Abstract
Background
Certain symptoms associated with infertility are associated with cardiovascular disease, including menstrual cycle irregularity, early menopause, and obesity; however, few studies have investigated the association between infertility and cardiovascular disease risk.
Methods and Results
Participants in the NHSII (Nurses' Health Study II) who reported infertility (12 months of trying to conceive without success, including women who subsequently conceived) or who were gravid, with no infertility were followed from 1989 until 2017 for development of incident, physician‐diagnosed coronary heart disease (CHD) (myocardial infarction, coronary artery bypass grafting, angioplasty, stent) and stroke. Time‐varying Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% CIs and were adjusted a priori for potential confounding variables. Among 103 729 participants, 27.6% reported having ever experienced infertility. Compared with gravid women who had not reported infertility, women with a history of infertility had greater risk of CHD (HR, 1.13 [95% CI, 1.01–1.26]) but not stroke (HR, 0.91 [95% CI, 0.77–1.07]). The association between history of infertility and CHD was strongest among women who reported infertility at an earlier age (HR for infertility first reported at ≤25 years, 1.26 [95% CI, 1.09–1.46]; HR at 26–30 years, 1.08 [95% CI, 0.93–1.25]; HR at >30 years, 0.91 [95% CI, 0.70–1.19]). When we investigated specific infertility diagnoses, elevated risk of CHD was observed among women whose infertility was attributed to an ovulatory disorder (HR, 1.28 [95% CI, 1.05–1.55]) or endometriosis (HR, 1.42 [95% CI, 1.09–1.85]).
Conclusions
Women with infertility may be at an increased risk of CHD. Risk differed by age at first infertility diagnosis and was restricted to ovulatory‐ and endometriosis‐related infertility.
Keywords: cardiovascular disease, coronary heart disease, endometriosis, infertility, myocardial infarction, polycystic ovary syndrome, stroke
Subject Categories: Epidemiology, Pregnancy, Women
Nonstandard Abbreviations and Acronyms
- NHSII
Nurses' Health Study II
- PCOS
polycystic ovary syndrome
- SWAN
Study of Women's Health Across the Nation
Clinical Perspective.
What Is New?
We observed that women with infertility may be at an increased risk of experiencing coronary heart disease later in life, and this risk varied by age at first infertility diagnosis and appears to be restricted to infertility related to ovulation disorders and endometriosis.
What Are the Clinical Implications?
Our findings support the growing body of literature on the importance of paying attention to female‐specific risk factors when studying coronary heart disease.
If our findings are robustly replicated, then information on reproductive and gynecologic history should be discussed with medical providers to better counsel patients on future coronary heart disease risk.
Cardiovascular disease is known to present differently among men and women, including later age of onset for women, higher risk of stroke for women, and a significant influence of women's reproductive factors and obstetric history. 1 , 2 Although prior research among women has suggested an association between female‐specific risk factors (eg, adverse pregnancy outcomes, age at menopause, miscarriage) and cardiovascular disease (CVD) risk, 3 , 4 , 5 , 6 , 7 , 8 , 9 few studies have investigated the association between overall infertility (trying to conceive for ≥12 months without success, including women who conceive thereafter) in relation to CVD risk. 10 , 11 Symptoms associated with certain infertility diagnoses are known to be associated with CVD, including menstrual cycle irregularity, 12 early menopause, 13 and obesity. 14 Moreover, several studies suggest that infertile women may have a worse CVD risk factor profile, including having a higher body mass index (BMI), larger waist circumference, and elevated triglycerides, than women without infertility. 15 , 16
Although there has been limited research on the relation between infertility and CVD, the research that does exist suggests that severity of infertility and certain infertility diagnoses, such as polycystic ovary syndrome (PCOS), confer the greatest risk. The largest study to date, using Swedish registry data, observed that parous women who experienced ≥5 years of infertility had a 19% greater risk of CVD compared with women who did not experience infertility. 17 This is supported by recent findings from the Norwegian Mother, Father and Child Cohort Study, which observed that gravid women who experienced ≥12 months of trying to conceive had a 14% greater risk of CVD. 18 Cross‐sectional data from the National Health and Nutrition Examination Survey found that women with a history of infertility had ≈1.8 times higher odds of experiencing metabolic syndrome and of having a cardiovascular event. 19 However, the existing research on this topic has been restricted to analyses that are among parous women, cross sectional, or with limited follow‐up time for the development of CVD events (≤12 years on average). Additionally, certain infertility diagnoses, such as PCOS, endometriosis, and unexplained infertility, have been observed to be associated with CVD risk and adverse cardiometabolic profiles (eg, hypertension, high cholesterol, type 2 diabetes), 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 which has not been thoroughly investigated in the current literature. The objective of the current study was therefore to investigate the association of infertility (overall, cause‐specific infertility diagnoses) with CVD using data from the NHSII (Nurses' Health Study II), a prospective cohort study of >116 000 female nurses followed for nearly 30 years. We hypothesized that women with a history of infertility will have a greater risk of CVD compared with gravid women without infertility and that this elevated risk will be highest among women whose infertility is attributed to tubal factor, endometriosis, or ovulatory disorders as opposed to women with infertility attributed to cervical disorders, male factor, and cause unknown.
Methods
Enrollment for the NHSII began in 1989; 116 429 registered nurses, who were between the ages of 25 to 42 years, were enrolled if they returned a mailed questionnaire. Every 2 years, follow‐up questionnaires were sent to participants to collect detailed information on a variety of health conditions as well as risk factors. Questionnaire completion implied consent. The study was approved by the Institutional Review Board of Brigham and Women's Hospital and Michigan State University. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Channing Division of Network Medicine at nhsaccess@channing.harvard.edu.
Infertility Definition
Participants self‐reported whether they had “tried to become pregnant for >1 year without success” on every questionnaire between 1989 and 2001 and then every other questionnaire until 2009 (when women were between the ages of 45 and 62). Participants were defined as having a history of infertility if they indicated they had tried to conceive for ≥1 year without success at any point in time, including women who concurrently or subsequently reported becoming pregnant. Infertility diagnoses were updated until age 45. 29 Women were also asked about the cause of their infertility and could choose from the following: tubal blockage, ovulatory disorder, endometriosis, cervical mucus factors, male factor infertility, not investigated, not found, and other. Women could report multiple causes for infertility. In sensitivity analyses, ovulatory infertility was further classified by symptoms associated with PCOS, based on the self‐reported presentation of menstrual cycle irregularity and excess androgens 30 , 31 Specifically, participants who reported ovulatory infertility were further stratified by menstrual cycle length (<32, ≥32 days), regularity (very regular or regular versus irregular or no period), and hirsutism (yes/no). Self‐reported recall of ovulatory infertility has been previously validated using a supplemental questionnaire (93% concurrence) and medical records (95% concurrence) and was found to have high validity, 32 as was self‐reported endometriosis (97% concordance with medical records). 33
Outcomes
In each follow‐up questionnaire, participants were asked to report new diagnoses of myocardial infarction (MI), stroke (cerebrovascular accident or transient ischemic attack), and coronary artery bypass grafting/angioplasty/stent. We included cases from enrollment in 1989 until the 2017 questionnaire cycle (which ended in May 2017). Following a self‐reported MI or stroke, participants or next of kin were asked for permission to obtain and review medical records or death certificates, which were then reviewed by study clinicians blinded to the questionnaire information. Confirmed MIs met the criteria of the World Health Organization: symptoms and either diagnostic electrocardiographic changes or raised cardiac enzymes 34 (International Classification of Diseases, Eighth Revision [ICD‐8] codes 410 and 412). Stroke was classified as ischemic or hemorrhagic by the National Survey of Stroke criteria (atypical neurological deficit of rapid or sudden onset lasting ≥24 hours or until death attributable to a vascular cause 35 ) (ICD‐8 codes 430–437). For reports of stroke for which medical records were not available/permitted, strokes were corroborated by nurse participant information. Information on physician‐diagnosed coronary artery bypass grafting, angioplasty, and stent was based on self‐report.
Statistical Analysis
Time‐varying Cox proportional hazard models were jointly stratified by age (months) and calendar time (months) and used to calculate the hazard ratio (HR) of CVD. The proportional hazard assumptions were tested and met. Person‐months at risk were calculated from age at return date of the questionnaire in which the participant reported either first report of infertility or first pregnancy until incident CVD incidence; death; or June 30, 2017. Participants who never reported a pregnancy attempt lasting >12 months or who never reported a pregnancy were excluded (n=10 455) (FigureFigure). Participants who reported having experienced MI or stroke before enrollment into the NHSII in 1989 were excluded at baseline (n=875), as were participants who never returned subsequent questionnaires (n=1370). Our primary analysis compared women with a history of any type of infertility (exposed) with gravid women with no history of infertility (unexposed). In secondary analyses, we investigated differences by different self‐reported causes of infertility. Multivariable model 1 adjusted for potential confounding factors, including age at menarche (<12, 12, 13, ≥14 years), White race (yes, no), marital status (ever/currently married, never), BMI at age 18 years (<19, 20.5–21.9, 22–24.9, 25–29.9, ≥30 kg/m2), time‐varying daily aspirin use (yes, no), time‐varying oral contraceptive use (never, past, current), time‐varying gravidity (≤1, 2, 3, ≥4), and time‐varying breastfeeding duration (<3, 3–12, or >12 months). Model 2 additionally adjusted for cardiovascular risk factors, including time‐varying covariates (updated every 2–4 years) of BMI (<24.9, 25–29.9, 30–34.9, or ≥35 kg/m2), smoking status (never, former, current 1–34 cigarettes/d, or current ≥35 cigarettes/d), physical activity (0, 0.1–1.0, 1.1–2.4, 2.5–5.9, or ≥6 h/wk), and Alternative Healthy Eating Index 2010 diet quality score (quintiles). For covariates with missing data, a missing indicator variable was created. Cumulative missingness ranged from <1% for BMI at age 18 to 10% for diet.
Figure . Flowchart of study population.
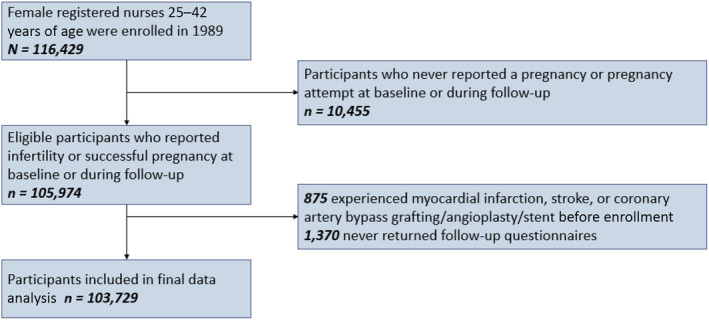
We additionally investigated the impact of timing of infertility experience on CVD risk by classifying infertility history on the basis of age at reporting first infertility (≤25, 26–30, >30 years), as well as investigating the association with specific infertility diagnoses. We examined effect modification by smoking status (never, ever/current), time‐varying BMI (<25, ≥25 kg/m2), nulliparity (yes, no), history of hypertensive disorders of pregnancy (yes, no), and BMI at age 18 years (<25, ≥25 kg/m2) by testing for interaction using likelihood ratio tests.
In sensitivity analyses, we stratified by whether infertility was primary (occurring before gravidity) or secondary (occurring after proven gravidity). Additionally, in sensitivity analyses, we restricted our infertility definition to women experiencing infertility before age 37 36 and excluded women reporting infertility only attributable to male partners. Finally, we conducted mediation analyses to quantify the proportion of the association between infertility and coronary heart disease (CHD) mediated by specific covariates 37 that may be on the causal pathway between infertility and CHD, including type 2 diabetes, hypertension, and hypercholesterolemia. 38 , 39
Results
In our study sample, 28 611 women reported having ever experienced infertility (27.6%) (Table 1), and there were 782 incident MIs, 984 incident revascularization events, and 762 incident stroke events (210 ischemic). Compared with gravid women without a history of infertility, women with a history of infertility were less likely to be gravid or parous at cohort baseline in 1989, but were more likely to have experienced gestational diabetes or hypertensive disorders of pregnancy, to be obese, to have a parent with a history of myocardial infarction or stroke, and to be never smokers. Among those who had their infertility investigated, the cause of infertility was attributed to ovulatory disorders (39.1%), spouse/male factor infertility (25.7%), other cause (21.8%), endometriosis (21.5%), or cause not found (30.5%).
Table 1.
Age‐Standardized Baseline (1989) Characteristics Among 103 729 Women in the Nurses' Health Study II, by Infertility History Across Follow‐Up*
| Characteristics in 1989 | Ever reported infertility | |
|---|---|---|
| No | Yes | |
| No. | 75 118 | 28 611 |
| Age, y† | 34.8 (4.7) | 34.9 (4.6) |
| Race, White, % | 92.2 | 91.2 |
| Ever married, % | 92.6 | 90.2 |
| Body mass index at age 18 y, kg/m2 | 21.1 (3) | 21.3 (3.6) |
| Body mass index, kg/m2, % | ||
| <18.5 | 1.3 | 1.8 |
| 18.5–24.9 | 39.5 | 36.6 |
| 25–29.9 | 30.9 | 29.6 |
| ≥30 | 28.3 | 32.0 |
| Past/current oral contraceptive use, % | 88.7 | 89.1 |
| Total physical activity, mean (SD), h/wk | 3.3 (4.9) | 3.4 (5.1) |
| Regular aspirin user in 1989,‡ % | 10.9 | 10.7 |
| History of cigarette smoking, % | ||
| Never | 66.3 | 65.3 |
| Past | 21.0 | 19.6 |
| Current 1–34 cigarettes/d | 11.6 | 13.8 |
| Current ≥35 cigarettes/d | 1.0 | 1.2 |
| Parental history of myocardial infarction or stroke, % | 14.5 | 16.0 |
| Gravidity, mean (SD) | 2.4 (1.4) | 1.8 (1.6) |
| Parous, % | 85.0 | 61.8 |
| Gestational diabetes,§ % | 3.6 | 5.6 |
| Hypertensive disorders of pregnancy,§ % | 14.2 | 19.6 |
| Breastfeeding duration,§ mo | 13.1 (13.4) | 11.3 (11.8) |
| Causes of infertility, no. (%)|| | ||
| Cause not investigated | … | 7761 (27.1) |
| Among those investigated | ||
| Ovulatory disorder | … | 8149 (39.1) |
| Endometriosis | … | 4497 (21.5) |
| Cervical mucus disorder | … | 1567 (7.6) |
| Tubal blockage | … | 3073 (14.7) |
| Male factor/spouse | … | 5353 (25.7) |
| Other cause | … | 4544 (21.8) |
| Cause not found | … | 6356 (30.5) |
Values are given as means (SDs) or percentages and are standardized to the age distribution of the study population.
Value is not age‐adjusted.
Aspirin or aspirin‐containing products used at least once per week in the past 2 years.
Among parous participants.
Attributed causes of infertility, which were not mutually exclusive, were gathered at baseline and during follow‐up among those who reported having tried to conceive unsuccessfully for at least 12 months up to age 40. N=1011 (3.5%) did not report if they had had an investigation or not or what the cause was attributed to if investigated.
We observed that women with a history of infertility were at greater risk of CHD in age‐adjusted models (HR, 1.16 [95% CI, 1.05–1.29]) and multivariable adjusted models (risk ratio [RR], 1.13 [95% CI, 1.01–1.26]) (Table 2). This association was predominantly driven by an association with the end point of coronary revascularization (HR, 1.18 [95% CI, 1.03–1.35]), with an attenuated, not statistically significant association between infertility and risk of MI (HR, 1.08 [95% CI, 0.93–1.27]). We observed no association between history of infertility and risk of stroke (HR, 0.91 [95% CI, 0.77–1.07]).
Table 2.
Hazard Ratios and 95% CIs for the Risk of CVD According to History of Infertility Among 103 729 Women (NHSII, 1989–2017)
| Ever reported infertility | ||
|---|---|---|
| No | Yes | |
| CVD§ , || , ¶ (stroke+CHD) | ||
| Events, no | 1489 | 761 |
| Person‐years | 1 675 650 | 730 114 |
| Age‐adjusted model* | 1.00 [Referent] | 1.08 (0.99–1.18) |
| Multivariable model 1† | 1.00 [Referent] | 1.09 (0.99–1.19) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.05 (0.96–1.15) |
| CHD§ , || | ||
| Events, n | 966 | 534 |
| Person‐years | 1 676 149 | 730 334 |
| Age‐adjusted model* | 1.00 [Referent] | 1.16 (1.05–1.29) |
| Multivariable model 1† | 1.00 [Referent] | 1.18 (1.06–1.32) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.13 (1.01–1.26) |
| MI§ | ||
| Events, n | 512 | 270 |
| Person‐years | 1 676 709 | 730 670 |
| Age‐adjusted model* | 1.00 [Referent] | 1.10 (0.95–1.28) |
| Multivariable model 1† | 1.00 [Referent] | 1.13 (0.96–1.31) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.08 (0.93–1.27) |
| Coronary revascularization|| | ||
| Events, n | 625 | 359 |
| Person‐years | 1 676 449 | 730 489 |
| Age‐adjusted model* | 1.00 [Referent] | 1.22 (1.07–1.39) |
| Multivariable model 1† | 1.00 [Referent] | 1.25 (1.09–1.43) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.18 (1.03–1.35) |
| Stroke¶ | ||
| Events, n | 531 | 231 |
| Person‐years | 1 676 650 | 730 691 |
| Age‐adjusted model* | 1.00 [Referent] | 0.92 (0.79–1.07) |
| Multivariable model 1† | 1.00 [Referent] | 0.93 (0.79–1.09) |
| Multivariable model 2‡ | 1.00 [Referent] | 0.91 (0.77–1.07) |
BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; ICD‐8, International Classification of Diseases, Eighth Revision; MI, myocardial infarction; and NHSII, Nurses’ Health Study II.
Models were jointly stratified by age (months) and calendar time (months).
Model 1 additionally adjusted for age at menarche (<12, 12, 13, ≥14 years of age), White Non‐Hispanic race and ethnicity (yes, no), marital status (ever married, never), daily aspirin use (yes, no), oral contraceptive use (never, past, current), gravidity (≤1, 2, 3, or ≥4 pregnancies), BMI at age 18 years (<19, 20.5–21.9, 22–24.9, 25–29.9, or ≥30 kg/m2), and breastfeeding duration (<3, 3–12, or >12 months).
Model 2 is additionally adjusted for time‐varying BMI (<24.9, 25–29.9, 30–34.9, or ≥35 kg/m2), cigarette smoking status and current dose (never, past, current 1–34 cigarettes/d, current ≥35 cigarettes/d), physical activity (0, 0.1–1.0, 1.1–2.4, 2.5–5.9, or ≥6 h/wk), and Alternative Healthy Eating Index 2010 diet quality score (quintiles).
Fatal and nonfatal MI and fatal coronary heart disease (ICD‐8: 410, 412).
Self‐reported coronary artery bypass grafting/angioplasty/stent.
Fatal and nonfatal cerebrovascular accident or transient ischemic attack (ICD‐8: 430–437).
The association between infertility and risk of CHD was strongest among women who reported experiencing infertility at younger ages (Table 3). Women who were ≤25 years old when they first experienced infertility had a 26% greater risk of CHD compared with gravid women who never experienced infertility (RR, 1.26 [95% CI, 1.09–1.46]), while women who first experienced infertility after age 30 years were not at greater risk of CHD (RR, 0.91 [95% CI, 0.70–1.19]).
Table 3.
Hazard Ratios and 95% CIs for the Risk of CHD and Stroke According to Age at First Report of Infertility Among 103 729 Women (NHSII, 1989–2017)
| No infertility | Age at first report of infertility | P value, test for trend§ | |||
|---|---|---|---|---|---|
| ≤25 y | 26–30 y | >30 y | |||
| CHD|| | |||||
| Events, n | 966 | 230 | 240 | 64 | |
| Person‐years | 1 676 149 | 238 335 | 376 255 | 115 744 | |
| Age‐adjusted model* | 1.00 [Referent] | 1.43 (1.24–1.65) | 1.07 (0.92–1.23) | 0.87 (0.68–1.13) | <0.001 |
| Multivariable model 1† | 1.00 [Referent] | 1.40 (1.21–1.63) | 1.10 (0.95–1.28) | 0.88 (0.68–1.14) | <0.001 |
| Multivariable model 2‡ | 1.00 [Referent] | 1.26 (1.09–1.46) | 1.08 (0.93–1.25) | 0.91 (0.70–1.19) | 0.004 |
| CVD¶ | |||||
| Events, n | 1489 | 311 | 351 | 99 | |
| Person‐years | 1 675 650 | 238 263 | 376 140 | 115 711 | |
| Age‐adjusted model* | 1.00 [Referent] | 1.27 (1.12–1.44) | 1.01 (0.90–1.13) | 0.87 (0.71–1.07) | 0.004 |
| Multivariable model 1† | 1.00 [Referent] | 1.25 (1.10–1.42) | 1.04 (0.92–1.17) | 0.87 (0.70–1.07) | 0.003 |
| Multivariable model 2‡ | 1.00 [Referent] | 1.14 (1.01–1.29) | 1.02 (0.90–1.15) | 0.90 (0.73–1.11) | 0.08 |
| Stroke | |||||
| Events, n | 531 | 83 | 111 | 37 | |
| Person‐years | 1676 650 | 238 514 | 376 389 | 115 787 | |
| Age‐adjusted model* | 1.00 [Referent] | 0.98 (0.77–1.23) | 0.89 (0.73–1.10) | 0.89 (0.64–1.25) | 0.43 |
| Multivariable model 1† | 1.00 [Referent] | 0.97 (0.77–1.23) | 0.91 (0.74–1.12) | 0.89 (0.63–1.25) | 0.50 |
| Multivariable model 2‡ | 1.00 [Referent] | 0.91 (0.72–1.15) | 0.90 (0.73–1.12) | 0.91 (0.64–1.28) | 0.28 |
BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; ICD‐8, International Classification of Diseases, Eighth Revision; MI, myocardial infarction; and NHSII, Nurses’ Health Study II.
Models were jointly stratified by age (months) and calendar time (months).
Model 2 additionally adjusted for age at menarche (<12, 12, 13, ≥14 years of age), White Non‐Hispanic race and ethnicity (yes, no), marital status (ever married, never), daily aspirin use (yes, no), oral contraceptive use (never, past, current), gravidity (≤1, 2, 3, or ≥4 pregnancies), BMI at age 18 years (<19, 20.5–21.9, 22–24.9, 25–29.9, or ≥30 kg/m2), and breastfeeding duration (<3, 3–12, or >12 months).
Model 3 is additionally adjusted for current BMI (<24.9, 25–29.9, 30–34.9, or ≥35 kg/m2), cigarette smoking status and current dose (never, past, current 1–34 cigarettes/d, current ≥35 cigarettes/d), physical activity (0, 0.1–1.0, 1.1–2.4, 2.5–5.9, or ≥6 h/wk), and Alternative Healthy Eating Index 2010 diet quality score (quintiles).
Tests for linear trend were conducted by modeling categories of age at first report of infertility as an ordinal variable assigning integer values.
CHD includes fatal and nonfatal MI, fatal coronary heart disease (ICD‐8: 410, 412), and self‐reported coronary artery bypass grafting/angioplasty/stent.
CVD includes CHD and fatal and nonfatal cerebrovascular accident or transient ischemic attack (ICD‐8: 430–437).
We observed heterogeneity in the association with CHD by the underlying cause of infertility (Table 4). Women whose infertility was attributed to ovulatory disorders had a 28% greater risk of CHD (RR, 1.28 [95% CI, 1.05–1.55]), and women whose infertility was attributed to endometriosis had a 42% greater risk of CHD (RR, 1.42 [95% CI, 1.09–1.85]) compared with gravid women with no history of infertility. No other infertility diagnoses were associated with the risk of CHD.
Table 4.
Hazard Ratios and 95% CIs for the Risk of CHD According to Conditions That Were Reported to be the Cause of Infertility (NHSII, 1989–2017)
| Attributed causes of infertility | Ever reported infertility diagnosis | |
|---|---|---|
| No§ | Yes|| | |
| Ovulatory disorder | ||
| Events, no. | 966 | 161 |
| Age‐adjusted model* | 1.00 [Referent] | 1.35 (1.14–1.60) |
| Multivariable model 1† | 1.00 [Referent] | 1.32 (1.11–1.56) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.28 (1.05–1.55) |
| Endometriosis | ||
| Events, n | 966 | 87 |
| Age‐adjusted model* | 1.00 [Referent] | 1.25 (1.00–1.56) |
| Multivariable model 1† | 1.00 [Referent] | 1.36 (1.08–1.70) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.42 (1.09–1.85) |
| Cervical mucus disorder | ||
| Events, n | 966 | 22 |
| Age‐adjusted model* | 1.00 [Referent] | 0.93 (0.61–1.43) |
| Multivariable model 1† | 1.00 [Referent] | 1.00 (0.65–1.53) |
| Multivariable model 2‡ | 1.00 [Referent] | 0.98 (0.61–1.57) |
| Tubal blockage | ||
| Events, n | 966 | 49 |
| Age‐adjusted model* | 1.00 [Referent] | 0.97 (0.73–1.30) |
| Multivariable model 1† | 1.00 [Referent] | 0.99 (0.74–1.32) |
| Multivariable model 2‡ | 1.00 [Referent] | 0.95 (0.69–1.31) |
| Spouse or partner factors | ||
| Events, n | 966 | 92 |
| Age‐adjusted model* | 1.00 [Referent] | 1.11 (0.90–1.38) |
| Multivariable model 1† | 1.00 [Referent] | 1.16 (0.93–1.45) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.12 (0.87–1.44) |
| Other cause | ||
| Events, n | 966 | 84 |
| Age‐adjusted model* | 1.00 [Referent] | 1.25 (1.00–1.56) |
| Multivariable model 1† | 1.00 [Referent] | 1.27 (1.01–1.59) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.20 (0.94–1.54) |
| Cause not found | ||
| Events, n | 966 | 93 |
| Age‐adjusted model* | 1.00 [Referent] | 0.97 (0.78–1.20) |
| Multivariable model 1† | 1.00 [Referent] | 1.01 (0.82–1.26) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.01 (0.81–1.27) |
| Cause not investigated | ||
| Events, n | 966 | 129 |
| Age‐adjusted model* | 1.00 [Referent] | 1.11 (0.92–1.34) |
| Multivariable model 1† | 1.00 [Referent] | 1.10 (0.91–1.32) |
| Multivariable model 2‡ | 1.00 [Referent] | 1.04 (0.86–1.26) |
CHD includes fatal and nonfatal MI (ICD‐8: 410), fatal coronary heart disease (ICD‐8: 412), and self‐reported coronary artery bypass grafting/angioplasty/stent. BMI indicates body mass index; CHD, coronary heart disease; ICD‐8, International Classification of Diseases, Eighth Revision; MI, myocardial infarction; and NHSII, Nurses’ Health Study II.
Models were jointly stratified by age (months) and calendar time (months).
Model 2 additionally adjusted for age at menarche (<12, 12, 13, ≥14 years of age), White Non‐Hispanic race and ethnicity (yes, no), marital status (ever married, never), daily aspirin use (yes, no), oral contraceptive use (never, past, current), gravidity (≤1, 2, 3, or ≥4 pregnancies), BMI at age 18 years (<19, 20.5–21.9, 22–24.9, 25–29.9, or ≥30 kg/m2), and breastfeeding duration (<3, 3–12, or >12 months).
Model 3 is additionally adjusted for current BMI (<24.9, 25–29.9, 30–34.9, or ≥35 kg/m2), cigarette smoking status and current dose (never, past, current 1–34 cigarettes/d, current ≥35 cigarettes/d), physical activity (0, 0.1–1.0, 1.1–2.4, 2.5–5.9, or ≥6 h/wk), Alternative Healthy Eating Index 2010 diet quality score (quintiles), and mutually adjusted for other infertility diagnoses.
Comparison group is gravid women without a history of any kind of infertility.
Attributed causes of infertility are not mutually exclusive.
We observed no statistically significant effect modification by smoking status, parity, hypertensive disorders of pregnancy, or BMI at age 18 years (Table S1). However, we did observe differences in the association between infertility and CHD by updating adult BMI (P value, test for heterogeneity=0.005). Among women with BMI ≥25 kg/m2, infertility was associated with an increased risk of CHD (RR, 1.24 [95% CI, 1.09–1.41]). No association was observed between infertility and CHD among women with a BMI <25 kg/m2 (RR, 0.88 [95% CI, 0.70–1.11]).
In sensitivity analyses, we observed no meaningful differences between individuals with primary compared with secondary infertility (Table S2). Our findings also did not appreciably change in analyses in which infertility was not updated after age 37 years or when we excluded participants with only infertility attributed to male factor (Table S3). We observed that 16.3% (95% CI, 3.6%–50.2%) of the association between infertility and risk of CHD was mediated through development of type 2 diabetes; however, CIs were wide, while the history of hypertension and hypercholesterolemia did not meaningfully mediate the association. When we investigated infertility diagnoses and risk of stroke (Table S4), endometriosis was associated with a 27% increased risk of stroke; however, it did not reach the threshold of statistical significance, nor did any other infertility diagnoses. In sensitivity analyses restricted to women with ovulatory infertility, we did not observe meaningful differences between women who did and did not express common PCOS symptoms and risk of CHD (Table S5).
Discussion
Overall, we observed that women with infertility were at increased risk of CHD, and this risk was strongest among women who experienced infertility at a younger age. When we investigated differences by cause‐specific infertility, we observed that the greatest risk of CHD was observed among women whose infertility was attributed to ovulatory disorders or endometriosis. The association between infertility and CHD was observed only among women who were overweight or obese and not among women who were underweight or normal weight.
Prior research has suggested that reproductive factors may be associated with the risk of CVD. For example, nulliparous women and women who had 1 birth had a modestly increased risk of cardiovascular disease compared with women with 2 births (0 births: RR, 1.11; 1 birth: RR, 1.10). 4 Prior research from our group observed that women who experienced pregnancy loss had an increased risk of CVD (HR, 1.21) 8 and CVD risk factors. 40 Research from the Women's Health Initiative observed a similar pattern between pregnancy loss, CVD risk (HR, 1.11), and CVD risk factors (hypertension, type 2 diabetes) after 16 years of follow‐up6. However, there has been limited prior research into the relationship between overall infertility and the risk of cardiovascular disease. 29
One of the largest studies to investigate this question used data from the Swedish Birth Registry. 17 They observed that years of trying to conceive without success, potentially a marker for infertility severity, was associated with a risk of CVD. Parous women who had experienced ≥5 years of subfertility had a 19% greater risk of CVD in models adjusted for birth year, age at first delivery, education level, income, country of birth, hypertension, diabetes, preterm birth, small for gestational age birth, smoking, and parity compared with other parous women. A similar pattern of association has been observed in smaller cross‐sectional analyses using the National Health and Nutrition Examination Survey (OR, 1.71), 19 in the Women's Health Initiative (HR, 1.13), 11 Trøndelag Health Study (HR, 1.10), 41 and among Norwegian women who experienced a longer time to conception (HR, 1.14). 18 In the current study, we observed that women with infertility were at a modestly increased risk of CHD (HR, 1.13) and that this association was strongest among women who reported having experienced infertility early in life (HR for women experiencing infertility ≤25 years, 1.26). Infertility at a younger age may be a marker of infertility severity, which is consistent with findings from the Swedish Birth Registry that observed elevated CVD risk among women with infertility lasting >5 years. 17
Notably, we did not observe an association between infertility and risk of stroke (HR, 0.91 [95% CI, 0.77–1.07]). A recent meta‐analysis that included 5 prior studies on infertility and risk of stroke concluded that existing research was inconsistent and inconclusive (meta‐analysis HR, 1.07 [95% CI, 0.87–1.32]). 42 Indeed, subsequent analyses have also produced mixed findings. The InterLACE pooling project observed a 14% increased risk of stroke, 43 while a subsequent analysis by the SWAN (Study of Women's Health Across the Nation) observed no association for their composite CVD measurement (stroke, MI, angina: HR, 0.79 [95% CI, 0.52–1.21]). 44 Differences in findings may be influenced by variability in exposure definitions (eg, experiencing infertility versus using treatment for infertility, which is also influenced by access to care), outcome definitions (ischemic versus hemorrhagic stroke, fatal versus nonfatal stroke), and differences in the demographics of the population sampled. Indeed, focusing on ischemic stroke only, prior research among women enrolled in the Taiwan National Health Insurance program observed a protective effect for women using infertility treatment (HR, 0.82) 45 ; however, a harmful effect was observed for women with a history of infertility in the InterLACE pooling project (HR, 1.15). 43
Research from the Framingham Heart Study observed that women with infertility had increased risk factors for CVD, such as higher BMI, waist circumference, and triglyceride levels, and lower levels of high‐density lipoprotein cholesterol. 15 While we were able to a priori adjust for adult BMI and BMI at age 18 years, as well as take into account high cholesterol in mediation analyses, we observed that the association between infertility and risk of CHD varied by BMI (P value, test for heterogeneity=0.005). Among women with an overweight or obese BMI, women with a history of infertility were at greater risk of CHD (HR, 1.24 [95% CI, 1.09–1.41]); however, we observed no association among women with a BMI <25 kg/m2, suggesting that BMI may be an important modifiable risk factor for women with infertility. These data also suggest that history of infertility among women with large body size may be a marker of insulin insensitivity and could be a target for early therapeutics or intervention.
When investigating specific infertility diagnoses, we observed that the risk of CHD was greatest among women whose infertility was attributed to ovulatory disorders (HR, 1.28) or endometriosis (HR, 1.42). The diagnosis of ovulatory infertility includes the diagnosis of PCOS, which is known to be associated with cardiometabolic outcomes. A recent meta‐analysis investigating the association between PCOS and cardiovascular disease observed that women with PCOS had a greater risk of nonfatal cerebrovascular disease (8 studies; RR, 1.41 [95% CI, 1.02–1.94]) and nonfatal coronary events (6 studies; HR, 1.78 [95% CI, 0.99–3.23]). 28 Women with PCOS were also observed to have a greater risk of hypertension (RR, 1.75), type 2 diabetes (RR, 3.00), and adverse lipid profiles (higher serum concentrations of total cholesterol and lower serum concentrations of high‐density lipoprotein). 28 PCOS may also be an extreme manifestation of the more common symptom of menstrual cycle irregularity that has been associated with CVD risk and CVD mortality. 46 Women with endometriosis have also been shown in prior research to have an elevated risk of hypertension, 26 hypercholesterolemia, 26 , 47 , 48 , 49 and subclinical atherosclerosis 50 compared with women without endometriosis, suggesting an adverse vascular profile among these women. 51 , 52 Additionally, data from our research team observed a 62% greater risk of CHD 27 and a 34% greater risk of stroke 53 among women with laparoscopically confirmed endometriosis independent of history of infertility. Diagnoses associated with infertility may therefore confer risk of CHD through different mechanisms; PCOS manifests through ovulatory dysfunction and hyperandrogenic milieu, 30 , 54 while endometriosis has been associated with higher levels of systemic inflammation and changes in endogenous hormones, 55 , 56 which may influence CVD risk. Future research into infertility and CHD should further investigate informative heterogeneity by infertility diagnoses and infertility severity.
A recent scientific statement from the American Heart Association 57 underscores the importance of knowing patient history of adverse pregnancy outcomes and early menopause when counseling patients for prevention of CVD events and appropriate therapeutic interventions for CVD. Clinically, our findings suggest the need to pay attention to female‐specific risk factors when studying CHD. If our findings are robustly replicated, then information on reproductive and gynecologic history should be discussed with medical providers to better counsel patients on future CHD risk.
Although this study has many strengths, including its large sample size, longitudinal follow‐up, and detailed information on infertility symptoms, we must also recognize its limitations. Information on infertility and infertility diagnoses and confounding variables (eg, BMI) were based on self‐report and therefore may be prone to misclassification; however, prior research has suggested that women are able to recall these with high accuracy. 32 , 33 Therefore, we would expect any misclassification to be nondifferential with respect to our outcome and therefore attenuate any associations. The population under study was relatively homogenous in terms of racial or ethnic background and education level; therefore, these results may not be generalizable to other groups who may have different risks for cardiometabolic outcomes. However, this may increase internal validity and reduce the risk of residual confounding by these covariates. For covariates with missing data, a missing indicator variable was used, which will bias the results if data are missing not at random. We lacked sufficient sample size to be able to differentiate between ischemic and hemorrhagic stroke, which may have different mechanisms of association with infertility.
In summary, we observed that women with a history of infertility had greater risk of CHD, and this risk was greatest among women with infertility attributed to ovulatory problems or endometriosis. These findings are consistent with other studies that have suggested that women with severe infertility, PCOS, and endometriosis have poorer cardiometabolic health. Future research should continue to investigate informative heterogeneity by infertility symptom presentation, as this may lead to a better understanding of mechanisms of association and identify groups that may benefit from targeted screening or interventions. Additionally, future research should investigate the contribution of knowing infertility history on top of established CVD risk factors and disentangling the mechanism of association between infertility and CVD.
Sources of Funding
This work was supported by grants HD096033 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development; and U01 CA176726 and U01 HL145386 from the National Cancer Institute.
Disclosures
None.
Supporting information
Tables S1–S5
Acknowledgments
The authors thank the participants in the Nurses' Health Study cohorts and Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, as home of the Nurses' Health Study II.
Drs Farland and Missmer conceived, designed, and supervised the study. Dr Y.‐X. Wang performed the statistical analysis. Drs Farland, Y.‐X. Wang, Gaskins, Rich‐Edwards, S. Wang, Magnus, Chavarro, Rexrode, and Missmer drafted and critically reviewed the manuscript and approved the final version.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027755
For Sources of Funding and Disclosures, see page 8.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/cir.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 2. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2:e000298. doi: 10.1136/bmjgh-2017-000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Søndergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, Gemmill A, Van Horn L, Park K, Salmoirago‐Blotcher E, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. 2020;5:1390–1398. doi: 10.1001/jamacardio.2020.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later‐life maternal cardiovascular disease. Am Heart J. 2010;159:215–221.e216. doi: 10.1016/j.ahj.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 5. Niemczyk NA, Catov JM, Barinas‐Mitchell E, McClure CK, Roberts JM, Tepper PG, Sutton‐Tyrrell K. Nulliparity is associated with less healthy markers of subclinical cardiovascular disease in young women with overweight and obesity. Obesity (Silver Spring). 2015;23:1085–1091. doi: 10.1002/oby.21044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall PS, Nah G, Vittinghoff E, Parker DR, Manson JE, Howard BV, Sarto GE, Gass ML, Sealy‐Jefferson SM, Salmoirago‐Blotcher E, et al. Relation of pregnancy loss to risk of cardiovascular disease in parous postmenopausal women (from the Women's Health Initiative). Am J Cardiol. 2019;123:1620–1625. doi: 10.1016/j.amjcard.2019.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, Lewis CE, Loucks EB, Parker DR, Rillamas‐Sun E, et al. Reproductive risk factors and coronary heart disease in the Women's Health Initiative observational study. Circulation. 2016;133:2149–2158. doi: 10.1161/circulationaha.115.017854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang YX, Mínguez‐Alarcón L, Gaskins AJ, Wang L, Ding M, Missmer SA, Rich‐Edwards JW, Manson JE, Chavarro JE. Pregnancy loss and risk of cardiovascular disease: the Nurses' Health Study II. Eur Heart J. 2022;43:190–199. doi: 10.1093/eurheartj/ehab737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peters SA, Woodward M. Women's reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104:1069–1075. doi: 10.1136/heartjnl-2017-312289 [DOI] [PubMed] [Google Scholar]
- 10. Lau ES, Wang D, Roberts M, Taylor CN, Murugappan G, Shadyab AH, Schnatz PF, Farland LV, Wood MJ, Scott NS, et al. Infertility and risk of heart failure in the Women's Health Initiative. J Am Coll Cardiol. 2022;79:1594–1603. doi: 10.1016/j.jacc.2022.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murugappan G, Leonard SA, Farland LV, Lau ES, Shadyab AH, Wild RA, Schnatz P, Carmichael SL, Stefanick ML, Parikh NI. Association of infertility with atherosclerotic cardiovascular disease among postmenopausal participants in the Women's Health Initiative. Fertil Steril. 2022;117:1038–1046. doi: 10.1016/j.fertnstert.2022.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solomon CG, Hu FB, Dunaif A, Rich‐Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87:2013–2017. doi: 10.1210/jcem.87.5.8471 [DOI] [PubMed] [Google Scholar]
- 13. Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause‐specific mortality and total life expectancy. Epidemiology. 2005;16:556–562. doi: 10.1097/01.ede.0000165392.35273.d4 [DOI] [PubMed] [Google Scholar]
- 14. Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, Willett WC, Hu FB. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318:255–269. doi: 10.1001/jama.2017.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahalingaiah S, Sun F, Cheng JJ, Chow ET, Lunetta KL, Murabito JM. Cardiovascular risk factors among women with self‐reported infertility. Fertil Res Pract. 2017;3:7. doi: 10.1186/s40738-017-0034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Xiong X, Bazzano L, Harville EW. Childhood cardiovascular health and subfertility: the Bogalusa Heart Study. Pediatr Res. 2018;84:625–631. doi: 10.1038/s41390-018-0032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Hum Reprod. 2012;27:568–575. doi: 10.1093/humrep/der400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Magnus MC, Fraser A, Rich‐Edwards JW, Magnus P, Lawlor DA, Håberg SE. Time‐to‐pregnancy and risk of cardiovascular disease among men and women. Eur J Epidemiol. 2021;36:383–391. doi: 10.1007/s10654-021-00718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gleason JL, Shenassa ED, Thoma ME. Self‐reported infertility, metabolic dysfunction, and cardiovascular events: a cross‐sectional analysis among U.S. women. Fertil Steril. 2019;111:138–146. doi: 10.1016/j.fertnstert.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 20. Cibula D, Cifkova R, Fanta M, Poledne R, Zivny J, Skibova J. Increased risk of non‐insulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Hum Reprod. 2000;15:785–789. doi: 10.1093/humrep/15.4.785 [DOI] [PubMed] [Google Scholar]
- 21. Merz CN, Shaw LJ, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper‐DeHoff RM, Johnson BD, et al. Cardiovascular disease and 10‐year mortality in postmenopausal women with clinical features of polycystic ovary syndrome. J Womens Health (Larchmt). 2016;25:875–881. doi: 10.1089/jwh.2015.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, Blackledge H, Khunti K, Howlett TA. Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20‐year retrospective cohort study. Clin Endocrinol. 2013;78:926–934. doi: 10.1111/cen.12068 [DOI] [PubMed] [Google Scholar]
- 23. Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long‐term follow‐up. J Clin Epidemiol. 1998;51:581–586. doi: 10.1016/S0895-4356(98)00035-3 [DOI] [PubMed] [Google Scholar]
- 24. Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long‐term follow‐up: a retrospective cohort study. Clin Endocrinol. 2000;52:595–600. doi: 10.1046/j.1365-2265.2000.01000.x [DOI] [PubMed] [Google Scholar]
- 25. Verit FF, Yildiz Zeyrek F, Zebitay AG, Akyol H. Cardiovascular risk may be increased in women with unexplained infertility. Clin Exp Reprod Med. 2017;44:28–32. doi: 10.5653/cerm.2017.44.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mu F, Rich‐Edwards J, Rimm EB, Spiegelman D, Forman JP, Missmer SA. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension. 2017;70:59–65. doi: 10.1161/HYPERTENSIONAHA.117.09056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mu F, Rich‐Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9:257–264. doi: 10.1161/circoutcomes.115.002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wekker V, van Dammen L, Koning A, Heida KY, Painter RC, Limpens J, Laven JSE, Roeters van Lennep JE, Roseboom TJ, Hoek A. Long‐term cardiometabolic disease risk in women with PCOS: a systematic review and meta‐analysis. Hum Reprod Update. 2020;26:942–960. doi: 10.1093/humupd/dmaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y‐X, Farland LV, Wang S, Gaskins AJ, Wang L, Rich‐Edwards JW, Tamimi R, Missmer SA, Chavarro JE. Association of infertility with premature mortality among US women: prospective cohort study. The Lancet Regional Health ‐ Americas. 2022;7:100122. doi: 10.1016/j.lana.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Azziz R, Carmina E, Dewailly D, Diamanti‐Kandarakis E, Escobar‐Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178 [DOI] [PubMed] [Google Scholar]
- 31. Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 32. Rich‐Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0 [DOI] [PubMed] [Google Scholar]
- 33. Shafrir AL, Wise LA, Palmer JR, Shuaib ZO, Katuska LM, Vinayak P, Kvaskoff M, Terry KL, Missmer SA. Validity of self‐reported endometriosis: a comparison across four cohorts. Hum Reprod. 2021;36:1268–1278. doi: 10.1093/humrep/deab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rose GA, Blackburn H. Cardiovascular survey methods. 2nd ed. World Health Organization ; 1982. WHO monograph series No 58. [PubMed] [Google Scholar]
- 35. Walker AE, Robins M, Weinfeld FD. The national survey of stroke. Clinical findings. Stroke. 1981;12:I13–I44. [PubMed] [Google Scholar]
- 36. Female age‐related fertility decline . Committee opinion No. 589. Obstet Gynecol. 2014;123:719–721. doi: 10.1097/01.Aog.0000444440.96486.61 [DOI] [PubMed] [Google Scholar]
- 37. Farland LV, Correia KFB, Dodge LE, Modest AM, Williams PL, Smith LH, Toth TL, Hacker MR, Missmer SA. The importance of mediation in reproductive health studies. Hum Reprod. 2020;35:1262–1266. doi: 10.1093/humrep/deaa064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology. 2011;22:582–585. doi: 10.1097/EDE.0b013e31821db37e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hertzmark E, Pazaris M, Spiegelman D. The SAS mediate macro. Harvard University; 2018. Accessed June 2, 2022. https://cdn1.sph.harvard.edu/wp‐content/uploads/sites/271/2012/08/mediate.pdf [Google Scholar]
- 40. Horn J, Tanz LJ, Stuart JJ, Markovitz AR, Skurnik G, Rimm EB, Missmer SA, Rich‐Edwards JW. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: a prospective cohort study. BJOG. 2019;126:33–42. doi: 10.1111/1471-0528.15452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skåra KH, Åsvold BO, Hernáez Á, Fraser A, Rich‐Edwards JW, Farland LV, Næss Ø, Lawlor DA, Brumpton B, Magnus MC. Risk of cardiovascular disease in women and men with subfertility: the Trøndelag Health Study. Fertil Steril. 2022;118:537–547. doi: 10.1016/j.fertnstert.2022.05.038 [DOI] [PubMed] [Google Scholar]
- 42. Liang C, Chung HF, Dobson AJ, Mishra GD. Infertility, miscarriage, stillbirth, and the risk of stroke among women: a systematic review and meta‐analysis. Stroke. 2022;53:328–337. doi: 10.1161/strokeaha.121.036271 [DOI] [PubMed] [Google Scholar]
- 43. Liang C, Chung HF, Dobson AJ, Hayashi K, van der Schouw YT, Kuh D, Hardy R, Derby CA, El Khoudary SR, Janssen I, et al. Infertility, recurrent pregnancy loss, and risk of stroke: pooled analysis of individual patient data of 618 851 women. BMJ. 2022;377:e070603. doi: 10.1136/bmj-2022-070603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cairncross ZF, Ahmed SB, Dumanski SM, Nerenberg KA, Metcalfe A. Infertility and the risk of cardiovascular disease: findings from the Study of Women's Health Across the Nation (SWAN). CJC open. 2021;3:400–408. doi: 10.1016/j.cjco.2020.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ge SQ, Tao X, Cai LS, Deng XY, Hwang MF, Wang CL. Associations of hormonal contraceptives and infertility medications on the risk of venous thromboembolism, ischemic stroke, and cardiovascular disease in women. J Invest Med. 2019;67:729–735. doi: 10.1136/jim-2018-000750 [DOI] [PubMed] [Google Scholar]
- 46. Wang YX, Arvizu M, Rich‐Edwards JW, Stuart JJ, Manson JE, Missmer SA, Pan A, Chavarro JE. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ. 2020;371:m3464. doi: 10.1136/bmj.m3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Melo AS, Rosa‐e‐Silva JC, Rosa‐e‐Silva AC, Poli‐Neto OB, Ferriani RA, Vieira CS. Unfavorable lipid profile in women with endometriosis. Fertil Steril. 2010;93:2433–2436. doi: 10.1016/j.fertnstert.2009.08.043 [DOI] [PubMed] [Google Scholar]
- 48. Verit FF, Erel O, Celik N. Serum paraoxonase‐1 activity in women with endometriosis and its relationship with the stage of the disease. Hum Reprod. 2008;23:100–104. doi: 10.1093/humrep/dem340 [DOI] [PubMed] [Google Scholar]
- 49. Turgut A, Ozler A, Goruk NY, Tunc SY, Evliyaoglu O, Gul T. Copper, ceruloplasmin and oxidative stress in patients with advanced‐stage endometriosis. Eur Rev Med Pharmacol Sci. 2013;17:1472–1478. [PubMed] [Google Scholar]
- 50. Pretta S, Remorgida V, Abbamonte LH, Anserini P, Ragni N, Del Sette M, Gandolfo C, Ferrero S. Atherosclerosis in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;132:226–231. doi: 10.1016/j.ejogrb.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 51. Yu E, Rimm E, Qi L, Rexrode K, Albert CM, Sun Q, Willett WC, Hu FB, Manson JE. Diet, lifestyle, biomarkers, genetic factors, and risk of cardiovascular disease in the Nurses' Health Studies. Am J Public Health. 2016;106:1616–1623. doi: 10.2105/ajph.2016.303316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Farland LV, Harris HR. Long‐term health consequences of endometriosis—pathways and mediation by treatment. Curr Obstet Gynecol Rep. 2020;9:79–88. doi: 10.1007/s13669-020-00287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farland LV, Degnan WJ III, Bell ML, Kasner SE, Liberman AL, Shah DK, Rexrode KM, Missmer SA. Laparoscopically confirmed endometriosis and risk of incident stroke: a prospective cohort study. Stroke. 2022;53:3166–3122. doi: 10.1161/strokeaha.122.039250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217 [DOI] [PubMed] [Google Scholar]
- 55. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76:1–10. doi: 10.1016/S0015-0282(01)01816-7 [DOI] [PubMed] [Google Scholar]
- 56. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7 [DOI] [PubMed] [Google Scholar]
- 57. Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902–e916. doi: 10.1161/cir.0000000000000961 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


