Key Points
-
•
Lete-cel was well tolerated and showed antigen-specific antitumor activity in patients with relapsed/refractory multiple myeloma.
-
•
Lete-cel is a targeted therapy that showed HLA-restricted and antigen-specific (NY-ESO-1/LAGE-1A) antitumor activity in patients with relapsed/refractory multiple myeloma.
Visual Abstract
Abstract
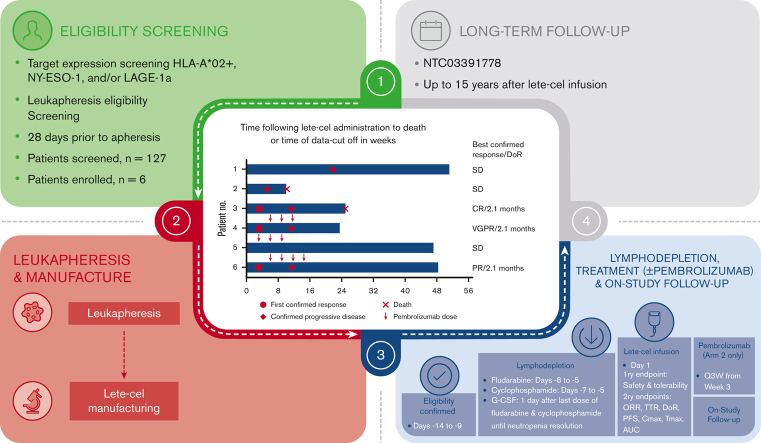
This pilot study assessed the safety and efficacy of letetresgene autoleucel (lete-cel; GSK3377794), a genetically modified autologous T-cell therapy targeting New York esophageal squamous cell carcinoma-1 (NY-ESO-1)/L antigen family member 1 isoform A (LAGE-1a)–positive myeloma cells, alone or in combination with pembrolizumab in patients with relapsed/refractory multiple myeloma. Eligible patients expressed NY-ESO-1 and/or LAGE-1a and either HLA-A∗02:01, ∗02:05, or ∗02:06. Patients received lete-cel single infusion alone (arm 1) or with pembrolizumab (arm 2). 127 patients were screened, and 6 patients (3 per arm) were enrolled; patients in arm 1 and 2 received lete-cel alone, or with pembrolizumab, respectively. All patients exhibited grade 3/4 cytopenias, which resolved or improved to grade 1. One patient (arm 1) had grade 3/4 lete-cel–related adverse events (AEs); 2 patients (arm 2) had grade 3/4 AEs related to lete-cel and lymphodepletion. Three patients with grade 1/2 cytokine release syndrome (CRS) exhibited elevated post–lete-cel interleukin-6 levels versus those without CRS. Pooled overall response rate was 50% including 1 patient each with confirmed clinical response, very good clinical response, and partial response, and progression-free survival ranged from 1.3 to 5.2 months. Responders (arm 1: n = 1; arm 2: n = 2) had a time-to-response of 3 weeks, duration of response of 2.1 months. Two responders, but no nonresponders, exhibited elevated cytokine levels after lete-cel infusion. Lete-cel had a manageable safety profile and demonstrated clear but transient antitumor activity in patients with relapsed/refractory multiple myeloma. This trial was registered at www.clinicaltrials.gov as #NCT03168438.
Introduction
Despite advances in treatment, novel therapeutic approaches for patients with relapsed/refractory multiple myeloma (RRMM) are needed.1 Patients with penta-refractory disease (ie, disease refractory to 2 proteasome inhibitors [PIs], 2 immunomodulatory agents, and an anti-CD38 monoclonal antibody [mAb]) have a median overall survival (OS) of only 5.6 months.1,2
One emerging therapeutic approach utilizes genetically modified T cells, including chimeric antigen receptor T-cell (CAR-T) therapy and T-cell receptor (TCR) T-cell therapy.3 CAR T cells are engineered to express synthetic receptors that bind to a specific surface antigen leading to activation, proliferation, and myeloma-directed cytotoxicity.4 Currently, 2 CAR-T therapies are approved for RRMM, which target B-cell maturation antigen (BCMA; ciltacabtagene autoleucel and idecabtagene vicleucel).5,6 In a pooled meta-analysis of 20 MM clinical trials with patients with RRMM the overall response rate (ORR) was 84% (range: 78%-89%) and median duration of response (DoR) was 11 months.2,3 TCR T-cell therapy is another form of T cell–based autologous therapy that, unlike CAR-T therapies, recognizes intracellular epitopes presented by HLA on a target cell.7 One such therapy is the investigational TCR T-cell therapy letetresgene autoleucel (lete-cel; GSK3377794), which consists of autologous T cells, genetically modified to target the 9-mer epitope presented on HLA-A∗02 that is shared by New York esophageal squamous cell carcinoma-1 (NY-ESO-1) and L antigen family member 1 isoform A (LAGE-1a).8 NY-ESO-1 and LAGE-1a are intracellular cancer-testis antigens that are highly immunogenic and potent inducers of cellular and humoral immunity.9,10 NY-ESO-1 and LAGE-1a are commonly detected on MM cells and have been linked to poor clinical outcomes, making them target antigens of interest in MM.11, 12, 13, 14, 15, 16, 17
In a phase 1 study (NCT01343043) of patients with advanced synovial sarcoma, lete-cel infusion after lymphodepletion chemotherapy demonstrated persistent, clinically meaningful activity with a manageable safety profile.18 In a prior study of lete-cel in 25 patients with advanced MM who had received an autologous hematopoietic cell transplant (HCT) 2 days before T-cell infusion, promising tumor antigen–specific antimyeloma activity was observed with 80% (20/25) of patients achieving a partial response (PR) or better at day 42, with a median progression-free survival (PFS) of 13.5 months and median OS of 35.1 months.19,20 However, the direct effect of lete-cel independent of concurrent HCT in MM remains unclear.
Programmed cell death receptor-1 (PD-1) expression may limit the innate and adaptive immune response in MM.21,22 PD-1 expression has also been observed in patients with RRMM after lete-cel treatment.19 Although single-agent PD-1 inhibition has demonstrated limited efficacy in MM clinical trials,23,24 the combination of antigen-targeting autologous T cells with a PD-1 inhibitor, such as pembrolizumab, could potentially improve treatment efficacy and durability. Therefore, the primary aim of this pilot study was to assess the efficacy as well as safety and tolerability of lete-cel, alone or in combination with pembrolizumab, in patients with RRMM.
Materials
Study design
This was an open-label, 2-arm (noncomparative), phase 1 study (NCT03168438) in adults with RRMM conducted at 5 centers in the United States. Up to 20 patients were to be enrolled into 2 study arms to receive lete-cel monotherapy (arm 1) or lete-cel in combination with pembrolizumab (arm 2). For the first 5 patients, enrollment was randomized 1:1 to arm 1 and arm 2. Following a protocol amendment, 1 further patient was enrolled into arm 1 with the intent of completing enrollment in arm 1 followed by enrollment into arm 2. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines after approval of the protocol and amendments by ethics committees and institutional review boards at each study site. All patients provided written informed consent.
Patients
Eligible patients were adults (aged ≥18 years) with primary refractory MM or with RRMM having received ≥2 prior regimens (containing ≥1 immunomodulatory agent and PI [alone or in combination]), with a response to ≥1 prior therapy but who were refractory to the most recent therapy. Patients had histologically confirmed secretory MM, defined as measurable myeloma protein (M-protein) in the serum (M-protein ≥0.5 g/dL for immunoglobulin G [IgG], IgM, IgA, or ≥0.05 g/dL for IgD) or urine (M-protein ≥200 mg per 24 hours) or measurable free light chain (FLC) in the serum (involved FLC ≥10 mg/dL and an abnormal FLC ratio [<0.26 or >1.65]).
Eligible patients had HLA-A∗02:01, HLA-A∗02:05, and/or HLA-A∗02:06 (assessed by high-resolution sequence-based blood typing) conducted at a central laboratory. Marrow myeloma cell expression of NY-ESO-1 and LAGE-1a were assessed by real-time polymerase chain reaction (a positive result was defined as a delta cycle threshold [ΔCT] of ≤5.0 for either NY-ESO-1 or LAGE-1a or, a ΔCT of ≤5.0 for both antigens and an average CT value of ≤35) conducted at a central laboratory. Patients had an Eastern Cooperative Oncology Group performance status score of ≤1, were fit for leukapheresis, and had adequate vital organ function.
Patients who received previous allogeneic HCT or gene therapy using a lentiviral vector were excluded. In arm 2 (pembrolizumab combination arm), patients who received prior therapy with anti–PD-1 or anti–PD-ligand (L)1/2 inhibitors were also excluded.
Procedures
Eligible patients were enrolled and had leukapheresis to supply T cells for lete-cel manufacture at a central laboratory (supplemental Figure 1). To manufacture lete-cel, patient T cells were enriched, stimulated with anti-CD3/CD28 mAbs, and transduced with a self-inactivating lentiviral vector expressing an affinity-enhanced NY-ESO-1/LAGE-1a–specific TCR.25 The planned lymphodepletion regimen was fludarabine (30 mg/m2 per day) on days −8, −7, −6, and −5, and cyclophosphamide (900 mg/m2 per day) on days −7, −6, and −5 by IV infusion (over 1 hour) followed by granulocyte colony-stimulating factor, 24 hours after the last dose of chemotherapy. Dose modification of lymphodepletion regimens was permitted for patients with a documented history of severe and prolonged cytopenias, advanced age (≥60 years), and/or renal impairment. For patients with progressive disease, bridging therapy was permitted between screening and leukapheresis and between leukapheresis and lymphodepletion.
Patients in both arms received between 1 × 109 and 8 × 109 transduced cells via IV infusion on day 1. Patients in arm 2 received the transduced cells followed by pembrolizumab (200 mg IV) every 3 weeks (Q3W) starting on week 3 after lete-cel infusion, up to the protocol-specified maximum study duration of 108 weeks. Patients were to be subsequently enrolled in a separate long-term follow-up study for up to 15 years after lete-cel infusion and completion of the study treatment phase (NCT03391778).
Assessments
The primary end point of this study was safety and tolerability. Adverse events (AEs), including treatment-limiting toxicities (TLTs) and serious AEs, were graded per the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0.26 Laboratory (chemistry, hematology, and coagulation) and cardiac assessments (by electrocardiogram) were collected throughout the study. TLTs were assessed Q3W and only in arm 2. Refer to the supplemental Methods for TLT definitions. AEs of special interest included hematopoietic cytopenias, cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), graft-versus-host disease (GVHD), and Guillain-Barré syndrome.
Secondary end points included efficacy outcomes. Investigator-assessed ORR was defined as a best overall confirmed response of a PR or better per the International Myeloma Working Group Uniform Response Criteria 2016.27 PFS was defined as the time between lete-cel infusion and earliest date of confirmed disease progression or death due to any cause. Refer to the supplemental Methods for additional details regarding clinical response assessments as well as secondary and exploratory end points including T-cell kinetics and serum cytokine measurements.
Statistics
The intention-to-treat (ITT) population included all patients who underwent leukapheresis. The modified ITT population included all participants in the ITT population who received lete-cel infusion and was the primary analysis population for efficacy and safety end points.
The ORR was estimated for each arm using 2-sided exact (Clopper-Pearson) 95% confidence intervals (CIs). PFS, DoR, and time to response were listed by patient. The study was not powered to conduct statistical comparisons. Refer to the supplemental Methods for further details of statistical treatment of response, survival, and biomarker analysis.
Results
Patients
Of 127 patients screened, 6 patients (3 patients per treatment arm) were enrolled (ITT population) and treated with lete-cel (modified ITT population) (supplemental Figure 2). Because of protracted enrollment and a shifting treatment landscape, the study was terminated early after the enrollment of these 6 patients without reaching its originally intended sample size.
All 6 patients completed the treatment phase of the study and ultimately had disease progression; 1 patient withdrew before study completion. Among the 5 remaining patients, 2 patients (arm 1) died after study completion and 3 patients (1 in arm 1 [patient 1] and 2 in arm 2 [patients 4 and 5]) transferred to the long-term follow-up study (NCT03391778). The 2 deaths in arm 1 after study completion occurred >30 days after lete-cel infusion, because of disease progression, and were considered unrelated to study treatment. The remaining 4 patients were still alive at the time of transfer to the long-term follow-up study or study withdrawal, with the last patient on study as of 5 February 2021. Follow-up for these 4 patients ranged from 5.9 to 12.8 months after lete-cel infusion.
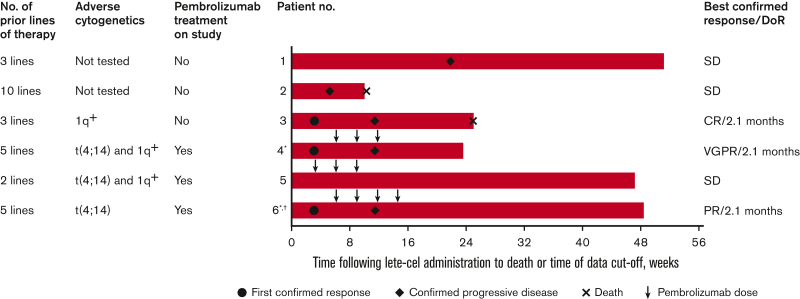
All patients were White males, with a median age of 63.0 years (Table 1). The median time from initial diagnosis to screening was 50.7 months. Two patients had t(4;14) and 3 patients had 1q amplification, both considered high-risk cytogenetics.28 Three patients had ≥5 prior lines of systemic therapy before study enrollment. All patients received prior treatment with immunomodulatory agents (lenalidomide, pomalidomide), PIs (bortezomib, carfilzomib), and an anti-CD38 mAb (daratumumab). Two patients (1 in each arm) had a history of autologous HCT, each between 4 and 5 years before lete-cel infusion. Four patients required bridging therapy before lete-cel infusion.
Table 1.
Patient demographics and baseline disease/clinical characteristics
| Arm 1 (lete-cel) (N = 3) | Arm 2 (lete-cel + pembrolizumab) (N = 3) | Total (N = 6) | |
|---|---|---|---|
| Median age, y (range) | 60.0 (59-79) | 64.0 (62-67) | 63.0 (59-79) |
| Male sex, n (%) | 3 (100) | 3 (100) | 6 (100) |
| HLA status, n (%) | |||
| HLA-A∗02:01–positive | 3 (100) | 3 (100) | 6 (100) |
| NY-ESO-1/LAGE-1a status, n (%)∗ | |||
| NY-ESO-1–positive | 2 (67) | 2 (67) | 4 (67) |
| LAGE-1a–positive | 3 (100) | 2 (67) | 5 (83) |
| Median time from initial diagnosis to screening, mo (range)† | n = 1 50.7 (50.7-50.7) |
n = 2 30.2 (7.3-53.1) |
n = 3 50.7 (7.3-53.1) |
| Disease stage at initial diagnosis, n (%) | |||
| Stage I/II | 1 (33) | 0 | 1 (17) |
| Stage III | 1 (33) | 1 (33) | 2 (33) |
| Unknown | 1 (33) | 2 (67) | 3 (50) |
| History of autologous HCT, n (%) | |||
| Yes | 1 (33) | 1 (33) | 2 (33) |
| Adverse cytogenetics, n (%)‡ | n = 1 | n = 3 | n = 4 |
| t(4;14) | 0 | 2 (67) | 2 (50) |
| 1q amplification | 1 (100) | 2 (67) | 3 (75) |
| Prior number of systemic therapy regimens, n (%) | |||
| 2 | 0 | 1 (33) | 1 (33) |
| 3 | 2 (67) | 0 | 2 (67) |
| 5 | 0 | 2 (67) | 2 (67) |
| ≥6 | 1 (33) | 0 | 1 (33) |
| Prior exposure to, n (%) | |||
| Immunomodulatory agents | |||
| Lenalidomide | 3 (100) | 3 (100) | 6 (100) |
| Pomalidomide | 3 (100) | 3 (100) | 6 (100) |
| PIs | |||
| Bortezomib | 3 (100) | 3 (100) | 6 (100) |
| Carfilzomib | 3 (100) | 3 (100) | 6 (100) |
| Anti-CD38 mAb | |||
| Daratumumab | 3 (100) | 3 (100) | 6 (100) |
Patient demographics and disease characteristics at screening for the mITT population, including all patients who received lete-cel.
mITT, modified ITT; N, patients in mITT population; n, patients with available data.
Eligible patients could have NY-ESO-1– and/or LAGE-1a–positive status.
Median time from diagnosis to screening is only provided for patients with complete diagnosis dates. Three patients had partial diagnosis dates and therefore were excluded from this row.
Patients could have more than 1 adverse cytogenetic feature.
Treatment
Patients in each arm received a single infusion containing similar numbers of transduced lete-cel T cells (ranging from 1.0 × 109 to 5.6 × 109 transduced cells). In arm 2, 2 patients received 3 doses of pembrolizumab, and 1 patient received 4 doses. The start of pembrolizumab dosing was delayed to week 6 in 2 patients because of ongoing grade 4 pancytopenia in 1 patient and grade 3 mucositis in the second patient, both related to lete-cel infusion and lymphodepleting chemotherapy.
Safety
All patients had at least 1 treatment-related AE, which included lymphodepletion-related AEs (n = 6) or lete-cel–related AEs (n = 5) (Table 2). The most common treatment-related AEs (ie, occurring in ≥50% [n ≥ 3 of 6] of patients) of any grade were anemia, leukopenia, and thrombocytopenia, which occurred in all patients, lymphopenia and neutropenia (83% each), and CRS (50%). All patients experienced at least 1 grade ≥3 treatment-related hematopoietic cytopenia, such as leukopenia, lymphopenia, or neutropenia. Cytopenias resolved in 4 patients or improved to grade 1 in 2 patients by data cutoff. There were no grade 5 AEs and no TLTs (assessed in arm 2 only); no deaths occurred within 30 days after lete-cel infusion.
Table 2.
Summary of treatment-related AEs
| Patients, n (%)∗ | Arm 1 (lete-cel) (N = 3) | Arm 2 (lete-cel + pembrolizumab) (N = 3) | Total (N = 6) |
|---|---|---|---|
| Treatment-related AEs | 3 (100) | 3 (100) | 6 (100) |
| Lymphodepletion-related | 3 (100) | 3 (100) | 6 (100) |
| Lete-cel–related | 2 (67) | 3 (100) | 5 (83) |
| Treatment-related SAEs | 1 (33) | 1 (33) | 2 (33) |
| Lymphodepletion-related | 1 (33) | 1 (33) | 2 (33) |
| Lete-cel–related | 1 (33) | 1 (33) | 2 (33) |
| Treatment-related AE grade ≥3† | |||
| Leukopenia | 3 (100) | 3 (100) | 6 (100) |
| Lymphopenia | 3 (100) | 2 (67) | 5 (83) |
| Neutropenia | 3 (100) | 2 (67) | 5 (83) |
| Anemia | 2 (67) | 2 (67) | 4 (67) |
| Thrombocytopenia | 2 (67) | 2 (67) | 4 (67) |
| Pancytopenia | 1 (33) | 1 (33) | 2 (33) |
| Atrial fibrillation | 0 | 1 (33) | 1 (17) |
| Stomatitis | 0 | 1 (33) | 1 (17) |
Treatment-related AEs in the mITT population, including all patients who received lete-cel. Safety assessed per NCI-CTCAE version 4.0.26
mITT, modified ITT; N, patients in mITT population; n, patients with available data; SAEs, serious AEs.
Study treatment-related AEs includes those related to lymphodepletion or lete-cel infusion and are defined as AEs with definite, probable, and possible study drug relationship; AEs are listed in descending order of total frequency. No patients experienced a grade 5 AE.
Cytopenias were reported as pooled terms: anemia included anemia/red blood cell count decrease; leukopenia included leukopenia/white blood cell decrease; thrombocytopenia included thrombocytopenia/platelet count decrease; lymphopenia included lymphopenia/lymphocyte count decrease; and neutropenia included neutropenia/neutrophil count decrease.
Treatment-emergent serious AEs are summarized in supplemental Table 1. Notably, 2 patients experienced grade ≥3 protracted pancytopenia, which was considered related to lymphodepletion and lete-cel infusion.
Three patients experienced grade 1/2 CRS, all of whom experienced a myeloma clinical response. One patient in arm 1 had grade 2 CRS (patient 3, with a best confirmed response of a complete response [CR]), 1 patient in arm 2 had grade 2 CRS (patient 4, with a best response of a very good PR [VGPR]), and 1 patient in arm 2 had grade 1 CRS (patient 6, with a best response of a PR). Patients with CRS (patients 3, 4, and 6) had elevated levels of interleukin-6 (IL-6) starting on day 3 after lete-cel infusion compared with patients with no CRS (patients 1, 2, and 5) (supplemental Figure 3A). All episodes of CRS were considered related to lete-cel and responded to treatment with tocilizumab, corticosteroids, and/or IV fluids.
In arm 2, 1 patient had grade 1 GVHD-like rash of the skin with symptoms of anemia, neutropenia, and rash; no symptom-specific treatment was administered. Another patient in arm 2 had grade 1 ICANS. Both GVHD and ICANS events resolved and were considered lete-cel related.
In addition, chemistry laboratory test-confirmed grade 3 AEs postbaseline were observed: hyperglycemia (arm 2: n = 1), increased alkaline phosphatase (arm 2: n = 1), hypophosphatemia (arm 2: n = 1), and hyponatremia (arm 1: n = 2). During the treatment period, 1 patient in arm 2 had a clinically significant abnormality on their electrocardiogram (QTcB interval of 502 milliseconds). At data cutoff, no additional AEs were reported for this patient after transfer to long-term follow-up.
Efficacy
The combined ORR across both arms (n = 6) was 50.0% (95% CI, 11.8-88.2; supplemental Table 2). The ORR was 33.3% (95% CI, 0.8-90.6) in arm 1 (which included patient 3 with a best response of a CR) and 66.7% (95% CI, 9.4-99.2) in arm 2 (which included patient 4 with a best response of a VGPR and patient 6 with a best response of a PR). Response over time for individual patients is shown in Figure 1. All responders had a time-to-response of 3 weeks and a DoR of 2.1 months. Clinical responses were observed only in patients who experienced CRS.
Figure 1.
Response outcomesover time for all patients. The best overall response per IMWG uniform response criteria 2016 after lete-cel administration in the mITT population, including all patients who received lete-cel. ∗Pembrolizumab initiation was delayed to week 6 in patients 4 and 6 due to ongoing AEs; †Pending decision to transfer to long-term follow-up study (NCT03391778) at time of data cutoff. IMWG, International Myeloma Working Group; mITT, modified ITT.
Both responses in arm 2 (patients 4 and 6) occurred before pembrolizumab initiation at week 6 (Figure 1). Before pembrolizumab initiation, patient 4 experienced a decline in FLC and serum M-protein, and patient 6 had a decline in FLC (supplemental Figure 4). In nonresponders (patients 1, 2, and 5), serum or urine M-protein and FLC levels were similar to, or increased from, baseline.
Several proinflammatory cytokines were also increased in responders (patients 3, 4, and 6) compared with nonresponders (patients 1, 2, and 5) starting at day 3 after lete-cel infusion (supplemental Figure 3). Evaluated granulocyte-macrophage colony-stimulating factor, interferon gamma, and IL-8 levels subsequently decreased after day 3, whereas IL-6 and, to a lesser degree, IL-2Rα levels remained elevated in responders relative to nonresponders through week 6.
In arm 1, individual patients achieved a PFS of 1.3, 2.8, and 5.2 months, respectively. In arm 2, 2 patients achieved a PFS of 2.8 months and 1 patient was censored and follow-up ended at 2.1 months (date of last adequate assessment). OS data were not mature at the time of data analysis.
Antigen expression and T-cell kinetics
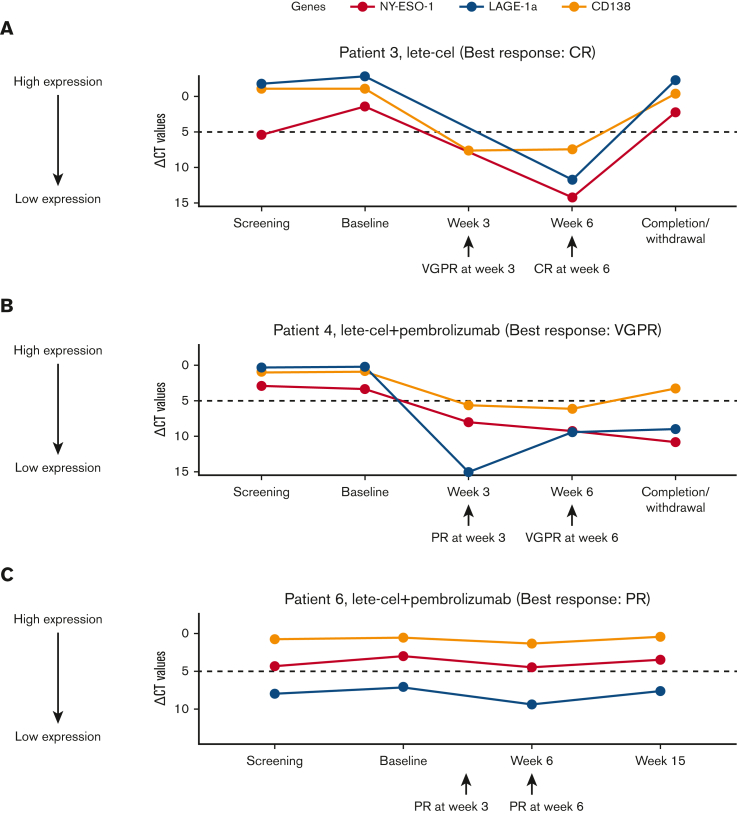
Two of the 3 responders (1 patient in arm 1 [patient 3; best response: CR] and 1 patient in arm 2 [patient 4; best response: VGPR]) exhibited clearance of antigen-positive myeloma cells in the bone marrow for up to 6 weeks after lete-cel infusion (Figure 2).
Figure 2.
Antigen expression over time in responders. Antigen expression (NY-ESO-1 and LAGE-1a) in bone marrow over time after lete-cel infusion in responders (patients who achieved a ≥PR per IMWG Uniform Response Criteria 2016). Dotted horizontal line at ΔCT (relative antigen expression) value = 5.0 represents GSK scoring threshold for antigen expression. IMWG, International Myeloma Working Group; ∆CT, delta cycle threshold (relative antigen expression).
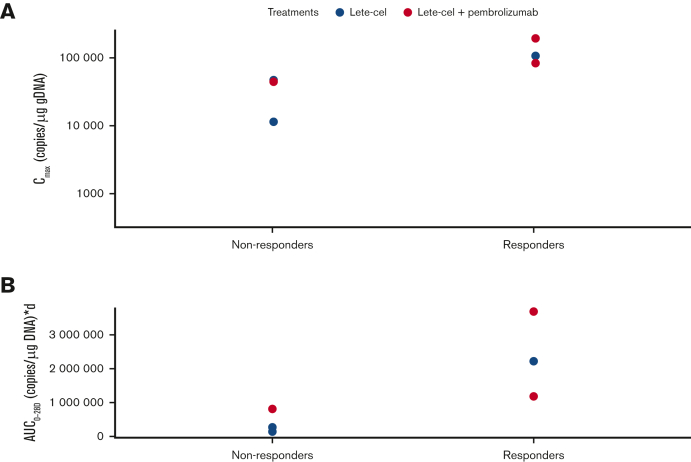
Responders exhibited a trend toward higher T-cell expansion (Cmax) compared with nonresponders (Figure 3A). Responders also showed a trend of higher T-cell exposure over the first 28 days after lete-cel infusion (AUC0-28d) compared with nonresponders (Figure 3B). T-cell persistence over time in individual patients is shown in supplemental Figure 5.
Figure 3.
T-cell expansion and exposure. (A) T-cell expansion (Cmax) in responders (patients who achieved a ≥PR per IMWG Uniform Response Criteria 2016) and nonresponders. (B) T-cell exposure shown (AUC0-28d) in responders vs nonresponders. AUC0-28d, area under the curve from day 0 to day 28; Cmax, peak T-cell expansion; gDNA, genomic DNA; IMWG, International Myeloma Working Group.
Case example
Among the 6 patients assessed, additional clinical characteristics, histology samples, and imaging scans were available for 1 patient in arm 1 (patient 3). This case is from a 79-year-old male who was diagnosed 3 years before study enrollment with symptomatic MM. At screening, this patient had 1q amplification, was positive for HLA-A∗02:01 and LAGE-1a expression, and had serum kappa light chain levels of 1239 mg/L. Radiologic assessments revealed extensive osteolytic bone disease as well as large soft tissue plasmacytomas on the left forearm and right side of the forehead. He had penta-refractory disease after 3 prior lines of therapy. Due to rapidly progressing disease, the patient received 3 separate lines of bridging therapy (line 1: daratumumab, carfilzomib, and pomalidomide [1 cycle]; line 2: bortezomib, low-dose melphalan, and dexamethasone [1 cycle]; line 3: daratumumab, bortezomib, doxorubicin, and low-dose melphalan [1 cycle]), and 2 rounds of radiotherapy (to the right femur and posterior skull) and surgery to the femur to stabilize a pathological fracture.
At the baseline visit (63 days after screening visit), the patient had serum kappa light chain levels of 3198 mg/L. Given his advanced age, the duration of fludarabine was reduced from 4 to 3 days and the dose of cyclophosphamide was reduced from 900 to 600 mg/m2 per day. The patient received lete-cel 5.6 × 109 transduced cells on day 1, along with granulocyte colony-stimulating factor (480 μg/d).
The patient experienced grade 2, nonserious, lete-cel–related CRS starting on day 1, with symptoms of gait disorder/tremor and hypotension, which was treated with tocilizumab (700 mg IV infusion). On days 2 to 5, he developed hypotension and hypoxia, which was treated with tocilizumab, corticosteroids, IV fluids, and supplemental oxygen. IL-6 levels peaked on day 3 (1426 ng/L) and day 5 (1380 ng/L) and gradually declined through week 1 (178 ng/L) and week 2 (127 ng/L) (supplement Figure 3A, patient 3). On day 6, the CRS was considered resolved.
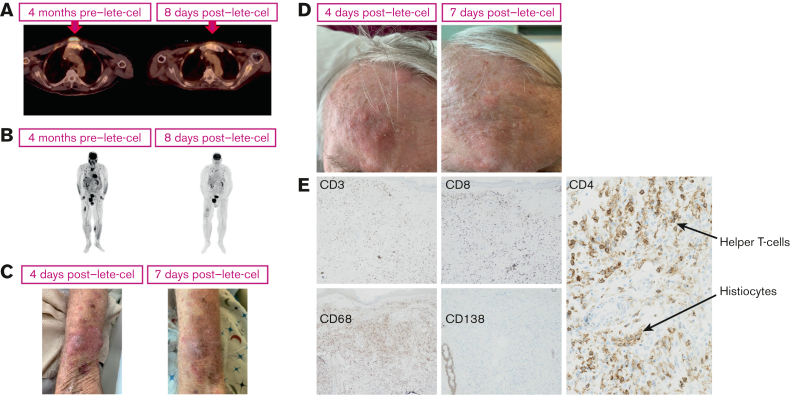
Representative positron emission tomography scan images from this patient demonstrated an overall reduction in tumor burden 1 week after lete-cel infusion compared with 4 months before lete-cel infusion (Figure 4A-B). The soft tissue plasmacytomas in the forearm and forehead also reduced in appearance from days 4 to 7 (Figure 4C-D). Biopsy of the forearm soft tissue plasmacytoma on day 7 after lete-cel infusion demonstrated an absence of CD138+ plasma cells and an abundance of tumor-infiltrating CD4+/CD8+ T cells and CD68+ histiocytes (Figure 4E).
Figure 4.
PET scans, photographs, and histology of MM lesions from the example case. (A) Axial and (B) coronal whole-body PET scan images demonstrating changes in tumor burden before (left) and after (right) lete-cel infusion. The arrow denotes prominent lesion shrinkage in the anterior, respiratory region. Images demonstrating the disappearance of soft tissue plasmacytomas on the patient’s left forearm (size at day 4: medial lesion [∼3 cm]; lateral lesion [∼2 cm]); (C) and left forehead (aggregate size at day 4: ∼5 cm); (D) from days 4 to 7 after lete-cel infusion. Biopsy of left forearm soft tissue plasmacytoma on day 7 after lete-cel infusion and IHC for T cells (CD3; original magnification ×100), T-helper cells (CD4; original magnification ×400), T-effector cells (CD8; original magnification ×100), histiocytes (CD68; original magnification ×100, also shown in CD4 panel [original magnification ×400]), and plasma cells (CD138; original magnification ×40) (E). Antibodies for IHC obtained from Roche Ventana, Oro Valley, AZ. Patient consent was obtained for use of all images. CD, cluster of differentiation; IHC, immunohistochemistry; PET, positron emission tomography.
At week 3 after lete-cel infusion, a VGPR was observed, with a serum kappa light chain level of 2 mg/L. A best response of CR was observed at 6 weeks, with a serum kappa light chain level of 5 mg/L. This response was maintained for 2.1 months. The patient was diagnosed with progressive disease on day 85 (serum kappa light chain levels to 609 mg/L) and died from myeloma on day 177.
Discussion
Genetically modified T-cell therapies, including CAR-T and TCR T-cell therapies, have shown promising results for patients with RRMM.2,20 CAR-T therapies have demonstrated an ORR of ∼84% with durable remissions achieved3; however, cures remain elusive because of a variety of resistance mechanisms, including immune escape and T-cell exhaustion.2,21 Current US Food and Drug Administration–approved CAR-T therapies for RRMM (ciltacabtagene autoleucel and idecabtagene vicleucel) target the surface antigen BCMA but may elicit potentially severe AEs, including fatal or life-threatening CRS and ICANS.2,5,6 TCR T-cell therapies are engineered to recognize intracellular antigens, which offer a broader pool of potential target tumor antigens compared with surface proteins.7 However, TCR T-cell therapy is HLA-restricted and depends on antigen presentation by HLA molecules for target recognition and T-cell activation.28 In addition, the use of normal physiologic T-cell signaling by TCR T cells could in theory improve the safety profile compared with CAR T cells.28
In this pilot study, the TCR T-cell therapy lete-cel demonstrated a manageable safety profile, consistent with the previous study in MM.20 Grade 1/2 CRS and ICANS events were observed but resolved with standard treatments, including tocilizumab and glucocorticoids. The frequency of these low-grade events compares favorably to the rates of CRS and neurotoxicity of any grade reported for CAR-T therapies in hematologic malignancies (CRS: 18%-100%; neurotoxicity: 20%-64%).29,30 To our knowledge, this is the first study to report clinically significant CRS and ICANS after TCR T-cell therapy in patients with RRMM. These AEs may reflect the higher levels of proinflammatory cytokines observed after lete-cel infusion, particularly in patients who achieved a clinical response. However, it should be noted that a significantly higher dose of transduced TCR T cells was used in this study (1.0 × 109 to 5.6 × 109 transduced cells) compared with the recommended dose for approved BCMA-directed CAR-T therapy (300 × 106 to 400 × 106 cells for idecabtagene vicleucel and 1 × 108 cells for ciltacabtagene autoleucel).5,6 Although the relationship between the dose of transduced cells and toxicity profile is unclear, some studies have shown that the severity of CRS and neurotoxicity events is influenced by the dose of transduced cells.30
This study, to the best of our knowledge, is the first to demonstrate lete-cel clinical activity and safety in patients with RRMM without concurrent autologous HCT.20 Lete-cel demonstrated modest but clear and rapid antimyeloma activity with or without pembrolizumab in 3 of 6 patients who achieved a PR or better. Treatment responses occurred early (within 1 month) after lete-cel administration, which was before the addition of pembrolizumab in patients who received combination treatment. Biomarker analyses supported an early-onset treatment response, with inflammatory biomarker levels peaking at day 3 in responders and declining over time. The DoR was 2.1 months in all responders in both treatment arms, PFS ranged from 1.3 to 5.2 months, and OS data were not mature at the data cutoff.
The 3 patients in this study who transferred into the long-term follow-up study survived ≥5.9 months after lete-cel infusion. One explanation for the lack of a clinical response in patients 1 and 5 and the short-lived clinical response in patient 4 (but a survival of almost 6 months in these 3 patients) may be defective trafficking of transduced T cells to the disease sites outside of the bone marrow. For example, upon relapse, patient 4 exhibited disease progression only in extramedullary sites (including soft tissue and later the pleural space) but not in the bone marrow for up to 1.5 years after lete-cel infusion (clinical observation; data not shown). A similar pattern of relapse also occurred in 1 patient with RRMM in the earlier study of lete-cel and autologous HCT20 who achieved a 1-year stringent CR and then had disease reappearance only in extramedullary soft tissue sites, which was associated with prominent stromal reactions (clinical observation; data not shown). During the 2-year follow-up after posttransplant lete-cel infusion, this patient had detectable gene-modified T cells in the blood. These clinical observations suggest potential ongoing effective immunosurveillance of the blood and bone marrow, which may not extend to extramedullary soft tissue sites.
Negative expression of NY-ESO-1 and LAGE-1a antigens after infusion in 2 responders suggests temporary clearance or suppression of antigen-positive malignant plasma cells from the bone marrow. A higher trend of T-cell kinetics in responders compared with nonresponders was also notable and is consistent with observations from lete-cel trials in patients with 2 types of soft tumor sarcomas: advanced synovial sarcoma (NCT01343043) or myxoid/round cell liposarcoma (NCT02992743).31,32 Limited TCR T-cell expansion and persistence, T-cell exhaustion, or other factors leading to T-cell dysfunction may also be mechanisms underlying the lack of response in the nonresponders, rather than a loss of antigen expression, which is a mechanism of resistance pertinent to CAR-T therapies.33 These observations suggest that better clinical responses after TCR-T therapy might be associated with enhanced in vivo expansion and exposure from more intensive lymphodepletion.33
A key challenge of this study was the patient eligibility in the United States, given the requirements for antigen expression and HLA criteria. NY-ESO-1 and LAGE-1a antigens are expressed in ∼50% and ∼30% of MM patient samples, respectively.11,16 In patients with cancer in the United States, between ∼42% and 46% carry the HLA-A∗02:01, ∗02:05, or ∗02:06 haplotypes, with HLA-A∗02:01 as the most common subtype regardless of race.34
A final challenge came from the rapidly changing treatment landscape in MM. Before initiation of this study, promising antimyeloma activity had been observed with the combination of pembrolizumab plus a PI or immunomodulatory agent in the phase 2 KEYNOTE-023 study.35,36 However, in subsequent pembrolizumab phase 3 trials (KEYNOTE-183 and -185), an unfavorable benefit-to-risk profile was observed with these combinations, and this led to the termination of these studies and halted additional investigation of PD-1/PD-L1 inhibitors in MM.35, 36, 37
Overall, these findings indicate that lete-cel may elicit a clinical benefit in patients with RRMM who carry the appropriate HLA antigens and sufficient NY-ESO-1/LAGE-1 expression. Importantly, this study, in a small proportion of patients with RRMM, shows that meaningful clinical responses may occur by targeting overexpressed intracellular tumor-associated antigens in myeloma by TCR T-cell therapy, which significantly expands the repertoire of potential immunotherapeutic targets.
Conflict-of-interest disclosure: T.N. receives/has received research funding from Novartis and Karyopharm and is a member of the advisory committee for Medexas but has declined to receive compensation. A.H., G.S.K., I.K., S.Z., J.W.D., W.D., T.F., A.U., and S.S. are employees of and/or hold stocks/shares in GSK. M.C. is an employee of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co, Inc, Rahway, NJ, US, and holds stocks/shares in Merck & Co, Inc, Rahway, NJ, US. A.P.R. declares no conflicts of interest other than support received as site principal investigator for this study. The remaining author declares no competing financial interests.
Acknowledgments
The authors acknowledge the clinical nurses and research staff, including Sunita Philip of the University of Maryland, for their care and support, as well as the courageous patients who participated in the study and their families. The authors also acknowledge Michael Kallen of the University of Maryland for patient pathology and immunohistochemistry slides and Charlotte Snape and Amit Jain from the Statistical and Programming team at GlaxoSmithKline (GSK).
This study (GSK study 208470; NCT03168438) was funded by GSK and is in collaboration with Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ. A.P.R was supported by the National Cancer Institute, Cancer Center Support Grant #P30CA134274 and by funds through the Maryland Department of Health’s Cigarette Restitution Fund Program. Editorial support was provided by Sarah Hauze and Mary Clare Cathcart of Fishawack Indicia Ltd UK, part of Fishawack Health, and Evangelia Giannakouri and Travis Taylor of Scion, London, UK, and was funded by GSK.
Authorship
Contribution: T.N. and J.E.H. were responsible for investigation, formal analysis, and reviewing and editing the manuscript; A.H., G.S.K., I.E., S.Z., T.F., A.U., and M.C. were responsible for study conceptualization, formal analysis, and reviewing and editing the manuscript; J.W.D. and S.S. were responsible for formal analysis and reviewing and editing the manuscript; and A.P.R. was responsible for study conceptualization, investigation formal analysis, and reviewing and editing the manuscript.
Footnotes
GlaxoSmithKline makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GlaxoSmithKline sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an inquiry via the website. Information about GlaxoSmithKline’s data-sharing commitments and access requests to anonymized individual participant data and associated documents can be requested for further research from ClinicalStudyDataRequest.com.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266–2275. doi: 10.1038/s41375-019-0435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SF, Xing L, Anderson KC, Tai YT. Promising antigens for the new frontier of targeted immunotherapy in multiple myeloma. Cancers (Basel) 2021;13(23):6136. doi: 10.3390/cancers13236136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagelmann N, Riecken K, Wolschke C, et al. Development of CAR-T cell therapies for multiple myeloma. Leukemia. 2020;34(9):2317–2332. doi: 10.1038/s41375-020-0930-x. [DOI] [PubMed] [Google Scholar]
- 4.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ABECMA (idecabtagene vicleucel) Bristol-Myers Squibb; 2021. US prescribing information. [Google Scholar]
- 6.CARVYKTI (ciltacabtagene autoleucel) Janssen Biotech, Inc.; 2022. US prescribing information. [Google Scholar]
- 7.Chandran SS, Klebanoff CA. T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunol Rev. 2019;290(1):127–147. doi: 10.1111/imr.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Angelo SP, Melchiori L, Merchant MS, et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 (c259)T cells in synovial sarcoma. Cancer Discov. 2018;8(8):944–957. doi: 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purbhoo MA, Sutton DH, Brewer JE, et al. Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J Immunol. 2006;176(12):7308–7316. doi: 10.4049/jimmunol.176.12.7308. [DOI] [PubMed] [Google Scholar]
- 10.Thomas R, Al-Khadairi G, Roelands J, et al. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol. 2018;9:947. doi: 10.3389/fimmu.2018.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrade VC, Vettore AL, Felix RS, et al. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 2008;8:2. [PMC free article] [PubMed] [Google Scholar]
- 12.Atanackovic D, Arfsten J, Cao Y, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109(3):1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 13.Condomines M, Hose D, Raynaud P, et al. Cancer/testis genes in multiple myeloma: expression patterns and prognosis value determined by microarray analysis. J Immunol. 2007;178(5):3307–3315. doi: 10.4049/jimmunol.178.5.3307. [DOI] [PubMed] [Google Scholar]
- 14.de Carvalho F, Vettore AL, Inaoka RJ, et al. Evaluation of LAGE-1 and NY-ESO-1 expression in multiple myeloma patients to explore possible benefits of their homology for immunotherapy. Cancer Immun. 2011;11:1. [PMC free article] [PubMed] [Google Scholar]
- 15.Jungbluth AA, Ely S, DiLiberto M, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106(1):167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 16.van Baren N, Brasseur F, Godelaine D, et al. Genes encoding tumor-specific antigens are expressed in human myeloma cells. Blood. 1999;94(4):1156–1164. [PubMed] [Google Scholar]
- 17.van Rhee F, Szmania SM, Zhan F, et al. NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood. 2005;105(10):3939–3944. doi: 10.1182/blood-2004-09-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Angelo SP DG, Van Tine BA, Druta M, et al. 298 final analysis of the phase 1 trial of NY-ESO-1–specific T-cell receptor (TCR) T-cell therapy (letetresgene autoleucel; GSK3377794) in patients with advanced synovial sarcoma (SS) J Immunother Cancer. 2020;8(Suppl 3):A1–A559. [Google Scholar]
- 19.Stadtmauer EA, Faitg TH, Lowther DE, et al. Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma. Blood Adv. 2019;3(13):2022–2034. doi: 10.1182/bloodadvances.2019000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa F, Marchica V, Storti P, Malavasi F, Giuliani N. PD-L1/PD-1 axis in multiple myeloma microenvironment and a possible link with CD38-mediated immune-suppression. Cancers (Basel) 2021;13(2):164. doi: 10.3390/cancers13020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura H, Ishibashi M, Yamashita T, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27(2):464–472. doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 23.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribrag V, Avigan DE, Green DJ, et al. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br J Haematol. 2019;186(3):e41–e44. doi: 10.1111/bjh.15888. [DOI] [PubMed] [Google Scholar]
- 25.Silk JD, Abbott RJM, Adams KJ, et al. Engineering cancer antigen-specific T cells to overcome the immunosuppressive effects of TGF-beta. J Immunol. 2022;208(1):169–180. doi: 10.4049/jimmunol.2001357. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. 2009. [Google Scholar]
- 27.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250. doi: 10.3389/fimmu.2019.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimabukuro-Vornhagen A, Godel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Angelo SP, Druta M, Van Tine BA, et al. Safety and efficacy of letetresgene autoleucel (lete-cel; GSK3377794) in advanced myxoid/round cell liposarcoma (MRCLS) following high lymphodepletion (Cohort 2): interim analysis. J Clin Oncol. 2021;39(15 suppl):11521. [Google Scholar]
- 32.Gyurdieva A, Zajic S, Chang YF, et al. Biomarker correlates with response to NY-ESO-1 TCR T cells in patients with synovial sarcoma. Nat Commun. 2022;13(1):5296. doi: 10.1038/s41467-022-32491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchsl F, Krackhardt AM. Adoptive cellular therapy for multiple myeloma using CAR- and TCR-transgenic T cells: response and resistance. Cells. 2022;11(3):410. doi: 10.3390/cells11030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel N, Carroll C, Culver K, et al. Population coverage of HLA-A∗02:01, ∗02:05, ∗02:06 across selected cancer types in the United States. J Immunother Cancer. 2021;9 [Google Scholar]
- 35.Mateos MV, Orlowski RZ, Ocio EM, et al. Pembrolizumab combined with lenalidomide and low-dose dexamethasone for relapsed or refractory multiple myeloma: phase I KEYNOTE-023 study. Br J Haematol. 2019;186(5):e117–e121. doi: 10.1111/bjh.15946. [DOI] [PubMed] [Google Scholar]
- 36.Moreau P, Ghori R, Farooqui M, Marinello P, San Miguel J. Pembrolizumab combined with carfilzomib and low-dose dexamethasone for relapsed or refractory multiple myeloma: cohort 2 of the phase I KEYNOTE-023 study. Br J Haematol. 2021;194(1):e48–e51. doi: 10.1111/bjh.17448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gormley NJ, Pazdur R. Immunotherapy combinations in multiple myeloma - known unknowns. N Engl J Med. 2018;379(19):1791–1795. doi: 10.1056/NEJMp1803602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.