Abstract
Background
Fragmented QRS (fQRS) morphology as a surrogate marker of the possible presence of myocardial scarring has been shown to confer a higher risk in patients with reduced ejection fraction heart failure. We aimed to investigate the pathophysiological correlates and prognostic implications of fQRS in patients with heart failure with preserved ejection fraction (HFpEF).
Methods and Results
We consecutively studied 960 patients with HFpEF (76.4±12.7 years, men: 37.2%). fQRS was assessed using a body surface ECG during hospitalization. QRS morphology was available and classified into 3 categories among 960 subjects with HFpEF as non‐fQRS, inferior fQRS, and anterior/lateral fQRS groups. Despite comparable clinical features in most baseline demographics among the 3 fQRS categories, anterior/lateral fQRS showed significantly higher B‐type natriuretic peptide/troponin levels (both P<0.001), with both the inferior and anterior/lateral fQRS HFpEF groups demonstrating a higher degree of unfavorable cardiac remodeling, greater extent of myocardial perfusion defect, and slower coronary flow phenomenon (all P<0.05). Patients with anterior/lateral fQRS HFpEF exhibited significantly altered cardiac structure/function and more impaired diastolic indices (all P<0.05). During a median of 657 days follow‐up, the presence of anterior/lateral fQRS conferred a doubled HF re‐admission risk (adjusted hazard ratio 1.90, P<0.001), with both inferior and anterior/lateral fQRS having a higher risk of cardiovascular and all‐cause death (all P<0.05) by using Cox regression models.
Conclusions
The presence of fQRS in HFpEF was associated with more extensive myocardial perfusion defects and worsened mechanics, which possibly denotes a more severe involvement of cardiac damage. Early recognition in such patients with HFpEF likely benefits from targeted therapeutic interventions.
Keywords: fragmented QRS complex, heart failure with preserved ejection fraction, mortality, QRS duration
Subject Categories: Heart Failure, Electrocardiology (ECG), Clinical Studies
Nonstandard Abbreviations and Acronyms
- fQRS
fragmented QRS
- HFpEF
heart failure with preserved ejection fraction
- HHF
hospitalization for heart failure
Clinical Perspective.
What Is New?
The presence of fragmented QRS in heart failure with preserved ejection fraction, irrespective of location, was associated with a higher degree of unfavorable cardiac remodeling, greater extent of myocardial perfusion defect, and slower coronary flow phenomenon.
Patients with heart failure with preserved ejection fraction with fragmented QRS, especially for anterior/lateral fragmented QRS, may experience more clinical cardiovascular events, including more heart failure re‐admission risk and death during follow‐up.
What Are the Clinical Implications?
The presence of fragmented QRS likely serves as a useful clinical prognosticator in patients with heart failure with preserved ejection fraction and may warrant more intensive treatments for comorbid underlying conditions.
Fragmented QRS (fQRS) refers to various QRS morphologies that are typically <120 ms in duration in the presence or absence of the Q‐wave on body surface 12‐lead ECG. It is characterized by an R' or notching in the nadir of the S wave, and there can also be more than 1 R' in 2 contiguous leads corresponding to a major coronary artery territory. 1 , 2 fQRS complexes are new ECG signals associated with varied conduction abnormalities and are assumed to originate from the delay in peri‐infarct conduction because of myocardial scarring or necrosis. 1 , 2 This specific ECG morphology is observed in patients with coronary artery diseases, cardiomyopathies, structural heart diseases, heart rhythm disturbances, and cardiac sarcoidosis. 3 , 4 , 5 Several studies have shown that the presence of fQRS is a predictor of sudden cardiac death in patients with cardiomyopathy and heart failure (HF). 4 , 6 Furthermore, it has been shown to be a prognostic marker of cardiac events and mortality in patients with coronary artery disease (CAD) 7 , 8 and a predictor of dysrhythmia in patients with Brugada syndrome. 9
As a clinical HF phenotype accompanied by a high clinical comorbidity burden (ie, coronary heart disease or hypertrophy from hypertension) with unfavorable myocardial remodeling, patients with heart failure with preserved ejection fraction (HFpEF) may theoretically have a higher chance of developing a longer QRS duration. 10 However, reports investigating the prevalence and significance of fQRS in HFpEF or the general population were either small in sample size or with study limitations. 11 , 12 , 13
In this study, we sought to investigate the prevalence and long‐term prognostic significance of fQRS and QRS duration in patients with HFpEF.
METHODS
Deidentified participant data with corresponding data dictionary of the data underlying the current article will be made available upon reasonable request to the corresponding author, Prof. Chung‐Lieh Hung (jotaro3791@gmail.com). Data will be shared with external researchers for scientific noncommercial purposes after approval of the proposal by the MMH IRB steering committee, including a signed data access agreement.
Study Subjects
Our current study was retrospective in the study design. The study workflow including setting, study participants selection, and exclusion criteria have been published previously and further detailed 14 (Figure S1, Data S1). In brief, we investigated 1120 consecutively discharged patients aged >20 years with HFpEF who were discharged with a main clinical diagnosis of discharge HF from a tertiary medical center located in Northern Taiwan (from March 2012 to December 2014). Medical information regarding the diagnosis, medical history, complete 12‐lead ECG, and laboratory data were all obtained in study participants. A total of 960 patients with HFpEF had complete body surface ECG information for fQRS categorization in the current analysis. This study was conducted following the guidelines of the Declaration of Helsinki and was approved by the Mackay Memorial Hospital Institutional Review Board (15MMHIS015). All study participants were waived from informed consent from retrospective study design.
Definition of fQRS
Standard 12‐lead ECGs were collected and analyzed. The fQRS in the current study was limited to ECGs with QRS duration <120 ms and was defined by the presence of various RSR patterns, including the presence of an additional R wave (R), a notching of the R wave, a notching in the nadir of the S wave, or the presence of more than 1 R in at least 2 contiguous leads, corresponding to a specific and major coronary artery territory. 7 Bundle branch block was excluded from the definition of fQRS. Furthermore, when notching was confined to the terminal QRS accompanied by a J‐point elevation of at least 0.1 mV, it was classified as early repolarization rather than fQRS. 15
fQRS was then further categorized by anatomic locations and coronary territory distributions. fQRS was classified into anterior (leads V1–V5), lateral (leads I, aVL, and V6), or inferior (leads II, III, and aVF). 16 Among the 1120 admitted patients with HFpEF, 960 had ECG‐defined QRS morphology data available and were classified into the 3 categories: 657 non‐fQRS, 220 inferior fQRS, and 83 subjects with fQRS on anterior or lateral leads (anterior/lateral). We also classified our study participants into 2 groups according to QRS duration cutoff: ≤110 ms and those with >110 ms.
Echocardiography
Comprehensive echocardiography was performed within the first 3 days of HF admission. Two‐dimensional cine loops and Doppler images are acquired in 3 consecutive heartbeats during the hospital stay or on arrival at the emergency department. Our current study mainly focused on parameters of left ventricular (LV) structure (wall thickness, internal dimension, LV mass index, LV end‐diastolic and end‐systolic volume) and geometry as recommended by a standardized protocol, with left ventricular ejection fraction determined by using biplane Simpson's method. 17 Right ventricular (RV) structure/function (RV end‐diastolic area and RV end‐systolic area) and RV function (RV fractional area change, as %) were also determined. LV diastolic function including mitral E/A ratio, deceleration time, interventricular relaxation time, early mitral annulus tissue Doppler velocity e′ and s′, LV filling E divided by e′ (E/e′), and bi‐atrial (right atrium and left atrium) volume index were all analyzed and quantified using contemporary guidelines. 18
Single‐Photon Emission Computed Tomography and Coronary Flow Angiography
Overall, 413 out of 960 (43.0%) patients with HFpEF underwent coronary angiography because of known CAD (including myocardial infarction; n=325) or symptom‐driven (n=87) indications. Among 325 out of 960 study participants with CAD, 210 (64.6% of all known CAD population) had post–percutaneous coronary intervention radiopharmaceutical single‐photon emission computed tomography (SPECT; technetium‐99m sestamibi) myocardial scan data available within 6 months of the study enrollment date. SPECT myocardial perfusion burden was quantified using quantitative gated SPECT and quantitative perfusion SPECT software. Among the symptom‐driven 634 non‐CAD participants, 203 (32.0%) underwent coronary angiography. Coronary flow grades for the symptom‐driven 203 non‐CAD participants were assessed using the corrected thrombolysis in myocardial infarction frame count method, which represents the total of cine‐frames required for contrast to first reach standard distal coronary landmarks. The coronary slow flow in the current study was defined as thrombolysis in myocardial infarction frame count >27 frames in the current study. 19
Clinical Outcomes
The primary clinical outcome measures of the current study included prespecified clinical end points: hospitalization for heart failure (HHF), cardiovascular mortality, all‐cause mortality, and the composite end points of HHF and any death during follow‐up after the study indexed date. Follow‐up periods of primary clinical outcomes were measured from the index date of HFpEF diagnosis to the occurrence date of cardiovascular events (HHF, cardiovascular mortality, and all‐cause death). All participants were tracked until death or loss to follow‐up or the end of June 30, 2016. These clinical end points were further adjudicated by 2 experienced cardiologists (SCC and CLH), mainly based on the extracted electronic data capture information.
Statistical Analysis
Comparisons of the continuous variables between the 2 groups were performed using an unpaired 2‐tailed t test, with nominal/categorical variables compared by a χ2 analysis or Fisher exact test, as appropriate. Continuous variables among the different clinical categories of more than 3 groups were performed using a 1‐way ANOVA or Kruskal–Wallis test with post hoc paired comparisons as appropriate.
Multivariate Cox regression models were used to examine the associations of region‐specific fQRS (non‐fQRS as reference group) with clinical end points, including HHF, cardiovascular mortality, all‐cause mortality, and the composite end point of HHF and any death. Individual hazard ratio (HR) and correspondent 95% CIs were performed. Key clinical covariates including age, sex, body size, medical histories of hypertension, diabetes, hyperlipidemia treatment, atrial fibrillation, stroke, known coronary artery disease, cerebrovascular event, estimated glomerular filtration rate, and left ventricular ejection fraction from echocardiography data served as confounders. The survival curve was plotted using the Kaplan–Meier method for time to events (HHF, cardiovascular death, all‐cause mortality, and composite end point) for a certain amount of time after enrollment. Log‐rank test was used to determine significances (whether survival distributions among groups may differ statistically) of these cardiovascular events across different fQRS strata. Statistical significance was set at P<0.05 using STATA software (version 13.1).
RESULTS
Baseline Characteristics
Among the original 1120 patients with HFpEF enrolled, we identified a total of 960 patients with HFpEF (76.4±12.7 years of age, men: 37.2%) with fQRS classification available as non‐fQRS (68.4%), inferior fQRS (22.9%), and anterior/lateral fQRS (8.7%) without overt bundle branch block and after application of our exclusion criteria (Table 1). Compared with those classified as non‐fQRS, patients with inferior fQRS had higher systolic blood pressure and were more likely to have diabetes; patients with anterior/lateral fQRS had higher serum potassium level and were more likely to have atrial fibrillation (Table 1), while other baseline demographics were comparable. We also observed graded and significantly higher B‐type natriuretic peptide and troponin I levels across the 3 categories (non‐fQRS, inferior fQRS, anterior/lateral fQRS, B‐type natriuretic peptide: 554 versus 581 versus 893 pg/mL; troponin‐I: 0.04 versus 0.04 versus 0.06 as median [interquartile range] ranges, all trend P<0.01). Baseline demographics, according to QRS duration strata cutoff of 110 ms, are detailed in Table S1.
Table 1.
Baseline Characteristics of Study Subjects Categorized by the Location of fQRS
| fQRS groups | Non‐fQRS (N=657) | Inferior fQRS (N=220) | Anterior/lateral fQRS (N=83) | P value (ANOVA) |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 76.7±12.8 | 75.8±12.5 | 75.6±12.6 | 0.41 |
| Sex, male (%) | 238 (36.2%) | 86 (39.1%) | 33 (39.8%) | 0.52 |
| BMI, kg/m2 | 24.6±5.5 | 25.3±5.7 | 24.4±11.2 | 0.04 |
| SBP, mm Hg | 139.5±32.3 | 146.1±32.8* | 138.8±34.3 | 0.02 |
| DBP, mm Hg | 72.6±17.5 | 74.4±18.8 | 73.5±19.8 | 0.41 |
| HR, bpm | 90.4±2.3 | 88.8±19.3 | 94.7±4.9 | 0.09 |
| History, n (%) | ||||
| Prior heart failure | 362 (55.1%) | 118 (53.6%) | 46 (55.4%) | 0.89 |
| Hypertension | 469 (71.4%) | 171 (77.7%) | 61 (73.5%) | 0.22 |
| Diabetes | 315 (47.9%) | 127 (58.0%) | 39 (47.0%) | 0.03 |
| Coronary artery disease | 215 (32.7%) | 76 (34.7%) | 34 (41.0%) | 0.31 |
| ESRD/hemodialysis | 72 (11.0%) | 26 (11.8%) | 16 (19.3%) | 0.16 |
| Hyperlipidemia treatment | 118 (18.0%) | 47 (21.4%) | 12 (14.5%) | 0.31 |
| Stroke | 111 (16.9%) | 39 (17.7%) | 17 (20.5%) | 0.71 |
| Atrial fibrillation | 132 (20.1%) | 36 (16.4%) | 24 (28.9%)† | 0.09 |
| PAD | 44 (6.7%) | 16 (7.3%) | 5 (6.0%) | 0.91 |
| Laboratory | ||||
| Fasting glucose, mg/dL | 170.1±108.1 | 184.5±114.6 | 192.4±131.3 | 0.09 |
| eGFR, mL/min per 1.73 m2 | 42.1±32.9 | 39.0±32.9 | 39.0±32.4 | 0.41 |
| ALT, μ/L | 52.7±108.0 | 78.6±200.4 | 87.3±261.1 | 0.03 |
| Serum sodium, mmol/L | 131.0±8.4 | 129.9±7.7 | 129.2±9.2 | 0.10 |
| Serum potassium, mmol/L | 5.2±1.2 | 5.4±1.3 | 5.6±1.3* | 0.007 |
| BNP, pg/mL (n=917)‡ | 554 [256, 1170] | 581 [246, 1330] | 893 [478, 2740]* , † | <0.001 |
| Troponin I (n=887)‡ | 0.03 [0.02, 0.1] | 0.04 [0.02, 0.12] | 0.06 [0.03, 0.23]* , † | 0.008 |
| QRS duration, ms | 92.3±15.0 | 94.2±15.3 | 97.8±17.3* | 0.005 |
| NYHA Fc | 0.061 | |||
| ≤II | 14.3% | 14.5% | 12.0% | |
| III | 62.2% | 60.0% | 49.4% | |
| IV | 23.4% | 25.4% | 38.6% | |
| Measurement | ||||
| LVEF, % | 64.7±6.3 | 64.8±6.7 | 64.1±7.1 | 0.66 |
| Prognostic nutritional score | 41.3±9.1 | 41.3±8.9 | 40.0±9.4 | 0.43 |
| Medications, n (%) | ||||
| ACEI/ARB | 220 (33.5%) | 83 (37.7%) | 27 (32.5%) | 0.45 |
| β‐Blocker | 133 (20.2%) | 48 (21.8%) | 14 (16.8%) | 0.73 |
| Aldosterone antagonists | 105 (16.0%) | 39 (17.7%) | 11 (13.3%) | 0.61 |
| Digoxin | 34 (5.2%) | 14 (6.4%) | 7 (8.4%) | 0.43 |
| Diuretics | 297 (45.2%) | 105 (47.7%) | 28 (33.7%) | 0.05 |
Data are expressed as mean±SD or percentage. ACEI indicates angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II‐receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; bpm, beats per minute; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; fQRS, fragmented QRS; HR, heart rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral arterial disease; and SBP, systolic blood pressure.
P<0.05 vs non‐fQRS.
P<0.05 vs inferior fQRS.
Troponin‐I and BNP are expressed as median [25th percentile, 75th percentile].
Cardiac Structure and Function
Compared with those classified as non‐fQRS, patients with HFpEF with anterior/lateral fQRS had a substantially larger LV end‐diastolic volume index (48.9±17.0 versus 44.2±13.8 mL, P<0.05), greater LV mass‐to‐volume ratio (2.21±0.68 versus 2.19±0.84, P<0.05), and had significantly lower myocardial relaxation velocity tissue Doppler imaging (TDI)‐e′ (4.8±1.9 versus 5.7±1.8 cm/s, P<0.05). Both patient groups with inferior and anterior/lateral fQRS presented with a significantly larger LV mass (174.9±60.7 and 180.2±75.1 g versus 162.8±58.6 g, trend P=0.004), and those with anterior/lateral fQRS had significantly larger indexed LV mass and were more likely to have left ventricular hypertrophy compared with non‐fQRS (39.6% and 49.4% versus 34.7%, trend P=0.027). Furthermore, compared with those classified as non‐fQRS/inferior fQRS, patients with HFpEF with anterior/lateral fQRS showed a substantially lower myocardial systolic velocity TDI‐s′ (trend P=0.001), higher LV filling E/e′ (20.4±9.8 versus 16.1±7.0 and 16.5±7.1, trend P<0.001), higher TR velocity (3.2±0.6 m/s versus 3.0±0.4 and 3.0±0.4 m/s, trend P=0.010), larger right atrium/LA indexed volumes (both trend P<0.05; LA indexed volume: 33.0±15.0 mL/m2 versus 28.3±13.3 and 28.0±11.9 mL/m2, trend P=0.010), and a greater RV end‐diastolic/systolic area (both trend P<0.001; Table 2). In addition, compared with non‐fQRS, those presenting with anterior/lateral fQRS had significantly lower RV fractional area change (P=0.015). Overall, more unfavorable cardiac structural and functional indices were found in those presenting wider (>110 ms) compared with those with smaller QRS duration (≤110 ms), though these differences were less prominent compared with fQRS strata (Table S1).
Table 2.
The LV/RV Structure and Function in Non‐fQRS, Inferior fQRS, and Anterior/Lateral fQRS Groups
| fQRS groups | Non‐fQRS (N=657) | Inferior fQRS (N=220) | Anterior/lateral fQRS (N=83) | P value (ANOVA) |
|---|---|---|---|---|
| LV structure | ||||
| Septal wall thickness, mm | 9.9±2.0 | 10.2±2.0 | 10.4±1.9 | 0.015 |
| Posterior wall thickness, mm | 10.0±2.0 | 10.3±2.0 | 10.3±1.9 | 0.039 |
| LV internal dimension, mm | 46.3±6.1 | 47.0±6.2 | 47.7±6.7 | 0.080 |
| LV EDV index, mL/m2 | 44.2±13.8 | 45.2±15.5 | 48.9±17.0* | 0.028 |
| LV ESV index, mL/m2 | 15.0±8.7 | 15.1±9.9 | 17.3±11.8 | 0.120 |
| LVEF, % | 67.9±13.6 | 69.2±14.2 | 67.6±15.4 | 0.450 |
| LV mass, g | 162.8±58.6 | 174.9±60.7* | 180.2±75.1* | 0.004 |
| LV mass index, g/m2 | 94.2±30.8 | 99.4±32.7 | 105.3±39.5* | 0.004 |
| Presence of LVH, % | 216 (32.9%) | 84 (38.2%) | 39 (47.0%) | 0.027 |
| LV M/V ratio | 2.2±0.8 | 2.3±1.0 | 2.2±0.7* | 0.684 |
| LV function | ||||
| Mitral E/A ratio | 1.2±1.5 | 1.1±0.8 | 1.2±0.8 | 0.370 |
| DT, ms | 210.8±77.3 | 211.1±74.8 | 206.7±79.9 | 0.892 |
| IVRT, ms | 85.9±33.4 | 87.5±30.5 | 91.5±34.3 | 0.312 |
| TDI‐e′, cm/s | 5.7±1.8 | 5.4±1.8 | 4.8±1.9* | 0.001 |
| TDI‐s′, cm/s | 5.7±1.4 | 5.6±1.3 | 5.0±1.3* , † | 0.001 |
| Mitral E/TDI‐e′ | 16.1±7.0 | 16.5±7.1 | 20.4±9.8* , † | <0.001 |
| TR velocity, cm/s | 3.0±0.4 | 3.0±0.5 | 3.2±0.6* , † | 0.010 |
| RV structure and function | ||||
| RV EDA, cm2 | 30.5±13.1 | 31.9±13.5 | 37.3±16.3* , † | <0.001 |
| RV ESA, cm2 | 16.1±8.7 | 16.9±9.0 | 20.9±11.9* , † | <0.001 |
| RV FAC, % | 48.4±7.9 | 47.9±8.4 | 45.5±8.4* | 0.015 |
| Atrial structure | ||||
| LA volume index, mL/m2 | 28.3±13.3 | 28.0±11.9 | 33.0±15.0* , † | 0.010 |
| RA volume index, mL/m2 | 22.9±14.8 | 22.4±20.3 | 28.3±18.2* , † | 0.022 |
Data are expressed as mean±SD or percentage. All expressions are listed in Table 1. DT indicates deceleration time; EDA, end‐diastolic area; EDV, end‐diastolic volume; ESA, end‐systolic area; ESV, end‐systolic volume; FAC, fractional area change; fQRS, fragmented QRS; IVRT, interventricular relaxation time; LA, left atrial; LV, left ventricular mass to volume ratio; LV, left ventricular; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; RA, right atrial; RV, right ventricular; TDI, tissue Doppler imaging; and TR, tricuspid regurgitation.
P<0.05 vs non‐fQRS.
P<0.05 vs inferior fQRS.
Myocardial Perfusion and Coronary Flow Findings
Despite comparable CAD prevalence, patients with HFpEF categorized into inferior fQRS and anterior/lateral fQRS had a graded increase in poststress total myocardial perfusion defect when compared with the non‐fQRS group (Figure 1A, trend P=0.001). By examining individual myocardial perfusion defects according to coronary artery territories, patients with HFpEF categorized into anterior/lateral fQRS were more likely to have fixed myocardial perfusion defects from the left anterior descending artery/left circumflex artery coronary territory compared with the right coronary artery territory (40%, 40% versus 13.3%), while those patients with HFpEF categorized into inferior fQRS tend to have fixed perfusion defects close to left circumflex artery/right coronary artery coronary territory compared with the left anterior descending artery (38.1%, 33.3% versus 19%). HFpEF classified as non‐fQRS was less likely to present myocardial perfusion defects on 3 coronary arterial territories (all<20%; Figure 1B through 1D).
Figure 1. Relationship between the percentage of cardiac ischemic area and different coronary arteries.
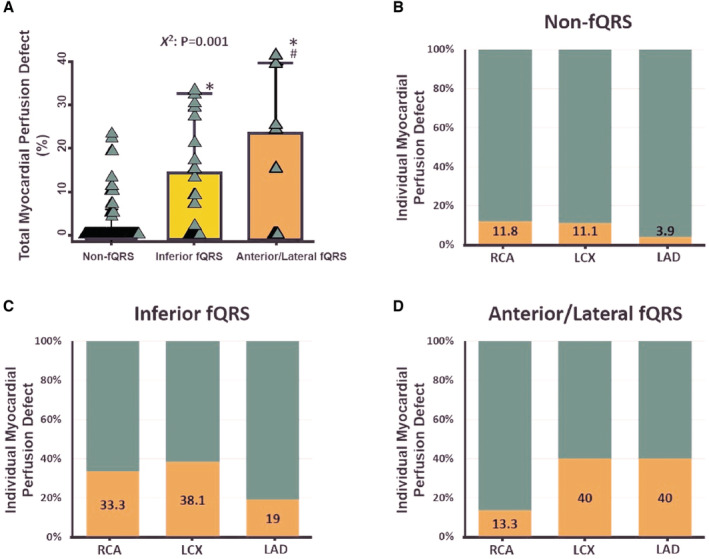
A, The percentage of total myocardial perfusion defects in non‐fQRS, inferior and anterior/lateral fQRS among 210 out of 326 (64.4%) study participants with known CAD. B through D, The percentage of myocardial perfusion defects in the RCA, LCX, and LAD in non‐fQRS, inferior and anterior/lateral fQRS. CAD indicates coronary artery disease; fQRS, fragmented QRS; LAD, left anterior descending artery; LCX, left circumflex artery; and RCA, right coronary artery. *p<0.05 vs non‐fQRS; # p<0.05 vs inferior fQRS.
Illustrations of myocardial perfusion deficit by SPECT are displayed in Figure 2A through 2C. The burden of myocardial perfusion defect was inversely correlated with TDI‐s' (r=−0.15, P=0.02), showing a borderline reverse relationship with TDI‐e′ (r=−0.11, P=0.08), with a nonsignificant positive relationship with E/TDI‐e′ (r=0.05, P=0.46; Figure 2D through 2F). Overall, 67 out of 203 (33%) symptom‐driven patients with HFpEF did not have a significant CAD manifested slow flow phenomenon, with a graded and significantly higher proportion observed across non‐fQRS, inferior fQRS, and anterior/lateral fQRS (28%, 50%, and 83.3%, respectively, X 2<0.001; Figure 3A). Interestingly, substantially lower myocardial TDI‐e′ and TDI‐s′ were associated with a higher LV filling pressure (E/TDI‐e′) in those patients with HFpEF without CAD manifesting slow coronary flow (Figure 3B through 3D).
Figure 2. Myocardial perfusion defects in (A) non‐fQRS, (B) inferior fQRS, and (C) anterior/lateral fQRS with SPECT imaging.
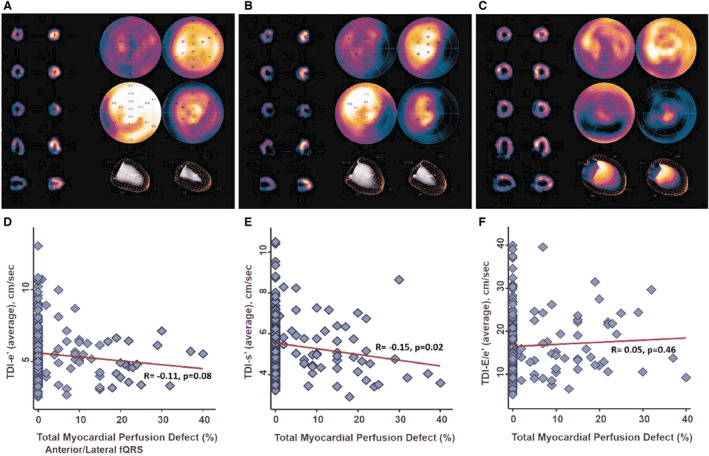
D through F, Correlation between total myocardial perfusion defect and TDI‐derived myocardial early relaxation (TDI‐e′) velocity, systolic (TDI‐s′) velocities, and LV filling pressure E/e′. fQRS indicates fragmented QRS; LV, left ventricular; SPECT, myocardial perfusion single‐photon emission computed tomography; and TDI, tissue Doppler imaging.
Figure 3. Association between the presence of coronary slow flow and cardiac function.
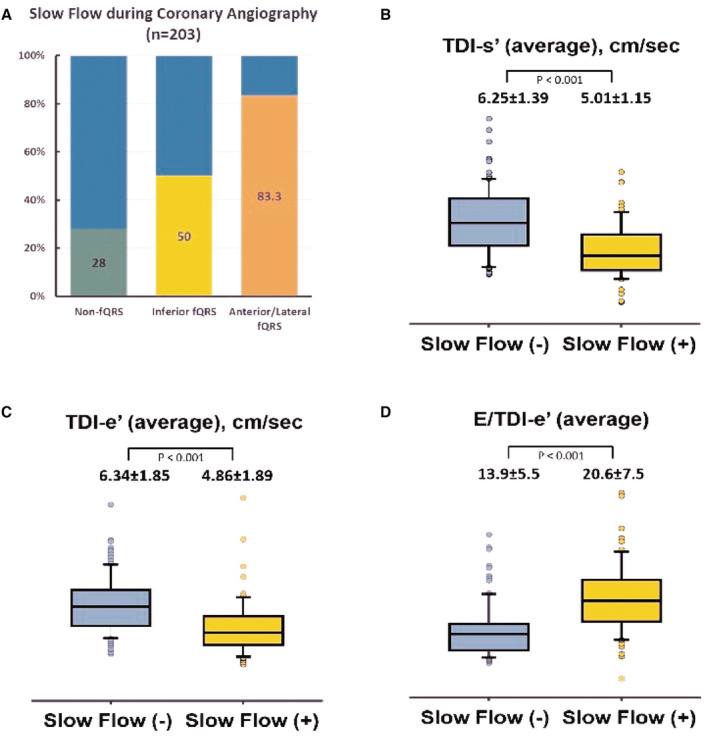
A, The percentage of coronary slow flow during coronary angiography among 203 out of 634 (32.0%) non‐CAD study participants. B through D, Comparisons of the TDI‐s′, TDI‐e′, and average E/e′ in normal versus slow coronary flow non‐CAD study participants. CAD indicates coronary artery disease; fQRS, fragmented QRS; and TDI, tissue Doppler imaging.
Clinical Outcomes
During a median follow‐up of 657 days (interquartile range: 70–1274 days), 314 patients (32.7%) were hospitalized for HF, 178 (18.5%) experienced cardiovascular death, 337 (35.1%) experienced all‐cause death, and 585 (60.9%) had composite events of HF hospitalization and mortality irrespective of any cause. A Cox proportional hazard regression analysis for adverse events is shown in Figure 4 and Table S1. By using non‐fQRS as the reference group, the presence of anterior/lateral fQRS was associated with a higher risk of HHF (adjusted HR, 1.90 [1.34–2.69], P<0.001), with the presence of both inferior fQRS and anterior/lateral fQRS associated with a higher risk of cardiovascular (adjusted HR, 1.44 [1.02–2.05] and 2.00 [1.26–3.16] for inferior and anterior/lateral fQRS, respectively), and all‐cause mortality (adjusted HR, 1.35 [1.04–1.74] and 1.84 [1.32–2.56] for inferior and anterior/lateral fQRS, respectively) in multivariate model with adjustment for age, sex, body mass index, hypertension, diabetes, HF, hyperlipidemia, cardiovascular disease, estimated glomerular filtration rate, and LV ejection fraction. However, the risk of HHF did not reach a significant difference in the inferior fQRS group in crude and multivariate models compared with the non‐fQRS group as the reference (adjusted HR, 1.09 [0.83–1.42] P=0.053). Although the presence of inferior fQRS was associated with a significantly higher risk of composite end point in the crude model (crude HR, 1.27 [1.05–1.53], P=0.015), this association was not statistically significant in the multivariable model (adjusted HR, 1.21 [1.00–1.47], P=0.055). Kaplan–Meier survival curves for relevant clinical outcomes according to fQRS strata are displayed in Figure 5 (left; all log‐rank P<0.001). Clinical outcomes according to QRS duration strata (cutoff: 110 ms) are detailed in Table S1, where risk stratification by QRS duration cutoff (110 ms) were less prominent compared with fQRS. The patients with known CAD and presence of anterior/lateral fQRS had significantly higher B‐type natriuretic peptide level (920.5 versus 558 pg/mL, P=0.003), higher troponin‐I level (0.14 versus 0.04, P=0.002), larger LV end‐diastolic volume index (54.2±17.3 versus 46.1±14.5, P=0.038), greater LA volume index (34.8±13.9 versus 28.4±12.7, P=0.027), and higher LV filling pressure (mitral E/TDI‐e′: 22.7±13.3 versus 16.8±7.7, P=0.012) compared with the counterparts with non‐fQRS (Table S1). In addition, the presence of anterior/lateral fQRS was associated with a higher risk of all‐cause death (adjusted HR, 2.27 [1.35–3.82], P=0.002) and cardiovascular death (adjusted HR, 2.60 [1.29–5.11], P=0.007) by using non‐fQRS as reference group, which is shown in Table S1.
Figure 4. Crude and adjusted hazard ratio with corresponding 95% CIs for 3 fQRS strata (non‐fQRS as the reference) on hospitalization for heart failure, cardiovascular mortality, all‐cause mortality, and composite end point.
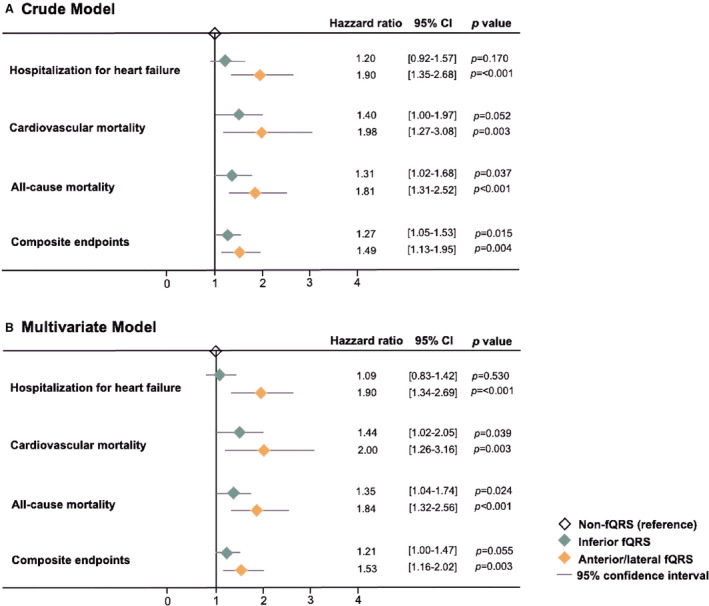
The multivariate model was adjusted for age, sex, body mass index, hypertension, diabetes, heart failure, hyperlipidemia, cardiovascular disease, estimated glomerular filtration rate, and left ventricular ejection fraction. fQRS indicates fragmented QRS.
Figure 5. Kaplan–Meier survival curves of clinical outcomes according to fQRS strata (left).
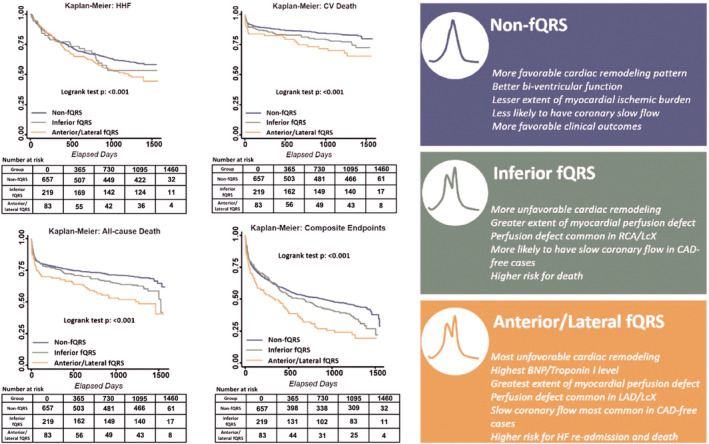
Condensed illustrations and graphical abstract about the clinical features, functional correlates, and outcomes of 3 fQRS strata in HFpEF (right). BNP indicates B‐type natriuretic peptide; CAD, coronary artery disease; CV, cardiovascular; fQRS, fragmented QRS; HF, heart failure; HHF, hospitalization for heart failure; HFpEF, heart failure with preserved ejection fraction; LAD, left anterior descending artery; and LCX, left circumflex artery.
DISCUSSION
In the present study, we examined the associations of QRS fragmentation utilizing standard 12‐lead body surface ECGs in a large‐scale patient population with HFpEF and further related these measures to a variety of clinical end points, including HF hospitalization and death. We found that fQRS was not uncommon in HFpEF (31.6%), with inferior fQRS nearly tripling the prevalence of anterior/inferior QRS. Despite comparable global LV ejection fractions, patients with HFpEF manifesting fQRS, particularly those with anterior/lateral fQRS, were associated with a more diseased myocardium, including distinct yet larger areas of myocardial perfusion defect, more unfavorable cardiac systolic/diastolic properties, and a higher prevalent coronary slow flow than those without fQRS. Anterior/lateral fQRS was further associated with a higher risk of HF hospitalization, with all HFpEF presenting with fQRS associated with a higher rate of mortality events during follow‐up.
Fragmentation of the QRS Complex
Boineau and Cox 20 first demonstrated the occurrence of fractionated electrograms within ischemic regions using an experimental canine heart model after acute ischemia. 21 Autopsy findings in patients with myocardial infarction have confirmed that islands of viable myocardial tissue interspersed in abundant fibrous tissue within myocardial necrotic regions may generate depolarized and depressed action potential upstroke velocities, resulting in slower electrical activation. 22 This feature, termed fQRS phenotype by ECG, is responsible for inhomogeneous activation and altered depolarization of the ventricles and duplicate features of left bundle branch block complicated by HF. 23 , 24 , 25 Higher myocardial perfusion defects in those with known CAD along with an impaired coronary flow reserve in non‐CAD patients with HFpEF likely represented a higher total burden of macro/microvascular dysfunction, resulting in more extensive myocardial fibrosis and perturbed myocardial electrical spread. Notably, markedly diminished systolic and early myocardial relaxation velocities (s′ and e′) representing overall more deteriorated intrinsic cardiac mechanics were observed when anterior/lateral fQRS existed in patients with HFpEF. Our finding was in accordance with another recent work with a relatively small number of patients utilizing speckle‐tracking‐based techniques. 11 Higher B‐type natriuretic peptide, higher troponin I levels, and more unfavorable LV remodeling in our patients with HFpEF complicated by fQRS likely represents higher LV wall stress, excessive extracellular matrix turnover/fibrotic replacement, and more extensive myocardial involvement. 26
Relationships Between fQRS and Cardiac Outcomes
The prognostic value of QRS fragmentation during acute coronary syndrome has been well demonstrated. 27 , 28 fQRS was prevalent in up to 30% of subjects with CAD manifesting preserved LV function, with those presenting fQRS demonstrating a more dilated LV dimension, lower left ventricular ejection fraction, higher wall motion abnormality, and reduced global 2‐dimensional circumferential strains as compared with those without fQRS. 29 In another study, the presence of fQRS in a healthy population was associated with reduced LV global longitudinal strain compared with those without. 11 However, these 2 studies were limited by their small sample size and lack of cardiac outcomes. In 1 large epidemiological study conducted among 10 904 individuals from the Finnish general population, fQRS was prevalent in 19.7% of subjects, including 15.7% in inferior leads, 0.8% in lateral leads, and 2.9% in anterior leads, 12 with presence of lateral fQRS rather than anterior or inferior fQRS associated with higher all‐cause, arrhythmic, and cardiac deaths. Interestingly, the presence of fQRS also conferred a higher risk of ventricular tachyarrhythmia in individuals with hypertrophic cardiomyopathy. 30 Notably, our data also showed that morphological fQRS phenotypes may serve as a better functional and survival indicator for HFpEF compared with QRS duration (cutoff: 110 ms). Our study is the first and, thus far, the largest to show the prevalence of fQRS in patients with HFpEF (≈31.6%), with inferior fQRS being more common than anterior/lateral fQRS, though the presence of anterior/lateral fQRS (8.6%) was associated with a higher HF rehospitalization rate all‐cause mortality.
The condensed illustrations of the 3 fQRS strata about the clinical features, functional correlates, and outcomes in patients with HFpEF are demonstrated in Figure 5 (right).
Limitations
Our study has several limitations. First, our findings may be limited by the retrospective study design; the anterior/lateral fQRS had a relatively smaller sample size (total number: 83) than non‐fQRS (total number: 657) and inferior fQRS (total number: 220). Also, less than half of patients with HFpEF (43%) underwent coronary angiography, and only 210 patients (21%) had a post–percutaneous coronary intervention SPECT myocardial scan. Second, myocardial perfusion defects, as assessed by the SPECT method, did not clarify the exact underlying causes: for example, irreversible myocardial damage (such as scar formation), residual incomplete revascularization, or restenosis in smaller caliber coronary vessels after intervention. Third, the causal relationship between QRS fragmentation and the observed perfusion defect cannot be ascertained, because it has been reported that disorganized myocardial electrical conduction through the myocardium may, on the other hand, result in an abnormal myocardial perfusion pattern.
Despite these limitations, our findings demonstrated that patients manifesting QRS fragmentation may represent a specific HFpEF phenotype of undertreated macro/microvascular consequences from multiple comorbidities (such as hypertension, diabetes, and coronary artery disease) leading to regional myocardial dyskinesia. 31 Further advanced functional and myocardial characterization imaging using magnetic resonance imaging may be more helpful in future studies to solve these issues.
CONCLUSIONS
The presence of fQRS in HFpEF, especially those manifesting anterior or lateral fQRS, was associated with more severe involvement of cardiac damage. These may include a more extensive deficit in myocardial perfusion and worsened myocardial functional properties, which likely translate into more unfavorable clinical outcomes. Early recognition of such a patient population may be helpful for more intensive targeted therapeutic interventions.
Sources of Funding
This research was supported by the Ministry of Science and Technology (Taiwan; grants NSC‐101‐2314‐B‐195‐020, NSC103‐2314‐B‐010‐005‐MY3, 103‐2314‐B‐195‐001‐MY3, 101‐2314‐B‐195‐020‐MY1, MOST 103‐2314‐B‐195‐006‐MY3, NSC102‐2314‐B‐002‐046‐MY3, and 106‐2314‐B‐195‐008‐MY2), MacKay Memorial Hospital (10271, 10248, 10220, 10253, 10375, 10358, E‐102003), and the Taiwan Foundation for Geriatric Emergency and Critical Care.
Disclosures
We have nothing to disclose.
Supporting information
Data S1
Tables S1–S6
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028105
Preprint posted on Research Square October 25, 2021. doi: https://doi.org/10.21203/rs.3.rs‐943276/v1.
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Jen‐Yuan Kuo, Email: jykuo5813@gmail.com.
Chung‐Lieh Hung, Email: jotaro3791@gmail.com.
References
- 1. Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892 [DOI] [PubMed] [Google Scholar]
- 2. Das MK, Suradi H, Maskoun W, Michael MA, Shen C, Peng J, Dandamudi G, Mahenthiran J. Fragmented wide QRS on a 12‐lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1:258–268. doi: 10.1161/CIRCEP.107.763284 [DOI] [PubMed] [Google Scholar]
- 3. Fares H, Heist K, Lavie CJ, Kumbala D, Ventura H, Meadows R, Carter W, Deitelzweig S, Ray IB. Fragmented QRS complexes‐a novel but underutilized electrocardiograhic marker of heart disease. Crit Pathw Cardiol. 2013;12:181–183. doi: 10.1097/HPC.0b013e31829e005d [DOI] [PubMed] [Google Scholar]
- 4. Das MK, Zipes DP. Fragmented QRS: a predictor of mortality and sudden cardiac death. Heart Rhythm. 2009;6(Supplement):S8–S14. doi: 10.1016/j.hrthm.2008.10.019 [DOI] [PubMed] [Google Scholar]
- 5. Schuller JL, Olson MD, Zipse MM, Schneider PM, Aleong RG, Wienberger HD, Varosy PD, Sauer WH. Electrocardiographic characteristics in patients with pulmonary sarcoidosis indicating cardiac involvement. J Cardiovasc Electrophysiol. 2011;22:1243–1248. doi: 10.1111/j.1540-8167.2011.02099.x [DOI] [PubMed] [Google Scholar]
- 6. Torigoe K, Tamura A, Kawano Y, Shinozaki K, Kotoku M, Kadota J. The number of leads with fragmented QRS is independently associated with cardiac death or hospitalization for heart failure in patients with prior myocardial infarction. J Cardiol. 2012;59:36–41. doi: 10.1016/j.jjcc.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 7. Das MK, Saha C, Masry HE, Peng J, Dandamudi G, Mahenthiran J, McHenry P, Zipes DP. Fragmented QRS on a 12‐lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–1392. doi: 10.1016/j.hrthm.2007.06.024 [DOI] [PubMed] [Google Scholar]
- 8. Pourafkari L, Ghaffari S, Nader ND. Prognostic value of fragmented QRS on admission in non‐ST‐elevation myocardial infarction. Ann Noninvasive Electrocardiol. 2017;22:e12344. doi: 10.1111/anec.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, Ohe T, Zipes DP, Wu J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917 [DOI] [PubMed] [Google Scholar]
- 10. Pandey A, Vaduganathan M, Arora S, Qamar A, Mentz RJ, Shah SJ, Chang PP, Russel SD, Rosamond WD, Caughey MC. Temporal trends in prevalence and prognostic implications of comorbidities among patients with acute decompensated heart failure: the ARIC study community surveillance. Circulation. 2020;142:230–243. doi: 10.1161/CIRCULATIONAHA.120.047019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dehghani MR, Rostamzadeh A, Abbasnezhad A, Shariati A, Nejatisafa S, Rezaei Y. Fragmented QRS and subclinical left ventricular dysfunction in individuals with preserved ejection fraction: a speckle‐tracking echocardiographic study. J Arrhythm. 2020;36:335–340. doi: 10.1002/joa3.12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terho HK, Tikkanen JT, Junttila JM, Anttonen O, Kenttä TV, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Prevalence and prognostic significance of fragmented QRS complex in middle‐aged subjects with and without clinical or electrocardiographic evidence of cardiac disease. Am J Cardiol. 2014;114:141–147. doi: 10.1016/j.amjcard.2014.03.066 [DOI] [PubMed] [Google Scholar]
- 13. Ollitrault P, Pellissier A, Champ‐Rigot L, Junqua N, Chequel M, Reboursiere E, Saloux É, Milliez P, Hodzic A. Prevalence and significance of fragmented QRS complex in lead V1 on the surface electrocardiogram of healthy athletes. Europace. 2020;22:649–656. doi: 10.1093/europace/euaa037 [DOI] [PubMed] [Google Scholar]
- 14. Chien SC, Lo CI, Lin CF, Sung KT, Tsai JP, Huang WH, Yun CH, Hung TC, Lin JL, Liu CY, et al. Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. Eur J Heart Fail. 2019;6:953–964. doi: 10.1002/ehf2.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Narayanan K, Zhang L, Kim C, Uy‐Evanado A, Teodorescu C, Reinier K, Zheng ZJ, Gunson K, Jui J, Chugh SS. QRS fragmentation and sudden cardiac death in the obese and overweight. J Am Heart Assoc. 2015;4:e001654. doi: 10.1161/JAHA.114.001654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hung CL, Lai YJ, Chi PC, Chen LC, Tseng YM, Kuo JY, Lin CI, Chen YC, Lin SJ, Yeh HI. Dose‐related ethanol intake, Cx43 and Nav1.5 remodeling: exploring insights of altered ventricular conduction and QRS fragmentation in excessive alcohol users. J Mol Cell Cardiol. 2018;114:150–160. doi: 10.1016/j.yjmcc.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 18. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 19. Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.CIR.93.5.879 [DOI] [PubMed] [Google Scholar]
- 20. Boineau JP, Cox JL. Slow ventricular activation in acute myocardial infarction. A source of re‐entrant premature ventricular contractions. Circulation. 1973;48:702–713. doi: 10.1161/01.cir.48.4.702 [DOI] [PubMed] [Google Scholar]
- 21. Gardner PI, Ursell PC, Fenoglio JJ Jr, Wit AL. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72:596–611. doi: 10.1161/01.CIR.72.3.596 [DOI] [PubMed] [Google Scholar]
- 22. Friedman PL, Fenoglio JJ Jr, Wit AL. Time course for reversal of electrophysiological and ultrastructural abnormalities in subendocardial Purkinje fibers surviving extensive myocardial infarction in dogs. Circ Res. 1975;36:127–144. doi: 10.1161/01.RES.36.1.127 [DOI] [PubMed] [Google Scholar]
- 23. Hatala R, Savard P, Tremblay G, Pagé P, Cardinal R, Molin F, Kus T, Nadeau R. Three distinct patterns of ventricular activation in infarcted human hearts. An intraoperative cardiac mapping study during sinus rhythm. Circulation. 1995;91:1480–1494. doi: 10.1161/01.CIR.91.5.1480 [DOI] [PubMed] [Google Scholar]
- 24. Wiener I, Mindich B, Pitchon R. Endocardial activation in patients with coronary artery disease: effects of regional contraction abnormalities. Am Heart J. 1984;107:1146–1152. doi: 10.1016/0002-8703(84)90270-9 [DOI] [PubMed] [Google Scholar]
- 25. Cate TJFT, Kelder JC, Plokker HWM, Verzijlbergen JF, Hemel NMV. Myocardial perfusion SPECT identifies patients with left bundle branch block patterns at high risk for future coronary events. J Nucl Cardiol. 2010;17:216–224. doi: 10.1007/s12350-009-9183-9 [DOI] [PubMed] [Google Scholar]
- 26. Thawabi M, Hawatmeh A, Studyvin S, Habib H, Shamoon F, Cohen M. Cardiac troponin and outcome in decompensated heart failure with preserved ejection fraction. Cardiovasc Diagn Ther. 2017;7:359–366. doi: 10.21037/cdt.2017.03.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Das MK, Michael MA, Suradi H, Peng J, Sinha A, Shen C, Mahenthiran J, Kovacs RJ. Usefulness of fragmented QRS on a 12‐lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104:1631–1637. doi: 10.1016/j.amjcard.2009.07.046 [DOI] [PubMed] [Google Scholar]
- 28. Pietrasik G, Goldenberg I, Zdzienicka J, Moss AJ, Zareba W. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q‐wave myocardial infarction. Am J Cardiol. 2007;100:583–586. doi: 10.1016/j.amjcard.2007.03.063 [DOI] [PubMed] [Google Scholar]
- 29. Yan GH, Wang M, Yiu KH, Lau CP, Zhi G, Lee SWL, Siu CW, Tse HF. Subclinical left ventricular dysfunction revealed by circumferential 2D strain imaging in patients with coronary artery disease and fragmented QRS complex. Heart Rhythm. 2012;9:928–935. doi: 10.1016/j.hrthm.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 30. Kang KW, Janardhan AH, Jung KT, Lee HS, Lee MH, Hwang HJ. Fragmented QRS as a candidate marker for high‐risk assessment in hypertrophic cardiomyopathy. Heart Rhythm. 2014;11:1433–1440. doi: 10.1016/j.hrthm.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 31. Hedeer F, Ostenfeld E, Hedén B, Prinzen FW, Arheden H, Carlsson M, Engblom H. To what extent are perfusion defects seen by myocardial perfusion SPECT in patients with left bundle branch block related to myocardial infarction, ECG characteristics, and myocardial wall motion? J Nucl Cardiol. 2021;6:2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S6
Figure S1


