Abstract
Background
Sleep irregularity has been linked to incident cardiovascular disease. Less is known about associations of sleep regularity with atherosclerosis. We examined cross‐sectional associations of actigraphy‐assessed sleep duration and sleep timing regularity with subclinical atherosclerosis in the community‐based MESA (Multi‐Ethnic Study of Atherosclerosis).
Methods and Results
MESA Sleep Ancillary Study participants (N=2032; mean age, 68.6±9.2 years; 37.9% White) completed 7‐day wrist actigraphy. Participants underwent assessments of coronary artery calcium, carotid plaque presence, carotid intima‐media thickness, and the ankle‐brachial index. Sleep regularity was quantified by the 7‐day with‐in person SD of sleep duration and sleep onset timing. Relative risk regression models were used to calculate prevalence ratios and 95% CIs. Models are adjusted for demographics, cardiovascular disease risk factors, and other objectively assessed sleep characteristics including obstructive sleep apnea, sleep duration, and sleep fragmentation. After adjustment, compared with participants with more regular sleep durations (SD ≤60 minutes), participants with greater sleep duration irregularity (SD >120 minutes) were more likely to have high coronary artery calcium burden (>300; prevalence ratio, 1.33 [95% CI, 1.03–1.71]) and abnormal ankle‐brachial index (<0.9; prevalence ratio, 1.75 [95% CI, 1.03–2.95]). Compared with participants with more regular sleep timing (SD ≤30 minutes), participants with irregular sleep timing (SD >90 minutes) were more likely to have high coronary artery calcium burden (prevalence ratio, 1.39 [95% CI, 1.07–1.82]). Associations persisted after adjustment for cardiovascular disease risk factors and average sleep duration, obstructive sleep apnea, and sleep fragmentation.
Conclusions
Sleep irregularity, particularly sleep duration irregularity, was associated with several measures of subclinical atherosclerosis. Sleep regularity may be a modifiable target for reducing atherosclerosis risk. Future investigation into cardiovascular risk reduction interventions targeting sleep irregularity may be warranted.
Keywords: cardiovascular disease, circadian rhythms, lifestyle, risk factors
Subject Categories: Cardiovascular Disease, Epidemiology, Lifestyle, Risk Factors
Nonstandard Abbreviations and Acronyms
- cIMT
carotid intima‐media thickness
- MESA
Multi‐Ethnic Study of Atherosclerosis
- OSA
obstructive sleep apnea
- PR
prevalence ratio
Clinical Perspective.
What Is New?
In this large, racially and ethnically diverse, community‐based cohort, sleep duration irregularity (variation in sleep duration >120 minutes in a week) was associated with measures of subclinical atherosclerosis.
Our findings suggest that irregular sleep patterns may play a role in the pathophysiologic development of cardiovascular disease.
What Are the Clinical Implications?
Encouraging regular sleep schedules may be an important part of clinical lifestyle recommendations for the prevention of cardiovascular disease.
Poor sleep, including poor quality, abnormal quantity, and fragmented sleep, is associated with cardiovascular risk factors, incident cardiovascular disease (CVD), and CVD‐related mortality. 1 , 2 , 3 , 4 , 5 , 6 Questions remain about which dimensions of sleep may drive the pathophysiologic development of CVD 7 and be promising intervention targets for improving cardiovascular health. 1 To explore mechanisms through which poor sleep may lead to CVD development, researchers have examined associations of various dimensions of sleep with noninvasive measures of atherosclerosis, which have shown to be strongly associated with incident CVD. 8 , 9 , 10 , 11 , 12 There is a consistent line of cross‐sectional 13 , 14 , 15 and prospective 14 , 16 evidence to suggest adults with abnormal sleep characteristics are more likely to have significant subclinical atherosclerosis.
An emerging area of interest is whether sleep irregularity, estimated by variation in sleep durations and sleep timing across nights, 1 , 17 is associated with CVD risk. Irregular sleep patterns and night‐to‐night variations in sleep timing may be indicators of circadian misalignment, or desynchronization of sleep–wake timing, which has been linked to cardiometabolic risk factors. 18 , 19 , 20 Initial studies on regularity of sleep patterns and CVD risk were focused on nurses and shift workers with pronounced variations in sleep schedules. 21 , 22 , 23 However, recent evidence has connected irregular sleep patterns to cardiovascular health in the general population. 20 , 24 , 25 , 26 , 27
It remains unknown whether irregularity in sleep durations and timing plays a role in the development of atherosclerosis. 27 The MESA (Multi‐Ethnic Study of Atherosclerosis) includes objectively measured sleep data and numerous noninvasive measures of subclinical atherosclerosis, assessed in a diverse sample of older adults. In this study, we tested the hypotheses that more irregular sleep durations and sleep timing were associated with subclinical atherosclerosis (including prevalent coronary artery calcium [CAC], prevalent carotid plaque, abnormal carotid intima‐media thickness [cIMT], and abnormal ankle‐brachial index [ABI]), after adjustment for cardiovascular risk factors and objectively measured obstructive sleep apnea (OSA), sleep duration, and sleep fragmentation. We were also interested in understanding whether associations were consistent across several subclinical markers.
METHODS
Requests to access data sets from qualified researchers trained in human subject confidentiality protocols should be made through the MESA internal site at https://mesa‐nhlbi.org.
Study Design
The MESA is a multisite longitudinal cohort study designed to investigate the prevalence and progression of subclinical CVD and to identify risk factors for incident CVD in a racially and ethnically diverse community‐based sample. 28 Between 2000 and 2002, 6814 men and women aged 45 to 84 years, who identified as White, Black/African American, Hispanic American, or Chinese American and were free of clinical CVD, were recruited from 6 US communities (St. Paul, MN; Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; and Northern Manhattan and the Bronx, NY).
All 4077 MESA participants who took part in exam 5 (2010–2012) were invited to participate in the MESA Sleep Ancillary Study (2010–2013). Interested participants who did not report regular use of oral devices, nocturnal oxygen, or nightly positive airway pressure devices and who did not live too far away from the MESA clinics were eligible. Of the interested and eligible participants, 2261 took part in the MESA Sleep Ancillary study (59.7%). The study protocol and procedures were approved by the institutional review board of each study field center, and all participants provided written informed consent to participate in the study.
Of the 2261 participants, 2147 had actigraphy wear and available data on at least 1 subclinical marker. We further excluded participants who had <5 days of actigraphy wear (n=18) or had extreme values for sleep duration regularity or sleep onset regularity (n=73), resulting in an available analytic sample of 2032 participants. The final analytic sample differed for each outcome on the basis of participation in subclinical measure assessment (Figure 1).
Figure 1. Flow chart of MESA Sleep participants in analytic sample.
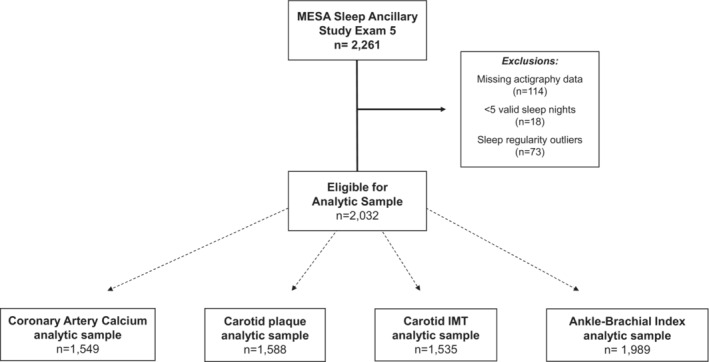
Of 2261 eligible participants, 2147 had valid actigraphy and subclinical marker data. Participants were excluded who had <5 days of actigraphy wear (n=18) and extreme sleep duration regularity or sleep onset regularity (n=73). Final sample sizes varied by subclinical marker.
Objective Sleep Assessment
Participants wore the Actiwatch Spectrum wrist actigraph (Philips Respironics, Murrysville, PA) on the nondominant wrist for 7 consecutive days, while concurrently completing a sleep diary over the same period. The 30‐second epoch actigraph data were scored using the Actiware‐Sleep version 5.59 analysis software (MiniMitter Co, Inc, Bend, OR), after technicians at the Brigham and Women's Hospital Sleep Reading Center annotated the rest periods using sleep diaries and information from light, markers, and movement. 26 A validated sleep/wake algorithm was used in which each epoch was classified as sleep or wake on the basis of all activity counts in the surrounding 2‐minute period of time (eg, ±2 min). 29 The median time interval between MESA exam 5 assessment and objective sleep assessment was 1 year.
Sleep duration was estimated as the sum of the minutes classified as sleep during the main sleep period by the algorithm minus all minutes classified as wake after sleep onset. Sleep onset time was defined as the time of the first epoch classified as sleep during the main sleep period. Consistent with previous MESA analyses, 30 we estimated the regularity in sleep duration and sleep onset timing as the within‐person SD of these variables across the 7‐day wear period. Also consistent with previous MESA analyses, 30 participants were categorized by 30‐minute increments on the basis of the data distributions in the sample (≤60, 61–90, 91–120, >120 minutes for sleep duration regularity and ≤30, 31–60, 61–90, and >90 minutes for sleep timing regularity). The sleep fragmentation index is a measure of sleep continuity and estimates disruption in sleep throughout the sleep period. The fragmentation index is calculated as the sum of (1) the percentage of the sleep period with movement (>2 activity counts) and (2) the percentage of the sleep period with immobile phases that are ≤1 minute. 31 , 32
According to a previously developed protocol, participants also completed a 1‐night in‐home polysomnography study with the 15‐channel Compumedics Somte system (Compumedics, Abbottsvile, Australia), which measures sleep‐disordered breathing, sleep stages, wake after sleep onset, and heart rate. 33 The polysomnography recording provided quantitative assessments of levels of overnight hypoxemia, apneas and hypopneas, and sleep stage distributions. For this analysis, OSA severity was quantified using the Apnea‐Hypopnea Index, composed of the sum of all obstructive apneas plus hypopneas associated with ≥4% oxygen desaturation. Participants with an Apnea‐Hypopnea Index ≥30 events/hour were categorized as having severe OSA. 34
Subclinical Markers of CVD
Study outcomes include noninvasive measures of subclinical CVD collected at exam 5 including prevalent CAC, carotid plaque presence, abnormal cIMT, and abnormal ABI.
MESA scanning centers at all 6 sites measured CAC by cardiac computed tomography, using a standardized protocol. 35 A cardiologist or radiologist at a central reading center (Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center), blinded to participant data, read all cardiac computed tomography scans using an interactive scoring system. The phantom adjusted Agatston score, a continuous score derived from the densities and areas of plaque in the coronary arteries, 36 was quantified for each participant. This standardized measure of CAC has shown to have high intra‐ and interreader reproducibility in MESA (kappa >0.90). 35 For this study, Agatston scores were categorized as >0 for presence of CAC (reference 0). Agatston scores >300 (reference ≤300) represented high CAC burden.
To measure cIMT and carotid plaque, trained technicians in each field center performed B‐mode ultrasonography of the right and left near and far walls of the internal carotid and common carotid arteries. Technicians recorded artery images with the Logiq 700 ultrasound device (General Electric Medical Systems, Waukesha, WI). The recorded images were reviewed at the University of Wisconsin Atherosclerosis Imaging Research Program MESA Carotid Ultrasound Reading Center. For this analysis, cIMT was calculated as the mean of the left mean and the right mean distal common carotid artery wall thickness. 15 Abnormal cIMT was defined as >0.9 mm. Carotid plaques in the images of the right or left walls of the internal carotid artery, common carotid arteries, or bulb were identified and scored. 37 Carotid plaque identified in any location indicated carotid plaque presence (1 versus 0).
The ABI, a measure of systemic atherosclerosis and stiffness in the blood vessels, is a ratio of the systolic blood pressure at the ankle to the systolic blood pressure in the arm. To derive ABI estimates, MESA technicians obtained systolic blood pressure measurements from the bilateral brachial, dorsalis pedis, and posterior tibial arteries using a hand‐held Doppler instrument with a 5‐mHz probe with the participant in the supine position. 11 For each leg, the ABI numerator was the highest pressure recorded from that leg (dorsalis pedis or posterior tibial), while the denominator was the higher of the 2 arm pressures. Abnormal ABI was defined when at least 1 leg had a value <0.90, whereas “normal” was defined when both legs had values 0.90≤ABI≤1.40. Previous evidence suggests that ABI >1.40 may be associated with greater CVD risk. 38 Thus, those with ABI >1.40 were excluded from ABI analyses.
Covariates
Relevant covariates were obtained from the MESA sleep questionnaire, the MESA exam 5 questionnaire, and exam 5 physiologic assessments. Covariates in this analysis included age, sex, race and ethnicity, study site, education, yearly income, work schedule, smoking status, alcohol consumption, physical activity, body mass index (BMI), systolic blood pressure, diastolic blood pressure, antihypertensive medication use, statin medication use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and prevalent diabetes.
Interview‐administered questionnaires were used to assess education, yearly income, smoking status, alcohol consumption, and physical activity. Education was categorized as high school graduate or less, some college, or college graduate plus. Participants average yearly income was categorized as <$20 000; $20 000 to <$50 000; and ≥$50 000. Participants were asked if they currently smoke cigarettes or drink alcohol and categorized as current, former, or never. Participants were asked the number of days (and hours and minutes per day) they participated in various physical activities (including household chores, walking, sports activities, etc) in a typical week in the past month. Total metabolic equivalent minutes of moderate to vigorous physical activity were calculated to represent weekly average physical activity. At the MESA sleep exam, participants also completed a sleep questionnaire to report sleep habits and usual work schedules. Usual work schedule was grouped as “do not work,” “day shift,” and “other shift” that included responses of “afternoon shift,” “night shift,” “split shift,” “irregular shift/on‐call,” and “rotating shifts.”
Trained staff collected height, weight, blood pressure, and fasting blood measures. BMI was calculated as weight (kilogram) divided by height (meter) squared. Blood pressure was measured 3 times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL), and the final 2 measurements were averaged. Participants taking antihypertensive medications were coded as 1 for antihypertensive medication use (0: no antihypertensive medications). Participants taking statin medications were coded as 1 for statin medication use (0: no statin medication use). Serum assays were processed (Fairview University Medical Center, Minneapolis, MN), and high‐density lipoprotein cholesterol was assessed in EDTA plasma using the cholesterol oxidase method (Roche Diagnostics, Risch‐Rotkreuz, Switzerland). Low‐density lipoprotein cholesterol was calculated in plasma specimens using the Friedewald formula. Prevalent diabetes was defined as fasting blood glucose concentration ≥126 mg/dL, nonfasting glucose concentration ≥200 mg/dL, self‐reported physician diagnosis, or pharmacological treatment for diabetes.
Statistical Analysis
Means and proportions of participant characteristics were calculated and compared according to categories of sleep duration regularity and sleep timing regularity.
A series of progressively adjusted relative risk regression models were run to examine associations between categories of sleep duration regularity and sleep timing regularity with each subclinical marker of CVD. Prevalence ratios (PRs) and 95% CIs according to sleep regularity categories were calculated using modified Poisson regression with robust error variances. 39 Because the outcomes investigated in this study were common, we considered this relative risk regression approach more appropriate than calculating prevalence odds ratios. 40
Progressive model adjustments included adjustment for basic demographics in model 1, including age, sex, race and ethnicity, and site. Model 2 included additional adjustments for lifestyle characteristics that are potential confounders of the examined association, including education, yearly income, work schedule, smoking status, alcohol consumption, physical activity, and BMI. To further evaluate whether the associations persisted after adjustment for known CVD risk factors, model 3 included additional adjustments for systolic blood pressure, diastolic blood pressure, antihypertensive medication use, statin medication use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and prevalent diabetes.
We performed a series of sensitivity analyses. Because sleep duration, 3 OSA, 41 and sleep fragmentation 13 are also associated with CVD risk, we assessed if the association between sleep irregularity and atherosclerosis remained after adjusting for these other relevant dimensions of sleep health. We reran all models separately, excluding participants who had prevalent CVD (n=107), those with severe OSA (n=386), and those who identified as other shift workers (n=246). Because of the potential influence of seasonality on our exposure, we explored the additional adjustment for season of actigraphy data collection. We also examined sleep regularity defined by variation in only weekday sleep duration. Finally, we explored potential effect modification by adding an age*sleep regularity and sex*sleep regularity cross‐product term to the models.
RESULTS
Table 1 presents the participant characteristics of the 2032 participants in the eligible sample overall and by categories of sleep duration regularity. The mean age of participants was 68.6 (±9.2) years. Just over half of the participants were women (53.6%), and 37.9% identified as White, 11.1% as Chinese American, 27.6% as Black or African American, and 23.4% as Hispanic American. Approximately 40% of participants had a college degree, and 53% had an average yearly income ≥$50 000. Over 56% of the sample reported not currently working, while 12.1% (n=246) reported working a shift other than a day shift, of whom 12% (n=30) reported night shift work. For OSA severity, 15% of the sample had normal breathing during sleep, 31% had mild OSA, 25% moderate OSA, and 19% severe OSA.
Table 1.
MESA Sleep Participant Characteristics by Categories of Sleep Duration Regularity (N=2032)
| Overall | Sleep duration regularity (nightly within‐person SD) | P value | ||||
|---|---|---|---|---|---|---|
| ≤60 min | 61–90 min | 91–120 min | >120 min | |||
| n=696 | n=558 | n=420 | n=358 | |||
| Demographics | ||||||
| Age, y | 68.6±9.2 | 68.8±8.9 | 68.1±9.1 | 69.0±9.6 | 68.5±9.4 | 0.44 |
| Female sex, n (%) | 1090 (53.6) | 357 (51.3) | 320 (57.3) | 228 (54.3) | 185 (51.7) | 0.19 |
| Race and ethnicity, n (%) | <0.001 | |||||
| White | 770 (37.9) | 346 (49.7) | 197 (35.3) | 129 (30.7) | 98 (27.4) | |
| Chinese American | 226 (11.1) | 78 (11.2) | 53 (9.5) | 49 (11.7) | 46 (12.8) | |
| Black, African American | 561 (27.6) | 132 (19.0) | 153 (27.4) | 152 (36.2) | 124 (34.6) | |
| Hispanic American | 475 (23.4) | 140 (20.1) | 155 (27.8) | 90 (21.4) | 90 (25.1) | |
| Education, n (%) | 0.04 | |||||
| ≤HS graduate | 613 (30.2) | 188 (27.0) | 198 (35.5) | 122 (29.0) | 105 (29.3) | |
| Some college | 615 (30.3) | 204 (29.3) | 163 (29.2) | 137 (32.6) | 111 (31.0) | |
| College graduate plus | 800 (39.4) | 302 (43.4) | 197 (35.3) | 160 (38.1) | 141 (39.4) | |
| Average yearly income | <0.01 | |||||
| <$20 000 | 383 (18.8) | 101 (14.5) | 115 (20.6) | 85 (20.2) | 82 (22.9) | |
| $20 000 to <$50 000 | 502 (24.7) | 167 (24.0) | 139 (24.9) | 99 (23.6) | 97 (27.1) | |
| ≥$50 000 | 1086 (53.4) | 408 (58.6) | 290 (52.0) | 224 (53.3) | 164 (45.8) | |
| Work schedule, n (%) | 0.01 | |||||
| Do not work | 1148 (56.5) | 406 (58.3) | 295 (52.9) | 235 (56.0) | 212 (59.2) | |
| Day shift | 617 (30.4) | 213 (30.6) | 189 (33.9) | 134 (31.9) | 81 (22.6) | |
| Other shift | 246 (12.1) | 74 (10.6) | 68 (12.2) | 47 (11.2) | 57 (15.9) | |
| Lifestyle characteristics | ||||||
| Current smoker, n (%) | 142 (7.0) | 35 (5.0) | 35 (6.3) | 36 (8.6) | 36 (10.1) | 0.02 |
| Current alcohol consumer, n (%) | 881 (43.4) | 335(48.1) | 235 (42.1) | 177 (42.1) | 134 (37.4) | 0.02 |
| MVPA, MET min/wk | 5380±6220 | 5300±4850 | 5440±6120 | 5680±8320 | 5080±5890 | 0.39 |
| BMI, kg/m2 | 28.8±5.6 | 28.0±5.1 | 29.2±5.7 | 29.0±5.8 | 29.4±5.8 | <0.01 |
| Cardiovascular risk factors | ||||||
| Systolic blood pressure, mm Hg | 123.0±20.0 | 121.0±19.1 | 124.0±20.7 | 124.0±19.1 | 125.0±21.6 | 0.02 |
| Diastolic blood pressure, mm Hg | 68.3±9.8 | 67.3±9.5 | 68.6±9.9 | 68.7±9.2 | 69.4±10.8 | 0.01 |
| Antihypertensive medication use, n (%) | 1090 (53.6) | 358 (51.4) | 293 (52.5) | 230 (54.8) | 209 (58.4) | 0.18 |
| Statin medication use, n (%) | 775 (38.1) | 278 (39.9) | 200 (35.8) | 158 (37.6) | 139 (38.8) | 0.51 |
| LDL‐C, mg/dL | 106.0±32.4 | 105.0±31.3 | 108.0±31.4 | 107.0±35.3 | 105.0±32.7 | 0.39 |
| HDL‐C, mg/dL | 55.6±16.5 | 55.5±15.4 | 56.9±17.3 | 55.4±16.8 | 54.0±16.8 | 0.10 |
| Prevalent diabetes, n (%) | 397 (19.5) | 102 (14.7) | 100 (17.9) | 97 (23.1) | 98 (27.4) | <0.001 |
| Severe obstructive sleep apnea, n (%) | 386 (19.0) | 112 (16.1) | 102 (18.3) | 83 (19.8) | 89 (24.9) | 0.17 |
| Average sleep fragmentation | 20.2±7.0 | 18.8±6.6 | 20.1±7.0 | 20.9±6.6 | 22.0±7.8 | <0.001 |
Data presented as mean±SD unless specified. P values adjusted for multiple comparisons using false discovery rate method. BMI indicates body mass index; HDL‐C, high‐density lipoprotein cholesterol; HS, high school; LDL‐C, low‐density lipoprotein cholesterol; MESA, Multi‐Ethnic Study of Atherosclerosis; MET, metabolic equivalent; and MVPA, moderate to vigorous intensity physical activity.
Across the 7‐day data collection period, ≈38% of participants had sleep duration SD >90 minutes, and 18% of those participants had sleep duration SD >120 minutes. Participants with more irregular sleep durations (SD >120 minutes) were more likely to be non‐White, have lower average yearly incomes, were more likely to not work or work other shift schedules (including afternoon, night, split, irregular, or rotating shifts), and were more likely to be current smokers with higher average BMI. Participant characteristics are also presented by categories of sleep timing regularity (Table S1).
Sleep Duration Regularity
Compared with participants with regular sleep durations (SD <60 minutes), those with more irregular sleep durations had higher CAC scores and greater cIMT values, though differences were not statistically significant when these outcomes were explored as continuous measures (Table 2).
Table 2.
Average Subclinical Atherosclerosis Measures by Sleep Regularity Categories: the MESA Sleep Study (2010–2013)
| Sleep duration regularity (nightly within‐person SD) | |||||
|---|---|---|---|---|---|
| ≤60 min n=696 | 61–90 min n=558 | 91–120 min n=420 | >120 min n=358 | P value | |
| Subclinical measures, median (IQR) | |||||
| CAC, Agatston Score | 22.79 (213.95) | 30.86 (209.37) | 35.16 (290.60) | 69.16 (315.16) | 0.16 |
| Carotid IMT, mm | 0.81 (0.24) | 0.81 (0.22) | 0.85 (0.24) | 0.83 (0.22) | 0.13 |
| Ankle‐brachial index | 1.13 (0.15) | 1.13 (0.13) | 1.13 (0.14) | 1.12 (0.15) | 0.32 |
| Sleep timing regularity (nightly within‐person SD) | |||||
|---|---|---|---|---|---|
| ≤30 min n=420 | 31–60 min n=713 | 61–90 min n=443 | >90 min n=456 | P value | |
| Subclinical measures, median (IQR) | |||||
| CAC, Agatston Score | 37.38 (253.12) | 24.30 (208.90) | 35.01 (203.29) | 36.46 (287.76) | 0.44 |
| Carotid IMT, mm | 0.85 (0.27) | 0.81 (0.22) | 0.83 (0.23) | 0.82 (0.21) | 0.15 |
| Ankle‐brachial index | 1.12 (0.15) | 1.13 (0.15) | 1.13 (0.14) | 1.12 (0.15) | 0.09 |
P values calculated with Kruskal–Wallis rank‐sum test. CAC indicates coronary artery calcium; IMT, intima‐media thickness; IQR, interquartile range; and MESA, Multi‐Ethnic Study of Atherosclerosis.
Participants with more irregular sleep durations (SD >120 minutes) were 1.40 (95% CI, 1.09–1.81) times more likely to have high CAC burden (>300) compared with participants with regular sleep durations (SD ≤60 minutes), after adjustment for demographics, lifestyle characteristics, and BMI (Table 3). This association persisted after additional adjustment for prevalent CVD risk factors that may be mediators of the association (PR, 1.33 [95% CI, 1.03–1.71]). Compared with participants with regular sleep durations (SD ≤60 minutes), those with irregular sleep durations (SD >120 minutes) were also more likely to have carotid plaque (PR, 1.12 [95% CI, 1.01–1.23]) and abnormal ABI (PR, 1.91 [95% CI, 1.12–3.26]) after adjustment for demographics, lifestyle characteristics, and BMI. These associations were only slightly attenuated after additional adjustment for CVD risk factors (Figure 2). There was no association found between sleep duration regularity and abnormal cIMT.
Table 3.
Sleep Duration Regularity and Associations With Subclinical Markers of CVD: the MESA Sleep Study (2010–2013)
| Sleep duration regularity (nightly within‐person SD) | ||||
|---|---|---|---|---|
| ≤60 min | 61–90 min | 91–120 min | >120 min | |
| Prevalence ratio (95% CI) | ||||
| Coronary artery calcium (>0) | ||||
| n (%) | 363 (65.5) | 273 (65.5) | 211 (66.1) | 180 (69.5) |
| Model 1 | (Reference) | 1.06 (0.97–1.16) | 1.04 (0.95–1.14) | 1.10 (1.00–1.21)* |
| Model 2 | (Reference) | 1.05 (0.97–1.15) | 1.03 (0.94–1.13) | 1.08 (0.98–1.19) |
| Model 3 | (Reference) | 1.06 (0.98–1.16) | 1.03 (0.94–1.13) | 1.07 (0.98–1.18) |
| Coronary artery calcium (>300) | ||||
| n (%) | 113 (20.4) | 84 (20.1) | 78 (24.4) | 67 (25.9) |
| Model 1 | (Reference) | 1.16 (0.91–1.47) | 1.28 (1.01–1.61)* | 1.42 (1.10–1.84)* |
| Model 2 | (Reference) | 1.19 (0.94–1.51) | 1.28 (1.01–1.62)* | 1.40 (1.09–1.81)* |
| Model 3 | (Reference) | 1.26 (0.99–1.59) | 1.32 (1.05–1.67)* | 1.33 (1.03–1.71)* |
| Carotid plaque | ||||
| n (%) | 356 (64.1) | 289 (67.5) | 230 (69.3) | 192 (70.3) |
| Model 1 | (Reference) | 1.10 (1.01–1.20)* | 1.10 (1.00–1.21)* | 1.14 (1.03–1.25)* |
| Model 2 | (Reference) | 1.09 (1.00–1.20)* | 1.09 (0.99–1.19) | 1.12 (1.01–1.23)* |
| Model 3 | (Reference) | 1.10 (1.01–1.20)* | 1.09 (0.99–1.20) | 1.09 (0.99–1.21) |
| Carotid IMT (>0.9) | ||||
| n (%) | 183 (34.1) | 133 (32.2) | 119 (36.6) | 98 (37.5) |
| Model 1 | (Reference) | 1.04 (0.87–1.23) | 1.03 (0.86–1.22) | 1.09 (0.91–1.31) |
| Model 2 | (Reference) | 1.00 (0.84–1.19) | 0.99 (0.82–1.18) | 1.07 (0.89–1.29) |
| Model 3 | (Reference) | 0.99 (0.83–1.18) | 0.99 (0.83–1.18) | 1.03 (0.85–1.25) |
| Ankle‐brachial index (<0.9) | ||||
| n (%) | 24 (3.5) | 26 (4.8) | 28 (6.8) | 25 (7.3) |
| Model 1 | (Reference) | 1.37 (0.80–2.35) | 1.64 (0.97–2.75) | 1.85 (1.09–3.15)* |
| Model 2 | (Reference) | 1.43 (0.83–2.44) | 1.62 (0.96–2.73) | 1.91 (1.12–3.26)* |
| Model 3 | (Reference) | 1.50 (0.86–2.59) | 1.62 (0.98–2.70) | 1.75 (1.03–2.95)* |
Coronary artery calcium, n=1549; carotid plaque, n=1588; carotid IMT n=1535; ankle‐brachial index, n=1989. Model 1: age, sex, race and ethnicity, site; model 2: M1+ education, income, work schedule, smoking status, alcohol consumption, physical activity, and body mass index; model 3: M2+systolic blood pressure, diastolic blood pressure, antihypertension medication use, statin medication use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, prevalent diabetes. CVD indicates cardiovascular disease; IMT, intima‐media thickness; and MESA, Multi‐Ethnic Study of Atherosclerosis.
P value <0.05.
Figure 2. Sleep duration regularity and prevalent subclinical CVD in the MESA sleep study.
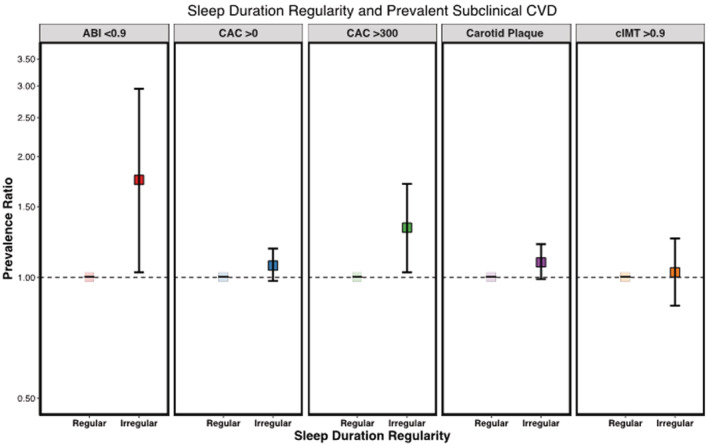
Adjusted for age, sex, race and ethnicity, site, education, yearly income, work schedule, smoking status, alcohol consumption, physical activity, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, statin medication use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and prevalent diabetes; regular sleep duration: ≤60 minutes; irregular sleep duration: >120 minutes. ABI indicates ankle‐brachial index; CAC, coronary artery calcium; cIMT, carotid intima‐media thickness; CVD, cardiovascular disease; and MESA, Multi‐Ethnic Study of Atherosclerosis.
Sleep Timing Regularity
Compared with participants with regular sleep timing (SD ≤30 minutes), participants with more irregular sleep timing (SD >90 minutes) were 1.43 (95% CI, 1.10–1.86) times more likely to have high CAC burden (>300) after adjustment for demographics, lifestyle characteristics, and BMI (Table 4). This association remained in the model adjusting for potential mediators (PR, 1.39 [95% CI, 1.07–1.82]) (Figure 3). There was little evidence that sleep timing regularity was associated with other subclinical markers of CVD.
Table 4.
Sleep Timing Regularity and Associations With Subclinical Markers of CVD: the MESA Sleep Study (2010–2013)
| Sleep timing regularity (nightly within‐person SD) | ||||
|---|---|---|---|---|
| ≤30 min | 31–60 min | 61–90 min | >90 min | |
| Prevalence ratio (95% CI) | ||||
| Coronary artery calcium (>0) | ||||
| n (%) | 234 (69.0) | 348 (64.1) | 223 (66.0) | 222 (67.5) |
| Model 1 | (Reference) | 1.02 (0.94–1.12) | 1.04 (0.94–1.15) | 1.08 (0.98–1.19) |
| Model 2 | (Reference) | 1.03 (0.94–1.12) | 1.04 (0.94–1.15) | 1.06 (0.96–1.17) |
| Model 3 | (Reference) | 1.04 (0.95–1.14) | 1.04 (0.94–1.14) | 1.05 (0.95–1.16) |
| Coronary artery calcium (>300) | ||||
| n (%) | 76 (22.4) | 114 (21.0) | 72 (21.3) | 80 (24.3) |
| Model 1 | (Reference) | 1.19 (0.93–1.52) | 1.15 (0.88–1.50) | 1.39 (1.07–1.82)* |
| Model 2 | (Reference) | 1.24 (0.97–1.57) | 1.19 (0.91–1.57) | 1.43 (1.10–1.86)* |
| Model 3 | (Reference) | 1.29 (1.01–1.65)* | 1.23 (0.93–1.62) | 1.39 (1.07–1.82)* |
| Carotid plaque | ||||
| n (%) | 240 (70.8) | 361 (64.6) | 230 (66.3) | 236 (68.8) |
| Model 1 | (Reference) | 0.98 (0.90–1.07) | 1.01 (0.91–1.11) | 1.05 (0.96–1.16) |
| Model 2 | (Reference) | 0.97 (0.89–1.06) | 1.00 (0.91–1.11) | 1.04 (0.94–1.14) |
| Model 3 | (Reference) | 0.98 (0.90–1.07) | 1.00 (0.90–1.10) | 1.02 (0.92–1.13) |
| Carotid IMT (>0.9) | ||||
| n (%) | 129 (39.6) | 176 (32.3) | 120 (35.7) | 108 (32.8) |
| Model 1 | (Reference) | 0.92 (0.77–1.09) | 0.96 (0.80–1.16) | 0.89 (0.73–1.08) |
| Model 2 | (Reference) | 0.90 (0.76–1.07) | 0.92 (0.76–1.11) | 0.87 (0.71–1.06) |
| Model 3 | (Reference) | 0.93 (0.78–1.10) | 0.94 (0.78–1.13) | 0.85 (0.69–1.05) |
| Ankle‐brachial index (<0.9) | ||||
| n (%) | 18 (4.4) | 31 (4.4) | 21 (4.9) | 33 (7.4) |
| Model 1 | (Reference) | 1.13 (0.64–1.99) | 1.23 (0.67–2.26) | 1.68 (0.97–2.93) |
| Model 2 | (Reference) | 1.08 (0.61–1.90) | 1.25 (0.69–2.27) | 1.72 (0.98–3.01) |
| Model 3 | (Reference) | 1.12 (0.64–1.98) | 1.18 (0.64–2.17) | 1.58 (0.92–2.74) |
Coronary artery calcium, n=1549; carotid plaque n=1588; carotid IMT, n=1535; ankle‐brachial index, n=1989. Model 1: age, sex, race and ethnicity, site. Model 2: M1+ education, income, work schedule, smoking status, alcohol consumption, physical activity, and body mass index; model 3: M2+systolic blood pressure, diastolic blood pressure, antihypertension medication use, statin medication use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, prevalent diabetes. CVD indicates cardiovascular disease; IMT, intima‐media thickness; and MESA, Multi‐Ethnic Study of Atherosclerosis.
P value <0.05.
Figure 3. Sleep timing regularity and prevalent subclinical CVD in the MESA Sleep Study.
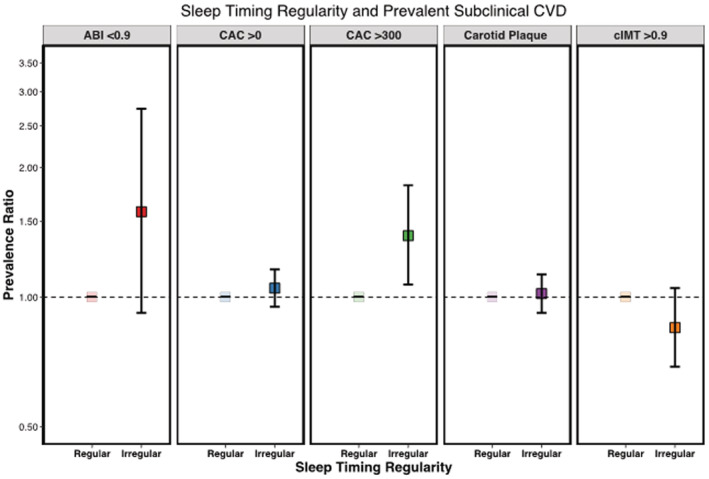
Adjusted for age, sex, race and ethnicity, site, education, yearly income, work schedule, smoking status, alcohol consumption, physical activity, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, statin medication use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and prevalent diabetes; regular sleep timing: ≤30 minutes; irregular sleep timing: >90 minutes. ABI indicates ankle‐brachial index; CAC, coronary artery calcium; cIMT, carotid intima‐media thickness; CVD, cardiovascular disease; and MESA, Multi‐Ethnic Study of Atherosclerosis.
In sensitivity analyses, the observed association of sleep duration regularity with high CAC burden and abnormal ABI persisted after additional adjustment for severe OSA, average nightly sleep duration, and average sleep fragmentation index (Table 5). Of note, when sleep duration was added to the model, all participants with more irregular sleep durations (SD >60 minutes) were more likely to have high CAC burden (>300) compared with participants with regular sleep durations (SD ≤60 minutes). When participants with prevalent CVD were excluded, results were slightly attenuated but remained mostly unchanged (Table S2). When participants with severe OSA were excluded, results remained unchanged (Table S3). All results remained unchanged when participants who reported other shift work, including night shift work, were excluded (data not shown). Adding additional adjustment for season of actigraphy data collection had no substantive impact on results (Table S4). When sleep regularity was calculated across weekdays only, fewer participants had a weekday sleep duration SD >90 minutes (33% versus 38% when compared with 7‐day SD) and a weekday sleep timing SD >60 minutes (36% versus 44% when compared with 7‐day SD). A similar association was observed between irregular weekday sleep duration and carotid plaque and abnormal ABI; however, the association with high CAC burden was attenuated and no longer statistically significant (Table S5). We found no meaningful interactions between sleep duration regularity or sleep timing regularity and any subclinical markers by age (all P>0.05), or sex (all P>0.05).
Table 5.
Sleep Duration Regularity and Subclinical Markers of CVD With Additional Adjustment for Severe OSA, Average Sleep Duration, and Sleep Fragmentation Index
| Sleep duration regularity (nightly within‐person SD) | ||||
|---|---|---|---|---|
| ≤60 min | 61–90 min | 91–120 min | >120 min | |
| Prevalence ratio (95% CI) | ||||
| Coronary artery calcium (>0) | ||||
| Model 3 | (Reference) | 1.06 (0.98–1.16) | 1.03 (0.94–1.13) | 1.07 (0.98–1.18) |
| Model 3+OSA | (Reference) | 1.06 (0.96–1.16) | 1.01 (0.91–1.11) | 1.09 (0.99–1.20) |
| Model 3+sleep duration | (Reference) | 1.06 (0.97–1.16) | 1.02 (0.93–1.13) | 1.06 (0.97–1.17) |
| Model 3+fragmentation | (Reference) | 1.05 (0.97–1.15) | 1.01 (0.92–1.11) | 1.05 (0.96–1.16) |
| Model 3+all sleep | (Reference) | 1.04 (0.95–1.15) | 0.99 (0.89–1.09) | 1.06 (0.96–1.17) |
| Coronary artery calcium (>300) | ||||
| Model 3 | (Reference) | 1.26 (0.99–1.59) | 1.32 (1.05–1.67)* | 1.33 (1.03–1.71)* |
| Model 3+OSA | (Reference) | 1.25 (0.98–1.61) | 1.29 (1.00–1.67)* | 1.34 (1.02–1.76)* |
| Model 3+sleep duration | (Reference) | 1.28 (1.01–1.62)* | 1.35 (1.07–1.71)* | 1.39 (1.07–1.81)* |
| Model 3+fragmentation | (Reference) | 1.24 (0.97–1.57) | 1.28 (1.01–1.62)* | 1.27 (0.98–1.65) |
| Model 3+all sleep | 1.26 (0.98–1.62) | 1.29 (0.99–1.68)* | 1.37 (1.03–1.82)* | |
| Carotid plaque | ||||
| Model 3 | (Reference) | 1.10 (1.01–1.20)* | 1.09 (0.99–1.20) | 1.09 (0.99–1.21) |
| Model 3+OSA | (Reference) | 1.10 (1.00–1.21)* | 1.08 (0.98–1.20) | 1.10 (0.99–1.22) |
| Model 3+sleep duration | (Reference) | 1.10 (1.01–1.21)* | 1.09 (0.99–1.20) | 1.10 (0.99–1.22) |
| Model 3+fragmentation | (Reference) | 1.10 (1.00–1.20)* | 1.08 (0.98–1.19) | 1.08 (0.98–1.20) |
| Model 3+all sleep | (Reference) | 1.10 (1.00–1.20)* | 1.08 (0.97–1.19) | 1.09 (0.98–1.22) |
| Carotid IMT (>0.9) | ||||
| Model 3 | (Reference) | 0.99 (0.83–1.18) | 0.99 (0.83–1.18) | 1.03 (0.85–1.25) |
| Model 3+OSA | (Reference) | 1.01 (0.84–1.22) | 1.03 (0.86–1.24) | 1.05 (0.85–1.28) |
| Model 3+sleep duration | (Reference) | 1.00 (0.84–1.19) | 1.00(0.84–1.20) | 1.06 (0.87–1.29) |
| Model 3+fragmentation | (Reference) | 0.99 (0.83–1.18) | 0.99 (0.82–1.18) | 1.03 (0.85–1.26) |
| Model 3+all sleep | (Reference) | 1.02 (0.84–1.23) | 1.04 (0.86–1.26) | 1.07 (0.87–1.32) |
| Ankle‐brachial index (<0.9) | ||||
| Model 3 | (Reference) | 1.50 (0.86–2.59) | 1.62 (0.98–2.70) | 1.75 (1.03–2.95)* |
| Model 3+OSA | (Reference) | 1.36 (0.76–2.44) | 1.52 (0.89–2.59) | 1.74 (1.00–3.04)* |
| Model 3+sleep duration | (Reference) | 1.50 (0.87–2.61) | 1.64 (0.98–2.74) | 1.77 (1.05–2.97)* |
| Model 3+fragmentation | (Reference) | 1.49 (0.86–2.59) | 1.62 (0.97–2.70) | 1.74 (1.03–2.95)* |
| Model 3+all sleep | (Reference) | 1.37 (0.76–2.47) | 1.54 (0.89–2.65) | 1.77 (1.02–3.07)* |
Model 3: age, sex, race and ethnicity, site, education, income, work schedule, smoking status, alcohol consumption, physical activity, body mass index, systolic blood pressure, diastolic blood pressure, antihypertension medication use, statin medication use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, prevalent diabetes. CVD indicates cardiovascular disease; IMT, intima‐media thickness; and OSA, obstructive sleep apnea.
P value <0.05.
DISCUSSION
In this racially and ethnically diverse sample of older adults, participants with greater sleep duration irregularity were more likely to have a high burden of atherosclerosis as measured across several subclinical markers, specifically higher CAC and an abnormally low ABI. These associations persisted after adjusting for BMI and prevalent CVD risk factors. When we examined sleep timing regularity, we observed an association between greater sleep timing irregularity and high CAC burden but not with other measures of subclinical CVD. Importantly, the associations observed also persisted after adjustment for severe OSA, average sleep duration, and sleep fragmentation. These results suggest that sleep regularity may have a unique etiologic link to subclinical CVD.
Sleep regularity has recently received increased attention as a relatively unexplored but relevant dimension of sleep health. 27 Day‐to‐day variations in sleep patterns appear to be prevalent in the general population, 42 , 43 with greater variation found across some racial and ethnic subgroups. 44 In our sample, more than one‐third of older adults had a sleep duration SD >90 minutes across a 7‐day period. While it is common for sleep patterns to change as adults age, 45 the proportion of the general population with irregular sleep patterns, who are not shift workers, may be concerning given the growing evidence linking sleep irregularity with CVD risk.
Importantly, we found a statistically significant association between sleep duration regularity and subclinical measures of atherosclerosis, even after adjustment for CVD risk factors, and objectively measured severe OSA, sleep duration, and sleep fragmentation. In previous MESA analyses, severe OSA was associated with prevalent CAC 13 but not with high CAC burden or abnormal ABI. 14 Additionally, MESA investigators reported associations between short (<6 hours) and long (>8 hours) sleep durations and an abnormal ABI, 14 but not CAC. 13 In the context of these prior findings, the consistency of the associations between greater sleep duration irregularity and high CAC burden and reduced ABI are compelling. The persistence of these associations after accounting for BMI, prevalent CVD risk factors, and objectively measured sleep duration and severe OSA suggests that sleep duration irregularity may have a unique etiologic role in atherosclerosis. Given that these associations are cross sectional, more research is warranted to better understand the role of sleep irregularity in the development of CVD.
Sleep regularity may be a modifiable dimension of sleep that can be targeted to reduce CVD risk in aging adults. 20 In fact, maintaining a regular sleep schedule and decreasing variability in sleep is a key component of clinical sleep hygiene recommendations. 46 Our findings suggest that these recommendations—that were developed to improve sleep—also may be useful as a cardiovascular health promotion strategy. Clinical trials, testing the role of interventions aimed at improving sleep duration regularity as a lifestyle component for CVD risk reduction intervention are warranted.
Several mechanisms have been suggested to explain the association between sleep regularity and atherosclerosis. It is hypothesized that irregular sleep patterns, including night‐to‐night variability in sleep duration and sleep timing, can cause desynchronization of sleep–wake timing and circadian disruption. 46 Almost all major cardiovascular functions, including heart rate, blood pressure, vascular tone, and endothelial functions, are regulated by circadian clock genes. 47 , 48 Disruption or misalignment of circadian rhythms can interrupt these important cardiovascular functions, resulting in the promotion of chronic inflammation, alterations in glucose metabolism, heightened sympathetic nervous system activation, and increases in arterial pressures, all predisposing to the risk of atherosclerosis progression. 22 These hypotheses are further supported by previous studies that have reported increased inflammatory markers, including interleukin‐6 and high‐sensitivity C‐reactive protein, in individuals with irregular sleep patterns 49 , 50 and increased white blood cell count in individuals with excessive sleep disruption. 51 It is also plausible that irregular sleep patterns may be a marker of irregular health behaviors in general, including irregular timing of meals or exercise, which may contribute to circadian disruption. 20 , 52
Strengths of this study include the objective assessment of sleep over a 7‐day period and the inclusion of several complementary noninvasive measures of subclinical CVD. Further, the MESA cohort includes a diverse representation of older adults, extending the generalizability of our study findings. Studies show that sleep characteristics differ across racial and ethnic groups. 34 , 53 Diverse representation in study samples is necessary to better understand associations between poor sleep and CVD across the population. Unfortunately, this study is cross sectional and observational and inferences regarding causality need to be confirmed in future studies. However, our results are novel and support the further investigation of sleep regularity in other samples with prospective assessment of CVD risk.
This study is one of the first studies to provide evidence that irregular sleep duration and timing are associated with measures of subclinical atherosclerosis. The development of atherosclerosis is a long process, allowing time for intervention before plaques have formed and hardened and can cause severe stenosis or rupture. Encouraging maintenance of regular sleep schedules with consistent sleep durations may be an important part of lifestyle recommendations provided in clinical practice for the prevention of CVD.
Sources of Funding
This research was supported by National Heart, Lung, and Blood Institute contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169; and by National Center for Advancing Translational Sciences grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420. The MESA Sleep Study was supported by National Heart, Lung, and Blood Institute grant HL56984. Dr Full was partially supported by National Heart, Lung, and Blood Institute training grant T32 HL007779 and Dr Lutsey by K24 HL159246. Dr Redline was partially supported by National Heart, Lung, and Blood Institute R35 HL 1351358181. Dr Huang was supported by K01HL143034. The funders had no role in the conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation or approval of the manuscript.
Disclosures
Dr Redline received research grants and consulting fees from Jazz Pharma and has received a consulting fee from Respircardia Inc. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027361
For Sources of Funding and Disclosures, see page 12.
References
- 1. Hall MH, Brindle RC, Buysse DJ. Sleep and cardiovascular disease: emerging opportunities for psychology. Am Psychol. 2018;73:994–1006. doi: 10.1037/amp0000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener‐West M, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 4. St‐Onge M‐P, Grandner MA, Brown D, Conroy MB, Jean‐Louis G, Coons M, Bhatt DL. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azarbarzin A, Sands SA, Stone KL, Taranto‐Montemurro L, Messineo L, Terrill P, Ancoli‐Israel S, Ensrud K, Purcell S, White DP, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease‐related mortality: the osteoporotic fractures in men study and the sleep heart health study. Eur Heart J. 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoevenaar‐Blom MP, Spijkerman AMW, Kromhout D, Van Den Berg JF, Verschuren WMM. Sleep duration and sleep quality in relation to 12‐year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–1492. doi: 10.5665/sleep.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aziz M, Ali SS, Das S, Younus A, Malik R, Latif MA, Humayun C, Anugula D, Abbas G, Salami J, et al. Association of subjective and objective sleep duration as well as sleep quality with non‐invasive markers of sub‐clinical cardiovascular disease (CVD): a systematic review. J Atheroscler Thromb. 2017;24:208–226. doi: 10.5551/jat.36194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Der Meer IM, Bots ML, Hofman A, Iglesias Del Sol A, Van Der Kuip DAM, Witteman JCM. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction. Circulation. 2004;109:1089–1094. doi: 10.1161/01.CIR.0000120708.59903.1B [DOI] [PubMed] [Google Scholar]
- 9. Gupta DK, Skali H, Claggett B, Kasabov R, Cheng S, Shah AM, Loehr LR, Heiss G, Nambi V, Aguilar D, et al. Heart failure risk across the spectrum of ankle‐brachial index: the ARIC study (atherosclerosis risk In communities). JACC Heart Fail. 2014;2:447–454. doi: 10.1016/j.jchf.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folsom AR. Coronary artery calcification compared with carotid intima‐media thickness in the prediction of cardiovascular disease incidence: the multi‐ethnic study of atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle‐brachial index and incident cardiovascular events in the MESA (multi‐ethnic study of atherosclerosis). J Am Coll Cardiol. 2010;56:1506–1512. doi: 10.1016/j.jacc.2010.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ali SS, Oni ET, Warraich HJ, Blaha MJ, Blumenthal RS, Karim A, Shaharyar S, Jamal O, Fialkow J, Cury R, et al. Systematic review on noninvasive assessment of subclinical cardiovascular disease in obstructive sleep apnea: new kid on the block! Sleep Med Rev. 2014;18:379–391. doi: 10.1016/j.smrv.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 13. Lutsey PL, McClelland RL, Duprez D, Shea S, Shahar E, Nagayoshi M, Budoff M, Kaufman JD, Redline S. Objectively measured sleep characteristics and prevalence of coronary artery calcification: the multi‐ethnic study of atherosclerosis sleep study. Thorax. 2015;70:880–887. doi: 10.1136/thoraxjnl-2015-206871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagayoshi M, Lutsey PL, Benkeser D, Wassel CL, Folsom AR, Shahar E, Iso H, Allison MA, Criqui MH, Redline S. Association of sleep apnea and sleep duration with peripheral artery disease: the multi‐ethnic study of atherosclerosis (MESA). Atherosclerosis. 2016;251:467–475. doi: 10.1016/j.atherosclerosis.2016.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao YY, Javaheri S, Wang R, Guo N, Koo BB, Stein JH, Korcarz CE, Redline S. Associations between sleep apnea and subclinical carotid atherosclerosis: the multi‐ethnic study of atherosclerosis. Stroke. 2019;50:3340–3346. doi: 10.1161/STROKEAHA.118.022184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon Y, Duprez DA, Jacobs DR Jr, Nagayoshi M, McClelland RL, Shahar E, Budoff M, Redline S, Shea S, Carr J, et al. Obstructive sleep apnea and progression of coronary artery calcium: the multi‐ethnic study of atherosclerosis study. J Am Heart Assoc. 2014;3:e001241. doi: 10.1161/JAHA.114.001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoopes EK, Witman MA, D'Agata MN, Berube FR, Brewer B, Malone SK, Grandner M, Patterson F. Rest‐activity rhythms in emerging adults: implications for cardiometabolic health. Chronobiol Int. 2021;38:543–556. doi: 10.1080/07420528.2020.1868490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zuraikat FM, Makarem N, Redline S, Aggarwal B, Jelic S, St‐Onge MP. Sleep regularity and cardiometabolic heath: is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Curr Diab Rep. 2020;20:38. doi: 10.1007/s11892-020-01324-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gohari A, Wiebe D, Ayas N. Shift working and cardiovascular health. Chronobiol Int. 2021;1–6. doi: 10.1080/07420528.2021.1933000 [DOI] [PubMed] [Google Scholar]
- 22. Reutrakul S, Knutson KL. Consequences of circadian disruption on cardiometabolic health. Sleep Med Clin. 2015;10:455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang F, Zhang L, Zhang Y, Zhang B, He Y, Xie S, Li M, Miao X, Chan EYY, Tang JL, et al. Meta‐analysis on night shift work and risk of metabolic syndrome. Obes Rev. 2014;15:709–720. doi: 10.1111/obr.12194 [DOI] [PubMed] [Google Scholar]
- 24. Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. [DOI] [PubMed] [Google Scholar]
- 25. Rosique‐Esteban N, Papandreou C, Romaguera D, Warnberg J, Corella D, Martinez‐Gonzalez MA, Diaz‐Lopez A, Estruch R, Vioque J, Aros F, et al. Cross‐sectional associations of objectively‐measured sleep characteristics with obesity and type 2 diabetes in the PREDIMED‐plus trial. Sleep. 2018;41:zsy190. [DOI] [PubMed] [Google Scholar]
- 26. Huang T, Redline S. Cross‐sectional and prospective associations of actigraphy‐assessed sleep regularity with metabolic abnormalities: the multi‐ethnic study of atherosclerosis. Diabetes Care. 2019;42:1422–1429. doi: 10.2337/dc19-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaput JP, Dutil C, Featherstone R, Ross R, Giangregorio L, Saunders TJ, Janssen I, Poitras VJ, Kho ME, Ross‐White A, et al. Sleep timing, sleep consistency, and health in adults: a systematic review. Appl Physiol Nutr Metab. 2020;45:S232–S247. [DOI] [PubMed] [Google Scholar]
- 28. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR Jr, Kronmal R, Liu K, et al. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 29. Oakley NR. Validation with polysomnography of the Sleepwatch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. Bend, OR: Mini Mitter, Cambridge Neurotechnology; 1997. [Google Scholar]
- 30. Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the multi‐ethnic study of atherosclerosis. J Am Coll Cardiol. 2020;75:991–999. doi: 10.1016/j.jacc.2019.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel SR, Weng J, Rueschman M, Dudley KA, Loredo JS, Mossavar‐Rahmani Y, Ramirez M, Ramos AR, Reid K, Seiger AN, et al. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;38:1497–1503. doi: 10.5665/sleep.4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross‐sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the coronary artery risk development in Young adults (CARDIA) sleep study. Diabetes Care. 2011;34:1171–1176. doi: 10.2337/dc10-1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 34. Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcantara C, Jackson CL, Williams MA, Redline S. Racial/ethnic differences in sleep disturbances: the multi‐ethnic study of atherosclerosis (MESA). Sleep. 2015;38:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of multi‐ethnic study of atherosclerosis (MESA) and coronary artery risk development in Young adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439 [DOI] [PubMed] [Google Scholar]
- 36. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 37. Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AEH, O'Leary DH. The value of carotid artery plaque and intima‐media thickness for incident cardiovascular disease: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all‐cause and cardiovascular disease mortality: the strong heart study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54 [DOI] [PubMed] [Google Scholar]
- 39. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 40. McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074 [DOI] [PubMed] [Google Scholar]
- 41. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, et al. Sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 42. Roenneberg T, Wirz‐Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679 [DOI] [PubMed] [Google Scholar]
- 43. Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra‐individual daily and yearly variability in actigraphically recorded sleep measures the CARDIA study. Sleep. 2007;30:793–796. doi: 10.1093/sleep/30.6.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dillon HR, Lichstein KL, Dautovich ND, Taylor DJ, Riedel BW, Bush AJ. Variability in self‐reported normal sleep across the adult age span. J Gerontol B Psychol Sci Soc Sci. 2015;70:46–56. doi: 10.1093/geronb/gbu035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edwards BA, O'Driscoll DM, Ali A, Jordan AS, Trinder J, Malhotra A. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31:618–633. doi: 10.1055/s-0030-1265902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23–36. doi: 10.1016/j.smrv.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skrlec I, Milic J, Steiner R. The impact of the circadian genes CLOCK and ARNTL on myocardial infarction. J Clin Med. 2020;9:484. doi: 10.3390/jcm9020484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martino TA, Young ME. Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology. J Biol Rhythms. 2015;30:183–205. doi: 10.1177/0748730415575246 [DOI] [PubMed] [Google Scholar]
- 49. Kwak HS, Park HO, Kim YO, Son JS, Kim CW, Lee JH, Shin YH, Park SH, Chung EY, Chae CH. The effect of shift work on high sensitivity C‐reactive protein level among female workers. Ann Occup Environ Med. 2019;31:e5. doi: 10.35371/aoem.2019.31.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okun ML, Reynolds CF 3rd, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M. Sleep variability, health‐related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73:142–150. doi: 10.1097/PSY.0b013e3182020d08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vallat R, Shah VD, Redline S, Attia P, Walker MP. Broken sleep predicts hardened blood vessels. PLoS Biol. 2020;18:e3000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gabriel BM, Zierath JR. Circadian rhythms and exercise ‐ re‐setting the clock in metabolic disease. Nat Rev Endocrinol. 2019;15:197–206. doi: 10.1038/s41574-018-0150-x [DOI] [PubMed] [Google Scholar]
- 53. Carnethon MR, De Chavez PJ, Zee PC, Kim KA, Liu K, Goldberger JJ, Ng J, Knutson KL. Disparities in sleep characteristics by race/ethnicity in a population‐based sample: Chicago area sleep study. Sleep Med. 2016;18:50–55. doi: 10.1016/j.sleep.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


