Abstract
Background
Vitamin D supplements may only be beneficial for the prevention of osteoporotic fractures when administered with calcium and in individuals with low blood levels of 25(OH)D, but possible hazards of calcium supplements on CVD cannot be excluded.
Objectives
We conducted a meta-analysis of all placebo-controlled randomized trials assessing the effects of calcium supplements alone or with vitamin D on CHD, stroke, and all-cause mortality.
Methods
A meta-analysis of 11 trials included 7 comparisons of calcium alone compared with control (n = 8634) and 6 comparisons of calcium plus vitamin D compared with control (n = 46,804). Aggregated study-level data were obtained from individual trials and combined using a fixed-effects meta-analysis. The main outcomes included MI, CHD death, any CHD, stroke, and all-cause mortality.
Results
Among trials of calcium alone (mean daily dose 1 g), calcium was not significantly associated with any excess risk of MI (RR, 1.15; 95% CI: 0.88, 1.51; n = 219 events), CHD death (RR, 1.24; 95% CI: 0.89, 1.73; n = 142), any CHD (RR, 1.01; 95% CI: 0.75, 1.37; n = 177), or stroke (RR, 1.15; 95% CI, 0.90, 1.46, n = 275). Among 6 trials of combined treatment, supplementation with calcium plus vitamin D was not significantly associated with any excess risk of MI (RR, 1.09; 95% CI: 0.95, 1.25; n = 854), CHD death (RR, 1.04; 95% CI: 0.85, 1.27; n = 391), any CHD (RR, 1.05; 95% CI: 0.93, 1.19; n = 1061), or stroke (RR, 1.02; 95% CI: 0.89, 1.17; n = 885). Likewise, calcium alone, or with vitamin D had no significant associations with all-cause mortality.
Conclusions
This meta-analysis demonstrated that calcium supplements were not associated with any significant hazard for CHD, stroke, or all-cause mortality and excluded excess risks above 0.3%–0.5% per year for CHD or stroke. Further trials of calcium and vitamin D are required in individuals with low blood levels of 25(OH)D for the prevention of fracture and other disease outcomes.
Keywords: calcium supplements, heart disease, randomized trials, stroke
Introduction
Calcium supplements are widely used for the prevention of osteoporotic fractures in middle-aged and older people [1]. Vitamin D is required for the maintenance of adequate blood levels of calcium and phosphate and vitamin D supplements are widely used for the prevention of osteoporotic fractures [2]. Randomized trials have demonstrated beneficial effects of vitamin D supplements for the prevention of hip or other osteoporotic fractures but only when administered together with calcium, and not when administered alone [[3], [4], [5], [6], [7], [8]]; with the maximum benefits reported in individuals with low plasma levels of 25(OH)D [[5], [6], [7], [8]]. However, some trials [9,10], but not others [11,12], have reported that calcium supplements may increase the risks of CVD.
A small trial of calcium supplements for prevention of fracture in 1471 older women reported that supplementation with calcium for 5 y was associated in a post hoc secondary analysis with a higher risk of CVD (a composite CVD outcome based on 51 compared with 35 women with events after adjudication and a HR of 1.21 (95% CI: 0.84, 1.74) when events from the national database were included [9]. Subsequently, a meta-analysis of 15 trials reported that calcium supplements were associated with 27% higher risks of MI (RR; 1.27; 95% CI, 1.01, 1.59), but the findings were now limited to only 296 MI outcomes and had no significant effects on a composite outcome of MI, stroke or sudden death (RR, 1.12; 95% CI: 0.97, 1.30) [10]. In contrast, other meta-analyses of trials of calcium supplements, involving much larger numbers of CVD outcomes reported no significant effects on risks of CVD [11,12].
A Mendelian randomization (MR) study using instrumental variants for plasma calcium levels in 60,801 CHD cases and 123,504 controls in the Coronary Artery Disease Genetics Consortium meta-analysis reported that a 1 SD (∼0.12 mmol/L) higher genetically-predicted plasma calcium levels was associated with a 25% higher risk of CHD (OR, 1.25; 95% CI: 1.08, 1.45), but the possibility of horizontal pleiotropy because of effects on other risk factors could not be excluded [13]. In contrast, an MR study involving 34,217 ischemic stroke cases and 404,630 controls in METASTROKE Consortium reported no significant effects of genetically-predicted calcium levels on ischemic stroke (OR, 1.03; 95% CI: 0.88, 1.21) [14]. Further meta-analyses of trials have reported no significant effects of calcium on CHD outcomes but lacked data on other potentially relevant CVD subtypes [15,16]. Recently, 2 additional trials of calcium supplements have reported their results [17,18], prompting the need for an updated meta-analysis of all trials addressing this topic. The aims of the present study were to conduct collaborative meta-analyses of placebo-controlled trials of calcium supplements alone or of calcium with vitamin D to assess the effects of supplementation with calcium on MI, any CHD, stroke, or all-cause mortality.
Methods
We conducted a systematic search for randomized placebo-controlled trials of calcium supplements reported in English in PubMed, Medline, or Embase datasets, and in the Cochrane Central Register of Controlled Trials, supplemented by hand-searching of reference lists of individual trials, review articles, or previous meta-analyses of calcium and risk of CHD, stroke, or all-cause mortality (Supplemental Figure 1). The key search terms included “calcium supplements,” “calcium intake,” and “cardiovascular disease” or “coronary heart disease” or “myocardial infarction,” or “stroke” or “death,” and “randomized controlled trial.” We contacted the lead authors of all the identified and eligible trials to seek collaboration and unpublished data if available. A protocol was agreed on with the collaborators and registered with the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020172517). Additional details of methods and study protocol are provided in the Supplementary material. The meta-analyses were reported according to the PRISMA guidelines [19,20].
The prespecified eligibility criteria included trials assessing calcium supplements alone or in combination with vitamin D compared with placebo or compared with no treatment, involving at least 500 participants that were treated for at least 1 y [4,5,17,18,[21], [22], [23], [24], [25], [26], [27], [28], [29]]. Cluster-randomized trials of calcium were excluded [30]. Data were obtained from individual trials by writing to the principal investigators seeking the number of participants in each treatment group who suffered either MI (ICD-10 codes I21-I23), death from CHD (ICD-10 codes I20-I25, I46, and R96), any CHD (defined as MI or coronary death), stroke (I60-I64), or all-cause mortality (Supplemental Table 1, Supplementary Methods).
All trials were assessed for risk of bias by 2 reviewers (X.H. and R.C.) using the Cochrane Collaboration risk of bias tool [19]. The risk of bias tool included assessment of random sequence generation, allocation concealment, masking of participants and personnel, masking of outcome assessment, completeness of outcome data, selective reporting, and other sources of bias. Each domain was classified as being either low risk, unclear risk, or high risk of bias. Individual trials had ethics approval from their respective institutional review boards, and all participants provided written informed consent. No additional ethics committee approval was required for the present meta-analysis.
Statistical analysis
The associations of calcium supplements with disease outcomes in individual trials were analyzed separately and aggregated study-level statistics were calculated for each trial using an intention-to-treat approach [20]. The summary statistics from the individual trials were combined using an inverse-variance weighted meta-analysis to provide summary estimates of the effects of treatment on disease outcomes in all trials. We used this “fixed-effects” meta-analysis to avoid providing undue weight to smaller trials with more extreme results [20].
The risk of bias was minimized by limiting subgroup analyses to a few prespecified analyses. For example, the meta-analysis of trials of calcium plus vitamin D was repeated after excluding participants in the Women’s Health Initiative (WHI) trial [4] who reported use of nonstudy calcium supplements prior to randomization (to minimize effects of reverse causality), and that of calcium alone compared with control was repeated after excluding the hypothesis-generating trial [9] that first reported an excess risk of CHD associated with calcium supplements (to assess replication of results in independent populations). Data included reported disease outcomes that were confirmed by clinical adjudication wherever such data were available. Risk of publication bias was assessed using a funnel plot or Egger test [19]. Analyses were conducted using SAS version 9.4 and R version 3.4.2 and results were considered statistically significant if 2-tailed P values were <0.05.
Results
Characteristics of individual trials
Study-level data were obtained from 11 randomized trials [4,5,17,[21], [22], [23], [24], [25], [26], [27], [28]] that fulfilled the inclusion criteria. The trials were conducted over 3 decades (TABLE 1, TABLE 2). One trial [18] did not respond to a request for data and did not report results for CVD outcomes and another trial [29] did not collect data on any CVD outcomes. Among the 11 trials, 2 had a full or partial 2 × 2 factorial-design (i.e., calcium, vitamin D, both, or neither) [17,22], and 1 trial [26] involved a 3-group comparison (i.e., calcium compared with calcium plus vitamin D compared with placebo). Hence, the present meta-analysis assessed the effects of calcium on CVD outcomes, including 7 comparisons of calcium alone compared with control and 6 comparisons of calcium plus vitamin D compared with control.
TABLE 1.
Characteristics of individual randomized clinical trials of supplementation with calcium alone or calcium plus vitamin D supplements vs. placebo or no treatment group
| Author (citation) | Country | No. ofparticipants | Criteria for selectionof trial participants | Daily doseof calcium (Ca)(g/d)/vitamin D (vitD) | Duration,y mean (SD) | Age,y mean (SD) | Female % |
|---|---|---|---|---|---|---|---|
| Trials of calcium supplements | |||||||
| Baron et al., 1999 [21] | United States | 930 | Colorectal adenoma | 1.2g/d Ca | 4 | 61 (9) | 28 |
| Grant et al., 2005 [22] | United Kingdom | 2643 | Prior fracture | 1g/d Ca | 3.8 | 77 (6) | 85 |
| Prince et al., 2006 [23] | Australia | 1460 | Healthy women | 1.2g/d Ca | 5 | 75 (3) | 100 |
| Reid et al., 2006 [24] | New Zealand | 1471 | Healthy women | 1g/d Ca | 5 | 74 (4) | 100 |
| Bonnick et al., 2007 [25] | United States | 563 | Healthy women | 1g/d Ca | 2 | 66 (9) | 100 |
| Lappe et al., 2007 [26] | Unites States | 733 | Healthy women | 1.4–1.5g/d Ca | 4 | 67 (7) | 100 |
| Baron et al., 2015 [17] | Unites States | 834 | Colorectal adenoma | 1.2g/d Ca | 3.7 | 58 (7) | 15 |
| All | 8634 | 4.1 (1) | 71 (6) | 79 | |||
| Trials of calcium with vitamin D supplements | |||||||
| Chapuy et al., 1992 [5] | France | 3270 | Institutionalized women | 1.2g/d Ca + 800 IU/d VitD | 1.5 | 84 (6) | 100 |
| Chapuy et al., 2002 [27] | France | 583 | Institutionalized women | 1.2g/d Ca + 800 IU/d VitD | 2 | 85 (7) | 100 |
| Grant et al., 2005 [22] | United Kingdom | 2638 | Prior fracture | 1g/d Ca + 800 IU/d VitD | 3.8 | 77 (6) | 85 |
| Jackson et al., 2006 [4] | United States | 36,282 | Healthy women | 1g/d Ca + 400 IU/d VitD | 7 | 62 (7) | 100 |
| Salovaara et al., 2010 [28] 1 | Finland | 3195 | Healthy women | 1g/d Ca + 800 IU/d VitD | 3 | 67 (2) | 100 |
| Baron et al., 2015, [27] | United States | 836 | Colorectal adenoma | 1g/d Ca + 1000 IU/d VitD | 3.7 | 58 (7) | 15 |
| All | 46,804 | 6.0 (3) | 65 (6.5) | 98 | |||
All trials used placebo except Salovaara, where the control group was allocated to receive no treatment.
TABLE 2.
Distribution of vascular disease outcomes in the randomized trials of calcium alone or calcium plus vitamin D vs. placebo or no treatment
| No. of participants |
No. of disease outcomes |
||||||
|---|---|---|---|---|---|---|---|
| Author, y (citation) | Total No. of participants | No. of treated/control | Any MI1 | CHD Death | Any CHD | Any stroke | All-cause mortality |
| Comparisons of calcium supplements | |||||||
| Baron et al., 1999 [21] | 930 | 464/466 | 35 | 16 | 46 | 20 | 47 |
| Grant et al., 2005 [22] | 2643 | 1311/1332 | 84 | 99 | NA2 | 104 | 455 |
| Prince et al., 2006 [23] | 1460 | 730/730 | 40 | 22 | 122 | 78 | 92 |
| Reid et al., 2006 [24] | 1471 | 732/739 | 52 | NA | NA | 59 | 63 |
| Bonnick et al., 2007 [25] | 563 | 282/281 | 0 | NA | NA | 3 | 3 |
| Lappe et al., 2007 [26] | 733 | 445/288 | 4 | NA | NA | 9 | NA |
| Baron et al., 2015 (17) | 834 | 419/415 | 4 | 5 | 9 | 2 | 13 |
| ALL | 8634 | 4383/4251 | 219 | 142 | 177 | 275 | 673 |
| Comparisons of calcium plus vitamin D supplements | |||||||
| Chapuy et al., 1992 [5] | 3270 | 1634/1636 | NA | NA | NA | NA | 532 |
| Chapuy et al., 2002 [27] | 583 | 389/194 | NA | NA | NA | NA | 116 |
| Grant et al., 2005 [22] | 2638 | 1306/1332 | 83 | 83 | NA | 108 | 437 |
| Jackson et al., 2006 [4] | 36,282 | 18,176/18,106 | 730 | 280 | 1010 | 726 | 1584 |
| Salovaara et al., 2010 [28] | 3195 | 1586/1609 | 35 | 5 | 38 | 46 | 33 |
| Baron et al., 2015 [27] | 836 | 421/415 | 6 | 7 | 13 | 5 | 15 |
| ALL | 46,804 | 23,512/23,292 | 854 | 391 | 1061 | 885 | 2717 |
Any MI includes fatal MI and nonfatal MI.
N/A denotes not assessed.
Calcium alone compared with placebo comparisons
All of the trials of calcium alone were placebo-controlled [17,[21], [22], [23], [24], [25], [26]], and included a total of 8634 participants. The number of participants in individual trials varied from 563 to 2643 (Table 1). However, 2 trials were unable to provide all relevant CVD outcomes [25,26]. The mean (SD) age of trial participants was 71 (6) y, and 6849 (79.3%) were women. The daily doses of elemental calcium varied from 1.0 to 1.5 g/d. The mean (SD) treatment duration was 4.1 (1) y (range: 2–5 y).
Calcium plus vitamin D compared with control comparisons
Among the trials of calcium plus vitamin D, 6 trials [4,5,17,22,26,27] involved blinded comparisons with placebo, and the other trial used an open design [28]. Overall, the meta-analysis included a total of 46,804 participants, the number of participants in individual trials varied from 583 to 36,282. However, 3 trials [5,26,27] were unable to provide results for some CVD outcomes and 2 trials [5,27], only reported data on all-cause mortality. The mean (SD) age of trial participants was 65 (6.5) y, and 45,689 (97.6%) were women. The median daily dose of elemental calcium varied from 1 to 1.5 g/d, and the dose for vitamin D varied from 400 to 2000 IU/d. The mean duration of treatment was 6.0 (3) y (range: 1.5–7 y) (Table 1).
Risks of bias
Overall, 5 (45.5%) trials had a low risk of bias and 3 (27.3%) trials had an uncertain risk of bias. Three trials [5,27,28] (27.3%) had a high risk of bias (Supplemental Figure 2), each of which involved comparisons of calcium plus vitamin D compared with control. One trial did not report allocation concealment [28], and reporting was incomplete in 2 trials (with risk of selective reporting) [27,28]. Table 2 shows the number of CVD outcomes in the individual trials and indicated if any data were unavailable.
Effects of calcium supplementation on vascular disease and all-cause mortality
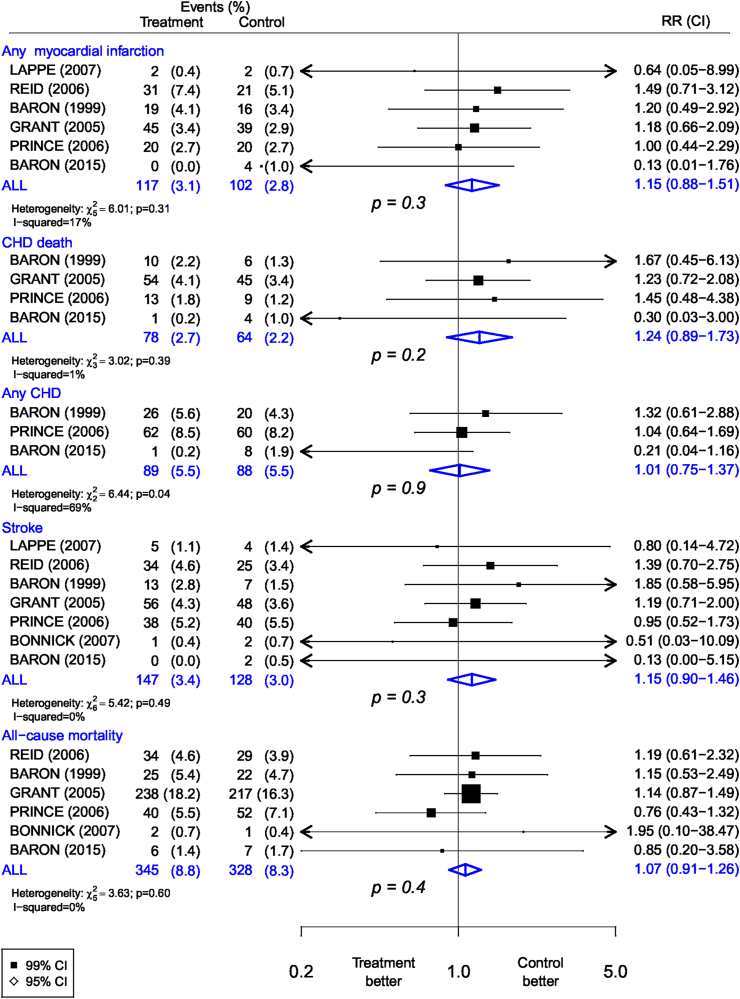
The numbers of events and RRs for the different trials are shown in Figure 1. The number of vascular disease outcomes in the individual trials was small. The top panel shows that the overall allocation to calcium supplements alone compared with placebo was associated with a nonsignificant excess risk of MI (117 compared with 102 in the calcium and placebo groups, respectively: RR, 1.15; 95% CI: 0.88, 1.51; P = 0.3). Similar nonsignificant excess risks of CVD were observed, albeit based on small numbers, for CHD death, any CHD, stroke, and all-cause mortality. There was no significant heterogeneity among the results of individual trials for MI, fatal CHD, stroke, or all-cause mortality (Figure 1). Among the trials of calcium supplements, the contour-enhanced funnel plots were broadly symmetric (Supplemental Figure 3) and Egger test results demonstrated no evidence of any significant publication bias for any MI (P = 0.06), stroke (P = 0.24), or all-cause mortality (P = 0.81).
FIGURE 1.
Meta-analysis of randomized trials of supplementation with calcium alone vs. placebo for the prevention of vascular disease outcomes.
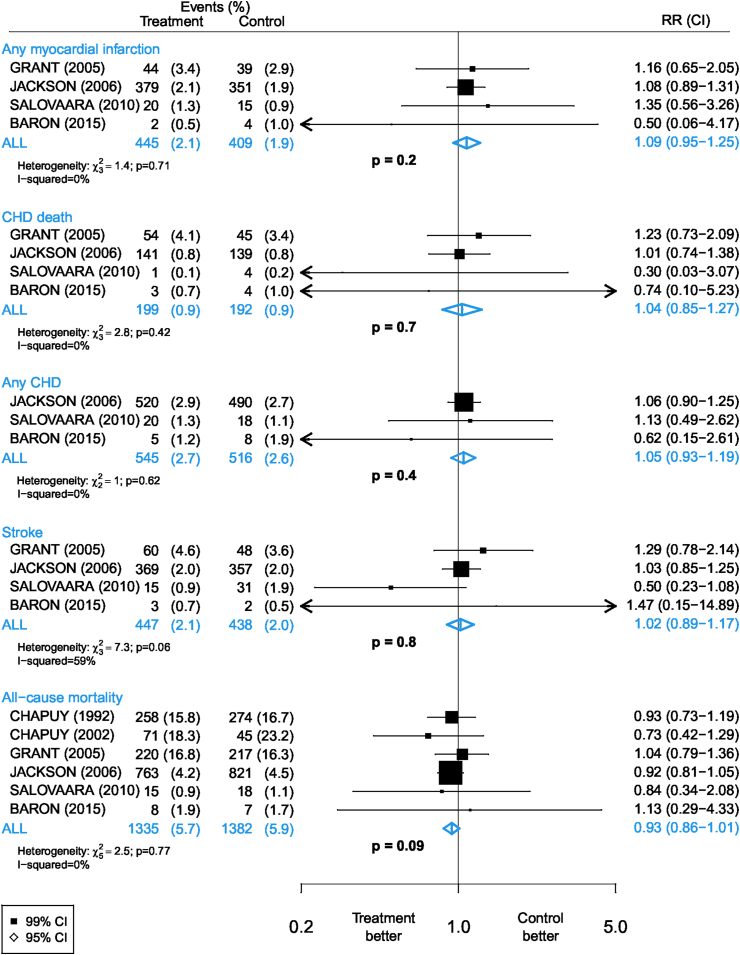
Effects of calcium plus vitamin D supplementation on vascular disease and all-cause mortality
Figure 2 shows the number of disease outcomes and results for the comparisons of allocation to calcium plus vitamin D supplements compared with control where available. The number of disease outcomes in each category was dominated by the WHI trial [4], which accounted for 97% of all trial participants and 95% of all CHD outcomes. No significant excess risks were observed, but the RRs were >1.0 for MI (RR, 1.09; 95% CI: 0.95, 1.25; P = 0.2), CHD death, any CHD, and stroke (Figure 2). For all-cause mortality, the RR was <1.0, an outcome that included a high proportion of nonvascular deaths. There was no significant heterogeneity between the results of individual trials of calcium plus vitamin D compared with control on any of the disease outcomes. A contour-enhanced funnel plot demonstrated no evidence of asymmetry and the Egger test indicated no evidence of publication bias (P = 0.87).
FIGURE 2.
Meta-analysis of randomized trials of supplementation with calcium plus vitamin D vs. placebo or no treatment for the prevention of vascular disease outcomes.
Sensitivity analyses
The results of sensitivity analyses, excluding participants who reported use of calcium supplements at baseline in the WHI trial, [4] did not differ from the overall findings with no significant excess risks of MI, fatal CHD, any CHD, stroke, or all-cause mortality (Supplemental Figure 4). The RR (95% CI) associated with calcium supplements were 1.15 (95% CI: 0.96, 1.37) for MI and 1.04 (95% CI: 0.89, 1.23) for any CHD. In a further sensitivity analysis after excluding the hypothesis-generating trial [24] that first reported the excess risks of CHD with calcium supplements, the RR (95% CI) associated with calcium supplements were 1.06 (95% CI: 0.78, 1.45) for MI and 1.01 (95% CI: 0.75, 1.37) for any CHD (Supplemental Figure 5). The results were unaltered by additional sensitivity analyses involving sequential exclusion of individual trials with high risk of bias (Supplemental Tables 2 and 3).
Discussion
Historically, vitamin supplements (including vitamin D and calcium) were administered to individuals with the insufficiency of the relevant vitamins, but advances in precision medicine prompted the need for randomized evidence on the efficacy and safety of vitamin supplements for prevention of disease outcomes in a wider proportion of the population. The present report, involving 2 meta-analyses, 1 involving 7 trials of calcium alone (including 8634 participants) and 1 involving 6 trials of calcium with vitamin D (including 46,804 participants compared with control) demonstrated no significant adverse effects of calcium on CHD or stroke, but the numbers of these CVD types were insufficient to exclude absolute excess risks of 0.3%–0.5% per year for CHD or stroke associated with calcium supplements in the study populations. The randomized comparisons of calcium alone compared with placebo (n = 8634) assessed the hypothesis that calcium might increase risk of CHD or stroke, but even for MI, the CHD outcome with the most events, the numbers of outcomes (117 compared with 102) were insufficient to yield reliable conclusions. Likewise, for stroke, the numbers of outcomes (147 compared with 128) were insufficient to yield reliable conclusions.
The comparisons of calcium plus vitamin D with control included a larger number of participants and disease outcomes (reflecting the inclusion of the WHI trial), than those for calcium alone. Consequently, the meta-analysis could only exclude the possibility that calcium supplements might cause an excess risk of CVD greater than 0.3%–0.5% per year (equivalent to a 20%–30% higher-RRs) for any CHD or any stroke. Trials of vitamin D alone have not reported any adverse effects on CVD outcomes and a meta-analysis of 8 trials, involving 42,637 individuals, of vitamin D alone compared with placebo reported no overall effect on major vascular events (RR, 1.00; 95% CI: 0.93, 1.07) [31].
A recent genetic study reported that there was evidence of a causal association of low plasma levels of 25(OH)D with all-cause mortality when restricted to individuals with low plasma levels of 25(OH)D (<40 nmol/L) [32]. The available evidence cannot exclude the possibility that vitamin D supplements may have some beneficial effects on CVD in people with vitamin D deficiency which could offset any adverse effects of calcium when administered in combination. In contrast, MR studies have suggested that genetically-predicted higher plasma calcium levels were associated with higher risks of CHD [13], but not with ischemic stroke [14], although the possibility of horizontal pleiotropy (where the associations were because of factors other than calcium levels) could not be excluded. Furthermore, comparing intermittent postmedication hypercalcemia in later life with life-long mild hypercalcemia related to genetic predisposition is problematic.
The present meta-analysis had several strengths and limitations. None of the trials included in this meta-analysis had prespecified CVD outcomes as their primary outcome and none had a sufficient number of CVD outcomes to exclude a small, but potentially important hazard for risk of CVD. The meta-analysis was constrained by heterogeneity in reported CVD types reported in the individual trials. The inclusion of the additional unpublished trial results for 2 trials has enabled more reliable estimates of any associations of calcium supplements with CVD subtypes. The inclusion criteria and vascular disease outcomes used in this meta-analysis (which were prespecified) differ from those used in previous similar meta-analyses of subsets of these trials or related smaller trials [11,12,33,34]. The meta-analysis minimized the risks of publication bias (and other bias) by restricting the inclusion criteria to trials involving ≥500 participants and a duration of treatment ≥12 mo and avoiding the conduct of subgroup analyses.
The results of previous meta-analyses [[10], [11], [12],[34], [35], [36], [37]] of calcium supplements and risk of CHD or stroke have been difficult to interpret, partly because of selection biases (exclusion of participants from W HI [16]), selective emphasis on MI rather than any CHD [10], failure to stratify trials by those that assessed the effects by calcium alone from those that assessed combined effects of calcium and vitamin D, and use of random effects models to estimate summary measures of effect of calcium on risk of CHD and stroke outcomes [[10], [11], [12],16,34]. Because the number of participants in individual trials differs by about 60-fold, use of random effects models to combine the results of all trials is likely to yield unreliable results because such models assign disproportionate weight to the smaller trials. In contrast, the present meta-analysis prespecified the primary disease outcomes, stratified trials that reported effects of calcium alone from those trials of calcium plus vitamin D, and use fixed effects (i.e., assumption-free inverse-variance weighted method) rather than random effects models to combine the results of the individual trials.
The 2016 guidelines of the National Osteoporosis Foundation and the American Society for Preventive Cardiology advocate that daily intake of calcium with or without vitamin D from supplements or food should not exceed the tolerable upper level of intake of 2–2.5 g/d, which they suggest should not be associated with adverse effects on CVD outcomes [38]. The United States Preventive Task Force concluded that the available evidence was insufficient to assess the balance of the benefits and hazards of combined calcium and vitamin D supplementation on CVD outcomes [2].
The present meta-analysis of calcium trials, involving 55,438 participants demonstrated that calcium supplements were not associated with any significant excess hazards for CHD and stroke outcomes, but still included too few CHD and stroke outcomes to exclude absolute excess risks of any CHD or stroke of 0.3%–0.5% per year because of use of calcium supplements. Hence, for people with low-bone density and low absolute risks of CVD, the present report demonstrates no concern about excess CVD risks associated with calcium supplements. However, further large trials are needed to assess the efficacy and safety of combined supplementation with calcium and vitamin D for the prevention of osteoporotic fracture in older people at high risk of CVD.
Acknowledgments
We would like to thank Dr Mark Bolland, Dr Ian Reid, and Dr Alison Avenell for providing data from their trials and for comments on earlier versions of this report. The authors’ responsibilities were as follows—XH, RC,and JA: designed the protocol for this meta-analysis. XH: conducted a literature search and prepared the data requests, data entry, data checking, and verification of outcomes and corresponded with the trial investigators. JH: conducted the statistical analyses. RJ, AH, RP, JL, JAB, HK, and RS: conducted the individual trials and provided summary data from their trials for this meta-analysis. Although ZH wrote the first draft of the report, all authors provided critical comments on revised versions of the report. All authors: read and approved the final manuscript.
Members of Calcium Treatment Trialists’ Collaboration
Xiqian Huo, Robert Clarke, Jim Halsey, Rebecca Jackson, Amy Lehman, Richard Prince, Joshua Lewis, John A Baron, Heikki Kroger, Reijo Sund, and Jane Armitage.
Footnotes
Members of consortium are shown at the end of the report.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdnut.2023.100046.
Data Availability
Summary data collected from individual trials that are included in this report can be shared with bona fide researchers upon reasonable requests by contacting the corresponding author of this report.
Funding
Robert Clarke and Jane Armitage are members of the Nuffield Department of Population Health at the University of Oxford which receives core support from UK Medical Research Council (MC_UU_0017/1) and British Heart Foundation (H6R00450) and British Heart Foundation Centre for Research Excellence.
Author disclosures
JRL was supported by the National Heart Foundation of Australia Future Leader Fellowship (ID 102817). JA and RC were supported by the British Heart Foundation and British Heart Foundation Centre for Research Excellence. All other authors report no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Del Valle H.B., Yaktine A.L., Taylor C.L., Ross A.C. National Academies Press; 2011. Dietary reference intakes for calcium and vitamin D. [PubMed] [Google Scholar]
- 2.Kahwati L.C., Weber R.P., Pan H., Gourlay M., LeBlanc E., Coker-Schwimmer M., et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(15):1600–1612. doi: 10.1001/jama.2017.21640. [DOI] [PubMed] [Google Scholar]
- 3.Yao P., Bennett D., Mafham M., Lin X., Chen Z., Armitage J., et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw. Open. 2019;2(12) doi: 10.1001/jamanetworkopen.2019.17789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson R.D., LaCroix A.Z., Gass M., Wallace R.B., Robbins J., Lewis C.E., et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 5.Chapuy M.C., Arlot M.E., Duboeuf F., Brun J., Crouzet B., Arnaud S., et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992;327(23):1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J.G., Zeng X.T., Wang J., Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318(24):2466–2482. doi: 10.1001/jama.2017.19344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolland M.J., Grey A., Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847–858. doi: 10.1016/S2213-8587(18)30265-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhu K., Devine A., Dick I.M., Wilson S.G., Prince R.L. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J. Clin. Endocrinol. Metab. 2008;93(3):743–749. doi: 10.1210/jc.2007-1466. [DOI] [PubMed] [Google Scholar]
- 9.Bolland M.J., Barber P.A., Doughty R.N., Mason B., Horne A., Ames R., et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336(7638):262–266. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolland M.J., Avenell A., Baron J.A., Grey A., MacLennan G.S., Gamble G.D., et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao P.J., Zhang C., Tang L., Xian Y.Q., Li Y.S., Wang W.D., et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int. J. Cardiol. 2013;169(2):106–111. doi: 10.1016/j.ijcard.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 12.Lewis J.R., Radavelli-Bagatini S., Rejnmark L., Rejnmark L., Chen J.S., Simpson J.M., et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J. Bone. Miner. Res. 2015;30(1):165–175. doi: 10.1002/jbmr.2311. [DOI] [PubMed] [Google Scholar]
- 13.Larsson S.C., Burgess S., Michaëlsson K. Association of genetic variants related to serum calcium levels with coronary artery disease and myocardial infarction. JAMA. 2017;318(4):371–380. doi: 10.1001/jama.2017.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson S.C., Traylor M., Burgess S., et al. Serum magnesium and calcium levels in relation to ischemic stroke: Mendelian randomization study. Neurology. 2019;92(9):e944–e950. doi: 10.1212/WNL.0000000000007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michos E.D., Cainzos-Achirica M., Heravi A.S., Appel L.J. Vitamin D, calcium supplements, and implications for cardiovascular health: JACC Focus seminar. J. Am. Coll. Cardiol. 2021;77(4):437–449. doi: 10.1016/j.jacc.2020.09.617. [DOI] [PubMed] [Google Scholar]
- 16.Bolland M.J., Grey A., Avenell A., Gamble G.D., Reid I.R. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron J.A., Barry E.L., Mott L.A., Rees J.R., Sandler R.S., Snover D.C., et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N. Engl. J. Med. 2015;373(16):1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lappe J., Watson P., Travers-Gustafson D., Recker R., Garland C., Gorham E., et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317(12):1234–1243. doi: 10.1001/jama.2017.2115. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P., Altman D.G., Gøtzsche P.C., Juni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baigent C. Large-scale randomized evidence: trials and metaanalyses of trials. Oxf. Textbook. Med. 2010:31–45. [Google Scholar]
- 21.Baron J.A., Beach M., Mandel J.S., van Stolk R.U., Haile R.W., Sandler R.S., et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N. Engl. J. Med. 1999;340(2):101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 22.Grant A.M., Avenell A., Campbell M.K., McDonald A.M., MacLennan G.S., McPherson G.C., et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial [Record] Lancet. 2005;365(9471):1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 23.Prince R.L., Devine A., Dhaliwal S.S., Dick I.M. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch. Intern. Med. 2006;166(8):869–875. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 24.Reid I.R., Mason B., Horne A., Ames R., Reid H.E., Bava U., et al. Randomized controlled trial of calcium in healthy older women. Am. J. Med. 2006;119(9):777–785. doi: 10.1016/j.amjmed.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Bonnick S., Broy S., Kaiser F., Teutsch C., Rosenberg E., DeLucca P., et al. Treatment with alendronate plus calcium, alendronate alone, or calcium alone for postmenopausal low bone mineral density. Curr. Med. Res. Opin. 2007;23(6):1341–1349. doi: 10.1185/030079907X188035. [DOI] [PubMed] [Google Scholar]
- 26.Lappe J.M., Travers-Gustafson D., Davies K.M., Recker R.R., Heaney R.P. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am. J. Clin. Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 27.Chapuy M.C., Pamphile R., Paris E., Kempf C., Schlichting M., Arnaud S., et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos. Int. 2002;13(3):257–264. doi: 10.1007/s001980200023. [DOI] [PubMed] [Google Scholar]
- 28.Salovaara K., Tuppurainen M., Kärkkäinen M., Rikkonen T., Sandini L., Sirola J., et al. Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: a population-based 3-year randomized, controlled trial--the OSTPRE-FPS. J. Bone Miner. Res. 2010;25(7):1487–1495. doi: 10.1002/jbmr.48. [DOI] [PubMed] [Google Scholar]
- 29.Porthouse J., Cockayne S., King C., Saxon L., Steele E., Aspray T., et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330(7498):1003. doi: 10.1136/bmj.330.7498.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen E.R., Mosekilde L., Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J. Bone Miner. Res. 2004;19(3):370–378. doi: 10.1359/JBMR.0301240. [DOI] [PubMed] [Google Scholar]
- 31.Barbarawi M., Kheiri B., Zayed Y., Barbarawi O., Dhillon H., Swaid B., et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA. Cardiol. 2019;4(8):765–776. doi: 10.1001/jamacardio.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emerging Risk Factors Collaboration/EPIC. CVD/vitamin D studies collaboration. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and Mendelian randomization analyses. Lancet Diabetes Endocrinol. 2021;12:837–846. doi: 10.1016/S2213-8587(21)00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Yang C., Shi X., Xia H., Yang X., Liu H., Pan D., et al. The evidence and controversy between dietary calcium intake and calcium supplementation and the risk of cardiovascular disease: a systematic review and meta-analysis of cohort studies and randomized controlled trials. J. Am. Coll. Nutr. 2020;39(4):352–370. doi: 10.1080/07315724.2019.1649219. [DOI] [PubMed] [Google Scholar]
- 34.Myung S.K., Kim H.B., Lee Y.J., Choi Y.J., Oh S.W. Calcium supplements and risk of cardiovascular disease: a meta-analysis of clinical trials. Nutrients. 2021;13(2) doi: 10.3390/nu13020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Manson J.E., Sesso H.D. Calcium intake and risk of cardiovascular disease: a review of prospective studies and randomized clinical trials. Am. J. Cardiovasc. Drugs. 2012;12(2):105–116. doi: 10.2165/11595400-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins D.J.A., Spence J.D., Giovannucci E.L., et al. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment: JACC Focus seminar. J. Am. Coll. Cardiol. 2021;77(4):423–436. doi: 10.1016/j.jacc.2020.09.619. [DOI] [PubMed] [Google Scholar]
- 37.Khan S.U., Khan M.U., Riaz H., Valavoor S., Zhao D., Vaughan L., et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann. Intern. Med. 2019;171(3):190–198. doi: 10.7326/M19-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kopecky S.L., Bauer D.C., Gulati M., Nieves J.W., Singer A.J., Toth P.P., et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the national Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann. Intern. Med. 2016;165(12):867–868. doi: 10.7326/M16-1743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summary data collected from individual trials that are included in this report can be shared with bona fide researchers upon reasonable requests by contacting the corresponding author of this report.