Abstract
Background
Limited evidence is available on sex differences about the association between hypertension and incident atrial fibrillation (AF).
Methods and Results
We used a nationwide health checkup and claims database to analyze 3 383 738 adults (median age, 43 (36–51) years, 57.4% men). We investigated the relationship between hypertension and incident AF in men and women using a Cox regression model. We used restricted cubic spline functions to identify the association of blood pressure (BP) as a continuous parameter with incident AF. We categorized the men and women into 4 groups according to the 2017 American College of Cardiology/American Heart Association BP guidelines. During a mean follow‐up of 1199±950 days, 13 263 AF diagnoses were recorded. The incidence (95% CI) of AF was 15.8 (15.5–16.1) per 10 000 person‐years in men and 6.1 (5.9–6.3) per 10 000 person‐years in women. Compared with normal BP, elevated BP, stage 1 hypertension, and stage 2 hypertension were associated with an increased risk AF in both men and women. However, the hazard ratios were greater in women than in men, and the P value for interactions in the multivariable model was 0.0076. The models using restricted cubic spline showed that the risk of AF associated with elevated systolic BP increased steeply above an approximate threshold of systolic BP of 130 mm Hg in men and 100 mm Hg in women. Although our primary findings were consistent across subgroup analyses, this association was most significant in younger individuals.
Conclusions
Although the incidence of AF was higher in men, the association between hypertension and incident AF was more pronounced in women than in men, suggesting a potential sex difference in the relationship between hypertension and incident AF.
Keywords: atrial fibrillation, blood pressure, hypertension, sex difference
Subject Categories: Hypertension
Nonstandard Abbreviations and Acronyms
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
Clinical Perspective.
What Is New?
Our analysis of a large‐scale real‐world data set including >3 million people demonstrated that elevated blood pressure, stage 1 hypertension, and stage 2 hypertension were all associated with a greater risk of developing atrial fibrillation in men and women, and this relationship was more pronounced in women than in men.
What Are the Clinical Implications?
Women may be more susceptible to increasing blood pressure than men in the development of atrial fibrillation, and we need to recognize that the risk of atrial fibrillation was higher even in elevated blood pressure compared with normal blood pressure in both men and women.
Atrial fibrillation (AF) is increasing in prevalence. 1 , 2 , 3 In the United States, the number of patients with AF is estimated to increase from ≈5.2 million in 2010 to 12.1 million in 2030. 4 Similarly, the prevalence of AF is projected to increase from 8.8 million in 2010 to 17.9 million in 2060 among people aged >55 years in the European Union. 5 Given this epidemiological background, the primary prevention of AF is needed from the public health perspective. For this end, hypertension is one of the important risk factors for cardiovascular disease including AF, 6 , 7 and the risk stratification of AF using blood pressure (BP) status is clinically important. Regarding the potential sex difference, although the incidence of AF is known to be higher in men than in women, 2 , 8 , 9 once AF occurs, women could have adverse clinical events (eg, recurrence, stroke, death). 10 , 11 , 12 Therefore, even if the incidence is low, preventing AF in women is essential. Furthermore, considering the possible sex difference in the relationship between the risk factors and incident cardiovascular events, 13 risk stratification for AF by sex would also be required. However, data on the potential sex difference in the influence of hypertension on incident AF have been limited. In the present study, we examined the association of hypertension with incident AF stratified by sex using a nationwide health checkup and administrative claims data set and aimed to clarify whether sex differences are present in the relationship between hypertension and the risk of developing AF.
METHODS
We used the JMDC Claims Database, commercially available for anyone who would purchase it, from JMDC Inc. (JMDC Inc., Tokyo, Japan; https://www.jmdc.co.jp/en/). JMDC Inc. is a health care venture corporation in Japan.
Study Population
In this retrospective observational cohort study, we used the JMDC Claims Database, consisting of a combined database of health checkup and administrative claims database (both outpatient and inpatient settings) in Japan. 6 , 14 , 15 The JMDC Claims Database covered individuals who were mainly employees and their family members in Japan between January 2005 and April 2021. Japan has a universal health insurance system, and the JMDC Claims Database consists of administrative claims records reimbursed by insurance (eg, medical diagnoses, pharmacological prescriptions) from >60 insurers, and medical diagnoses are registered in the form of International Classification of Diseases, 10th Revision (ICD‐10) coding. We identified 4 534 334 individuals with available health checkup data on BP and blood test results. Subsequently, we excluded individuals aged <20 years; those with a history of myocardial infarction, angina pectoris, stroke, heart failure, and AF; those with a history of renal replacement therapy; those taking BP‐lowering medications; and those with missing data on cigarette smoking and alcohol consumption. Finally, we obtained 3 383 738 participants in this study (Figure 1). In this study, we used data on BP and the status of BP‐lowering medication use only at the initial health checkup.
Figure 1. Flowchart.
Study population after applying inclusion and exclusion criteria.
Ethics
This study was performed in accordance with the ethical guidelines of the University of Tokyo (approval by the Ethical Committee of the University of Tokyo: 2018‐10862) and the principles of the Declaration of Helsinki. The requirement for informed consent was waived because all data in the JMDC Claims Database were anonymized and deidentified.
BP Measurement
In Japan, employers are obligated to perform health checkups (generally once a year) for their employees, and we used BP data at health checkup. BP was measured during a health checkup according to the protocol recommended by the Japanese Ministry of Health, Labor, and Welfare by health care professionals after a 5‐minute rest (see Data S1). 6 , 14 , 15
BP Categorization
The participants were categorized into 4 groups based on BP at initial health checkup: normal BP (systolic BP (SBP) <120 mm Hg and diastolic BP (DBP) <80 mm Hg), elevated BP (SBP of 120 to 129 mm Hg and DBP <80 mm Hg), stage 1 hypertension (SBP of 130–139 mm Hg or DBP of 80–89 mm Hg), and stage 2 hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg). 16 Furthermore, we also categorized study participants into normal/elevated BP that was defined as SBP <130 mm Hg and DBP <80 mm Hg, isolated diastolic hypertension defined as SBP <130 mm Hg and DBP ≥80 mm Hg, isolated systolic hypertension defined as SBP ≥130 mm Hg and DBP <80 mm Hg, and systolic diastolic hypertension defined as SBP ≥130 mm Hg and DBP ≥80 mm Hg.
Risk Factors Other Than BP
Data on body mass index (BMI), and fasting laboratory values were obtained using standardized protocols during the health checkups. Information on cigarette smoking (current or noncurrent) and alcohol consumption (every day or not every day) were collected from self‐reported questionnaires at the health checkup. Overweight and obesity were defined as BMI ≥25 kg/m2. Diabetes was defined as having a fasting glucose level ≥126 mg/dL or using glucose‐lowering medications. Dyslipidemia was defined as low‐density lipoprotein cholesterol level ≥140 mg/dL, high‐density lipoprotein cholesterol level <40 mg/dL, triglyceride level ≥150 mg/dL, or using lipid‐lowering medications.
Outcomes
Information on the outcomes was collected between January 2005 and April 2021. The primary outcome was AF (ICD‐10 code: I480–I484, and I489).
Statistical Analysis
We analyzed the study population stratified by sex. The data are expressed as median (Q1–Q3) for continuous variables or number (percentage) for categorical variables. The statistical significance of differences between men and women on clinical characteristics were assessed using unpaired t test and Chi‐squared test, for continuous variables and for categorical variables, respectively. We performed analyses using Cox regression to examine the association between BP categories and incident AF. We calculated hazard ratios (HRs) in an unadjusted model (Model 1), an age‐adjusted model (Model 2), and after adjustment for age, BMI, diabetes, dyslipidemia, cigarette smoking, and alcohol consumption using the forced entry method (Model 3). To examine whether the BP category had a differential relationship with incident AF by sex, multiplicative interaction terms for sex were calculated. We performed a stratified subgroup analysis by age (≥50 versus <50 years) or alcohol consumption (every day versus not every day). We checked the proportional hazard assumption for Cox proportional hazard models using Schoenfeld residuals.
We evaluated the dose–response relationship of SBP or DBP with the risk of developing AF using a restricted cubic spline regression model. We used 4 knots for SBP and DBP (5th, 35th, 65th, and 95th percentiles), with the reference point set at SBP of 120 mm Hg and DBP of 80 mm Hg. The HR of SBP or DBP as a continuous variable was adjusted for age, BMI, diabetes, dyslipidemia, cigarette smoking, and alcohol consumption.
Four sensitivity analyses were conducted. First, we included individuals taking BP‐lowering medications at the initial health checkup; these individuals had been excluded from the main analysis as shown in Figure 1. Second, we imputed missing data on cigarette smoking and alcohol consumption and obtained results, as previously described. 17 , 18 Briefly, we performed the analysis using the multiple imputation by the chained equation method with 20 iterations described by Aloisio et al, 19 and obtained the HRs with standard errors based on Rubin's rules. 20 Third, because death could be regarded as a competing risks with AF events, we also conducted a competing risks analysis using cause‐specific Cox proportional hazard modeling. 21 , 22 Fourth, we added use of sex hormones or modulators of genital system medications (ATC code of G03) to multivariable Cox regression model (model 3).
Statistical significance was set at a P value <0.05. All statistical analyses were conducted using STATA version 17 (StataCorp LLC, College Station, TX, USA).
RESULTS
Clinical Characteristics
The clinical characteristics of the study participants are summarized in Table 1. The median (interquartile range) age was 43 (36–51) years, and 1 943 708 participants (57.4%) were men. The median age was 44 years in women, and 43 years in men. The SBP and DBP were higher in men than in women. Accordingly, the prevalence of stage 1 and stage 2 hypertension were higher in men than in women. Moreover, men had a higher BMI and a higher prevalence of diabetes, dyslipidemia, cigarette smoking, and alcohol consumption than women.
Table 1.
Baseline Characteristics
| Men (n=1 943 708) | Women (n=1 440 030) | P value | |
|---|---|---|---|
| Age, y | 43 (36–51) | 44 (36–51) | <0.001 |
| Body mass index, kg/m2 | 23.1 (21.1–25.3) | 20.9 (19.2–23.2) | <0.001 |
| Overweight/obesity, n (%) | 548 872 (28.2) | 210 716 (14.6) | <0.001 |
| Systolic blood pressure, mm Hg | 120 (111–129) | 111 (102–122) | <0.001 |
| Diastolic blood pressure, mm Hg | 75 (67–82) | 68 (61–76) | <0.001 |
| Blood pressure category | <0.001 | ||
| Normal blood pressure, n (%) | 851 494 (43.8) | 971 120 (67.4) | |
| Elevated blood pressure, n (%) | 336 104 (17.3) | 167 421 (11.6) | |
| Stage 1 hypertension, n (%) | 491 293 (25.3) | 199 296 (13.8) | |
| Stage 2 hypertension, n (%) | 264 817 (13.6) | 102 193 (7.1) | |
| Diabetes, n (%) | 65 133 (3.4) | 16 038 (1.1) | <0.001 |
| Dyslipidemia, n (%) | 875 301 (45.0) | 387 728 (26.9) | <0.001 |
| Low‐density lipoprotein cholesterol, mg/dL | 122 (101–144) | 113 (94–135) | <0.001 |
| High‐density lipoprotein cholesterol, mg/dL | 56 (48–67) | 70 (61–81) | <0.001 |
| Triglycerides, mg/dL | 94 (65–140) | 64 (48–89) | <0.001 |
| Fasting plasma glucose, mg/dL | 93 (87–100) | 89 (84–94) | <0.001 |
| Cigarette smoking, n (%) | 707 446 (36.4) | 159 791 (11.1) | <0.001 |
| Alcohol consumption, n (%) | 552 688 (28.4) | 172 932 (12.0) | <0.001 |
Data are reported as medians (interquartile range) or numbers (percentage), where appropriate.
BP Category and AF
During a mean follow‐up period of 1199±950 days, 10 601 AF diagnoses were recorded in men, and the incidence of AF was 15.8 (95% CI, 15.5–16.1) per 10 000 person‐years. In women, 2662 AF events were recorded, and the incidence of AF was 6.1 (95% CI, 5.9–6.3) per 10 000 person‐years. The risk of AF increases with increasing BP. Compared with normal/elevated BP, HRs of elevated BP, stage 1 hypertension, and stage 2 hypertension for AF were 1.10 (95% CI, 1.03–1.17), 1.17 (95% CI, 1.11–1.23), and 1.55 (95% CI, 1.47–1.63) in men, respectively. HRs of elevated BP, stage 1 hypertension, and stage 2 hypertension for AF were 1.22 (95% CI, 1.08–1.37), 1.37 (95% CI, 1.23–1.52), and 1.89 (95% CI, 1.68–2.12) in women, respectively. The P value for interaction was 0.0076 in the model 3, suggesting that the significant association of the BP category with incident AF was modified by sex (Table 2). We checked the proportional hazard assumption by Schoenfeld residual tests, and there was no breach of this hypothesis (P=0.060 for men, P=0.462 for women).
Table 2.
Blood Pressure Category and Atrial Fibrillation Stratified by Sex
| Men | Women | P for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal blood pressure | Elevated blood pressure | Stage 1 hypertension | Stage 2 hypertension | Normal blood pressure | Elevated blood pressure | Stage 1 hypertension | Stage 2 hypertension | ||
| No. of individuals | 851 494 | 336 104 | 491 293 | 264 817 | 971 120 | 167 421 | 199 296 | 102 193 | … |
| No. of events | 3326 | 1490 | 3160 | 2625 | 1345 | 371 | 532 | 414 | … |
| Incidence per 10 000 person‐years | 11.4 (11.1–11.8) | 13.2 (12.6–13.9) | 17.8 (17.2–18.4) | 29.1 (28.0–30.3) | 4.6 (4.3–4.8) | 7.2 (6.5–8.0) | 8.7 (8.0–9.5) | 14.3 (13.0–15.7) | … |
| Model 1 | 1 [Reference] | 1.15 (1.08–1.23) | 1.54 (1.47–1.62) | 2.54 (2.41–2.67) | 1 [Reference] | 1.57 (1.40–1.77) | 1.91 (1.73–2.12) | 3.16 (2.83–3.53) | <0.001 |
| Model 2 | 1 [Reference] | 1.13 (1.06–1.20) | 1.23 (1.17–1.29) | 1.68 (1.59–1.76) | 1 [Reference] | 1.22 (1.09–1.37) | 1.38 (1.24–1.52) | 1.91 (1.70–2.14) | 0.1525 |
| Model 3 | 1 [Reference] | 1.10 (1.03–1.17) | 1.17 (1.11–1.23) | 1.55 (1.47–1.63) | 1 [Reference] | 1.22 (1.08–1.37) | 1.37 (1.23–1.52) | 1.89 (1.68–2.12) | 0.0076 |
The association between blood pressure category and atrial fibrillation stratified by sex is summarized. The incidence is presented in 10 000 person‐years. Model 1=unadjusted model. Model 2=adjusted for age. Model 3=Adjusted for age, body mass index, diabetes, dyslipidemia, cigarette smoking, and alcohol consumption.
The BP category was associated with the incidence of AF in men and women, not only in people aged ≥50 years, but also in those aged <50 years. The P value for the interaction assessing the association of BP category with incident AF between men and women was statistically significant in people aged <50 years, but not in those aged ≥50 years. The association of hypertension with incident AF was greater in women than in men among both people with and without alcohol consumption. However, the statistically significant interaction was more robust in people with alcohol consumption (Table 3).
Table 3.
Subgroup Analysis of Blood Pressure Category and Atrial Fibrillation Stratified by Sex
| Men | Women | P for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal blood pressure | Elevated blood pressure | Stage 1 hypertension | Stage 2 hypertension | Normal blood pressure | Elevated blood pressure | Stage 1 hypertension | Stage 2 hypertension | ||
| Age ≥50 y | |||||||||
| No. of individuals | 186 761 | 76 258 | 174 353 | 127 596 | 211 545 | 65 042 | 89 931 | 61 020 | … |
| No. of events | 1751 | 808 | 1943 | 1773 | 588 | 208 | 315 | 304 | … |
| Incidence per 10 000 person‐years | 26.6 (25.4–27.8) | 30.8 (28.8–33.0) | 31.9 (30.6–33.4) | 43.1 (41.1–45.1) | 9.4 (8.7–10.2) | 11.2 (9.8–12.9) | 12.3 (11.0–13.7) | 18.7 (16.7–20.9) | … |
| Model 1 | 1 [Reference] | 1.16 (1.07–1.26) | 1.20 (1.12–1.28) | 1.62 (1.52–1.73) | 1 [Reference] | 1.19 (1.02–1.39) | 1.30 (1.13–1.49) | 1.99 (1.74–2.29) | 0.0630 |
| Model 2 | 1 [Reference] | 1.10 (1.01–1.19) | 1.16 (1.09–1.24) | 1.51 (1.41–1.61) | 1 [Reference] | 1.07 (0.91–1.25) | 1.18 (1.03–1.35) | 1.71 (1.48–1.96) | 0.3495 |
| Model 3 | 1 [Reference] | 1.07 (0.98–1.16) | 1.12 (1.05–1.19) | 1.41 (1.32–1.51) | 1 [Reference] | 1.06 (0.90–1.24) | 1.16 (1.01–1.33) | 1.65 (1.43–1.91) | 0.2274 |
| Age <50 y | |||||||||
| No. of individuals | 664 733 | 259 846 | 316 940 | 137 221 | 759 575 | 102 379 | 109 365 | 41 173 | … |
| No. of events | 1575 | 682 | 1217 | 852 | 757 | 163 | 217 | 110 | … |
| Incidence per 10 000 person‐years | 7.0 (6.7–7.4) | 7.9 (7.3–8.5) | 10.4 (9.9–11.0) | 17.4 (16.3–18.6) | 3.2 (3.0–3.5) | 4.9 (4.2–5.7) | 6.2 (5.4–7.0) | 8.7 (7.2–10.4) | … |
| Model 1 | 1 [Reference] | 1.12 (1.02–1.23) | 1.45 (1.35–1.57) | 2.44 (2.24–2.65) | 1 [Reference] | 1.50 (1.26–1.77) | 1.87 (1.61–2.18) | 2.66 (2.18–3.25) | 0.0025 |
| Model 2 | 1 [Reference] | 1.17 (1.07–1.27) | 1.29 (1.20–1.39) | 2.00 (1.84–2.17) | 1 [Reference] | 1.39 (1.18–1.65) | 1.66 (1.43–1.94) | 2.20 (1.80–2.70) | 0.0201 |
| Model 3 | 1 [Reference] | 1.13 (1.03–1.23) | 1.21 (1.12–1.31) | 1.79 (1.64–1.96) | 1 [Reference] | 1.41 (1.19–1.68) | 1.69 (1.45–1.98) | 2.26 (1.83–2.80) | 0.0008 |
| Alcohol consumption: every day | |||||||||
| No. of individuals | 192 742 | 85 481 | 166 928 | 107 537 | 101 316 | 21 325 | 32 171 | 18 120 | … |
| No. of events | 1165 | 551 | 1394 | 1254 | 146 | 57 | 92 | 66 | … |
| Incidence per 10 000 person‐years | 16.1 (15.2–17.0) | 17.4 (16.0–18.9) | 21.9 (20.8–23.1) | 33.4 (31.6–35.3) | 4.9 (4.1–5.7) | 9.1 (7.0–11.8) | 10.1 (8.2–12.4) | 13.8 (10.9–17.6) | … |
| Model 1 | 1 [Reference] | 1.08 (0.98–1.20) | 1.36 (1.26–1.47) | 2.09 (1.93–2.26) | 1 [Reference] | 1.86 (1.37–2.52) | 2.07 (1.60–2.69) | 2.87 (2.15–3.84) | 0.0016 |
| Model 2 | 1 [Reference] | 1.04 (0.94–1.15) | 1.14 (1.05–1.23) | 1.48 (1.37–1.61) | 1 [Reference] | 1.55 (1.14–2.11) | 1.66 (1.28–2.17) | 2.02 (1.49–2.72) | 0.0172 |
| Model 3 | 1 [Reference] | 1.02 (0.92–1.13) | 1.11 (1.02–1.20) | 1.41 (1.30–1.54) | 1 [Reference] | 1.53 (1.12–2.09) | 1.64 (1.25–2.14) | 1.96 (1.45–2.66) | 0.0150 |
| Alcohol consumption: not every day | |||||||||
| No. of individuals | 658 752 | 250 623 | 324 365 | 157 280 | 869 804 | 146 096 | 167 125 | 84 073 | … |
| No. of events | 2161 | 939 | 1766 | 1371 | 1199 | 314 | 440 | 348 | … |
| Incidence per 10 000 person‐years | 9.9 (9.5–10.3) | 11.6 (10.9–12.3) | 15.5 (14.8–16.3) | 26.1 (24.8–27.5) | 4.5 (4.3–4.8) | 6.9 (6.2–7.7) | 8.5 (7.7–9.3) | 14.4 (13.0–16.0) | … |
| Model 1 | 1 [Reference] | 1.17 (1.08–1.26) | 1.55 (1.46–1.65) | 2.62 (2.45–2.81) | 1 [Reference] | 1.53 (1.35–1.73) | 1.87 (1.68–2.09) | 3.20 (2.84–3.61) | 0.0002 |
| Model 2 | 1 [Reference] | 1.17 (1.08–1.26) | 1.25 (1.17–1.33) | 1.76 (1.65–1.89) | 1 [Reference] | 1.18 (1.04–1.33) | 1.33 (1.19–1.49) | 1.89 (1.67–2.14) | 0.6910 |
| Model 3 | 1 [Reference] | 1.14 (1.06–1.23) | 1.20 (1.13–1.28) | 1.65 (1.54–1.77) | 1 [Reference] | 1.17 (1.03–1.33) | 1.33 (1.18–1.48) | 1.88 (1.65–2.14) | 0.2729 |
The incidence is presented in 10 000 person‐years. Model 1=unadjusted model. Model 2=Adjusted for age. Model 3=adjusted for age, body mass index, diabetes, dyslipidemia, cigarette smoking, and alcohol consumption. In a subgroup analysis stratified by alcohol consumption, alcohol consumption was excluded from covariate.
Furthermore, compared with the normal/elevated BP, isolated diastolic hypertension, isolated systolic hypertension, and systolic diastolic hypertension were associated with an increased risk of developing AF in both men and women, and this relationship was more pronounced in women than in men (Table S1). The significant interaction assessing the association of this alternative BP category with incident AF between men and women was only observed in people aged <50 years (Table S2).
Restricted Cubic Spline
Restricted cubic spline showed that the risk of AF associated with elevated systolic BP increased steeply above an approximate threshold of systolic BP of 130 mm Hg in men and 100 mm Hg in women. In men, the risk of AF as a function of DBP was flat (ie, did not increase with respect to DBP) when DBP <80 mm Hg but increased steadily above a threshold of DBP >80 mm Hg. In women, the risk of AF increased steadily throughout the range of DBP, without threshold (Figure 2).
Figure 2. Restricted cubic spline.
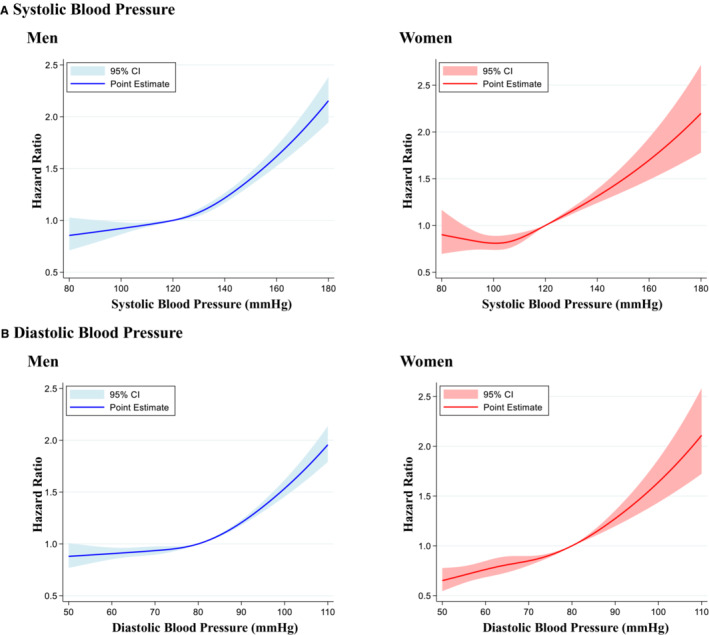
Restricted cubic spline of systolic blood pressure (A) and diastolic blood pressure (B) in the incidence of atrial fibrillation. We used 4 knots for change in systolic blood pressure (5, 35, 65, and 95 percentiles), with the reference point set at systolic blood pressure of 120 mm Hg and diastolic blood pressure of 80 mm Hg. The hazard ratio of systolic or diastolic blood pressure as a continuous variable was adjusted for age, obesity, diabetes, dyslipidemia, cigarette smoking, and alcohol consumption.
Sensitivity Analyses
First, we added 301 915 individuals taking BP‐lowering medications to the population of the main analysis (n=3 383 738) and analyzed 3 685 653 individuals in this sensitivity analysis. In this population, the main results were almost unchanged (Table S3). Second, after multiple imputations, 15 803 AF events were recorded in 3 981 393 individuals. As also seen in this analysis, the association between BP category and incident AF was greater in women than in men (Table S4). Third, our primary results were consistent with those of a competing risks model (Table S5). Fourth, after adding use of sex hormones or modulators of genital system medications to model 3, our primary findings were unchanged (Table S6).
DISCUSSION
The current analyses using a large‐scale health checkup and administrative claims database including >3 000 000 individuals demonstrated that hypertension was associated with a higher risk of developing AF in both men and women, and the relationship between hypertension and incident AF was more pronounced in women than in men. This relationship is consistent across a variety of sensitivity analyses. This is the first epidemiological data suggesting a potential sex difference in the association between hypertension and the subsequent risk of AF using a large‐scale real‐world data set.
In agreement with preceding studies, 2 , 8 , 9 the incidence of AF was higher in men than in women. Furthermore, the status of cardiovascular risk factors, including BP, was much better in women than in men. Nevertheless, our analyses demonstrated that the relationship between hypertension and incident AF was more pronounced in women than that in men. The restricted cubic spline suggested that the risk of AF began to increase with SBP or DBP earlier in women than in men. Women may be more susceptible to BP than men are in terms of the risk of developing AF. Several possible explanations for this have been suggested. First, the baseline risk of developing AF is lower in women than in men. Therefore, the influence of hypertension could be more pronounced in women. Accordingly, as shown in Table 3, among older individuals, we did not find the P value for the interaction statistically significant. In the older people, the baseline AF risk markedly increases in both men and women, and the menopause‐associated changes also increase the risk of AF in women, 9 which would have attenuated the sex difference in the hypertension‐AF relationship. Second, there are various sex differences in the pathological mechanisms of AF (including structural, electrophysiological, and hormonal factors), 23 which may explain our results. For example, sex differences in atrial anatomy or tissue fibrosis are involved in sex‐specific responses to hypertension in the development of AF. 24 , 25 Third, recent advances in basic research have also identified possible sex differences in the pathology of hypertension. 26 For example, the anti‐inflammatory profile is greater in females as a compensatory mechanism for hypertension, and this enhanced anti‐inflammatory response would be mediated by angiotensin type 2 receptor. 26 Disruption of such regulatory mechanisms may be greater in women than in men, and induce not only hypertension, but also the development of AF. The underlying pathological link between hypertension and AF could explain the sex difference in the relationship between hypertension and incident AF.
Our study has several strengths and clinical implications. Our data set included a large sample size with high retention attributable to the linkage of insurance records, which enabled various sensitivity analyses that strengthened the robustness of our results. Although the incidence of AF was lower in women, our results underscore the importance of BP control in women for the primary AF prevention and should not underestimate the clinical significance of BP in both men and women. Results of subgroup analyses are also important. Subgroup analysis stratified by age showed that the interaction between sex and BP as risk factors for incident AF was most significant in the subgroup of individuals <50 years of age. This might be attributable to a potential complex interaction on the risk of hypertension and AF between age and sex, 27 , 28 and we need further investigations regarding this point. Subgroup analysis stratified by alcohol consumption showed that the sex‐specific relationship between medication‐naïve BP and incident AF was more pronounced in people with daily alcohol consumption. Unfortunately, our data set lacks detailed information on alcohol consumption (eg, amount of alcohol consumption), and thus, it is difficult to deepen this result any further. However, given that alcohol drinking is involved in the pathogenesis of both hypertension and AF, and given the results from our subgroup analysis, further studies are needed to explore sex differences in the cardiovascular effects of alcohol consumption. It is also essential that in both men and women, the risk of AF started to increase from BP values much lower than the SBP/DBP of 140/90 mm Hg, which is the classical cut‐off value for diagnosing hypertension. Indeed, SPRINT (Systolic Blood Pressure Intervention Trial) demonstrated that intensive BP‐lowering treatment in patients with hypertension reduced the risk of AF. 29 Furthermore, the results of cubic spline suggest that the optimal BP value would be lower in women than in men. Therefore, our results would be helpful in determining target BP values from the point of view of AF prevention. Because of the retrospective nature of this study, we cannot conclude a causal relationship, and further investigations are required to identify adequate BP management for both men and women from the perspective of primary AF prevention.
This study had several limitations that should be addressed. Most limitations of this study are common to other studies using this health checkup and administrative claims database, as we described previously. 6 , 14 , 15 We used BP data measured at health checkups and conducted BP measurement on a single occasion, which may not fully represent the BP phenotype of study participants. Although health care professionals (eg, nurses) are requested to measure BP according to the standardized protocol of the Ministry of Health, Labor, and Welfare, adherence to this protocol might be lenient in a busy clinical setting. Because the JMDC Claims Database primarily includes employed working‐age individuals, a selection bias (healthy worker bias) should be considered, and our results need to be validated by other independent data sets. In addition, when compared with countries with a diversity of ethnicities such as the United States, Japan is a relatively homogeneous country; this fact should be considered when our results are applied to other populations. Although we performed multivariable Cox regression analyses, we could not eliminate potential unmeasured confounders and residual bias (eg, salt intake, socioeconomic status, and psychological factors). Moreover, while the accuracy of recorded diagnoses in an administrative claims data set was reported to be high in Japan, 30 , 31 the recorded diagnoses of administrative data sets should generally be considered as less well‐validated. In this study, we focused on the sex difference in the association between medication‐naïve BP and the risk of developing AF. However, it is interesting that this sex difference was seemingly attenuated if we included people taking BP‐lowering medications as shown in Table S3 and we need further investigations regarding the potential sex difference in the association of BP on BP‐lowering treatment and the subsequent risk of AF. Although we used a large‐scale database, the number of AF events in women was relatively small, and thus, the CIs for the results in women were wide.
CONCLUSIONS
We analyzed a large‐scale health checkup and administrative claims database and found that elevated BP, stage 1 hypertension, and stage 2 hypertension were associated with a higher subsequent risk of AF in both men and women. However, the relationship between the BP category and incident AF was more pronounced in women than in men, suggesting a sex difference in the association between hypertension and incident AF. Therefore, we may need to recognize the significance of hypertension, particularly in women, in the primary prevention of AF.
Sources of Funding
This work was supported by the grants from the Ministry of Health, Labor and Welfare, Japan (21AA2007), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907, 21H03159, and 21K08123). The funding sources were not related to the current study.
Disclosures
Research funding and scholarship funds (Hidehiro Kaneko and Katsuhito Fujiu) were provided by Medtronic Japan Co., LTD, Boston Scientific Japan Co., LTD, Biotronik Japan, Simplex QUANTUM Co., LTD, and Fukuda Denshi, Central Tokyo CO., LTD.
Supporting information
Data S1
Tables S1–S6
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026240
For Sources of Funding and Disclosures, see page 9.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140 [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 4. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063 [DOI] [PubMed] [Google Scholar]
- 5. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, Fujiu K, Michihata N, Jo T, Takeda N, et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with risk of heart failure and atrial fibrillation. Circulation. 2021;143:2244–2253. doi: 10.1161/CIRCULATIONAHA.120.052624 [DOI] [PubMed] [Google Scholar]
- 7. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9 [DOI] [PubMed] [Google Scholar]
- 8. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ko D, Rahman F, Martins MA, Hylek EM, Ellinor PT, Schnabel RB, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: treatment. Nat Rev Cardiol. 2017;14:113–124. doi: 10.1038/nrcardio.2016.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scheuermeyer FX, Mackay M, Christenson J, Grafstein E, Pourvali R, Heslop C, MacPhee J, Ward J, Heilbron B, McGrath L, et al. There are sex differences in the demographics and risk profiles of emergency department (ED) patients with atrial fibrillation and flutter, but no apparent differences in ED management or outcomes. Acad Emerg Med. 2015;22:1067–1075. doi: 10.1111/acem.12750 [DOI] [PubMed] [Google Scholar]
- 11. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- 12. Volgman AS, Manankil MF, Mookherjee D, Trohman RG. Women with atrial fibrillation: greater risk, less attention. Gend Med. 2009;6:419–432. doi: 10.1016/j.genm.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 13. Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241:211–218. doi: 10.1016/j.atherosclerosis.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 14. Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Takeda N, et al. Association of isolated diastolic hypertension based on the cutoff value in the 2017 American College of Cardiology/American Heart Association blood pressure guidelines with subsequent cardiovascular events in the general population. J Am Heart Assoc. 2020;9:e017963. doi: 10.1161/JAHA.120.017963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuoka S, Kaneko H, Yano Y, Itoh H, Fukui A, Morita K, Kiriyama H, Kamon T, Fujiu K, Seki H, et al. Association between blood pressure classification using the 2017 ACC/AHA blood pressure guideline and retinal atherosclerosis. Am J Hypertens. 2021;34:1049–1056. doi: 10.1093/ajh/hpab074 [DOI] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484–e594. doi: 10.1161/CIR.0000000000000596 [DOI] [PubMed] [Google Scholar]
- 17. Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, Morita K, Michihata N, Jo T, Morita H, et al. Association of body weight gain with subsequent cardiovascular event in non‐obese general population without overt cardiovascular disease. Atherosclerosis. 2020;308:39–44. doi: 10.1016/j.atherosclerosis.2020.05.015 [DOI] [PubMed] [Google Scholar]
- 18. Yagi M, Yasunaga H, Matsui H, Morita K, Fushimi K, Fujimoto M, Koyama T, Fujitani J. Impact of rehabilitation on outcomes in patients with ischemic stroke: a nationwide retrospective cohort study in Japan. Stroke. 2017;48:740–746. doi: 10.1161/STROKEAHA.116.015147 [DOI] [PubMed] [Google Scholar]
- 19. Aloisio KM, Swanson SA, Micali N, Field A, Horton NJ. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J. 2014;14:863–883. doi: 10.1177/1536867X1401400410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubin DB, Schenker N. Multiple imputation in health‐care databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410 [DOI] [PubMed] [Google Scholar]
- 21. Morita K, Ono S, Ishimaru M, Matsui H, Naruse T, Yasunaga H. Factors affecting discharge to home of geriatric intermediate care facility residents in Japan. J Am Geriatr Soc. 2018;66:728–734. doi: 10.1111/jgs.15295 [DOI] [PubMed] [Google Scholar]
- 22. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Odening KE, Deiss S, Dilling‐Boer D, Didenko M, Eriksson U, Nedios S, Ng FS, Roca Luque I, Sanchez Borque P, Vernooy K, et al. Mechanisms of sex differences in atrial fibrillation: role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace. 2019;21:366–376. doi: 10.1093/europace/euy215 [DOI] [PubMed] [Google Scholar]
- 24. Forleo GB, Tondo C, De Luca L, Dello Russo A, Casella M, De Sanctis V, Clementi F, Fagundes RL, Leo R, Romeo F, et al. Gender‐related differences in catheter ablation of atrial fibrillation. Europace. 2007;9:613–620. doi: 10.1093/europace/eum144 [DOI] [PubMed] [Google Scholar]
- 25. Cochet H, Mouries A, Nivet H, Sacher F, Derval N, Denis A, Merle M, Relan J, Hocini M, Haissaguerre M, et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed‐enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol. 2015;26:484–492. doi: 10.1111/jce.12651 [DOI] [PubMed] [Google Scholar]
- 26. Gillis EE, Sullivan JC. Sex differences in hypertension: recent advances. Hypertension. 2016;68:1322–1327. doi: 10.1161/HYPERTENSIONAHA.116.06602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825 [DOI] [PubMed] [Google Scholar]
- 28. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017–2018. NCHS Data Brief. 2020;364:1–8. [PubMed] [Google Scholar]
- 29. Soliman EZ, Rahman AF, Zhang ZM, Rodriguez CJ, Chang TI, Bates JT, Ghazi L, Blackshear JL, Chonchol M, Fine LJ, et al. Effect of intensive blood pressure lowering on the risk of atrial fibrillation. Hypertension. 2020;75:1491–1496. doi: 10.1161/HYPERTENSIONAHA.120.14766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27:476–482. doi: 10.1016/j.je.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujihara K, Yamada‐Harada M, Matsubayashi Y, Kitazawa M, Yamamoto M, Yaguchi Y, Seida H, Kodama S, Akazawa K, Sone H. Accuracy of Japanese claims data in identifying diabetes‐related complications. Pharmacoepidemiol Drug Saf. 2021;30:594–601. doi: 10.1002/pds.5213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S6


