Abstract
Background
High burden of premature ventricular complex (PVC) leads to increased cardiovascular mortality. A recent nationwide population‐based study demonstrated that PVC is associated with an increased risk of atrial fibrillation (AF). However, the relationship between PVC burden and new‐onset AF has not been investigated. The purpose of the study is to elucidate whether PVC burden is associated with new‐onset AF.
Methods and Results
We designed a single‐center, retrospective, large population‐based cohort study to evaluate the role of PVC burden and new‐onset AF in Taiwan. Patients who were AF naïve with PVC were divided into the low burden group (<1000/day) and moderate‐to‐high burden group (≥1000/day) based on the 24‐h Holter ECG report. New‐onset AF was defined as a new or first detectable event of either a persistent or paroxysmal AF. A total of 16 030 patients who were AF naïve and underwent 24‐h Holter ECG monitoring were enrolled in this study, with a mean follow‐up time of 973 days. A propensity score‐matched analysis demonstrated that the moderate‐to‐high burden PVC group had a higher risk of developing new‐onset AF than that of the low burden PVC group (4.91% versus 2.73%, P<0.001). Multivariate Cox regression analysis showed that moderate‐to‐high burden of PVC is an independent risk factor for new‐onset AF. The Kaplan–Meier analysis demonstrated that patients with moderate‐to‐high PVC burden were associated with higher risk of new‐onset AF (log‐rank P<0.001).
Conclusions
PVC burden is associated with new‐onset AF. Patients with moderate‐to‐high PVC burden are at a higher risk of new‐onset AF.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03877614.
Keywords: 24‐h Holter ECG, new‐onset atrial fibrillation, premature ventricular complex, premature ventricular complex‐induced atrial fibrillation
Subject Categories: Arrhythmias, Atrial Fibrillation, Electrophysiology
Nonstandard Abbreviation and Acronym
- PVC
premature ventricular complex
Clinical Perspective.
What Is New?
This is a single‐center, retrospective, large population‐based cohort study confirming the association between premature ventricular complex burden and new‐onset atrial fibrillation.
Younger patients with moderate‐to‐high premature ventricular complex burden are at a higher risk of new‐onset atrial fibrillation.
What Are the Clinical Implications?
Patients with high premature ventricular complex burden should be monitored closely for new‐onset atrial fibrillation.
Our study provides insights on the possible mechanisms between premature ventricular complex, atrial fibrillation, heart failure, and ischemic stroke.
Premature ventricular complex (PVC) is one of the most common cardiac arrhythmias and is associated with cardiovascular morbidity and mortality. 1 A high PVC burden may induce PVC‐induced cardiomyopathy, which is detrimental to the left ventricular systolic function. 2 Moderate and high burdens of PVC also increase the risk of cardiovascular mortality compared to that of low burden of PVC. 3 , 4 In addition to cardiovascular complications, PVC is also associated with ischemic stroke. 5 Some case reports have demonstrated that PVC can trigger atrial fibrillation (AF) via intracardiac tracing. 6 , 7 Recently, a nationwide population‐based study demonstrated that PVC, which was identified based on International Classification of Diseases, Tenth Revision (ICD‐10) codes, is associated with an increased risk of AF. 8 However, the relationship between high PVC burden and increased risk of new‐onset AF has not been investigated. Our study aimed to evaluate whether a high PVC burden induces new‐onset AF by analyzing the daily burden of PVC using the 24‐h Holter ECG and electronic medical record databases of a single hospital.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
Patients who underwent 24‐h Holter ECG monitoring between July 1, 2011, and December 31, 2018, were enrolled. The indication for 24‐h Holter ECG monitoring included a clinical diagnosis of arrhythmia and transient loss of consciousness with suspected syncope and palpitation. If there were multiple Holter studies of the same patient, the index Holter study was defined as the study with the largest PVC burden and naïve to medical treatment. We excluded patients with incomplete chart records or Holter studies, aged <18 years old, inadequately followed up for >12 months, a history of AF, and ever having underwent PVC ablation. The study design was validated and described in our previous studies. 3 , 9 , 10 , 11 The study protocol and clinical data were approved by the institutional review board of National Cheng‐Kung University Hospital (approval number B‐ER‐108‐290). Informed consent was not required because the database enrolled in this study was deidentified. This study was registered at ClinicalTrials.gov (http://clinicaltrials.gov/ct2/show/NCT03877614).
Baseline Characteristics and Clinical Outcomes
Baseline characteristics of the patients were collected from the National Cheng‐Kung University Hospital electronic medical record database, which was retrieved using a data‐mining technique from the electronic medical record system of National Cheng‐Kung University Hospital. To ensure accuracy of the database, 3 independent cardiologists validated the clinical diagnosis using a sample survey with an accuracy of 99%. The baseline characteristics included age, sex, and disease status such as diabetes, hypertension, heart failure (HF; defined as a left ventricular ejection fraction of <50%), and coronary artery disease (CAD). The clinical outcome was new‐onset AF, and the evidence of new‐onset AF was retrieved from the medical records of our hospital.
Definition and Ascertainment of New‐Onset Atrial Fibrillation
New‐onset clinical AF was defined as the first detectable episode of AF. The devices used to detect AF included a 12‐lead ECG, 24‐h Holter ECG, event recorder, implantable loop recorder, and ECG smartwatch. Clinical AF was defined as a standard 12‐lead ECG recording or single‐lead ECG tracing showing heart rhythms with irregular RR intervals and no discernible P waves that last for more than 30 seconds. 12 The diagnosis of AF was further confirmed by 2 independent cardiologists. Disagreements on AF diagnosis were further evaluated by a third cardiologist.
Twenty‐Four‐Hour Holter ECG Variables
Data were collected from the Holter database of National Cheng‐Kung University Hospital. The PVC burden was recorded using a 3‐channel 24‐h Holter device (NorthEast Monitoring, Inc., DR200/HE Digital Recorder). The raw data from the Holter monitor were analyzed and edited by 4 experienced technicians. Five senior cardiologists reviewed the arrhythmic episodes and unknown strips and confirmed the final reports. ECG variables included PVC burden, premature atrial complex, supraventricular tachycardia, and ventricular tachycardia. Incomplete and uninterpretable Holter studies were excluded.
Statistical Analysis
Baseline clinical characteristics and Holter variables were presented as mean±SD for continuous variables, median (interquartile range) for not normally distributed variables, and percentages for categorical variables. Student's t‐test was used to analyze continuous variables, and the chi‐square test with Yates' correction was used to analyze categorical variables. Univariate and multivariate Cox regression models were used to analyze the contribution of clinical and Holter variables to new‐onset AF. Adjusted variables included age, sex, diabetes, hypertension, HF, and CAD. A propensity score matching method was used to analyze new‐onset AF in patients with low (<1000/day) versus moderate‐to‐high burden (>1000–10 000/day). Unadjusted survival probabilities of new‐onset AF from Kaplan–Meier survival curves were compared between the 2 groups using the log‐rank test. Predefined subgroup analyses and interaction tests were performed on complete cases in younger population (<60 years old) versus older population (≥60 years old), men versus women, in patients with versus without diabetes, in patients with versus without HF, and in patients with versus without CAD.
RESULTS
Baseline Characteristics, Premature Ventricular Complex Burden, and New‐Onset Atrial Fibrillation of the Study Population
A total of 25 398 patients were enrolled in this study. Patients with incomplete chart records, a history of AF and PVC ablation, aged <18 years old, and not followed up or followed up for <6 months were excluded from this study. The flow chart of the study selection process is shown in Figure 1. Based on our previous studies, the patients were divided into 2 groups: low PVC burden (PVC daily count <1000) and moderate‐to‐high PVC burden (PVC daily count ≥1000). In comparison with moderate and high PVC burdens, patients with low PVC burden were younger (moderate and high versus low=59.9±16.2 versus 56.9±17.7, P<0.001). Patients with a high PVC burden tended to have diabetes (moderate and high versus low=25.8% versus 19.5%, P<0.001), hypertension (moderate and high versus low=49.7% versus 44.0%, P<0.001), HF (moderate and high versus low=18.0% versus 7.3%, P<0.001), CAD (moderate and high versus low=17.2% versus 9.0%, P<0.001), and chronic kidney disease (moderate and high versus low=15.4% versus 22.3%, P<0.001). Patient in the moderate and high burden group used more antiplatelet drugs, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, statin, beta blockers, nondihydropyridine calcium channel blocker, and antiarrhythmia drugs in comparison with the low burden group. In terms of the Holter data, the premature atrial complex counts in both groups were similar (1 versus 23, P=0.205). Regarding the echocardiographic data, the left ventricular ejection fraction is 65.1±13.2 in the moderate and high burden group, and 70.3%±9.2% in the low burden group (P<0.001). The incidence of new‐onset AF was higher in the moderate and high burden groups than that of the low burden group (moderate and high versus low=5.3% versus 2.4%, P<0.001) after a mean follow‐up of 973 days. The incidence of new‐onset ischemic stroke was similar in both groups. The results are presented in Table 1.
Figure 1. Flow chart of the study selection process.
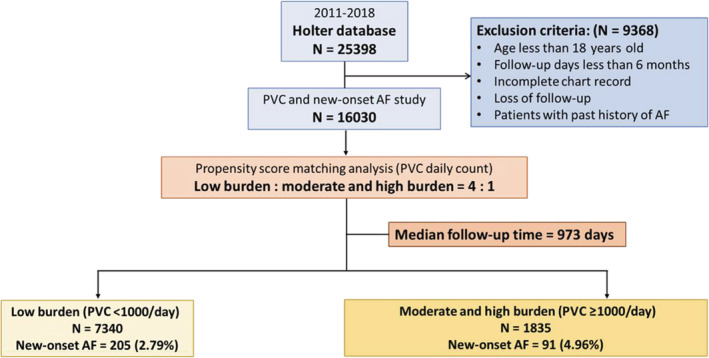
Initially, 25 398 patients were screened. After excluding patients who met the exclusion criteria, 16 030 patients were enrolled in the study. A propensity score‐matched analysis was used to compare the low burden (PVC daily count <1000) and moderate and high burden (PVC daily count >1000) groups, with a ratio of 4:1. After a median follow‐up time of 973 days, the incidences of new‐onset atrial fibrillation were 2.79% and 4.96% in the low‐burden and moderate‐to‐high‐burden groups, respectively (P<0.001). AF indicates atrial fibrillation; and PVC, premature ventricular complex.
Table 1.
Baseline Characteristics, Medication History, Holter Data, and New‐Onset Atrial Fibrillation Based on PVC Burdens
| PVC daily count <1000 (N=14 097) | PVC daily count ≥1000 (N=1933) | P value | |
|---|---|---|---|
| Median (IQR)/N (%)/Mean±SD | Median (IQR)/N (%)/Mean±SD | ||
| Clinical characteristics | |||
| Age, y | |||
| <60 | 7537 (53.47) | 912 (47.18) | <0.001 |
| ≥60 | 6560 (46.53) | 1021 (52.82) | |
| Mean ± SD | 56.9±17.7 | 59.9±16.2 | <0.001 |
| Male | 6208 (44.04) | 1024 (52.97) | <0.001 |
| Diabetes | 2743 (19.46) | 498 (25.75) | <0.001 |
| Hypertension | 6209 (44.04) | 961 (49.72) | <0.001 |
| Heart failure | 1029 (7.30) | 328 (16.97) | <0.001 |
| Coronary artery disease | 1272 (9.02) | 332 (17.18) | <0.001 |
| Stroke | 993 (7.04) | 158 (8.17) | 0.071 |
| Chronic kidney disease | 2168 (15.38) | 431 (22.30) | <0.001 |
| Alcoholism | 61 (0.43) | 13 (0.67) | 0.201 |
| Hyperthyroidism | 312 (2.21) | 38 (1.97) | 0.539 |
| Sleep apnea | 257 (1.82) | 36 (1.86) | 0.976 |
| Medication | |||
| Aspirin | 2689 (19.07) | 447 (23.12) | <0.001 |
| P2Y12 inhibitor | 962 (6.82) | 208 (10.76) | <0.001 |
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 1988 (14.10) | 401 (20.74) | <0.001 |
| Statin | 2396 (17.00) | 405 (20.95) | <0.001 |
| Class Ia AAD | 6 (0.04) | 1 (0.05) | 0.856 |
| Class Ib AAD | 588 (4.17) | 524 (27.11) | <0.001 |
| Class Ic AAD | 75 (0.53) | 20 (1.03) | 0.007 |
| Class III AAD | 197 (1.40) | 195 (10.09) | <0.001 |
| Beta blocker | 2711 (19.23) | 503 (26.02) | <0.001 |
| Nondihydropyridine calcium channel blocker | 607 (4.31) | 358 (18.52) | <0.001 |
| Holter data | |||
| PVC count | 4 (1–30) | 4685 (2032–11 421) | <0.001 |
| PVC burden | 0.01 (0.01–0.03) | 4.6 (20.1–11.2) | <0.001 |
| Premature atrial complex count | 15 (4–73) | 23 (4–159) | 0.205 |
| Subsequent Holter monitoring | 1.2±0.6 | 1.7±1.2 | <0.001 |
| Echocardiographic data | |||
| Left ventricular ejection fraction | 70.3±9.2 | 65.1±13.2 | <0.001 |
| Left atrium diameter | 3.4±0.6 | 3.6±0.7 | <0.001 |
| Outcome | |||
| New‐onset atrial fibrillation | 339 (2.40) | 103 (5.33) | <0.001 |
| New‐onset ischemic stroke | 649 (4.60) | 104 (5.38) | 0.146 |
AAD indicates antiarrhythmic drug; IQR, interquartile range; and PVC, premature ventricular complex.
Premature Ventricular Complex Burden Is Associated With New‐Onset Atrial Fibrillation
To demonstrate the association between PVC burden and new‐onset AF, a propensity score‐matched analysis adjusting for baseline characteristics that were considered to be associated with AF development, including age, diabetes, hypertension, HF, CAD, alcoholism, hyperthyroidism, and sleep apnea were used to analyze the incidence of new‐onset AF between the 2 groups (Table 2). Two hundred patients (2.73%) in the moderate and high burden groups developed new‐onset AF, whereas only 90 patients (4.91%) in the low‐burden group developed new‐onset AF during the follow‐up period (P<0.001). Multivariable Cox regression analysis was used to further analyze the contribution of PVC burden and other clinical factors to new‐onset AF (Table 3). Higher PVC burden of PVC daily count ≥1000 (adjusted hazard ratio [HR], 1.42 [95% CI, 1.09–1.84]; P=0.009), older population (≥60 years, adjusted HR, 3.71 [95% CI, 2.70–5.11]; P<0.001), HF (adjusted HR, 2.42 [95% CI, 1.83–3.20]; P<0.001), alcoholism (adjusted HR, 7.30 [95% CI, 2.68–19.85]; P<0.001), and use of antiarrhythmia drugs (adjusted HR, 2.02 [95% CI, 1.41–2.91]; P<0.001) were associated with new‐onset AF. PVC daily count in different cutoff values (1000 ≤PVC count <5000 group, adjusted HR, 1.48 [95% CI, 1.09–2.00]; P=0.011; PVC count ≥5000 group, adjusted HR, 1.66 [95% CI, 1.23–3.23]; P=0.001) and PVC daily burden in percentile (adjusted HR, 8.05 [95% CI, 1.79–36.29]; P=0.007) was also associated with new‐onset AF; these data are shown in Table S1 and Table S2. Kaplan–Meier analysis was used to evaluate the prognostic effect of PVC burden on new‐onset AF (Figure 2). The analysis demonstrated that moderate and high PVC burdens led to higher incidences of new‐onset AF (log‐rank P<0.001) than that of the low PVC burden group.
Table 2.
New‐Onset Atrial Fibrillation After Propensity Score‐Matched Analysis of Different PVC Burdens (4:1 Match)
| Variables | PVC daily count <1000 (N=7328) | PVC daily count ≥1000 (N=1832) | P value |
|---|---|---|---|
| n (%) | n (%) | ||
| Age, y | |||
| <60 | 3560 (48.58) | 892 (48.69) | 0.954 |
| ≥60 | 3768 (51.42) | 940 (51.31) | |
| Mean±SD | 58.7±17.5 | 59.4±16.3 | 0.097 |
| Male | 3646 (49.75) | 932 (50.87) | 0.406 |
| Diabetes | 1732 (23.64) | 432 (23.58) | 0.985 |
| Hypertension | 3567 (48.68) | 882 (48.14) | 0.703 |
| Heart failure | 793 (10.82) | 227 (12.39) | 0.062 |
| Coronary artery disease | 1020 (13.92) | 237 (12.94) | 0.291 |
| Alcoholism | 39 (0.53) | 9 (0.49) | 0.971 |
| Hyperthyroidism | 131 (1.79) | 37 (2.02) | 0.572 |
| Sleep apnea | 125 (1.71) | 32 (1.75) | 0.984 |
| Outcome | |||
| New‐onset atrial fibrillation | 200 (2.73) | 90 (4.91) | <0.001 |
| New‐onset ischemic stroke | 395 (5.39) | 90 (4.91) | 0.448 |
PVC indicates premature ventricular complex.
Table 3.
Univariable and Multivariable Cox Regression Models for New‐Onset AF After Propensity Score‐Matched Analysis
| Variable | Univariate model | Multivariable model 1 | Multivariable model 2 | Multivariable model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| PVC count ≥1000 | 1.49 (1.15–1.91) | 0.002 | 1.50 (1.17–1.94) | 0.002 | 1.52 (1.18–1.97) | 0.001 | 1.42 (1.09–1.84) | 0.009 |
| Age ≥60 | 3.26 (2.41–4.40) | <0.001 | 3.39 (2.47–4.64) | <0.001 | 3.49 (2.54–4.79) | <0.001 | 3.71 (2.70–5.11) | <0.001 |
| Male | 1.15 (0.91–1.46) | 0.232 | 1.07 (0.85–1.36) | 0.563 | 1.07 (0.85–1.36) | 0.564 | 1.03 (0.81–1.32) | 0.787 |
| Diabetes | 1.48 (1.16–1.90) | 0.002 | 1.18 (0.91–1.52) | 0.218 | 1.15 (0.89–1.49) | 0.296 | 1.17 (0.90–1.51) | 0.241 |
| Hypertension | 1.81 (1.41–2.33) | <0.001 | 1.05 (0.80–1.38) | 0.712 | 1.07 (0.82–1.42) | 0.609 | 1.07 (0.82–1.41) | 0.612 |
| Heart failure | 2.45 (1.88–3.18) | <0.001 | 2.76 (2.11–3.59) | <0.001 | 2.76 (2.12–3.61) | <0.001 | 2.42 (1.83–3.20) | <0.001 |
| Coronary artery disease | 1.37 (1.02–1.84) | 0.035 | 1.16 (0.85–1.57) | 0.354 | 1.16 (0.86–1.58) | 0.331 | 1.17 (0.86–1.59) | 0.318 |
| Alcoholism | 4.05 (1.51–10.89) | 0.006 | 6.97 (2.56–18.94) | <0.001 | 7.30 (2.68–19.85) | <0.001 | ||
| Hyperthyroidism | 1.54 (0.82–2.90) | 0.183 | 1.70 (0.88–3.28) | 0.112 | 1.52 (0.78–2.96) | 0.218 | ||
| Use of antiarrhythmia drugs | 2.26 (1.61–3.18) | <0.001 | 2.02 (1.41–2.91) | <0.001 | ||||
AF indicates atrial fibrillation; HR, hazard ratio; and PVC, premature ventricular complex.
Figure 2. Kaplan–Meier survival plot of different burdens of premature ventricular complex and new‐onset atrial fibrillation.
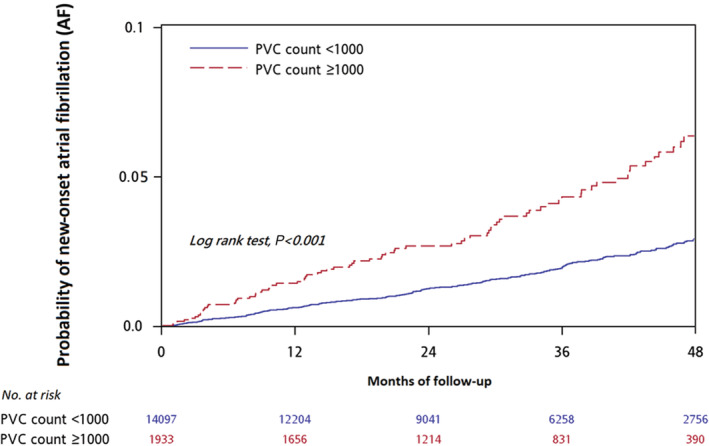
A PVC burden of ≥1000/day was associated with a higher risk of new‐onset AF (log‐rank test, P<0.001). AF indicates atrial fibrillation; and PVC, premature ventricular complex.
Effect of Catheter Ablation to the Highest PVC Burden Group
To clarify the effect of PVC ablation on new‐onset AF in the highest PVC burden group (PVC daily count >10 000/day), we enrolled the other PVC ablation cohort. In this ablation group, 126 patients with highest PVC burden (>10 000/day) underwent successful elimination of PVC by catheter ablation. In comparison with the 675 patients in the index study cohort with highest PVC burden, the rate of new‐onset AF is 0.79% (1/126) in the ablation group and 3.9% (26/675) in the without‐ablation group (P=0.08). Although not statistically significant, the ablation group had a trend to decrease the development of new‐onset AF.
Subgroup Analysis of High Premature Ventricular Complex Burden and New‐Onset Atrial Fibrillation
Figure 3 shows the subgroup analysis of adjusted HRs for new‐onset AF between the moderate and high PVC burden groups and low PVC burden group. Moderate and high PVC burdens led to higher incidences of new‐onset AF irrespective of sex and underlying diseases, including diabetes, HF, and CAD. However, the younger population (<60 years old) with moderate and high PVC burdens (adjusted HR, 2.76 [95% CI, 1.70–4.49]) had a higher risk of developing new‐onset AF than that of the older population (≥60 years old, adjusted HR, 1.37 [95% CI, 1.05–1.77]; P for interaction=0.009).
Figure 3. Subgroup analysis of the effect of baseline characteristics on new‐onset atrial fibrillation in the moderate and high premature ventricular complex groups (PVC daily count ≥1000/day).
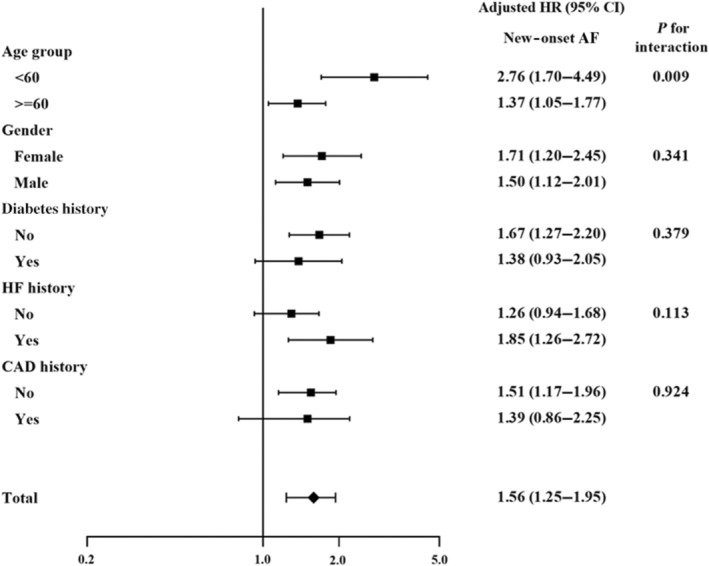
Younger patients (<60 years old) in the moderate and high PVC burden groups were at a higher risk of new‐onset AF, whereas sex, history of diabetes, heart failure, and coronary artery disease did not have an impact on new‐onset AF. AF indicates atrial fibrillation; CAD, coronary artery disease; HF, heart failure; HR, hazard ratio; and PVC, premature ventricular complex.
Effect of Time on New‐Onset AF in Moderate and High Premature Ventricular Complex Burden Groups
A Cox regression model analyzing follow‐up time intervals was used to demonstrate the effect of time on new‐onset AF in moderate and high PVC burden groups (Table 4). The adjusted HR of new‐onset AF was similar but slightly elevated with time. There were risks of developing new‐onset AF in patients with moderate and high PVC burdens after ≤12 months (adjusted HR, 1.60 [95% CI, 1.02–2.50], P=0.039), ≤36 months (adjusted HR, 1.62 [95% CI, 1.22–2.17], P=0.001), and ≤48 months (adjusted HR, 1.67 [95% CI, 1.28–2.16], P=0.001).
Table 4.
Univariable and Multivariable Cox Regression Models for New‐Onset AF Based on Follow‐Up Time Interval
| Follow‐up time | Univariate model | Multivariable model | ||
|---|---|---|---|---|
| Crude HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| ≤12 mo | ||||
| PVC daily count ≥1000 | 2.38 (1.54–3.68) | <0.001 | 1.60 (1.02–2.50) | 0.040 |
| ≤36 mo | ||||
| PVC daily count ≥1000 | 2.26 (1.71–3.00) | <0.001 | 1.62 (1.22–2.17) | 0.001 |
| ≤48 mo | ||||
| PVC daily count ≥1000 | 2.25 (1.74–2.90) | <0.001 | 1.66 (1.28–2.16) | <0.001 |
Multivariable model: adjusted for age, sex, diabetes, hypertension, heart failure, coronary artery disease, alcoholism, and hyperthyroidism. AF indicates atrial fibrillation; HR, hazard ratio; and PVC, premature ventricular complex.
DISCUSSION
Main Findings
The main findings of our study were as follows: (1) moderate and high PVC burdens led to higher incidences of new‐onset AF compared to that of the low PVC burden group, (2) the younger population with moderate and high PVC burdens had a higher risk of developing new‐onset AF than that of the older population, and (3) the risk of developing new‐onset AF in patients with moderate and high PVC burdens was similar at different follow‐up time intervals.
Premature Ventricular Complex Burden and Cardiovascular Complications
High PVC burden is a predictive factor for long‐term all‐cause mortality. 13 , 14 Medically fragile populations, such as those with cancer and frequent PVC, are associated with mortality irrespective of cancer and performance status. 15 Besides the medically fragile population, frequent PVC induced during an exercise test is also an indicator of long‐term cardiovascular mortality in asymptomatic individuals. 16 To stratify the risk of PVC, our previous work demonstrated that moderate (1000–10 000/day) and high PVC burdens (≥10 000/day) were associated with cardiovascular mortality. Although PVC‐induced cardiomyopathy is hypothesized to cause the structural changes in the heart, not every cardiovascular death can be explained through frequent PVC‐induced HF. Thus, the question of whether PVC can cause cardiac complications other than HF has become an important research topic. A recent nationwide study found that the risk of new‐onset AF increases in patients with a clinical diagnosis of PVC. 8 Our study further demonstrated that PVC burden is associated with new‐onset AF. Of note, in this study, new‐onset AF may still be underestimated in the moderate and high PVC groups because of the prevalence of antiarrhythmic drug use in this group.
Possible Mechanisms of Premature Ventricular Complex‐Induced Atrial Fibrillations
We postulated several mechanisms that can lead to a PVC‐induced AF. First, a high PVC burden has been shown to contribute to PVC‐induced cardiomyopathy, and HF causes AF. 13 , 14 , 17 , 18 AF is the most common type of arrhythmia in patients with HF with reduced ejection fraction. The prevalence of AF increased as the severity of HF increased, reaching 49.8% in patients with severe HF with reduced ejection fraction with a New York Heart Association functional class IV. 17 , 19 Common structural changes in HF, including pressure and volume overload, result in tissue stretch with a shorter atrial refractory period and longer atrial conduction time, leading to atrial chamber enlargement and arrhythmogenesis in the atrium and predisposition to the development of AF. 20 In addition, neurohormonal activation in HF also affects the synthesis and degradation of the atrial extracellular matrix, resulting in atrial tissue fibrosis, which leads to the development and maintenance of AF. Second, evidence and mechanism of PVC‐induced AF can be directly observed in intracardiac tracing. PVC‐associated atrial activation by either retrograde atrioventricular nodal or accessory pathway conduction has been reported to trigger AF. 6 , 21 In patients with an implantable device, retrograde atrial activation induced by PVC during the postventricular atrial refractory period and subsequent atrial pacing also resulted in a long–short sequence and induced AF. 7 Thus, PVC ablation may be a possible adjuvant strategy for AF ablation that could eliminate all potential AF triggers. 21 Based on the evidence provided here, it is reasonable to suggest that patients with a high PVC burden may have a higher risk of new‐onset AF.
Younger Patients With Moderate and High Premature Ventricular Complex Burdens Are at a Higher Risk of Developing New‐Onset Atrial Fibrillation
In the subgroup analysis, we found that, among patients with moderate and high PVC burdens, the younger population (<60 years old) had a higher risk of new‐onset AF than that of the older population. According to a previous genetic study, disease‐associated genetic variants, including cardiomyopathy and ventricular tachyarrhythmia, were found in 10% of early‐onset AF. 22 A total of 22 genes responsible for cardiomyopathy and arrhythmia could be detected by commercial panels in this study. This means that patients with a genetic background of cardiomyopathy may have higher PVC burden as the first clinical manifestation, followed by AF. A recent genome‐wide area study also demonstrated that PKN2 (protein kinase N2), which was previously reported to be responsible for atrial fibrosis and development of AF, was associated with calcium homeostasis and ventricular arrhythmia. 23 , 24 Therefore, we hypothesized that frequent PVC with moderate to large burdens may share common genetic pathological variants with AF. Moreover, in comparison with the low burden group, the adjusted HRs of the moderate and high PVC burden groups for new‐onset AF at different follow‐up time intervals were similar. This phenomenon implies that certain genetically high‐risk groups may have a higher chance of developing new‐onset AF regardless of the follow‐up period. However, the hypothesis of whether new‐onset AF share similar genetic background with high burden of PVC requires further investigation.
Prognosis of Premature Ventricular Complex Associated New‐Onset Atrial Fibrillation
Patients with PVC are at a higher risk of ischemic stroke. Four studies reported that PVC is associated with ischemic stroke. 1 , 5 , 25 , 26 A meta‐analysis of these 4 studies demonstrated that patients with a clinical diagnosis of PVC had a pooled relative risk of 1.31 (95% CI, 1.07–1.60) for ischemic stroke. However, these studies did not present a causal relationship between PVC burden, new‐onset AF, and new‐onset ischemic stroke. In this study, we did not provide evidence that higher PVC burden increases ischemic stroke, which could be attributed to insufficient follow‐up time of this study. Further, intensive continuous monitoring to detect subclinical AF in patients with PVC and ischemic stroke should be considered.
This study had several limitations. First, this was a single‐center retrospective cohort study. Most of the study population was Asian, and those who were enrolled were from southern Taiwan. The applicability of the study results to other races remains uncertain. Second, serial echocardiographic data were not available for this study. Thus, it is unclear whether patients had PVC‐induced cardiomyopathy, and the actual timing of PVC‐induced cardiomyopathy is unknown. Third, missing data of body weight and body mass index are significant in this study. Therefore, these 2 variables could not be included in the regression model. Finally, the mean follow‐up duration was 973 days. Patients may have an AF or ischemic stroke after the follow‐up period, which was a significant limitation of this study.
CONCLUSIONS
Overall, our study showed that PVC burden is associated with new‐onset AF. Patients with moderate‐to‐high PVC burden are at a higher risk of developing new‐onset AF than those with low PVC burden.
Sources of Funding
This study was supported by grants 108‐2314‐B‐006‐098‐MY3, 111‐2634‐F‐006‐007 from the Ministry of Science and Technology of Taiwan, the grant of D111‐G2512 from Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University, and the research grant from the Ministry of Health and Welfare (MOHW111‐TDU‐B‐211‐134003). It was also supported by National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH‐11104046).
Disclosures
None.
Supporting information
Table S1–S2
Acknowledgments
We are grateful to Ms. Chi‐Hui Hsu and Prof. Sheng‐Hsian Lin for providing the statistical consulting services from the Biostatistics Consulting Center, Clinical Medicine Research Center, National Cheng Kung University Hospital.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027674
For Sources of Funding and Disclosures, see page 9.
See Editorial by Lacharite‐Roberge et al.
Contributor Information
Sheng‐Hsian Lin, Email: shlin922@mail.ncku.edu.tw.
Ping‐Yen Liu, Email: larry@mail.ncku.edu.tw.
References
- 1. Lin CY, Chang SL, Lin YJ, Lo LW, Chung FP, Chen YY, Chao TF, Hu YF, Tuan TC, Liao JN, et al. Long‐term outcome of multiform premature ventricular complexes in structurally normal heart. Int J Cardiol. 2015;180:80–85. doi: 10.1016/j.ijcard.2014.11.110 [DOI] [PubMed] [Google Scholar]
- 2. Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13:612–632. doi: 10.1177/1747493018778713 [DOI] [PubMed] [Google Scholar]
- 3. Lee P‐T, Huang T‐C, Huang M‐H, Hsu L‐W, Su P‐F, Liu Y‐W, Hung M‐H, Liu P‐Y. The burden of ventricular premature complex is associated with cardiovascular mortality. Front Cardiovasc Med. 2022;8:797976. doi: 10.3389/fcvm.2021.797976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee YH, Zhong L, Roger VL, Asirvatham SJ, Shen WK, Slusser JP, Hodge DO, Cha YM. Frequency, origin, and outcome of ventricular premature complexes in patients with or without heart diseases. Am J Cardiol. 2014;114:1373–1378. doi: 10.1016/j.amjcard.2014.07.072 [DOI] [PubMed] [Google Scholar]
- 5. Ofoma U, He F, Shaffer ML, Naccarelli GV, Liao D. Premature cardiac contractions and risk of incident ischemic stroke. J Am Heart Assoc. 2012;1:e002519. doi: 10.1161/jaha.112.002519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peinado R, Merino JL, Gnoatto M, Arias MA. Atrial fibrillation triggered by postinfarction ventricular premature beats in a patient with Wolff‐Parkinson‐White syndrome. Europace. 2005;7:221–224. doi: 10.1016/j.eupc.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 7. Friedman DJ, Chasten T, Anderson K, Mullenix J, Rider K, Sun AY. Premature ventricular contraction response‐induced new‐onset atrial fibrillation. HeartRhythm Case Rep. 2019;5:120–123. doi: 10.1016/j.hrcr.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim YG, Han KD, Choi JI, Choi YY, Choi HY, Shim J, Kim YH. Premature ventricular contraction is associated with increased risk of atrial fibrillation: a nationwide population‐based study. Sci Rep. 2021;11:1601. doi: 10.1038/s41598-021-81229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu LW, Chen PW, Chang WT, Lee WH, Liu PY. The role of ROCK in platelet‐monocyte collaborative induction of thromboinflammation during acute coronary syndrome. Thromb Haemost. 2020;120:1417–1431. doi: 10.1055/s-0040-1714278 [DOI] [PubMed] [Google Scholar]
- 10. Huang MS, Wang CS, Chiang JH, Liu PY, Tsai WC. Automated recognition of regional wall motion abnormalities through deep neural network interpretation of transthoracic echocardiography. Circulation. 2020;142:1510–1520. doi: 10.1161/circulationaha.120.047530 [DOI] [PubMed] [Google Scholar]
- 11. Huang TC, Lee PT, Huang MS, Su PF, Liu PY. Higher premature atrial complex burden from the Holter examination predicts poor cardiovascular outcome. Sci Rep. 2021;11:12198. doi: 10.1038/s41598-021-91800-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 13. Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, Armstrong W, Good E, Chugh A, Jongnarangsin K, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036 [DOI] [PubMed] [Google Scholar]
- 14. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66:101–109. doi: 10.1016/j.jacc.2015.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anker MS, von Haehling S, Coats AJS, Riess H, Eucker J, Porthun J, Butler J, Karakas M, Haverkamp W, Landmesser U, et al. Ventricular tachycardia, premature ventricular contractions, and mortality in unselected patients with lung, colon, or pancreatic cancer: a prospective study. Eur J Heart Fail. 2021;23:145–153. doi: 10.1002/ejhf.2059 [DOI] [PubMed] [Google Scholar]
- 16. Refaat MM, Gharios C, Moorthy MV, Abdulhai F, Blumenthal RS, Jaffa MA, Mora S. Exercise‐induced ventricular ectopy and cardiovascular mortality in asymptomatic individuals. J Am Coll Cardiol. 2021;78:2267–2277. doi: 10.1016/j.jacc.2021.09.1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2d–8d. doi: 10.1016/s0002-9149(02)03373-8 [DOI] [PubMed] [Google Scholar]
- 18. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/circulationaha.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Effects of enalapril on mortality in severe congestive heart failure. Results of the cooperative north Scandinavian enalapril survival study (CONSENSUS). N Engl J Med. 1987;316:1429–1435. doi: 10.1056/nejm198706043162301 [DOI] [PubMed] [Google Scholar]
- 20. Van den Berg MP, Tuinenburg AE, Crijns HJ, Van Gelder IC, Gosselink AT, Lie KI. Heart failure and atrial fibrillation: current concepts and controversies. Heart (British Cardiac Society). 1997;77:309–313. doi: 10.1136/hrt.77.4.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bocchese M, Mangrolia H, Basil A, Gangireddy C, Cronin E, Yesenosky GA, Greenberg RM, Cooper JM, Whitman IR. Atrial fibrillation triggered by premature ventricular complexes: an under‐recognized trigger. JACC Case Rep. 2020;2:2244–2248. doi: 10.1016/j.jaccas.2020.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoneda ZT, Anderson KC, Quintana JA, O'Neill MJ, Sims RA, Glazer AM, Shaffer CM, Crawford DM, Stricker T, Ye F, et al. Early‐onset atrial fibrillation and the prevalence of rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol. 2021;6:1371–1379. doi: 10.1001/jamacardio.2021.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang C, Wang P, Yu D, Zhang X, Gou D, Liang L, Bai X, Xie W, Li H, Pu J, et al. Genome‐wide association study for idiopathic ventricular tachyarrhythmias identifies key role of CCR7 and PKN2 in calcium homeostasis and cardiac rhythm maintenance. Circ Genom Precis Med. 2022;15:e003603. doi: 10.1161/circgen.121.003603 [DOI] [PubMed] [Google Scholar]
- 24. Zhang P, Wang W, Wang X, Wang X, Song Y, Han Y, Zhang J, Zhao H. Protein analysis of atrial fibrosis via label‐free proteomics in chronic atrial fibrillation patients with mitral valve disease. PLoS One. 2013;8:e60210. doi: 10.1371/journal.pone.0060210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agarwal SK, Chao J, Peace F, Judd SE, Kissela B, Kleindorfer D, Howard VJ, Howard G, Soliman EZ. Premature ventricular complexes on screening electrocardiogram and risk of ischemic stroke. Stroke. 2015;46:1365–1367. doi: 10.1161/strokeaha.114.008447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Im SI, Kim SH, Kim BJ, Cho KI, Kim HS, Heo JH. Association of frequent premature ventricular complex >10% and stroke‐like symptoms without a prior diagnosis of stroke or transient ischemic attack. Int J Cardiol Heart Vasc. 2018;19:58–62. doi: 10.1016/j.ijcha.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S2


