Abstract
Background
Patients with chronic kidney disease (CKD) can experience acute coronary syndromes (ACS) with high morbidity and mortality. Early invasive management of ACS is recommended for most high‐risk patients; however, choosing between an early invasive versus conservative management approach may be influenced by the unique risk of kidney failure for patients with CKD.
Methods and Results
This discrete choice experiment measured the preferences of patients with CKD for future cardiovascular events versus acute kidney injury and kidney failure following invasive heart procedures for ACS. The discrete choice experiment, consisting of 8 choice tasks, was administered to adult patients attending 2 CKD clinics in Calgary, Alberta. The part‐worth utilities of each attribute were determined using multinomial logit models, and preference heterogeneity was explored using latent class analysis. A total of 140 patients completed the discrete choice experiment. The mean age of patients was 64 years, 52% were male, and mean estimated glomerular filtration rate was 37 mL/min per 1.73 m2. Across the range of levels, risk of mortality was the most important attribute, followed by risk of end‐stage kidney disease and risk of recurrent myocardial infarction. Latent class analysis identified 2 distinct preference groups. The largest group included 115 (83%) patients, who placed the greatest value on treatment benefits and expressed the strongest preference for reducing mortality. A second group of 25 (17%) patients was identified who were procedure averse and had a strong preference toward conservative management of ACS and avoiding acute kidney injury requiring dialysis.
Conclusions
The preferences of most patients with CKD for management of ACS were most influenced by lowering mortality. However, a distinct subgroup of patients was strongly averse to invasive management. This highlights the importance of clarifying patient preferences to ensure treatment decisions are aligned with patient values.
Keywords: acute coronary syndrome, chronic kidney disease, discrete choice experiment, patient preferences, shared decision‐making
Subject Categories: Quality and Outcomes, Treatment, Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- AKI
acute kidney injury
- CAIC
Consistent Akaike Information Criteria
- DCE
discrete choice experiment
- ESKD
end‐stage kidney disease
Clinical Perspective.
What Is New?
In this discrete choice experiment measuring the preferences of patients with chronic kidney disease for management of acute coronary syndrome, most patients with chronic kidney disease placed the greatest emphasis on lowering mortality, followed by avoiding end‐stage kidney disease and reducing recurrent myocardial infarction.
However, the study also identified a subgroup of almost one fifth of patients with a stronger preference for conservative over invasive management who placed more value on avoiding acute kidney injury requiring dialysis.
What Are the Clinical Implications?
The findings of the study illustrate the importance of clarifying the preferences of patients with chronic kidney disease to ensure treatment decisions related to use of invasive versus conservative management are aligned with their values.
Chronic kidney disease (CKD) is associated with a high prevalence of coronary artery disease and high risk of mortality and cardiovascular events following an acute coronary syndrome (ACS). 1 , 2 Guidelines for treatment of high‐risk individuals with a non‐ST elevation ACS recommend early invasive management, involving coronary angiography followed by percutaneous or surgical revascularization if appropriate, including among patients with CKD. 3 Nonetheless, observational studies suggest patients with CKD eligible for such interventions receive them at only half the rate of those patients without CKD. 2 , 4 , 5 Choosing between an early invasive versus conservative management approach presents unique challenges for patients with CKD, where decisions must balance the risk of future cardiovascular events against the risk of iatrogenic kidney injury and kidney failure. 2 , 5 , 6
Shared decision‐making between patients and health care providers is an important component of patient‐centered care and requires patients to have an opportunity to express their preferences and values and for these preferences to inform treatment decisions. 7 Characterization of patients' values and preferences is also important for implementing clinical practice guidelines, 8 especially when recommendations are weak or conditional. The preferences of patients with CKD for invasive versus conservative ACS management is unclear.
A discrete choice experiment (DCE) is a method for measuring patient preferences based on utility theory, which allows for quantification of the preference weights for the relative importance of individual attributes of a treatment option. 9 , 10 , 11 DCEs can characterize how patients weigh competing risks and benefits of treatment options and help to understand the amount of variability in patient preferences. The objective of this DCE was to measure the preferences of patients with CKD for routine invasive versus conservative treatment of ACS. We also sought to identify whether important between‐patient differences exist in their preferences for ACS treatment. 5 , 12
Methods
We designed this DCE according to the recommendations of the International Society for Pharmacoeconomics and Outcomes Research and those of Lancsar and Louviere for conducting DCEs. 10 , 13 Approval was obtained from the University of Calgary, Conjoint Health Research Ethics Board. Informed consent was obtained from all participants. Data are available on request from the corresponding author.
Survey Design
During the design of the study, we identified attributes of management of ACS relevant to patients with CKD through semistructured interviews with 20 patients with CKD and 10 cardiologists. Interviews included questions that asked participants to describe the attributes they considered to be the most important. 14 , 15 The top 5 attributes most frequently identified by participants were use of an early invasive versus conservative management approach (early receipt of an angiogram), risk of death, risk of acute kidney injury (AKI) requiring dialysis, risk of end‐stage kidney disease (ESKD), and risk of recurrent myocardial infarction (MI). For these attributes included in the DCE, we determined levels of risk for each attribute from reports of randomized controlled trials of early invasive versus conservative management strategies for non‐ST elevation ACS, as well as cohort studies that reported ranges in the incidence of these outcomes following ACS among patients with varying stages of CKD (Table S1). 2 , 16 , 17
We engaged 2 patient partners with lived experience with CKD and heart disease (C.C. and W.E.P.) in the design and development of the patient survey for the DCE. Our patient partners contributed to the development by providing advice on the study information for patients and the survey design, participating in iterative refinement of the DCE during its development, and contributing to this article.
The design of this survey was further informed by piloting the DCE with 43 patients. 14 During the initial interviews, participants were encouraged to talk through the survey. Research coordinators recorded patient questions and comments related to the introductory material and choice questions. Based on knowledge gained from patients participating in the pilot study, we modified introductory information about the clinical problem and treatment options, added graphics to aid communication of risk (shaded boxes out of 100) in addition to written information on the number of events out of 100 people, expanded the choice of media for patients by adding the option of a pencil and paper format for those who may prefer it over the computer format, and revised the description of AKI requiring acute dialysis to more clearly distinguish acute dialysis from permanent kidney replacement therapy.
After incorporating the revisions to the DCE, we generated new choice sets. The paper survey option consisted of 10 different versions of the 8 choice tasks randomly chosen from the 100 electronic versions. For the final DCE, a sample size of 190 survey responses was determined to provide sufficient power to ensure statistical efficiency for estimating main effects part‐worth utilities (standard errors for estimating attribute/level combinations were <0.05 for main effects). 14 , 18 , 19 The survey was developed using Lighthouse Studio, Sawtooth Software (Orem, UT). The introductory material, final questions, and an example of one version of the DCE choice tasks are available in Data S1.
Patient Recruitment and Survey Administration
We recruited patients from 2 CKD clinics in Calgary, Alberta. Eligible patients were 18 years of age or older, with estimated glomerular filtration rate <45 mL/min per 1.73 m2, not receiving dialysis, and able to communicate in English. Eligibility criteria did not require that patients had a history of cardiac disease or a prior ACS. Nursing staff from clinics identified patients who were potentially eligible for the study and research coordinators explained the details of the study, obtained informed consent, and assisted patients with completion of the survey. Patients had the option of completing the survey in clinic or from home with follow‐up provided by research coordinators within 2 weeks of their clinic visit. Patient information collected as part of the survey included self‐reported age, sex, race or ethnicity, kidney and cardiovascular disease history, whether they had received education about dialysis modality options, decision‐making preferences, and goals of care. Most recent estimated glomerular filtration rate measurements were obtained directly from patient electronic medical records.
Statistical Analysis
We described participant characteristics using means and SDs for continuous variables and numbers and percentages for dichotomous variables. We assessed the internal validity of the DCE, based on naturally occurring tests, using the following measures: (1) within‐set dominated pairs, where participants choose the alternative that is worse for all attributes; (2) across‐set dominated pairs, where the alternative chosen in 2 different choice sets is logically inconsistent; and (3) straight‐lining, where participants choose the alternative in the same position (rightmost or leftmost) for each question. 18 Additionally, we also evaluated attribute dominance, where the better level of a single attribute is chosen in all scenarios. Although responses showing attribute dominance were not considered to be a test of internal validity, we compared their frequency to characterize responses. The within‐set dominated pairs test included only those choice tasks where the treatment approach attribute was equivalent (ie, both were invasive management or both were conservative management) as we did not, a priori, assume one treatment approach provided higher utility than the other. In addition, the within‐set dominated pairs test was not incorporated into the design of the study; however, some versions of the survey included a dominated choice task that arose naturally from the survey generated by the software. Four of the 10 versions of the survey had a dominated pairs test; therefore, not all patients received a survey where the dominated pairs test could be assessed.
To estimate the preference weights patients placed on each attribute and level, we fit a main‐effects multinomial logit model and hierarchical Bayes model. Attributes were modeled as categorical variables and assessed for linearity across the range of attribute levels specified. Linearity was assessed visually and by comparing the change in part‐worth utility for each attribute between the lowest risk level and moderate risk level, divided by the change in levels, and the moderate risk level and the highest risk level, divided by the change in levels. Model fit was compared based on onsistent Akaike information criteria (CAIC) and Bayesian information criteria. We chose the CAIC for model selection because of its widespread use in determining the number of latent class segments to accept and its superiority to AIC for selecting parsimonious models. 19 As a sensitivity analysis we estimated preference weights, using the multinomial logit model, excluding those participants who showed evidence of straight‐lining in case these were invalid responses.
Utilities and importances were scaled such that the difference of the mean utility between the highest and lowest levels for each attribute summed to 100. Larger positive values indicated greater utility, and larger negative values indicated lower utility. We also calculated the marginal rate of substitution for each attribute relative to mortality, which can be defined as the absolute percentage increase in the risk of each undesirable attribute patients were willing to accept relative to a 1% decrease in the risk of mortality. The marginal rate of substitution was estimated from the multinomial logit model by dividing the mean utility for a 1% increase in the risk of the undesirable attribute by the mean utility for a 1% decrease in the risk of mortality. SEs for the marginal rate of substitution were estimated using the delta method. 20
We investigated preference heterogeneity using latent class analysis for a 2‐class model and a 3‐class model and compared models using the CAIC and patterns of part‐worth utilities between the models. Latent class methodology iteratively estimates the utilities for each group and the probability respondents belong in each group, allowing patterns of similar preferences to be identified in the data. Using the model with the lowest CAIC, we compared the characteristics of respondents within the groups using univariate analysis, with chi‐square tests for categorical variables and t tests for continuous variables.
Share Preference Calculator
To facilitate future research, we developed a share preference calculator using the Hierarchical Bayes model and the Choice Simulator in Lighthouse Studio, Sawtooth Software. The choice simulator takes the individual part‐worth utilities for each respondent in the DCE to model preference shares for treatment options. The share preference calculator takes, as inputs, estimated risk of the attributes presented in the DCE and treatment approach. This allows for an estimate of the preference for a treatment option for a future respondent based on predicted risk estimates of the attributes in the model.
RESULTS
Patient Recruitment
A total of 146 patients consented to participate in the DCE, and 140 (96%) completed the survey between January 2019 and March 2020. We were unable to reach our intended sample size of 190 patients because of introduction of restrictions related to the COVID‐19 pandemic. The paper option was chosen by 115 patients (82%), 112 (97%) completed the survey in clinic with the assistance of the research coordinator, and 3 (3%) completed the survey later from home. Three patients missed 1 question and 2 patients missed 2 questions. There were missing data for some patient variables; 92 (66%) reported their goals of care designation, and 127 (91%) patients reported the duration of their CKD diagnosis. Response rates for the remaining patient characteristics ranged from 96% to 100% (Table 1).
Table 1.
Characteristics of Patients Completing the Discrete Choice Experiment Examining Preferences of Patients With Chronic Kidney Disease for Invasive Versus Conservative Treatment of Acute Coronary Syndrome
| Characteristic | Result (N=140) |
|---|---|
| Age, mean (SD), y | 64 (16) |
| Male sex, n (%) | 72 (52) |
| Estimated glomerular filtration rate, mean (SD), mL/min per 1.73 m2 | 37 (26) |
| Duration of chronic kidney disease,* mean (SD), y | 9 (6) |
| Prior invasive procedure, n (%) | 39 (28) |
| Prior heart attack, n (%) | 19 (14) |
| Prior stroke, n (%) | 10 (7) |
| History of acute kidney injury, n (%) | 12 (9) |
| Prior dialysis treatment, n (%) | 5 (4) |
| Received dialysis modality education, n (%) | 32 (24) |
| Have made a dialysis treatment choice, n (%) | 78 (56) |
| Goals of care designation,† n (%) | |
| Includes resuscitation | 61 (66) |
| No resuscitation | 31 (34) |
Based on 127 respondents.
Based on 92 respondents.
Internal Validity
Analysis for naturally occurring within‐set dominated pairs in the survey showed that out of 55 participants who received an option with a dominated pairs test, 10 (18%) participants chose the dominated choice profile (the alternative that is worse for all attributes). Evidence of straight‐lining occurred with 6 (4%) participants choosing the alternative in the same position for all 8 tasks. The frequency of these internal validity measures were similar to those of other published DCEs 18 (Table S2). There was evidence of attribute dominance for 49 (35%) participants, with 17 (12%) of these choosing the option with the same treatment type or with the lower level of risk of a single attribute in all 8 choice tasks (4 based on treatment approach, 1 based on heart attack risk, 9 based on risk of death, and 3 based on ESKD risk) and 32 choosing the treatment option with the same treatment type or with the lower level of risk of a single attribute in 7 out of 8 choice tasks (2 based on treatment approach, 2 based on heart attack risk, 17 based on risk of death, 6 based on risk of AKI requiring dialysis, and 5 based on ESKD risk).
Patient Preferences
The model coefficients for the categorical multinomial logit model are shown in Table S3 and for the continuous multinomial model in Table S4. The continuous model provided the lowest CAIC, indicating a better fit to the data. The part‐worth utilities representing the relative importance of each attribute based on the categorical multinomial logit model are illustrated in Figure 1. Among all participants, the risk of death within 1 year was the most important attribute across the range of levels specified, followed by avoiding ESKD. The treatment approach was the least important attribute. Based on the continuous model, for each 1% absolute increase in risk, mortality remained the most important attribute whereas ESKD and recurrent MI were of similar secondary importance. When the marginal rate of substitution was calculated relative to a 1% decrease in the annual risk of mortality (to quantify the risk of other outcomes a patient would be willing to accept to gain a 1% decrease in risk of death), participants were willing to accept a 1.4% (95% CI, 1.0%–1.9%) increased absolute risk of ESKD within 1 year, 1.4% (95% CI, 0.8%–2.0%) increase in the risk of another heart attack within 1 year, and a 1.9% (95% CI, 1.2%–2.5%) increase in the risk of AKI requiring dialysis (Table 2).
Figure 1. Part‐worth utilities for each attribute and level from a discrete choice experiment examining preferences of patients with chronic kidney disease for invasive versus conservative treatment of acute coronary syndrome.
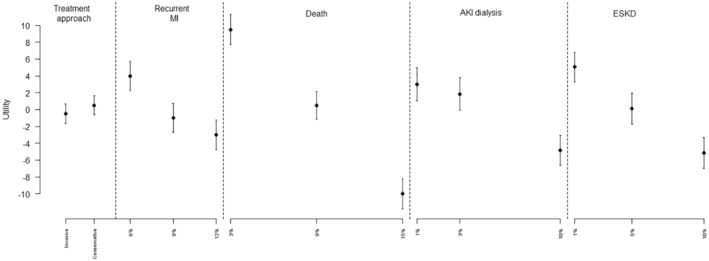
Part‐worth utilities are calculated as means +/− 95% CIs and scaled from −10 to +10, with +10 indicating a strong preference for the attribute level and −10 indicating a strong aversion to the attribute level. AKI indicates acute kidney injury; ESKD, end‐stage kidney disease; and MI, myocardial infarction.
Table 2.
Trade‐Offs in the Absolute Risk of Various Attributes That Patients Would be Willing to Accept Relative to a 1% Decrease in Risk of Death Within 1 Year from a Discrete Choice Experiment Examining Preferences of Patients With Chronic Kidney Disease for Invasive Versus Conservative Treatment of Acute Coronary Syndrome
| Attribute | Marginal rate of substitution relative to a 1% decrease in the risk of death, % (95% CI) |
|---|---|
| Treatment approach (invasive versus conservative) | 1.7 (0.0–3.7) |
| Risk of another heart attack within 1 year | 1.4 (0.8–2.0) |
| Risk of AKI requiring dialysis | 1.9 (1.2–2.5) |
| Risk of ESKD | 1.4 (1.0–1.9) |
The marginal rate of substitution for the treatment approach indicates that, for patients to accept an invasive over conservative approach, it would have to correspond to a 1.7% or more decrease in the risk of death. For the attributes of risk of recurrent myocardial infarction, risk of AKI, and risk of ESKD, the marginal rate of substitution indicates the absolute percentage increase in the risk of the listed attribute that patients are willing to accept with a choice that achieves a 1% decrease in the risk of death within 1 year. AKI indicates acute kidney injury; and ESKD, end‐stage kidney disease.
Latent Class Analysis
Latent class analysis with 2 subgroups of patients provided the best fit to the data based on the model fit statistics. One hundred fifteen (83%) patients had the highest probability (average probability of group membership: 97.7%) of belonging to a group that placed the most value on treatment benefit, assigning the highest importance on lower risk of death, with lower risk of ESKD being of secondary importance. However, 25 (17%) patients had the highest probability of belonging to a second group (average probability of group membership: 87.1%) that were more procedure averse, with a strong preference for conservative management, followed by high importance for minimizing the risk of AKI requiring dialysis (Figure 2 and Figure 3). Among the 25 patients in the second group, 8 showed dominated preferences with 6 choosing the option with conservative treatment in at least 7 of 8 tasks and 2 choosing the option with the lower risk of AKI requiring dialysis in 7 of 8 tasks.
Figure 2. Relative importance of attributes for the overall cohort and the 2 latent class groups over the range of levels from a discrete choice experiment examining preferences of patients with chronic kidney disease for invasive versus conservative treatment of acute coronary syndrome.
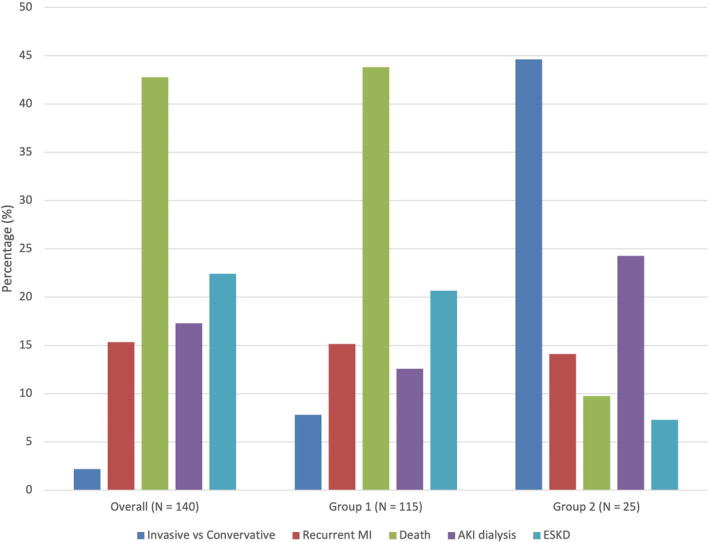
AKI indicates acute kidney injury; ESKD, end‐stage kidney disease; and MI, myocardial infarction.
Figure 3. Part‐worth utilities for each attribute and level in the 2‐group latent class analysis from a discrete choice experiment examining preferences of patients with chronic kidney disease for invasive versus conservative treatment of acute coronary syndrome.
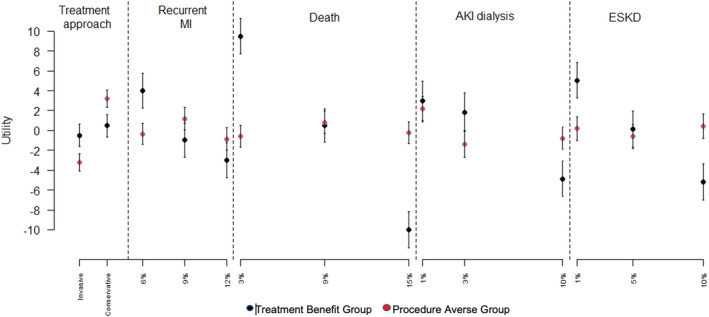
Part‐worth utilities are calculated as means +/− 95% CIs and scaled from −10 to +10, with +10 indicating a strong preference for the attribute level and −10 indicating a strong aversion to the attribute level. AKI indicates acute kidney injury; ESKD, end‐stage kidney disease; and MI, myocardial infarction.
Univariate analysis identified no statistically significant differences in age, sex, history of AKI, or prior experience with treatment with dialysis between the 2 groups (Table 3). However, more patients in the procedure‐averse group had attended dialysis modality sessions, and this group also had a lower average estimated glomerular filtration rate (29 mL/min per 1.73 m2 versus 39 mL/min per 1.73 m2). Furthermore, among 92 patients that had completed a Goals of Care Designation, 71% had specified they would accept resuscitation in the treatment benefit group, whereas a minority (47%) in the procedure‐averse group had specified they would accept resuscitation within their goals of care.
Table 3.
Characteristics of Patients in 2‐Group Latent Class Analysis From a Discrete Choice Experiment Examining Preferences of Patients With Chronic Kidney Disease for Invasive Versus Conservative Treatment of Acute Coronary Syndrome
| Treatment benefit group (N=115) | Procedure‐averse group (N=25) | P value | |
|---|---|---|---|
| Age, y, mean (SD) | 64 (16) | 63 (16) | 0.66 |
| Male sex, n (%) | 62 (54) | 10 (40) | 0.19 |
| Estimated glomerular filtration rate, mean (SD), mL/min 1.73 m2 | 39 (27) | 29 (20) | 0.09 |
| Duration of chronic kidney disease, mean (median), y | 9 (6) | 9 (6) | 0.97 |
| Prior invasive procedure, n (%) | 29 (25) | 10 (40) | 0.14 |
| Prior heart attack, n (%) | 14 (13) | 5 (22) | 0.25 |
| Prior stroke, n (%) | 7 (6) | 3 (14) | 0.23 |
| History of acute kidney injury, n (%) | 10 (10) | 2 (9) | 0.83 |
| Prior dialysis treatment, n (%) | 4 (4) | 1 (4) | 0.90 |
| Received dialysis modality education, n (%) | 23 (21) | 9 (38) | 0.08 |
| Have made a dialysis treatment choice, n (%) | 63 (55) | 15 (60) | 0.63 |
| Goals of care designation,* n (%) | |||
| Includes resuscitation | 53 (71) | 8 (47) | |
| No resuscitation | 22 (29) | 9 (53) | 0.06 |
Based on 92 respondents.
In sensitivity analyses that excluded the 6 patients who showed evidence of straight‐lining (5 from the treatment benefit preference group and 1 from the procedure‐averse preference group), the average importances were similar to those from the main analysis, and 2 distinct preference groups were again identified, with the first group including 117 (87%) patients who placed the most value on treatment benefit and the second group including 17 (13%) patients who were procedure averse (Figure S1).
Shared Preference Calculator
The share preference calculator based on the continuous hierarchical Bayes model (Table S5) provides a comparison of preferences for treatment options with varying levels of risk of attributes. Three examples of treatment choice scenarios and estimated preferences for each option obtained using the share preference calculator are shown in Table S6. The examples illustrate how patient preferences may differ with varying attributes of the treatment choice.
Discussion
This DCE quantified the preferences of patients with CKD for invasive versus conservative treatment of ACS. Overall, we found the preferences of most participants were driven by treatment benefit, with lower risk of death the most important attribute, followed by lower risk of progression to ESKD and lower risk of recurrent MI of equal secondary importance. However, there was preference heterogeneity among respondents, with a smaller group of patients identified who were procedure averse and placed highest importance on avoiding invasive management and AKI requiring dialysis.
We are not aware of any other studies investigating the management preferences of patients with CKD and ACS; however, studies have been conducted on the broader population of patients experiencing ACS. Similar to our study, a recent study from China on preferences for treatment following acute MI found mortality to be the most important attribute. Latent class analyses found 3 distinct groups with varying preferences based on treatment cost, treatment duration, and mortality risk. 21 Muhlbacher et al. conducted a DCE to evaluate patient preferences for medication options following ACS. 22 These authors also found reduction in mortality to be the most important attribute, followed by prevention of new MI. 23 The findings from our DCE for patients with CKD are consistent with these studies, with mortality being the most important attribute overall; however, we also discovered 2 distinct groups of patients with varying preferences; one focused on treatment benefit, and a second group, made up of about one‐fifth of respondents, being procedure averse. We investigated the possibility that the identification of this group may have resulted from participants who failed the internal validity tests. However, only 1 of the 25 patients in the procedure‐averse group failed the straight‐lining test (chose the alternative in the same position for all 8 tasks), suggesting this group was identified from valid responses to the survey. In addition, in a sensitivity analysis that excluded all 6 patients who failed the straight‐lining test, we found consistent results to the main analysis. We did not identify any significant differences in patient characteristics to explain these differences in preferences. However, in the procedure‐averse group, more patients had received dialysis modality education and this group had a lower average estimated glomerular filtration rate, suggesting that stated preferences of participants for ACS management may align with knowledge about the impact of kidney failure on quality of life. This indicates that a sizable group of patients may value quality over length of life and that patient preferences may in part appropriately contribute to the lower use of invasive heart procedures following ACS among people with CKD that has been reported in previous studies. 2 , 4 , 24 We expect there are other factors contributing to preferences, which vary between individuals and were not measured in our DCE. For example, some patients may be opposed to invasive treatment based on anecdotal examples of negative outcomes experienced by a family member or friend.
The strengths of our study include the rigorous process to establish valid and important attributes through semistructured interviews with patients and physicians 15 and the incorporation of findings from published randomized control trials and observational studies of early versus select invasive strategies for non‐ST elevation ACS to ensure the attribute levels were appropriate and relevant. 2 , 16 We used a range of risks for each of the attributes obtained from published literature to ensure the risk of outcomes were plausible for people across the stages the CKD. Input from our patient research partners with lived experience ensured the DCE survey design was relevant and understandable from a patient perspective. In addition, we followed the International Society for Pharmacoeconomics and Outcomes Research guidelines for DCE design to ensure methodological rigor. 13 , 25 As many of the respondents were elderly, with lower technological literacy, the paper‐based survey option and research coordinator support allowed for more inclusive recruitment. We expect the use of dedicated research coordinators to administer the survey supported respondents in carefully considering the choice questions, as well as ensuring a high proportion of completed surveys. In addition, the validity of responses obtained from participants was supported by the test of internal validity, which was consistent with other published DCE studies. 18
There are limitations to this DCE. We could not include risks for all possible treatment outcomes, including risk of stroke and bleeding as attributes in this DCE, which may be other relevant attributes for those facing an invasive versus conservative treatment decision. Because of the COVID‐19 pandemic, we were unable to reach our intended sample size of 190 patients. Although the average importance of attributes met our a priori expectations, the precision of our estimates were reduced as the SEs of model coefficients were higher with a smaller than intended sample size. Further, the generalizability of this DCE may be limited as recruitment occurred in 2 clinics in Calgary, Canada. Results likely reflect preferences in other high‐income countries with universal access to health care such as invasive cardiac procedures and dialysis treatment. However, ideally such an experiment should be conducted in other regions to determine if the findings are externally valid. Given that latent class analyses tend to require large sample sizes, the results presented here might be underpowered to detect additional latent groups. Further research should seek to replicate these analyses in a larger sample. In addition, the introductory information to explain treatment options and attributes was quite complex, and 4 of the 5 attributes presented risks of the outcome. This may have been a potential limitation to patient understanding and the high proportion of risk attributes may have caused some to focus on only 1 or 2 attributes per choice task. However, our research coordinators worked one on one with most patients to complete the survey in clinic and were able to explain the introductory material and provide clarification on the choice tasks. DCEs are a stated preference method and patients may make different choices when they encounter these decisions in actual care. Use of other methodological approaches to understand preferences of patients with CKD at the time of ACS treatment decisions are warranted.
Our findings have implications for clinical care and future research on the management of ACS for patients with CKD. These findings can help raise clinician awareness of the need to understand patient preferences because they can differ, reveal the preference profiles and their frequency among patients that clinicians may encounter, and provide knowledge on variability in patient preferences that can strengthen clinician capacity to engage in shared decision‐making discussions with their patients. Furthermore, the quantification of relative weights for different treatment attributes can be used to weight competing outcomes such as death versus ESKD in quantitative studies according to patient preferences. Reduced kidney function is common in patients with ACS with about 1 in 4 patients having CKD, with a poorer prognosis than patients without CKD. However, studies have shown that patients with CKD are less likely to receive coronary angiography or revascularization following a coronary event than patients without CKD and this disparity increases as kidney function declines. 2 This may be because of fear of causing further kidney damage with invasive management despite the reduction in risks of adverse cardiovascular outcomes and death with their use. 4 , 24 By quantifying patient preferences through this DCE, we have determined that most patients with CKD identify the benefit of invasive management for reducing mortality risk as the most important feature influencing their treatment decision. However, the potential harms leading to EKSD remain an important consideration as we found that preferences vary among patients, and the preferences of some patients may appropriately account for lower overall use of invasive procedures among patients with CKD. This highlights the need for care providers to communicate risk information and elicit patient preferences when discussing treatment options as preferences cannot be assumed to be identical for all patients with CKD and are not simply based on patient demographic characteristics or comorbidities. Shared decision‐making approaches that include communicating the benefits and harms of each option and understanding each patient's treatment goals are important actions to ensure treatment decisions align with patient preferences. Identifying that 1 in 5 patients with CKD may be procedure averse highlights the need for tools and processes that allow for incorporation of preferences in patient decision aids. In addition, these results support the need to incorporate knowledge about patient preferences into treatment guidelines, as recommended by the Grading of Recommendations Assessment, Development and Evaluation working group. 26
Conclusions
In summary, this DCE has characterized the stated preferences of patients with CKD toward the benefits and harms of invasive versus conservative treatment options for ACS. Most patients with CKD were most concerned with lowering risk of mortality. Of secondary importance was lowering risk of ESKD and recurrent MI. Our findings provide new knowledge about important differences in preferences among patients, with a subgroup of patients being strongly averse to invasive management. This can facilitate improved shared decision‐making between patients with CKD and their care providers in the setting of ACS. Such knowledge can inform the development of patient decision aids and guidelines that address patient preferences within the decision‐making process to improve patient experiences and quality of care. 27
Sources of Funding
This study was supported by a Strategy for Patient‐Oriented Research Chronic Disease Network Grant: Can‐SOLVE Chronic Kidney Disease Network, from the Canadian Institutes of Health Research. Todd A. Wilson was supported by the Roy and Vi Baay Chair in Kidney Research Award, and Matthew T. James was supported by a Canadian Institutes of Health Research New Investigator Award.
Disclosures
Matthew T. James was the principal investigator of an investigator‐initiated research grant from Amgen Canada, outside the submitted work.
Supporting information
Data S1
Tables S1–S6
Figure S1
Acknowledgments
The authors thank their patient partners and research participants for their time and for sharing their values and preferences. The authors acknowledge Charlynn Ursu and Kristen Scott‐Douglas, the study coordinators, who recruited and worked with patients in the clinics.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028492
For Sources of Funding and Disclosures, see page 9.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 2. James MT, Tonelli M, Ghali WA, Knudtson ML, Faris P, Manns BJ, Pannu N, Galbraith PD, Hemmelgarn BR. Renal outcomes associated with invasive versus conservative management of acute coronary syndrome: propensity matched cohort study. BMJ. 2013;347:f4151. doi: 10.1136/bmj.f4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e663–e828. doi: 10.1161/CIR.0b013e31828478ac [DOI] [PubMed] [Google Scholar]
- 4. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD. Use of evidence‐based therapies in short‐term outcomes of ST‐segment elevation myocardial infarction and non‐ST‐segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chertow GM, Normand SL, McNeil BJ. "Renalism": inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15:2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B [DOI] [PubMed] [Google Scholar]
- 6. Wilson T, Miller J, Teare S, Penman C, Pearson W, Marlett NJ, Shklarov S, Galbraith DP, Southern DA, Knudtson ML, et al. Patient perspectives on engagement in decision‐making in early management of non‐ST elevation acute coronary syndrome: a qualitative study. BMC Med Inform Decis Mak. 2017;17:153. doi: 10.1186/s12911-017-0555-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barry MJ, Edgman‐Levitan S. Shared decision making‐‐pinnacle of patient‐centered care. New Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283 [DOI] [PubMed] [Google Scholar]
- 8. Harter M, van der Weijden T, Elwyn G. Policy and practice developments in the implementation of shared decision making: an international perspective. Z Evid Fortbild Qual Gesundhwes. 2011;105:229–233. doi: 10.1016/j.zefq.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 9. Hazlewood GS. Measuring patient preferences: an overview of methods with a focus on discrete choice experiments. Rheum Dis Clin North Am. 2018;44:337–347. doi: 10.1016/j.rdc.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 10. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics. 2008;26:661–677. doi: 10.2165/00019053-200826080-00004 [DOI] [PubMed] [Google Scholar]
- 11. Muhlbacher AC, Juhnke C. Patient preferences versus physicians' judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11:163–180. doi: 10.1007/s40258-013-0023-3 [DOI] [PubMed] [Google Scholar]
- 12. Wong JA, Goodman SG, Yan RT, Wald R, Bagnall AJ, Welsh RC, Wong GC, Kornder J, Eagle KA, Steg PG, et al. Temporal management patterns and outcomes of non‐ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J. 2009;30:549–557. doi: 10.1093/eurheartj/ehp014 [DOI] [PubMed] [Google Scholar]
- 13. Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J. Conjoint analysis applications in health‐‐a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 14. Wilson T, Javaheri P, Finlay J, Hazlewood G, Wilton SB, Sajobi T, Levin A, Pearson W, Connolly C, James MT. Treatment preferences for cardiac procedures of patients with chronic kidney disease in acute coronary syndrome: design and pilot testing of a discrete choice experiment. Can J Kidney Health Dis. 2021;8:2054358120985375. doi: 10.1177/2054358120985375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finlay J, Wilson T, Javaheri PA, Pearson W, Connolly C, Elliott MJ, Graham MM, Norris CM, Wilton SB, James MT. Patient and physician perspectives on shared decision‐making for coronary procedures in people with chronic kidney disease: a patient‐oriented qualitative study. CMAJ Open. 2020;8:E860–E868. doi: 10.9778/cmajo.20200039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fanning JP, Nyong J, Scott IA, Aroney CN, Walters DL. Routine invasive strategies versus selective invasive strategies for unstable angina and non‐ST elevation myocardial infarction in the stent era. Cochrane Database Syst Rev. 2016;2016:CD004815. doi: 10.1002/14651858.CD004815.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santopinto JJ, Fox KA, Goldberg RJ, Budaj A, Pinero G, Avezum A, Gulba D, Esteban J, Gore JM, Johson J, et al. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE). Heart. 2003;89:1003–1008. doi: 10.1136/heart.89.9.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson FR, Yang JC, Reed SD. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health. 2019;22:157–160. doi: 10.1016/j.jval.2018.07.876 [DOI] [PubMed] [Google Scholar]
- 19. Sawtooth Software Inc . The latent class technical paper. Sawtooth Software Technical Paper Series . 2021; Version 4.8.
- 20. Mott DJ, Chami N, Tervonen T. Reporting quality of marginal rates of substitution in discrete choice experiments that elicit patient preferences. Value Health. 2020;23:979–984. doi: 10.1016/j.jval.2020.04.1831 [DOI] [PubMed] [Google Scholar]
- 21. Dai W, Liu C, Liu J, Lin Y, Cheng Y, Ming WK. Preference of individuals in the treatment strategies of acute myocardial infarction in China: a discrete choice experiment. Health Qual Life Outcomes. 2020;18:217. doi: 10.1186/s12955-020-01466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muhlbacher AC, Bethge S. Reduce mortality risk above all else: a discrete‐choice experiment in acute coronary syndrome patients. PharmacoEconomics. 2015;33:71–81. doi: 10.1007/s40273-014-0223-1 [DOI] [PubMed] [Google Scholar]
- 23. Muhlbacher AC, Bethge S, Kaczynski A. Treatment after acute coronary syndrome: analysis of patient's priorities with analytic hierarchy process. Int J Technol Assess Health Care. 2016;32:284–291. doi: 10.1017/S0266462316000428 [DOI] [PubMed] [Google Scholar]
- 24. Bhatt DL, Roe MT, Peterson ED, Li Y, Chen AY, Harrington RA, Greenbaum AB, Berger PB, Cannon CP, Cohen DJ, et al. Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA. 2004;292:2096–2104. doi: 10.1001/jama.292.17.2096 [DOI] [PubMed] [Google Scholar]
- 25. Hauber AB, Gonzalez JM, Groothuis‐Oudshoorn CG, Prior T, Marshall DA, Cunningham C, MJ IJ, Bridges JF. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19:300–315. doi: 10.1016/j.jval.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 26. Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, Rind D, Montori VM, Brito JP, Norris S, et al. Grade guidelines: 15. Going from evidence to recommendation‐determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66:726–735. doi: 10.1016/j.jclinepi.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 27. Wilson T. Patient Preferences and Individualized Risk Prediction for Management of Acute Coronary Syndrome in Chronic Kidney Disease. Dissertation. University of Calgary. 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S6
Figure S1


