Abstract
Background
The COVID‐19 pandemic disrupted traditional health care; one fallout was a drastic decrease in blood pressure (BP) assessment. We analyzed the pandemic's impact on our existing remote hypertension management program's effectiveness and adaptability.
Methods and Results
This retrospective observational analysis evaluated BP control in an entirely remote management program before and during the pandemic. A team of pharmacists, nurse practitioners, physicians, and nonlicensed navigators used an evidence‐based clinical algorithm to optimize hypertensive treatment. The algorithm was adapted during the pandemic to simplify BP control. Overall, 1256 patients (605 enrolled in the 6 months before the pandemic shutdown in March 2020 and 651 in the 6 months after) were a median age of 63 years old, 57% female, and 38.2% non‐White. Among enrolled patients with sustained hypertension, 51.1% reached BP goals. Within this group, rates of achieving goal BP improved to 94.6% during the pandemic from 75.8% prepandemic (P<0.0001). Mean baseline home BP was 141.7/81.9 mm Hg during the pandemic and 139.8/82.2 prepandemic, and fell ≈16/9 mm Hg in both periods (P<0.0001). Maintenance during the pandemic was achieved earlier (median 11.8 versus 19.6 weeks, P<0.0001), with more frequent monthly calls (8.2 versus 3.1, P<0.0001) and more monthly home BP recordings per patient (32.4 versus 18.9, P<0.0001), compared with the prepandemic period.
Conclusions
A remote clinical management program was successfully adapted and delivered significant improvements in BP control and increased home BP monitoring despite a nationally observed disruption of traditional hypertension care. Such programs have the potential to transform hypertension management and care delivery.
Keywords: blood pressure, COVID‐19, hypertension, remote patient monitoring, telehealth, telemedicine
Subject Categories: High Blood Pressure, Hypertension, Quality and Outcomes, Digital Health
Nonstandard Abbreviations and Acronyms
- DBP
diastolic blood pressure
- HBPM
home blood pressure monitoring
- SBP
systolic blood pressure
Clinical Perspective.
What Is New?
In our entirely remote clinical management program, hypertension control improved during the COVID‐19 pandemic despite a nationally observed disruption of traditional hypertension care.
The program is team based and interprofessional: navigators delivered algorithm‐based medication titrations with the support of pharmacists, nurse practitioners, and physicians.
Patients measured home blood pressure frequently and consistently during the program, and blood pressure fell significantly.
What Are the Clinical Implications?
As virtual visits become more frequent, an appropriately designed remote management program is well positioned to optimize hypertension control.
Patient engagement in blood pressure control is challenging but necessary.
Hypertension is the single largest contributor to cardiovascular disease and the leading risk factor for death worldwide. 1 , 2 Alarmingly, current rates of blood pressure (BP) control are poor and have recently worsened. 3 Progress has been limited by patient medication and lifestyle nonadherence, 4 clinical inertia, 5 low rates of out‐of‐office BP monitoring (particularly home blood pressure monitoring [HBPM]), 6 and finite health system capacity for frequent visits. 7
The COVID‐19 pandemic threatened to exacerbate this trend by drastically reducing in‐person visits and thereby disrupting care delivery of chronic diseases like hypertension. A large portion of this decline in volume was offset by virtual ambulatory care in the early phase of the pandemic. 8 Some conditions have been effectively managed with virtual care. 9 , 10 In fact, society guidelines strongly recommend telehealth strategies for the accurate diagnosis and adjunctive management of hypertension. 11 Yet BP assessment occurred less frequently in the pandemic era, with the overall number of BP assessments falling dramatically in early 2020, 12 threatening hypertension control at a global level.
Different care models for the remote management of hypertension have been developed; 13 no study has demonstrated if and how they can operate during times of systemwide disruption. We tested the hypothesis that an entirely remote hypertension management program could be adapted and strengthened to achieve successful BP control during the COVID‐19 pandemic.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Design and Setting
In this pre‐ and postpandemic retrospective observational study, we identified patients enrolled in our remote hypertension management program during two 6‐month periods: the “prepandemic” period (September 15, 2019 to March 15, 2020) and the “pandemic” period (March 15, 2020 to September 15, 2020). The program was administered within Mass General Brigham, an integrated health system, and with the support of AllWays Health Partners, both in Boston, Massachusetts. Enterprise‐wide patient collection and analyses for this project were performed under approval from the Mass General Brigham Institutional Review Board, and patients provided verbal consent for clinical participation. The need for written informed consent was waived, as this was considered a quality improvement program delivering care according to practice standards.
Remote Hypertension Management Program
The structure and development of our entirely remote cardiovascular health program has been previously described. 14 , 15 , 16 Our umbrella program enrolls patients with low‐density lipoprotein cholesterol and/or BP above target identified either through direct referral or electronic health record (EHR) screening (with primary care provider assent). This study was limited to our remote hypertension management solution, where patient enrollment was predominantly through direct referral (75% during the prepandemic period and 83% during the pandemic period).
Adults (26–81 years old) with documented uncontrolled hypertension (defined as EHR‐identified systolic BP [SBP] ≥135 mm Hg and/or diastolic BP [DBP] ≥85 mm Hg on readings from at least 2 of the 3 most recent ambulatory encounters in the preceding 2 years; or at least 1 office SBP ≥130 mm Hg or DBP ≥80 mm Hg in the last 2 years with provider referral; or average 24‐hour ambulatory BP ≥130/80 mm Hg) followed actively in the Mass General Brigham system (defined as ≥1 ambulatory visit within the preceding 3 years) were eligible for the remote hypertension program. Key exclusion criteria were age younger than 26 years because of dependency on parents' insurance, confirmed or anticipated pregnancy, active breastfeeding, cognitive impairment, terminal medical condition, BP cuff‐weight incompatibility (male weight >290 pounds, female weight >270 pounds), and chronic kidney disease stages 4 and 5. A full list of inclusion and exclusion criteria is provided in Table S1.
Patients enrolled in the remote hypertension program were provided with a digitally connected (Bluetooth‐ or cellular‐enabled) home BP monitor mailed to their residences. The home BP monitors used in this program were A&D Deluxe BP Monitor/Cuff Med UA‐651BLE and SM UA 651BLE‐V, and BlipCare Model BP 800 with Cuff. Each of these models has been cleared by the Food and Drug Administration for home BP measurement. Additionally, the A&D devices met the US Validated Device Listing criteria after a review by independent experts (see www.validatebp.org). The BlipCare device offered the ability to transmit measurements over a cellular connection, providing an advantage to patients without access to home Wi‐Fi.
Patients were educated by program navigators on proper BP measurement technique, by telephone guidance supplemented with video resources. Following measurement, BP readings were automatically transmitted to an internally developed software suite used for analysis and clinical evaluation. Each patient's program baseline BP was obtained by averaging home readings obtained according to a guideline‐recommended schedule: BP was measured before medications, twice in the morning and twice in the evening for 1 week. 11 Goal BP was defined as mean SBP <130 and DBP <80 mm Hg for most, and SBP <135 and DBP<85 mm Hg for particular subsets, including age over 80 years old and frailty (use of assistive walking device and/or confirmed nonmechanical falls in the last 12 months). Patients whose program baseline home BP was at or below goal were defined as having white coat hypertension (if they were not taking antihypertensive medication) or white coat effect (if they were already taking antihypertensive medication). Patients whose program baseline home BP was above goal were defined as having sustained hypertension. Baseline laboratory values (renal function and electrolytes) were obtained if the patient's most recent measurements were older than 12 months before enrollment.
A clinical, evidence‐based algorithm for hypertension management (including medication initiation, dose escalation, and laboratory monitoring) was developed internally in accordance with society guideline recommendations 11 , 17 and has been previously described and is outlined in Figure S1A and Figure S2. 16 The algorithm recommended first‐line management with angiotensin receptor blockers (ARBs) or angiotensin‐converting enzyme inhibitors (ACEIs), or dihydropyridine calcium channel blockers, based on patient demographic and clinical characteristics.
The algorithm was implemented by teams of nonlicensed navigators, who were trained and supervised by pharmacists prescribing under a Mass General Brigham Collaborative Drug Therapy Management program. A physician disease expert oversaw the pharmacists and the entire program. Our suite of software applications provided electronic decision support and standardized workflows to facilitate automation of the clinical algorithm. The algorithm was implemented until patients reached “maintenance” phase, defined as cessation of active titration due to achievement or close approximation (within 1–2 mm Hg) of goal BP. Transitioning a patient who was not quite at goal BP to maintenance resulted from clinical discussions based on medical judgment and patient preference. The primary mode of communication between enrolled patients and navigators was the telephone. Additional communication modalities of messaging by text or through our secure electronic portal were used based on patient preference.
Pandemic‐Driven Program Adaptations
As the COVID‐19 caseload increased in Massachusetts, the algorithm was modified to address pandemic‐related challenges. Through the early stages of complete lockdown and beyond, patients were averse to leaving home to have blood drawn at a clinic or laboratory. Therefore, a decision was made to remove ARBs and ACEIs as first‐line drugs. Calcium channel blockers rose to the top of the prescribing algorithm for most patients. Importantly, this decision was not made because of concerns that ARBs and ACEIs might increase the risk of COVID‐19; our program followed professional society recommendations that these medications should not be discontinued because of concerns about COVID‐19. If a patient's BP was clearly hypertensive and treatment with an ARB, ACEI, or diuretic was required, conservative dosing was initialized to maximize safety. In addition, the mandate for laboratory testing was relaxed for patients being titrated to intermediate doses of drugs; laboratory values were always obtained when final doses were reached. Beta blockers and/or mineralocorticoid receptor antagonists were used if an additional agent was needed. These adaptations to our clinical algorithm are presented in Figure S1B.
Outcomes
The primary outcome of this study was mean change in home SBP and DBP with remote management. Outcome data were collected through internal program reporting and review of patient charts in the EHR through March 15, 2021 to allow for at least 6 months of program participation for any given patient. Secondary outcomes included the proportion of patients who achieved maintenance, the proportion of patients who were diagnosed with white coat hypertension/effect, and the average number of BP readings obtained throughout program enrollment and per month.
Statistical Analysis
Baseline demographic data and laboratory values were extracted from the EHR. Once a patient was enrolled in the program, all pertinent data were stored in a custom database and confirmed by chart review. All analyses of primary and secondary outcomes were performed in Tableau (2020.2.1) and R (version 4.1.2). Continuous variables are reported as means with SDs or medians with interquartile ranges and were tested for significance using 2‐tailed t tests. Categorical variables are reported as frequencies and proportions and were tested for significance using χ2 or binomial tests, as appropriate. The primary outcome (change in BP) was evaluated with paired t tests to account for the correlation between baseline and exit BP within a given subject. Additionally, a time to event analysis was performed to estimate the probability that a patient entered maintenance based upon a patient's time of enrollment into the program (prepandemic period versus postpandemic period) and duration of participation. The analysis was adjusted to censor for patients who actively dropped out or passively became unreachable.
Results
A total of 651 patients during the pandemic period and 605 patients during the prepandemic period were enrolled in the remote hypertension management program and recorded a week of BP readings to establish a baseline average. Of these, 477 (73.3%) pandemic and 512 (84.6%) prepandemic patients were determined to have sustained hypertension and met criteria for medication titration. Baseline characteristics of all enrolled patients were similar between the pandemic and prepandemic groups except for 3 striking differences: in the pandemic group, there was a greater proportion of white coat hypertension and/or effect (26.7 versus 15.4%, P<0.0001), non‐White race (46.5% versus 28.3%, P<0.001), and non‐English preferred language (18.6% versus 3.5%, P<0.001) compared with the prepandemic group (Tables 1 and 2). Comparing those who were found to have white coat hypertension and/or effect versus sustained hypertension after establishing a baseline home BP, the only difference significant in both periods was body mass index, which was within obesity range for patients with sustained hypertension (Table S2). Although there was a greater proportion of women with white coat hypertension, the difference did not reach statistical significance.
Table 1.
Demographic and Clinical Characteristics of All Enrolled Participants at Baseline
|
Prepandemic September 2019–March 2020 (n=605) |
Pandemic March 2020–September 2020 (n=651) |
P value | |
|---|---|---|---|
| Age, y, median (interquartle range) | 62 (18) | 64 (16) | 0.63 |
| Female sex, n (%) | 336 (55.5) | 380 (58.4) | 0.16 |
| Non‐White race, n (%) | 177 (29.3) | 303 (46.5) | <0.001 |
| Baseline renal function, mean (SD) | |||
| Most recent estimated glomerular filtration rate, mL/min per 1.73 m2 body surface area | 79.6 (18.9) | 79.3 (17.8) | 0.83 |
| Most recent serum creatinine, mg/dL | 0.92 (0.24) | 0.91 (0.24) | 0.56 |
| Most recent serum potassium, mmol/L | 4.19 (0.40) | 4.14 (0.41) | 0.04 |
| Comorbidities | |||
| Atherosclerotic cardiovascular disease, n (%) | 131 (21.2) | 160 (24.6) | 0.10 |
| Type 2 diabetes, n (%) | 157 (26.0) | 179 (27.5) | 0.41 |
| Hyperlipidemia based on uncontrolled low‐density lipoprotein cholesterol, n (%) | 81 (13.4) | 43 (6.6) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 31.8 (9.4) | 31.2 (7.0) | 0.19 |
| Non‐English preferred language, n (%) | 21 (3.5) | 121 (18.6) | <0.001 |
Table 2.
Demographic and Clinical Characteristics of Participants with Sustained Hypertension at Baseline
|
Prepandemic September 2019–March 2020 (N=512) |
Pandemic March 2020–September 2020 (N=477) |
P value | |
|---|---|---|---|
| Age, y, median (interquartile range) | 61 (19) | 64 (16) | 0.79 |
| Female sex, n (%) | 280 (54.7) | 270 (56.6) | 0.40 |
| Non‐White race, n (%) | 150 (29.3) | 232 (48.6) | <0.001 |
| Baseline renal function, mean (SD) | |||
| Most recent estimated glomerular filtration rate, mL/min per 1.73 m2 body surface area | 79.6 (19.2) | 78.4 (18.4) | 0.98 |
| Most recent serum creatinine, mg/dL | 0.92 (0.24) | 0.92 (0.25) | 0.69 |
| Most recent serum potassium, mmol/L | 4.18 (0.40) | 4.14 (0.42) | 0.73 |
| Comorbidities | |||
| Atherosclerotic cardiovascular disease, n (%) | 118 (23.0) | 124 (26.0) | 0.13 |
| Type 2 diabetes, n (%) | 139 (27.1) | 137 (28.7) | 0.46 |
| Hyperlipidemia based on uncontrolled low‐density lipoprotein cholesterol, n (%) | 73 (14.3) | 35 (7.3) | P<0.001 |
| Body mass index, kg/m2, mean (SD) | 32.2 (9.6) | 31.8 (7.2) | 0.50 |
| Non‐English preferred language, n (%) | 19 (3.7) | 93 (19.5) | P<0.001 |
Among all patients with sustained hypertension, mean baseline home SBP was 144.7 (14.7) mm Hg during the pandemic, significantly higher than prepandemic 141.8 (14.0 mm Hg; P=0.006), and fell ≈10/6 mm Hg in both groups (Table 3 and Figure 1A). Examining only the patients with sustained hypertension who reached the maintenance phase of the program (where medication titration ceased), target BP was reached by significantly more patients during the pandemic (94.6% versus 75.8%, P<0.0001). BP fell ≈16/9 mm Hg in both cohorts (Table 3 and Figure 1B). Patients who achieved maintenance during the pandemic took a mean of 1.5 (1.0) antihypertensive medications before enrollment in the program, underwent a mean of 1.6 (1.4) titrations throughout the program, and took a mean of 2.3 (1.0) medications at maintenance (P<0.00001). Patients who achieved maintenance during the prepandemic period took a mean of 1.5 (1.1) antihypertensive medications before enrollment, underwent a mean of 1.8 (1.9) titrations throughout the program, and took a mean of 1.9 (1.1) medications at maintenance. During the pandemic, maintenance was achieved earlier (median 11.8 [interquartile range 6, 21.3] versus 19.6 [6.4, 38.5] weeks, P<0.0001) with a greater frequency of phone calls per month between navigators and patients (mean 8.2 [7.1] versus 3.1 [6.8], P<0.0001) phone calls per month between patients and program navigators. Because patients enrolled late in the prepandemic period were partly managed during the pandemic period, we performed a time to maintenance analysis based on patients' enrollment period and duration of their participation, censoring patients who dropped out of the program either by actively withdrawing or passively becoming unreachable (Figure 2 and Table S3). We found that the probability of entering maintenance more than doubled at 6 months post enrollment and remained significantly higher for patients enrolled during the pandemic versus prepandemic period (respectively, 53.4% [95% CI, 48.4%–58.7%] versus 25.3% [95% CI, 21.0%–29.4%]).
Table 3.
Clinical Outcomes of the Remote Hypertension Management Program Among Patients With Sustained Hypertension
|
Prepandemic September 2019–March 2020 (n=512) |
Pandemic March 2020–September 2020 (n=475) |
|||||||
|---|---|---|---|---|---|---|---|---|
| All patients with documented baseline and exit BP* | Baseline | Exit | Change at exit, mm Hg (%) | P value | Baseline | Exit | Change at exit, mm Hg (%) | P value |
| SBP, mm Hg | 141.8 (14.0) | 131.9 (15.7) | −9.9 (12.2) | <0.0001 | 144.7 (14.7) | 134.6 (15.3) | −10.1 (7.0) | <0.0001 |
| DBP, mm Hg | 83.4 (9.7) | 77.3 (9.9) | −6.1 (6.9) | <0.0001 | 84.2 (9.3) | 78.6 (9.9) | −5.6 (6.7) | <0.0001 |
| Patients who reached maintenance† | Prepandemic (N=265) | Pandemic (N=241) | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP, mm Hg | 139.8 (11.8) | 123.7 (7.5) | −16.1 (11.1) | <0.0001 | 141.7 (12.1) | 125.4 (6.5) | −16.3 (11.5) | <0.0001 |
| DBP, mm Hg | 82.2 (8.6) | 73.5 (6.8) | −8.7 (6.7) | <0.0001 | 81.9 (8.2) | 73.1 (6.5) | −8.8 (10.7) | <0.0001 |
| Patients who exited without reaching maintenance | Prepandemic (N=244) | Pandemic (N=234) | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP, mm Hg | 145.6 (16.8) | 143.3 (17.0) | −2.3 (11.3) | 0.26 | 147.7 (16.4) | 144.0 (15.9) | −3.7 (2.5) | 0.013 |
| DBP, mm Hg | 85.8 (11.3) | 82.7 (11.0) | −3.1 (5.5) | 0.02 | 86.5 (9.7) | 84.3 (9.6) | −2.2 (2.5) | 0.013 |
BP indicates blood pressure; DBP, diastolic blood pressure; and SBP, systolic blood pressure. All data are presented as mean (SD).
P values associated with differences in baseline SBP and DBP in the pandemic versus prepandemic groups were 0.006 and 0.19 respectively.
There were 64 and 13 patients in the prepandemic and pandemic groups, respectively, who reached maintenance without reaching their goal BP. Among prepandemic patients, mean SBP was 136.4 (SD 9.4) at baseline and 128.8 mm Hg (SD 10.3) at exit (P=0.004); mean DBP was 82.8 (SD 6.9) at baseline and 78.6 (SD 5.7) mm Hg at exit (P=0.009). Among pandemic patients, mean SBP was 144.8 (SD 15.6) at baseline and 133.6 mm Hg at exit (P=0.02); mean DBP was 87.4 (SD 10.2) at baseline and 83.3 (SD 2.7) mm Hg at exit (P=0.15).
Figure 1. Blood pressure changes in participants with sustained hypertension in the remote hypertension management program before and during the COVID‐19 pandemic.
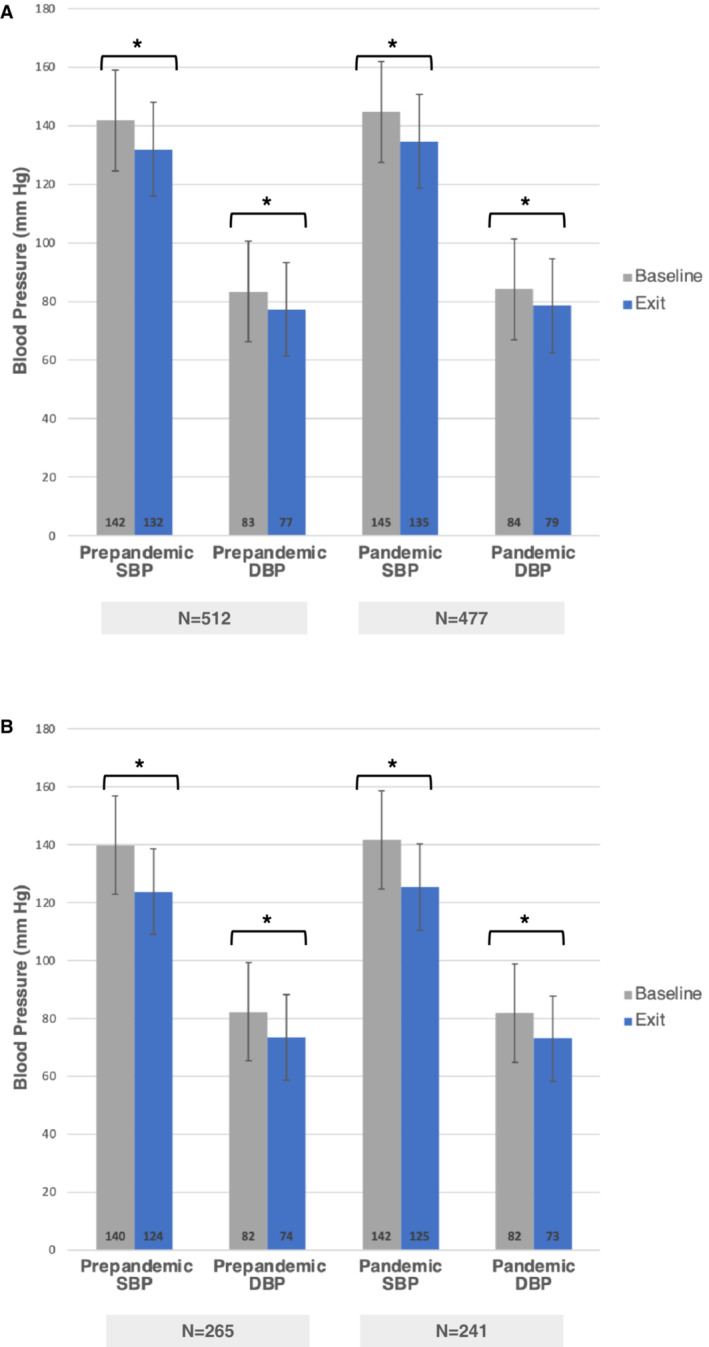
A, Changes in all participants who were found to have sustained hypertension. B, Changes only in those participants with sustained hypertension who reached maintenance. *P<0.0001. SBP indicates systolic blood pressure; and DBP, diastolic blood pressure.
Figure 2. Time to maintenance analysis by enrollment period.
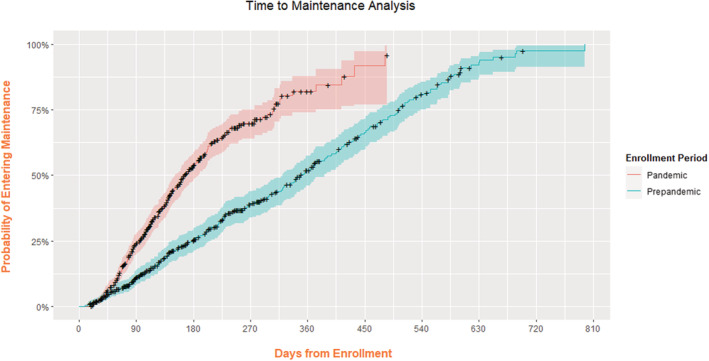
Each data point indicates the cumulative probability that a patient enters maintenance based on the duration of their participation in the program (days from enrollment). Patients who dropped out of the program are censored. Colored shading indicates 95% CIs.
There were 244 (47.7%) prepandemic and 234 (49.1%) pandemic patients with sustained hypertension who did not reach maintenance. Of these, the majority (76.8%) dropped out of the program, with 61 (16.6%) actively notifying the program of their decision to leave and the remaining patients 306 (83.4%) becoming unreachable by telephone. During the pandemic, overall dropout rate fell, though this change did not reach statistical significance (35.6 during the pandemic period versus 38.5% in the prepandemic period, P=0.36). Among all patients who dropped out during the pandemic, the proportion of patients who actively notified the program of their decision to withdraw was similar to prepandemic rates (21.2% versus 12.7%, P=0.08) but lower among patients who became unreachable by telephone (78.8% versus 87.3%, P<0.0001). The remaining patients who did not reach maintenance (27.1% during the pandemic versus 19.3% prepandemic, P=0.06) were referred back to their providers or to a specialist because of medical complexity that included, for example, active severe or confounding illness, multiple medication intolerances, or resistant hypertension. Despite the fact that they did not reach maintenance, these patients had a notable fall in BP of 3.7/2.0 (10.1/5.6) mm Hg. Program and clinical outcomes are further detailed in Tables 3 and 4 and Figures 3A and 3B.
Table 4.
Overall Program Outcomes of the Remote Hypertension Management Program Among Participants With Sustained Hypertension
|
Prepandemic September 2019–March 2020 (N=512) |
Pandemic March 2020–September 2020 (N=477) |
P value | |
|---|---|---|---|
| Reached maintenance, n (%) | 265 (51.8) | 241 (50.5) | 0.60 |
| Reached BP goal | 201 (39.3) | 228 (47.8) | <0.001 |
| Did not reach BP goal | 64 (12.5) | 13 (2.7) | <0.001 |
| Exited without reaching maintenance, n (%) | 244 (47.7) | 234 (49.1) | 0.54 |
| Dropped out, n (%) | 197 (38.5) | 170 (35.6) | 0.36 |
| Referred back to referring provider, n (%) | 47 (9.2) | 64 (13.4) | 0.06 |
| Still under active titration at study end, n (%) | 3 (0.4) | 2 (0.4) | 0.48 |
“Maintenance” is defined as the phase of participation in which active titration terminates because of achievement or close approximation (within 1–2 mm Hg) of goal blood pressure.
Figure 3. Enrollment and follow‐up (through March 2021) of patients in the remote hypertension management program.
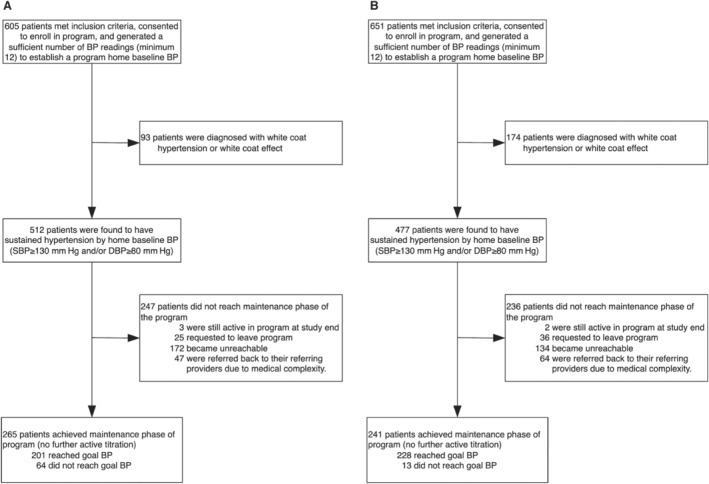
A, Enrollment during the prepandemic period (September 2019–March 2020). B, Enrollment during the pandemic period (March–September 2020).
Compared with the prepandemic period, patients in the pandemic period recorded a similar average volume of remote home BP readings (138.5 [151.9] versus 130.2 [147.3], P=0.33) even as the average volume of office BP readings fell (6.0 [6.0] versus 7.1 [8.1], P=0.003). On average, patients during the pandemic measured their BP at home more frequently: 32.4 (27.0) versus 18.9 (19.5) readings per patient per month (P<0.0001).
Discussion
In this pre‐ and postpandemic retrospective observational study of individuals with uncontrolled hypertension, we report several findings. An entirely remote hypertension management program was associated with significant and larger improvements in BP control despite systemic disruptions in care delivery because of the COVID‐19 pandemic. This program was additionally associated with significant increases in home BP monitoring. Critically, these results were achieved despite enrolling a more traditionally underserved patient population during the COVID‐19 pandemic.
Our remote care delivery program demonstrated striking results in achieving hypertension control. In our patients with sustained hypertension (whose BP required medication uptitration) and who reached maintenance, the proportion who had BP in target increased from 75.8% prepandemic to 94.6% during the pandemic, despite systemwide disruptions in care delivery during the first wave of the COVID‐19 pandemic. These outcomes contrast with data demonstrating a marked fall in national BP assessments during virtual versus office‐based visits and 50% fewer overall BP assessments during the early pandemic. 12 They also outpace recent national estimates of control (≈24% based on the 2017 American College of Cardiology/American Heart Association guidelines). 18 Furthermore, the decrease in mean SBP/DBP of ≈16/9 mm Hg among patients with sustained hypertension is much greater than typical decreases (3 to 9 mm Hg SBP) observed for contemporary telemedicine/telemonitoring hypertension interventions, 19 , 20 nonpharmacological interventions, 11 and even pharmacologic treatment. 21 SBP reductions of this magnitude are associated with ≈40% relative risk reduction in major cardiovascular events and all‐cause mortality, 22 and DBP is also independently associated with adverse cardiovascular events, especially in younger people. 23 , 24
Our program's effectiveness was most likely dependent upon our extensive experience with delivering fully remote hypertension care before the pandemic. Our program was piloted among 130 patients in 2017, scaled throughout 2018, and fully operational in 2019. 14 , 16 This growth permitted development of our full‐service delivery model's key evidence‐based features: frequent and active home BP monitoring; proactive, interdisciplinary teams including patient navigators as the main patient contacts and pharmacists; and frequent medication management supported by clinical algorithms. Each of these features has proven more effective in achieving BP control and modestly lowering BP than usual care, self‐monitoring, or telemonitoring without medication adjustment. 13 , 19 , 20 , 25 , 26 , 27 Our program's intervention combines many of these evidence‐based components, which may explain its association with larger reductions in BP. Experience building a completely remote hypertension care program also readied our team to adapt and refine our model when the COVID‐19 pandemic completely disrupted the broader health care system. As a prime example, we recognized severe patient hesitancy around laboratory testing because of fear of exposure to COVID‐19. The clinical algorithm was therefore modified to prioritize calcium channel blockers before ACEI/ARBs and thiazide diuretics, to avoid necessary laboratory monitoring of renal function and serum electrolytes. 28 In contrast to our team's nearly seamless transition to scaled, fully remote care, most systems were only minimally using telemedicine before the pandemic and were forced to launch new programs in days to weeks, without time to plan, pilot test, and refine. 29
As health systems turn to building sustainable remote or hybrid models of care for the ongoing pandemic era and beyond, successful examples can serve as templates. One particularly important driver of our observed outcomes was likely frequency of interaction. Our program was able to support ≈2 clinical interactions with patients per week because of the novel incorporation of nonlicensed navigators in our team structure. These navigators implemented medication management according to our clinical algorithm. Unlike advanced practice practitioners (eg, nurse practitioners and/or pharmacists) who are engaged in clinical duties, patient navigators had time to provide key continual support, including reminders to monitor BP at home and lifestyle counseling. 16 This type of frequent provider‐initiated interaction can improve adherence (typically a barrier for approximately one third of hypertensive patients managed in traditional settings) 30 through increased accountability and patient–provider trust. 31 In contrast, hypertension management through traditional models of care was characterized by infrequent visits before the COVID‐19 pandemic 32 , 33 and significantly reduced in‐office BP assessments during the pandemic. 12
Another major finding of our study is that remote hypertension management programs can yield a robust set of BP measurements through engaging patients in HBPM. Society guidelines recommend 1‐month in‐office reassessment intervals. 11 , 17 However, in practice, visits for hypertension management usually occur fewer than 5 times per year, 32 and office BP assessments decreased by 50% in the early phase of the COVID‐19 pandemic. 12 In contrast, the average number of BP measurements obtained by most patients who engage in HBPM is at least 1 per month. 34 HBPM has superior risk prediction of long‐term cardiovascular disease outcomes compared with office‐based BP measurement. 35 Increasing the volume of home BP measurements helps improve patient engagement and medication adherence, especially when combined with other strategies, such as telephone‐based counseling by nurses or pharmacists or app‐based coaching. 36 , 37 Increasing HBPM additionally facilitates better medical management of hypertension, enhancing providers' ability to assess response to therapy and potential adverse effects.
Implementation of HBPM was valuable in detecting the white coat phenomenon in 15% to 27% of our patients whose average baseline home BP was controlled. Recognizing this diagnosis prevented excessive medication and potential adverse effects in these patients, especially important during the pandemic, given limited access to laboratory monitoring. During the pandemic period, patients with white coat hypertension/effect in our study were more likely to be White and trended toward a greater likelihood of being female, consistent with previously established demographic and clinical profiles of patients with white coat hypertension. 38 The higher prevalence of white coat hypertension among our patients compared with other cohorts 39 may be related to lenient referral criteria (office BP ≥130/80 mm Hg) designed to support referring providers, especially during the pandemic.
A final major finding of our study was the expansion of the program's ability to reach vulnerable patient populations. During the pandemic period, the proportion of non‐White patients increased by almost 60%, and the proportion of non‐English speaking patients increased more than 5‐fold. This contrasts with data on the digital divide during the early pandemic, demonstrating that patients with these characteristics (among others such as older age and insurance type) were less likely to complete ambulatory telemedicine visits. 40 Our results were partly driven by concurrent expansion of the program's Spanish‐speaking navigator team. The program's more inclusive care was also supported by a strategic relationship developed with our health system's Population Health group and with interpreter services, which increased awareness of our program for non‐English speaking people among providers caring for underserved patients. We offered several types of devices, all at no cost, including cellular‐based units that did not require home Wi‐Fi, the downloading of apps, or pairing of devices, to facilitate use among patients of older age and low socioeconomic status who might have less comfort with digital technologies. These patients, in addition to members of racial and ethnic minority groups and non‐fluent English‐speaking patients, have been shown to have worse hypertension outcomes with traditionally delivered care. 41 , 42 , 43
Limitations to our study include a cohort within one health care system, small sample size, and observational design. Our program encountered challenges with patient dropout; about one‐third of patients dropped out of the program before reaching maintenance. Patient adherence with hypertension treatment is notoriously difficult, 30 with well‐known demographic, socioeconomic, medical, behavioral, and therapy‐related contributors. We surveyed a subset of patients who dropped out of our program and found these main reasons: belief that their condition was controlled, preference to work directly with their physicians, lack of comfort with intensification of medication therapy, and perception that the program was inconvenient. 44 Additional limitations inherent to this program may have hindered greater persistence. It relied predominantly upon telephone‐based communication during business hours (after‐hours phone calls were made when possible to accommodate specific requests, and text messaging was being introduced during this period). Given prevalent preferences for nontelephonic modes of communication, we have since expanded our text messaging capabilities. There was also significant variation in patient onboarding. Referrals to the program were expedited via orders placed directly in the EHR. Patient engagement would likely have been enhanced if all patients had the benefit of detailed explanation and discussion with their providers. Future work must focus on the implementation of strategies to create and maintain high levels of patient engagement. Potential limitations in generalizability must be acknowledged, given that providing home BP monitors may not be a financially viable option for all institutions. Finally, our program was unable to address patients with apparent resistant hypertension during this study. We have since developed “Hypertension Plus,” an intensified clinical pathway and extended algorithm that includes phone calls with a nurse practitioner (focusing on risk factors, lifestyle modification, and medication adherence), weekly rounding with a supervising physician, and evaluation for causes of secondary hypertension.
Conclusions
This observational study in individuals with uncontrolled hypertension demonstrates the ability to improve remote management of hypertension even during major disruptions like the COVID‐19 pandemic. Integrated remote hypertension management programs can dramatically improve BP control and home BP data quality. Such solutions have significant potential to transform the delivery of care for chronic diseases.
Sources of Funding
S.G. Lee is supported by the National Institutes of Health grant T32HL007604.
Disclosures
Dr Scirica receives institutional grants though Brigham and Women's Hospital from Pfizer, Merck, Eisai, NovoNordisk, and Novartis; consulting fees from Abbvie, Allergan, AstraZeneca, Boehringer Ingelheim, Eisai, Esperion, Hamni, Lexicon, Medtronic, Merck, and NovoNordisk; and has equity in Heath at Scale.
Dr Cannon receives research grants from Amgen, Better Therapeutics, Boehringer‐Ingelheim (BI), Bristol‐Myers Squibb (BMS), Daiichi Sankyo, Janssen, Merck, Novo Nordisk, Pfizer; and consulting fees from Aegerion/Amryt, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, BI, BMS, Eli Lilly, Janssen, Lexicon, Merck, Pfizer, Rhoshan, and Sanofi. He serves on Data and Safety Monitoring Boards for the Veterans Administration, Applied Therapeutics, and NovoNordisk.
Dr Fisher receives research funding from and is a consultant to Recor Medical and is a consultant to Medtronic and Aktiia.
Dr Gordon reports consulting income from the Office of the National Coordinator for Health IT and Novocardia, Inc., both outside the scope of this work.
Dr Blood receives research grants from Novo Nordisk and General Electric and reports consulting income from the Walgreens Health and Inlightened.
Supporting information
Tables S1–S3
Figures S1–S2
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027296
For Sources of Funding and Disclosures, see page 10.
References
- 1. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Abate KH, Akinyemiju TF, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990‐2015. JAMA. 2017;317:165–182. doi: 10.1001/JAMA.2016.19043 [DOI] [PubMed] [Google Scholar]
- 2. Zhou B, Carrillo‐Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B, Singleton RK, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999‐2000 to 2017‐2018. JAMA. 2020;324:1190–1200. doi: 10.1001/JAMA.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124:1124–1140. doi: 10.1161/CIRCRESAHA.118.313220 [DOI] [PubMed] [Google Scholar]
- 5. Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:5. doi: 10.1161/JAHA.116.004188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ostchega Y, Zhang G, Kit BK, Nwankwo T. Factors associated with home blood pressure monitoring among US adults: National Health and Nutrition Examination Survey, 2011–2014. Am J Hypertens. 2017;30:1126–1132. doi: 10.1093/AJH/HPX101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bodenheimer T, Pham HH. Primary care: current problems and proposed solutions. Health Aff (Millwood). 2010;29:799–805. doi: 10.1377/HLTHAFF.2010.0026 [DOI] [PubMed] [Google Scholar]
- 8. Patel SY, Mehrotra A, Huskamp HA, Uscher‐Pines L, Ganguli I, Barnett ML. Trends in outpatient care delivery and telemedicine during the COVID‐19 pandemic in the US. JAMA Intern Med. 2021;181:388–391. doi: 10.1001/jamainternmed.2020.5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García‐Lizana F, Muñoz‐Mayorga I. What about telepsychiatry? A systematic review. Prim Care Companion J Clin Psychiatry. 2010;12:PCC.09m00831. doi: 10.4088/PCC.09M00831WHI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JJ, 3rd English JC. Teledermatology: a review and update. Am J Clin Dermatology. 2018;19:253–260. doi: 10.1007/S40257-017-0317-6 [DOI] [PubMed] [Google Scholar]
- 11. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:E13–E115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 12. Alexander GC, Tajanlangit M, Heyward J, Mansour O, Qato DM, Stafford RS. Use and content of primary care office‐based vs telemedicine care visits during the COVID‐19 pandemic in the US. JAMA Netw Open. 2020;3:e2021476. doi: 10.1001/jamanetworkopen.2020.21476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Omboni S, McManus RJ, Bosworth HB, Chappell LC, Green BB, Kario K, Logan AG, Magid DJ, Mckinstry B, Margolis KL, et al. Evidence and recommendations on the use of telemedicine for the management of arterial hypertension. Hypertension. 2020;76:1368–1383. doi: 10.1161/HYPERTENSIONAHA.120.15873 [DOI] [PubMed] [Google Scholar]
- 14. Scirica BM, Cannon CP, Fisher NDL, Gaziano TA, Zelle D, Chaney K, Miller A, Nichols H, Matta L, Gordon WJ, et al. Digital care transformation: interim report from the first 5000 patients enrolled in a remote algorithm‐based cardiovascular risk management program to improve lipid and hypertension control. Circulation. 2021;143:507–509. doi: 10.1161/circulationaha.120.051913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plutzky J, Benson MD, Chaney K, Bui TV, Kraft M, Matta L, McPartlin M, Zelle D, Cannon CP, Dodek A, et al. Population health identification and management of low density lipoprotein cholesterol via a remote, algorithmic, navigator‐executed program. Am Heart J. 2021;243:15–27. doi: 10.1016/J.AHJ.2021.08.017 [DOI] [PubMed] [Google Scholar]
- 16. Fisher NDL, Fera LE, Dunning JR, Desai S, Matta L, Liquori V, Pagliaro J, Pabo E, Merriam M, MacRae CA, et al. Development of an entirely remote, non‐physician led hypertension management program. Clin Cardiol. 2019;42:285–291. doi: 10.1002/CLC.23141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi: 10.1001/JAMA.2013.284427 [DOI] [PubMed] [Google Scholar]
- 18. Ritchey MD, Gillespie C, Wozniak G, Shay CM, Thompson‐Paul AM, Loustalot F, Hong Y. Potential need for expanded pharmacologic treatment and lifestyle modification services under the 2017 ACC/AHA hypertension guideline. J Clin Hypertens. 2018;20:1377–1391. doi: 10.1111/JCH.13364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mills KT, Obst KM, Shen W, Molina S, Zhang HJ, He H, Cooper LA, He J. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta‐analysis. Ann Intern Med. 2018;168:110–120. doi: 10.7326/M17-1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team‐based care interventions for hypertension: a meta‐analysis. Arch Intern Med. 2009;169:1748–1755. doi: 10.1001/ARCHINTERNMED.2009.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blood Pressure Lowering Treatment Trialists' Collaboration BPLTT . Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta‐analysis of randomised trials. BMJ. 2008;336:1121–1123. doi: 10.1136/BMJ.39548.738368.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, He H, Chen J, Whelton PK, He J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta‐analysis. JAMA Cardiol. 2017;2:775–781. doi: 10.1001/JAMACARDIO.2017.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vishram‐Nielsen JKK, Kristensen AMD, Pareek M, Laurent S, Nilsson PM, Linneberg A, Greve SV, Palmieri L, Giampaoli S, Donfrancesco C, et al. Predictive importance of blood pressure characteristics with increasing age in healthy men and women. Hypertension. 2021;77:1076–1085. doi: 10.1161/HYPERTENSIONAHA.120.16354 [DOI] [PubMed] [Google Scholar]
- 24. Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMOA1803180 [DOI] [PubMed] [Google Scholar]
- 25. Shaw RJ, McDuffie JR, Hendrix CC, Edie A, Lindsey‐Davis L, Nagi A, Kosinski AS, Williams JW. Effects of nurse‐managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta‐analysis. Ann Intern Med. 2014;161:113–121. doi: 10.7326/M13-2567 [DOI] [PubMed] [Google Scholar]
- 26. Santschi V, Chiolero A, Colosimo AL, Platt RW, Taffé P, Burnier M, Burnand B, Paradis G. Improving blood pressure control through pharmacist interventions: a meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:3. doi: 10.1161/JAHA.113.000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, Earle K, George J, Godwin M, Green BB, et al. Self‐monitoring of blood pressure in hypertension: a systematic review and individual patient data meta‐analysis. PLoS Med. 2017;14:14. doi: 10.1371/JOURNAL.PMED.1002389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saqib MAN, Siddiqui S, Qasim M, Jamil MA, Rafique I, Awan UA, Haroon M, Afzal MS. Effect of COVID‐19 lockdown on patients with chronic diseases. Diabetes Metab Syndr Clin Res Rev. 2020;14:1621–1623. doi: 10.1016/J.DSX.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehrotra A, Ray K, Brockmeyer DM, Barnett ML, Bender JA. Rapidly converting to “virtual practices”: outpatient care in the era of Covid‐19. NEJM Catal. 2020;1:1–5. doi: 10.1056/CAT.20.0091 [DOI] [Google Scholar]
- 30. Chang TE, Ritchey MD, Park S, Chang A, Odom EC, Durthaler J, Jackson SL, Loustalot F. National rates of nonadherence to antihypertensive medications among insured adults with hypertension, 2015. Hypertension. 2019;74:1324–1332. doi: 10.1161/HYPERTENSIONAHA.119.13616 [DOI] [PubMed] [Google Scholar]
- 31. Netemeyer RG, Dobolyi DG, Abbasi A, Clifford G, Taylor H. Health literacy, health numeracy, and trust in doctor: effects on key patient health outcomes. J Consum Aff. 2020;54:3–42. doi: 10.1111/JOCA.12267 [DOI] [Google Scholar]
- 32. Guthmann R, Davis N, Brown M, Elizondo J. Visit frequency and hypertension. J Clin Hypertens. 2005;7:327–332. doi: 10.1111/J.1524-6175.2005.04371.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. King CC, Bartels CM, Magnan EM, Fink JT, Smith MA, Johnson HM. The importance of frequent return visits and hypertension control among US young adults: a multidisciplinary group practice observational study. J Clin Hypertens. 2017;19:1288–1297. doi: 10.1111/jch.13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Viera AJ, Cohen LW, Mitchell CM, Sloane PD. How and why do patients use home blood pressure monitors? Blood Press Monit. 2008;13:133–137. doi: 10.1097/MBP.0B013E32830263B7 [DOI] [PubMed] [Google Scholar]
- 35. Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192–204. doi: 10.7326/M14-1539 [DOI] [PubMed] [Google Scholar]
- 36. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens. 2006;8:174–180. doi: 10.1111/J.1524-6175.2006.04872.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gazit T, Gutman M, Beatty AL. Assessment of hypertension control among adults participating in a mobile technology blood pressure self‐management program. JAMA Netw Open. 2021;4:e2127008. doi: 10.1001/JAMANETWORKOPEN.2021.27008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas O, Shipman KE, Day K, Thomas M, Martin U, Dasgupta I. Prevalence and determinants of white coat effect in a large UK hypertension clinic population. J Hum Hypertens 2016 306. 2015;30:386–391. doi: 10.1038/JHH.2015.95 [DOI] [PubMed] [Google Scholar]
- 39. Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Smith N, Webber E, Perdue LA, Bigler KD, Whitlock EP. Screening for High Blood Pressure in Adults: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 121. AHRQ Publication No. 13‐05194‐EF‐1. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 40. Eberly LA, Kallan MJ, Julien HM, Haynes N, Khatana SAM, Nathan AS, Snider C, Chokshi NP, Eneanya ND, Takvorian SU, et al. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID‐19 pandemic. JAMA Netw Open. 2020;3:e2031640. doi: 10.1001/jamanetworkopen.2020.31640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bromfield SG, Bowling CB, Tanner RM, Peralta CA, Odden MC, Oparil S, Muntner P. Trends in hypertension prevalence, awareness, treatment, and control among US adults 80 years and older, 1988–2010. J Clin Hypertens. 2014;16:270–276. doi: 10.1111/JCH.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shahu A, Herrin J, Dhruva SS, Desai NR, Davis BR, Krumholz HM, Spatz ES. Disparities in socioeconomic context and association with blood pressure control and cardiovascular outcomes in ALLHAT. J Am Heart Assoc. 2019;8:8. doi: 10.1161/JAHA.119.012277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim EJ, Kim T, Paasche‐Orlow MK, Rose AJ, Hanchate AD. Disparities in hypertension associated with limited English proficiency. J Gen Intern Med 2017 326. 2017;32:632–639. doi: 10.1007/S11606-017-3999-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blood AJ, Cannon CP, Gordon WJ, Mailly C, MacLean T, Subramaniam S, Tucci M, Crossen J, Nichols H, Wagholikar KB, et al. Results of a remotely delivered hypertension and lipid program in more than 10 000 patients across a diverse health care network. JAMA Cardiol. 2023. 2;8:12–21. doi: 10.1001/JAMACARDIO.2022.4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2


