014;57:8652–8656. doi: 10.1021/jm501168q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajasekaran D., Zierow S., et al. Lolis E.J. Targeting distinct tautomerase sites of D-DT and MIF with a single molecule for inhibition of neutrophil lung recruitment. Faseb. J. 2014;28:4961–4971. doi: 10.1096/fj.14-256636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantouris G., Bucala R., Lolis E.J. Structural plasticity in the C-terminal region of macrophage migration inhibitory factor-2 is associated with an induced fit mechanism for a selective inhibitor. Biochemistry. 2018;57:3599–3605. doi: 10.1021/acs.biochem.8b00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Z., Osipyan A., et al. Dekker F.J. Thieno[2,3-d]pyrimidine-2,4(1H,3H)-dione derivative inhibits d-dopachrome tautomerase activity and suppresses the proliferation of non-small cell lung cancer cells. J. Med. Chem. 2022;65:2059–2077. doi: 10.1021/acs.jmedchem.1c01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantouris G., Ho J., et al. Lolis E.J. Nanosecond dynamics regulate the MIF-induced activity of CD74. Angew. Chem., Int. Ed. Engl. 2018;57:7116–7119. doi: 10.1002/anie.201803191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen E., Reiss K., et al. Lisi G.P. A structurally preserved allosteric site in the MIF superfamily affects enzymatic activity and CD74 activation in D-dopachrome tautomerase. J. Biol. Chem. 2021;297:101061. doi: 10.1016/j.jbc.2021.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaglio F., Grzesiek S., et al. Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 31.Lee W., Tonelli M., Markley J.L. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics. 2015;31:1325–1327. doi: 10.1093/bioinformatics/btu830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLano W.L. Delano Scientific, San Carlos; 2002. The PyMOL Molecular Graphics System. [Google Scholar]
- 33.Pettersen E.F., Goddard T.D., et al. Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 34.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 35.Vanommeslaeghe K., Hatcher E., et al. Mackerell A.D., Jr. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips J.C., Hardy D.J., et al. Tajkhorshid E. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020;153:044130. doi: 10.1063/5.0014475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange O.F., Grubmüller H. Generalized correlation for biomolecular dynamics. Proteins. 2006;62:1053–1061. doi: 10.1002/prot.20784. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Spoel D., Lindahl E., et al. Berendsen H.J.C. GROMACS: fast, flexible, and free. J. Comput. Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 39.Luo J., Bruice T.C. Ten-nanosecond molecular dynamics simulation of the motions of the horse liver alcohol dehydrogenase.PhCH2O- complex. Proc. Natl. Acad. Sci. USA. 2002;99:16597–16600. doi: 10.1073/pnas.262667599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arakawa T., Kita Y., Timasheff S.N. Protein precipitation and denaturation by dimethyl sulfoxide. Biophys. Chem. 2007;131:62–70. doi: 10.1016/j.bpc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Fenwick R.B., Orellana L., et al. Salvatella X. Correlated motions are a fundamental property of β-sheets. Nat. Commun. 2014;5:4070. doi: 10.1038/ncomms5070. [DOI] [PubMed] [Google Scholar]
- 42.Lee N.Y., Koland J.G. Conformational changes accompany phosphorylation of the epidermal growth factor receptor C-terminal domain. Protein Sci. 2005;14:2793–2803. doi: 10.1110/ps.051630305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumann F., Hoffmeister H., et al. Kalbitzer H.R. Ca2+-dependent conformational changes in a C-terminal cytosolic domain of polycystin-2. J. Biol. Chem. 2009;284:24372–24383. doi: 10.1074/jbc.M109.025635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mechaly A.E., Haouz A., et al. Bellinzoni M. Conformational changes upon ligand binding in the essential class II fumarase Rv1098c from Mycobacterium tuberculosis. FEBS Lett. 2012;586:1606–1611. doi: 10.1016/j.febslet.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 45.Deprez C., Lloubès R., et al. Blanchard L. Solution structure of the E.coli TolA C-terminal domain reveals conformational changes upon binding to the phage g3p N-terminal domain. J. Mol. Biol. 2005;346:1047–1057. doi: 10.1016/j.jmb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Ćelić A.S., Petri E.T., et al. Boggon T.J. Calcium-induced conformational changes in C-terminal tail of polycystin-2 are necessary for channel gating. J. Biol. Chem. 2012;287:17232–17240. doi: 10.1074/jbc.M112.354613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisselev O.G., Downs M.A., et al. Hargrave P.A. Conformational changes in the phosphorylated C-terminal domain of rhodopsin during rhodopsin arrestin interactions. J. Biol. Chem. 2004;279:51203–51207. doi: 10.1074/jbc.M407341200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.