Abstract
Background
Myocardial revascularization has been advocated to improve myocardial function and prognosis in ischemic cardiomyopathy (ICM). We discuss the evidence for revascularization in patients with ICM and the role of ischemia and viability detection in guiding treatment.
Methods and Results
We searched for randomized controlled trials evaluating the prognostic impact of revascularization in ICM and the value of viability imaging for patient management. Out of 1397 publications, 4 randomized controlled trials were included, enrolling 2480 patients. Three trials (HEART [Heart Failure Revascularisation Trial], STICH [Surgical Treatment for Ischemic Heart Failure], and REVIVED [REVascularization for Ischemic VEntricular Dysfunction]‐BCIS2) randomized patients to revascularization or optimal medical therapy. HEART was stopped prematurely without showing any significant difference between treatment strategies. STICH showed a 16% lower mortality with bypass surgery compared with optimal medical therapy at a median follow‐up of 9.8 years. However, neither the presence/extent of left ventricle viability nor ischemia interacted with treatment outcomes. REVIVED‐BCIS2 showed no difference in the primary end point between percutaneous revascularization or optimal medical therapy. PARR‐2 (Positron Emission Tomography and Recovery Following Revascularization) randomized patients to imaging‐guided revascularization versus standard care, with neutral results overall. Information regarding the consistency of patient management with viability testing results was available in ≈65% of patients (n=1623). No difference in survival was revealed according to adherence or no adherence to viability imaging.
Conclusions
In ICM, the largest randomized controlled trial, STICH, suggests that surgical revascularization improves patients' prognosis at long‐term follow‐up, whereas evidence supports no benefit of percutaneous coronary intervention. Data from randomized controlled trials do not support myocardial ischemia or viability testing for treatment guidance. We propose an algorithm for the workup of patients with ICM considering clinical presentation, imaging results, and surgical risk.
Keywords: coronary artery bypass surgery, hibernation, ischemic cardiomyopathy, myocardial ischemia, myocardial revascularization, myocardial viability, percutaneous coronary intervention
Subject Categories: Chronic Ischemic Heart Disease, Heart Failure
Nonstandard Abbreviations and Acronyms
- CCS
chronic coronary syndrome
- ICM
ischemic cardiomyopathy
- MR
mitral regurgitation
- OMT
optimal medical therapy
- PARR
Positron Emission Tomography and Recovery Following Revascularization
- REVIVED‐BCIS2R
Revascularization for Ischemic Ventricular Dysfunction
- SCAAR
Swedish Coronary Angiography and Angioplasty Registry
- SPECT
single‐photon emission computed tomography
- STICH
Surgical Treatment for Ischemic Heart Failure
Clinical Perspective.
What Is New?
Myocardial revascularization has been advocated to improve myocardial function and prognosis in ischemic cardiomyopathy and is widely practiced, with an increasing proportion of revascularization being done, but evidence for its benefits and for the role of ischemia and viability detection in guiding treatment is very limited.
What Are the Clinical Implications?
Neither the extent of myocardial viability nor the extent of left ventricular inducible ischemia appear to interact with treatment strategies in favorably affecting outcomes.
The role of pretreatment assessment of myocardial viability in guiding treatment and predicting individual patients' benefit from myocardial revascularization can be neither affirmed nor refuted.
Available evidence, however, supports surgical revascularization in patients with coronary artery disease and severely depressed left ventricular systolic function, although the benefit of coronary bypass surgery over optimal medical therapy appears to become significant only late during a long‐term follow‐up.
Ischemic heart disease, most often a result of underlying obstructive epicardial coronary artery disease (CAD), is the most frequent cause of left ventricular (LV) systolic dysfunction, ultimately leading to heart failure (HF). 1 , 2 In this setting, the term “ischemic cardiomyopathy” (ICM) is generally used to identify the presence of significant LV systolic dysfunction coexisting with—and thought to be caused by—severe CAD (ie, significant left‐main or multivessel CAD with or without prior myocardial infarction [MI]). 3 Although most of the available evidence on ICM has been derived from patients with HF and “severely” reduced ejection fraction (EF <35%–40%), 4 , 5 , 6 it is now well appreciated that HF with mildly reduced EF (EF=41%–49%) also portends increased cardiovascular risk, those patients having a similar clinical profile and sharing the same diagnostic and treatment algorithms as patients with HF with reduced EF. 7 The presence of ICM has classically identified a category of patients at high risk of adverse cardiac events, with historical data demonstrating an almost 30% worse 5‐year survival in such patients compared with those with nonischemic LV systolic dysfunction. 8 Yet, the most appropriate management strategy here is still debated. Patients with ICM have been systematically excluded from the most recent randomized controlled trials (RCTs) on the management of subjects with chronic coronary syndrome (CCS), 9 , 10 leaving significant uncertainty about the applicability of results from those studies to this specific high‐risk population. For example, despite data accumulated from multiple RCTs showing the lack of prognostic benefit of myocardial revascularization (largely percutaneous coronary intervention [PCI]) in a large proportion of patients with CCS and stable CAD, and pointing to optimal medical treatment (OMT) alone as the most appropriate initial treatment management strategy, 9 , 10 , 11 current guideline recommendations still favor—and actually recommend—“complete” myocardial revascularization in patients with ICM, without specifying how to achieve it. 12 , 13
Most of the evidence favoring myocardial revascularization (here we prefer the term “myocardial” instead of the often used “coronary” revascularization) in ICM derives from older observational studies, 14 , 15 with much more equivocal data coming from RCTs. 4 , 5 , 6 , 16 , 17 Similarly, although recent evidence has questioned the role of functional cardiac imaging for risk stratification and management of patients with CCS and preserved LV systolic function, 10 , 18 , 19 , 20 a systematic assessment of myocardial viability is still advocated in patients with ICM to guide clinical decision‐making and the need for myocardial revascularization. 12 , 21 , 22 The purported evidence derived from historical observational data in support of a prognostic benefit of myocardial revascularization in patients with ICM and significant myocardial viability 15 is counterbalanced by conflicting results from more contemporary RCTs that have largely questioned the role of an imaging‐guided approach in this high‐risk population. 23 , 24
With these considerations in mind, we undertook a systematic review of the existing evidence deriving from dedicated RCTs to appraise (1) the role of myocardial revascularization in treating patients with ICM; and (2) the assessment of myocardial viability and ischemia in guiding treatment options, eventually interpreting the available evidence and translating it into a practical approach. This review is an extended and updated version of a recently published ViewPoint of ours on the same subject. 25
Methods
The authors declare that all supporting data are available within the article and its online supplementary file.
We conducted a systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. 26 , 27 We identified eligible studies through search of the PubMed bibliographical database, the Cochrane Library, and the Scopus database (end‐of‐search date: October 2022). We also searched reference lists of studies included for further relevant publications that could have been missed in the original search. The literature search strategy was executed by 2 independent investigators (R.L., R.D.C.) using the following algorithm based on the combinations of the terms “myocardial/coronary revascularization,” “heart failure,” and/or “left ventricular dysfunction” using Medical Subject Headings. Finally, we manually retrieved and further reviewed references of eligible articles on myocardial revascularization in ICM. We screened titles and abstracts of all publications first according to the eligibility criteria, resolving discrepancies through consensus whenever necessary, and then analyzed screened trials in detail.
Eligibility Criteria
For the present systematic review, publications were eligible if they reported on RCTs enrolling patients with ICM submitted to either myocardial revascularization or OMT. Specifically, only original studies reporting on demographics, clinical characteristics, myocardial functional data, and outcomes of patients with ICM undergoing myocardial revascularization were eligible. We excluded (1) papers published in languages other than English, (2) nonrandomized studies, (3) animal studies, and (4) reviews and meta‐analyses. We applied no sample size restriction for eligible studies.
Data Extraction and Tabulation
Publications identified in the screening phase were then reviewed in the full texts, and major data were extracted and tabulated using a standardized, predefined, form. Data extraction parameters were determined a priori and included year of publication, years of recruitment, sample size, inclusion and exclusion criteria, patient characteristics (age, sex, New York Heart Association functional class, cardiac functional data, EF), and type of revascularization. From each study we also retrieved data on cardiac imaging modalities used for patients' characterization, with special attention to the assessment of myocardial viability and the presence/extent of ischemia. Outcome measures of all‐cause death and cardiac death were also extracted whenever available. When viability imaging was performed, information on the adherence of patients' management to the presence of myocardial viability was retrieved from each study. Patient management was deemed adherent to viability imaging if myocardial revascularization was performed only in the presence of significant myocardial viability—as defined by the trial inclusion criteria. 23 , 28 , 29 All other combinations of management were considered nonadherent to the results of viability imaging.
Statistical Analysis
We assessed the impact of adherence to viability imaging on patients' outcome. Data were analyzed according to the intention to treat. Results of all studies were combined using a random‐effects model to minimize between‐groups heterogeneity and confirmed by a fixed‐effects model to avoid overweighting of smaller studies. Heterogeneity was assessed using the Cochran's Q tests. To limit heterogeneity of the analysis, for each study included we considered events occurring at a comparable follow‐up duration (≈4–5 years). Trial‐level and pooled estimates are reported as hazard ratios (HR) and 95% CIs. Risk distribution is presented by forest plots with weighting and shows both random‐ and fixed‐effects models. For summary estimates, a P≤0.05 (2 tailed) was considered statistically significant. Analyses were conducted using the R Project version 4.0.2 software for statistical computing and the Comprehensive Meta‐Analysis Biostat Software 2.0.
Literature Search Outcomes
A flow chart depicting the selection process of the studies is shown in Figure 1. 27 The initial search yielded 1397 full publications. Of these, 18 were duplicates. After initial review by the authors, 1354 papers were excluded based on the abstract because they did not satisfy the inclusion criteria. Overall, 25 studies satisfied the predetermined search criteria, of which an additional 21 were excluded after revision of the full text because they were either papers reporting a study design or were subanalyses of the main RCTs. Four studies were therefore ultimately included in the present review.
Figure 1. Study selection according to the preferred reporting items for systematic reviews and meta‐analyses: The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses Statement. 27 .
RCT indicates randomized controlled trial.
Table 1 summarizes the characteristics of the 4 selected studies. The trials took place between 2000 and 2021 and enrolled a total of 2480 patients with ICM and LV systolic dysfunction (EF <35%). The majority of patients (49%) were enrolled from the STICH (Surgical Treatment for Ischemic Heart Failure) trial, 16 28% were enrolled from the REVIVED (Revascularization for Ischemic Ventricular Dysfunction)‐BCIS2 trial, 17 17% were enrolled from the PARR‐2 (Positron Emission Tomography and Recovery Following Revascularization) trial, 6 and only 6% from the HEART (Heart Failure Revascularisation Trial). 5 In STICH, REVIVED‐BCIS2, and HEART, all patients were randomized to either myocardial revascularization on top of OMT or OMT alone, whereas PARR‐2 compared an imaging‐guided management approach versus standard care. In REVIVED‐BCIS2 and HEART, viability imaging was required in all patients, whereas in STICH, the decision to perform viability imaging was left to the discretion of treating physicians, such that treatment was not randomized according to imaging results.
Table 1.
Characteristics of the Studies Included in the Systematic Review
| Trial acronym | HEART | PARR‐2 | STICH | REVIVED‐BCIS2 |
|---|---|---|---|---|
| Reference | Cleland et al 5 |
Beanlands et al 6 McArdle et al 28 |
Velazquez et al 4 Velazquez et al 16 |
Perera et al 17 |
| Number of patients enrolled | 138 | 430 | 1212 | 700 |
| Primary outcome | All‐cause mortality | Cardiovascular death, MI, hospital admission due to cardiac cause | All‐cause mortality | All‐cause mortality, hospitalization for HF |
| Secondary outcome | Time to primary outcome, time to cardiovascular death | Cardiovascular death, death by any cause, hospitalization for cardiovascular cause | LVEF at 6 and 12 mo, quality‐of‐life scores, New York Heart Association class, Canadian Cardiovascular Society class, cardiovascular death, MI, unplanned revascularization, change in NT‐proBNP, major bleeding, health resource use | |
| Inclusion criteria | Heart failure ≥6 wks on diuretics with history of MI, LVEF ≤35%, ≥5 viable segments with reduced contractility | LVEF ≤35%, suspect for CAD based on coronary angiogram, previous revascularization, previous MI, positive stress perfusion imaging | LVEF ≤35%, CAD deemed suitable for revascularization | LVEF ≤35%, extensive coronary artery disease (British Cardiovascular Intervention Society jeopardy score of ≥6), viability in at least 4 dysfunctional myocardial segments amenable to revascularization with PCI |
| Exclusion criteria | Recent acute coronary syndrome or stroke, revascularization required for angina or valve surgery, ventricular arrhythmias requiring device therapy. Life‐limiting comorbidity | Patients with final revascularization strategy decision already made, fluorodeoxyglucose viability info already known, recent (<4 wks) MI, unsuitable for revascularization, emergency revascularization, surgery for valvular heart disease needed, severe comorbidities | Aortic disease needing surgery; cardiogenic shock within 72 hours of randomization planned PCI; recent MI causing left ventricular dysfunction; history of >1 past CABG; life expectancy <3 y for noncardiac causes; likely poor treatment adherence; previous heart, kidney, liver, or lung transplantation | MI in the 4 wks before randomization, acute decompensated HF or sustained ventricular arrhythmias within 72 h before randomization, valve disease requiring imminent intervention, glomerular filtration rate <25 mL/min (unless on dialysis) |
| LVEF at enrollment | 24% | 27% | 28% | 27% |
| Imaging technique allowed | Stress echo, PET, SPECT | PET | Stress echo, SPECT | Stress echo, cardiac magnetic resonance, SPECT/PET |
| Revascularization technique | CABG (n=30) and PCI (n=15) | CABG (n=71, 68%) and PCI (n=33, 32%) | CABG | PCI |
| Median follow‐up | 59 mo | 5 y | STICH: 4.7 y | |
| 41 mo | ||||
| STICH long‐term: 9.8 y | ||||
| Outcome | No significant benefit of revascularization over OMT | No significant difference between PET‐guided and standard strategies | No benefit of revascularization over OMT at 5 y | No significant benefit of revascularization over OMT quality‐of‐life scores at 6 and 12 mo appeared to favor the PCI group, but the difference had diminished at 24 mo |
| At 10 y CABG was associated with a lower all‐cause mortality than OMT (0.72 [95% CI, 0.64–0.82]; P<0.001) | ||||
| Pitfalls | Underpowered, smaller enrollment than planned (138 out of 800 planned patients) | Low protocol adherence (24% did not adhere to PET findings) | Significant crossover (17% of medical therapy group underwent CABG), small sample, no left main disease in medical therapy alone group | Relatively underpowered, largely pauci‐symptomatic patients (97% with no or mild angina and 73% with mild dyspnea) |
CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; HEART, Heart Failure Revascularisation Trial; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OMT, optimal medical therapy; PARR‐2, Positron Emission Tomography and Recovery Following Revascularization; PCI, percutaneous coronary intervention; PET, positron emission tomography; REVIVED‐BCIS2, Study of Efficacy and Safety of Percutaneous Coronary Intervention to Improve Survival in Heart Failure; SPECT, single‐photon computed tomography; and STICH, Surgical Treatment for Ischemic Heart Failure.
A detailed description of the 4 studies is provided here.
The STICH Trial
STICH randomized 1012 patients with CAD and severe LV systolic dysfunction (EF <35%) to either surgical revascularization on top of OMT or OMT alone. 16 After a median follow‐up of 56 months, no significant difference between the 2 arms in the primary outcome was evident in the prespecified intention‐to‐treat analysis. However, curves depicting the accrual of events over time crossed over at ≈2 years, with a signal for a lower event rate in the coronary artery bypass graft (CABG) arm in the long run. 30 There was a significant crossover between the 2 study arms, with 17% of the patients originally randomized to OMT ultimately undergoing myocardial revascularization. At the per‐protocol analysis, the CABG arm showed a rate of all‐cause death 24% lower than the OMT arm (P=0.005). 16
More recently, the results of the STICH Extended Study (STICHES; median follow‐up 9.8 years) showed a 16% lower all‐cause mortality in the CABG group compared with the OMT group (P=0.02). 4 In subgroup analyses, patients with either 3‐vessel CAD (P=0.04) or a severely remodeled LV (LV end‐systolic volume index >78 mL/m2 or EF <27%: P=0.03) appeared to benefit the most from revascularization. 31 , 32
In 18% of the STICH patients a moderate‐to‐severe mitral regurgitation (MR) was also present. 16 While in these patients CABG had practically no prognostic impact over OMT (overall survival 48% versus 50%; HR, 0.92 [95% CI, 0.63–1.35]), 33 a significant interaction between simultaneous surgical MR correction and the long‐term event‐rate was observed. In fact, only patients also treated with mitral valve repair benefitted from surgical revascularization (overall death rate 43%; HR versus OMT, 0.68 [95% CI, 0.41–1.12]), whereas those undergoing isolated CABG did not (HR versus OMT, 1.20 [95% CI, 0.77–1.87]). 33
Two prespecified substudies of STICH evaluated the interaction between myocardial perfusion patterns and the impact of revascularization. In the viability substudy, 601 patients underwent myocardial viability assessment with either single‐photon emission computed tomography (SPECT) imaging or dobutamine stress echocardiography. 23 After multivariable adjustment, no association between the presence of myocardial viability and patients' prognosis was observed (P=0.21). 23 , 32 Moreover, at an extended follow‐up (10.4 years), no significant interaction between myocardial viability and treatment allocation was observed, both regarding all‐cause (P for interaction 0.34) and cardiovascular mortality (P for interaction 0.07). 34
The second substudy included 399 patients who underwent myocardial ischemia imaging with either SPECT or dobutamine stress echocardiography, 199 of whom had moderate‐to‐severe myocardial perfusion abnormalities (ie, involving >10% of the LV). 24 There was no difference in all‐cause mortality between patients with and without inducible ischemia (P=0.28). Similarly, no interaction was found between study treatment (revascularization or OMT) and the presence or absence of ischemia.
REVIVED‐BCIS2 Trial
REVIVED‐BCIS2 enrolled 700 patients with LVEF ≤35%, extensive CAD (as defined as a British Cardiovascular Intervention Society jeopardy score of ≥6), and ascertained viability in at least 4 dysfunctional myocardial segments amenable to percutaneous revascularization. 17 Ninety‐five (14%) and 408 (58%) patients had a significant left main or proximal left anterior descending stenosis, respectively, with most of the enrolled subjects showing multivessel CAD (89%). Patients were randomized to either OMT alone or PCI on top of OMT, with the aim of revascularizing all diseased proximal coronary vessels subtending areas of viable myocardium. After a median of 41 months, no difference in the primary end point (all‐cause death and hospitalization for HF) was evident between the 2 arms (HR, 0.99 [95% CI, 0.78–1.27]; P=0.96). Similarly, after prolonged follow‐up no significant difference in the secondary outcomes was observed between treatment arms, including no interaction between treatment allocation and either symptoms status, quality‐of‐life measures, or LVEF changes at follow‐up (mean difference at 12 months, 0.9% [95% CI, −1.7 to 3.4]). Moreover, no interaction between the completeness of coronary revascularization (median anatomical completeness 71%) and patients' prognosis was observed.
The PARR‐2 Trial
PARR‐2 included 430 patients with ICM and LVEF <35% randomized to either an imaging‐guided management, or standard care. 6 Patients randomized to the imaging strategy underwent cardiac positron emission tomography (PET) with fluorodeoxyglucose for the evaluation of myocardial viability and prediction of the likelihood of EF recovery following revascularization. 35 , 36 Hence, an invasive management was mandated in patients with high‐to‐moderate likelihood of recovery after myocardial revascularization and discouraged in those with low likelihood of recovery. A significantly higher proportion of patients enrolled in the PET arm than in the standard‐care arm underwent surgical revascularization (48% versus 35%, P=0.007). Overall, the study could not demonstrate significantly less cardiac events in patients randomized to the PET‐guided management versus standard care (relative risk [RR], 0.82 [95% CI, 0.59–1.14]; P=0.16). However, in ≈25% of the patients randomized to PET, the final management was not adherent to imaging results, mostly because of inappropriately deferred revascularization despite the demonstration of extensive myocardial viability. When those patients were excluded from the survival analysis, in the remaining 138 patients who adhered to the PET‐guided management, a prognostic benefit was observed over standard care (RR, 0.73 [95% CI, 0.54–0.99]; P=0.042). 28 However, the event rate difference was primarily driven by the “soft” cardiac rehospitalization end point (39% in the PET arm versus 46% in the standard‐care arm; P=0.04), with lack of impact of viability‐guided management on major end points (overall and cardiac mortality). In post hoc analyses, those with more extensive myocardial viability (>7% of hibernating myocardium) appeared to benefit the most from myocardial revascularization (1‐year event rate 13% versus 56% in nonrevascularized patients; P=0.015), 36 as well as those in an experienced center with ready access to (18)F‐fluorodeoxyglucose and integration with imaging, HF, and revascularization teams. 37
The HEART Trial
HEART was originally meant to enroll 800 patients with established ICM, severely reduced LVEF (<35%), and a significant burden of residual myocardial viability on preenrollment imaging. 5 , 29 Patients were randomized to either OMT or an invasive management with the intent to proceed to revascularization. The study was, however, halted prematurely because of slow recruitment, and only 138 patients were ultimately randomized. In this largely underpowered cohort, no significant difference was found between patients randomized to either management strategies.
Detailed information on the cardiac imaging substudies of STICH and PARR‐2 is summarized in Table 2.
Table 2.
Characteristics of the Studies Evaluating the Interaction Between Myocardial Viability or Ischemia and Coronary Revascularization
| STICH viability substudy | STICH ischemia substudy | PARR‐2 | |
|---|---|---|---|
| Number of patients | 601 | 399 | 392 |
| Primary outcome | All‐cause mortality | All‐cause mortality | Cardiovascular death, myocardial infarction, cardiac rehospitalization |
| Secondary outcome | Cardiovascular death, death by any cause, hospitalization for cardiovascular cause | Cardiovascular death, death by any cause, hospitalization for cardiovascular cause | |
| LVEF at enrollment | 27% | 26% | 25% |
| Imaging | Stress echo, SPECT | Stress echo, SPECT | PET (197 pts) vs standard of care (195 pts) |
| Parameter used to stratify patients (extent of viability or ischemia) | Stress echo: ≥5 disfunctional segments showing recovery during dobutamine infusion SPECT: ≥11 viable segments | Stress echo: ischemic response ≥2 left ventricle segments | LVEF recovery likelihood: |
| SPECT: summed difference score ≥4 | ‐ Low: predicted LVEF increase ≤3% | ||
| ‐ Moderate: 3%–5% | |||
| ‐ High: ≥5% | |||
| Revascularization technique | CABG | CABG | CABG and percutaneous coronary intervention |
| Median follow‐up, y |
5.1 10.4 (extended follow‐up analysis) |
4.7 | 5 |
| Outcome | No correlation between viability and CABG‐related benefit vs mortality (P for interaction 0.34) | No correlation between inducible ischemia and benefit from CABG vs medical therapy (HR, 0.83 [95% CI, 0.56–1.23]) | Only in 138/197 patients was the management adherent to PET findings. Significant reduction in cardiac events in PET‐adherent patients compared with standard care (51% vs 57%; HR, 0.73 [95% CI, 0.54–0.99]; P=0.042) |
| Reference |
Bonow et al 23 Panza et al 34 |
Panza et al 24 |
McArdle et al 28 D'Egidio et al 36 |
CABG indicates coronary artery bypass grafting; HR, hazard ratio; LVEF, left ventricular ejection fraction; PARR‐2, Positron Emission Tomography and Recovery Following Revascularization; PET, positron emission tomography; SPECT, single‐photon emission cardiac tomography; and STICH, Surgical Treatment for Ischemic Heart Failure.
Outcomes According to Viability Demonstration
Information regarding the adherence of patient management to results of viability imaging was available in only 1623 subjects. Most of those patients were enrolled in REVIVED‐BCIS2 (N=700; 43%) 17 and STICH (N=601; 37%), 23 with only 11% and 9% of patients enrolled in PARR‐2 28 and HEART, 5 respectively. Patient management was guided by the results of viability imaging in 858 patients (53%), whereas it was independent of imaging results in 765 patients. As shown in Figure 2, 5 , 17 , 23 , 28 there was no significant difference in patient mortality according to adherence or nonadherence to viability imaging (odds ratio [OR], 0.92 [95% CI, 0.75–1.14]), with a similar long‐term event rate in the 2 patients' categories (34% in adherent versus 36% in nonadherent). No significant between‐study heterogeneity was revealed.
Figure 2. Effect of viability‐guided management on all‐cause and cardiac mortality in randomized controlled trials of patients with ischemic cardiomyopathy. 5 , 17 , 23 , 28 .
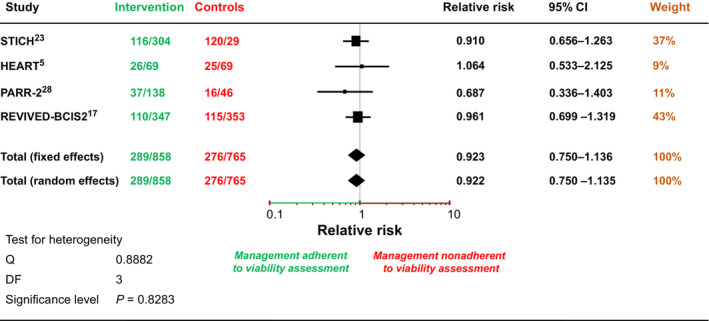
No difference in the event rate was observed in patients in whom revascularization was performed in adherence to the results of viability assessment versus in those labeled as nonadherent. HEART indicates Heart Failure Revascularisation Trial; PARR‐2, Positron Emission Tomography and Recovery Following Revascularization; REVIVED‐BCIS2, Study of Efficacy and Safety of Percutaneous Coronary Intervention to Improve Survival in Heart Failure; and STICH, Surgical Treatment for Ischemic Heart Failure.
Overall Appraisal
Current evidence on the role of revascularization in patients with ICM and severe LV systolic dysfunction is derived from a limited number of RCTs. We found that available evidence, recently reinforced by the results of the REVIVED‐BCIS2 trial, 17 supports surgical revascularization rather than PCI in patients with CAD and severely depressed LV systolic function, although the benefit of CABG over OMT appears to become significant only late during a long‐term follow‐up. Importantly, neither the extent of myocardial viability nor the extent of LV inducible ischemia appears to interact with treatment strategies in favorably affecting outcomes. Moreover, the role of pretreatment assessment of myocardial viability in guiding treatment and predicting individual patients' benefit from myocardial revascularization can be neither affirmed nor refuted.
The Role of Viability Assessment
LV dysfunction in patients with CAD is not necessarily an irreversible condition, as it may improve after myocardial revascularization 3 , 38 with the rescuing of hibernating myocardium—but also with intensive, targeted secondary prevention through OMT—by enhancing reverse remodeling. 39 Therefore, evaluation of the presence and extent of myocardial viability has been traditionally advocated in the assessment of patients with ICM and generally considered a predictor of LV functional improvement after revascularization. 40 In this regard, the meta‐analysis by Allman et al., including 24 small single‐center studies with a cumulative population of 573 patients with CAD and LV systolic dysfunction (mean LVEF 33%, range 27%–46%), showed that the presence of myocardial viability predicted a large and significant benefit from myocardial revascularization (% death rate/year: 16% with OMT and 3.2% with myocardial revascularization). 41 However, included studies were limited by their retrospective design, heterogeneous methodologies and definition of myocardial viability, nonuniform correction for baseline variables, nonstandardized treatment protocols, and the absence of randomization, this last limiting the possibility of avoiding systematic bias.
The STICH trial for the first time provided prospective evidence on the relationship between myocardial viability and myocardial revascularization in patients with ICM. 23 , 34 Although the long‐term results of the trial favored myocardial revascularization, 4 the prespecified viability substudy did not show any interaction between extent of viable LV myocardium and patient treatment allocation (Table 1 and Table 2). 23 , 32 , 34 Moreover, although the presence of myocardial viability was associated with a marginal improvement of LVEF (+2.29±0.56%) during a long‐term follow‐up, this was neither related to treatment allocation nor did it affect the overall survival, 34 a finding further supported by recent retrospective evidence suggesting the absence of a prognostic interaction between surgical revascularization and postoperative changes of LVEF in patients with CAD and LV systolic dysfunction at large. 42 There were, however, limitations of the STICH substudy, including the nonrandomized design and the inhomogeneity of imaging protocols used for the evaluation of viability (eg, 5 SPECT protocols allowed), 16 , 23 with a rather liberal definition of viability thresholds. 43 In addition, because STICH did not incorporate the more accurate noninvasive imaging modalities—such as cardiac magnetic resonance (cMRI) imaging and PET—there were inherent limitations in the accuracy of myocardial viability assessment. 40 , 43 , 44
In this regard, earlier retrospective data had suggested that the presence of extensive (>15%–20% of the LV mass) hibernating myocardium on PET imaging could predict patients who benefit from myocardial revascularization. 15 Those data are apparently supported by the results of the small Ottawa substudy of PARR‐2 (with 111 patients enrolled), which reported significantly lower cardiac events with PET‐guided myocardial revascularization versus standard care (HR, 0.34 [95% CI, 0.16–0.72]; P=0.005). 37 Nevertheless, the study was not powered to assess differences in overall or cardiac mortality, significantly limiting its generalizability. However, in disagreement with those findings, the recent REVIVED‐BCIS2 trial excluded a favorable functional impact of coronary revascularization over OMT in contemporary patients with ICM and extensive myocardial viability (no significant difference on LVEF at follow‐up between PCI [33.8%] and OMT [32.9%], P=not significant), as mostly demonstrated by state‐of‐the‐art imaging techniques (cMRI performed in ≈70% and SPECT/PET in ≈4%), 17 further downplaying the possible impact of viability assessment in such patients.
In our meta‐analysis including all the available evidence deriving from the major RCTs performed in ICM to date, there was no significant incremental value of viability‐guided management (ie, myocardial revascularization in patients with significant viability plus OMT versus OMT alone) on survival (Figure 2), with similar event rates whether or not patients' revascularization was guided by the results of viability imaging. Although in real‐life treatment decisions are likely to be also influenced by the presence of underlying clinical confounders (eg, comorbidities or frailty) 45 , 46 —possibly affecting the expected results of surgical revascularization—the present results seem of particular relevance in patients with ICM and (more) severe LV impairment, where clinical evolution of the disease becomes progressively dominated by HF symptoms and pathophysiology rather than by angina, 47 and where events secondary to the natural progression of LV systolic dysfunction at one point outweigh those related to CAD and to the risk of future ischemic events. 4 , 30 This inconclusive evidence for a viability‐guided management is at odds with previous data suggesting a survival benefit with revascularization compared with OMT alone in patients with viable myocardium (RR, 0.31 [95% CI, 0.25–0.39]; P<0.05), 48 a concept also supported by recent retrospective results showing that revascularization‐associated EF improvement—as the likely result of rescued hibernating myocardium—may be associated with a significant survival advantage in ICM (10% reduction in the rate of death and HF hospitalization for each 5% improvement of EF at 5‐year follow‐up). 42 Although deriving from nonrandomized studies, those data could somehow support viability‐guided revascularization in selected patients with ICM, particularly if at high surgical risk, vouching for the need of dedicated RCTs to provide a solid conclusion on the topic, at the moment still the subject of clinical speculation.
The Role of Ischemia Testing in Guiding Revascularization
Myocardial revascularization currently remains a widely recognized class I recommendation for patients with multivessel CAD, inducible myocardial ischemia, and severely reduced LVEF to improve prognosis. 12 , 13 However, these recommendations are based largely on historical data reporting a dismal outcome of medically managed patients with multivessel CAD and myocardial ischemia in an era before the advent of modern OMT. 49 The MASS II study (Medicine, Angioplasty, or Surgery Study), reported in 2007, randomized 611 patients with 2‐ or 3‐vessel CAD to myocardial revascularization, by either PCI or CABG, or to OMT. 50 Of these patients, 270 had objective evidence of ischemia at an exercise stress test. Over a 10‐year follow‐up, patients randomized to OMT had a significantly higher event rate (total mortality, Q‐wave MI, and angina requiring revascularization) than those undergoing CABG (59% versus 35%, respectively). However, this difference was largely explained by a higher rate of nonfatal MI and urgent revascularization, whereas total mortality did not differ significantly between treatment arms. 51 Documented myocardial ischemia was neither associated with improved patient outcomes nor did it interact with treatment (HR, 1.00 [95% CI, 0.80–1.27]; P=0.95). 52 Additionally, a widely cited report by Hachamovitch et al, including observational data from >13 000 patients who had undergone SPECT imaging, concluded for the absence of any prognostic relevance of inducible myocardial ischemia in patients with extensive myocardial scar (ie, >10% of the LV myocardium), 53 a condition frequently encountered in ICM. Notably, this study was nonrandomized and antedated the modern era of OMT, which may have biased outcomes in favor of revascularization.
In line with these findings, data from the nuclear substudy of the STICH trial also could not document any beneficial impact of ischemia‐guided myocardial revascularization in patients with ICM, both as to the incidence of all‐cause death (35% versus 33%; P=0.64) and as to the secondary end point (all‐cause death or hospitalization for cardiovascular causes) (57% versus 58%; P=0.90). 24 Those results were recently further confirmed by a nonprespecified post hoc analysis of the same trial, demonstrating that presence or absence of ischemia does not interact with the prognostic benefit derived from CABG compared with OMT. 54 At partial disagreement with previous evidence, a subanalysis of the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial has suggested a benefit of myocardial revascularization in patients with HF. 55 However, in that study positive effects of revascularization were evident only in a subgroup of 28 patients with HF and mild‐to‐moderately reduced LVEF (35%–45%), making the findings, at best, only hypothesis generating.
Although these reports are entirely consistent with recent evidence questioning the role of an ischemia‐guided management in patients with CAD at large, 10 to date no RCT has objectively evaluated the prognostic impact of myocardial ischemia in patients with ICM, leaving very relevant doubts on the role of ischemia testing in this category of patients. Thus, based on existing data, there is currently no compelling justification for myocardial revascularization in patients with ICM and suitable coronary anatomy based on the presence and extent of inducible myocardial ischemia alone.
Type of Revascularization: PCI Versus CABG Surgery
Old data from the CASS (Coronary Artery Surgery Study) registry more than 3 decades ago in the pre‐OMT era showed that medically managed patients with CAD and LV systolic dysfunction (EF <35%) had a dismal prognosis at a long‐term follow‐up compared with those submitted to myocardial revascularization (5‐year survival 54% versus 68%; P=0.0007). 56 In this setting, the surgical benefit was most apparent for patients with EF ≤25% (43% 5‐year survival with the medical therapy available at that time versus 63% with CABG). Conversely, the long‐term survival of patients with less severe LV functional impairment (EF 31%–35%) was not influenced by the management strategy (64% with medical therapyversus 73% with CABG). 56
Those pioneering results were confirmed by more contemporary data obtained in patients with ICM, supporting the existence of a significant interaction between the extent and severity of both CAD and LV systolic dysfunction on the one hand and the benefits associated with surgical revascularization on the other. 4 , 31 In the STICH era of almost contemporary OMT, however, only patients with 3‐vessel CAD benefitted from CABG (overall survival of CABG versus OMT: HR, 0.79 [95% CI, 0.63–0.99]; P=0.046), whereas those with more limited CAD extent did not (HR, 0.98, [95% CI, 0.73–1.32]; P=0.906). 31
Regarding the best revascularization strategy to be pursued in patients with ICM, some initial data seemed to suggest a comparable event rate in patients undergoing either PCI or CABG (HR, 1.01 [95% CI, 0.81–1.28]; P=0.91), at least during a short‐term follow‐up (median 2.9 years). 57 However, more prolonged observations (median follow‐up of 5.1 years) documented the superiority of CABG over PCI in the CREDO‐Kyoto (Coronary Revascularization Demonstrating Outcome Study in Kyoto) registry, with an event rate in patients with ICM higher with PCI than CABG (HR, 1.49 [95% CI, 1.04–2.14]; P=0.03). 58 The APPROACH (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease) study showed that in patients with CAD and severe LV dysfunction the prognostic benefit of CABG over PCI was maintained even at the 15‐year follow‐up (HR, 1.21 [95% CI, 1.00–1.46]). 59 Similar results come from the retrospective analysis of the Ontario Registry in 12 113 patients with LVEF <35% and left anterior descending, left main, or multivessel CAD (with or without left anterior descending coronary artery involvement) who underwent PCI or CABG, showing a survival benefit of CABG versus PCI across different subgroups, including patients with left anterior descending–only disease. 60 These data are similar to those arguing for a superiority of CABG over PCI on adverse cardiac events in patients with multivessel CAD at large, particularly in the presence of higher anatomical complexity (eg, a SYNTAX score >23), 61 where CABG achieves a rate of complete revascularization significantly higher than PCI. The missed achievement of “complete” revascularization is a condition strongly associated with unfavorable outcomes in the long‐term (all cause death: 50% at a 10‐year follow‐up versus 22% in patients achieving complete revascularization; P<0.001). 62 Similarly, available evidence supports the choice of surgical revascularization over PCI also in the presence of a heightened clinical risk, such as in the presence of peripheral artery disease, 63 or, most important, in those with diabetes, as in the randomized FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trial, where subjects with multivessel CAD and diabetes had a better survival in the long‐term follow‐up (median 7.5, range 0–13.2 years) with CABG than with PCI (10.9% versus 16.3%; P=0.049). 64 The relative superiority of CABG over PCI in patients with ICM has been confirmed also by a recent meta‐analysis, including >10 000 patients, demonstrating a higher rate of all‐cause death with PCI versus CABG (HR, 1.43 [95% CI, 1.07–1.90]). 65 These data support the concept that “surgical collateralization” of the bypassed vessels allows the sparing of at least some recurrent coronary events despite CAD progression, likely explaining the prognostic advantage of CABG over PCI in patients with complex coronary anatomy. 25 , 66 , 67 , 68 It is plausible that revascularization with CABG reduces the occurrence of spontaneous MI (as observed in the BARI [Bypass Angioplasty Revascularization Investigation]‐2D trial and FREEDOM) because CABG bypasses both stenotic epicardial segments as well as more proximal nonobstructive rupture‐prone vulnerable coronary plaques. After PCI, conversely, the majority of spontaneous MI events occur as a consequence of progression of atherosclerotic disease in non‐flow‐limiting (and non instrumented) coronary segments remote from stented target lesion(s). 67 , 69 The superiority of CABG over PCI on prognosis in this setting is also supported by a report from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry), including over 2500 patients with multivessel CAD and reduced EF, where CABG was associated with a lower risk of death than PCI (HR, 0.62 [95% CI, 0.41–0.96]; P=0.031). This translated on average into 0.5 years more of event‐free survival time over 10 years. 70 Interestingly, the risk of death increased linearly with quintiles of hospitals in which PCI was the preferred method for revascularization, with a consistently shorter survival (≈10 months less) for patients treated in hospitals with a higher preference for PCI over CABG. As observed in STICH, 4 survival curves in SCAAR also started to separate after 4 years, because of an early increase in periprocedural mortality with CABG, offset by beneficial effects only later in the follow‐up. 70 More recently, the REVIVED‐BCIS2 trial provided long awaited, prospective evidence on the possible role of PCI in patients with ICM, 17 showing no significant prognostic impact of percutaneous revascularization over OMT, with a similar overall death rate in the 2 treatment strategies (31.7% for PCI and 32.6% for OMT, P=not significant). Among the secondary outcome measures, the rate of MI at follow‐up was not affected by patients' treatment allocation (10.7% in PCI and 10.8% in OMT patients), with a modest numerically higher prevalence of nonfatal spontaneous MI in patients randomized to OMT alone (N=33 versus 18 in PCI patients). Interestingly, after a median follow‐up of 41 months, no relevant difference in symptoms status was observed between patient groups, with only a limited number of those randomized to OMT undergoing an unplanned coronary revascularization (10.5% versus 2.9% in PCI), thus confirming the positive impact of contemporary medical therapy (>85% of patients on a statin, beta blocker, and renin‐angiotensin system inhibitor) and cardiac device therapy (≈50% of the enrolled patients with a cardiac resynchronization/implantable cardioverter‐defibrillator device) on quality of life and events prevention. Altogether, these data argue for the inadequacy of the wording “coronary/myocardial revascularization,” looping together intervention modalities, such as CABG or PCI, with profoundly different impact on myocardial biology. 71 , 72
A preference for CABG over PCI has been traditionally reserved to patients with ICM and “significant” functional MR, a condition independently associated with a dismal prognosis in patients with HF with reduced EF at large (>70% higher relative risk for death). 73 Despite current guidelines consider combined CABG and mitral valve repair the treatment of choice in patients with ICM and severe functional MR, 74 , 75 evidence on the prognostic benefit of such approach is, however, not univocal, 33 , 76 and well‐powered RCTs are still lacking. In patients with high surgical risk, PCI would here appear an option based on data showing a significant reduction of MR severity in ≈1/3 of revascularized subjects, 77 possibly owing to (but not conclusively associated with) the functional recovery of viable myocardium adjacent to the dysfunctional papillary muscle(s). 78 In cases of persistent severe MR after PCI, percutaneous mitral valve correction would remain the most logical approach, based on recent evidence obtained with transcatheter edge‐to‐edge repair. 79 Patients with ICM and moderate MR, conversely, will not likely benefit from mitral valve surgery and should be submitted to coronary revascularization alone, 80 with a preference for CABG over PCI. 81 , 82
We must recognize that prospective evidence on the role of surgical revascularization in patients with ICM dates back more than a decade, with no direct comparison with currently available OMT approaches. The consistent advances in recent pharmacological therapies for the management of HF (mostly) and CCS 83 , 84 , 85 have likely narrowed the gap between OMT and myocardial revascularization also in patients with ICM. This emphasizes the compelling need for novel RCTs in this category of patients. In particular, contemporary data suggest that innovative therapies available for the management of patients with HF (ie, sacubitril/valsartan and sodium‐glucose transporter 2 inhibitors) have a consistent beneficial effect on myocardial reverse remodeling, 86 , 87 ultimately associating with improved outcomes. Moreover, because the REVIVED‐BCIS2 trial 17 did not include a CABG arm, a new prospective head‐to‐head comparison of PCI and CABG versus contemporary OMT in ICM is urgently needed.
Limitations of Available Evidence
The paucity of available data derived from RCTs regarding the role of myocardial revascularization in patients with ICM limits definitive conclusions regarding its benefits. The same applies to the treatment of categories generally excluded from RCTs, such as patients with significant left main CAD, whose management is more frequently based on common sense than on modern evidence. Similarly, to date the most solid evidence on the use of viability imaging for the management of patients with ICM comes from the nonrandomized subanalyses of 2 RCTs, namely the STICH 23 , 34 and the PARR‐2 6 , 28 studies, limiting definitive conclusions. The evidence presented here in is in accord with previous, albeit limited, data from observational studies and generally supportive of surgical revascularization in patients with ICM. Such evidence is still far from robust or definitive and leaves considerable uncertainty about the utility and value of noninvasive ischemia and viability assessments in such patients. 34 , 54
Conclusions
What to Do in Practice and Where Do We Go From Here?
Over the past few years, there have been remarkable advances in OMT that have resulted in a significant improvement of prognosis in patients with HF. Nevertheless, ICM still identifies a category of patients at particularly high risk of future cardiac events. In addition to the role of medical and device therapy in improving prognosis, the prognostic impact of myocardial revascularization remains controversial. Limited and relatively outdated evidence from an earlier era suggests a benefit of surgical myocardial revascularization over pharmacological therapy during extended follow‐up. More recent data point to the absence of benefit of PCI in the same category of patients, 17 indicating the urgent need of dedicated RCTs comparing the available revascularization strategies (CABG versus PCI) in combination with OMT versus OMT alone in well‐characterized patients with ICM to define the best diagnostic and therapeutic approaches to clinical decision‐making. Until newer and more relevant evidence emerges, surgical revascularization, when technically feasible, and after carefully weighting of the perioperative risk, would appear to be the best option for selected patients with ICM. Such an approach has also been recently reported to be cost effective over OMT. 88 Viability or ischemia testing appears not to be mandatory based on current evidence. Documentation of viability, today possible at its best with cMRI or PET, would still appear, however, a logical step, no matter if not based on strict evidence, but with the aim of tilting the balance in favor of surgery in cases where perioperative risk makes decisions particularly difficult. Conversely, the practical impact of ischemia testing on clinical decision making, particularly in the absence of ischemic symptoms, would appear less robust, although recent retrospective evidence in patients not selected for LVEF suggests a possible benefit of ischemia‐guided revascularization in the presence of a (very) severe ischemic burden (>15% of the LV myocardium). 89 Such data need confirmation in dedicated RCTs.
Considering the recent results of the REVIVED‐BCIS2 trial, 17 indications to PCI in patients with ICM would remain limited to patients in whom ischemia‐related symptoms, such as angina, are refractory to OMT and predominant over HF‐related symptoms. A summary of our proposed therapeutic algorithms provided in Figure 3, where one can distinguish a few sequential steps, as follows:
Are anginal symptoms present? In such cases—a minority in our experience—it is possible that myocardial ischemia dominates the course of disease, certainly in terms of quality of life. Detection of the extent of myocardial ischemia would be justified, and stress imaging—perfusion stress SPECT or dobutamine stress echocardiography as the easiest available techniques; stress PET or stress cMRI as alternative—should be pursued. In the presence of severe inducible ischemia (>15% of LV mass according to recent evidence 89 ), this would prompt revascularization, with a preference for CABG also to improve prognosis, but with a role also for PCI in cases of high surgical risk mainly to improve symptoms (inference derived from CAD out of ICM, 10 and now also, to some extent, in patients with ICM 17 ). Even in the presence of a more limited myocardial ischemic burden, coronary revascularization would still remain an option, particularly with anginal symptoms refractory to OMT. Also in these cases, available evidence supports CABG as the primary revascularization strategy 24 , 54 —provided a multidisciplinary evaluation of the surgical risk is done—reserving ischemia‐guided PCI to high‐surgical risk patients in whom symptom control would become the main therapeutic target. An important percentage of patients may have diabetes, masking the occurrence of angina: inferring from patients with non‐ICM who had ischemia, 64 CABG would be still the best option in patients with multivessel CAD (if feasible and with acceptable perioperative risk after a multidisciplinary team evaluation), considering the expected lower rate of complete myocardial revascularization that can be achieved with PCI in such patients. 62
In cases where HF symptoms predominate—the majority of patients in our experience—an assessment of the extent of myocardial viability would still appear opportune, considering the absolute and relative extent of myocardial scar versus viable hibernating myocardium, as well as surgical risk, as factors favoring OMT. It would appear wise here to set a decision‐making threshold for the extent of hibernating myocardium around 7% of LV mass, 36 beyond which one would possibly favor (surgical) revascularization. PET scanning and cMRI are here the methods of choice, according to local availability and expertise. It has to be borne in mind that evidence on this topic is quite disputed. 23 , 32 , 34 Regarding the revascularization modality, despite the limited and relatively outdated prospective evidence available, CABG should remain the standard of treatment. With the mildly favorable results of the REVIVED‐BCIS2 trial 17 in terms of quality of life improvement with coronary revascularization in patients with ICM (limited benefit in the first 6–12 months of follow‐up disappearing beyond 2 years), PCI could still be pursued in patients with ICM at high surgical risk and refractory symptoms after a multidisciplinary team discussion, aiming at complete coronary revascularization if technically feasible.
An evaluation of procedural risk is always mandatory: here both the Society of Thoracic Surgeons risk score and Euro‐SCORE‐2, which have been tested specifically in STICH patients, 90 would appear suitable to predict the 30‐day postoperative mortality of CABG, with a preference for the latter. 90 As an indication, a 30‐day perioperative mortality <4% would appear to be a reasonable green light to prompt surgery. 90 In patients deemed at higher risk for CABG, the SYNTAX score/SYNTAX score II 91 may be also useful to tilt the balance toward PCI, which may remain an option, although not supported by clinical evidence, in patients at low anatomical complexity and low PCI procedural risk after multidisciplinary team discussion.
Figure 3. Proposal of a management algorithm for patients with ischemic cardiomyopathy.
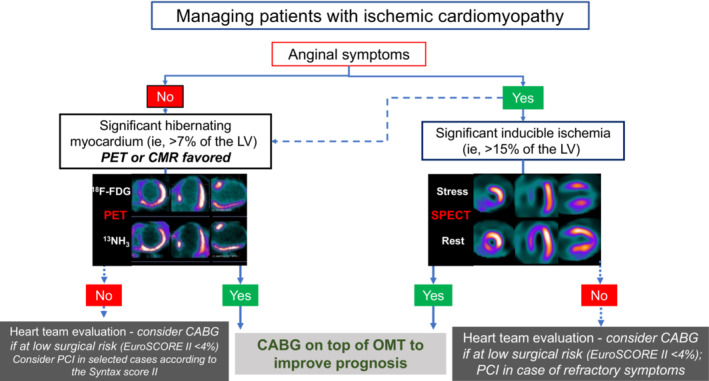
In patients with anginal symptoms, complete coronary revascularization with CABG should “probably” be performed in the presence of significant inducible myocardial ischemia (>15% of the LV) to improve prognosis. 89 CABG should “probably” also be performed in patients with evidence of extensive hibernating myocardium (>7% of the LV) 36 on noninvasive imaging (PET or cMRI if available). A preference to CABG should also be given in patients with diabetes. 64 In the absence of significant hibernating myocardium, a heart team evaluation should be performed with preference to OMT and consideration for coronary revascularization (surgical if feasible) 4 only if with low surgical risk. PCI may be considered factoring the SYNTAX scores in selected cases when surgical risk is unacceptable. CABG indicates coronary artery bypass graft; cMRI, cardiac magnetic resonance imaging; F‐FDG, F‐fluorodeoxyglucose; LV, left ventricle; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; PET, positron emission tomography; and SPECT, single‐photon emission computed tomography.
This scheme, as depicted in Figure 3, has to be regarded as “work‐in‐progress,” as a reasonable way of action at the moment, and possibly be operatively tested prospectively in future trials.
Sources of Funding
None.
Disclosures
None.
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Briceno N, Schuster A, Lumley M, Perera D. Ischaemic cardiomyopathy: pathophysiology, assessment and the role of revascularisation. Heart. 2016;102:397–406. doi: 10.1136/heartjnl-2015-308037 [DOI] [PubMed] [Google Scholar]
- 2. Gheorghiade M, Fonarow GC. Management of post‐myocardial infarction patients with left ventricular systolic dysfunction. Am J Med. 2007;120:109–120. doi: 10.1016/j.amjmed.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 3. Cabac‐Pogorevici I, Muk B, Rustamova Y, Kalogeropoulos A, Tzeis S, Vardas P. Ischaemic cardiomyopathy. Pathophysiological insights, diagnostic management and the roles of revascularisation and device treatment. Gaps and dilemmas in the era of advanced technology. Eur J Heart Fail. 2020;22:789–799. [DOI] [PubMed] [Google Scholar]
- 4. Velazquez EJ, Lee KL, Jones RH, Al‐Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, et al. Coronary‐artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cleland JG, Calvert M, Freemantle N, Arrow Y, Ball SG, Bonser RS, Chattopadhyay S, Norell MS, Pennell DJ, Senior R. The Heart Failure Revascularisation Trial (HEART). Eur J Heart Fail. 2011;13:227–233. doi: 10.1093/eurjhf/hfq230 [DOI] [PubMed] [Google Scholar]
- 6. Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, Gulenchyn KY, Garrard L, deKemp R, Guo A, et al. F‐18‐fluorodeoxyglucose positron emission tomography imaging‐assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR‐2). J Am Coll Cardiol. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 7. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 8. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/S0735-1097(01)01738-7 [DOI] [PubMed] [Google Scholar]
- 9. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829 [DOI] [PubMed] [Google Scholar]
- 10. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, Chaitman BR, Senior R, López‐Sendón J, Alexander KP, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. doi: 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 13. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622 [DOI] [PubMed] [Google Scholar]
- 14. Tarakji KG, Brunken R, McCarthy PM, Al‐Chekakie MO, Abdel‐Latif A, Pothier CE, Blackstone EH, Lauer MS. Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation. 2006;113:230–237. doi: 10.1161/circulationaha.105.541664 [DOI] [PubMed] [Google Scholar]
- 15. Ling LF, Marwick TH, Flores DR, Jaber WA, Brunken RC, Cerqueira MD, Hachamovitch R. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging. 2013;6:363–372. doi: 10.1161/circimaging.112.000138 [DOI] [PubMed] [Google Scholar]
- 16. Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, et al. Coronary‐artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perera D, Clayton T, O'Kane PD, Greenwood JP, Weerackody R, Ryan M, Morgan HP, Dodd M, Evans R, Canter R, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387:1351–1360. doi: 10.1056/NEJMoa2206606 [DOI] [PubMed] [Google Scholar]
- 18. Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/circulationaha.107.743963 [DOI] [PubMed] [Google Scholar]
- 19. Mancini GBJ, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, Maron DJ, Teo K, Sedlis SP, Chaitman BR, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7:195–201. doi: 10.1016/j.jcin.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 20. Neglia D, Liga R, Caselli C, Carpeggiani C, Lorenzoni V, Sicari R, Lombardi M, Gaemperli O, Kaufmann PA, Scholte A, et al. Anatomical and functional coronary imaging to predict long‐term outcome in patients with suspected coronary artery disease: the EVINCI‐outcome study. Eur Heart J Cardiovasc Imaging. 2020;21:1273–1282. doi: 10.1093/ehjci/jez248 [DOI] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 22. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 23. Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne‐Nickens P, Drozdz J, Farsky PS, Feldman AM, Doenst T, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panza JA, Holly TA, Asch FM, She L, Pellikka PA, Velazquez EJ, Lee KL, Borges‐Neto S, Farsky PS, Jones RH, et al. Inducible myocardial ischemia and outcomes in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2013;61:1860–1870. doi: 10.1016/j.jacc.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Caterina R, Liga R, Boden WE. Myocardial revascularization in ischaemic cardiomyopathy: routine practice vs. scientific evidence. Eur Heart J. 2021;43:387–390. doi: 10.1093/eurheartj/ehab680 [DOI] [PubMed] [Google Scholar]
- 26. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mc Ardle B, Shukla T, Nichol G, deKemp RA, Bernick J, Guo A, Lim SP, Davies RA, Haddad H, Duchesne L, et al. Long‐term follow‐up of outcomes with F‐18‐fluorodeoxyglucose positron emission tomography imaging‐assisted management of patients with severe left ventricular dysfunction secondary to coronary disease. Circ Cardiovasc Imaging. 2016;9:e004331. doi: 10.1161/circimaging.115.004331 [DOI] [PubMed] [Google Scholar]
- 29. Cleland JGF, Freemantle N, Ball SG, Bonser RS, Camici P, Chattopadhyay S, Dutka D, Eastaugh J, Hampton J, Large S, et al. The Heart Failure Revascularisation Trial (HEART): rationale, design and methodology. Eur J Heart Fail. 2003;5:295–303. [DOI] [PubMed] [Google Scholar]
- 30. Carson P, Wertheimer J, Miller A, O'Connor CM, Pina IL, Selzman C, Sueta C, She L, Greene D, Lee KL, et al. The STICH trial (Surgical Treatment for Ischemic Heart Failure): mode‐of‐death results. JACC Heart Fail. 2013;1:400–408. doi: 10.1016/j.jchf.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panza JA, Velazquez EJ, She L, Smith PK, Nicolau JC, Favaloro RR, Gradinac S, Chrzanowski L, Prabhakaran D, Howlett JG, et al. Extent of coronary and myocardial disease and benefit from surgical revascularization in ischemic LV dysfunction [corrected]. J Am Coll Cardiol. 2014;64:553–561. doi: 10.1016/j.jacc.2014.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonow RO, Castelvecchio S, Panza JA, Berman DS, Velazquez EJ, Michler RE, She L, Holly TA, Desvigne‐Nickens P, Kosevic D, et al. Severity of remodeling, myocardial viability, and survival in ischemic LV dysfunction after surgical revascularization. J Am Coll Cardiol Img. 2015;8:1121–1129. doi: 10.1016/j.jcmg.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, Jain AR, Leng Chua Y, Daly R, Senni M, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–2648. doi: 10.1161/circulationaha.111.072256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panza JA, Ellis AM, Al‐Khalidi HR, Holly TA, Berman DS, Oh JK, Pohost GM, Sopko G, Chrzanowski L, Mark DB, et al. Myocardial viability and long‐term outcomes in ischemic cardiomyopathy. N Engl J Med. 2019;381:739–748. doi: 10.1056/NEJMoa1807365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beanlands R, Nichol G, Ruddy TD, deKemp RA, Hendry P, Humen D, Racine N, Ross H, Benard F, Coates G, et al. Evaluation of outcome and cost‐effectiveness using an FDG PET‐guided approach to management of patients with coronary disease and severe left ventricular dysfunction (PARR‐2): rationale, design, and methods. Control Clin Trials. 2003;24:776–794. doi: 10.1016/S0197-2456(03)00106-5 [DOI] [PubMed] [Google Scholar]
- 36. D'Egidio G, Nichol G, Williams KA, Guo A, Garrard L, deKemp R, Ruddy TD, DaSilva J, Humen D, Gulenchyn KY, et al. Increasing benefit from revascularization is associated with increasing amounts of myocardial hibernation: a substudy of the PARR‐2 trial. J Am Coll Cardiol Img. 2009;2:1060–1068. doi: 10.1016/j.jcmg.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 37. Abraham A, Nichol G, Williams KA, Guo A, deKemp RA, Garrard L, Davies RA, Duchesne L, Haddad H, Chow B, et al. 18F‐FDG PET imaging of myocardial viability in an experienced center with access to 18F‐FDG and integration with clinical management teams: the Ottawa‐FIVE substudy of the PARR 2 trial. J Nuclear Med. 2010;51:567–574. doi: 10.2967/jnumed.109.065938 [DOI] [PubMed] [Google Scholar]
- 38. Rahimtoola SH. The hibernating myocardium. Am Heart J. 1989;117:211–221. doi: 10.1016/0002-8703(89)90685-6 [DOI] [PubMed] [Google Scholar]
- 39. Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103–114. doi: 10.1161/circulationaha.107.702993 [DOI] [PubMed] [Google Scholar]
- 40. Parikh K, Choy‐Shan A, Ghesani M, Donnino R. Multimodality imaging of myocardial viability. Curr Cardiol Rep. 2021;23:5. doi: 10.1007/s11886-020-01433-8 [DOI] [PubMed] [Google Scholar]
- 41. Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta‐analysis. J Am Coll Cardiol. 2002;39:1151–1158. doi: 10.1016/S0735-1097(02)01726-6 [DOI] [PubMed] [Google Scholar]
- 42. Velagaleti RS, Vetter J, Parker R, Kurgansky KE, Sun YV, Djousse L, Gaziano JM, Gagnon D, Joseph J. Change in left ventricular ejection fraction with coronary artery revascularization and subsequent risk for adverse cardiovascular outcomes. Circ Cardiovasc Interv. 2022;15:e011284. doi: 10.1161/CIRCINTERVENTIONS.121.011284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anavekar NS, Chareonthaitawee P, Narula J, Gersh BJ. Revascularization in patients with severe left ventricular dysfunction: is the assessment of viability still viable? J Am Coll Cardiol. 2016;67:2874–2887. doi: 10.1016/j.jacc.2016.03.571 [DOI] [PubMed] [Google Scholar]
- 44. Brunken RC, Mody FV, Hawkins RA, Nienaber C, Phelps ME, Schelbert HR. Positron emission tomography detects metabolic viability in myocardium with persistent 24‐hour single‐photon emission computed tomography 201Tl defects. Circulation. 1992;86:1357–1369. doi: 10.1161/01.CIR.86.5.1357 [DOI] [PubMed] [Google Scholar]
- 45. Ambrosy AP, Stevens SR, Al‐Khalidi HR, Rouleau JL, Bouabdallaoui N, Carson PE, Adlbrecht C, Cleland JGF, Dabrowski R, Golba KS, et al. Burden of medical co‐morbidities and benefit from surgical revascularization in patients with ischaemic cardiomyopathy. Eur J Heart Fail. 2019;21:373–381. doi: 10.1002/ejhf.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stewart RA, Szalewska D, She L, Lee KL, Drazner MH, Lubiszewska B, Kosevic D, Ruengsakulrach P, Nicolau JC, Coutu B, et al. Exercise capacity and mortality in patients with ischemic left ventricular dysfunction randomized to coronary artery bypass graft surgery or medical therapy: an analysis from the STICH trial (surgical treatment for ischemic heart failure). JACC Heart Failur. 2014;2:335–343. doi: 10.1016/j.jchf.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, Braun O, Savarese G, Dahlström U, Lund LH. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail. 2017;10:10. doi: 10.1161/circheartfailure.117.003875 [DOI] [PubMed] [Google Scholar]
- 48. Orlandini A, Castellana N, Pascual A, Botto F, Cecilia Bahit M, Chacon C, Luz Diaz M, Diaz R. Myocardial viability for decision‐making concerning revascularization in patients with left ventricular dysfunction and coronary artery disease: a meta‐analysis of non‐randomized and randomized studies. Int J Cardiol. 2015;182:494–499. doi: 10.1016/j.ijcard.2015.01.025 [DOI] [PubMed] [Google Scholar]
- 49. Hueb W, Soares PR, Gersh BJ, César LA, Luz PL, Puig LB, Martinez EM, Oliveira SA, Ramires JA. The medicine, angioplasty, or surgery study (MASS‐II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one‐year results. J Am Coll Cardiol. 2004;43:1743–1751. doi: 10.1016/j.jacc.2003.08.065 [DOI] [PubMed] [Google Scholar]
- 50. Hueb W, Lopes NH, Gersh BJ, Soares P, Machado LA, Jatene FB, Oliveira SA, Ramires JA. Five‐year follow‐up of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2007;115:1082–1089. doi: 10.1161/circulationaha.106.625475 [DOI] [PubMed] [Google Scholar]
- 51. Hueb W, Lopes N, Gersh BJ, Soares PR, Ribeiro EE, Pereira AC, Favarato D, Rocha AS, Hueb AC, Ramires JA. Ten‐year follow‐up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122:949–957. doi: 10.1161/circulationaha.109.911669 [DOI] [PubMed] [Google Scholar]
- 52. Garzillo CL, Hueb W, Gersh B, Rezende PC, Lima EG, Favarato D, Franchini Ramires JA, Kalil FR. Association between stress testing‐induced myocardial ischemia and clinical events in patients with multivessel coronary artery disease. JAMA Intern Med. 2019;179:1345–1351. doi: 10.1001/jamainternmed.2019.2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LEJ, Friedman JD, Hayes SW, Cohen I, Germano G, Berman DS. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress‐rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012–1024. doi: 10.1093/eurheartj/ehq500 [DOI] [PubMed] [Google Scholar]
- 54. O'Fee K, Panza JA, Brown DL. Association of inducible myocardial ischemia with long‐term mortality and benefit from coronary artery bypass graft surgery in ischemic cardiomyopathy: ten‐year follow‐up of the STICH trial. Circulation. 2021;143:205–207. doi: 10.1161/circulationaha.120.050734 [DOI] [PubMed] [Google Scholar]
- 55. Lopes RD, Alexander KP, Stevens SR, Reynolds HR, Stone GW, Piña IL, Rockhold FW, Elghamaz A, Lopez‐Sendon JL, Farsky PS, et al. Initial invasive versus conservative management of stable ischemic heart disease in patients with a history of heart failure or left ventricular dysfunction: insights from the ISCHEMIA trial. Circulation. 2020;142:1725–1735. doi: 10.1161/circulationaha.120.050304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alderman EL, Fisher LD, Litwin P, Kaiser GC, Myers WO, Maynard C, Levine F, Schloss M. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation. 1983;68:785–795. doi: 10.1161/01.cir.68.4.785 [DOI] [PubMed] [Google Scholar]
- 57. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Hannan EL. Revascularization in patients with multivessel coronary artery disease and severe left ventricular systolic dysfunction: everolimus‐eluting stents versus coronary artery bypass graft surgery. Circulation. 2016;133:2132–2140. doi: 10.1161/circulationaha.115.021168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marui A, Nishiwaki N, Komiya T, Hanyu M, Tanaka S, Kimura T, Sakata R. Comparison of 5‐year outcomes after coronary artery bypass grafting in heart failure patients with versus without preserved left ventricular ejection fraction (from the CREDO‐Kyoto CABG registry Cohort‐2). Am J Cardiol. 2015;116:580–586. doi: 10.1016/j.amjcard.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 59. Nagendran J, Norris CM, Graham MM, Ross DB, Macarthur RG, Kieser TM, Maitland AM, Southern D, Meyer SR. Coronary revascularization for patients with severe left ventricular dysfunction. Ann Thorac Surg. 2013;96:2038–2044. doi: 10.1016/j.athoracsur.2013.06.052 [DOI] [PubMed] [Google Scholar]
- 60. Sun LY, Gaudino M, Chen RJ, Bader Eddeen A, Ruel M. Long‐term outcomes in patients with severely reduced left ventricular ejection fraction undergoing percutaneous coronary intervention vs coronary artery bypass grafting. JAMA Cardiol. 2020;5:631–641. doi: 10.1001/jamacardio.2020.0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fearon WF, Zimmermann FM, De Bruyne B, Piroth Z, van Straten AHM, Szekely L, Davidavičius G, Kalinauskas G, Mansour S, Kharbanda R, et al. Fractional flow reserve‐guided PCI as compared with coronary bypass surgery. N Engl J Med. 2022;386:128–137. doi: 10.1056/NEJMoa2112299 [DOI] [PubMed] [Google Scholar]
- 62. Takahashi K, Serruys PW, Gao C, Ono M, Wang R, Thuijs D, Mack MJ, Curzen N, Mohr FW, Davierwala P, et al. Ten‐year all‐cause death according to completeness of revascularization in patients with three‐vessel disease or left main coronary artery disease: insights from the SYNTAX extended survival study. Circulation. 2021;144:96–109. doi: 10.1161/circulationaha.120.046289 [DOI] [PubMed] [Google Scholar]
- 63. Hlatky MA, Boothroyd DB, Baker L, Kazi DS, Solomon MD, Chang TI, Shilane D, Go AS. Comparative effectiveness of multivessel coronary bypass surgery and multivessel percutaneous coronary intervention: a cohort study. Ann Intern Med. 2013;158:727–734. doi: 10.7326/0003-4819-158-10-201305210-00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Farkouh ME, Domanski M, Dangas GD, Godoy LC, Mack MJ, Siami FS, Hamza TH, Shah B, Stefanini GG, Sidhu MS, et al. Long‐term survival following multivessel revascularization in patients with diabetes: the FREEDOM follow‐on study. J Am Coll Cardiol. 2019;73:629–638. doi: 10.1016/j.jacc.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cui K, Zhang D, Lyu S, Song X, Yuan F, Xu F, Zhang M. Meta‐analysis comparing percutaneous coronary revascularization using drug‐eluting stent versus coronary artery bypass grafting in patients with left ventricular systolic dysfunction. Am J Cardiol. 2018;122:1670–1676. doi: 10.1016/j.amjcard.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 66. Serruys PW, Morice M‐C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, et al. Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 67. Doenst T, Haverich A, Serruys P, Bonow RO, Kappetein P, Falk V, Velazquez E, Diegeler A, Sigusch H. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol. 2019;73:964–976. doi: 10.1016/j.jacc.2018.11.053 [DOI] [PubMed] [Google Scholar]
- 68. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 69. Boden WE, Taggart DP. Diabetes with coronary disease‐‐a moving target amid evolving therapies? N Engl J Med. 2009;360:2570–2572. doi: 10.1056/NEJMe0904090 [DOI] [PubMed] [Google Scholar]
- 70. Völz S, Redfors B, Angerås O, Ioanes D, Odenstedt J, Koul S, Haraldsson I, Dworeck C, Hofmann R, Hansson E, et al. Long‐term mortality in patients with ischaemic heart failure revascularized with CABG or PCI: insights from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2021;42:2657–2664. doi: 10.1093/eurheartj/ehab273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bakaeen FG, Gaudino M, Whitman G, Doenst T, Ruel M, Taggart DP, Stulak JM, Benedetto U, Anyanwu A, Chikwe J, et al. 2021: the American Association for Thoracic Surgery expert consensus document: coronary artery bypass grafting in patients with ischemic cardiomyopathy and heart failure. J Thorac Cardiovasc Surg. 2021;162:829–850.e821. doi: 10.1016/j.jtcvs.2021.04.052 [DOI] [PubMed] [Google Scholar]
- 72. Doenst T, Bonow RO, Bhatt DL, Falk V, Gaudino M. Improving terminology to describe coronary artery procedures: JACC review topic of the week. J Am Coll Cardiol. 2021;78:180–188. doi: 10.1016/j.jacc.2021.05.010 [DOI] [PubMed] [Google Scholar]
- 73. Goliasch G, Bartko PE, Pavo N, Neuhold S, Wurm R, Mascherbauer J, Lang IM, Strunk G, Hülsmann M. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J. 2018;39:39–46. doi: 10.1093/eurheartj/ehx402 [DOI] [PubMed] [Google Scholar]
- 74. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, ||| , Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the Management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35‐e71. doi: 10.1161/cir.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 75. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 76. Samad Z, Shaw LK, Phelan M, Ersboll M, Risum N, Al‐Khalidi HR, Glower DD, Milano CA, Alexander JH, O'Connor CM, et al. Management and outcomes in patients with moderate or severe functional mitral regurgitation and severe left ventricular dysfunction. Eur Heart J. 2015;36:2733–2741. doi: 10.1093/eurheartj/ehv343 [DOI] [PubMed] [Google Scholar]
- 77. Yousefzai R, Bajaj N, Krishnaswamy A, Goel SS, Agarwal S, Aksoy O, Aggarwal B, Duarte VE, Anabtawi A, Parashar A, et al. Outcomes of patients with ischemic mitral regurgitation undergoing percutaneous coronary intervention. Am J Cardiol. 2014;114:1011–1017. doi: 10.1016/j.amjcard.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 78. Penicka M, Linkova H, Lang O, Fojt R, Kocka V, Vanderheyden M, Bartunek J. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation. 2009;120:1474–1481. doi: 10.1161/circulationaha.108.842104 [DOI] [PubMed] [Google Scholar]
- 79. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640 [DOI] [PubMed] [Google Scholar]
- 80. Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, Hung JW, Parides MK, Ailawadi G, Perrault LP, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188. doi: 10.1056/NEJMoa1410490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Castleberry AW, Williams JB, Daneshmand MA, Honeycutt E, Shaw LK, Samad Z, Lopes RD, Alexander JH, Mathew JP, Velazquez EJ, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: a 20‐year experience. Circulation. 2014;129:2547–2556. doi: 10.1161/circulationaha.113.005223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fan Q, Liu J, Xu Y, Ni R, Xi R, Wang F, Hu J, Sun H, Yang Z, Zhou M, et al. Real‐world outcomes of revascularization strategies in patients with left ventricular dysfunction and three‐vessel coronary disease stratified by mitral regurgitation. Front Cardiovasc Med. 2021;8:675722. doi: 10.3389/fcvm.2021.675722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin–Neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 84. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 85. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 86. Gori M, Januzzi JL, D'Elia E, Lorini FL, Senni M. Rationale for and practical use of sacubitril/valsartan in the patient's journey with heart failure and reduced ejection fraction. Card Fail Rev. 2021;7:e06. doi: 10.15420/cfr.2020.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mason T, Coelho‐Filho OR, Verma S, Chowdhury B, Zuo F, Quan A, Thorpe KE, Bonneau C, Teoh H, Gilbert RE, et al. Empagliflozin reduces myocardial extracellular volume in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol Img. 2021;14:1164–1173. doi: 10.1016/j.jcmg.2020.10.017 [DOI] [PubMed] [Google Scholar]
- 88. Chew DS, Cowper PA, Al‐Khalidi H, Anstrom KJ, Daniels MR, Davidson‐Ray L, Li Y, Michler RE, Panza JA, Piña IL, et al. Cost effectiveness of coronary‐artery bypass surgery versus medicine in ischemic cardiomyopathy: the STICH randomized clinical trial. Circulation. 2022;145:819–828. doi: 10.1161/CIRCULATIONAHA.121.056276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Miller RJH, Bonow RO, Gransar H, Park R, Slomka PJ, Friedman JD, Hayes S, Thomson L, Tamarappoo B, Rozanski A, et al. Percutaneous or surgical revascularization is associated with survival benefit in stable coronary artery disease. Eur Heart J Cardiovasc Imaging. 2020;21:961–970. doi: 10.1093/ehjci/jeaa083 [DOI] [PubMed] [Google Scholar]
- 90. Bouabdallaoui N, Stevens SR, Doenst T, Petrie MC, Al‐Attar N, Ali IS, Ambrosy AP, Barton AK, Cartier R, Cherniavsky A, et al. Society of Thoracic Surgeons risk score and EuroSCORE‐2 appropriately assess 30‐day postoperative mortality in the STICH trial and a contemporary cohort of patients with left ventricular dysfunction undergoing surgical revascularization. Circ Heart Fail. 2018;11:e005531. doi: 10.1161/circheartfailure.118.005531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, Kappetein AP, Colombo A, Holmes DR Jr, Mack M, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381:639–650. doi: 10.1016/s0140-6736(13)60108-7 [DOI] [PubMed] [Google Scholar]


