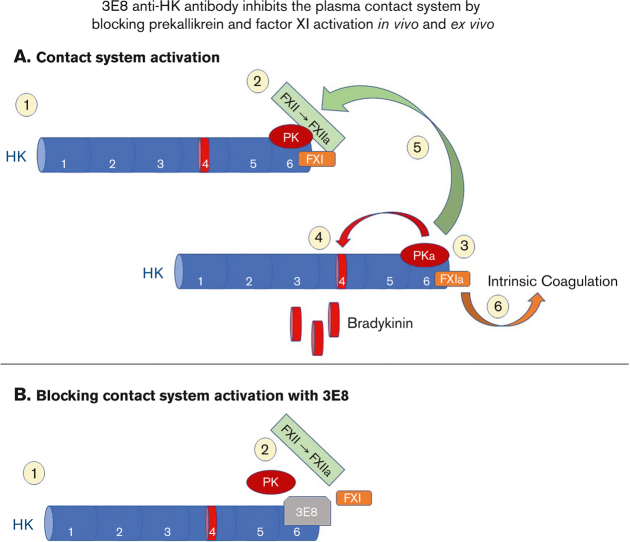
Key Points
-
•
3E8 anti-HK antibody inhibits thrombotic and inflammatory pathways of the contact system ex vivo and in vivo.
-
•
3E8 blocks the binding of PK and FXI to HK both ex vivo and in vivo.
Visual Abstract
Abstract
A dysregulated plasma contact system is involved in various pathological conditions, such as hereditary angioedema, Alzheimer disease, and sepsis. We previously showed that the 3E8 anti–high molecular weight kininogen (anti-HK) antibody blocks HK cleavage and bradykinin generation in human plasma ex vivo. Here, we show that 3E8 prevented not only HK cleavage but also factor XI (FXI) and prekallikrein (PK) activation by blocking their binding to HK in mouse plasma in vivo. 3E8 also inhibited contact system–induced bradykinin generation in vivo. Interestingly, FXII activation was also inhibited, likely because of the ability of 3E8 to block the positive feedback activation of FXII by kallikrein (PKa). In human plasma, 3E8 also blocked PK and FXI binding to HK and inhibited both thrombotic (FXI activation) and inflammatory pathways (PK activation and HK cleavage) of the plasma contact system activation ex vivo. Moreover, 3E8 blocked PKa binding to HK and dose-dependently inhibited PKa cleavage of HK. Our results reveal a novel strategy to inhibit contact system activation in vivo, which may provide an effective method to treat human diseases involving contact system dysregulation.
Introduction
The plasma contact system, which is mainly composed of factor XII (FXII), prekallikrein (PK), FXI, and high molecular weight kininogen (HK), plays important roles in both thrombosis and inflammation.1, 2, 3, 4 Many factors and pathological conditions can activate FXII.5, 6, 7, 8, 9 Activation of FXI by activated FXII (FXIIa) triggers the intrinsic clotting pathway, leading to thrombin generation and fibrin clot formation.7,10,11 FXIIa also activates PK to kallikrein (PKa), which leads to the release of bradykinin from its precursor HK and subsequent activation of inflammatory processes.7,12, 13, 14, 15, 16, 17 PKa can positively feedback to activate FXII to amplify contact system activation. This system works most efficiently when PK and FXI are bound to HK.18, 19, 20, 21, 22
Dysregulation of the contact system is involved in various disease conditions, such as sickle cell anemia,23 hereditary angioedema,24, 25, 26 inflammatory bowel disease,27,28 Alzheimer disease,7,29,30 sepsis,31 lupus,32 arthritis,33,34 cancer metastasis,35 and other pathological conditions.3,16,36, 37, 38, 39, 40, 41, 42, 43 Importantly, patients with a deficient contact system do not bleed,11 making it an ideal target for developing inhibitors.
HK is a nonenzymatic cofactor that allows for optimal activation of the contact system; it circulates in the blood complexed with PK or FXI,21,44 thus playing an important role in both the inflammatory and thrombotic pathways. Therefore, targeting HK may provide additional options to interfere with contact system activation.
To study the effects of targeting HK in contact system activation, we generated a hamster monoclonal antibody (clone 3E8) against a 20–amino acid region of HK’s domain 6 (the PK/FXI binding site).45 The 3E8 anti–HK antibody blocks both dextran sulfate (DXS)– and amyloid-β (Aβ)–induced HK cleavage and bradykinin generation in human plasma ex vivo.13 The 20-residue peptide abolishes the protective effect of 3E8 from contact system activation–induced HK cleavage, confirming that 3E8’s effect occurs because of binding to the PK/FXI binding site on HK. 3E8 has high binding affinity for human HK (KD = 168 ± 38 pM) and pulls down HK but not FXII or PK from normal human plasma (NHP). It disassembles HK/PK and HK/FXI complexes in NHP in the absence of a contact system activator, but it does not affect HK binding to negatively-charged surfaces such as Aβ.46
In this study, we focused on the in vivo effects of 3E8 and the mechanisms by which 3E8 inhibits contact system activation. Our results show that 3E8 effectively blocked HK cleavage and bradykinin generation in vivo, and the enzymes responsible for plasma contact system activation, such as FXII, FXI, and PK, were inhibited by 3E8 IV injection in vivo. Therefore, to our knowledge, our results reveal a novel mechanism and strategy to inhibit plasma contact system activation that works effectively both ex vivo and in vivo, which may provide an additional option for treating human diseases involving contact system dysregulation.
Materials and methods
Antibodies and materials
The 3E8 anti–HK antibody was prepared as described previously.45 For all reagent sources and preparations, see supplemental Materials and Methods.
Animals
All animal experiments were conducted in accordance with the guidelines of the US National Institutes of Health guide for the care and use of laboratory animals and with approval from the animal care and use committee of The Rockefeller University. For details, see supplemental Materials and Methods.
Mouse plasma preparation
Blood was collected through either retro-orbital (RO) plexus or by tail clipping.12 For RO bleeding, mice were anesthetized and treated as described in supplemental Materials and Methods, their RO plexus was penetrated with the coated capillary tube, and blood was collected into EDTA-coated tubes. Platelet poor plasma was prepared and kept at −80°C until analysis. For tail clipping, a tiny piece of soft tissue at the end of the tail was clipped. Blood was collected into EDTA-coated tubes for plasma preparation. Pulldown and western blot experiments are described in “Pulldown experiments” and “Western blotting.”
Human blood collection and plasma preparation
Collection and preparation of human plasma was approved by The Rockefeller University institutional review board. Blood from healthy human donors (n = 8) who had given informed, written consent was drawn with 21-gauge 0.75 inch butterfly needles with a multiadapter for S-Monovette into S-Monovette tubes containing 1:10 volume 105 mM trisodium citrate solution or EDTA (final concentration, 5 mM) at The Rockefeller University Hospital. Platelet poor plasma was prepared as described previously.45
Pulldown experiments
Biotinylated human HK (B-hHK)/phosphate-buffered saline (PBS), B-hHK/3E8 (1:3 M ratio), and B-hHK/hamster immunoglobulin G (IgG, 1:3 M ratio) mixtures were incubated with kininogen-deficient (KN-DF) plasma or NHP at 37°C for 20 minutes (90 μg hHK/mL plasma total).47 In 2B7 anti–HK antibody pulldown experiment, NHP was incubated with 3E8/PBS/IgG, then incubated with B-2B7 at 37°C for 20 minutes. In B-hHK injected mouse plasma pulldown experiments, mouse plasma collected 30 minutes after the injection was used for the study. Dynabeads M-280 Streptavidin was used to pull down the B-hHK or hHK/bound protein complex from human or mouse plasmas, according to the manufacturer’s instructions. Samples were eluted with sodium dodecyl sulfate sample buffer and analyzed by western blotting.
DXS treatment
KN-DF human plasma was incubated with hHK/PBS, hHK/3E8, or hHK/hamster IgG at 37°C for 20 minutes. DXS was added and incubated at 37°C for 60 minutes. For NHP, PBS, 3E8, or hamster IgG was added and incubated at 37°C for 20 minutes, followed by DXS for 60 minutes at 37°C. Samples were then analyzed by western blotting.
Western blotting
Western blots were performed as described previously.48 For details, see supplemental Materials and Methods.
FXIIa, PKa, and FXIa activity assays
FXIIa and PKa activities were measured using chromogenic substrate S2302.49 FXIa activity was measured using chromogenic substrate S2366.50 For details on these assays, see supplemental Materials and Methods.
Statistical analyses
All statistical analyses were performed using GraphPad Prism software. Comparisons among multiple groups were performed using 1-way analysis of variance followed by Newman-Keuls multiple comparison test.
Results
3E8 anti–HK antibody inhibits DXS-induced hHK cleavage and bradykinin production in KN-1 knockout (KN1-KO) mice in vivo
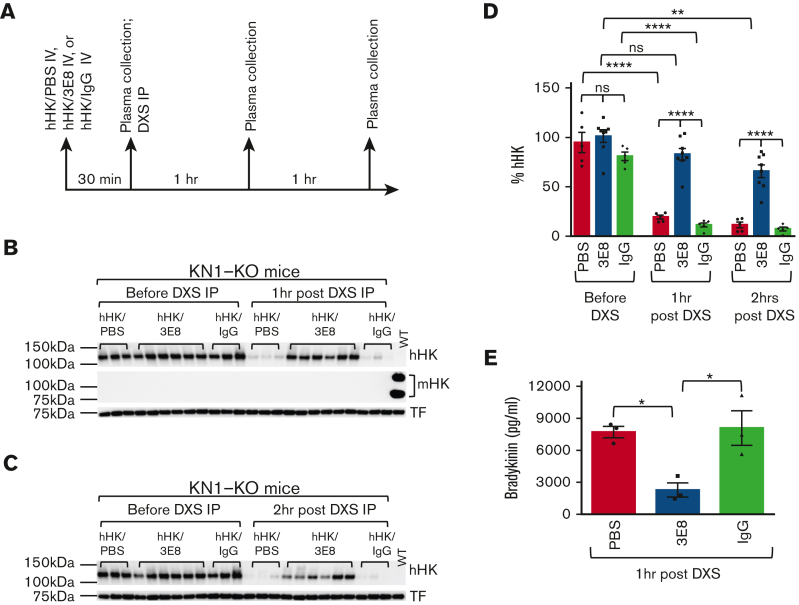
We showed previously that the 3E8 anti–HK antibody blocks DXS- or Aβ-induced HK cleavage in NHP ex vivo.13 This antibody was raised against hHK, which shares only ∼56% homology to mouse HK (mHK) (data not shown), and therefore does not recognize mHK (data not shown). To analyze whether 3E8 can inhibit hHK cleavage in vivo, we used KN1-KO mice51 and IV-injected hHK with PBS, 3E8 anti–HK antibody, or hamster IgG. Thirty minutes after hHK injection, plasma was collected and then DXS, a strong contact system activator,13,52,53 was intraperitoneally injected to induce mouse contact system activation. One or 2 hours after DXS injection, mouse plasma was prepared for western blot analysis (Figure 1A). Our results show that the amount of hHK in mouse plasma was similar among PBS-, 3E8-, or IgG-injected mice before DXS administration (Figure 1B-D). One or 2 hours after DXS injection, hHK was cleaved and intact hHK was dramatically decreased in hHK/PBS- and hHK/IgG-injected mice. However, in hHK/3E8-injected mice, hHK was protected from cleavage even 2 hours after DXS injection (Figure 1C-D). Because DXS is a very strong contact system activator, it is not surprising to observe some hHK cleavage 2 hours after DXS injection in the presence of 3E8 anti–HK antibody (Figure 1C-D). Plasma from 3E8-injected mice showed significantly less bradykinin, indicating 3E8 prevented DXS-induced HK cleavage and bradykinin production in vivo (Figure 1E). Because the half-life of hHK in mouse circulation is ∼24 hours (supplemental Figure 1), the disappearance of hHK in mouse blood after injection is a result of DXS-induced plasma contact system activation and not due to a short half-life of hHK. KN1-KO mouse plasma was confirmed with an anti-mouse HK antibody (Figure 1B), which does not crossreact with hHK. Plasma from a wild-type mouse was used as a control for anti-mouse and antihuman HK antibodies (Figure 1B). Although these results show that the 3E8 anti–HK antibody inhibited DXS-induced hHK cleavage and bradykinin generation in vivo, they also show that the mouse contact system, once activated, can cleave the injected hHK protein in vivo.
Figure 1.
3E8 anti–HK antibodyinhibitsDXS-induced hHK cleavage and bradykinin release in KN1-KO mice in vivo. (A) KN1-KO mice were IV injected with hHK along with either PBS, 3E8, or hamster IgG as described in “Methods” (n = 5-8 mice per group). After 30 minutes, blood was collected and plasma was prepared. DXS was then intraperitoneally injected, and 1 or 2 hours after DXS injection, blood was collected and plasma was prepared and analyzed by western blotting. (B) Representative western blot shows that injected hHK levels were consistent between groups before DXS injection. One hour after DXS injection, hHK was cleaved and its levels were decreased in hHK/PBS and hHK/hamster IgG groups, but hHK was protected from cleavage in hHK/3E8-injected mice. Mouse HK was not detected in KN1-KO mouse plasma but was present in wild-type mouse plasma. The membrane was stripped and reprobed for transferrin (TF) for normalization. (C) Representative western blot shows hHK changes before and 2 hours after DXS injection into KN1-KO mice. Two hours after DXS injection, hHK was cleaved and dramatically decreased in hHK/PBS and hHK/hamster IgG–injected mice but was protected from cleavage in hHK/3E8-treated mice. (D) Statistical analyses show that the injected hHK levels in groups of mice were similar before DXS injection, but 1 and 2 hours after DXS injection, hHK in 3E8-injected mouse plasma was significantly protected from cleavage compared with hHK in PBS or hamster IgG–injected plasmas. hHK signal was normalized to TF. n = 5 to 8/group. (E) Plasma bradykinin levels in DXS-injected KN1-KO mice infused with hHK/PBS, hHK/3E8, or hHK/IgG were measured according to manufacturer’s instructions. 3E8 significantly inhibited DXS-induced bradykinin generation. n = 3 per group. Data are denoted as mean ± standard error of the mean (SEM). ∗P<.05, ∗∗P≤.01, ∗∗∗∗P≤.0001, P>.05 was not significant (ns).
3E8 anti–HK antibody inhibits DXS-induced hHK cleavage by blocking mouse PK and mouse FXI from binding to hHK in vivo
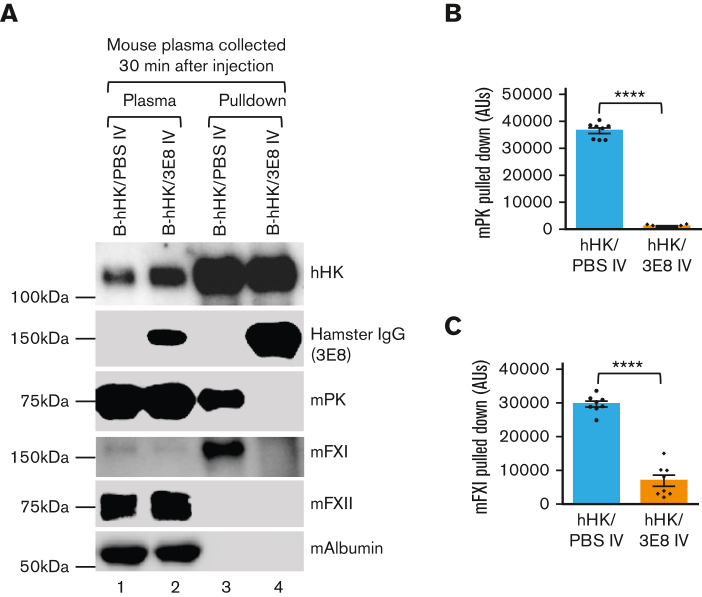
We analyzed the mechanism by which 3E8 anti–HK antibody inhibits DXS-induced hHK cleavage in vivo. B-hHK was IV injected with PBS or 3E8 anti–HK antibody in KN1-KO mice. Thirty minutes later, mouse plasma was collected and used to pull down B-hHK–bound proteins in mouse blood in the presence or absence of 3E8 anti–HK antibody. As expected, hHK was present in the injected mouse plasma, and hamster IgG was present in the 3E8 antibody–injected mouse plasma but not in PBS-injected mouse plasma (Figure 2A, lanes 1 and 2). Mouse PK (mPK), mFXI, mFXII, and mouse albumin (control) were similarly detected in B-hHK/PBS– and B-hHK/3E8–injected mouse plasma (Figure 2A, lanes 1 and 2). Streptavidin beads were used to pull down B-hHK from both hHK/PBS- and hHK/3E8-injected mouse plasma. The hamster IgG was only pulled down (through the binding of 3E8 to hHK) from B-hHK/3E8–injected mouse plasma, and mPK and mFXI were only pulled down in B-hHK/PBS–injected mouse plasma (Figure 2A, lanes 3 and 4). mFXII and mouse albumin were not pulled down in any plasma (Figure 2A, lanes 3 and 4). Statistical analysis showed that the 3E8 antibody blocking activity against mPK and mFXI binding to B-hHK is highly significant (Figure 2B-C). These results show that injected B-hHK binds to mPK and mFXI and that the 3E8 anti–HK antibody blocked this binding. Our results also show that B-hHK did not bind to mFXII or mouse albumin, indicating the specificity of B-hHK/mPK and B-hHK/mFXI binding.
Figure 2.
3E8 anti–HK antibody blocks binding of mPK and mFXI to hHK in KN1-KO mice in vivo. B-hHK with or without 3E8 anti–HK antibody was IV injected in KN1-KO mice (n = 7/group), and 30 minutes later mouse plasma was collected and prepared. (A) Lanes 1 and 2: western blot was used to analyze mouse plasma after injections. Plasma contained B-hHK, mPK, mFXI, and mFXII. Plasma from mice also injected with 3E8 showed presence of hamster IgG (lane 2). Mouse albumin was used as a loading control. Lanes 3 and 4: streptavidin beads pulled down B-hHK and any bound proteins, and samples were examined by western blot. B-hHK treated plasma pulled down hHK, mPK, and mFXI (through binding to HK). However, plasma treated with both B-hHK and 3E8 only pulled down hHK and IgG, because 3E8 blocked the binding of mPK and mFXI to HK. (B, C) Statistical analysis (n = 8 mice per group) showed 3E8 antibody significantly blocked mPK and mFXI binding to B-hHK. Data are denoted as mean ± SEM. ∗∗∗∗P≤.0001.
Blocking mPK or mFXI binding to hHK by 3E8 anti–HK antibody inhibits DXS-induced mouse contact system thrombotic and inflammatory pathway activation in vivo
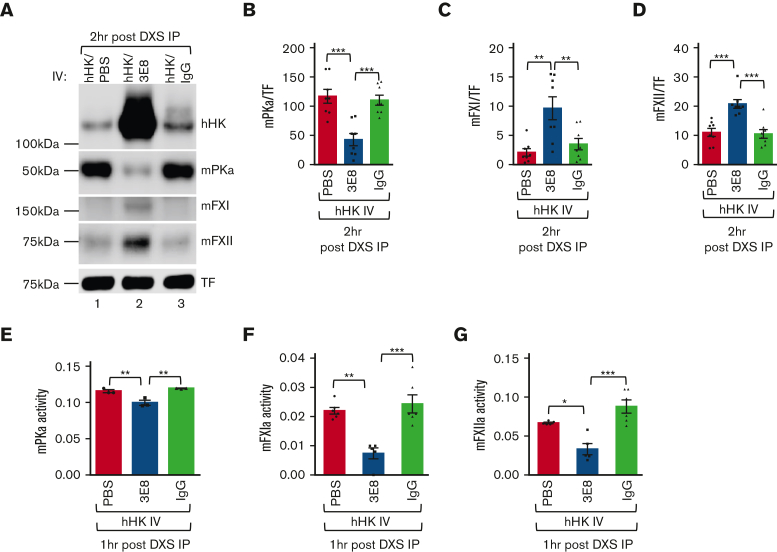
We further analyzed the effects of blocking hHK/mPK and hHK/mFXI binding on DXS-induced mouse contact system activation in vivo. Similar to that in Figure 1, hHK cleavage was protected in hHK/3E8-injected mice but neither in hHK/PBS- nor in hHK/hamster IgG–injected mice 2 hours after DXS injection (Figure 3A). 3E8 injection also inhibited mPK, mFXI, and mFXII activation in mouse plasma (Figure 3A, lane 2 vs lanes 1 and 3, Figure 3A-D). mPKa (cleaved and activated from PK) was significantly less in hHK/3E8-injected mouse plasma than in hHK/PBS- or hHK/hamster IgG–injected mouse plasma (Figure 3A-B), indicating less contact activation and mPK cleavage in the presence of 3E8. There was significantly more mFXI in hHK/3E8-injected mouse plasma than in hHK/PBS- or hHK/IgG-injected mouse plasma (Figure 3A,C), indicating less mFXI was cleaved and activated in the presence of 3E8. Interestingly, even though mFXII did not directly bind to hHK (Figure 2A), the presence of 3E8 prevented mFXII activation by DXS, because there was significantly more mFXII in plasma from hHK/3E8-injected mice than that from hHK/PBS- or hHK/IgG-injected mice, indicating less mFXII cleavage and activation in hHK/3E8-injected mice (Figure 3A,D). We also found that 3E8 significantly reduced the activities of PKa, FXIa, and FXIIa in mouse plasma after DXS injection when compared with those in PBS or IgG groups (Figure 3E-G; representative kinetic activity curves are shown in supplemental Figure 2). These results show that 3E8 can inhibit (1) HK cleavage and bradykinin production in vivo and (2) FXI and PK binding to HK in vivo, which subsequently prevents FXII, FXI, and PK activation. Therefore, the 3E8 antibody inhibits activation of both the inflammatory (via inhibition of mPK activation and HK cleavage) and thrombotic (via inhibition of mFXI activation) pathways of the plasma contact system in vivo.
Figure 3.
3E8 blocks binding of mPK or mFXI to hHK and inhibits mouse contact system thrombotic and inflammatory pathways in vivo. KN1-KO mice were IV injected with hHK along with either PBS, 3E8, or hamster IgG, and then intraperitoneally injected with DXS as described in “Methods”. (A) Two hours after DXS injection, plasma was prepared and analyzed by western blotting. Cleavage of hHK, mFXI, and mFXII was protected in mice treated with 3E8 antibody, but not in PBS- or hamster IgG–treated mice. mPKa levels were lower in 3E8-treated mice, as 3E8 protected against activation of PK. TF was used for normalization. (B-D) Quantification of mPKa, mFXI, and mFXII levels from western blot analyses. mPKa levels (cleaved and activated prekallikrein) were significantly less in 3E8-injected mouse plasma than in PBS- or hamster IgG–injected mouse plasma, indicating less mPK cleavage and activation by 3E8. mFXI levels were significantly higher in 3E8-injected mouse plasma than those in PBS- or IgG-injected mouse plasma, indicating less mFXI cleavage and activation because of 3E8 protection. Levels of mFXII were significantly higher in 3E8 vs PBS or IgG-treated mouse plasma, indicating less mFXII cleavage and activation because of 3E8 treatment. (E-G) 3E8 antibody significantly inhibited mouse PKa activity (E), mouse FXIa activity (F), and mouse FXIIa activity (G) when compared with PBS or IgG-treated mouse plasma. Activity bar graphs were prepared from cumulative data at 15 minute measurements. n = 3 to 8 per group. Data are denoted as mean ± SEM. ∗P<.05, ∗∗P≤.01, ∗∗∗P≤.001.
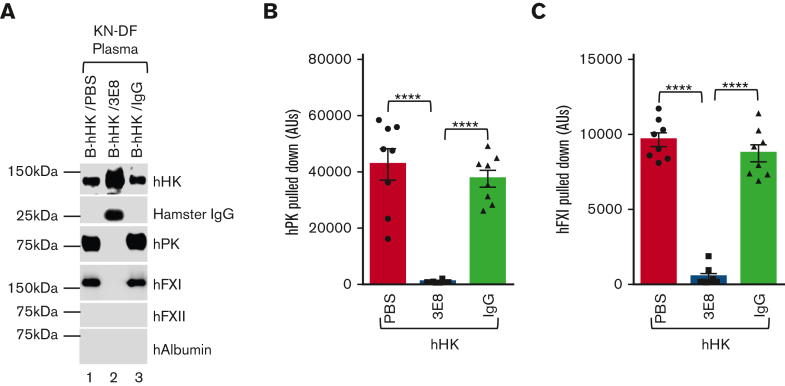
3E8 anti–HK antibody blocks hPK and hFXI binding to hHK and inhibits human contact system activation and HK cleavage in KN-DF human plasma ex vivo
Because 3E8 inhibited the mouse contact system and hHK cleavage by blocking mPK and mFXI binding to hHK in vivo, we further analyzed the effects of 3E8 on hHK binding to hPK and hFXI in KN-DF human plasma ex vivo. B-hHK in the presence or absence of 3E8 anti–HK antibody or hamster IgG was incubated with KN-DF human plasma at 37°C for 20 minutes. Streptavidin was used to pull down B-hHK and any bound proteins. As expected, hHK was pulled down under all conditions (Figure 4A). Hamster IgG was also pulled down from KN-DF plasma incubated with B-hHK/3E8, but hamster IgG was not pulled down in KN-DF plasma incubated with B-hHK/IgG, indicating the specific binding of 3E8 antibody to B-hHK (Figure 4A; Hamster IgG band, lane 2 vs lanes 1 and 3). hPK and hFXI were pulled down from B-hHK/PBS- and B-hHK/IgG-treated KN-DF plasma but not from B-hHK/3E8-treated KN-DF plasma, indicating that hPK and hFXI binding to B-hHK was blocked by 3E8 anti–HK antibody. hFXII and human albumin were not pulled down from any of the samples (Figure 4A), indicating the specificity of hHK/hPK and hHK/hFXI binding. Statistical analysis showed that the ability of 3E8 anti–HK antibody to block hHK/hPK and hHK/hFXI binding was highly significant (Figures 4B-C).
Figure 4.
Exogenous purified hHK binds to hPK and hFXI, and 3E8 anti–HK antibody blocked this binding in KN-DF human plasma ex vivo. B-hHK along with either PBS, 3E8, or hamster IgG were added to KN-DF human plasma and incubated at 37°C for 20 minutes. Streptavidin was used to pull down B-hHK–bound proteins complex. (A) Representative western blot shows that B-hHK was pulled down from all samples. B-hHK pulled down 3E8 anti–HK antibody (as denoted by hamster IgG band), but B-hHK did not pull down hamster IgG in the absence of 3E8 (indicating specificity of the antibody; lane 2 vs lanes 1 and 3). hPK and hFXI were pulled with B-hHK only in the absence of 3E8 in KN-DF plasma, suggesting that 3E8 blocked the binding of hPK and hFXI to B-hHK. hFXII and human albumin were not pulled down from any of the samples. (B,C) Quantification of amount of hPK and hFXI pulled down under all KN-DF plasma conditions. n = 8 per group, Data are denoted as mean ± SEM. ∗∗∗∗P ≤ .0001.
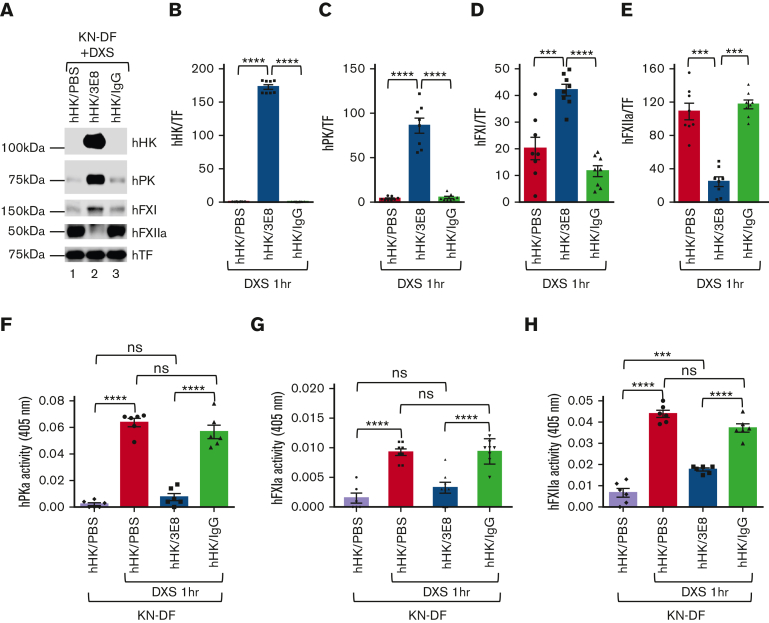
We then examined if 3E8’s ability to block hHK/hPK and hHK/hFXI binding also prevented activation of hPK and hFXI after DXS administration in KN-DF plasma ex vivo. hHK/PBS, hHK/3E8, or hHK/hamster IgG was added to KN-DF human plasma and incubated at 37°C for 20 minutes, and then DXS was added and incubated at 37°C for additional 60 minutes to activate the contact system. After treatment, samples were analyzed by western blotting and chromogenic activity assays. As expected, hHK cleavage was blocked in the presence of 3E8, but not PBS- or hamster IgG–treated KN-DF human plasma (Figure 5A-B). More interestingly, activation of hPK, hFXI, and hFXII was inhibited in 3E8-treated KN-DF human plasma (Figure 5A, compare lane 2 with lanes 1 and 3, and 5C-E). There is significantly more hPK and hFXI (inactive forms) in hHK/3E8-treated KN-DF human plasma than in hHK/PBS- or hHK/IgG-treated plasma (Figure 5A,C,D). Furthermore, there was significantly less hPKa and hFXIa enzymatic activity in hHK/3E8-treated KN-DF human plasma compared with that in hHK/PBS- or hHK/IgG-treated plasma. Levels of hPKa and hFXIa after hHK/3E8 and DXS treatment were similar to the levels of KN-DF plasma without DXS treatment (Figure 5F-G, first and third bars; supplemental Figure 3A-B, representative kinetic activity curves). Together, these data indicate that 3E8 prevented activation of hPK and hFXI by DXS. There was significantly less activated hFXII (hFXIIa) in hHK/3E8-treated KN-DF human plasma compared with that in hHK/PBS- or hHK/IgG-treated plasma (Figure 5A,E). In DXS-treated samples, the activity of hFXIIa in hHK/3E8-treated KN-DF plasma was significantly lower than that in hHK/PBS- or hHK/IgG-treated plasma, but was significantly higher than plasma without DXS treatment (Figure 5H; supplemental Figure 3C, representative kinetic activity curve), indicating that 3E8 inhibits positive feedback activation of FXII by PKa but does not affect DXS-induced FXII autoactivation (Figure 5H).
Figure 5.
3E8 blocks binding of of hPK or hFXI to hHK and inhibits contact system thrombotic and inflammatory pathway activation in KN-DF human plasma ex vivo. hHK was added to KN-DF human plasma along with either PBS, 3E8, or hamster IgG. After incubation at 37°C for 20 minutes, DXS was added for an additional 60 minutes at 37°C to activate plasma contact system. (A) Representative western blot of samples. TF was used for normalization. (B) As expected, hHK cleavage was blocked by 3E8 antibody, but not by PBS or IgG in KN-DF human plasma. (C,D) Levels of hPK and hFXI were significantly higher in 3E8-treated plasma than in PBS- or IgG-treated KN-DF human plasma, indicating that 3E8 inhibited binding and activation of hPK and hFXI. (E) Levels of hFXIIa were significantly lower in 3E8-treated plasma than in PBS- or IgG-treated KN-DF human plasma, suggesting that the prevention of hPK and hFXI activation by 3E8 also hinders hFXII activation through the feedback mechanism. (F-H) The 3E8 antibody significantly inhibited PKa activity (F), FXIa activity (G), and FXIIa activity (H) when compared with PBS- or IgG-treated KN-DF plasma. Activity bar graphs were generated from cumulative data at 15 minute measurements. n = 7 to 8 per group, Data are denoted as mean ± SEM. ∗∗∗P≤.001, ∗∗∗∗P≤.0001.
To determine whether the 3E8 anti–HK antibody can block PKa binding to HK and therefore directly inhibit PKa cleavage of HK, HK/PKa binding was analyzed by enzyme-linked immunosorbent assay. The presence of 3E8 dramatically reduced HK binding to PKa (supplemental Figure 4A). To determine whether 3E8 could directly prevent HK cleavage by PKa, PKa was added to PK-DF human plasma in the presence of different concentrations of 3E8. We found that 3E8 dose-dependently inhibited PKa cleavage of HK (supplemental Figure 4B-C). Taken together, these results suggest that 3E8 blocks both PK and PKa from binding to HK, inhibits PK activation, and prevents PKa from cleaving HK.
3E8 anti–HK antibody inhibits activation of plasma contact system thrombotic and inflammatory pathways by blocking binding of hPK and hFXI to domain 6 of hHK in NHP ex vivo
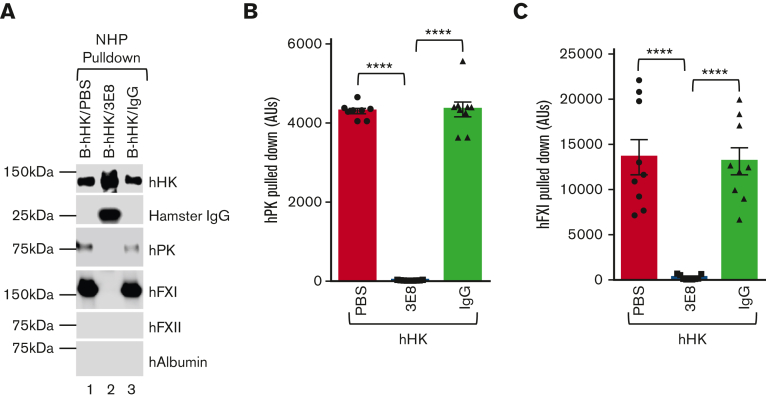
Addition of 3E8 anti–HK antibody to KN-DF human plasma, which lacks HK 1) blocked hPK and hFXI binding to exogenously added hHK and 2) prevented DXS-induced contact system activation and hHK cleavage. We then investigated how 3E8 affects B-hHK interactions with contact system proteins in NHP, which has normal levels of endogenous HK. B-hHK/PBS, B-hHK/3E8, or B-hHK/hamster IgG was added to NHP and incubated at 37°C for 20 minutes. Streptavidin was used to pull down B-hHK and any bound proteins. As expected, B-hHK was pulled down from all treated NHP groups. 3E8 was pulled down with B-hHK, but control hamster IgG was not, indicating specificity of 3E8 antibody (Figure 6A, Hamster IgG blot, lane 2 vs lanes 1 and 3). hPK and hFXI were pulled down from B-hHK/PBS– and B-hHK/IgG–incubated NHP, but not from B-hHK/3E8-incubated NHP, indicating that 3E8 blocked the binding of hPK and hFXI to B-hHK in NHP (Figure 6A-C). hFXII and human albumin were not pulled down from any of the NHP groups (Figure 6A). These results indicate that in NHP, which has normal levels of HK, addition of exogenous B-hHK can still bind hPK and hFXI, but 3E8 anti–HK antibody can block this binding.
Figure 6.
3E8 anti–HK antibody blocks binding of exogenously added hHK to hPK and hFXI and displaces hPK and hFXI binding to hHK in NHP ex vivo. B-hHK was added to NHP with PBS, 3E8, or hamster IgG and incubated at 37°C for 20 minutes. Streptavidin was used to pull down B-hHK-bound proteins complex. (A) Representative western blot results of the pulldown experiments. hHK was pulled down in all samples. The 3E8 anti–HK antibody was pulled down with B-hHK, but control hamster IgG was not. (B,C) hPK and hFXI were pulled from PBS- and hamster IgG–treated NHP but not from 3E8-incubated KN-DF plasma, indicating that binding of hPK and hFXI to B-hHK was blocked by 3E8 anti–HK antibody. hFXII and human albumin were not pulled down from any of the samples. (D-G) NHP was incubated with PBS, 3E8, or hamster IgG at 37°C for 20 minutes. B-2B7 anti–HK antibody was added and incubated for additional 20 minutes. Streptavidin was used to pull down B-2B7–bound proteins. (D) Representative western blot results of the pulldown experiments. Although hHK was pulled down in all samples, hFXI and hPK were only pulled down in PBS- or IgG-treated plasma (Lanes 1 and 3 vs Lane 2). (E) 3E8 anti–HK antibody protected hHK cleavage during pulldown processes. (F & G) hFXI and hPK were pulled down from PBS- and hamster IgG–treated, but not 3E8-treated NHP, indicating that binding of hPK and hFXI to hHK was displaced by the 3E8 anti–HK antibody. hFXII and human albumin were not pulled down in any of the samples. n = 8 to 9/group. Data are denoted as mean ± SEM. ∗∗P ≤ .01, ∗∗∗∗P ≤ .0001.
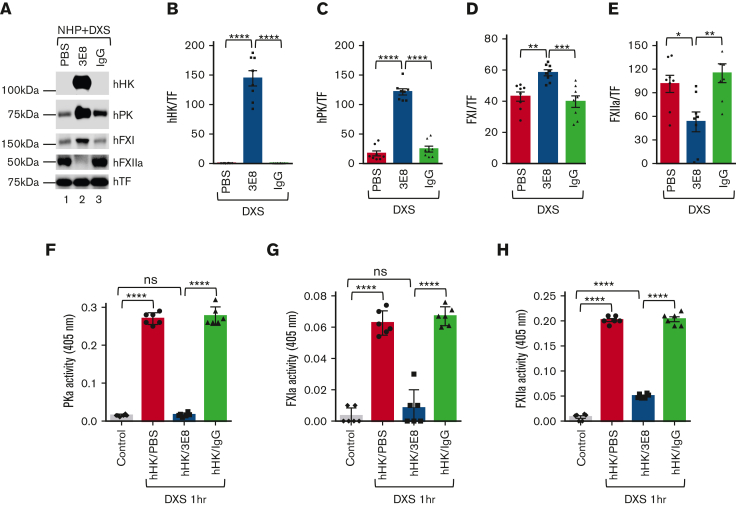
We then investigated the effects of 3E8 on activation of hPK, hFXI, and hFXII in NHP after DXS treatment. NHP was incubated with PBS, 3E8 antibody, or hamster IgG at 37°C for 20 minutes, then DXS was added to induce contact system activation. Samples were analyzed. As shown previously, 3E8 blocked hHK cleavage12 (Figure 7A-B). Moreover, 3E8 inhibited activation of hPK, hFXI, and hFXII activation (Figure 7A, lane 2 vs lanes 1 and 3,C-E). Levels of inactive hPK and hFXI are significantly lower in NHP treated with PBS or IgG because DXS is activating them to hPKa and hFXIa (Figure 7A,C,D). Furthermore, the enzymatic activities of hPKa and FXIa were significantly less in 3E8-treated NHP than those in PBS or IgG groups and were similar to the levels of control plasma without DXS treatment (Figure 7F-G, first and third bars; supplemental Figure 5A-B, representative kinetic activity curves), indicating that 3E8 inhibited DXS-induced PK and FXI activation and maintained levels of hPK and hFXI. The expression level of hFXIIa was significantly less in 3E8-treated NHP than that in PBS- or IgG-treated NHP, suggesting 3E8’s protection against activation of FXII in NHP (Figure 7A,E). In DXS-treated samples, the activity of hFXIIa in 3E8-treated NHP was significantly lower than that in PBS- or IgG-treated plasma but was significantly higher than that in plasma without DXS treatment (Figure 7H; supplemental Figure 5C, representative kinetic activity curve), indicating that 3E8 inhibited positive feedback activation of FXII by PKa but did not affect DXS-induced FXII autoactivation, which is independent of PKa (Figure 7H).
Figure 7.
Blocking hPK or hFXI binding to hHK inhibits activation of both contact system thrombotic and inflammatory pathways in NHP ex vivo. hHK as well as either PBS, 3E8, or hamster IgG were added to NHP and incubated at 37°C for 20 minutes. DXS was then added and incubated at 37°C for additional 60 minutes to activate the plasma contact system. (A) Samples were analyzed via western blotting. TF was used for normalization. (B) As expected, hHK cleavage by DXS-induced contact system activation was blocked by 3E8 antibody but occurred in plasma samples containing PBS or IgG. (C,D) hPK and hFXI levels were significantly higher in NHP treated with 3E8 rather than in PBS or IgG, indicating that 3E8 prevented hPK and hFXI cleavage and activation by DXS. (E) Levels of hFXIIa were significantly lower in NHP treated with 3E8 than with PBS or hamster IgG, indicating that 3E8 indirectly allows for less hFXII cleavage and activation in NHP ex vivo. (F-H) The 3E8 antibody significantly inhibited PKa (F), FXIa (G), and FXIIa (H) activities when compared with PBS- or IgG-treated NHP. Activity bar graphs were prepared from cumulative data at 15 minute measurements. n = 8 per group. Data are denoted as mean ± SEM. ∗P≤.05, ∗∗P≤.01, ∗∗∗P≤.001, ∗∗∗∗P≤.0001.
We further analyzed the 3E8 and FXII interaction by testing the binding between biotinylated 3E8 and purified HK or FXII. We found that biotinylated 3E8 binds to HK but not FXII (supplemental Figure 6A). In addition, DXS-induced purified FXII autoactivation, but 3E8 did not affect DXS-mediated FXII autoactivation (supplemental Figure 6B-C). These results further show that 3E8 only blocked PKa-mediated feedback activation of FXII (Figures 5H and 7H; supplemental Figure 6B-C).
3E8 does not recognize the KN isoform that lacks domain 6 (low molecular weight KN[LK]),46 indicating that its effect on contact activation is through its binding to HK’s domain 6. We performed a competition experiment using B-HK and molar excess of nonbiotinylated HK or nonbiotinylated LK. The binding of B-HK to 3E8 was dramatically decreased in the presence of nonbiotinylated HK, but LK did not have an effect on B-HK/3E8 binding (supplemental Figure 7A). This result indicates 3E8 only interacts with KN isoforms that have domain 6.
KN-DF human plasma lacks both HK and LK.46 DXS induced a significant increase in FXII activation in KN-DF plasma compared with that in KN-DF plasma without DXS treatment, indicating DXS-mediated FXII autoactivation, which is independent of HK. Addition of purified HK to KN-DF plasma at its physiological concentration (666 nM) further increased DXS-induced FXII activation significantly. This additional increase in FXII activation indicates the positive feedback activation of FXII by PKa, which depends on the binding of PK to HK. However, this FXII activation by addition of HK is blocked by 3E8 antibody (supplemental Figure 7B). Addition of LK to KN-DF plasma did not have an effect on DXS-induced FXII activation, and 3E8 did not have an effect on LK in DXS-mediated FXII activation (supplemental Figure 7B). These results show that 3E8 inhibited contact system activation through binding to HK’s domain 6, whereas 3E8 had no effect on LK, which lacks domain 6.
We also investigated the effects of 3E8 on plasmin-3,43 or kaolin54-induced HK cleavage in NHP. Our results show that 3E8 inhibited plasmin-induced HK cleavage (supplemental Figure 8), and kaolin-induced HK cleavage and PK and FXI activation (supplemental Figure 9). Furthermore, 3E8 blocked PKa-mediated feedback activation of FXII in kaolin-induced contact system activation (supplemental Figure 9). These results show that 3E8 not only inhibited DXS-induced contact system activation but also other reagent-mediated HK cleavage or contact system activation.
Discussion
hHK consists of 6 domains (designated D1-D6). D6 contains the PK and FXI binding sites, which partially overlap. Previous studies identified a 31–amino acid residue region of HK as the binding site for PK and FXI and showed that antibodies raised against this region inhibit DXS-induced contact system activation ex vivo.55,56 Our present work focuses on analyzing the in vivo effects of the 3E8 anti–HK antibody on contact system activation in mouse models and the in vivo mechanism by which 3E8 blocks HK cleavage and inhibits DXS-induced contact system activation.
Our results show that DXS can activate the mouse contact system and cleave injected hHK, and the 3E8 anti–HK antibody can prevent hHK cleavage and bradykinin production by the DXS-induced activated mouse contact system in vivo. These data suggest that this mouse model could be used to further analyze the mechanism of the effects of 3E8 on the plasma contact system (Figure 1). Experiments using B-hHK showed that mPK and mFXI bind to injected hHK, and the presence of 3E8 blocked these bindings in vivo (Figure 2). Moreover, IV injection of 3E8 also inhibited DXS-induced activation of mFXII, mPK, and mFXI in vivo (Figure 3). Examining whether anti-HK antibody 3E8 has a beneficial antithrombotic/anti-inflammatory effect in animal models in vivo will be the focus of future studies.
Ex vivo analyses using KN-DF and NHP revealed that 3E8 blocked binding of hPK and hFXI to hHK, which also inhibited hPK and hFXI activation by DXS (Figure 4., Figure 5., Figure 6., Figure 7.). Interestingly, FXII activation was also inhibited by 3E8 both ex vivo and in vivo (Figures 3, 5, and 7), likely because of inhibition of PKa’s positive feedback activation of FXII, which depends on PK’s binding to HK.
Based on our ex vivo and in vivo results, we hypothesize the following model for the effects of 3E8 anti–HK antibody on plasma contact system activation: (1) In normal plasma, PK and FXI can independently bind to HK;44,57 (2) FXII binds to an activator and is autoactivated to FXIIa, which initiates contact system activation; (3) FXIIa activation of FXI (to FXIa) triggers the intrinsic coagulation pathway, leading to clot formation, whereas FXIIa activation of PK (to PKa) leads to the cleavage of HK and release of bradykinin and subsequent activation of bradykinin receptors and inflammatory processes; (4) PKa can positively feedback to activate FXII,40 and then FXIIa can further activate the downstream pathways and amplify contact system activation; (5) In the presence of 3E8, 3E8 binds to HK at domain 645 and blocks the binding of PK or FXI to HK (Figures 2, 4, and 6); (6) DXS can initiate FXII autoactivation, but DXS-bound FXIIa does not have access to FXI and PK on HK and therefore cannot effectively activate PK or FXI (Figures 3A-C,E,F; 5A,C,D,F,G; and 7A,C,D,F,G); (7) Because PK is not activated, it cannot feedback to activate FXII, and FXII remains autoactivated by DXS (Figures 3A,D,G; 5A,E,H; and 7A,E,H). As a result, HK cleavage is blocked (Figures 1, 5A-B; and 7A-B).
HK has important functions in many pathophysiological conditions.4,58, 59, 60 KN1-KO mice are protected from ischemic brain damage without an increase in infarct-associated hemorrhage.61 However, its contributions to common human thrombotic processes is unknown. Bradykinin, which is released from HK when it is cleaved, is involved in many pathologies in a variety of systems and organs such as stroke, allergic reaction, diabetic retinopathy, Alzheimer disease, sepsis, colitis, and so on.3,36,37,62, 63, 64, 65, 66, 67
Our work shows that an antibody targeting HK effectively blocks HK cleavage and bradykinin release13 and inhibits activation of both the thrombotic and inflammatory pathways of the plasma contact system ex vivo and in vivo, indicating that targeting HK may provide an additional strategy for treatment of contact system–related pathological conditions.
Conflict-of-interest disclosure: The 3E8 and 2B7 anti–HK antibodies have been licensed to MilliporeSigma.
Acknowledgments
The authors thank members of the Strickland laboratory for helpful discussions and Neranjan De Silva for technical assistance.
This work was supported by the National Institutes of Health grants NS102721 and AG069987, Alzheimer’s Association, Robertson Therapeutic Development Fund, Samuel Newhouse Foundation, John A. Herrmann, and May and Samuel Rudin Family Foundation. This work was also supported by UL1TR001866 from the National Center for Advancing Translational Sciences, National Institutes of Health Clinical and Translational Science Award program.
Authorship
Contribution: Z.-L.C. designed the study, performed experiments, analyzed data, and wrote the manuscript; P.K.S. and K.H. performed experiments and analyzed data; M.R.C. and S.K. performed experiments; K.R.M. provided KN1-KO mice for the studies; S.S. designed the study and participated in data analysis; and E.H.N. designed the study, participated in data analysis, and wrote the manuscript.
Footnotes
Data are available on request from the corresponding author, Erin H. Norris (enorris@rockefeller.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Jukema BN, de Maat S, Maas C. Processing of factor XII during inflammatory reactions. Front Med (Lausanne) 2016;3 doi: 10.3389/fmed.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14(1):28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 3.Simao F, Ustunkaya T, Clermont AC, Feener EP. Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke. Blood. 2017;129(16):2280–2290. doi: 10.1182/blood-2016-09-740670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y. Contact pathway of coagulation and inflammation. Thromb J. 2015;13 doi: 10.1186/s12959-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas C, Govers-Riemslag JW, Bouma B, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118(9):3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibayama Y, Joseph K, Nakazawa Y, Ghebreihiwet B, Peerschke EI, Kaplan AP. Zinc-dependent activation of the plasma kinin-forming cascade by aggregated beta amyloid protein. Clin Immunol. 1999;90(1):89–99. doi: 10.1006/clim.1998.4621. [DOI] [PubMed] [Google Scholar]
- 7.Zamolodchikov D, Renne T, Strickland S. The Alzheimer's disease peptide beta-amyloid promotes thrombin generation through activation of coagulation factor XII. J Thromb Haemost. 2016;14(5):995–1007. doi: 10.1111/jth.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Li Y, Bhattacharya A, Zhang Y. A plasma proteolysis pathway comprising blood coagulation proteases. Oncotarget. 2016;7(27):40919–40938. doi: 10.18632/oncotarget.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y. The plasma contact system as a modulator of innate immunity. Curr Opin Hematol. 2018;25(5):389–394. doi: 10.1097/MOH.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed BM, Matafonov A, Ivanov I, et al. An update on factor XI structure and function. Thromb Res. 2018;161:94–105. doi: 10.1016/j.thromres.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renne T. In: Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Marder VJ, Aird WC, Bennett JS, Schulman S, White GC, editors. Wolters Kluwer/Lippincott, Williams & Wilkins; 2013. The Factor XII-Driven Plasma Contact System; pp. 242–253. [Google Scholar]
- 12.Chen ZL, Revenko AS, Singh P, MacLeod AR, Norris EH, Strickland S. Depletion of coagulation factor XII ameliorates brain pathology and cognitive impairment in Alzheimer disease mice. Blood. 2017;129(18):2547–2556. doi: 10.1182/blood-2016-11-753202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen ZL, Singh P, Wong J, Horn K, Strickland S, Norris EH. An antibody against HK blocks Alzheimer's disease peptide beta-amyloid-induced bradykinin release in human plasma. Proc Natl Acad Sci U S A. 2019;116(46):22921–22923. doi: 10.1073/pnas.1914831116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalaria RN. Small vessel disease and Alzheimer's dementia: pathological considerations. Cerebrovasc Dis. 2002;13(Suppl 2):48–52. doi: 10.1159/000049150. [DOI] [PubMed] [Google Scholar]
- 15.Maas C, Renne T. Coagulation factor XII in thrombosis and inflammation. Blood. 2018;131(17):1903–1909. doi: 10.1182/blood-2017-04-569111. [DOI] [PubMed] [Google Scholar]
- 16.Murugesan N, Fickweiler W, Clermont AC, Zhou Q, Feener EP. Retinal proteome associated with bradykinin-induced edema. Exp Eye Res. 2019;186:107744. doi: 10.1016/j.exer.2019.107744. [DOI] [PubMed] [Google Scholar]
- 17.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson VH, Glueck HI, Miller MA, Movat HZ, Habal F. Kininogen deficiency in Fitzgerald trait: role of high molecular weight kininogen in clotting and fibrinolysis. J Lab Clin Med. 1976;87(2):327–337. [PubMed] [Google Scholar]
- 19.Griffin JH, Cochrane CG. Mechanisms for the involvement of high molecular weight kininogen in surface-dependent reactions of Hageman factor. Proc Natl Acad Sci U S A. 1976;73(8):2554–2558. doi: 10.1073/pnas.73.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CY, Scott CF, Bagdasarian A, Pierce JV, Kaplan AP, Colman RW. Potentiation of the function of Hageman factor fragments by high molecular weight kininogen. J Clin Invest. 1977;60(1):7–17. doi: 10.1172/JCI108770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandle RJ, Colman RW, Kaplan AP. Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc Natl Acad Sci U S A. 1976;73(11):4179–4183. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier HL, Pierce JV, Colman RW, Kaplan AP. Activation and function of human Hageman factor. The role of high molecular weight kininogen and prekallikrein. J Clin Invest. 1977;60(1):18–31. doi: 10.1172/JCI108754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparkenbaugh EM, Kasztan M, Henderson MW, et al. High molecular weight kininogen contributes to early mortality and kidney dysfunction in a mouse model of sickle cell disease. J Thromb Haemost. 2020;18(9):2329–2340. doi: 10.1111/jth.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorkqvist J, Sala-Cunill A, Renne T. Hereditary angioedema: a bradykinin-mediated swelling disorder. Thromb Haemost. 2013;109(3):368–374. doi: 10.1160/TH12-08-0549. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan AP, Joseph K. Pathogenesis of hereditary angioedema: the role of the bradykinin-forming cascade. Immunol Allergy Clin North Am. 2017;37(3):513–525. doi: 10.1016/j.iac.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Schmaier AH. The hereditary angioedema syndromes. J Clin Invest. 2019;129(1):66–68. doi: 10.1172/JCI125378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadnicki A, Gonciarz M, Niewiarowski TJ, et al. Activation of plasma contact and coagulation systems and neutrophils in the active phase of ulcerative colitis. Dig Dis Sci. 1997;42(11):2356–2366. doi: 10.1023/a:1018891323205. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Yang A, Zhao Z, et al. The plasma kallikrein-kininogen pathway is critical in the pathogenesis of colitis in mice. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strickland S. Blood will out: vascular contributions to Alzheimer's disease. J Clin Invest. 2018;128(2):556–563. doi: 10.1172/JCI97509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh PK, Badimon A, Chen ZL, Strickland S, Norris EH. The contact activation system and vascular factors as alternative targets for Alzheimer's disease therapy. Res Pract Thromb Haemost. 2021;5(4):e12504. doi: 10.1002/rth2.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silasi R, Keshari RS, Regmi G, et al. Factor XII plays a pathogenic role in organ failure and death in baboons challenged with Staphylococcus aureus. Blood. 2021;138(2):178–189. doi: 10.1182/blood.2020009345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanarsa K, Henderson J, Soomro S, et al. Upregulation of proinflammatory bradykinin peptides in systemic lupus erythematosus and rheumatoid arthritis. J Immunol. 2020;205(2):369–376. doi: 10.4049/jimmunol.1801167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang A, Zhou J, Wang B, et al. A critical role for plasma kallikrein in the pathogenesis of autoantibody-induced arthritis. FASEB J. 2017;31(12):5419–5431. doi: 10.1096/fj.201700018R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hargreaves KM, Troullos ES, Dionne RA, Schmidt EA, Schafer SC, Joris JL. Bradykinin is increased during acute and chronic inflammation: therapeutic implications. Clin Pharmacol Ther. 1988;44(6):613–621. doi: 10.1038/clpt.1988.202. [DOI] [PubMed] [Google Scholar]
- 35.Hilfenhaus G, Mompeon A, Freshman J, et al. A high-content screen identifies drugs that restrict tumor cell extravasation across the endothelial barrier. Cancer Res. 2021;81(3):619–633. doi: 10.1158/0008-5472.CAN-19-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sala-Cunill A, Bjorkqvist J, Senter R, et al. Plasma contact system activation drives anaphylaxis in severe mast cell-mediated allergic reactions. J Allergy Clin Immunol. 2015;135(4):1031–1043. e1036. doi: 10.1016/j.jaci.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Feener EP. Plasma kallikrein-kinin system and diabetic retinopathy. Biol Chem. 2013;394(3):319–328. doi: 10.1515/hsz-2012-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flick MJ, LaJeunesse CM, Talmage KE, et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117(11):3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. doi: 10.1182/blood-2018-07-818211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renne T, Stavrou EX. Roles of factor XII in innate immunity. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Maat S, Joseph K, Maas C, Kaplan AP. Blood clotting and the pathogenesis of types I and II hereditary angioedema. Clin Rev Allergy Immunol. 2021;60(3):348–356. doi: 10.1007/s12016-021-08837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stavrou EX. Factor XII in inflammation and wound healing. Curr Opin Hematol. 2018;25(5):403–409. doi: 10.1097/MOH.0000000000000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henderson MWS E, Noubouossie D, Mailer R, et al. High molecular weight kininogen but not factor XII deficiency attenuates acetaminophen-induced liver injury in mice. Blood. 2019;134(suppl 1):3621–3623. [Google Scholar]
- 44.Thompson RE, Mandle R, Jr., Kaplan AP. Association of factor XI and high molecular weight kininogen in human plasma. J Clin Invest. 1977;60(6):1376–1380. doi: 10.1172/JCI108898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto-Imoto H, Zamolodchikov D, Chen ZL, et al. A novel detection method of cleaved plasma high-molecular-weight kininogen reveals its correlation with Alzheimer’s pathology and cognitive impairment. Alzheimers Dement (Amst) 2018;10:480–489. doi: 10.1016/j.dadm.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen ZL, Singh PK, Horn K, Strickland S, Norris EH. Anti-HK antibody reveals critical roles of a 20-residue HK region for Abeta-induced plasma contact system activation. Blood Adv. 2022;6(10):3090–3101. doi: 10.1182/bloodadvances.2021006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleniewski J. Plasma high molecular weight kininogen concentration in health and in chosen impairments of haemostasis. Evidence that plasmin uncovers a new antigenic site in high molecular weight kininogen. Thromb Haemost. 1979;42(3):1046–1055. [PubMed] [Google Scholar]
- 48.Chen ZL, Yao Y, Norris EH, et al. Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J Cell Biol. 2013;202(2):381–395. doi: 10.1083/jcb.201212032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverberg M, Dunn JT, Garen L, Kaplan AP. Autoactivation of human Hageman factor. demonstration utilizing a synthetic substrate. J Biol Chem. 1980;255(15):7281–7286. [PubMed] [Google Scholar]
- 50.Scott CF, Pixley RA, Colman RW. A new assay for high molecular weight kininogen in human plasma using a chromogenic substrate. Thromb Res. 1987;48(6):685–700. doi: 10.1016/0049-3848(87)90434-8. [DOI] [PubMed] [Google Scholar]
- 51.Merkulov S, Zhang WM, Komar AA, et al. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111(3):1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansen HT, Buoo L, Karlsrud TS, Aasen AO. Dextran sulphate activation of the contact system in plasma and ascites. Thromb Res. 1994;76(4):363–371. doi: 10.1016/0049-3848(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 53.Siebeck M, Cheronis JC, Fink E, et al. Dextran sulfate activates contact system and mediates arterial hypotension via B2 kinin receptors. J Appl Physiol (1985) 1994;77(6):2675–2680. doi: 10.1152/jappl.1994.77.6.2675. [DOI] [PubMed] [Google Scholar]
- 54.Zhu S, Diamond SL. Contact activation of blood coagulation on a defined kaolin/collagen surface in a microfluidic assay. Thromb Res. 2014;134(6):1335–1343. doi: 10.1016/j.thromres.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddigari SR, Kaplan AP. Monoclonal antibody to human high-molecular-weight kininogen recognizes its prekallikrein binding site and inhibits its coagulant activity. Blood. 1989;74(2):695–702. [PubMed] [Google Scholar]
- 56.Schmaier AH, Schutsky D, Farber A, Silver LD, Bradford HN, Colman RW. Determination of the bifunctional properties of high molecular weight kininogen by studies with monoclonal antibodies directed to each of its chains. J Biol Chem. 1987;262(3):1405–1411. [PubMed] [Google Scholar]
- 57.Thompson RE, Mandle R, Jr., Kaplan AP. Studies of binding of prekallikrein and factor XI to high molecular weight kininogen and its light chain. Proc Natl Acad Sci U S A. 1979;76(10):4862–4866. doi: 10.1073/pnas.76.10.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y, Dai J, Schmuckler NG, et al. Cleaved high molecular weight kininogen inhibits tube formation of endothelial progenitor cells via suppression of matrix metalloproteinase 2. J Thromb Haemost. 2010;8(1):185–193. doi: 10.1111/j.1538-7836.2009.03662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang A, Dai J, Xie Z, et al. High molecular weight kininogen binds phosphatidylserine and opsonizes urokinase plasminogen activator receptor-mediated efferocytosis. J Immunol. 2014;192(9):4398–4408. doi: 10.4049/jimmunol.1302590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang A, Xie Z, Wang B, Colman RW, Dai J, Wu Y. An essential role of high-molecular-weight kininogen in endotoxemia. J Exp Med. 2017;214(9):2649–2670. doi: 10.1084/jem.20161900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langhauser F, Gob E, Kraft P, et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood. 2012;120(19):4082–4092. doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albert-Weissenberger C, Siren AL, Kleinschnitz C. Ischemic stroke and traumatic brain injury: the role of the kallikrein-kinin system. Prog Neurobiol. 2013;101-102:65–82. doi: 10.1016/j.pneurobio.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Ashby EL, Love S, Kehoe PG. Assessment of activation of the plasma kallikrein-kinin system in frontal and temporal cortex in Alzheimer's disease and vascular dementia. Neurobiol Aging. 2012;33(7):1345–1355. doi: 10.1016/j.neurobiolaging.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 64.Gauberti M, Potzeha F, Vivien D, Martinez de Lizarrondo S. Impact of Bradykinin Generation During Thrombolysis in Ischemic Stroke. Front Med (Lausanne) 2018;5 doi: 10.3389/fmed.2018.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Isordia-Salas I, Pixley RA, Sainz IM, Martinez-Murillo C, Colman RW. The role of plasma high molecular weight kininogen in experimental intestinal and systemic inflammation. Arch Med Res. 2005;36(1):87–95. doi: 10.1016/j.arcmed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Nicola H. The role of contact system in septic shock: the next target? An overview of the current evidence. J Intensive Care. 2017;5 doi: 10.1186/s40560-017-0228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nokkari A, Abou-El-Hassan H, Mechref Y, et al. Implication of the kallikrein-kinin system in neurological disorders: quest for potential biomarkers and mechanisms. Prog Neurobiol. 2018;165-167:26–50. doi: 10.1016/j.pneurobio.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.