Abstract
Background
A parental history of cardiovascular disease (CVD) confers greater risk of future CVD among offspring. Whether the presence of parental modifiable risk factors contribute to or modify CVD risk in offspring is unclear.
Methods and Results
We studied 6278 parent–child trios in the multigenerational longitudinal Framingham Heart Study. We assessed parental history of CVD and modifiable risk factors (smoking, hypertension, diabetes, obesity, and hyperlipidemia). Multivariable Cox models were used to evaluate the association of parental history and future CVD among offspring. Among 6278 individuals (mean age 45±11 years), 44% had at least 1 parent with history of CVD. Over a median follow‐up of 15 years, 353 major CVD events occurred among offspring. Parental history of CVD conferred 1.7‐fold increased hazard of future CVD (hazard ratio [HR], 1.71 [95% CI, 1.33–2.21]). Parental obesity and smoking status were associated with higher hazard of future CVD (obesity: HR, 1.32 [95% CI, 1.06–1.64]; smoking: HR, 1.34 [95% CI, 1.07–1.68], attenuated after adjusting for offspring smoking status). By contrast, parental history of hypertension, diabetes, and hypercholesterolemia were not associated with future CVD in offspring (P>0.05 for all). Furthermore, parental risk factors did not modify the association of parental CVD history on future offspring CVD risk.
Conclusions
Parental history of obesity and smoking were associated with a higher hazard of future CVD in offspring. By contrast, other parental modifiable risk factors did not alter offspring CVD risk. In addition to parental CVD, the presence of parental obesity should prompt a focus on disease prevention.
Keywords: cardiovascular disease, family history, Framingham
Subject Categories: Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- FS
fractional shortening
- LVDD
left ventricular end‐diastolic dimension
Clinical Perspective.
What Is New?
Parental history of obesity and smoking were associated with an increased hazard of future CVD in offspring, though other parental modifiable risk factors did not alter offspring CVD risk.
What Are the Clinical Implications?
Our findings suggest that family history of CVD alone is generally sufficient to capture susceptibility to future CVD in offspring.
In addition to parental CVD, the presence of parental obesity should prompt a focus on disease prevention.
Cardiovascular disease (CVD) continues to be the leading cause of death worldwide. 1 In 2019, >870 000 deaths in the United States were attributable to heart disease. 2 Family history of CVD is often used as a marker of risk in offspring and is useful in risk assessment. 3 This risk can be further compounded by shared environments and engagement in adverse behaviors such as smoking, sedentary lifestyle, and poor diet. 4 A better understanding of CVD risk has been central to guiding preventive efforts and strategies.
Prior studies have evaluated the association of family history of CVD with future risk of CVD including myocardial infarction (MI) and heart failure (HF) among offspring. These studies were conducted in longitudinal observational cohorts 5 , 6 and case–control studies, relying on self‐reported data from offspring with limited ability to validate parental cardiovascular events. 7 , 8 , 9 , 10 , 11 While these prior studies have demonstrated that history of parental CVD confers elevated risk of future CVD among offspring, 1 major question that remains unknown is whether the presence of parental modifiable risk factors is similarly associated with CVD risk in offspring. In clinical practice, for example, it remains unclear whether concomitant parental smoking history or obesity may alter or refine how we think about parental CVD as a risk enhancer among offspring.
In this context, we sought to evaluate whether the presence of parental modifiable risk factors, including parental history of smoking, hypertension, diabetes, obesity, and hyperlipidemia may be associated with CVD risk in offspring. Furthermore, we sought to investigate whether these parental risk factors may modify the effect of family history of CVD on future CVD risk in offspring. To assess parental exposures and subsequent cardiovascular outcomes among offspring, we leveraged the unique setting of the Framingham Heart Study, which has conducted cardiovascular risk assessment across 3 generations of participants.
Methods
The data supporting the study findings have been made publicly available and can be accessed through the National Institutes of Health database of Genotypes and Phenotypes (https://www.ncbi.nlm.nih.gov/gap/).
Study Population
The Framingham Heart Study began in 1948 with enrollment of 5209 participants from Framingham, Massachusetts without symptoms suggestive of CVD or sequelae such as stroke or MI. 12 , 13 Thereafter, the Offspring Study was established in 1971, comprising children from the original Framingham Heart Study cohort as well as their spouses, followed by the Third Generation in 2002, which enrolled grandchildren of the original cohort. 14 For our study, parent–child trios were confirmed using pedigree information across the 3 generations of Framingham Heart Study participants. The baseline examination was defined as the earliest examination attended by “offspring” during Offspring examination 2 (1978–1982), 6 (1995–1998), and Third Generation examination 1 (2002–2005). Offspring were eligible for the study if they were free of prevalent CVD at their baseline examination, and if both parents were Framingham Heart Study participants. Of 6568 parent–child trios, we excluded n=96 with prevalent CVD in the offspring, and n=134 with missing key covariate data, yielding a final sample of 6278 trios. The study protocol was approved by the appropriate Institutional Review Boards, and all participants provided written informed consent.
Clinical Assessment and Imaging
At each follow‐up visit, a clinical assessment was performed by a physician. The assessment included medical history, physical examination, anthropometric data, and a 12‐lead ECG. Blood and urine samples were collected to assess for conditions such as hypercholesterolemia, hyperglycemia, myocardial injury, and renal insufficiency. 15 Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications. Obesity was defined as a body mass index ≥30 kg/m2. Diabetes was defined as fasting glucose ≥126 mg/dL or use of antihyperglycemic medications. Hypercholesterolemia was defined as total cholesterol ≥240 mg/dL or use of lipid‐lowering medications.
A parental history of traditional risk factors (smoking, obesity, hypertension, diabetes, and hyperlipidemia) was defined as the presence of these risk factors in either or both parents any time before the offspring baseline examination. Individuals with unknown parental history for both parents were excluded from the analysis.
Participants (“offspring”) underwent transthoracic echocardiography with Doppler color‐flow imaging at baseline examination. Cardiac structure and function were assessed including left ventricular end‐diastolic (LVDD) and left ventricular end‐systolic dimensions, left ventricular wall thickness, and left atrial diameter. Left ventricular wall mass was calculated according to the formula proposed by Devereaux and colleagues: (0.8×[1.04 (LVDD+LV posterior wall thickness+LV septal wall thickness3)−LVDD3]+0.6 g). 16 Fractional shortening (FS) was calculated using the formula: ([LVDD −left ventricular end‐systolic dimension]/LVDD)×100, and LV systolic dysfunction defined as FS <29%, which is estimated to correspond to an LV ejection fraction of 50% or less. 17 Cardiac mechanics were assessed using speckle tracking including global longitudinal strain. 18
A subset of participants (n=3529) underwent computed tomography imaging for assessment of coronary artery calcification (CAC) between the years of 1998 and 2005. 19
Clinical Outcomes
All participants were followed longitudinally for the occurrence of incident CVD. Events were adjudicated by a 3‐clinician end point review committee upon review of all available medical records. The primary outcome was incident major CVD, which included coronary death, coronary insufficiency, and stroke. 20 Secondary outcomes included MI, HF, and atrial fibrillation (AF). Parental history of CVD was defined as an adjudicated CVD event occurring in either parent before the offspring baseline examination. Follow‐up for offspring participants was censored at 15 years, and participants without events at 15 years were eligible to be re‐entered at the subsequent baseline examination for another observation period as described previously. 21
Statistical Analysis
Baseline characteristics of the offspring study sample were summarized according to parental history of CVD. Data were reported as means with SDs or medians with interquartile ranges for continuous variables and frequencies with percentages for dichotomous variables. In our primary analysis, we examined the association of parental history of CVD as well as parental history of risk factors (smoking, hypertension, diabetes, obesity, and hyperlipidemia) with incident CVD in the offspring using the Kaplan–Meier method. Log‐rank P values were presented for overall differences among groups. We then examined the association of parental history and future CVD events in offspring using Cox proportional hazards regression models. Primary analyses were adjusted for age and sex. In secondary analyses, we further adjusted Cox models for the presence of the risk factor of interest in the offspring (eg, for parental history of smoking, we adjusted the model for smoking status in the offspring). In exploratory analyses, we evaluated for effect modification where addition of the offspring risk factor attenuated the primary association using multiplicative interaction terms (parental history of CVD×parental history of risk factor of interest) that were added to the Cox models. We also examined additive interaction terms. 22 In secondary analyses, we examined the following outcomes: AF, HF, and MI. We confirmed that the proportional hazards assumption was not violated using Schoenfeld residuals. All Cox models included a strata variable to indicate generation and examination cycle. In exploratory analyses, we examined the effect of having 0, 1, or 2 parents with history of CVD or traditional risk factors on offspring risk of subsequent CVD.
In cross‐sectional analyses, we used multivariable linear regression models to examine the association of parental history with echocardiographic cardiac structure, function, and CAC as measured by computed tomography. Primary analyses focused on parental history of HF for echocardiographic measures, and parental history of CVD for CAC measures, with secondary analyses on parental history of CVD. All models were adjusted for age and sex, then additionally for systolic blood pressure, hypertension treatment, diabetes, body mass index, smoking, and total cholesterol/high‐density lipoprotein ratio in the offspring. Effect modification by parental risk factors was also examined in all cross‐sectional analyses. CAC scores were natural log‐transformed after adding 1 unit to raw values to account for the skewness in distribution and normal values of zero. A 2‐sided P value <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (Cary, NC).
Results
Among n=6278 offspring individuals included with mean age 45±11 years and 53% women, 44% had at least 1 parent with a history of CVD and 56% had no known parental history of CVD. Individuals with parental history of CVD were older, with a greater burden of traditional CVD factors such as higher blood pressure, cholesterol, body mass index, diabetes, and smoking (P<0.01 for all, Table 1). Furthermore, among participants with a parental history of CVD, traditional CVD risk factors also were more prevalent among parents, including parental history of smoking in 35%, hypertension in 65%, and diabetes in 23%. By contrast, among those without parental CVD history, these risk factors were less prevalent including parental history among 28% of smoking, 54% hypertension, and 10% diabetes (P<0.001 for all between‐group differences). Parental history of obesity was similar among groups (P=0.41). Baseline characteristics stratified by offspring baseline examination, as well as number of parents with CVD and maternal versus paternal history are available in Tables S1 through S5.
Table 1.
Baseline Characteristics of Offspring Stratified by Parental History of CVD
| Characteristics | Total | PHx of CVD | No PHx of CVD | P value* |
|---|---|---|---|---|
| n=6278 | n=2778 | n=3500 | ||
| Age, y | 45 (11) | 50 (11) | 41 (10) | <0.001 |
| Women, n (%) | 3298 (53) | 1443 (52) | 1855 (53) | 0.47 |
| Body mass index, kg/m2 | 26.8 (5.2) | 27.3 (5.1) | 26.3 (5.2) | <0.001 |
| Systolic blood pressure, mm Hg | 120 (17) | 125 (18) | 117 (15) | <0.001 |
| Diastolic blood pressure, mm Hg | 76 (10) | 77 (10) | 75 (10) | <0.001 |
| Total cholesterol, mg/dL | 197 (39) | 204 (40) | 191 (36) | <0.001 |
| High‐density lipoprotein, mg/dL | 52 (16) | 51 (16) | 53 (15) | <0.001 |
| Hypertension treatment, n (%) | 781 (12) | 530 (19) | 251 (7) | <0.001 |
| Diabetes, n (%) | 2226 (4) | 152 (5) | 74 (2) | <0.001 |
| Smoking, n (%) | 1433 (23) | 689 (25) | 744 (21) | 0.01 |
| Hypercholesterolemia, n (%) | 962 (15) | 541 (19) | 421 (12) | <0.001 |
| Parental history of modifiable risk factors | ||||
| Smoking, n (%) | 1827 (31) | 919 (35) | 908 (28) | <0.001 |
| Hypertension, n (%) | 3681 (59) | 1794 (65) | 1887 (54) | <0.001 |
| Obesity, n (%) | 2489 (40) | 1026 (37) | 1463 (42) | 0.41 |
| Diabetes, n (%) | 977 (16) | 633 (23) | 344 (10) | <0.001 |
| Hypercholesterolemia, n (%) | 2826 (45) | 1254 (45) | 1572 (45) | <0.001 |
Family history is dichotomous and represents respective condition in either one of the parents. Data are shown as mean (SD) or otherwise noted. CVD indicates cardiovascular disease; and PHx, parental history.
Between‐group differences were compared using ANCOVA or Cochran–Mantel–Haenszel test as appropriate.
Over a median follow‐up of 15 years (Q1‐Q3: 14.7–15 years), we observed 353 major CVD events among offspring. This included 90 with HF and 181 with MI. In addition, 264 had incident AF. Offspring with a parental history of CVD had greater risk of future CVD, with incidence rate of 6.8 per 1000 person‐years when compared with 1.8 per 1000 person‐years among offspring without a parental history of CVD. We found similar differences for MI (3.5 versus 0.9 per 1000 person‐years), HF (1.9 versus 0.3 per 1000 person‐years), and AF (5.01 versus 1.4 per 1000 person‐years).
Modifiable Risk Factors in Parents and Association With Future CVD in Offspring
We examined the association of parental history of each of the modifiable risk factors with future risk of CVD among offspring. Figure 1 displays cumulative incidence of CVD in offspring, stratified by those with and without parental history of CVD and absence or presence of modifiable parental risk factors. We found greater risk of major CVD events in offspring with a parental history of CVD regardless of parental smoking history (Figure 1A). Similar findings were observed when stratified by other parental modifiable risk factors (Figure 1B through 1E).
Figure 1. Cumulative incidence of major CVD, stratified by parental history of CVD and modifiable risk factors.
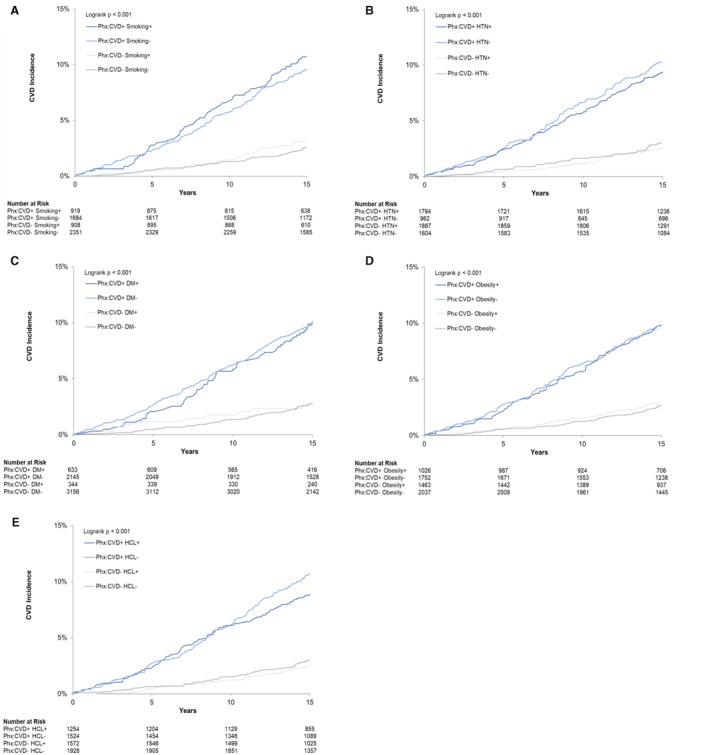
Kaplan–Meier cumulative incidence plots for offspring with and without parental history of CVD, stratified by absence or presence of modifiable parental risk factors. Depicted in smoking (A), HTN (B), DM (C), obesity (D), and HCL (E). CVD indicates cardiovascular disease; DM, diabetes mellitus; HCL, hypercholesterolemia; HTN, hypertension; and PHx, parental history.
When stratified by parental modifiable risk factors, individuals with parental history of concomitant CVD and smoking had an incidence rate of 7.45 per 1000 person‐years for future CVD, and individuals with parental history of CVD in the absence of smoking had an incidence rate of 6.64 per 1000 person‐years for future CVD, though there was no statistically significant difference (P=0.38, Table S4). Among individuals without parental history of CVD, incidence rates of CVD were also similar among those with and without parental history of smoking (2.08 and 1.68 per 1000 person‐years, respectively, P=0.37). We observed similar findings for other parental history of modifiable risk factors, including hypertension, diabetes, obesity, and hyperlipidemia. Specifically, CVD incidence rates were higher among those with parental CVD history, with comparable incidence rates in the concomitant presence or absence of parental modifiable risk factors (Table S4). Age‐adjusted analyses revealed similar findings of higher CVD incidence rates in individuals with parental CVD history compared with those without and were overall not statistically significant except for smoking (incidence rate of 4.20 versus 3.16, P=0.03). Incidence rates were also comparable in the presence or absence of modifiable risk factors in parents (Table S5).
Multivariable‐Adjusted Analyses of Modifiable Risk Factors in Parents and Association With Future CVD in Offspring
We next examined the association of parental history of CVD and modifiable cardiovascular risk factors with incidence of CVD in offspring using multivariable Cox models (Table 2). Parental history of CVD conferred a 1.7‐fold increased hazard of future CVD in the offspring (hazard ratio [HR], 1.71 [95% CI, 1.33–2.21]). Parental history of smoking was associated with 34% higher hazard of future CVD (HR, 1.34 [95% CI, 1.07–1.68]). In addition, parental history of obesity was associated with 32% higher hazard of future CVD (HR, 1.32 [95% CI, 1.06–1.64]). There was no association of parental history of hypertension, diabetes, or hyperlipidemia with future CVD in offspring (P>0.05 for all).
Table 2.
Association Between Parental History of Major CVD/CVD Risk Factors and Incidence of Cardiovascular Outcomes in Offspring
| Primary model | Secondary model | ||||||
|---|---|---|---|---|---|---|---|
| Parental history | Age‐ and sex‐adjusted | Age‐, sex‐, and risk factor–adjusted* | |||||
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Primary outcome | |||||||
| CVD | CVD | 1.71 | 1.33–2.21 | <0.001 | … | … | … |
| n=353† | Smoking | 1.34 | 1.07–1.68 | 0.01 | 1.21 | 0.96–1.51 | 0.11 |
| Hypertension | 1.05 | 0.85–1.31 | 0.64 | 1.01 | 0.81–1.25 | 0.94 | |
| Diabetes | 1.18 | 0.91–1.53 | 0.22 | 1.08 | 0.83–1.41 | 0.57 | |
| Obesity | 1.32 | 1.06–1.64 | 0.01 | 1.27 | 1.02–1.58 | 0.04 | |
| Hypercholesterolemia | 1.14 | 0.92–1.42 | 0.23 | 1.12 | 0.90–1.40 | 0.30 | |
| Secondary outcomes | |||||||
| AF | CVD | 1.60 | 1.19–2.15 | 0.002 | … | … | … |
| n=264† | Smoking | 1.17 | 0.90–1.53 | 0.25 | 1.09 | 0.83–1.43 | 0.52 |
| Hypertension | 1.17 | 0.91–1.52 | 0.22 | 1.14 | 0.88–1.48 | 0.31 | |
| Diabetes | 0.87 | 0.63–1.21 | 0.41 | 0.82 | 0.58–1.14 | 0.24 | |
| Obesity | 1.15 | 0.90–1.49 | 0.27 | 1.07 | 0.82–1.38 | 0.62 | |
| Hypercholesterolemia | 1.06 | 0.82–1.37 | 0.64 | 1.06 | 0.82–1.37 | 0.63 | |
| HF | CVD | 2.16 | 1.23–3.81 | 0.008 | … | … | … |
| n=90† | Smoking | 1.34 | 0.86–2.09 | 0.19 | 1.27 | 0.81–1.98 | 0.30 |
| Hypertension | 1.03 | 0.67–1.57 | 0.90 | 0.98 | 0.64–1.49 | 0.91 | |
| Diabetes | 1.20 | 0.72–2.02 | 0.49 | 1.05 | 0.62–1.79 | 0.85 | |
| Obesity | 1.23 | 0.79–1.90 | 0.36 | 1.03 | 0.66–1.61 | 0.89 | |
| Hypercholesterolemia | 1.16 | 0.75–1.79 | 0.50 | 1.15 | 0.74–1.77 | 0.53 | |
| MI | CVD | 2.21 | 1.54–3.19 | <0.001 | … | … | … |
| n=181† | Smoking | 1.04 | 0.76–1.44 | 0.79 | 0.92 | 0.66–1.27 | 0.61 |
| Hypertension | 1.01 | 0.75–1.37 | 0.93 | 0.98 | 0.72–1.32 | 0.88 | |
| Diabetes | 1.43 | 1.01–2.02 | 0.045 | 1.38 | 0.97–1.96 | 0.08 | |
| Obesity | 1.17 | 0.86–1.58 | 0.33 | 1.16 | 0.85–1.58 | 0.35 | |
| Hypercholesterolemia | 1.14 | 0.84–1.54 | 0.39 | 1.12 | 0.82–1.51 | 0.48 | |
AF indicates atrial fibrillation; CVD, cardiovascular disease; HF, heart failure; HR, hazard ratio; and MI, myocardial infarction.
Adjusted for age, sex, and the same risk factor in the offspring.
n refers to the number of observed outcomes for each respective event.
In secondary analyses, we additionally adjusted for the presence of the risk factor of interest in the offspring and found that the association of parental obesity status with incident CVD in offspring remained similar (HR, 1.27 [95% CI, 1.02–1.58]). By contrast, the association of parental smoking status after adjusting for offspring smoking status was attenuated (HR, 1.21 [95% CI, 0.96–1.51]). In exploratory mediation analyses, we estimated up to 41% (95% CI, 12–70%, P=0.006) of the effect of parental smoking status on future CVD was mediated by offspring smoking status.
In exploratory analyses, we examined the effect of having 0, 1, or 2 parents with exposures deemed significant in primary analyses (history of CVD, obesity, or smoking status) as displayed in Table S6. Lastly, we observed that parental risk factors did not modify the effect of parental CVD history on the future CVD risk in offspring when examining multiplicative interaction or additive interaction terms (P>0.05 for all).
Modifiable Risk Factors in Parents and Association With Future AF, HF, and MI in Offspring
In secondary analyses, we examined parental history of CVD and modifiable risk factors and their association with secondary outcomes including AF, HF, and MI (Table 2). Parental history of CVD was associated with future risk of AF (HR, 1.60 [95% CI, 1.19–2.15]), HF (HR, 2.16 [95% CI, 1.23–3.81]), and MI (HR, 2.21 [95% CI, 1.54–3.19]). By contrast, the presence of parental modifiable risk factors was not associated with future risk of AF, HF, or MI in the offspring, with the exception of parental diabetes history, which was associated with future risk of MI in age‐ and sex‐adjusted analyses (HR, 1.43 [95% CI, 1.01–2.02]), though this relationship was no longer significant after accounting for offspring diabetes status (P>0.05), with no evidence for effect modification (P=0.24).
Association of Parental HF and Modifiable Risk Factors With Cardiac Structure and Function in Offspring
Measures of cardiac structure, function, and CAC scores in the offspring stratified by parental history of HF or CVD are presented in Table S7. Offspring with a family history of HF had a larger left atrial diameter, LV wall thickness, and LV mass (P<0.001 for all).
In multivariable‐adjusted analyses, we found that parental history of HF was associated with greater left atrial diameter, LV wall thickness, and LV mass (P<0.05 for all, Table 3). By contrast, there was no association of parental history of HF with LVEDD, FS, or global longitudinal strain. When examining parental history of modifiable risk factors, parental history of obesity was associated with greater LVEDD (P=0.03), and parental history of hypercholesterolemia was associated with greater LV wall thickness (P=0.04). Other parental modifiable risk factors were not associated with echocardiographic traits (P>0.05 for all, Table 3).
Table 3.
Associations of Parental History of HF, CVD, and Risk Factors With Cardiac Structure and Function in Offspring
| Parental history | β estimate | SE | P value* | Interaction with PHx of HF P value | |
|---|---|---|---|---|---|
| Outcomes | |||||
| LAD | HF | 0.06 | 0.01 | <0.001 | … |
| Smoking | 0.02 | 0.01 | 0.14 | 0.01 | |
| Hypertension | 0.01 | 0.01 | 0.26 | 0.05 | |
| Diabetes | −0.001 | 0.01 | 0.94 | 0.98 | |
| Obesity | 0.01 | 0.01 | 0.19 | 1.00 | |
| Hypercholesterolemia | 0.002 | 0.01 | 0.83 | 0.54 | |
| LVEDD | HF | 0.02 | 0.01 | 0.23 | … |
| Smoking | −0.01 | 0.01 | 0.39 | 0.26 | |
| Hypertension | −0.02 | 0.01 | 0.10 | 0.84 | |
| Diabetes | −0.01 | 0.01 | 0.34 | 0.58 | |
| Obesity | 0.02 | 0.01 | 0.03 | 0.10 | |
| Hypercholesterolemia | −0.004 | 0.01 | 0.67 | 0.44 | |
| LVWT | HF | 0.016 | 0.007 | 0.04 | … |
| Smoking | 0.001 | 0.006 | 0.85 | 0.52 | |
| Hypertension | −0.006 | 0.005 | 0.25 | 0.37 | |
| Diabetes | −0.015 | 0.007 | 0.04 | 0.89 | |
| Obesity | 0.005 | 0.005 | 0.38 | 0.03 | |
| Hypercholesterolemia | −0.011 | 0.005 | 0.04 | 0.51 | |
| LV mass | HF | 2.91 | 1.22 | 0.02 | … |
| Smoking | −0.65 | 0.91 | 0.47 | 0.76 | |
| Hypertension | −1.44 | 0.86 | 0.09 | 0.34 | |
| Diabetes | −2.45 | 1.16 | 0.03 | 0.73 | |
| Obesity | 1.54 | 0.88 | 0.08 | 0.02 | |
| Hypercholesterolemia | −1.38 | 0.86 | 0.11 | 0.96 | |
| LVFS | HF | 0.04 | 0.15 | 0.77 | … |
| Smoking | −0.16 | 0.12 | 0.18 | 0.58 | |
| Hypertension | 0.04 | 0.11 | 0.68 | 0.98 | |
| Diabetes | −0.08 | 0.15 | 0.59 | 0.53 | |
| Obesity | −0.14 | 0.11 | 0.20 | 0.64 | |
| Hypercholesterolemia | −0.06 | 0.11 | 0.59 | 0.40 | |
| GLS† | HF | 0.12 | 0.13 | 0.35 | … |
| Smoking | 0.03 | 0.10 | 0.79 | 0.64 | |
| Hypertension | 0.15 | 0.09 | 0.11 | 0.46 | |
| Diabetes | 0.16 | 0.12 | 0.20 | 0.38 | |
| Obesity | 0.09 | 0.09 | 0.32 | 0.27 | |
| Hypercholesterolemia | 0.13 | 0.09 | 0.16 | 0.21 | |
| CAC‡ | HF | 0.24 | 0.08 | 0.002 | … |
| Smoking | −0.06 | 0.08 | 0.48 | 0.09 | |
| Hypertension | 0.05 | 0.08 | 0.55 | 0.13 | |
| Diabetes | 0.25 | 0.09 | 0.008 | 0.01 | |
| Obesity | 0.03 | 0.08 | 0.69 | 0.06 | |
| Hypercholesterolemia | 0.11 | 0.07 | 0.12 | 0.79 | |
CAC indicates coronary artery calcification; CVD, cardiovascular disease; GLS, global longitudinal strain; HF, heart failure; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic dimension; LVFS, left ventricular fractional shortening; LVWT, left ventricular wall thickness; and PHx, parental history.
Multivariable analyses adjusted for age, sex, cohort, systolic blood pressure, hypertension treatment, body mass index, diabetes, smoking status, and total cholesterol/high‐density lipoprotein ratio.
Models for GLS were adjusted additionally for heart rate. β estimate represents between‐group differences in each outcome.
CAC scores were natural log‐transformed.
In exploratory analyses, we examined whether parental risk factors modified the association of parental history of HF on echocardiographic traits and found 3 suggestive interactions: (1, 2) concomitant parental obesity was associated with more pronounced relation of parental HF with offspring LV mass and LV wall thickness (Pint=0.02 and Pint=0.03, respectively), and (3) parental smoking status was associated with a greater relation of parental HF with offspring left atrial diameter. In secondary analyses, we did not observe that parental history of CVD was associated with echocardiographic measurements in offspring (Figure 2).
Figure 2. Parental history of HF and CVD, and association with cardiac structure and function in offspring.
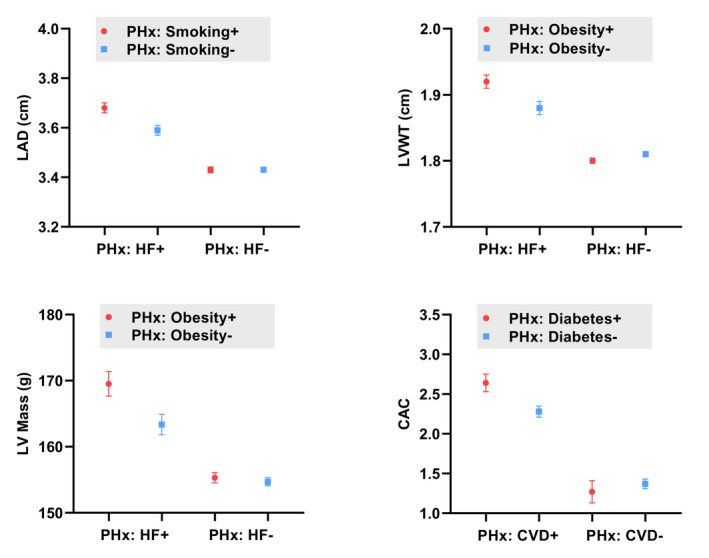
Point estimates represent adjusted means of LAD, LV mass, LVWT, and natural log‐transformed CAC, with error bars representing SE. Stratified by 4 groups based on parental history. Means adjusted for age, sex, cohort, systolic blood pressure, hypertension treatment, body mass index, diabetes, smoking status, and total cholesterol/high‐density lipoprotein ratio. CAC indicates coronary artery calcification; CVD, cardiovascular disease; HF, heart failure; LAD, left atrial diameter; LV Mass, left ventricular mass; LVWT, left ventricular wall thickness; and PHx, parental history.
Lastly, parental history of CVD was associated with higher CAC scores compared with those without (multivariable‐adjusted P=0.002). In addition, parental history of diabetes was associated with higher CAC scores and modified the association of parental history of CVD with CAC score (Pint=0.01). Specifically, those with concomitant parental history of diabetes and CVD had higher CAC scores compared with individuals with only parental history of CVD (Figure 2). Other parental modifiable risk factors were not associated with CAC scores.
Discussion
Our study leveraged a unique multigenerational longitudinal community‐based cohort to examine parental history of modifiable risk factors and the association with long‐term cardiovascular risk in the offspring. We observed that parental history of smoking and obesity was associated with future CVD in the offspring. Intriguingly, family history of obesity was associated with a higher risk of future CVD regardless of obesity status in the offspring. By contrast, the association of parental smoking status with offspring CVD appeared to be mediated by smoking status in the offspring. While parental history of CVD conferred a higher risk of CVD and specific cardiac events including MI, HF, and AF, this association was not modified by parental risk factors. In cross‐sectional analyses, parental history of HF was associated with measures of cardiac structure and function, with associations with left atrial diameter and LV mass that were magnified in the setting of parental smoking and obesity status, respectively. Taken together, these results suggest that obesity, smoking, as well as parental history of overt CVD confer cardiovascular risk among offspring.
These results have potential clinical implications; for example, patients may attribute a positive family history of CVD to concomitant parental smoking status or parental comorbidities including diabetes or hypertension. Our findings suggest that family history of obesity and smoking in particular do matter. Interestingly, for parental smoking status, much of the effect appears to be mediated by offspring smoking status. By contrast, for other modifiable risk factors including diabetes, hypertension, and hyperlipidemia, parental CVD alone is generally sufficient to capture susceptibility to future CVD in offspring. While parental modifiable risk factors may matter less in those circumstances, it is well‐described that offspring lifestyle factors appear to be independent of genetic CVD risk. 23 , 24 , 25 Furthermore, results from the Physician's Health Study cohort suggests that adherence to a healthy lifestyle in children with a positive family history of premature MI was associated with a lower risk of HF with antecedent MI; this association appeared to be stronger in the younger population. 26 Taken together, our study argues for a strong focus on preventive measures such as lifestyle modifications among individuals with a family history of CVD. We also found that presence of parental obesity was associated with CVD risk in offspring. Whether this may be because of genetic or heritable components of obesity versus contributions of environmental factors or other cardiovascular risk enhancers remains unclear, and future work is needed to validate these results and investigate underlying mechanisms.
Our analysis builds upon previous reports in the Framingham Heart Study showing the association of parental CVD with future offspring risk events 5 , 27 , 28 , 29 by evaluating the contribution of parental risk factors to disease risk. Other studies have evaluated self‐reported family history and show similar associations with cardiovascular risk in offspring: Colditz and colleagues found that among male health professionals of the Health Professionals Follow up Study, family history of MI in either parent was associated with an increased risk of CAD. 11 The risk of MI was noted to be higher in offspring whose fathers had had a MI at a younger age. Like other studies, they relied on self‐report questionnaires to ascertain family history. In a large multinational case–control study, a parental history of MI was independently associated with risk of MI in offspring. 7 Furthermore, other case–control studies found a similar trend of increased risk of CVD with a positive family history. 8 , 9 , 10
Although our study was not powered to detect differences between number and sex of parents affected with outcomes in the offspring, we did observe a potential dose–response effect based on CVD and number of parents affected. Similar to prior studies such as the Health Professionals Follow‐up Study cohort and Stockholm Heart Epidemiology Program, our findings suggest that risk of CVD is higher when more than 1 first‐degree relative is affected. 8 , 11 Our investigation now adds complementary information to parental CVD history by examining contributions of parental modifiable risk factors, with some suggestion that having 2 versus 1 parent with risk factors confers even greater risk.
Our findings with respect to cardiac structure and function are notable, in that parental smoking and obesity history appear to amplify the association of parental HF history with important precursors to HF, including left atrial size, and LV mass. It is important to acknowledge that a previous study by Lee et al observed that parental history of HF was associated with LV systolic dysfunction, increased LV mass, and end‐diastolic dimensions, though in multivariable‐adjusted models, this relation was maintained for systolic dysfunction only. 6 They also found that a family history of HF was associated with a 70% increased risk of HF in comparison to those without. In contrast, we observed that family history of HF was related to increased LV mass, left atrial diameter, and LV wall thickness in multivariable‐adjusted models. Our larger sample size via inclusion of additional cohorts may have allowed for detection of more subtle associations. We did not assess systolic dysfunction directly but did evaluate FS, a measure of ventricular ejection fraction. 30 We observed a higher mean FS in offspring with a family history of HF, though there was no statistically significant difference in multivariable models.
We also report that parental CVD history was associated with greater CAC scores in offspring. We now also expand upon prior findings by showing that parental history of diabetes was associated with CAC scores, and that individuals with concomitant parental CVD and diabetes history had the highest CAC scores. Interestingly, Cohen et al found that in the MESA (Multi‐Ethnic Study of Atherosclerosis) cohort, even in asymptomatic individuals with a CAC score of zero, a positive family history of heart disease conferred increased risk of future CVD. 31
Our study leveraged multigenerational data with rigorously ascertained parental lifestyle factors and CVD that minimized recall bias, coupled with long‐term follow‐up to evaluate subsequent risk in the offspring. However, several limitations deserve mention. First, no standardized definition of CVD family history exists. 32 For example, prior studies have described family history as important only if premature CVD occurred at younger parental ages, whereas others find it noteworthy regardless of the age of onset. 5 , 33 Furthermore, family history of CVD has ranged from composite CVD to specific cardiovascular end points including coronary heart disease, HF, and stroke, and in some cases has been expanded to include siblings. 34 Thus, despite the known association of family history with offspring cardiovascular risk, it has not been incorporated into risk prediction models such as the Framingham risk score, pooled cohort equations, or European Systematic COronary Risk Evaluation. 35 , 36 , 37 Recently, Patel and colleagues found that a single‐question assessment of family history in a first‐degree relative of any age may be just as useful as more complex definitions. 38 For purposes of our analysis, we used a conservative definition of parental history of adjudicated CVD that occurred before the baseline assessment of the offspring, a definition that may not be easily translated to other samples. Furthermore, our sample size limited the power to detect nuances between the affected number of parents and outcomes in the offspring. We acknowledge that although our findings cannot imply causality because of the observational nature of our study, the findings strengthen the literature on family history of CVD and risk. Lastly, the Framingham sample utilized for our study is primarily composed of individuals who are White, limiting generalizability to other racial and ethnic groups such as Black, Hispanic, and Asian. Future studies are needed to determine whether our findings are replicable in more racially and ethnically diverse populations.
In summary, our study demonstrates that parental history of CVD captures susceptibility to future CVD in offspring. In addition, parental modifiable risk factors including family history of obesity and smoking are risk factors of future CVD, with evidence for mediation by offspring smoking status for the latter. By contrast, parental history of diabetes, hypertension, and hypercholesterolemia do not seem to appreciably alter this risk. When considering cross‐sectional findings, family history of smoking and obesity appear to amplify the effect of parental HF history on important precursors to HF, including LA size and LV mass. These findings add nuance to previous studies that highlight the importance of family history of CVD on disease susceptibility and suggest that parental obesity may affect offspring CVD risk. Irrespective of parental modifiable risk factors, the presence of parental CVD should prompt a focus on disease prevention.
Sources of Funding
The Framingham Heart Study was supported by contracts from the National Heart, Lung, and Blood Institute NO1‐HC‐25195, HHSN268201500001I, and 75N92019D00031. Dr Lau is supported by grants from the National Institutes of Health K23‐HL159243 and the American Heart Association #853922. Dr Ho is supported by grants from the National Institutes of Health R01‐HL134893, R01‐HL140224, R01‐HL160003, and K24‐HL153669. Dr Benjamin is supported by the National Institutes of Health R01‐HL092577 grant. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclosures
None.
Supporting information
Tables S1–S7
Acknowledgments
None.
C. N. Taylor and D. Wang contributed equally.
For Sources of Funding and Disclosures, see page 10.
References
- 1. World Health Organization . The top 10 causes of death. December 9, 2020. Accessed March 12, 2022. https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death
- 2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3. McCusker ME, Yoon PW, Gwinn M, Malarcher AM, Neff L, Khoury MJ. Family history of heart disease and cardiovascular disease risk‐reducing behaviors. Genet Med. 2004;6:153–158. doi: 10.1097/01.gim.0000127271.60548.89 [DOI] [PubMed] [Google Scholar]
- 4. Riegel B, Moser DK, Buck HG, Dickson VV, Dunbar SB, Lee CS, Lennie TA, Lindenfeld J, Mitchell JE, Treat‐Jacobson DJ, et al. Self‐care for the prevention and management of cardiovascular disease and stroke: a scientific statement for healthcare professionals from the American Heart Association. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.117.006997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lloyd‐Jones DM, Nam BH, D'Agostino RB Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O'Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle‐aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204 [DOI] [PubMed] [Google Scholar]
- 6. Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O'Donnell CJ, Nam BH, Larson MG, D'Agostino RB, Vasan RS. Association of parental heart failure with risk of heart failure in offspring. New Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948 [DOI] [PubMed] [Google Scholar]
- 7. Pohjola‐Sintonen S, Rissanen A, Liskola P, Luomanmaki K. Family history as a risk factor of coronary heart disease in patients under 60 years of age. Eur Heart J. 1998;19:235–239. doi: 10.1053/euhj.1997.0543 [DOI] [PubMed] [Google Scholar]
- 8. Leander K, Hallqvist J, Reuterwall C, Ahlbom A, de Faire U. Family history of coronary heart disease, a strong risk factor for myocardial infarction interacting with other cardiovascular risk factors: results from the Stockholm Heart Epidemiology Program (SHEEP). Epidemiology. 2001;12:215–221. doi: 10.1097/00001648-200103000-00014 [DOI] [PubMed] [Google Scholar]
- 9. Roncaglioni MC, Santoro L, D'Avanzo B, Negri E, Nobili A, Ledda A, Pietropaolo F, Franzosi MG, La Vecchia C, Feruglio GA, et al. Role of family history in patients with myocardial infarction. An Italian case‐control study. GISSI‐EFRIM investigators. Circulation. 1992;85:2065–2072. doi: 10.1161/01.cir.85.6.2065 [DOI] [PubMed] [Google Scholar]
- 10. Chow CK, Islam S, Bautista L, Rumboldt Z, Yusufali A, Xie C, Anand SS, Engert JC, Rangarajan S, Yusuf S. Parental history and myocardial infarction risk across the world: the INTERHEART Study. J Am Coll Cardiol. 2011;57:619–627. doi: 10.1016/j.jacc.2010.07.054 [DOI] [PubMed] [Google Scholar]
- 11. Colditz GA, Rimm EB, Giovannucci E, Stampfer MJ, Rosner B, Willett WC. A prospective study of parental history of myocardial infarction and coronary artery disease in men. Am J Cardiol. 1991;67:933–938. doi: 10.1016/0002-9149(91)90163-f [DOI] [PubMed] [Google Scholar]
- 12. Andersson C, Johnson AD, Benjamin EJ, Levy D, Vasan RS. 70‐year legacy of the Framingham Heart Study. Nat Rev Cardiol. 2019;16:687–698. doi: 10.1038/s41569-019-0202-5 [DOI] [PubMed] [Google Scholar]
- 13. Andersson C, Nayor M, Tsao CW, Levy D, Vasan RS. Framingham Heart Study: JACC focus seminar, 1/8. J Am Coll Cardiol. 2021;77:2680–2692. doi: 10.1016/j.jacc.2021.01.059 [DOI] [PubMed] [Google Scholar]
- 14. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021 [DOI] [PubMed] [Google Scholar]
- 15. Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–1813. doi: 10.1093/ije/dyv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x [DOI] [PubMed] [Google Scholar]
- 17. Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, Levy D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham Heart Study. JAMA. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252 [DOI] [PubMed] [Google Scholar]
- 18. Ho JE, McCabe EL, Wang TJ, Larson MG, Levy D, Tsao C, Aragam J, Mitchell GF, Benjamin EJ, Vasan RS, et al. Cardiometabolic traits and systolic mechanics in the community. Circ Heart Fail. 2017;10:10. doi: 10.1161/CIRCHEARTFAILURE.116.003536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preis SR, Hwang SJ, Fox CS, Massaro JM, Levy D, Hoffmann U, O'Donnell CJ. Eligibility of individuals with subclinical coronary artery calcium and intermediate coronary heart disease risk for reclassification (from the Framingham Heart Study). Am J Cardiol. 2009;103:1710–1715. doi: 10.1016/j.amjcard.2009.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 21. Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new‐onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. doi: 10.1161/CIRCHEARTFAILURE.112.972828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72. doi: 10.1515/em-2013-0005 [DOI] [Google Scholar]
- 23. Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank study. JAMA Cardiol. 2018;3:693–702. doi: 10.1001/jamacardio.2018.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hasbani NR, Ligthart S, Brown MR, Heath AS, Bebo A, Ashley KE, Boerwinkle E, Morrison AC, Folsom AR, Aguilar D, et al. American Heart Association's Life's Simple 7: lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation. 2022;145:808–818. doi: 10.1161/CIRCULATIONAHA.121.053730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khawaja O, Kotler G, Gaziano JM, Djousse L. Usefulness of desirable lifestyle factors to attenuate the risk of heart failure among offspring whose parents had myocardial infarction before age 55 years. Am J Cardiol. 2012;110:326–330. doi: 10.1016/j.amjcard.2012.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myers RH, Kiely DK, Cupples LA, Kannel WB. Parental history is an independent risk factor for coronary artery disease: the Framingham Study. Am Heart J. 1990;120:963–969. doi: 10.1016/0002-8703(90)90216-k [DOI] [PubMed] [Google Scholar]
- 28. Fox CS, Parise H, D'Agostino RB Sr, Lloyd‐Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851 [DOI] [PubMed] [Google Scholar]
- 29. Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, Villalon ML, Vasan RS, Pencina MJ, Levy D, et al. Association between familial atrial fibrillation and risk of new‐onset atrial fibrillation. JAMA. 2010;304:2263–2269. doi: 10.1001/jama.2010.1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MH, Laragh JH. Assessment of left ventricular function by the midwall fractional shortening/end‐systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23:1444–1451. doi: 10.1016/0735-1097(94)90390-5 [DOI] [PubMed] [Google Scholar]
- 31. Cohen R, Budoff M, McClelland RL, Sillau S, Burke G, Blaha M, Szklo M, Uretsky S, Rozanski A, Shea S. Significance of a positive family history for coronary heart disease in patients with a zero coronary artery calcium score (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2014;114:1210–1214. doi: 10.1016/j.amjcard.2014.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bittencourt MS. Family history of cardiovascular disease: how detailed should it be? Mayo Clin Proc. 2018;93:1167–1168. doi: 10.1016/j.mayocp.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 33. Barrett‐Connor E, Khaw K. Family history of heart attack as an independent predictor of death due to cardiovascular disease. Circulation. 1984;69:1065–1069. doi: 10.1161/01.cir.69.6.1065 [DOI] [PubMed] [Google Scholar]
- 34. Murabito JM, Pencina MJ, Nam BH, D'Agostino RB Sr, Wang TJ, Lloyd‐Jones D, Wilson PW, O'Donnell CJ. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle‐aged adults. JAMA. 2005;294:3117–3123. doi: 10.1001/jama.294.24.3117 [DOI] [PubMed] [Google Scholar]
- 35. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180 [DOI] [PubMed] [Google Scholar]
- 36. Thomsen T. HeartScore: a new web‐based approach to European cardiovascular disease risk management. Eur J Cardiovasc Prev Rehabil. 2005;12:424–426. doi: 10.1097/01.hjr.0000186617.29992.11 [DOI] [PubMed] [Google Scholar]
- 37. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 38. Patel J, Al Rifai M, Scheuner MT, Shea S, Blumenthal RS, Nasir K, Blaha MJ, McEvoy JW. Basic vs more complex definitions of family history in the prediction of coronary heart disease: the Multi‐Ethnic Study of Atherosclerosis. Mayo Clin Proc. 2018;93:1213–1223. doi: 10.1016/j.mayocp.2018.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7


