Abstract
Background
Mitochondrial abnormalities exist in gastrocnemius muscle of people with peripheral artery disease (PAD). Whether abnormalities in mitochondrial biogenesis and autophagy are associated with greater ischemia or walking impairment in PAD is unknown.
Methods and Results
Protein markers of mitochondrial biogenesis and autophagy and the abundance of mitochondrial electron transport chain complexes were quantified in gastrocnemius muscle biopsies from people with and without PAD. Their 6‐minute walk distance and 4‐m gait speed were measured. Sixty‐seven participants (mean age 65.0 years [±6.8], 16 [23.9%] women, 48 [71.6%] Black) were enrolled, including 15 with moderate to severe PAD (ankle brachial index [ABI] <0.60), 29 with mild PAD (ABI 0.60–0.90), and 23 without PAD (ABI 1.00–1.40). Abundance of all electron transport chain complexes was significantly higher in participants with lower ABI (eg, complex I: 0.66, 0.45, 0.48 arbitrary units [AU], respectively, P trend=0.043). Lower ABI values were associated with a higher LC3A/B II‐to‐LC3A/B I (microtubule‐associated protein 1A/1B‐light chain 3) ratio (2.54, 2.31, 2.15 AU, respectively, P trend=0.017) and reduced abundance of the autophagy receptor p62 (0.71, 0.69, 0.80 AU, respectively, P trend=0.033). The abundance of each electron transport chain complex was positively and significantly associated with 6‐minute walk distance and 4‐m gait speed at usual and fast pace only among participants without PAD (eg, complex I: r=0.541, P=0.008; r=0.477, P=0.021; r=0.628, P=0.001, respectively).
Conclusions
These results suggest that accumulation of electron transport chain complexes in gastrocnemius muscle of people with PAD may be because of impaired mitophagy in the setting of ischemia. Findings are descriptive, and further study in larger sample sizes is needed.
Keywords: ankle‐brachial index, electron transport chain, mitochondrial biogenesis, mtDNA damage, muscle
Subject Categories: Peripheral Vascular Disease
Nonstandard Abbreviations and Acronyms
- ETC
electron transport chain
- LC3A/B
microtubule‐associated protein 1A/1B‐light chain 3
- mtDNA
mitochondrial DNA
- NRF1
nuclear respiratory factor 1
- PGC‐1α
peroxisome proliferator‐activated receptor‐gamma coactivator
- PINK1
phosphatase and tensin homolog‐induced kinase 1
- SPPB
short physical performance battery
- TFAM
mitochondrial transcription factor A
Clinical Perspective.
What Is New?
Lower ankle‐brachial index values were associated with greater abundance of mitochondrial electron transport chain complexes in gastrocnemius muscle biopsies.
The ratio between nonlipidated (cytosolic) and lipidated (membrane‐bound) forms of the macroautophagy marker LC3A/B (microtubule‐associated protein 1A/1B) and the protein content of the autophagy receptor p62 were, respectively, higher and lower in participants with lower ankle‐brachial index values.
Abundance of mitochondrial electron transport chain complexes was positively associated with 6‐minute walk distance and 4‐m gait speed only in participants without peripheral artery disease.
What Are the Clinical Implications?
These results suggest the possibility that mitophagy is impaired in the setting of ischemia, resulting in accumulation of damaged mitochondrial complexes.
Findings also suggest that macroautophagy is upregulated in the setting of ischemia, possibly as an attempt to cope with mitophagy impairment.
Results are cross‐sectional, and further study is needed to corroborate these initial findings and determine whether restoring leg blood flow ameliorates mitochondrial turnover in people with peripheral artery disease.
Mitochondrial structural and functional abnormalities have been described in lower extremity muscle biopsies from people with PAD. 1 For example, reduced activity of electron transport chain (ETC) complexes, 2 , 3 possible impairment of mitochondrial autophagy (mitophagy), 4 increased oxidative stress, 5 , 6 , 7 and greater mitochondrial DNA (mtDNA) content 8 and heteroplasmy 9 have been documented in people with lower extremity ischemia. These alterations have been attributed, at least partly, to overproduction of reactive oxygen species by mitochondria following recurrent cycles of ischemia–reperfusion. 10 , 11 However, the relationship between mitochondrial abnormalities and walking impairment in people with PAD is unclear. 8 , 9 , 12
Mitochondrial quality control consists of coordinated processes including mitochondrial dynamics (mitochondrial fusion/fission), mitochondrial biogenesis, and mitophagy. 13 These processes serve to maintain a well‐functioning pool of mitochondria within the cell and protect against accumulation of mtDNA mutations and defective organelles. 14 , 15 , 16 An alteration in any mitochondrial quality control pathways or the loss of their coordination might reduce efficiency of mitochondrial bioenergetics and, hence, tissue function. Whether markers of mitochondrial quality control are altered in muscle of people with PAD and an association of these markers with muscle dysfunction exists in PAD is currently unclear. In the present study, markers of mtDNA damage, mitochondrial biogenesis, including mtDNA copy number and the abundance of ETC complexes, and mitophagy were measured in gastrocnemius muscle biopsies from people with and without PAD. We hypothesized that people with PAD would show increased markers of mitochondrial biogenesis and diminished markers of mitophagy compared with those without PAD. These alterations were expected to be linked with increased levels of mtDNA damage owing to the combination of defective mitophagic removal of mitochondria carrying mutated genomes and their proliferation. We further hypothesized that greater abnormalities in mitochondrial quality control markers would be associated with greater walking impairment in people with PAD.
METHODS
The data that support the findings of this study are available from co‐investigator M.M.M. (mdm608@northwestern.edu) upon reasonable request.
Study Participants
This study used gastrocnemius samples collected from people with and without PAD from research studies at Northwestern University (Chicago, IL). Each study had institutional review board approval at Northwestern University and all participants signed written informed consent. Participants were recruited using postcards mailed to people 50 and older living in the Chicago area, advertisements by the Chicago transit authority, letters mailed to patients with PAD at the Northwestern's medical center, a database of participants from prior studies of a co‐investigator (M.M.M.), physician referral, newspaper advertisements, and friend referral. Some participants were recruited from among patients at the Jesse Brown Veterans Administration in Chicago. Muscle samples from 2 participants with PAD were matched by age, sex, and race with a muscle sample from 1 participant without PAD.
Eligibility Criteria
Eligibility criteria were described in detail in prior publications. 17 , 18 , 19 , 20 PAD was defined as ABI <0.90 in either leg. 20 PAD was defined as mild if ABI was 0.60 to 0.90 and moderate to severe if ABI was <0.60. Absence of PAD was defined as a resting ABI of 1.00 to 1.40. People with diabetes were excluded. Additional exclusion criteria were presence of foot ulcer or chronic limb threatening ischemia, below‐knee or above‐knee amputation, wheelchair confinement, use of a walking aid other than a cane, significant visual or hearing impairment, major surgery or lower extremity revascularization in the previous 3 months, and cognitive impairment as documented by a Mini‐Mental State Examination 21 score <23. A muscle biopsy from the medial head of the gastrocnemius was collected as described previously. 12
General Characteristics, Anthropometry, and Ankle‐Brachial Index Measurement
Information on age, sex, race or ethnicity, smoking habits, and medical history were collected through patient report. 17 , 18 , 19 , 20 Body mass index was calculated as the ratio between body mass (kg) and the square of height (m2). The ABI was measured as previously described. 22 Briefly, systolic pressures of right and left brachial, dorsalis pedis, and posterior tibial arteries were measured twice using a handheld Doppler probe (Nicolet Vascular Pocket Dop II, Nicolet Biomedical Inc., Golden, CO). The ABI was calculated in each leg as the ratio between the mean of the dorsal pedis and posterior tibial pressures and the mean of 4 brachial pressures. 22 The mean systolic pressure in the arm with the higher pressure was used in cases when one brachial pressure was higher than the other side in both measurement sets and the 2 brachial pressures differed by ≥10 mm Hg in 1 measurement set. 22
Assessment of Physical Performance
Six ‐Minute Walk
The 6‐minute walking test was performed as detailed elsewhere. 18 , 23 , 24 Briefly, participants were asked to walk up and down a 30‐m hallway for 6 minute to cover as much distance as possible at their preferred walking speed. The total distance walked (m) was recorded.
Usual and Fast 4‐Meter Gait Speed
Gait speed over 4 meters was measured at usual and fast pace. 25 For these tests, participants were asked to walk at their normal pace as if they were “walking down the street to go to the store,” and, separately, to walk as fast as they could over 4 m. Each test was performed twice, and the fastest trial (m/s) was used for the analysis.
Short Physical Performance Battery
The short physical performance battery (SPPB) consists of 3 components: standing balance, gait speed at usual pace over 4 meters, and 5‐repetition chair rise. 26 For the standing balance test, participants were asked to stand in 3 increasingly difficult positions for 10 seconds each: a side‐by‐side feet standing position, a semitandem position, and a full‐tandem position. Gait speed was measured over a 4‐m course at the participant's usual pace. The faster of 2 trials (m/s) was used for test scoring. For the chair rise test, participants were asked to perform 5 repetitions of standing up and sitting down from a chair with arms folded across their chest as quickly as possible and the performance was timed. Each of the 3 SPPB subtasks was scored on a scale of 0 to 4, with 0 representing inability to do the test and 4 corresponding to the highest level of performance. 26 Scores were summed to obtain a total score ranging from 0 to 12, with higher scores indicating better physical performance. 26
Biochemical Analyses in Muscle Biopsies
Determination of Selected Protein Markers of Mitochondrial Quality Control by Immunodetection
Protein levels of markers of macroautophagy (LC3A/B [microtubule‐associated protein 1A/1B‐light chain 3] and p62), mitophagy (PINK1 [phosphatase and tensin homolog‐induced kinase 1] and Parkin), 27 mitochondrial biogenesis (NRF1 [nuclear respiratory factor 1], PGC‐1α [peroxisome proliferator‐activated receptor‐gamma coactivator 1 alpha], and TFAM [mitochondrial transcription factor A]), and those of ETC complex I to V were measured in gastrocnemius muscle samples of all participants. The protein content of LC3A/B and ETC complexes I to V was quantified by Western blot, as described previously. 28 An automated capillary‐based immunoassay was used to measure protein levels of NRF1, p62, Parkin, PGC‐1α, PINK1, and TFAM on a Jess system (ProteinSimple, San Jose, CA).
The protocol followed for the preparation of whole‐tissue extracts and the quantification of target proteins as well as technical specifications of primary and secondary antibodies used are detailed in Data S1.
Quantification of mtDNA Copy Number and Damage
mtDNA copy number and damage (ie, mtDNA common deletion, strand breaks, and oxidized purines) were measured in gastrocnemius muscle samples by quantitative real‐time polymerase chain reaction using SYBR Green chemistry on a CFX96 Touch™ Real‐Time PCR Detection System (Bio‐Rad Laboratories Inc., Hercules, CA), as previously described. 12 Strand breaks were assayed within the following mtDNA regions: ND1/2 (NADH dehydrogenase subunit 1/2), ND4/5 (NADH dehydrogenase subunit 4/5), COII ATPase 6/8 (cytochrome oxidase subunit II and ATPase subunit 6/8), cytochrome B6, and displacement loop. The abundance of mtDNA oxidized purines was quantified within ND4/5, cytochrome B6, and displacement loop regions. All reactions were run in triplicate. mtDNA copy number was calculated as the ratio between amplicons corresponding to ND1 and ND5 relative to nuclear glyceraldehyde 3‐phosphate dehydrogenase, as previously described. 12 The abundance of the mtDNA common deletion (mtDNA4977), strand breaks, and oxidized purines was normalized to mtDNA copy number and differences among groups were calculated according to the Pfaffl mathematical model using the formula R=2ΔΔCt. 29
Statistical Analysis
Characteristics of study participants are presented as means and SDs for continuous variables and counts and proportions for categorical variables. Descriptive characteristics of participants with moderate‐to‐severe PAD (ABI <0.60), mild PAD (ABI 0.60–0.90), and without PAD (ABI 1.0–1.40) were compared by 1‐way ANOVA and χ 2 or Fisher's exact tests for continuous and categorical variables, respectively. Linear regressions were used to compare the distributions of markers of mitochondrial quality control, ETC complex abundance, and measures of mtDNA content and damage across participant groups. The independent variable in the model was an ordinal variable with 3 levels, coded as 1, 2 and 3, denoting the 3 ABI categories. Models were adjusted for age, sex, race or ethnicity, and smoking status. Pearson's correlation coefficients were calculated to estimate the associations between biochemical markers and measures of physical performance. Prior work showed differences in markers of mitochondrial biogenesis and bioenergetics or their association with physical performance in people with severe PAD (ie, ABI <0.60) relative to those with mild‐to‐moderate PAD (ie, ABI 0.60–0.90). 3 , 8 Therefore, because ABI values were expected to alter the association between biochemical markers and physical performance measures, separate analyses were conducted for moderate to severe PAD, mild PAD, and non‐PAD groups. The 95% CI of the correlation coefficients was estimated, and the null hypothesis of null correlation was tested. The estimated correlations were graphed as heat maps that were generated using R software (RStudio, PBC, Boston, MA). For all tests statistical significance was set at a 2‐sided 0.05 level. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Study Participants
A total of 67 participants, 15 with moderate to severe PAD, 29 with mild PAD, and 23 without PAD were included. Characteristics of participants according to the presence and severity of PAD are shown in Table 1. Overall mean age was 65.0±6.8 years, 16 (23.9%) were women, and 48 (71.6%) were Black. Age, sex distribution, race or ethnicity, body mass index, prevalence of comorbid conditions, 4‐m gait speed at either usual or fast pace, and the SPPB score were not significantly different between participants with and without PAD regardless of disease severity. Participants with PAD, either mild or moderate to severe, had a higher prevalence of current or former smokers and walked a significantly shorter distance on the 6‐minute walk test than those without PAD.
Table 1.
Main Characteristics of Study Participants According to the Presence and Severity of Peripheral Artery Disease
| Total | PAD | No PAD | P | ||
|---|---|---|---|---|---|
| (n=67) | Moderate to severe (ABI <0.60; n=15) | Mild (ABI 0.60–0.90; n=29) | (n=23) | ||
| Characteristic | |||||
| Age, y | 65.0 (6.8) | 64.9 (7.8) | 65.4 (6.6) | 64.6 (6.6) | 0.903 |
| Sex, female | 16 (23.9%) | 3 (20.0%) | 7 (24.1%) | 6 (26.1%) | 0.911 |
| Race, Black | 48 (71.6%) | 10 (66.7%) | 23 (79.3%) | 15 (65.2%) | 0.475 |
| Current or former smoking | 59 (88.1%) | 15 (100.0%) | 28 (96.6%) | 16 (69.6%) | 0.004 |
| Body mass index, kg/m2 | 28.2 (5.4) | 28.6 (4.6) | 28.1 (5.7) | 28.2 (5.8) | 0.951 |
| ABI | 0.83 (0.26) | 0.50 (0.08) | 0.74 (0.08) | 1.14 (0.08) | <0.001 |
| Comorbidities | |||||
| Myocardial infarction | 8 (12.1%) | 2 (13.3%) | 4 (13.8%) | 2 (9.1%)† | 0.897 |
| Angina | 6 (9.1%) | 2 (13.3%) | 3 (10.3%) | 1 (4.5%)† | 0.649 |
| Congestive heart failure | 5 (7.6%) | 0 (0.0%) | 4 (13.8%) | 1 (4.5%)† | 0.285 |
| Cancer | 9 (13.4%) | 4 (26.7%) | 3 (10.3%) | 2 (8.7%) | 0.306 |
| Chronic obstructive pulmonary disease | 12 (17.9%) | 3 (20.0%) | 5 (17.2%) | 4 (17.4%) | 1.000 |
| Physical performance | |||||
| 6‐minute walk distance, m | 367.0 (122.5) | 302.7 (95.2) | 366.4 (96.3) | 409.8 (150.9) | 0.029 |
| 4‐m gait speed at usual pace, m/s | 0.85 (0.22) | 0.85 (0.17) | 0.85 (0.19)* | 0.84 (0.28) | 0.959 |
| 4‐m gait speed at fast pace, m/s | 1.19 (0.32) | 1.20 (0.22) | 1.21 (0.28)* | 1.15 (0.41) | 0.774 |
| Short physical performance battery summary score | 9.8 (2.5) | 10.7 (1.6) | 9.7 (2.3)* | 9.2 (3.1)† | 0.222 |
Data are mean (SD) or n (%). ABI indicates ankle‐brachial index; and PAD, peripheral artery disease.
n=28.
n=22.
Selected Markers of Mitochondrial Quality Control and Damage
Markers of Macroautophagy and Mitophagy
After adjustment for potential confounders (ie, age, sex, race or ethnicity, and smoking status), lower ABI values were associated with a higher LC3A/B II‐to‐LC3A/B I ratio (P trend=0.017) and lower content of the autophagy receptor p62 (P trend=0.033; Table 2). Lower ABI values were also associated with lower abundance of the mitophagy marker PINK1 (P trend=0.017; Table 2). However, this association was no longer significant after adjustment for potential confounders (P trend=0.070). Protein levels of cleaved PINK1 and Parkin were not significantly different across ABI categories.
Table 2.
Protein Levels of Selected Markers of Macroautophagy, Mitophagy, and Mitochondrial Biogenesis, Including mtDNA Copy Number, in Gastrocnemius Muscle Biopsies According to the Presence and Severity of Peripheral Artery Disease
| PAD | No PAD | P trend | |||
|---|---|---|---|---|---|
| Moderate to severe (ABI <0.60; n=15) | Mild (ABI 0.60–0.90; n=29) | (n=23) | Unadjusted | Adjusted* | |
| Macroautophagy | |||||
| LC3A/B I, cytosolic | 0.60 (0.19) | 0.53 (0.17) | 0.62 (0.25) | 0.598 | 0.590 |
| LC3A/B II, membrane‐bound | 1.49 (0.56) | 1.24 (0.53) | 1.32 (0.60) | 0.450 | 0.261 |
| LC3 II/LC3 I | 2.54 (0.82) | 2.31 (0.61) | 2.15 (0.60) | 0.079 | 0.017 |
| p62 | 0.71 (0.18) | 0.69 (0.19) | 0.80 (0.23) | 0.122 | 0.033 |
| Mitophagy | |||||
| PINK1 | 0.62 (0.14) | 0.79 (0.30) | 0.92 (0.52) | 0.017 | 0.070 |
| Cleaved PINK1 | 0.24 (0.11) | 0.21 (0.08) | 0.23 (0.09) | 0.770 | 0.566 |
| Parkin | 1.18 (0.42) | 1.17 (0.49) | 1.18 (0.39) | 0.990 | 0.468 |
| Mitochondrial biogenesis | |||||
| mtDNA copy number, log‐transformed | 6.54 (0.43) | 6.44 (0.39) | 6.37 (0.48) | 0.222 | 0.187 |
| Nuclear respiratory factor 1 | 0.23 (0.07) | 0.26 (0.10) | 0.27 (0.14) | 0.270 | 0.287 |
| Peroxisome proliferator‐activated receptor gamma coactivator 1 alpha | 0.51 (0.26) | 0.40 (0.16) | 0.49 (0.25) | 0.931 | 0.639 |
| Mitochondrial transcription factor A | 0.22 (0.17) | 0.21 (0.13) | 0.17 (0.11) | 0.241 | 0.113 |
| Complex I | 0.66 (0.23) | 0.45 (0.23) | 0.48 (0.29) | 0.085 | 0.043 |
| Complex II | 0.88 (0.24) | 0.70 (0.17) | 0.72 (0.25) | 0.061 | 0.032 |
| Complex III | 0.73 (0.28) | 0.49 (0.20) | 0.53 (0.25) | 0.031 | 0.022 |
| Complex IV | 1.18 (0.43) | 0.74 (0.36) | 0.84 (0.39) | 0.034 | 0.017 |
| Complex V | 0.70 (0.15) | 0.57 (0.10) | 0.59 (0.13) | 0.015 | 0.018 |
Data are mean (SD). Spot density values are normalized to the amount of protein loaded except for LC3 II/LC3 I, and are expressed as arbitrary units. ABI indicates ankle brachial index; LC3B, microtubule‐associated protein 1A/1B‐light chain 3 nonlipidated (I) and lipidated (II) forms; PINK1, phosphatase and tensin homolog‐induced kinase 1.
Adjusted for age, sex, race or ethnicity, and smoking status.
Markers of Mitochondrial Biogenesis
The mtDNA copy number and protein expression of markers of mitochondrial biogenesis (NRF1, PGC‐1α, TFAM) were not significantly different across ABI categories (Table 2). Lower ABI values were associated with a significantly higher abundance of all ETC complexes (all P trend values <0.05; Table 2).
mtDNA Damage
There were no statistically significant differences in the abundance of mtDNA4977 deletion, mtDNA strand breaks, or oxidized purines across ABI categories within any of the assayed regions (Table 3).
Table 3.
Relative Abundance of mtDNA4977 Deletion, Strand Breaks, and Oxidized Purines in Gastrocnemius Muscle Biopsies According to the Presence and Severity of Peripheral Artery Disease
| PAD | No PAD | P trend | |||
|---|---|---|---|---|---|
| Moderate to severe (ABI <0.60; n=15) | Mild (ABI 0.60–0.90; n=29) | (n=23) | Unadjusted | Adjusted* | |
| mtDNA4977 deletion | 1.04 (0.42–7.67) | 1.96 (0.56–6.16) | 1.20 (0.60–6.03) | 0.872 | 0.7961 |
| Strand breaks | |||||
| ND1/2 | 1.94 (1.49–2.82) | 3.12 (2.09–5.32) | 2.66 (1.52–4.77) | 0.397 | 0.1798 |
| ND4/5 | 2.37 (1.88–3.99) | 3.40 (2.40–5.47) | 3.34 (2.59–5.27) | 0.164 | 0.1042 |
| Cytochrome oxidase subunit II and ATPase subunit 6/8 | 2.04 (1.79–3.96) | 2.88 (2.05–5.14) | 2.60 (1.83–4.41) | 0.677 | 0.2374 |
| CytB6 | 1.83 (0.79–3.17) | 2.09 (1.26–4.81) | 2.06 (1.11–4.83) | 0.406 | 0.3728 |
| Displacement loop | 0.58 (0.45–1.98) | 1.95 (0.61–3.99) | 0.92 (0.42–2.13) | 0.766 | 0.4531 |
| Oxidized purines | |||||
| ND4/5 | 2.03 (1.58–3.09) | 3.61 (1.77–8.40) | 4.24 (1.78–5.79) | 0.122 | 0.1194 |
| CytB6 | 2.84 (0.95–4.28) | 4.22 (2.19–30.31) | 5.53 (2.29–10.12) | 0.184 | 0.1380 |
| Displacement loop | 2.30 (1.17–3.51) | 3.35 (2.18–5.86) | 4.16 (1.94–6.55) | 0.136 | 0.1496 |
Data are median (interquartile range). Values are expressed as arbitrary units. ABI indicates ankle brachial index; CytB6, cytochrome B6; ND1/2, NADH dehydrogenase subunit 1/2; and ND4/5, NADH dehydrogenase subunit 4/5.
Adjusted for age, sex, race or ethnicity, and smoking status.
Relationship Between Markers of Mitochondrial Quality Control and Measures of Physical Performance
Results of correlation analyses between markers of mitochondrial quality control in gastrocnemius muscle samples and measures of physical performance in participants without PAD and in those with mild or moderate to severe PAD are shown in Figure 1 and Table S1.
Figure 1. Heat maps of correlation between markers of mitochondrial quality control in gastrocnemius muscle samples and measures of physical performance in participants with and without peripheral artery disease.
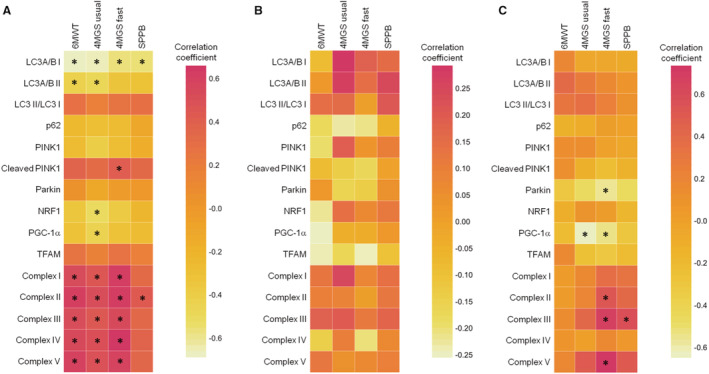
A, Participants without peripheral artery disease (PAD); B, participants with mild PAD (ankle‐brachial index, ABI 0.60–0.90); C, participants with moderate to severe PAD (ABI <0.60). *P<0.05. 4MGS fast indicates 4‐m gait speed at fast pace; 4MGS usual, 4‐m gait speed at usual pace; 6MWT, 6‐minute walking test; LC3B, microtubule‐associated protein 1A/1B‐light chain 3 nonlipidated (I), and lipidated (II) forms; NRF1, nuclear respiratory factor 1; PGC‐1α, peroxisome proliferator‐activated receptor gamma coactivator 1 alpha; PINK1, phosphatase and tensin homolog‐induced kinase 1; SPPB, short physical performance battery; and TFAM, mitochondrial transcription factor A.
In participants without PAD, the abundance of LC3A/B I was negatively associated with 6‐minute walk distance (r=−0.686; P<0.001), 4‐m gait speed at both usual (r=−0.675; P<0.001) and fast pace (r=−0.575; P=0.004), and the SPPB score (r=−0.568; P=0.006) (Figure 1A). A negative association was also observed between the abundance of LC3A/B II and 6‐minute walk distance (r=−0.438; P=0.037) and 4‐m gait speed at usual pace (r=−0.484; P=0.019). A positive association was found between protein levels of cleaved PINK1 and 4‐m gait speed at fast pace (r=0.419; P=0.047). The content of NRF1 and PGC‐1α was negatively and significantly associated with 4‐m gait speed at usual pace (r=−0.525; P=0.010 and r=−0.451; P=0.031, respectively). Finally, among people without PAD, the abundance of all ETC complexes was positively associated with 6‐minute walk distance (complex I: r=0.541; complex II: r=0.623; complex III: r=0.530; complex IV: r=0.557; complex V: r=0.597; all P values <0.05) and 4‐m gait speed at both usual (complex I: r=0.477; complex II: r=0.532; complex III: r=0.452; complex IV: r=0.510; complex V: r=0.500; all P values <0.05) and fast pace (complex I: r=0.628; complex II: r=0.640; complex III: r=0.554; complex IV: r=0.660; complex V: r=0.590; all P values <0.05). The content of complex II was also positively associated with the SPPB score (r=0.439; P=0.041).
In participants with mild PAD, no significant associations were observed between the protein content of any of the assayed mitochondrial quality control markers and measures of physical performance (Figure 1B).
In participants with moderate to severe PAD, negative associations were observed between protein abundance of Parkin and PGC‐1α and 4‐m gait speed at fast pace (r=−0.574; P=0.025 and r=−0.553; P=0.033, respectively; (Figure 1C). PGC‐1α was also negatively associated with 4‐m gait speed at usual pace (r=−0.646; P=0.009). Positive associations were observed between protein levels of complex II (r=0.543; P=0.037), III (r=0.693; P=0.004), and V (r=0.735; P=0.002) and 4‐m gait speed at fast pace. The content of complex III was also positively associated with the SPPB score (r=0.586; P=0.022).
DISCUSSION
Results from the present study indicate that most biomarkers of mitochondrial quality control were not significantly different across ABI categories in a cohort of 67 people with and without PAD. However, lower ABI values were associated with a higher abundance of all 5 ETC complexes. Protein levels of mitochondrial biogenesis transcription factors (NRF1, PGC‐1α, and TFAM) and markers of mitophagy (PINK1, cleaved PINK1, and Parkin) did not significantly differ by ABI. In contrast, lower ABI values were associated with a higher LC3A/B II‐to‐LC3A/B I ratio and lower content of the autophagy receptor p62, suggesting upregulation of macroautophagy in the setting of ischemia.
The removal of damaged mitochondria and their replenishment with newly synthesized organelles are accomplished via the coordination of mitophagy and mitochondrial biogenesis to maintain a functional pool of mitochondria and meeting cell's energy demands. 30 Intracellular signals and environmental factors (eg, nutrient availability, growth factors and hormones, toxins, oxygen fluctuations) operate a spatiotemporal control over mitochondrial biogenesis and mitophagy. Under normal conditions, mitochondrial biogenesis signaling stimulates mitophagy via Parkin, an E3 ubiquitin ligase that triggers the tagging and clearance of damaged or unnecessary mitochondria. 31 , 32 Although higher protein levels of the 5 ETC complexes were associated with lower ABI values, upregulation of mitochondrial biogenesis transcription factors was not observed. The canonical pathway of mitochondrial biogenesis is controlled by the transcriptional regulators PGC‐1α and NRFs. 33 Their activation stimulates the expression of nuclear genes encoding mitochondrial transcription factors, including TFAM. 33 Upon import into mitochondria, TFAM triggers mtDNA transcription and replication. Mitochondria can also upregulate the synthesis of ETC complexes independent of PGC‐1α and NRFs by enhancing the binding of TFAM to mtDNA. 34 For instance, mice lacking PGC‐1α retain the ability to upregulate mitochondrial biogenesis in response to wheel‐running exercise. 35 In the absence of PGC‐1α upregulation, the expression of nuclear encoded ETC subunits may be sustained through alternative signaling pathways involving, for instance, other PGC‐1 isoforms (eg, PGC‐1β). 36 These observations might explain the greater abundance of ETC complexes observed in muscles of participants with moderate to severe PAD without concomitant increases in the expression of PGC‐1α, NRF1, or TFAM. Alternatively, the accumulation of ETC complexes in participants with moderate‐to‐severe PAD may have resulted from defective mitochondrial disposal by mitophagy. In either case, mitochondrial activity becomes impaired if the extent of damage exceeds the ability to remove dysfunctional mitochondria and generate high‐quality organelles. 6 , 37 In this scenario, the increase in the LC3A/B II‐to‐LC3A/B I ratio and the lower protein content of p62 in participants with lower ABI values might be indicative of a compensatory upregulation of macroautophagy in the setting of ischemia. 27 However, the accumulation of ETC complexes in the absence of upregulation of mitochondrial biogenesis markers suggests that mitophagy is not stimulated in those with lower ABI values. This view is consistent with the hypothesis of defective mitochondrial removal in PAD and is also in line with previous data showing altered mitophagy signaling in the gastrocnemius muscle of people with PAD. 4 In this prior work, cavities devoid of mitochondria and with lack of colocalization of LC3 with lysosome‐associated membrane protein 2 were identified in approximately 17% of type I muscle fibers of people with PAD. 4 The main function of mitophagy is to remove dysfunctional, depolarized mitochondria. The loss of mitochondrial membrane potential is induced by a variety of insults, including accumulation of mutated, deleted, or otherwise damaged mtDNA. 38 Unexpectedly, the abundance of mtDNA4977 deletion, mtDNA strand breaks, and oxidized purines was not increased in participants with lower ABI values. The possibility cannot be ruled out that, in our sample, the lack of upregulation of mitophagy might have been compensated by overactivation of mtDNA repair systems. However, the expression or the activity of mtDNA repair enzymes was not measured in this study.
Taken as a whole, data from this study suggest that lower extremity ischemia may induce accumulation of ETC complexes, possibly as a consequence of lack of mitophagy upregulation, as an attempt to sustain mitochondrial bioenergetics in the setting of reduced blood supply. In this scenario, diminished muscle perfusion, rather than accumulation of mitochondrial damage, may be the major factor limiting walking performance. This hypothesis is supported by the lack of an association between the content of any ETC complexes in muscle and the distance walked during 6 minutes in participants with moderate to severe PAD, in spite of higher levels of all 5 complexes relative to both those with mild PAD and participants without PAD (Figure 1, Table 2). This finding is in agreement with the negative association between mtDNA abundance (a proxy for mitochondrial mass) in gastrocnemius muscle and 6‐minute walk distance in people with PAD, as described in a prior study. 12
Limitations
This study has limitations. First, the study was cross‐sectional and no causal inferences can be made. This study did not include data from preclinical models that tested specific mechanistic hypotheses. The generation of such models is warranted to gain mechanistic information on the relationship between mitochondrial quality control and physical performance in PAD and inform the design of future human studies. Second, available muscle specimens were frozen, which did not allow mitochondrial functional assessments, measurement of mitophagy flux, or mitochondrial imaging to be conducted. Third, no markers of mitochondrial dynamics were measured, which precluded obtaining a more comprehensive appraisal of mitochondrial quality control. Fourth, the sample size was relatively small and included multiple comparisons. Some of the statistically significant findings may have been owing to chance. Findings reported here should be replicated in a population with a larger sample size. Fifth, comparisons that did not reach statistical significance may have lacked statistical power.
CONCLUSIONS
In gastrocnemius muscle of people with and without PAD, lower ABI values were associated with greater abundance of ETC complexes and upregulation of macroautophagy. No significant differences in the abundance of mitochondrial biogenesis transcription factors or mitophagy markers were detected across ABI categories. These findings suggest that lower extremity ischemia may interfere with the proper activation of mitophagy, which might result in accumulation of aged mitochondrial proteins. However, the study was cross‐sectional and descriptive, and additional research is needed to replicate these findings in a larger sample size. Further study is also needed to determine whether restoring leg blood flow improves muscle mitochondrial turnover in people with PAD.
Sources of Funding
The work was partly supported by the American Heart Association Strategically Focused Research Network in Vascular Disease (AHA SFRN 18SFRN33900136), Intramural Research Program of the National Institute on Aging, Intramural Research Grants from the Università Cattolica del Sacro Cuore (D1.2020 and D1.2022), and the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon.”
Disclosures
E.M. reports personal fees from Nestlè and Nutricia outside the submitted work. The other authors declare no potential conflicts of interest.
Supporting information
Acknowledgments
We thank Brian B. Bouverat and Kevin Wu from the Metabolism and Translational Science Core of the Claude D. Pepper Older Americans Independence Center at the University of Florida (Gainesville, FL) for their technical assistance with muscle sample handling and processing.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027088
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Emanuele Marzetti, Email: emanuele.marzetti@policlinicogemelli.it.
Christiaan Leeuwenburgh, Email: cleeuwen@ufl.edu.
References
- 1. McDermott MM, Ferrucci L, Gonzalez‐Freire M, Kosmac K, Leeuwenburgh C, Peterson CA, Saini S, Sufit R. Skeletal muscle pathology in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40:2577–2585. doi: 10.1161/ATVBAHA.120.313831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Baxter BT, Lynch TG, Dodd SL. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med. 2006;41:262–269. doi: 10.1016/j.freeradbiomed.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 3. Ryan TE, Yamaguchi DJ, Schmidt CA, Zeczycki TN, Shaikh SR, Brophy P, Green TD, Tarpey MD, Karnekar R, Goldberg EJ, et al. Extensive skeletal muscle cell mitochondriopathy distinguishes critical limb ischemia patients from claudicants. JCI Insight. 2018;3:e123235. doi: 10.1172/jci.insight.123235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White SH, McDermott MM, Sufit RL, Kosmac K, Bugg AW, Gonzalez‐Freire M, Ferrucci L, Tian L, Zhao L, Gao Y, et al. Walking performance is positively correlated to calf muscle fiber size in peripheral artery disease subjects, but fibers show aberrant mitophagy: an observational study. J Transl Med. 2016;14:284. doi: 10.1186/s12967-016-1030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhat HK, Hiatt WR, Hoppel CL, Brass EP. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation. 1999;99:807–812. doi: 10.1161/01.cir.99.6.807 [DOI] [PubMed] [Google Scholar]
- 6. Brass EP, Wang H, Hiatt WR. Multiple skeletal muscle mitochondrial DNA deletions in patients with unilateral peripheral arterial disease. Vasc Med. 2000;5:225–230. doi: 10.1191/135886300701568513 [DOI] [PubMed] [Google Scholar]
- 7. Koutakis P, Weiss DJ, Miserlis D, Shostrom VK, Papoutsi E, Ha DM, Carpenter LA, McComb RD, Casale GP, Pipinos II. Oxidative damage in the gastrocnemius of patients with peripheral artery disease is myofiber type selective. Redox Biol. 2014;2:921–928. doi: 10.1016/j.redox.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDermott MM, Peterson CA, Sufit R, Ferrucci L, Guralnik JM, Kibbe MR, Polonsky TS, Tian L, Criqui MH, Zhao L, et al. Peripheral artery disease, calf skeletal muscle mitochondrial DNA copy number, and functional performance. Vasc Med. 2018;23:340–348. doi: 10.1177/1358863X18765667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalez‐Freire M, Moore AZ, Peterson CA, Kosmac K, McDermott MM, Sufit RL, Guralnik JM, Polonsky T, Tian L, Kibbe MR, et al. Associations of peripheral artery disease with calf skeletal muscle mitochondrial DNA heteroplasmy. J Am Heart Assoc. 2020;9:e015197. doi: 10.1161/JAHA.119.015197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andrienko TN, Pasdois P, Pereira GC, Ovens MJ, Halestrap AP. The role of succinate and ROS in reperfusion injury – a critical appraisal. J Mol Cell Cardiol. 2017;110:1–14. doi: 10.1016/j.yjmcc.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saini SK, McDermott MM, Picca A, Li L, Wohlgemuth SE, Kosmac K, Peterson CA, Tian L, Ferrucci L, Guralnik JM, et al. Mitochondrial DNA damage in calf skeletal muscle and walking performance in people with peripheral artery disease. Free Radic Biol Med. 2020;160:680–689. doi: 10.1016/j.freeradbiomed.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 13. Stotland A, Gottlieb RA. Mitochondrial quality control: easy come, easy go. Biochim Biophys Acta. 2015;1853:2802–2811. doi: 10.1016/j.bbamcr.2014.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Picca A, Mankowski RT, Burman JL, Donisi L, Kim J‐SS, Marzetti E, Leeuwenburgh C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol. 2018;15:543–554. doi: 10.1038/s41569-018-0059-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joseph A‐M, Adhihetty PJ, Wawrzyniak NR, Wohlgemuth SE, Picca A, Kujoth GC, Prolla TA, Leeuwenburgh C. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS One. 2013;8:e69327. doi: 10.1371/journal.pone.0069327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Picca A, Sirago G, Pesce V, Lezza AMS, Calvani R, Bossola M, Villani ER, Landi F, Leeuwenburgh C, Bernabei R, et al. Administration of enalapril started late in life attenuates hypertrophy and oxidative stress burden, increases mitochondrial mass, and modulates mitochondrial quality control signaling in the rat heart. Biomolecules. 2018;8:177. doi: 10.3390/biom8040177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDermott MM, Ferrucci L, Tian L, Guralnik JM, Lloyd‐Jones D, Kibbe MR, Polonsky TS, Domanchuk K, Stein JH, Zhao L, et al. Effect of granulocyte‐macrophage colony‐stimulating factor with or without supervised exercise on walking performance in patients with peripheral artery disease: the PROPEL randomized clinical trial. J Am Med Assoc. 2017;318:2089–2098. doi: 10.1001/jama.2017.17437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDermott MM, Leeuwenburgh C, Guralnik JM, Tian L, Sufit R, Zhao L, Criqui MH, Kibbe MR, Stein JH, Lloyd‐Jones D, et al. Effect of resveratrol on walking performance in older people with peripheral artery disease the restore randomized clinical trial. JAMA Cardiol. 2017;2:902–907. doi: 10.1001/jamacardio.2017.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDermott MM, Criqui MH, Domanchuk K, Ferrucci L, Guralnik JM, Kibbe MR, Kosmac K, Kramer CM, Leeuwenburgh C, Li L, et al. Cocoa to improve walking performance in older people with peripheral artery disease: the COCOA‐PAD pilot randomized clinical trial. Circ Res. 2020;126:589–599. doi: 10.1161/CIRCRESAHA.119.315600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDermott MM, Spring B, Tian L, Treat‐Jacobson D, Ferrucci L, Lloyd‐Jones D, Zhao L, Polonsky T, Kibbe MR, Bazzano L, et al. Effect of low‐intensity vs high‐intensity home‐based walking exercise on walk distance in patients with peripheral artery disease: the LITE randomized clinical trial. J Am Med Assoc. 2021;325:1266–1276. doi: 10.1001/jama.2021.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 22. McDermott MMG, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640 [DOI] [PubMed] [Google Scholar]
- 23. McDermott MM, Ades PA, Dyer A, Guralnik JM, Kibbe M, Criqui MH. Corridor‐based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg. 2008;48:1231–1237. doi: 10.1016/j.jvs.2008.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six‐minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130:61–68. doi: 10.1161/CIRCULATIONAHA.114.007002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDermott MM, Carroll TJ, Kibbe M, Kramer CM, Liu K, Guralnik JM, Keeling AN, Criqui MH, Ferrucci L, Yuan C, et al. Proximal superficial femoral artery occlusion, collateral vessels, and walking performance in peripheral artery disease. JACC Cardiovasc Imaging. 2013;6:687–694. doi: 10.1016/j.jcmg.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 27. Klionsky DJ, Abdel‐Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, Abeliovich H, Abildgaard MH, Abudu YP, Acevedo‐Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy. 2021;17:1–382. doi: 10.1080/15548627.2020.1797280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Picca A, Saini SK, Mankowski RT, Kamenov G, Anton SD, Manini TM, Buford TW, Wohlgemuth SE, Xiao R, Calvani R, et al. Altered expression of mitoferrin and frataxin, larger labile iron pool and greater mitochondrial DNA damage in the skeletal muscle of older adults. Cells. 2020;9:2579. doi: 10.3390/cells9122579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palikaras K, Lionaki E, Tavernarakis N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 2015;22:1399–1401. doi: 10.1038/cdd.2015.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, Matsumoto T. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet. 2006;15:883–895. doi: 10.1093/hmg/ddl006 [DOI] [PubMed] [Google Scholar]
- 32. Rothfuss O, Fischer H, Hasegawa T, Maisel M, Leitner P, Miesel F, Sharma M, Bornemann A, Berg D, Gasser T, et al. Parkin protects mitochondrial genome integrity and supports mitochondrial DNA repair. Hum Mol Genet. 2009;18:3832–3850. doi: 10.1093/hmg/ddp327 [DOI] [PubMed] [Google Scholar]
- 33. Picca A, Lezza AMS. Regulation of mitochondrial biogenesis through TFAM‐mitochondrial DNA interactions. Useful insights from aging and calorie restriction studies. Mitochondrion. 2015;25:67–75. doi: 10.1016/j.mito.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 34. Picca A, Fracasso F, Pesce V, Cantatore P, Joseph A‐M, Leeuwenburgh C, Gadaleta MN, Lezza AMS. Age‐ and calorie restriction‐related changes in rat brain mitochondrial DNA and TFAM binding. Age (Dordr). 2013;35:1607–1620. doi: 10.1007/s11357-012-9465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rowe GC, El‐Khoury R, Patten IS, Rustin P, Arany Z. PGC‐1α is dispensable for exercise‐induced mitochondrial biogenesis in skeletal muscle. PLoS One. 2012;7:e41817. doi: 10.1371/journal.pone.0041817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC‐1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. doi: 10.1016/j.cmet.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 37. Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med. 2000;5:55–59. doi: 10.1177/1358836X0000500109 [DOI] [PubMed] [Google Scholar]
- 38. Gilkerson RW, RLA DV, Lebot P, Wikstrom JD, Torgyekes E, Shirihai OS, Przedborski S, Schon EA. Mitochondrial autophagy in cells with mtDNA mutations results from synergistic loss of transmembrane potential and mTORC1 inhibition. Hum Mol Genet. 2012;21:978–990. doi: 10.1093/hmg/ddr529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fosang A, Colbran R. Transparency is the key to quality. J Biol Chem. 2015;290:29692–29694. doi: 10.1074/jbc.E115.000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thacker J, Yeung D, Staines W, Mielke J. Total protein or high‐abundance protein: which offers the best loading control for Western blotting? Anal Biochem. 2016;496:76–78. doi: 10.1016/j.ab.2015.11.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


