ABSTRACT
Cardiac sarcoidosis can mimic any cardiomyopathy in different stages. Noncaseating granulomatous inflammation can be missed, because of the nonhomogeneous distribution in the heart. The current diagnostic criteria show discrepancies and are partly nonspecific and insensitive. Besides the diagnostic pitfalls, there are controversies in the understanding of the causes, genetic and environmental background, and the natural evolution of the disease. Here, we review the current pathophysiological aspects and gaps that are relevant for future cardiac sarcoidosis diagnostics and research.
Keywords: cardiac inflammatory disease, cardiac sarcoidosis, myocarditis, sarcoidosis diagnostic criteria
Subject Categories: Inflammatory Heart Disease, Arrhythmias, Nuclear Cardiology and PET, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- CS
cardiac sarcoidosis
- PET‐CT
positron‐emission tomography–computed tomography
Cardiac sarcoidosis (CS) is a granulomatous inflammatory disease of the heart that is characterized by a patchy distribution almost invariably involving the septum. 1 Inflammation, fibrosis, and deterioration of the cardiac function are important features of CS. The course and the severity of the disease are variable, and the clinical presentation can resemble almost any cardiomyopathy. 2 Therefore, the key to diagnosis is the awareness and the careful interpretation of symptoms, laboratory and imaging findings that may be indicative of CS. Although confirmation of the diagnosis requires histological evidence of noncaseating granulomas, the sensitivity of endomyocardial biopsy is relatively low because of the patchy and midmyocardial distribution of the noncaseating (ie, nonnecrotizing) inflammation. 3 Recognizing the low sensitivity of the current criterion standard test for CS, different working groups proposed combinations of histological and clinical criteria to determine the probability of CS and to guide the immunosuppressive therapy. 4
The recognition of CS as an important cause of heart failure and arrhythmias fostered extensive experimental and clinical research during the past years. The main cell types, chemokines, and signal pathways that participate in the inflammation and fibrosis have been identified. 5 However, the triggers that start or curb the inflammation, and the mechanisms of healing or transition to a fibrotic stage still are not well understood. 5 Also, the role of environment and how it interacts with the genetic background are a matter of ongoing research. Because of the rising number of scientific publications and the increasing attention to CS, we decided to contemplate a short review that focuses on the current knowledge gaps in CS research (Figure 1).
Figure 1. Current knowledge gaps in cardiac sarcoidosis.
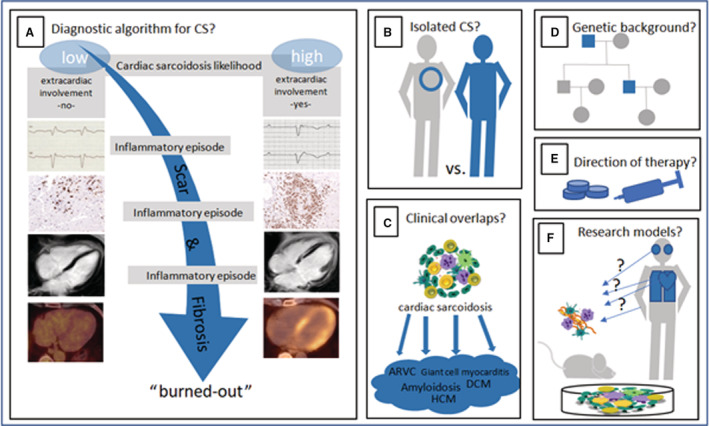
A, Is there a definite diagnostic algorithm for CS? (B) Is there an isolated CS phenotype? (C) Is the cardiac noncaseating granulomatous inflammation a unique feature of cardiac sarcoidosis? (D) Is there a genetic background that predisposes to CS? (E) How can the treatment of CS be directed? (F) Which models are useful to study CS? ARVC indicates arrhythmogenic right ventricular cardiomyopathy; CS, cardiac sarcoidosis; DCM, dilated cardiomyopathy; and HCM, hypertrophic cardiomyopathy.
Is There a Definitive Diagnostic Algorithm for CS?
What Is Known
Several diagnostic algorithms for CS make use of electrocardiographic criteria, different imaging modalities, and the response to immunosuppressive therapy to secure the diagnosis and at the same time recognize the low diagnostic yield of the endomyocardial biopsy 6 , 7 , 8 , 9 (Table 1). These multiple diagnostic tools partly allow diagnosis and treatment of CS in the absence of a positive endomyocardial biopsy. As pointed out in recent publications, the same person may fulfill the criteria to diagnose CS in 1 score but may fail to do so in another. 4 , 10 Classifying patients as having CS can be especially difficult in borderline cases where some criteria are met in 1 score but not fulfilled or nonexistent in another.
Table 1.
Comparison of Present Criteria in Expert Consensus Statements and Guidelines
| JCS 9 | HRS 7 | WASOG 8 | |
|---|---|---|---|
| Histological diagnosis of CS | |||
| EMB with noncaseating granulomas | x | x | X |
| Clinical diagnosis of CS | |||
| Histological diagnosis of extracardiac sarcoidosis mandatory for clinical diagnosis | X | x | |
| Mobitz type II 2nd‐degree heart block or 3rd‐degree heart block | x | X | x |
| Unexplained sustained (spontaneous or induced) VT | x | X | x |
| Patchy uptake on dedicated cardiac PET (pattern consistent with CS) | x | X | x |
| Late gadolinium enhancement on CMR (pattern consistent with CS) | x | X | x |
| Positive gallium uptake (pattern consistent with CS) | x | X | x |
| Steroid ± immunosuppressant‐responsive cardiomyopathy or heart block | X | x | |
| Unexplained reduced LVEF <40% | X | x | |
| Defect on perfusion scintigraphy or SPECT scan | x | x | |
| T2 prolongation on CMR | x | ||
| Reduced LVEF in the presence of other risk factors | x | ||
| Basal thinning of the ventricular septum or abnormal ventricular wall anatomy (ventricular aneurysm, thinning of the middle or upper ventricular septum, regional ventricular wall thickening) | x | ||
| Abnormal ECG findings (ventricular arrhythmias [nonsustained VT, multifocal or frequent PVCs], bundle branch block, axis deviation, abnormal Q wave) | x | ||
| Atrial dysrhythmias | x | ||
| EMB with monocyte infiltration, moderate or severe myocardial interstitial fibrosis | x | ||
CMR indicates cardiovascular magnetic resonance; CS, cardiac sarcoidosis; EMB, endomyocardial biopsy; HRS, Heart Rhythm Society; JCS, Japanese Circulation Society; LVEF, left ventricular ejection fraction; PET, positron‐emission tomography; PVCs, premature ventricular contractions; SPECT, single‐photon emission computed tomography; VT, ventricular tachycardia; and WASOG, World Association of Sarcoidosis and Other Granulomatous Diseases.
There are no specific diagnostic findings for CS, but some occur frequently:
ECG: signs of conduction disturbance (higher‐degree atrioventricular block), premature ventricular contractions, or ventricular tachycardia. 10 , 11
Echocardiography: reduced regional strain values, segmental postsystolic contraction, hypertrophy, septal thinning, and delayed time to peak strain. 12
Cardiovascular magnetic resonance imaging: multifocal late‐gadolinium enhancement with involvement of the interventricular septum, left lateral free wall, and right ventricle. 1 , 13
Positron‐emission tomography (F18‐fluorodeoxyglucose‐PET‐computed tomography [CT]): focal or focal on diffuse F18‐fluorodeoxyglucose uptake, if possible in combination with myocardial perfusion imaging. 13 , 14
All these findings reflect the severity and the recurring nature of the inflammation in CS, which explain the fluctuations (clinically active or silent disease) and the plethora of symptoms and imaging findings in the same patient over the course of disease (Figure 2). In addition, the limitations and suggestions for improvement of the aforementioned diagnostics need to be taken into account (eg, influence of different fast protocols from prior F18‐fluorodeoxyglucose‐PET‐CT studies that lead to different myocardial background uptake suppression 15 and electroanatomical mapping‐guided biopsy to enhance diagnostic yield). 16
Figure 2. Examples of diagnostic findings in patients with CS.
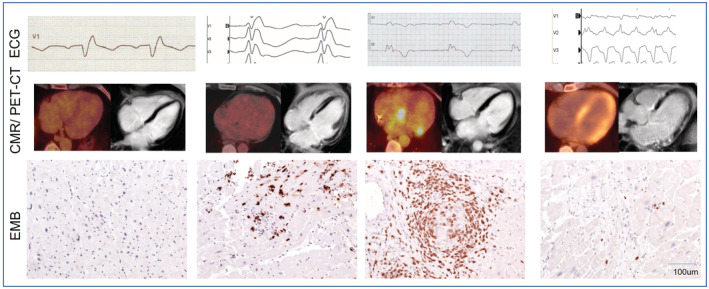
Fulfillment of current diagnostic criteria may vary because of different points in time of presentation (subclinical, active, quiescent, or burned‐out disease). All of the following findings are associated with patients with cardiac sarcoidosis and positive endomyocardial biopsy. Upper row (left to right): incomplete right bundle branch block, right bundle branch block, atrioventricular block III, and ventricular tachycardia. Middle row (left to right): no FDG uptake in PET‐CT and no late gadolinium enhancement in CMR, no FDG uptake in PET‐CT and late gadolinium enhancement in CMR, FDG uptake in PET‐CT and no late gadolinium enhancement in CMR, and FDG uptake in PET‐CT and late gadolinium enhancement in CMR. Bottom row (left to right): normal finding (sampling error), CD3+ T lymphocytes in lymphocytic myocarditis, CD3+ lymphocytes in noncaseating granuloma, and isolated CD3+ T lymphocytes and fibrosis; all images ×200. CMR indicates cardiovascular magnetic resonance; CS, cardiac sarcoidosis; EMB, endomyocardial biopsy; FDG, F18‐fluorodeoxyglucose; and PET‐CT, positron‐emission tomography‐computed tomography.
Awareness of CS is especially important in patients presenting with sudden cardiac death. Current data point to a 10.3% incidence of fatal or aborted sudden cardiac death and 24.6% with sudden cardiac death or sustained ventricular tachycardia as the first event in a cohort of patients with definite or probable CS in 5 years. 17
What Is Unknown
Since the endomyocardial biopsy remains frequently negative, the diagnosis relies on clinical criteria only in a significant number of cases. Data about outcome of patients meeting only the clinical diagnostic criteria, usually labeled as “suspected CS,” in comparison to histologically confirmed diagnosis, are scarce, 1 and whether both groups have a comparable natural evolution without immunosuppressive therapy is currently unknown. 18 , 19
Does Isolated CS Exist?
What Is Known
Myocardial granulomatous inflammation without an extracardiac involvement, the so‐called “isolated CS” phenotype, has been a subject of discussion for over 50 years, 20 , 21 contradicting the observations of sarcoidosis as a systemic inflammatory condition and suggesting a high rate of unrecognized or subclinical extracardiac foci. Possible explanations for a clinically isolated cardiac manifestation have been discussed by Birnie and colleagues, 21 , 22 who assumed the following:
Extracardiac lesions are, although present, not detected by CT or PET‐CT. 23
Extracardiac lesions form at a later stage of disease. 24
Disease progress leads to fibrosis without detectable inflammation. 25
The diagnosis of CS is wrong. 26
Although Kupari and colleagues argue that invisible extracardiac granulomas are clinically irrelevant, 27 it is important from a pathophysiological point of view to understand whether or not a truly isolated cardiac phenotype exists. If we assume the existence of isolated CS, we should stipulate the presence of a tissue‐specific myocardial predisposition as well as the nature of CS as a “second hit” disease in analogy to other acquired cardiomyopathies, such as alcoholic‐induced dilated cardiomyopathy 28 or acquired long QT syndrome 29 showing classical mutations in genes encoding different myocardial proteins. On the other hand, there are observations supporting the theory of misclassification, such as the report by Wiltshire and colleagues that showed recurrent isolated CS in a transplanted heart, 30 an unlikely finding in an isolated cardiac condition.
What Is Unknown
The number of reports about isolated CS is growing, despite the progress in multimodality imaging. 2 , 31 , 32 Moreover, there are multiple reports about isolated extracardiac sarcoidosis (eg, the spleen 33 or isolated hepatic sarcoidosis). 34 Whether all these represent misclassified cases with clinically nondetectable systemic disease is not clear to date. Findings of noncaseating granulomas in autopsies of people who died of other cardiac conditions question the role of unrecognized systemic inflammation in the cardiac pathophysiology. 35
Is the Cardiac Noncaseating Granulomatous Inflammation a Unique Feature of Cardiac Sarcoidosis?
What Is Known
Diagnosis of CS in histopathology is currently defined by “myocarditis with non‐necrotizing granulomas with macrophages and multinucleated giant cells, surrounded by fibrosis and a lymphocytic infiltrate.” 36 Although not completely understood, the initiation of the inflammatory process appears to be a combination of genetic predisposition and environmental triggers. The current understanding of pathophysiologic pathway of cardiac granuloma formation is depicted in Figure 3 (according to Drent et al 5 and Kato et al 37 ).
Figure 3. General proposed concept of granuloma formation in sarcoidosis.
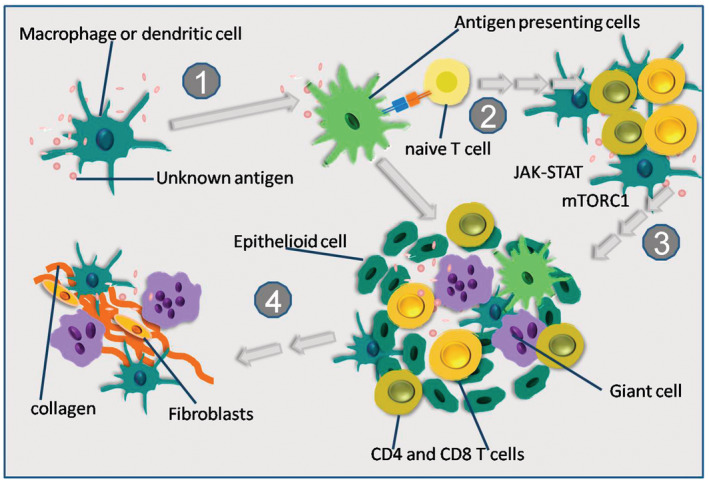
(1) Presentation of an unknown antigen by dendritic cells and macrophages to lymphocytes. (2) Recruitment of naive T cells and conversion to T helper 1 cells is followed by lymphocytic infiltration, accumulation of dendritic cells and macrophages caused by cytokines (ie, interleukin (IL‐2) and interferon‐γ. (3) M1 macrophages turn into epithelioid cells, epithelioid cells fuse to form multinucleated giant cells, granuloma formation including activated M1 macrophages, multinucleated giant cells, CD4+ helper T cells, and CD8+ cytotoxic T cells. (4) Transition from Th1 to Th2 predominance, induction of M2 macrophages, M2 macrophages potentially produce cytokine signals that stimulate fibrosis.
The clinical presentation of CS can mimic various cardiomyopathies such as amyloidosis, 38 hypertrophic cardiomyopathy, 2 or arrhythmogenic right ventricular cardiomyopathy. 39 , 40 However, the histological alterations in sarcoidosis show similarities to giant cell myocarditis. 41 The presence of cardiac noncaseating granulomas has been described as incidental findings and in patients with evidence for other cardiomyopathies (Table 2). 42 , 43 Thus, it is likely that the noncaseating granulomatous inflammation is the main pathophysiological pathway initiated in patients with sarcoidosis but is not unique for sarcoidosis.
Table 2.
There Are Several Cardiac Diseases With Reported Noncaseating Granulomas. The Distinct Histopathological Features Are Depicted Along With References
| Disease or clinical status | Histopathologic features | Refs. |
|---|---|---|
| ARVC | Cardiac fibrofatty infiltration and concomitant cardiac or extracardiac noncaseating granulomata | 35 |
| AL amyloidosis | Cardiac deposition of Ig lambda II light chain and noncaseating granulomas | 42 |
| Giant cell myocarditis | Mixed inflammatory infiltrate with histiocytes, histiocyte giant cells, and lymphocytes, but in second biopsy well‐formed, circumscribed, nonnecrotizing granulomas | 41 |
| Dilated cardiomyopathy, possible “end‐stage” cardiac sarcoidosis | Dilated LV cavity, former presence of noncaseating granulomas, no granulomas at explant of native heart but extensive scarring | 25 |
| Incidental finding | Nonnecrotizing granulomatous inflammation in excised valve tissue, no systemic disease identified | 43 |
AL amyloidosis indicates amyloid light chain or primary amyloidosis; ARVC, arrhythmogenic right ventricular cardiomyopathy; and LV, left ventricular.
What Is Unknown
To date, the histopathological definition of CS is usually based on histology, immunohistology, and molecular viral analysis with numerous protocols for immunostaining and classification of immune cell subtypes similarly to other types of myocarditis. 44 The interaction of many different subtypes of immune cells that interact over time to form the granuloma poses difficulties for understanding the pathogenesis of a noncaseating granulomatous inflammation. In the future, the application of microRNA profiling, 45 transcriptome‐based biomarker analysis, single‐cell‐sequencing, and analysis of anti‐heart and anti‐intercalated disc autoantibodies 46 may be helpful to define subtypes of noncaseating granulomatous diseases.
Is There a Genetic Background that Predisposes to CS?
What Is Known
The hypothesis for a genetic susceptibility to sarcoidosis is supported by studies on homozygotic twins, higher prevalence in certain ethnic groups, and familial clustering. 47 , 48 In a large national cohort, the risk of developing the disease was up to 4‐fold higher in first‐degree relatives of patients with sarcoidosis. 49 Like many multifactorial diseases, predisposition to CS most probably has a complex genetic architecture. Associations with loci of the human leukocyte antigen gene, with annexin A11, NOTCH4, and the X‐linked inhibitor of apoptosis‐associated factor 1 gene have been described for sarcoidosis. 50 A homozygous point mutation in exon 5 of the butyrophilin‐like 2 gene has been found in a patient with isolated CS, 51 and a small cohort study identified the association of the −857C/T and the −308G/A tumor necrosis factor‐α polymorphisms with cardiac involvement of patients with sarcoidosis. 52 These gene loci are implicated in pathways of apoptosis and T‐cell activation. Of note, the aforementioned studies showed some interesting associations but no causal relationship.
What Is Unknown
Although several genetic variations have been associated with CS in the past, it is unlikely that a single gene defect can cause predisposition to cardiac granulomatous inflammation. Nevertheless, there are data demonstrating significant inflammatory response in certain monogenic cardiomyopathies. Recently, Smith and colleagues observed an abnormal F18‐fluorodeoxyglucose ‐PET activity in patients with an arrhythmogenic right ventricular cardiomyopathy phenotype caused by a pathogenic desmoplakin mutation, suggesting some overlap between the monogenic cardiomyopathies and myocarditis. 26 Unfortunately, in many cases of primary cardiomyopathy, the diagnosis relies on a combination of clinical traits and a positive family history, without making use of the possibilities of genetic testing. The same applies for patients with CS in whom systematic testing for allelic variants in genes encoding the proteins of sarcomere, Z‐disc, cell‐to‐cell adhesion, or calcium handling are missing. 53 This poses the question of how some of these known allelic variants can influence the prognosis of the disease and whether they can predispose to a specific phenotypic expression of CS.
How Can the Treatment of CS Be Directed?
What Is Known
The current therapeutic options for CS include immunosuppressive agents, heart failure medication, cardiac electronic implantable devices, and ablation. 54 , 55 Not only is the treatment goal to stop active inflammation but also to prevent recurrence of inflammatory episodes and to reduce myocardial damage and prevent complications.
Experts advocate starting immunosuppressive therapy if signs of cardiac involvement are present. 6 , 54 These include arrhythmias, conduction disturbances, and heart failure symptoms, although precise criteria are lacking. Current retrospective studies and case series recommend steroid‐sparing regimens with the use of methotrexate, azathioprine, mycophenolate mofetil, leflunomide, cyclophosphamide, infliximab, and adalimumab. 54 , 56 Initiation of immunosuppressive treatment calls for predefined criteria for therapeutic response. Currently, no biomarker, single imaging technique, or clinical parameter has been validated to direct the long‐term treatment strategy. However, if initially abnormal, the following PET‐CT‐based follow‐up regimen has been suggested by Birnie and colleagues 57 :
PET‐CT baseline and after 3 months of treatment
If no abnormal uptake: PET‐CT 3 months after stopping treatment
If no relapse: evaluation at 6, 12, 24, 36 months (clinical, ECG, and echocardiogram)
If relapse occurs: intensification of treatment/more frequently PET‐CT
What Is Unknown
Although the use of immunosuppressive drugs is reasonable in the context of the inflammatory character of CS, one of the most important downsides is the lack of prospective, placebo‐controlled data showing better outcomes after treatment with immunosuppressives. 6 , 54 , 56 Severe cardiac manifestations may represent an end‐stage fibrotic remodeling that is not reversible with immunosuppressive drugs. In addition, the overall clinical benefit of immunosuppressive therapy in patients without life‐threatening symptoms (eg, premature ventricular contractions) is even more arguable. 5 Current recommendations on the induction corticosteroid dose and the use of steroid‐sparing agents are not uniform, although high‐dose corticosteroids are generally not recommended. 6 , 54 , 56 It is also unknown which tool is the most appropriate one for assessment of the therapeutic response. Different strategies based on the clinical evolution and advanced imaging techniques have been proposed. Finally, the duration of immunosuppressive therapy and whether it should be discontinued after achieving a remission or be continued indefinitely is also a matter of future research. 56
Which Models Are Useful to Study CS?
Animal and in‐vitro models can be useful to fill the knowledge gaps in the complex pathophysiology of CS and to identify possible targets for new treatments or diagnostic biomarkers. However, development of animal models in sarcoidosis is in an early stage and the in‐vivo models do not replicate all components of the histological features.
What Is Known
In animals, spontaneous sarcoidosis‐like granuloma formation has been described in horses, 58 dogs, 59 and brown Norwegian rats. 60 Artificial animal models have been created using bacteria or bacteria‐related agents, microparticles, or gene‐knockout models but without sufficient validation against human noncaseating granulomas. 61
As discussed by Locke and colleagues, the problem with unstimulated peripheral blood mononuclear cell–based models of patients with sarcoidosis is that unstimulated immune cells do not entirely replicate granuloma formation. 61 On the other hand, diseased tissue does not allow understanding of the early disease mechanisms but reflects the later stages of the disease. 61
The Kveim‐Siltzbach test, formerly labeled as “enigmatic”, 62 has been used for several decades in the diagnosis of sarcoidosis, but was abandoned because of lack of standardization and, more importantly, lack of pathophysiologic understanding. Initially described by Kveim in 1941, the test was performed using a sarcoid spleen‐ or lymph node–derived reagent that was inoculated into the skin of patients with suspected sarcoidosis, leading to granulomatous cutaneous response in ≈4 weeks. The sensitivity of the test was described as high as 80%. Despite extensive research, the culprit antigen, cell subtype, or cytokine responsible for Kveim reaction has not been identified and the test was abandoned when the Lancet published the Moratorium on the Kveim test because of fear of Creutzfeld‐Jacob disease or sarcoidosis transmission. 63 Although the Kveim test no longer plays a role as a diagnostic test, its effectiveness to induce sarcoid granulomas in humans makes it suitable as a research tool. 64 , 65
What Is Unknown
Because current pathophysiological models do not entirely reproduce the clinical human disease, it might be the right moment to step back and re‐evaluate some pitfalls of the previous research. Although the general principles of granuloma formation were studied in the past, the specific mechanisms of granuloma formation in different organs are not clear. In addition, most of the prior models focused on noncaseating granulomas in the lungs. Systematic studies comparing cell composition of granulomas in different organs of the same patient are lacking to date. Of note, the role of resident and recruited macrophages in the heart in homeostasis and under disease conditions, and immune cell heterogeneity in general, are still the subject of ongoing research. 66
In summary, most likely only a complex approach taking into account the genetic background, environmental factors, tissue‐specific immune responses exhibiting different intensity, and recruitment of different cell types will be successful to model this complex disease. Prospective registry data with deep phenotyping of patients will be important to complement the mechanistic studies.
Sources of Funding
None.
Disclosures
Gerhard Hindricks receives grants through the Leipzig Heart Institute from Boston Scientific (Boston Scientific Corporation, Marlborough, Massachusetts, USA) and Abbott/St. Jude Medical (Abbott Laboratories, Chicago, Illinois, USA). There are no personal payments to declare. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 7.
REFERENCES
- 1. Nabeta T, Kitai T, Naruse Y, Taniguchi T, Yoshioka K, Tanaka H, Okumura T, Sato S, Baba Y, Kida K, et al. Risk stratification of patients with cardiac sarcoidosis: the ILLUMINATE‐CS registry. Eur Heart J. 2022;43:3450–3459. doi: 10.1093/eurheartj/ehac323 [DOI] [PubMed] [Google Scholar]
- 2. Ueberham L, Paetsch I, Jahnke C, Klingel K, Dinov B. Right ventricular thickening and extensive late gadolinium enhancement in a patient with rare case of isolated cardiac sarcoidosis and initially negative biopsy. Eur Heart J Cardiovasc Imaging. 2017;18:1427–1428. doi: 10.1093/ehjci/jex226 [DOI] [PubMed] [Google Scholar]
- 3. Liang JJ, Hebl VB, DeSimone CV, Madhavan M, Nanda S, Kapa S, Maleszewski JJ, Edwards WD, Reeder G, Cooper LT, et al. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. JACC Heart Failure. 2014;2:466–473. doi: 10.1016/j.jchf.2014.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ueberham L, Jahnke C, Paetsch I, Klingel K, Kuehl M, Hindricks G, Dinov B. Current diagnostic criteria show a substantial disagreement in classification of patients with suspected cardiac sarcoidosis. JACC Clinical Electrophysiology. 2021;7:538–539. doi: 10.1016/j.jacep.2020.12.011 [DOI] [PubMed] [Google Scholar]
- 5. Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med. 2021;385:1018–1032. doi: 10.1056/NEJMra2101555 [DOI] [PubMed] [Google Scholar]
- 6. Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, Inomata T, Ishibashi‐Ueda H, Eishi Y, Kitakaze M, et al. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis–digest version. Circ J. 2019;83:2329–2388. doi: 10.1253/circj.CJ-19-0508 [DOI] [PubMed] [Google Scholar]
- 7. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 8. Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, Shigemitsu H, Culver DA, Gelfand J, Valeyre D, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 9. Terasaki F, Yoshinaga K. New guidelines for diagnosis of cardiac sarcoidosis in Japan. Ann Nucl Cardiol. 2017;3:42–45. doi: 10.17996/anc.17-00042 [DOI] [Google Scholar]
- 10. Ribeiro Neto ML, Jellis C, Hachamovitch R, Wimer A, Highland KB, Sahoo D, Khabbaza JE, Pande A, Bindra A, Southern BD, et al. Performance of diagnostic criteria in patients clinically judged to have cardiac sarcoidosis: is it time to regroup? Am Heart J. 2020;223:106–109. doi: 10.1016/j.ahj.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 11. Medor MC, Spence S, Nery PB, Beanlands R, Promislow S, Juneau D, de Kemp R, Ha AC, Rivard L, Gula L, et al. Treatment with corticosteroids is associated with an increase in ventricular arrhythmia burden in patients with clinically manifest cardiac sarcoidosis: insights from implantable cardioverter‐defibrillator diagnostics. J Cardiovasc Electrophysiol. 2020;31:2751–2758. doi: 10.1111/jce.14689 [DOI] [PubMed] [Google Scholar]
- 12. Polito MV, Stoebe S, Leifels L, Stumpp P, Solty K, Galasso G, Piscione F, Laufs U, Klingel K, Hagendorff A. Cardiac sarcoidosis: a challenging diagnosis. Clin Res Cardiol. 2018;107:980–986. doi: 10.1007/s00392-018-1265-8 [DOI] [PubMed] [Google Scholar]
- 13. Vita T, Okada DR, Veillet‐Chowdhury M, Bravo PE, Mullins E, Hulten E, Agrawal M, Madan R, Taqueti VR, Steigner M, et al. Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. 2018;11:e007030. doi: 10.1161/CIRCIMAGING.117.007030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, Birnie DH, Chen ES, Cooper LT, Tung RH, et al. Joint SNMMI‐ASNC expert consensus document on the role of (18)F‐FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med. 2017;58:1341–1353. doi: 10.2967/jnumed.117.196287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ozutemiz C, Koksel Y, Froelich JW, Rubin N, Bhargava M, Roukuz H, Cogswell R, Markowitz J, Perlman DM, Steinberger D. Comparison of the effect of three different dietary modifications on myocardial suppression in (18)F‐FDG PET/CT evaluation of patients for suspected cardiac sarcoidosis. J Nucl Med. 2021;62:1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Casella M, Dello Russo A, Bergonti M, Catto V, Conte E, Sommariva E, Gasperetti A, Vettor G, Tundo F, Sicuso R, et al. Diagnostic yield of electroanatomic voltage mapping in guiding endomyocardial biopsies. Circulation. 2020;142:1249–1260. doi: 10.1161/CIRCULATIONAHA.120.046900 [DOI] [PubMed] [Google Scholar]
- 17. Nordenswan HK, Poyhonen P, Lehtonen J, Ekstrom K, Uusitalo V, Niemela M, Vihinen T, Kaikkonen K, Haataja P, Kerola T, et al. Incidence of sudden cardiac death and life‐threatening arrhythmias in clinically manifest cardiac sarcoidosis with and without current indications for an implantable cardioverter defibrillator. Circulation. 2022;146:964–975. doi: 10.1161/CIRCULATIONAHA.121.058120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takaya Y, Nakamura K, Nishii N, Ito H. Clinical outcomes of patients with isolated cardiac sarcoidosis confirmed by clinical diagnostic criteria. Int J Cardiol. 2021;345:49–53. doi: 10.1016/j.ijcard.2021.10.150 [DOI] [PubMed] [Google Scholar]
- 19. Bobbio E, Hjalmarsson C, Bjorkenstam M, Polte CL, Oldfors A, Lindstrom U, Dahlberg P, Bartfay SE, Szamlewski P, Taha A, et al. Diagnosis, management, and outcome of cardiac sarcoidosis and giant cell myocarditis: a Swedish single center experience. BMC Cardiovasc Disord. 2022;22:192. doi: 10.1186/s12872-022-02639-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johansen A. Isolated myocarditis versus myocardial sarcoidosis. A contribution to the discussion regarding points of resemblance between these and a report of three illustrative cases. Acta Pathol Microbiol Scand. 1966;67:15–26. doi: 10.1111/apm.1966.67.1.15 [DOI] [PubMed] [Google Scholar]
- 21. Birnie DH, Nery PB, Beanlands RS. Counterpoint: should isolated cardiac sarcoidosis be considered a significant manifestation of sarcoidosis? No. Chest. 2021;160:38–42. doi: 10.1016/j.chest.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 22. Birnie DH, Nery PB. Debating the definition and incidence of isolated cardiac sarcoidosis. JACC Clin Electrophysiol. 2020;6:1190–1191. doi: 10.1016/j.jacep.2020.07.015 [DOI] [PubMed] [Google Scholar]
- 23. Petek BJ, Rosenthal DG, Patton KK, Behnia S, Keller JM, Collins BF, Cheng RK, Ho LA, Bravo PE, Mikacenic C, et al. Cardiac sarcoidosis: diagnosis confirmation by bronchoalveolar lavage and lung biopsy. Respir Med. 2018;144 S:S13–S19. [DOI] [PubMed] [Google Scholar]
- 24. Adamson P, Melton I, O'Donnell J, MacDonald S, Crozier I. Cardiac sarcoidosis: the Christchurch experience. Intern Med J. 2014;44:70–76. doi: 10.1111/imj.12314 [DOI] [PubMed] [Google Scholar]
- 25. Roberts WC, Roberts CC, Ko JM, Filardo G, Capehart JE, Hall SA. Morphologic features of the recipient heart in patients having cardiac transplantation and analysis of the congruence or incongruence between the clinical and morphologic diagnoses. Medicine. 2014;93:211–235. doi: 10.1097/MD.0000000000000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC, Agarwal PP, Arscott P, Dellefave‐Castillo LM, Vorovich EE, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kupari M, Lehtonen J. Rebuttal from Drs Kupari and Lehtonen. Chest. 2021;160:42–43. doi: 10.1016/j.chest.2020.12.039 [DOI] [PubMed] [Google Scholar]
- 28. Ware JS, Amor‐Salamanca A, Tayal U, Govind R, Serrano I, Salazar‐Mendiguchia J, Garcia‐Pinilla JM, Pascual‐Figal DA, Nunez J, Guzzo‐Merello G, et al. Genetic etiology for alcohol‐induced cardiac toxicity. J Am Coll Cardiol. 2018;71:2293–2302. doi: 10.1016/j.jacc.2018.03.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Itoh H, Crotti L, Aiba T, Spazzolini C, Denjoy I, Fressart V, Hayashi K, Nakajima T, Ohno S, Makiyama T, et al. The genetics underlying acquired long QT syndrome: impact for genetic screening. Eur Heart J. 2016;37:1456–1464. doi: 10.1093/eurheartj/ehv695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wiltshire S, Nadel J, Meredith T, Iglesias CK, Qiu MR, Macdonald P, Jabbour A. Twice bitten, thrice shy: a case of recurrent isolated cardiac sarcoidosis in the transplanted heart. JACC Case reports. 2021;3:427–432. doi: 10.1016/j.jaccas.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mathias IS, Oliveira Lima Filho JO, Culver DA, Rodriguez ER, Tan CD, Ribeiro Neto ML, Jellis CL. Case report of isolated cardiac sarcoidosis presenting as hypertrophic obstructive cardiomyopathy‐a clinical picture printed on lenticular paper. Eur Heart J Case Rep. 2021;5:ytab208. doi: 10.1093/ehjcr/ytab208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawai H, Sarai M, Kato Y, Naruse H, Watanabe A, Matsuyama T, Takahashi H, Motoyama S, Ishii J, Morimoto SI, et al. Diagnosis of isolated cardiac sarcoidosis based on new guidelines. ESC Heart Failure. 2020;7:2662–2671. doi: 10.1002/ehf2.12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zubair Ullah HM, Surya A, Morley N, Ahmad S. Isolated splenic sarcoidosis: a rare cause of hypercalcaemia in a patient with type 1 diabetes. BMJ Case Rep. 2021;14:e245987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arnfield E, Pattison DA. Identification of isolated hepatic sarcoidosis with 18F‐FDG PET/CT and MRI. Clin Nucl Med. 2021;46:e448–e450. [DOI] [PubMed] [Google Scholar]
- 35. Mussigbrodt A, Knopp H, Czimbalmos C, Jahnke C, Richter S, Husser D, Gradistanac T, Hindricks G. Exercise‐related sudden cardiac death of an American football player with arrhythmogenic right ventricular dysplasia/cardiomyopathy and sarcoidosis. Clin Case Rep. 2019;7:686–688. doi: 10.1002/ccr3.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seferovic PM, Tsutsui H, McNamara DM, Ristic AD, Basso C, Bozkurt B, Cooper LT, Filippatos G, Ide T, Inomata T, et al. Heart Failure Association, Heart Failure Society of America, and Japanese Heart Failure Society position statement on endomyocardial biopsy. J Card Fail. 2021;27:727–743. doi: 10.1016/j.cardfail.2021.04.010 [DOI] [PubMed] [Google Scholar]
- 37. Kato S, Sakai Y, Okabe A, Kawashima Y, Kuwahara K, Shiogama K, Abe M, Ito H, Morimoto S. Histology of cardiac sarcoidosis with novel considerations arranged upon a pathologic basis. J Clin Med. 2022;11:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takemura K, Nakamura R, Shimazu K, Sugimoto Y, Takase T, Ryogo M, Hiroe M. A case of cardiac sarcoidosis mimicking cardiac amyloidosis on cardiovascular magnetic resonance. ESC Heart Fail. 2018;5:306–310. doi: 10.1002/ehf2.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Omotoye S, Junpaparp P, McHugh J, Silva J, Kuk R, Sackett M, Tandri H. Cardiac sarcoidosis with prominent epsilon waves: a perfect phenocopy of ARVC. JACC Case Rep. 2021;3:1097–1102. doi: 10.1016/j.jaccas.2021.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asatryan B, Asimaki A, Landstrom AP, Khanji MY, Odening KE, Cooper LT, Marchlinski FE, Gelzer AR, Semsarian C, Reichlin T, et al. Inflammation and immune response in arrhythmogenic cardiomyopathy: state‐of‐the‐art review. Circulation. 2021;144:1646–1655. doi: 10.1161/CIRCULATIONAHA.121.055890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang GY, Cai Q, Grandin EW, Sabe MA. Fulminant cardiac sarcoidosis resembling giant cell myocarditis: a case report. Eur Heart J Case Rep. 2021;5:ytab042. doi: 10.1093/ehjcr/ytab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Treaba DO, Benson MD, Assad LW, Dainauskas JR. Sarcoidosis and immunoglobulin lambda II light‐chain amyloidosis diagnosed after orthotopic heart transplantation: a case report and review of the literature. Mod Pathol. 2005;18:451–455. doi: 10.1038/modpathol.3800277 [DOI] [PubMed] [Google Scholar]
- 43. Pichler Sekulic S, Sekulic M. Case report: isolated and focal non‐necrotizing granulomatous inflammation of mitral valves: a report of two cases. Front Cardiovasc Med. 2021;8:615707. doi: 10.3389/fcvm.2021.615707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tschope C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hubner N, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pattnaik B, Sryma PB, Mittal S, Agrawal A, Guleria R, Madan K. MicroRNAs in pulmonary sarcoidosis: a systematic review. Respir Investig. 2020;58:232–238. doi: 10.1016/j.resinv.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 46. Caforio ALP. Myocarditis: endomyocardial biopsy and circulating anti‐heart autoantibodies are key to diagnosis and personalized etiology‐directed treatment. Eur Heart J. 2021;42:1618–1620. doi: 10.1093/eurheartj/ehab024 [DOI] [PubMed] [Google Scholar]
- 47. Te HS, Perlman DM, Shenoy C, Steinberger DJ, Cogswell RJ, Roukoz H, Peterson EJ, Zhang L, Allen TL, Bhargava M. Clinical characteristics and organ system involvement in sarcoidosis: comparison of the University of Minnesota Cohort with other cohorts. BMC Pulm Med. 2020;20:155. doi: 10.1186/s12890-020-01191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sverrild A, Backer V, Kyvik KO, Kaprio J, Milman N, Svendsen CB, Thomsen SF. Heredity in sarcoidosis: a registry‐based twin study. Thorax. 2008;63:894–896. doi: 10.1136/thx.2007.094060 [DOI] [PubMed] [Google Scholar]
- 49. Rossides M, Grunewald J, Eklund A, Kullberg S, Di Giuseppe D, Askling J, Arkema EV. Familial aggregation and heritability of sarcoidosis: a Swedish nested case‐control study. Eur Respir J. 2018;52:1800385. [DOI] [PubMed] [Google Scholar]
- 50. Moller DR, Rybicki BA, Hamzeh NY, Montgomery CG, Chen ES, Drake W, Fontenot AP. Genetic, immunologic, and environmental basis of sarcoidosis. Ann Am Thorac Soc. 2017;14:S429–S436. doi: 10.1513/AnnalsATS.201707-565OT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meyer T, Lauschke J, Ruppert V, Richter A, Pankuweit S, Maisch B. Isolated cardiac sarcoidosis associated with the expression of a splice variant coding for a truncated BTNL2 protein. Cardiology. 2008;109:117–121. doi: 10.1159/000105552 [DOI] [PubMed] [Google Scholar]
- 52. Gialafos E, Triposkiadis F, Kouranos V, Rapti A, Kosmas I, Manali E, Giamouzis G, Elezoglou A, Peros I, Anagnostopoulou O, et al. Relationship between tumor necrosis factor‐α (TNFA) gene polymorphisms and cardiac sarcoidosis. In Vivo. 2014;28:1125–1129. [PubMed] [Google Scholar]
- 53. Gasperetti A, Rossi VA, Chiodini A, Casella M, Costa S, Akdis D, Buchel R, Deliniere A, Pruvot E, Gruner C, et al. Differentiating hereditary arrhythmogenic right ventricular cardiomyopathy from cardiac sarcoidosis fulfilling 2010 ARVC Task Force Criteria. Heart Rhythm. 2021;18:231–238. doi: 10.1016/j.hrthm.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 54. Baughman RP, Valeyre D, Korsten P, Mathioudakis AG, Wuyts WA, Wells A, Rottoli P, Nunes H, Lower EE, Judson MA, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58:2004079. [DOI] [PubMed] [Google Scholar]
- 55. Kouranos V, Sharma R. Cardiac sarcoidosis: state‐of‐the‐art review. Heart. 2021;107:1591–1599. doi: 10.1136/heartjnl-2019-316442 [DOI] [PubMed] [Google Scholar]
- 56. Obi ON, Lower EE, Baughman RP. Controversies in the treatment of cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2022;39:e2022015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605 [DOI] [PubMed] [Google Scholar]
- 58. Sloet van Oldruitenborgh‐Oosterbaan MM, Grinwis GC. Equine sarcoidosis: clinical signs, diagnosis, treatment and outcome of 22 cases. Vet Dermatol. 2013;24(218–24):e48. [DOI] [PubMed] [Google Scholar]
- 59. Hultman J, Rosati M, Gron TK, Matiasek K, Trangerud C, Jaderlund KH. Granulomatous interstitial polymyositis and intramuscular neuritis in a dog. Acta Vet Scand. 2021;63:14. doi: 10.1186/s13028-021-00579-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Noritake S, Ogawa K, Suzuki G, Ozawa K, Ikeda T. Pulmonary inflammation in brown Norway rats: possible association of environmental particles in the animal room environment. Exp Anim. 2007;56:319–327. doi: 10.1538/expanim.56.319 [DOI] [PubMed] [Google Scholar]
- 61. Locke LW, Schlesinger LS, Crouser ED. Current sarcoidosis models and the importance of focusing on the granuloma. Front Immunol. 2020;11:1719. doi: 10.3389/fimmu.2020.01719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reich JM. Eight fundamental unsolved problems in sarcoidosis. Eur J Intern Med. 2004;15:269–273. doi: 10.1016/j.ejim.2004.04.013 [DOI] [PubMed] [Google Scholar]
- 63. Wigley RD. Moratorium on Kveim tests. Lancet. 1993;341:1284. doi: 10.1016/0140-6736(93)91190-W [DOI] [PubMed] [Google Scholar]
- 64. Tercelj M, Salobir B, Rylander R. Beta‐glucan in the lymph nodes in sarcoidosis and in Kveim‐Siltzbach test reagent. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gaede KI, Kataria YP, Mamat U, Muller‐Quernheim J. Analysis of differentially regulated mRNAs in monocytic cells induced by in vitro stimulation with Kveim‐Siltzbach test reagent. Exp Lung Res. 2004;30:181–192. doi: 10.1080/01902140490276267 [DOI] [PubMed] [Google Scholar]
- 66. Corker A, Neff LS, Broughton P, Bradshaw AD, KY DL‐P. Organized chaos: deciphering immune cell heterogeneity's role in iInflammation in the heart. Biomolecules. 2021;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]


