Abstract
As the climate warms, wildfire activity is increasing, posing a risk to human health. Studies have reported on particulate matter (PM) in wildfire smoke, yet the chemicals associated with PM have received considerably less attention. Here, we analyzed 13 years (2006–2018) of PM2.5 chemical composition data from monitors in California on smoke-impacted days. Select chemicals (e.g., aluminum and sulfate) were statistically elevated on smoke-impacted days in over half of the years studied. Other chemicals, mostly trace metals harmful to human health (e.g., copper and lead), were elevated during particular fires only. For instance, in 2018, lead was more than 40 times higher on smoke days on average at the Point Reyes monitoring station due mostly to the Camp Fire, burning approximately 200 kilometers away. There was an association between these metals and the combustion of anthropogenic material (e.g., the burning of houses and vehicles). Although still currently rare, these infrastructure fires are likely becoming more common, and can mobilize trace metals in smoke far downwind, at levels generally unseen except in the most polluted areas of the country. We hope a better understanding of the chemicals in wildfire smoke will assist in the communication and reduction of public health risks.
Keywords: wildfire, Camp Fire, particulate matter, smoke, air quality, metals, lead, Pb
1. Introduction
The annual area burned by wildfires, total number of large fires, and fire season length have all been increasing in the western United States in recent decades.1, 2 Warmer average temperatures, reduced snowpack, and reduced warm-season precipitation contribute to greater wildfire activity, with climate change amplifying these trends.3 Beyond loss of life and destruction of property, wildfires profoundly affect air quality, even in relatively far downwind communities. The 2020 fire season, for instance, blanketed the entire West Coast of the United States (U.S.) in a thick layer of smoke for multiple days, and in some areas, for weeks (Figure 1a). These smoke events are becoming longer and more widespread as wildfires burn more acres.
Fig. 1.
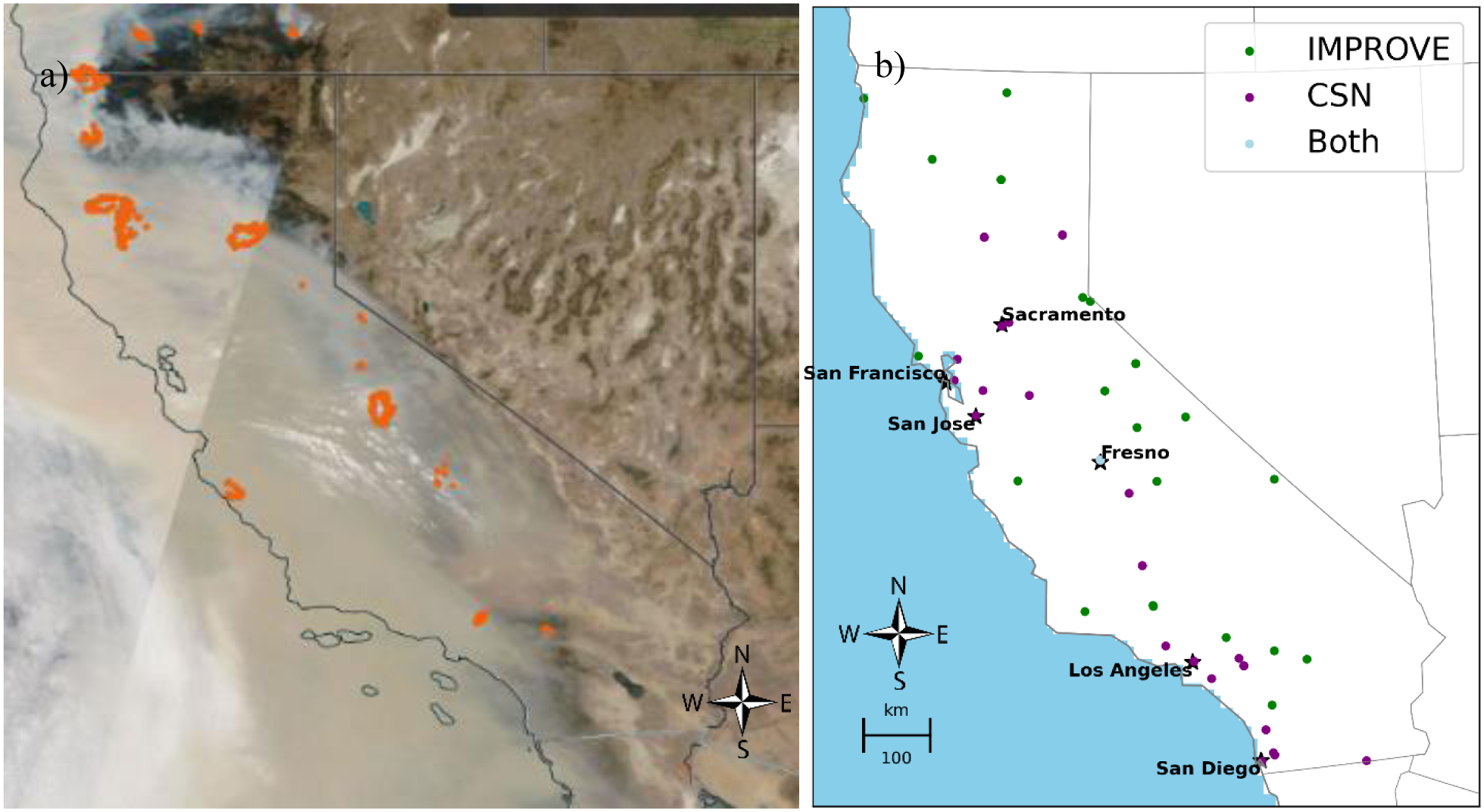
A satellite image of smoke covering California on September 10, 2020, with the orange dots of active fires superimposed (a); and a map of Air Quality System PM2.5 speciation network monitors (both the Interagency Monitoring of Protected Visual Environments (IMPROVE) (29 monitors) and Chemical Speciation Network (CSN) (35 monitors)) active at some point during 2006–2018 (b).
Fires impact air quality through the generation of a mixture of gaseous and particulate pollutants, with negative impacts to human health.4 The most well-studied pollutants are particulate matter (PM), ozone, and carbon monoxide.5–9 These criteria air pollutants (CAPs), as defined by the U.S. Environmental Protection Agency (EPA), are emitted in high quantities during wildfires, with important implications for human health.10–12 In contrast, less is known about the concentrations of specific chemicals found within wildfire smoke. Organic compounds can make up >90% of the PM in wildfire smoke.4 Beyond these compounds, however, PM from wildfires can contain traces of many chemicals, from toxic metals to nutrients. Even in small quantities, these chemicals can pose a threat to human health and impact ecosystems. There are studies reporting emission factors of these toxic chemicals, as well as studies looking at concentrations in post-fire ash,13–20 yet there are fewer studies focused on the actual concentrations in air quality samples associated with fire.21–24 Moreover, often studies reporting downwind air concentrations of these chemicals do so for only a handful of fires.21, 22 Long-term studies of these trace chemicals in smoke are lacking.
In addition to chemicals mobilized from natural fuels, there is an increasing concern that chemicals from burning infrastructure (e.g., vehicles, structures, etc.) will mobilize into the atmosphere or waterways as fires burn into urban areas. Wildfires burning into urban areas are likely becoming more common because of an increase in large fire frequency and an expansion of the wildland-urban interface (WUI).25, 26 The WUI is the intersection between the wildland vegetation and the urban housing. From 1990–2010, the WUI experienced a 41% growth in new houses and 33% growth in land area in the U.S., with the vast majority due to new housing.25 Fires are difficult to fight and often ignited by humans in the WUI.27, 28 These fires risk mobilization of a suite of pollutants from burning plastics, metals, and other human-made materials, in addition to property destruction and loss of life. There is very little information on emissions from fires in the urban environment,4 with studies generally limited to vehicle and tire fires showing elevated levels of select chemicals (e.g., flouride, calcium, sodium, lead, and zinc).29, 30 There is more information on structure fires derived from firefighter exposure measurements, where crustal elements (e.g., aluminum, calcium, iron, zinc) and antimony, chromium, copper, and nickel were most frequently observed in air samples.31 However, firefighter exposure measurements may include emissions from other sources (e.g., resuspended dust, diesel exhaust) in addition to the fire emissions.
To better understand the chemicals mobilized from fires, we analyzed over a decade of fire and air quality data from the U.S. state of California between 2006 and 2018. During this period, California experienced many destructive fires, especially in the last few years. In 2017 and 2018, for example, over 3.5 million hectares burned in California, with 34,506 structures destroyed.32, 33 Moreover, California has one of the most extensive networks of PM2.5 speciation stations in the U.S., facilitating a detailed analysis of smoke composition. We focused on individual chemicals in PM2.5 since  this size class poses the greatest risk to human health.11, 34 Thus, using over a decade of extensive data from California, we posed the following questions:
this size class poses the greatest risk to human health.11, 34 Thus, using over a decade of extensive data from California, we posed the following questions:
Beyond organic compounds, what chemicals in PM2.5 air quality samples are associated with smoke plumes from fires?
How much do these PM2.5 chemicals increase on smoke days versus non-smoke days?
Does distance between fires and monitors or total PM2.5 concentrations explain the potential variation in these chemicals in smoke?
Is there a unique air quality signature associated with fires burning infrastructure in the WUI?
Addressing these questions will help inform potential air quality risks posed by fire beyond that of PM mass, ozone, and carbon monoxide. It will also provide more information on chemicals emitted during infrastructure destroying fires and, if, and how, the smoke from these fires differs from that of natural fuels.
2. Data and Methods
a. Smoke plume identification
To answer Questions 1 and 2, we compared PM2.5 speciation air quality data inside and outside of smoke plumes. For smoke plume identification, we employed the Hazard Mapping System (HMS) fire and smoke product provided by the National Oceanic and Atmospheric Administration (NOAA).35, 36 This product uses multiple satellites to detect fire and smoke presence daily, with resolutions ranging from 2 kilometers (GOES-16 East and West geostationary satellites) down to 375 meters (S-NPP and NOAA-20 polar-orbiting satellites). The system operates year-round, and the automated fire detections utilize multi-spectral imagery, apply a form of temperature threshold, and evaluate each hotspot. The detected hotspots are then analyzed manually for validity by fire analysts, with the final daily smoke polygon and fire detection data made available to the public.
The HMS has been tested and validated by multiple studies.37, 38 After upgrades made to the system in 2006/2007, Schroeder, Ruminski, Csiszar, Giglio, Prins, Schmidt and Morisette38 compared the HMS fire detections to 30-m spatial resolution ASTER satellite data. They found an average 2% false positive rate, with a reduction in omission errors as additional fire locations were added by fire analysts. Commission errors, with true fires being automatically deleted from the HMS data accidentally, are more common. Additionally, satellite fire detection is typically best for detecting larger wildfires.39 Finally, the smoke plumes from the product are representative of smoke throughout the entire atmospheric column, not just at the surface. Yet, despite these inherent limitations, the HMS data provide a robust method for identifying daily smoke-plume impacts.
b. Particulate matter (PM2.5) measurements
In addition to the smoke plumes, we obtained PM2.5 24-hour concentration data for California from the EPA’s Air Quality System (AQS), specifically data from the PM2.5 Chemical Speciation Network (CSN) and the Interagency Monitoring of Protected Visual Environments (IMPROVE) network (Figure 1b).40, 41 The CSN stations are in more urban locations, generally higher in PM, while the IMPROVE network operates in National Parks and wilderness areas to track changes in visibility and typically record lower ambient PM2.5 concentrations. Depending upon the network and station, PM2.5 measurements are taken once every three to six days. Data from 2006–2018 were employed since these were the years with both complete HMS smoke polygon data and AQS PM2.5 speciation measurements.
Finally, we defined a fire season and cut down our data accordingly. To identify a fire season, we plotted the number of stations within a smoke plume each day from 2006–2018 in R version 4.0.3 using the sf and tidyverse packages. April 1 through December 31st contained ~99% of smoke plume measurements, so we used that to define our fire season, capturing both early and late season fires (Supplementary Figure 2).42
c. PM2.5 chemistry on smoke-impacted vs. non-smoke days
Using these input data, we compared PM2.5 chemicals concentrations within and outside of the HMS plume for all years from 2006–2018 at each station. PM2.5 measurements were labeled as smoke-impacted if the monitor location fell within an HMS smoke plume on the day of the measurement. For each station, we calculated the difference between the average PM2.5 chemical concentration on smoke-impacted days vs non-smoke impacted days. Comparing station-specific differences allowed us to control for non-smoke variation between stations (e.g., urban stations are often higher in certain PM2.5 chemicals than rural stations). We performed a permutation test for each PM2.5 chemical for each year grouped by station to test if differences between smoke and non-smoke days were significant.43 We used a non-parametric test to avoid the issues in our data violating the assumptions of a parametric test, including a skewed distribution and unequal sample sizes (i.e., the sample size of treatment ‘smoke days’ was smaller than the control ‘non-smoke days’).44
d. Chemical associations with distance and total PM2.5 concentrations
To explore the potential variation in PM2.5 chemical concentrations in smoke (Question 3), we compared chemical concentrations with distance to the closest fire and total PM2.5 concentrations within the smoke plume. For the distance analysis, we employed the fire source point HMS data. The fire points correspond to any fire burning during the 24-hour period of detection, and are not directly linked to the smoke polygons. We measured the distance between each AQS monitor site impacted by a smoke plume and the nearest HMS-detected fire point location. Days without any fires detected in the HMS fire point dataset were excluded from this analysis as there were no fires in which to measure distance.
The relationships between PM2.5 chemical concentrations within HMS smoke plumes and distance were investigated using a quantile regression model. We normalized the chemical concentration data through a robust scalar method, whereby we subtracted the median concentration for each chemical at every monitoring location for every year from its respective data and scaled the concentration distribution to the interquartile range. This accounted for variability in average PM2.5 composition across monitoring locations and time, while preserving the signature of extreme events in the data. A quantile regression model was fit to the normalized PM speciation data as a function of distance to the nearest HMS-detected fire point location,45 implemented in the Statsmodels Python package.46
Further, we tested the correlation between chemical concentrations and total PM2.5 concentrations by plotting the normalized concentrations of both on smoke-impacted days. Total PM2.5 was used as an index for smoke severity. If chemical concentrations were a function of higher smoke severity, we would expect a positive linear relationship with PM2.5, whereas if the concentrations did not follow PM2.5, this could be interpreted as a shift in the composition of the smoke. Both variables were normalized across all years and stations using the same robust scaling method, and the relationship was analyzed using linear regression.
e. Destructive fire air quality signature
Finally, we sought to identify any air quality signature associated with destructive fires. Here we define “destructive” as fires that burn infrastructure, for example, vehicles and houses (Question 4). Given the limited number of data points, we chose to use a case study approach to identify differences between a few fires of varying levels of destruction. The most destructive fire event we explored was the Camp Fire, which burned in November of 2018 and destroyed approximately 18,000 structures over two weeks. We also explored the Tubbs/Atlas/Nuns Fire complex, the Butte and Valley Fires, the Carr Fire, and the fires of June-July 2008, which destroyed approximately 7,000, 3,000, 1,500 and minimal structures (less than 100), respectively. We compared the chemical profiles of these fires along this spectrum of destructive fires to determine any patterns in concentrations of PM2.5 chemicals. We chose stations downwind of each fire event. The amount of smoke across the stations varied, but all sites fell within a smoke plume. For each station in the analysis, we subtracted the station-specific, non-fire average concentration for each chemical for the year (from April 1st through December 31st) from the concentration measured during each case study fire, in order to better characterize the chemistry of the smoke only.
All data processing and statistics were conducted in Python version 3.8.3, unless otherwise specifically noted. Results of all analyses were considered significant at a p-value of 0.05.
3. Results
Our results show that many chemicals (e.g., bromine, calcium, aluminum, iron, silicon, manganese, potassium, sulfate, and titanium) were statistically elevated on smoke-impacted days. In contrast, other chemicals were elevated only during select fires, mostly trace metals particularly toxic to humans and ecosystems (e.g., lead and copper). We used two different methods to analyze the monitoring data and both supported this conclusion. This section describes those analyses and findings in detail.
a. Chemical composition on smoke-impacted vs. non-smoke impacted days
Comparing smoke and non-smoke days for Questions 1 and 2, a third of the chemicals were consistently higher on smoke days for all the data combined when considering the median percent change across all stations averaged across all years (Supplementary Table 1; Figure 2). Thirteen of the 39 chemicals had a median percent change above 10% when comparing smoke to non-smoke days (bromine, potassium, manganese, calcium, titanium, silicon, sulfate, aluminum, iron, sulfur, copper, sodium, and zinc). The results from the permutation test similarly show a group of PM2.5 chemicals consistently elevated on smoke days (Supplementary Table 2). Bromine, calcium, aluminum, iron, silicon, manganese, potassium, sulfate, sulfur, and titanium were all significantly higher on smoke days for 8+ years in our 13-year study (Supplementary Table 1). Conversely, ammonium was significantly lower on smoke days for 7 of the 10 years, and on average across all the years (Supplementary Table 1; Figure 2). Nitrate was significantly lower on smoke impacted days for 9 of the 10 years, yet was slightly higher on average on smoke days across all years (ca. 6% higher) because of a few extremely high values on particular smoke days (Supplementary Table 1; Figure 2).
Fig. 2.
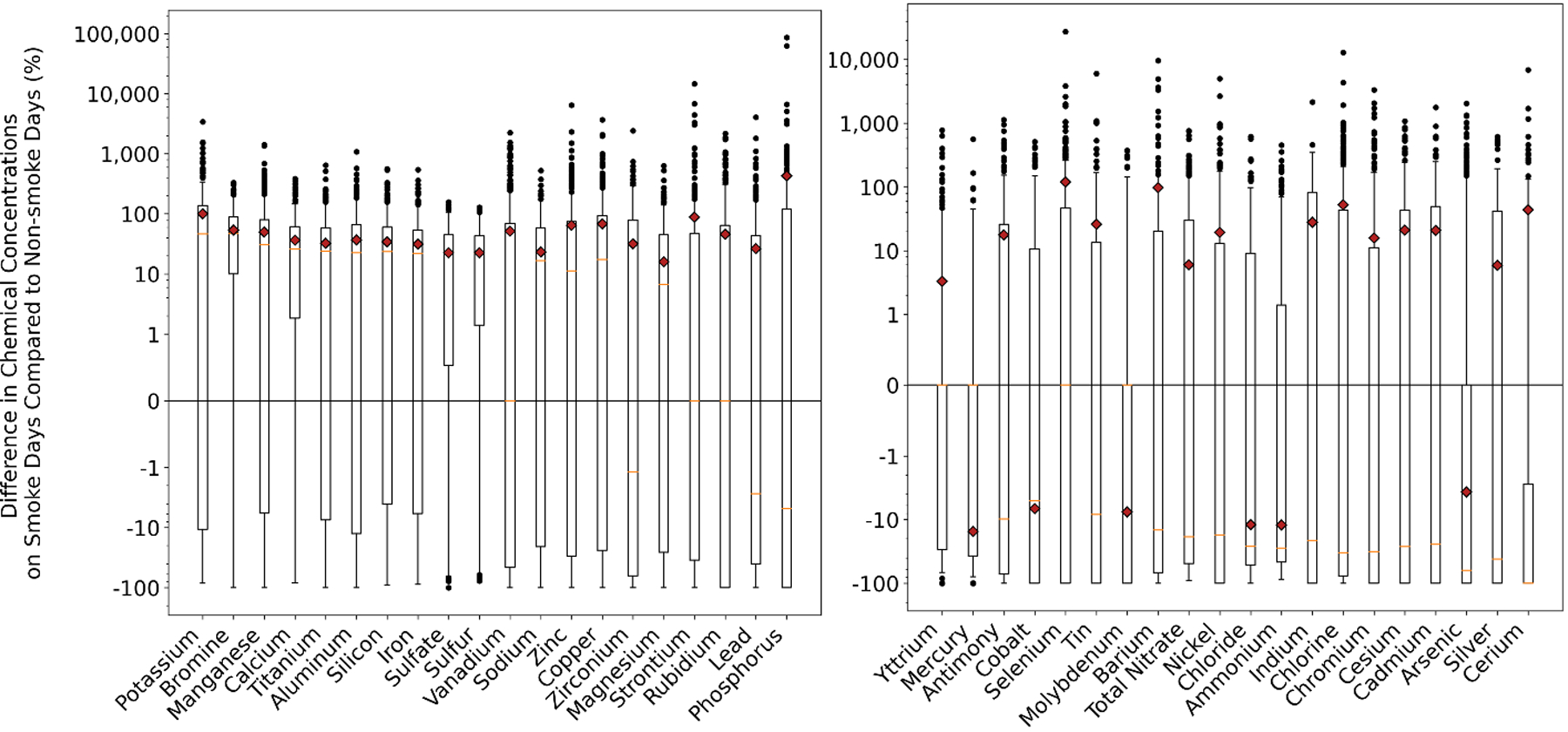
Percent differences in chemical concentrations on smoke days compared to non-smoke days at each monitoring station, for each chemical, across all years (2006–2018). Each black dot represents a single monitoring station for one year. The red diamonds are the average values across all stations, all years. The red horizonal lines are the median values across all monitoring stations, all years.
The maximum differences at individual stations for smoke and non-smoke days were much more variable, indicating that particular fires produce high levels of certain chemicals. For example, arsenic, chromium, copper, lead, nickel, and zinc all had maximum percent changes over 2,000% (Supplementary Table 1; Figure 2). Thus, concentrations on select smoke days were much higher than the highest concentrations on non-smoke days. Additionally, chemicals had multiple outlier events on smoke days (Figure 2).
As a result of this outlier pattern, we observed a second group of chemicals that were not higher on average on smoke days and only higher in a few of the years in the permutation test, yet still exhibited very high concentrations during select fire events. These chemicals include lead, copper, arsenic, and chromium (Supplementary Table 1). The magnitude of the chemical concentration of these episodically elevated individual events generally exceeded that of the chemicals almost always elevated. For example, comparing aluminum and lead, aluminum was consistently higher on smoke days with percent differences consistently above zero for all years (Figure 3a and b). By contrast, concentrations and percentages of lead were typically the same or lower on smoke days, on average, with only a few exceptions (Figure 3c). These exceptions were driven, however, by very high events, with the largest over 4,000% higher (Figure 3d).
Fig. 3.

Absolute and percentage concentration differences on smoke days compared to non-smoke days at each monitoring station for aluminum (a and b) and lead (c and d). The red diamonds are the average differences across all stations for each year. The red lines are the average median differences across all stations for each year. The black dots are outliers beyond the 5th and 95th percentiles.
b. Chemical associations with distance and total PM2.5 concentrations
In answering Question 3, we found that PM2.5 chemical concentrations generally had significant relationships with distance at the highest concentrations or quantiles but with relatively low goodness of fit (R2). High concentrations (defined as the 80th percentile or above) declined rapidly with distance for a group of chemicals, including aluminum, bromine, calcium, chloride, chlorine, iron, lead, magnesium, nickel, potassium, rubidium, selenium, strontium, total nitrate, vanadium, and zinc. (Supplementary Figure 4a, 4b). The remaining chemicals did not have a relationship significantly different from zero with distance at high concentrations (i.e., the 80th percentile) (Supplementary Table 4). At lower concentrations, declines with distance were either not significant or small compared to those at higher concentrations (Supplementary Table 4).
Similiarly, we observed a significant relationship, but with low goodness of fit, between normalized chemical concentrations and normalized total PM2.5 for most of the chemicals consistently elevated on smoke days (i.e., chemicals consistently higher 8 years or more; Supplementary Table 1). Normalized bromine concentrations, for example, had a significant relationship with normalized total PM2.5, with a low goodness of fit, (Supplementary Figure 5a). Likewise, normalized concentrations of nitrate and ammonium, consistently lower on smoke days, were also significantly related to normalized total PM2.5. For the remaining 27 chemicals (i.e., those not consistently higher or lower most years), slightly under half (12) had a significant relationship with normalized PM2.5, yet with extremely low R2 values (Supplementary Table 1); whereas, the other chemicals, such as sodium (Supplementary Figure 5b), did not have a significant relationship with normalized PM2.5 at all.
c. Chemical profile of destructive fires
Finally, for Question 4, we found distinct chemical profiles for destructive fires, burning structures and vehicles, especially in the production of trace metals (Figure 4 & Figure 5). The June 2008 fires (Figures 4a), burning minimal structures, exhibited relatively high sodium concentrations, followed by calcium and aluminum, the latter commonly elevated chemicals on smoke days (Supplementary Table 1). The Carr Fire (Figure 4b, c) burned a substantial area, but a small number of structures (~1,500). Its chemical profile mostly consisted of iron, calcium, and aluminum, commonly elevated chemicals (Supplementary Table 1), with a smaller fraction of zinc. Zinc was a metal only episodically elevated on smoke days, statistically significant in only four of the 10 years studied (Supplementary Table 1).The most destructive fire, the Camp Fire, produced these same chemicals, but the concentrations of zinc were relatively high compared to the other fires (Figure 4f). This occurred despite similar or lower total PM during the Camp Fire at several of the monitors relative to that measured during the Carr Fire (e.g., the Modesto vs. the Lava Beds monitors).
Fig. 4.
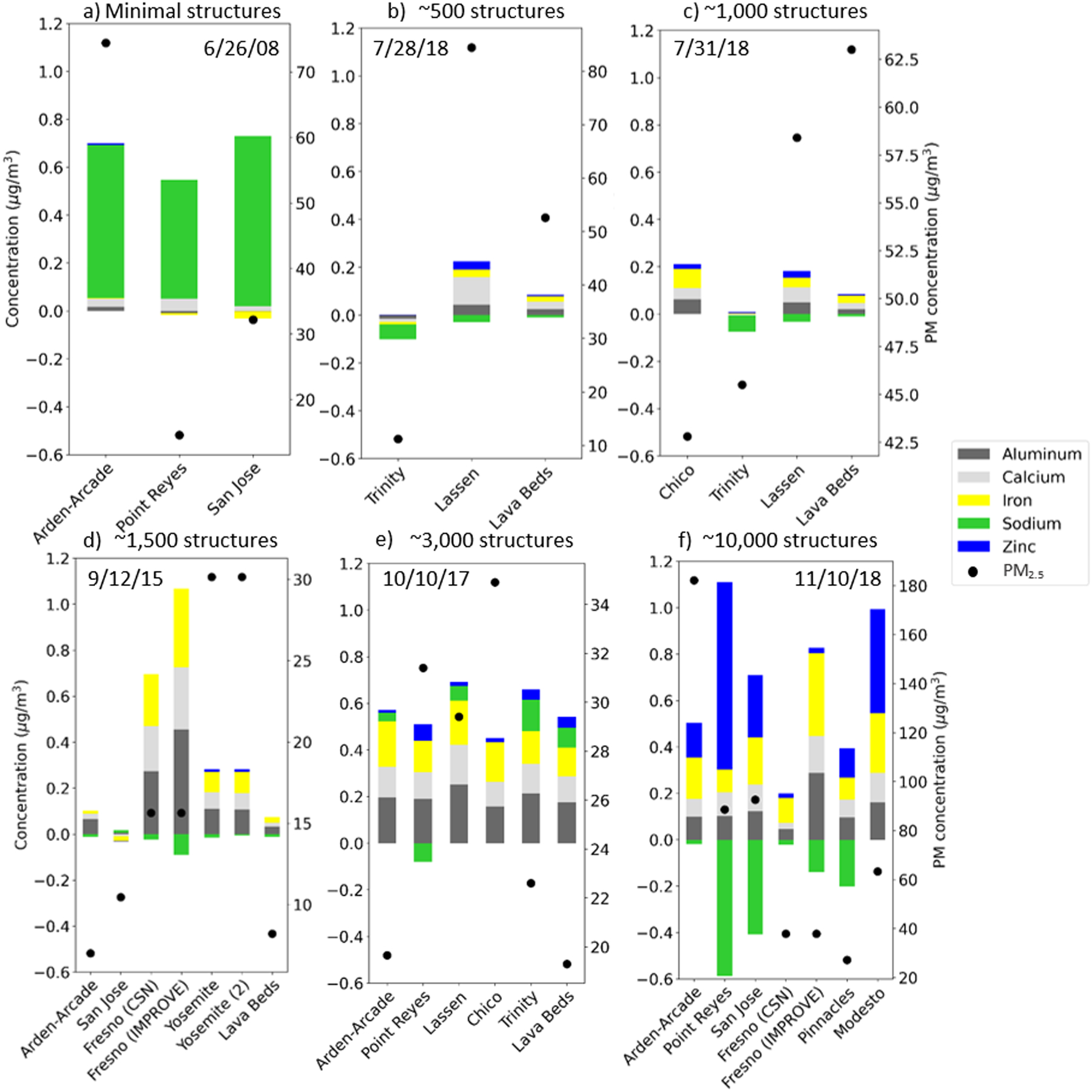
Chemical concentrations (in ug/m3) at smoke-impacted monitoring stations for six fire events in California: June 2008 Fires (a), the Carr Fire (b and c), the 2015 September Fires (Valley and Butte) (d), the October 2017 Fires (Tubbs, Atlas, Nuns, and Redwood Complex) (e), and the Camp Fire (f). Presented are the concentrations measured at each monitor on the fire event date noted in each panel minus the non-smoke day average concentration for each chemical at the corresponding station and year (April 1st through December 31st). Values are negative where the fire event concentration was exceeded by the corresponding non-smoke day average. Structures listed are approximate structures burned at time of measurement. The black dots are total PM2.5 concentration (in ug/m3) at each monitoring station.
Fig. 5.
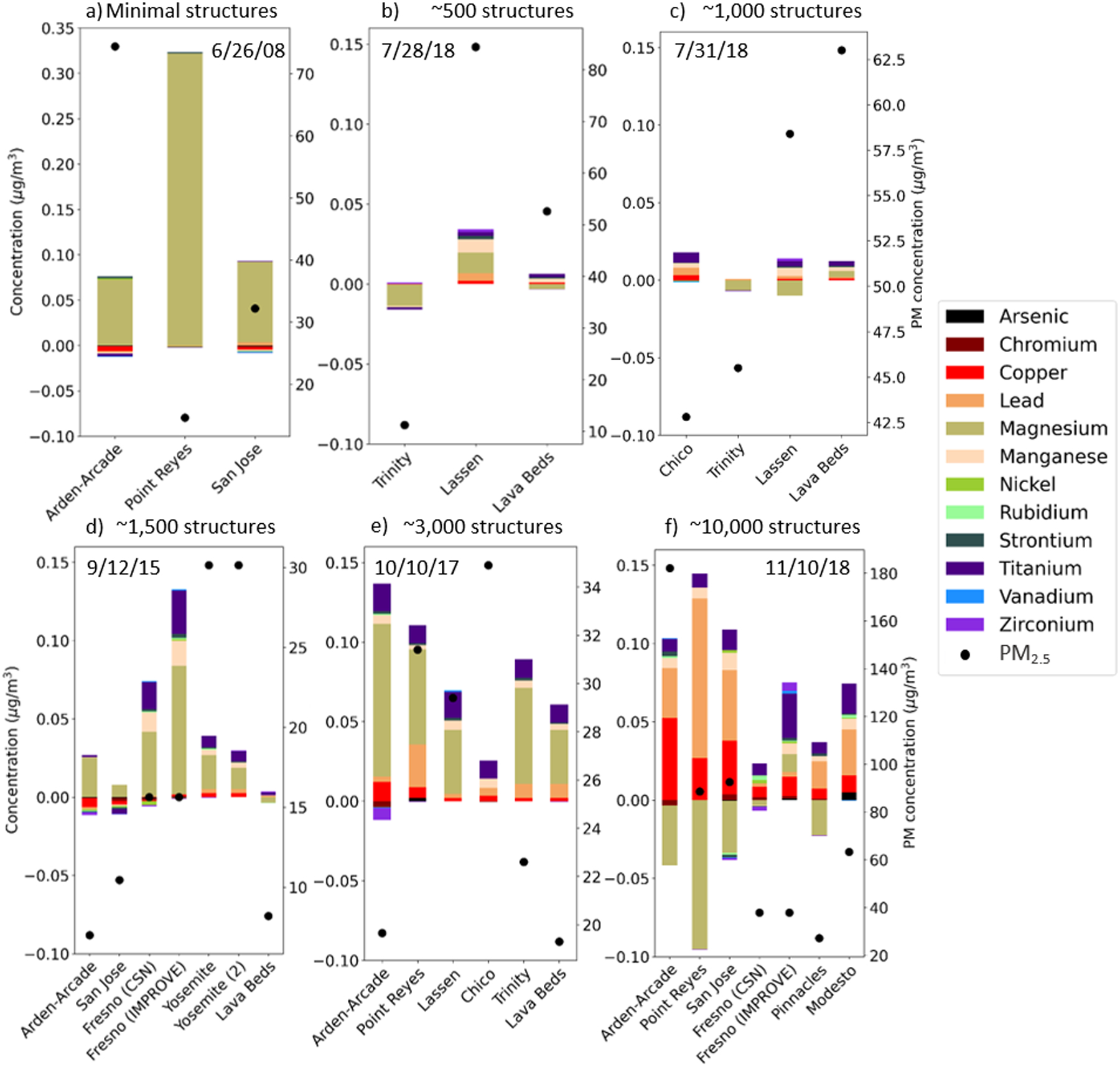
Profile of trace chemical concentrations (in ug/m3; totaling less than 0.5 ug/m3) at smoke-impacted monitoring stations for six fire events in California: June 2008 Fires (a), the Carr Fire (b and c), the 2015 September Fires (Valley and Butte) (d), the October 2017 Fires (Tubbs, Atlas, Nuns, and Redwood Complex) (e), and the Camp Fire (f). Presented are the concentrations measured at each monitor on the fire event date noted in each panel minus the non-smoke day average concentration for each chemical at the corresponding station and year (April 1st through December 31st). Values are negative where the fire event concentration was exceeded by the corresponding non-smoke day average. Structures listed are approximate structures burned at time of measurement. The black dots are total PM2.5 concentration (in ug/m3) at each monitoring station.
The distinct profile of more destructive fires was most evident in the trace chemicals, particularly lead and copper (totaling < 0.5 ug/m3; Figure 5). The 2008 Fire (Figure 5a), the least destructive, produced almost exclusively magnesium, while the Carr Fire (Figure 5b, c), the second least destructive, produced mainly manganese and titanium, with small amounts of lead and copper. The 2015 Fires (Figure 5d), intermediately destructive, produced mostly magnesium and titanium, with small concentrations of lead and copper. Lead and copper were only statistically elevated on smoke vs non-smoke days in one and four of the 10 years studied, respectively—the former in 2018 corresponding the Camp Fire. Indeed, the most destructive fires, the October 2017 and Camp Fires (Figure 5e, f), produced lead and copper in much higher concentrations compared to the other fires. The fires further mobilized additional chemicals such as arsenic, chromium, and nickel. This again was not driven by total PM. The Point Reyes station for example, during the Camp Fire, reported much higher concentrations of lead and copper than the Lassen station during the Carr Fire, despite similar PM values (Figure 5b, f).
As a potential indicator of destructive fires, we employed two chemicals found in destructive fires, lead and copper, divided by potassium, a typical marker of smoke. This ratio was lower during less destructive fires and a higher when more infrastructure was burning (Supplementary Figure 6).
4. Discussion
a. Chemical composition on smoke vs. non-smoke days
Overall, in answer to Question 1, a wide range of chemicals were associated with PM2.5 in smoke plumes from fires, including heavy metals. Our results echo the findings from a recent California state report47 raising this as an issue of concern. Exposure to PM2.5 is already associated with increased respiratory problems, such as asthma, reduced lung function and lung cancer, and cardiovascular disease, especially in pediatric patients.11 Further, there are likely ecosystem effects of mobilization of these chemicals, including the potential for bioaccumulation of metals or the eutrophication of terrestrial and aquatic systems from nutrient deposition.48
In general, there were two broad categories of these PM2.5-associated chemicals: those chemicals, like aluminum, consistently elevated on smoke vs. non-smoke days; and those chemicals, such as lead or copper, episodically elevated only during specific circumstances aligned with structural burning (e.g., houses, vehicles). As implied, this finding is likely influenced primarily by fuel source. Chemicals such as aluminum, calcium, iron, silicon, and potassium were found to be elevated for eight or more years out of the 13-years studied. Most of these chemicals are typically found in soil. These chemicals have been found to be elevated after fires in various mediums such as ash and air measurements.13, 14, 21, 23, 24, 49 Mobilized dust is one hypothesized reason for elevated soil-derived chemicals during wildfire events, since wildfires burn the most area during windy conditions, likely leading to the entrainment of dust into smoke plumes.
By contrast, ammonium and nitrate concentrations were generally lower on smoky days. This is likely because concentrations of ammonium nitrate in PM are typically higher on colder versus warmer days due to enhanced partitioning of ammonium nitrate into the particle phase.50 The enhanced partitioning is favored by lower temperatures and higher relative humidity.50 For example, Hasheminassab, Daher, Saffari, Wang, Ostro and Sioutas50 reported that seasonal contributions of ammonium nitrate to PM averaged over eight sites in California was 8.4 μg/m3 in winter and 3.2 μg/m3 in summer, and Hand, et al.51 also reported wintertime peaks of ammonium nitrate at both urban and rural monitors for nearly every region in the U.S., including California. We limited our analysis to smoky and non-smoky days for the period of April 1st through December 31st, and most of the high values for ammonium and nitrate occurred in November and December (Supplementary Figure 1). These months are relatively colder with higher relative humidity compared to the other months included and had fewer fires (Supplementary Figure 2), a combination likely explaining the generally higher ammonium and nitrate values on non-smoky days.
We also observed some chemicals only episodically elevated, such as lead, copper arsenic, nickel, chromium, and zinc (Supplementary Table 1). These are chemicals elevated for four years or less, especially in 2018. A few studies have shown the presence of remobilized lead from both natural and legacy sources in ash from wildfires in Australia and California.15, 16, 18, 52, 53 Thus, wildfire burning in the WUI or along roadways could remobilize lead deposited before the phase out of leaded gasoline.54, 55 Although this could have contributed to the signal we observed in this study, we found that episodic chemicals were most associated with destructive fires, particularly structural burning. The wildfire season of 2018 burned approximately 24,000 structures, with select destructive fires, like the Camp Fire, producing comparatively high amounts of lead, copper, zinc, and other metals relative to other fires (see part c below for more discussion on this topic).
In answer to Question 2, fires caused high concentration events for most chemicals measured. This was evident in the average values across all stations and all years, but even more so in the individual fire data, with “super-emitting” fires leading to extremely high average values at individual stations (Figure 3; Supplementary Figure 3). Averaging all stations for each chemical for each year, the chemicals that were statistically higher on smoke days in at least eight of the 13 years had median concentrations ranging around 20–50% higher on smoke days compared to non-smoke days (Figure 2). Comparatively, the remaining chemicals had median concentrations ranging from lower on smoke days to around 15% higher (Figure 2). Despite the lower average values, these chemicals had extremely high outliers, much higher than the elevated group, up to 150 times higher on smoke days at a given station and year (Figures 2 and 3; Supplementary Figure 3).
This was certainly the case, for example, at the Point Reyes monitoring station, where smoke days were more than 40 times higher in lead concentrations compared to non-smoke days on average because of the Camp Fire in 2018. This substantially increased the annual averages for lead at that station, located near the Pacific Ocean north of the San Francisco Bay area. The average for lead across all smoke and non-smoke days (from April 1st through December 31st) was almost 10 times higher than the average on non-smoke days alone at Point Reyes (0.00147 vs. 0.000157 ug/m3, respectively). Using our methods, we could not separate PM2.5 from wildfire vs. those from other sources, since non-fire sources often remain present on smoky days. Nonetheless, it has been estimated that almost half of PM2.5 originates from wildfires annually in the western U.S.56 This, plus the high percent increases shown here, suggests that fire can be a relatively large source of PM2.5-associated chemicals, such as lead, annually. This is likely particularly the case for areas like Point Reyes away from more traditional sources, such as utilities or industrial centers. Indeed, our results here indicate an episodic event, a wildfire burning WUI infrastructure, may mobilize a substantial proportion of lead measured in certain locations for the entire year in just a few days.
b. Chemical associations with distance and total PM2.5 concentration
In answering Question 3, we found that chemicals showed only a small decline with distance or no-relationship at all with distance at lower concentrations (below the 80th percentile). Even at higher concentrations where results with distance were generally significant, the relationships had low goodness of fit, meaning other factors, besides our measure of distance, explained the variation in concentrations. This finding may be explained, in part, by other fires, besides the nearest one by distance, influencing a measurement at a monitoring station. Identified fire points were often abundant, raising the prospect of other fires affecting a given measurement. Moreover, there were cases where monitors were covered by multiple smoke plumes, suggesting an influence of multiple fires. Furthermore, chemicals can also undergo atmospheric chemistry alterations downwind of fires, potentially affecting their relationship with distance.57 Despite this weak or lack of a relationship with distance, however, the very highest concentrations of most chemicals did decline rapidly with distance. This has implications for air quality managers and public health professionals since population centers, varying in distance from fire epicenters, would thus be impacted differently.
As for PM, we observed that chemicals almost always elevated (or lower in the case of nitrate and ammonium) on smoke days tended to have significant relationships with normalized total PM2.5, albeit with relatively low goodness of fit. By contrast, chemicals only episodically elevated tended to be more mixed in their relationship with normalized PM2.5, and even if significant, had extremely low R2 values. These results are perhaps unsurprising, given that we conducted the regression analysis on all smoke days, and if chemicals are not affected on most smoke days, then a relationship with normalized PM2.5 on those days is unlikely. Additionally, as in the distance analysis, the generally weak goodness-of-fit values also suggest that factors other than total PM2.5 affected chemical concentrations (Supplementary Table 1). This finding is consistent with the results from the destructive fires, where the amount of lead and other metals did not necessarily scale with total PM2.5 (Figure 5).
c. Burning of infrastructure
Finally, in answer to our fourth question, fires burning infrastructure do appear to have a unique air quality signature. Overall, both fires burning natural fuels and fires burning these fuels plus structures produce certain chemicals, such as magnesium, aluminum, and calcium. However, fires burning infrastructure in the WUI also produce other chemicals, such as copper lead and zinc, in significant quantities generally not observed from fires occurring where fewer structures were burned. Notably, these results are relatively rare. Most studies have examined measurements of metals in ash after fires, or emissions factors of pollutants from certain fuel types, and there are only a handful of papers that have examined concentrations of metals in air quality samples and linked them directly back to infrastructure-destroying wildfires.21–24 One study in Ottawa, Canada, did observe elevated lead and antimony resulting from structure fires, consistent with our findings for lead.58 Further, Sparks and Wagner59 focused on air emissions from the Camp Fire and found elevated levels of manganese, tin, copper, zinc, nickel, and lead, mostly in the PM10 size fraction, in wildfire smoke from that event. We observed similar results, with elevated lead, copper, zinc, and, to a lesser extent, nickel in the PM2.5 size fraction.
Since it can be difficult to link particular fires with downwind effects, as indicated by our distance analysis, an indicator may be helpful for smoke from destructive fires. More data are needed, but the ratio of lead and copper combined over potassium appears to be useful as an indicator, or tracer, of smoke from destructive fires (Supplementary Figure 6). The ratio roughly increases with number of structures burned. Ideally, we would regress this ratio against number of structures/vehicles burned, but we lacked sufficient data on structures/vehicles burned for most fires to perform this analysis. Further research on more individual fires is needed to determine whether this ratio is broadly indicative of destructive fires.
d. Limitations
As in any study, our results have limitations caused by the data and methods we employed. Both the NOAA HMS smoke and fire data and the AQS monitoring system measurements are subject to assumptions. First, the HMS data has a few limitations, consistent with satellite-based fire products. As noted in the methods, the HMS plumes are representative of smoke throughout the entire atmospheric column, not just at the surface. This likely reduced the magnitude of the smoke day versus non-smoke day results since the chemical concentration measurements of a given smoke plume high in the atmosphere would be low at the surface, even though it would be counted as a day with smoke. Thus, if this was accounted for, concentrations on smoke days for most chemicals would have been even more pronounced. Also, satellite products can miss smoke due to cloud coverage, which could mislabel some true smoke days as non-smoke days. Additionally, each smoke plume is labeled with a density of smoke (i.e., low, medium, or high density). We did not preserve this labeling system in our analysis when we treated all smoke plumes as equal. In our study, we considered using organic carbon as an indicator of smoke (along with the smoke plumes), but chose to use smoke plumes alone, as has been done in other studies.37 To attempt to account for these limitations, we compared chemical concentrations with total PM2.5 as a proxy for smoke at the surface. The HMS fire points impose limitations as well since a fire point is any detection of fire on the satellite image. This could be a misdetection or a small fire, which could end up being the closest fire to a station even if that fire is not producing the smoke above the station. This resulted in assumptions during the distance analysis, as discussed above.
The AQS data also have limitations. We chose to focus on PM2.5, but it must be noted that metals have been measured in PM size fractions larger than PM2.5 (i.e. PM2.5–10). Because PM10–2.5 is known to be inhalable, the total metal exposure impacts from wildfire events may be more extensive than those evaluated herein. Notably, the amount of data for each chemical also varied, with the number of non-zero smoke measurements ranging from 180 to 3356 in the chemicals analyzed (Supplementary Table 3). Thus, conclusions for certain chemicals were based on less data than others. However, despite this limitation, the ratio of non-zero smoke days to non-smoke days stayed relatively consistent, ranging from 7.9–12.9% of the available data for each chemical. This suggests our use of the ratio of smoke days to non-smoke days provided a valid comparison across all chemicals.
Finally, across the different chemicals and 13 years, the detection limit at monitoring sites varied due to measurement system changes and varying systems at different stations and is often not clearly reported in the AQS data. Additionally, there are differences in both detection limits and measurements around detection limits between the IMPROVE and CSN monitoring systems.60 Values below zero were set to zero, while positive values below the detection limit were retained and included in the analysis. The retention of low values below the detection limit likely predominantly affects chemicals with consistently low concentration measurements near or below the detection limit and few values (e.g., silver). This, however, likely does not impact the main results of this study because we focused almost entirely on median and the highest, outlier measurements even for trace chemicals.
e. Implications
Overall, this study has implications for both downwind communities and ecosystems. The difference in PM2.5 chemical composition breakdown in smoke observed in this study, specifically an increase in toxic metals due to structural burning, could have potential public health implications. As the WUI continues to grow into fire-prone wildlands,25 and as wildfire activity also increases, populations may suffer exposures to wildfire smoke that contains elevated concentrations of these metals. This has the potential to create additional health risks for fire-affected populations in general, and especially for more vulnerable populations, such as children. To date, studies of ambient PM2.5 exposure have not indicated that any individual chemical is more strongly related to health effects than PM2.5 mass, and less is known about whether combinations of specific chemicals could elicit greater health responses or differential risk11. While some epidemiologic studies have attempted to examine whether there are differences in the toxicity of wildfire-specific PM2.5 compared to ambient PM2.5, this is challenging and has been studied most extensively in experimental settings. In addition, studies of differences in toxicity have typically focused on natural fuel sources, with more limited data from fire burning human-made structures.61, 62 This research gap is significant considering the results of this study.
Also implied by this study is that fires are eroding past air quality gains from lowering anthropogenic emissions (e.g., from smokestacks and tailpipes). For example, we observed elevated lead and other metals from the Camp Fire at multiple sites despite measures to phase lead out from the environment.40 Since our results suggest that these extremely high concentrations decline with distance, these data may be able to be used for setting distance limits for exceptional event designations. Moreover, our findings suggest that emissions inventories may need to account for variable emission factors depending on fire type – for example, destructive versus non-destructive fires.63
Our study also shows that stationary monitors can be a powerful tool in understanding the chemistry of wildfire smoke. There has been an emphasis on more mobile monitors, particularly for PM, ozone, and CO, but the role of stationary monitors should not be overlooked. To better understand the impacts of smoke from different fires, full chemical speciation measurements are essential. These data have been routinely collected at stationary monitors over many years, both increasing the time frame of study and decreasing the need for additional expense. Moreover, wildfire-targeted and mobile studies tend to be limited by the availability of comparable non-fire data, required for demonstrating fire impacts. We suggest that future research could use stationary monitors to characterize PM composition on smoke days across the entire western U.S. to compare to the results found here for California.
Beyond human health, downwind ecosystems are also likely being impacted. Chemicals from fires can move into—and concentrate in—the ecological food chain, and nutrients can cause eutrophication. For example, we observed a high increase in PM2.5 phosphorus on smoke days. Thus, fires could be contributing to observed increases in lake phosphorus concentrations, particularly in the West.64 Likewise, a recent modeling paper by Koplitz, Nolte, Sabo, Clark, Horn, Thomas and Newcomer-Johnson48 found wildland fire emissions contributed up to 30% of total deposition for N and S in some areas, leading to levels of N that may affect tree survival and growth rates.
Overall, we analyzed over a decade of air chemistry data on smoke vs. non-smoke days for the state of California in this study. Certain chemicals are routinely found in higher concentrations in smoke, while are some are only episodically elevated. In particular, we conclude that infrastructure-destroying fires in the WUI can mobilize chemicals, especially metals such as lead, at concentrations far exceeding those typically observed and reaching a couple hundred kilometers or more from the fire center. With climate change increasing fire frequency, combined with the expansion of urban areas in fire-prone regions, it is even more urgent to understand how pollutants in smoke affect human health and downwind ecosystems. These findings emphasize the need for further studies of the health and environmental effects of fires, especially those burning infrastructure.
Supplementary Material
SYNOPSIS.
This study finds elevated concentrations of metals mobilized by wildfires destroying infrastructure in California over the last decade, raising potential health concerns for downwind communities.
Acknowledgments.
The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. We thank Peter Beedlow, Venkatesh Rao, Jason Sacks, and three anonymous reviewers for comments on earlier drafts of this manuscript. This research was supported by the U.S. EPA Air, Climate, and Energy Program within the Office of Research and Development. Katie Boaggio was supported by an Oak Ridge Institute for Science and Education fellowship while conducting this study.
Footnotes
Supporting Information.
Figures and tables labeled “Supplementary” in the text found in the Supporting Information section
Data Availability Statement.
Data analyzed in this study were a re-analysis of existing data, which are openly available at locations cited in the reference section. Further documentation about data processing is available at https://edg.epa.gov/metadata/catalog/main/home.page.
REFERENCES
- 1.Abatzoglou JT; Williams AP, Impact of anthropogenic climate change on wildfire across western US forests. Proceedings of the National Academy of Sciences 2016, 113, (42), 11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abatzoglou JT; Williams AP; Barbero R, Global Emergence of Anthropogenic Climate Change in Fire Weather Indices. Geophysical Research Letters 2019, 46, (1), 326–336. [Google Scholar]
- 3.Williams AP; Abatzoglou JT; Gershunov A; Guzman-Morales J; Bishop DA; Balch JK; Lettenmaier DP, Observed Impacts of Anthropogenic Climate Change on Wildfire in California. Earth’s Future 2019, 7, (8), 892–910. [Google Scholar]
- 4.Jaffe DA; O’Neill SM; Larkin NK; Holder AL; Peterson DL; Halofsky JE; Rappold AG, Wildfire and prescribed burning impacts on air quality in the United States. Journal of the Air & Waste Management Association 2020, 70, (6), 583–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseini S; Urbanski SP; Dixit P; Qi L; Burling IR; Yokelson RJ; Johnson TJ; Shrivastava M; Jung HS; Weise DR; Miller JW; Cocker Iii DR, Laboratory characterization of PM emissions from combustion of wildland biomass fuels. Journal of Geophysical Research: Atmospheres 2013, 118, (17), 9914–9929. [Google Scholar]
- 6.Jaffe DA; Wigder NL, Ozone production from wildfires: A critical review. Atmospheric Environment 2012, 51, 1–10. [Google Scholar]
- 7.McClure CD; Jaffe DA, US particulate matter air quality improves except in wildfire-prone areas. Proceedings of the National Academy of Sciences 2018, 115, (31), 7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson KM; Hsieh YP; Bugna GC, Fire environment effects on particulate matter emission factors in southeastern U.S. pine-grasslands. Atmospheric Environment 2014, 99, 104–111. [Google Scholar]
- 9.Schneising O; Buchwitz M; Reuter M; Bovensmann H; Burrows JP, Severe Californian wildfires in November 2018 observed from space: the carbon monoxide perspective. Atmos. Chem. Phys 2020, 20, (6), 3317–3332. [Google Scholar]
- 10.Suh HH; Bahadori T; Vallarino J; Spengler JD, Criteria air pollutants and toxic air pollutants. Environmental Health Perspectives 2000, 108 Suppl 4, (Suppl 4), 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Environmental Protection Agency, W., DC, Integrated Science Assessment for Particulate Matter (Final Report, Dec. 2019). 2019, EPA/600/R-19/188, 2019. [PubMed]
- 12.U.S. Environmental Protection Agency, W., DC, EPA/600/R-21/197, Comparative Assessment of the Impacts of Prescribed Fire Versus Wildfire (CAIF): A Case Study in the Western U.S. 2021, EPA/600/R-21/197.
- 13.Alexakis DE, Suburban areas in flames: Dispersion of potentially toxic elements from burned vegetation and buildings. Estimation of the associated ecological and human health risk. Environ Research 2020, 183, 109153. [DOI] [PubMed] [Google Scholar]
- 14.Campos I; Abrantes N; Keizer JJ; Vale C; Pereira P, Major and trace elements in soils and ashes of eucalypt and pine forest plantations in Portugal following a wildfire. Science of The Total Environment 2016, 572, 1363–1376. [DOI] [PubMed] [Google Scholar]
- 15.Odigie KO; Flegal AR, Pyrogenic Remobilization of Historic Industrial Lead Depositions. Environmental Science & Technology 2011, 45, (15), 6290–6295. [DOI] [PubMed] [Google Scholar]
- 16.Odigie KO; Flegal AR, Trace metal inventories and lead isotopic composition chronicle a forest fire’s remobilization of industrial contaminants deposited in the angeles national forest. PLoS One 2014, 9, (9), e107835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prichard SJ; O’Neill SM; Eagle P; Andreu AG; Drye B; Dubowy J; Urbanski S; Strand TM, Wildland fire emission factors in North America: synthesis of existing data, measurement needs and management applications. International Journal of Wildland Fire 2020, 29, (2), 132–147. [Google Scholar]
- 18.Wu L; Taylor M; Handley H, Remobilisation of industrial lead depositions in ash during Australian wildfires. Science of The Total Environment 2017, 599–600, 1233–1240. [DOI] [PubMed] [Google Scholar]
- 19.Alexakis DE, Contaminated Land by Wildfire Effect on Ultramafic Soil and Associated Human Health and Ecological Risk. Land 2020, 9, (11). [Google Scholar]
- 20.Panichev N; Mabasa W Fau - Ngobeni, P.; Ngobeni P Fau - Mandiwana, K.; Mandiwana K Fau - Panicheva, S.; Panicheva S, The oxidation of Cr(III) to Cr(VI) in the environment by atmospheric oxygen during the bush fires. Journal of Hazardous Materials 2007, 153, (0304–3894 (Print)). [DOI] [PubMed] [Google Scholar]
- 21.Anttila P; Makkonen U; Hellén H; Kyllönen K; Leppänen S; Saari H; Hakola H, Impact of the open biomass fires in spring and summer of 2006 on the chemical composition of background air in south-eastern Finland. Atmospheric Environment 2008, 42, (26), 6472–6486. [Google Scholar]
- 22.Isley CF; Taylor MP, Atmospheric remobilization of natural and anthropogenic contaminants during wildfires. Environmental Pollution 2020, 267, 115400. [DOI] [PubMed] [Google Scholar]
- 23.Ogulei D; Hopke PK; Zhou L; Patrick Pancras J; Nair N; Ondov JM, Source apportionment of Baltimore aerosol from combined size distribution and chemical composition data. Atmospheric Environment 2006, 40, 396–410. [Google Scholar]
- 24.Qureshi S; Dutkiewicz VA; Khan AR; Swami K; Yang KX; Husain L; Schwab JJ; Demerjian KL, Elemental composition of PM2.5 aerosols in Queens, New York: Solubility and temporal trends. Atmospheric Environment 2006, 40, 238–251. [Google Scholar]
- 25.Radeloff VC; Helmers DP; Kramer HA; Mockrin MH; Alexandre PM; Bar-Massada A; Butsic V; Hawbaker TJ; Martinuzzi S; Syphard AD; Stewart SI, Rapid growth of the US wildland-urban interface raises wildfire risk. Proceedings of the National Academy of Sciences 2018, 115, (13), 3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theobald D; Romme W, Expansion of the US wildland–urban interface. Landscape and Urban Planning 2007, 83, 340–354. [Google Scholar]
- 27.Balch JK; Bradley BA; Abatzoglou JT; Nagy RC; Fusco EJ; Mahood AL, Human-started wildfires expand the fire niche across the United States. Proceedings of the National Academy of Sciences 2017, 114, (11), 2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syphard AD; Radeloff VC; Keeley JE; Hawbaker TJ; Clayton MK; Stewart SI; Hammer RB, Human influence on California fire regimes. Ecological Applications 2007, 17, (5), 1388–1402. [DOI] [PubMed] [Google Scholar]
- 29.Lemieux PM; Ryan JV, Characterization of Air Pollutants Emitted from a Simulated Scrap Tire Fire. Air & Waste 1993, 43, (8), 1106–1115. [Google Scholar]
- 30.Lönnermark A; Blomqvist P, Emissions from an automobile fire. Chemosphere 2006, 62, (7), 1043–1056. [DOI] [PubMed] [Google Scholar]
- 31.Fabian TZ; Borgerson JL; Gandhi PD; Baxter CS; Ross CS; Lockey JE; Dalton JM, Characterization of Firefighter Smoke Exposure. Fire Technology 2014, 50, (4), 993–1019. [Google Scholar]
- 32.CALFIRE, 2017 Incident Archive. 2017.
- 33.CALFIRE, 2018 Incident Archive. 2018.
- 34.Stone S; Sacks J; Lahm P; Clune A; Radonovich L; D’Alessandro M; Wayland M; Mirabelli M, Wildfire Smoke: A Guide for Public Health Officials. 2019.
- 35.Ruminski M; Draxler RR; Kondragunta S; Zeng J, Recent Changes to the Hazard Mapping System. 2006.
- 36.National Oceanic and Atmospheric Administration, O. o. S. a. P. O., Hazard Mapping System.
- 37.Brey S; Ruminski M; Atwood S; Fischer E, Connecting smoke plumes to sources using Hazard Mapping System (HMS) smoke and fire location data over North America. Atmospheric Chemistry and Physics Discussions 2017, 18, 1–29. [Google Scholar]
- 38.Schroeder W; Ruminski M; Csiszar I; Giglio L; Prins E; Schmidt C; Morisette J, Validation analyses of an operational fire monitoring product: The Hazard Mapping System. International Journal of Remote Sensing 2008, 29, (20), 6059–6066. [Google Scholar]
- 39.Larkin NK; Raffuse SM; Huang S; Pavlovic N; Lahm P; Rao V, The Comprehensive Fire Information Reconciled Emissions (CFIRE) inventory: Wildland fire emissions developed for the 2011 and 2014 U.S. National Emissions Inventory. Journal of the Air & Waste Management Association 2020, 70, (11), 1165–1185. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Environmental Protection Agency, W., DC, Integrated Science Assessment (ISA) for Lead (Final Report, Jul 2013). 2013, EPA/600/R-10/075F, 2013.
- 41.Agency, U. E. P., Air Quality System
- 42.USFS, U. S. F. S., Wildfires in all seasons? 2019.
- 43.Edgington ES, Randomization Tests. In International Encyclopedia of Statistical Science, Lovric M, Ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 2011; pp 1182–1183. [Google Scholar]
- 44.Campbell M; Swinscow T, Statistics at Square One Wiley-Blackwell: BMJ Books; 2009, (11th ed.). [Google Scholar]
- 45.Koenker R; Basset G Jr., Regression Quantiles. The Econometric Society 1978, 46, 33–50. [Google Scholar]
- 46.Seabold S; Perktold J, Statsmodels: Econometric and Statistical Modeling with Python. Proc. of the 9th Python in Science Conference 2010. [Google Scholar]
- 47.California Air Resources Board, C., Camp Fire Air Quality Data Analysis. 2021.
- 48.Koplitz SN; Nolte CG; Sabo RD; Clark CM; Horn KJ; Thomas RQ; Newcomer-Johnson TA, The contribution of wildland fire emissions to deposition in the U S: implications for tree growth and survival in the Northwest. Environmental Research Letters 2021, 16, (2), 024028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bytnerowicz A; Arbaugh MJ; Riebau AR; Andersen C, Wildland Fires and Air Pollution. Elsevier; 2009, 8. [Google Scholar]
- 50.Hasheminassab S; Daher N; Saffari A; Wang D; Ostro BD; Sioutas C, Spatial and temporal variability of sources of ambient fine particulate matter (PM2.5) in California. Atmos. Chem. Phys 2014, 14, (22), 12085–12097. [Google Scholar]
- 51.Hand JL; Schichtel BA; Pitchford M; Malm WC; Frank NH, Seasonal composition of remote and urban fine particulate matter in the United States. Journal of Geophysical Research: Atmospheres 2012, 117, (D5). [Google Scholar]
- 52.Kristensen LJ; Taylor MP; Odigie KO; Hibdon SA; Flegal AR, Lead isotopic compositions of ash sourced from Australian bushfires. Environ Pollut 2014, 190, 159–65. [DOI] [PubMed] [Google Scholar]
- 53.Odigie KO; Khanis E; Hibdon SA; Jana P; Araneda A; Urrutia R; Flegal AR, Remobilization of trace elements by forest fire in Patagonia, Chile. Regional Environmental Change 2016, 16, (4), 1089–1096. [Google Scholar]
- 54.Laidlaw M; Zahran S; Mielke H; Taylor M; Filippelli G, Re-suspension of lead contaminated urban soil as a dominant source of atmospheric lead in Birmingham, Chicago, Detroit and Pittsburgh, USA. Atmospheric Environment 2012, 49, 302–310. [Google Scholar]
- 55.Laidlaw MAS; Filippelli GM; Brown S; Paz-Ferreiro J; Reichman SM; Netherway P; Truskewycz A; Ball AS; Mielke HW, Case studies and evidence-based approaches to addressing urban soil lead contamination. Applied Geochemistry 2017, 83, 14–30. [Google Scholar]
- 56.Burke M; Driscoll A; Heft-Neal S; Xue J; Burney J; Wara M, The changing risk and burden of wildfire in the United States. Proceedings of the National Academy of Sciences 2021, 118, (2), e2011048118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chai J; Dibb JE; Anderson BE; Bekker C; Blum DE; Heim E; Jordan CE; Joyce EE; Kaspari JH; Munro H; Walters WW; Hastings MG, Isotopic evidence for dominant secondary production of HONO in near-ground wildfire plumes. Atmos. Chem. Phys 2021, 21, (17), 13077–13098. [Google Scholar]
- 58.Keir JLA; Akhtar US; Matschke DMJ; White PA; Kirkham TL; Chan HM; Blais JM, Polycyclic aromatic hydrocarbon (PAH) and metal contamination of air and surfaces exposed to combustion emissions during emergency fire suppression: Implications for firefighters’ exposures. Science of The Total Environment 2020, 698, 134211. [DOI] [PubMed] [Google Scholar]
- 59.Sparks TL; Wagner J, Composition of particulate matter during a wildfire smoke episode in an urban area. Aerosol Science and Technology 2021, 55, (6), 734–747. [Google Scholar]
- 60.Gorham KA; Raffuse SM; Hyslop NP; White WH, Comparison of recent speciated PM2.5 data from collocated CSN and IMPROVE measurements. Atmospheric Environment 2021, 244, 117977. [Google Scholar]
- 61.Hargrove MM; Kim YH; King C; Wood CE; Gilmour MI; Dye JA; Gavett SH, Smoldering and flaming biomass wood smoke inhibit respiratory responses in mice. Inhalation Toxicology 2019, 31, (6), 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim Yong H; Warren Sarah H; Krantz QT; King C; Jaskot R; Preston William T; George Barbara J; Hays Michael D; Landis Matthew S; Higuchi M; DeMarini David M; Gilmour MI, Mutagenicity and Lung Toxicity of Smoldering vs. Flaming Emissions from Various Biomass Fuels: Implications for Health Effects from Wildland Fires. Environmental Health Perspectives 126, (1), 017011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.U.S. Environmental Protection Agency: Office of Air Quality Planning and Standards, A. Q. A. D., 2017 National Emissions Inventory: January 2021 Updated Release, Technical Support Document. 2021, EPA-454/R-21–001.
- 64.Stoddard JL, Long-Term Changes in Watershed Retention of Nitrogen. In Environmental Chemistry of Lakes and Reservoirs, American Chemical Society: 1994; Vol. 237, pp 223–284. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data analyzed in this study were a re-analysis of existing data, which are openly available at locations cited in the reference section. Further documentation about data processing is available at https://edg.epa.gov/metadata/catalog/main/home.page.


