Abstract
Analogues of 4-phosphoryloxy-N,N-dimethyltryptamine (psilocybin) are being sold on recreational drug markets and developed as potential medications for psychedelic-assisted therapies. Many of these tryptamine-based psilocybin analogues produce psychedelic-like effects in rodents and humans primarily by agonist activity at serotonin 2A receptors (5-HT2A). However, the comprehensive pharmacological target profiles for these compounds compared to psilocybin and its active metabolite 4-hydroxy-N,N-dimethyltryptamine (psilocin) are unknown. The present study determined the receptor binding profiles of various tryptamine-based psychedelics structurally related to psilocybin across a broad range of potential targets. Specifically, we examined tryptamine psychedelics with different 4-position (hydroxy, acetoxy, propionoxy) and N,N-dialkyl (dimethyl, methyl-ethyl, diethyl, methyl-propyl, ethyl-propyl, diisopropyl, methyl-allyl, diallyl) substitutions. Further, the psilocybin analogue 4-propionoxy-N,N-dimethyltryptamine (4-PrO-DMT) was administered to mice in experiments measuring head twitch response (HTR), locomotor activity, and body temperature. Overall, the present pharmacological profile screening data show that the tryptamine psychedelics target multiple serotonin receptors, including serotonin 1A receptors (5-HT1A). 4-Acetoxy and 4-propionoxy analogues of 4-hydroxy compounds displayed somewhat weaker binding affinities but similar target profiles across 5-HT receptors and other identified targets. Additionally, differential binding screen profiles were observed with N,N-dialkyl position variations across several non-5-HT receptor targets (i.e., alpha receptors, dopamine receptors, histamine receptors, and serotonin transporters), which could impact in vivo pharmacological effects of the compounds. In mouse experiments, 4-PrO-DMT displayed dose-related psilocybin-like effects to produce 5-HT2A-mediated HTR (0.3–3 mg/kg s.c.) as well as 5-HT1A-mediated hypothermia and hypolocomotion (3–30 mg/kg s.c.). Lastly, our data support a growing body of evidence that the 5-HT2A-mediated HTR induced by tryptamine psychedelics is attenuated by 5-HT1A receptor agonist activity at high doses in mice.
Keywords: tryptamines, psychedelics, psilocybin analogues, receptor binding, head twitch response
Classic psychedelics, such as 4-phosphoryloxy-N,N-dimethyltryptamine (4-PO-DMT or psilocybin), have traditionally been used by various indigenous groups as religious sacraments to facilitate mystical or spiritual experiences.1,2 Psychedelics are also used recreationally in various non-medical contexts, which can pose public health risks.3−5 More recently, psilocybin given in conjunction with psychotherapy is being investigated for the treatment of depression, obsessive compulsive disorder, chronic pain, substance use disorders, and psychological distress in terminally ill cancer patients.2,6−9
Aside from classic tryptamine-based psychedelics like psilocybin, many new psychoactive substances (NPS) from this drug class have emerged on recreational drug markets, and some of these compounds are used in scientific research.2,10−14 Many of these psilocybin analogues have substitutions at the 4-position (e.g., acetoxy, hydroxy) and various symmetrical or asymmetrical N,N-dialkyl groups (e.g., diethyl or methyl-ethyl).14,15 The psychedelic-like effects in rodent models and in vitro functional activities at serotonin 2 (5-HT2) receptors have been reported for a large number of tryptamines.15−17 However, the comprehensive pharmacological target profiles for many of the newer synthetic tryptamines are unknown, especially how they compare to the natural product psilocybin and its active metabolite 4-hydroxy-N,N-dimethyltryptamine (4-HO-DMT or psilocin).
4-Propionoxy-N,N-dimethyltryptamine (4-PrO-DMT) is an example of an obscure NPS sold as a psychedelic on recreational drug markets with little information available about its pharmacological activity.18 Similar to 4-acetoxy-N,N-dimethyltryptamine (4-AcO-DMT or psilacetin) and psilocybin, it has been postulated that 4-PrO-DMT is a prodrug for psilocin. Anecdotal reports from online sources indicate that consumption of purported 4-PrO-DMT samples induces subjective experiences that are similar to those produced by psilocin, psilocybin, and psilacetin.19,20
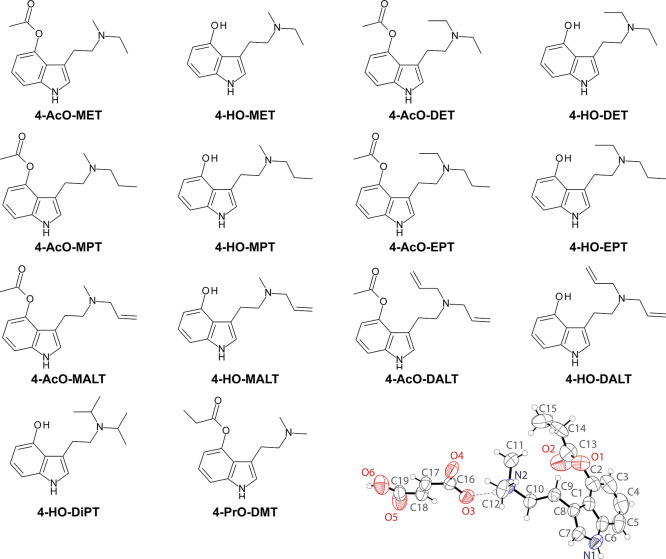
In this paper, we report the comprehensive target binding profiles and 5-HT2 receptor functional activities for several tryptamine psychedelic NPS with variations at the 4-position (hydroxy, acetoxy, propionoxy) and the N,N-dialkyl group (dimethyl, methyl-ethyl, diethyl, methyl-propyl, ethyl-propyl, diisopropyl, methyl-allyl, diallyl) (Figure 1). Further, 4-PrO-DMT was evaluated in mouse studies measuring acute effects of the drug on head twitch response (HTR), locomotor activity, and body temperature. Together the results from these experiments demonstrate structure–activity relationships (SARs) for in vitro pharmacological profiles and inhibition constants (Ki) at identified 5-HT receptors as well as non-5-HT targets of tryptamine psychedelics. Furthermore, the mouse studies demonstrate that 4-PrO-DMT is a psilocybin-like compound comparable to other tryptamine psychedelics.
Figure 1.
Chemical structures of tryptamine psychedelic NPS compounds included in the study along with the crystal structure of 4-PrO-DMT hydrofumarate. The freebase forms of the compounds are shown, though the samples studied were in a protonated state as either fumarate or hydrofumarate salts as outlined in the Methods.
Results and Discussion
Target Profiles of Tryptamine Psychedelics
Compounds were sent for comprehensive target profiling across 50 receptors and other targets of interest.58 The first stage is a “primary competition binding screen” where 10 μM concentrations of each compound are assessed in radioligand binding assays to determine potential targets. “Hits” for various targets were defined by >50% average inhibition of radioligand binding from a single experiment with quadruplicate determinations. The identified targets from the 10 μM screen for each compound are shown in Table 1. Across all compounds, the predominant targets identified were 5-HT receptors. Non-5-HT targets included kappa opioid receptors (KOR), histamine receptors (H1 and H2), alpha receptors (alpha2A, alpha2B, alpha2C), dopamine receptors (D2, D3, D4, D5), the dopamine transporter (DAT), the serotonin transporter (SERT), muscarinic receptors (M2, M3, M4), sigma receptors (sigma 1 and sigma 2), and NMDA glutamate receptors (NR2B subunit). The primary screening data also revealed potential differences between compounds regarding the number of targets identified. For instance, 4-hydroxy-N,N-diisopropyltryptamine (4-HO-DiPT) inhibited binding to fewer 5-HT receptors compared to the other compounds assessed.
Table 1. List of Target and Receptor Hits for Tryptamine Psychedelic NPS in Primary Competition Binding Screensa.
| Compound | Targets identified in competition binding screen at 10 μM |
|---|---|
| 4-PrO-DMT | 5HT1A,B,D,e, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, H1, KOR, NR2B, Sigma 2 |
| 4-HO-MET | 5HT1A,B,D,e, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, H1, SERT, NR2B, Alpha2A |
| 4-AcO-MET | 5HT1A,B,D,e, 5HT2A,B,C, 5HT6, 5HT7a, H1, D4, Alpha2B, Sigma 1 |
| 4-HO-DET | 5HT1A,B,D,e, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, H1, H2, M3, SERT, NR2B, Sigma 2 |
| 4-AcO-DET | 5HT1B,D,e, 5HT2B,C, 5HT6, 5HT7a, Alpha2A,B, NR2B, D2 |
| 4-HO-MPT | 5HT1A,B,D,e, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, H1, D3, Alpha2A, B, NR2B, SERT, Sigma 1 and 2 |
| 4-AcO-MPT | 5HT1B,D, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, H1, Alpha2A,B,C, Sigma 1 |
| 4-HO-EPT | 5HT1A,B,D,e, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, H1, D2, Alpha2A, NR2B, SERT, Sigma 1 and 2 |
| 4-AcO-EPT | 5HT1B, D, e, 5HT2A, B, C, 5HT6, 5HT7a, H1, D3, Alpha2A, B, C, NR2B, Sigma 2 |
| 4-HO-DiPT | 5HT1D, 5HT2A,B, Alpha2A,B, DAT, M4, SERT, Sigma 1 and 2 |
| 4-HO-MALT | 5HT1A,B,D,e, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, Alpha2A, D5, H1,2, SERT, Sigma 1 and 2 |
| 4-AcO-MALT | 5HT1A,D,e, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, Alpha2A,B, H1, Sigma 1 |
| 4-HO-DALT | 5HT1A,B,D,e, 5HT2A,B,C, 5HT5A, 5HT6, 5HT7a, Alpha 2A,B,C, D2,3,4, H1, M4, SERT, Sigma 1 and 2 |
| 4-AcO-DALT | 5HT1A,B,D,e, 5HT2A,B,C, 5HT6, 5HT7a, Alpha 2A,B,C, D2,3, H1, M2, Sigma 2 |
Data represent receptors and targets where compounds exhibited >50% average inhibition at the 10 μM test concentration.
Next, full concentration–effect curves for inhibition of radioligand binding for each compound were generated to determine the affinities (Ki) at each target identified in primary screens.58 Inhibition constants for 5-HT receptors assessed are shown in Table 2, while inhibition constants for the most common non-5-HT receptor targets are listed in Table 3 and Table 4. Many of the compounds displayed affinity for all 5-HT receptors assessed, similar to the findings for psilocin and psilacetin.21−ref24 In general, the receptor binding results show that 4-hydroxy compounds have higher affinity across all 5-HT receptor targets when compared to 4-acetoxy analogues with the same N-alkyl groups. We previously demonstrated that 4-hydroxy and 4-acetoxy analogues of psilocybin display higher affinity for more 5-HT receptor subtypes when compared to their 4-phosphoryloxy counterparts.22 Others have found similar potency trends for agonist activity at 5-HT2 receptors and HTR responses in mice for 4-hydroxy vs 4-acetoxy compounds with the same N,N-dialkyl substitutions.15 The 4-propionoxy analogue, 4-PrO-DMT, also displayed inhibition constants across 5-HT receptors more comparable to psilocin and psilacetin as opposed to psilocybin. These findings suggests that 4-PrO-DMT could be an active prodrug for psilocin, as has been suggested for psilacetin, depending on the speed of enzymatic hydrolysis in vivo.15
Table 2. Inhibition Constants of Tryptamine Psychedelic NPS in Competition Binding Assays for Human 5-HT Receptorsa.
|
Ki (nM) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| drug | 5-HT1A | 5-HT1B | 5-HT1D | 5-ht1e | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT5A | 5-HT6 | 5-HT7a |
| 4-PrO-DMT | 396 | 2,410 | 274 | 229 | 336 | 17 | 228 | 325 | 54 | 73 |
| 4-HO-MET | 135 | 331 | 197 | 161 | 177 | 12 | 164 | 304 | 70 | 60 |
| 4-AcO-MET | 950 | 960 | 667 | 500 | 514 | 17 | 370 | - | 345 | 310 |
| 4-HO-DET | 414 | 2,242 | 585 | 568 | 400 | 73 | 436 | 1,429 | 230 | 826 |
| 4-AcO-DET | - | 3,817 | 445 | 1,162 | - | 53 | 875 | - | 860 | 1,680 |
| 4-HO-MPT | 106 | 224 | 170 | 246 | 114 | 8 | 150 | 664 | 48 | 99 |
| 4-AcO-MPT | - | 9,108 | 1,900 | - | 830 | 22 | 694 | 1,805 | 383 | 220 |
| 4-HO-EPT | 163 | 1,097 | 644 | 591 | 546 | 62 | 1,272 | 1,576 | 284 | 438 |
| 4-AcO-EPT | - | 9,259 | 1,674 | 2,225 | 2,459 | 43 | 967 | - | 1,787 | 321 |
| 4-HO-DiPT | - | - | 1,860 | - | 922 | 85 | - | - | - | - |
| 4-HO-MALT | 402 | 1,500 | 159 | 165 | 357 | 20 | 392 | 492 | 195 | 179 |
| 4-AcO-MALT | 1,141 | - | 699 | 775 | 1,006 | 9 | 1,556 | 1,246 | 241 | 582 |
| 4-HO-DALT | 131 | 978 | 432 | 367 | 452 | 63 | 1,550 | 2,966 | 578 | 454 |
| 4-AcO-DALT | 582 | 2,689 | 2,099 | 2,116 | 958 | 137 | 824 | - | 479 | 406 |
Radioligands used, reference control compound used, and control Ki values for each 5-HT receptor were as follow: 5-HT1A = [3H]WAY100635 vs 8-HO-DPAT (Ki = 0.6–0.7 nM), 5-HT1B = [3H]GR125743 vs ergotamine tartrate (Ki = 4–12 nM), 5-HT1D = [3H]GR125743 vs ergotamine tartrate (Ki = 3–6 nM), 5-ht1e = [3H]5-HT vs 5-HT (Ki = 5–15 nM), 5-HT2A = [3H]ketanserin vs clozapine (Ki = 5–8 nM), 5-HT2B = [3H]LSD vs SB206553 (Ki = 7–12 nM), 5-HT2C = [3H]mesulergine vs ritanserin (Ki = 1–3 nM), 5-HT5A = [3H]LSD vs ergotamine tartrate (Ki = 12–37 nM), 5-HT6 = [3H]LSD vs clozapine (Ki = 7–23 nM), 5-HT7a = [3H]LSD vs clozapine (Ki = 12–36 nM). Control Ki values represent the range of inhibition constants across 3–4 experiments with triplicate determinations. Dash indicates <50% inhibition in the primary radioligand binding screen.
Table 3. Inhibition Constants of Tryptamine Psychedelic NPS in Competition Binding Assays for Non-5-HT Receptor Targetsa.
|
Ki (nM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | H1 | KOR | NR2B | Sigma 1 | Sigma 2 | Alpha2A | Alpha2B | Alpha2C | SERT |
| 4-PrO-DMT | 1,481 | 4,745 | 6,250 | - | 1,349 | - | - | - | - |
| 4-HO-MET | 483 | - | >10,000 | - | - | 1,666 | - | - | 1,830 |
| 4-AcO-MET | 1,720 | - | - | 7,077 | - | - | 1,681 | - | - |
| 4-HO-DET | 1,079 | - | 8,720 | - | 3,026 | - | - | - | 1,800 |
| 4-AcO-DET | - | - | 2,936 | - | - | 1,965 | 1,095 | - | - |
| 4-HO-MPT | 92 | - | 3,658 | 891 | 1,166 | 3,625 | 1,844 | - | 910 |
| 4-AcO-MPT | 493 | - | - | 1,512 | - | 1,300 | 453 | 1,447 | - |
| 4-HO-EPT | 406 | - | 5,947 | 1,400 | 1,773 | 2,073 | - | - | 1,257 |
| 4-AcO-EPT | 1,024 | - | 8,111 | - | 3,497 | 1,135 | 274 | 1,329 | - |
| 4-HO-DiPT | - | - | - | 1,063 | 2,215 | - | - | - | 816 |
| 4-HO-MALT | 815 | - | NT | 1,413 | 2,525 | 535 | - | - | 355 |
| 4-AcO-MALT | 2,603 | - | NT | 3,471 | - | 387 | 1,169 | - | - |
| 4-HO-DALT | 355 | - | - | 1,846 | 1,770 | 2,670 | 2,084 | 2,280 | 657 |
| 4-AcO-DALT | 874 | - | - | - | 4,190 | 482 | 214 | 803 | - |
Radioligands, reference control compound, and control Ki values for each radiolabeled binding site were as follow: H1 = [3H]pyrilamine vs chlorpheniramine maleate (Ki = 2–6 nM), KOR = [3H]U69593 (2007-07-27) vs Salvinorin A (Ki = 2–6 nM), NR2B = [3H]ifenprodil vs ifenprodil (Ki = 2–7 nM), sigma 1 = [3H]pentazocine vs haloperidol (Ki = 12–32 nM), sigma 2 = [3H]DTG vs haloperidol (Ki = 36–61 nM), alpha2A = [3H]rauwolscin vs oxymetazoline HCl (Ki = 5–14 nM), alpha2B = [3H]rauwolscin vs yohimbine (Ki = 6–10 nM), alpha2C = [3H]rauwolscin vs oxymetazoline HCl (Ki = 23–74 nM), SERT = [3H]citalopram vs amitriptyline (Ki = 12–43 nM). Control Ki values represent the range of inhibition constants across 2–3 experiments run in triplicate. NT = not tested. Dash indicates <50% inhibition in the primary radioligand binding screen.
Table 4. Inhibition Constants of Tryptamine Psychedelic NPS in Competition Binding Assays for Non-5-HT Receptor Targets (Continued)a.
|
Ki (nM) |
|||||||
|---|---|---|---|---|---|---|---|
| Drug | DAT | M4 | H2 | D2 | D3 | D4 | D5 |
| 4-PrO-DMT | - | - | - | - | - | - | - |
| 4-HO-MET | - | - | - | - | - | - | - |
| 4-AcO-MET | - | - | - | - | - | 1,371 | - |
| 4-HO-DET | - | - | 9,984 | - | - | - | - |
| 4-AcO-DET | - | - | - | 1,534 | - | - | - |
| 4-HO-MPT | - | - | - | - | 921 | - | - |
| 4-AcO-MPT | - | - | - | - | - | - | - |
| 4-HO-EPT | - | - | - | 3,010 | 985 | - | - |
| 4-AcO-EPT | - | - | - | - | - | - | - |
| 4-HO-DiPT | >10,000 | 1,725 | - | - | - | - | - |
| 4-HO-MALT | - | - | 1,448 | - | - | - | >10,000 |
| 4-AcO-MALT | - | - | - | - | - | - | - |
| 4-HO-DALT | - | 5,280 | - | >10,000 | 578 | 2,256 | - |
| 4-AcO-DALT | - | - | - | 4,522 | 1,907 | - | - |
Radioligands, reference control compound, and control Ki values for each radiolabeled binding site were: DAT = [3H]WIN35428 vs GBR12909 (Ki = 14 nM), M4 = [3H]QNB vs atropine (Ki = 0.2 nM), H2 = [125I]aminopotentidine vs asenapine maleate (Ki = 3 nM), D2 = [3H]N-methylspiperone vs haloperidol (Ki = 15 nM), D3 = [3H] N-methylspiperone vs nemonapride (Ki = 1 nM), D4 = [3H] N-methylspiperone vs nemonapride (Ki = 2 nM), D5 = [3H]SCH23390 vs SKF83566 (Ki = 5 nM). Dash indicates <50% inhibition in the primary radioligand binding screen.
Variations of the N,N-dialkyl constituents of the tested tryptamines had modest effects on the inhibition constants and receptor binding profiles across 5-HT receptors. Most compounds displayed comparable inhibition constants across 5-HT receptor subtypes in the ∼100–1,000 nM range, that were within 10-fold of one another. One exception was the finding that 4-PrO-DMT, 4-hydroxy-N-methyl-N-ethyltryptamine (4-HO-MET), and 4-hydroxy-N-methyl-N-propyltryptamine (4-HO-MPT) had higher inhibition constants at serotonin 6 receptors (5-HT6), which were more than 10-fold greater than the inhibition constants of 4-acetoxy-N,N-diethyltryptamine (4-AcO-DET) and 4-acetoxy-N-ethyl-N-propyltryptamine (4-AcO-EPT) at this site.
Additionally, inhibition constants at serotonin 7a (5-HT7a) for 4-PrO-DMT, 4-HO-MET, and 4-HO-MPT were more than 10-fold greater relative to the affinity of 4-AcO-DET. The present binding results are consistent with known differences in agonist activities (i.e., potency and efficacy) at 5-HT2 receptors and behavioral effects for many of the tested tryptamine analogues in mouse HTR studies.15 The present receptor binding data for 4-HO-DiPT also agree with the findings of others who showed weak serotonin 2C receptor (5-HT2C) agonist potency as well as binding affinity.15,ref24,23
Regarding inhibition constants for non-5-HT receptors and potential sites of action, most of the inhibition constants were >1,000 nM with a few exceptions. 4-HO-MET, 4-HO-MPT, 4-acetoxy-N-methyl-N-propyltryptamine (4-AcO-MPT), 4-HO-EPT, 4-hydroxy-N-methyl-N-allyltryptamine (4-HO-MALT), 4-hydroxy-N,N-diallyltryptamine (4-HO-DALT), and 4-acetoxy-N,N-diallyltryptamine (4-AcO-DALT) all had notable affinity at H1 which was comparable to inhibition constants at some 5-HT receptors including 5-HT2A. This finding is consistent with prior data for psilocin showing competition for receptor binding at H1 of ∼300–700 nM.21,22 4-HO-MALT and 4-acetoxy-N-methyl-N-allyltryptamine (4-AcO-MALT) also had mid nM inhibition constants at alpha2A, but not at other alpha receptors. 4-AcO-MPT and 4-AcO-EPT displayed low to mid nM affinities at alpha2B receptors in contrast to the corresponding 4-hydroxy analogues. 4-AcO-DALT competed for receptor binding at all 3 alpha receptors with inhibition constants from 200–800 nM, in contrast to its 4-hydroxy analogue which displayed weak μM affinities for these sites. At SERT, 4-HO-MPT, 4-HO-DiPT, 4-HO-MALT, and 4-HO-DALT had notable mid to high nM affinities. 4-PrO-DMT was the only compound to compete for binding at KOR, a site of action associated with the unique psychoactive effects of Salvinorin A, setting it apart from related 4-substituted analogues with the N,N-dimethyl moiety.22,24 4-HO-MPT, 4-HO-EPT, and 4-HO-DALT all had mid to high nM affinities for D3. Lastly, several of the compounds displayed inhibition constants for the NR2B subunit of NMDA receptors, both sigma receptors, H2, M4, D2, and D4, but inhibition constants were all >1,000 nM. The present data support the notion that psychedelic tryptamines are mostly serotonergic compounds, but each derivative has its own set of non-5-HT targets that might influence its subjective and physiological effects. The role of various non-5-HT receptors in modulating in vivo pharmacological effects of psychedelic tryptamine analogues is largely unexplored and warrants further investigation.
The present studies are the first to report the comprehensive receptor binding and target profiles for a set of tryptamine psychedelic NPS being used as alternatives to psilocybin in recreational drug markets and being explored as potential new medications, including 4-PrO-DMT.10−13 Partial receptor profiles for 4-HO-DALT, 4-AcO-DALT, 4-HO-DiPT, and 4-HO-MET have been reported previously.ref24,23,25 Prior results generally agree with the receptor binding affinity data reported here, but there are a few discrepancies worth noting. 4-HO-DALT was previously shown to have affinity at SERT of ∼5 μM in contrast to the affinity of ∼700 nM shown here.25 Additionally, another previous study found that 4-HO-MET had an affinity of ∼200 nM at SERT vs an affinity of ∼1.8 μM that we observed.23 Despite some differences, most of the receptor binding affinity data described in the present report are consistent with available SAR trends for the tested psychedelic tryptamines.
Agonist Potencies and Efficacies of Tryptamine Psychedelics at 5-HT2 Receptors
To confirm functional activities at 5-HT2 receptors, 4-PrO-DMT and several other 4-hydroxytryptamines (4-HO-MET, 4-HO-DET, 4-HO-EPT, 4-HO-MPT, 4-HO-MALT, 4-HO-DALT, 4-HO-DiPT) were also tested for agonist activity in vitro relative to the activity of 5-HT using a Gq-calcium mobilization assay.58 Concentration–response curves are shown in Figure S1, while potency and efficacy values are shown in Table S1. All tryptamine psychedelics displayed agonist activity in this assay at 5-HT2A (EC50 = 3–93 nM), 5-HT2B (EC50 = 3–72 nM), and 5-HT2C receptors (EC50 = 50–6,400 nM). All compounds also displayed partial to full agonist efficacies at 5-HT2A (Emax = 93–104%), 5-HT2B (Emax = 71–110%), and 5-HT2C (Emax = 57–91%) receptors. These data are generally consistent with other reports of agonist activities of these tryptamines at 5-HT2 receptors in the Gq-calcium mobilization assay.15 Agonist activity of 4-PrO-DMT has not previously been reported but is consistent with agonist actions of related compounds in this assay.15,22 As predicted by the present primary binding screen and previously reported by others,15,ref24 4-HO-DiPT had weak (EC50 ≈ 6,400 nM) potency and/or partial agonist efficacy (∼72%) at 5-HT2C relative to 5-HT and the other psychedelic tryptamines tested.
One limitation of our receptor screening data is the lack of functional assessments to determine efficacies for the compounds (i.e., agonist, antagonist, or inverse agonist) at the identified non-5-HT2receptors and targets. In addition to the present data showing agonist activities of several of the psychedelic tryptamines at 5-HT2 receptors (Figure S1, Table S1), functional agonist potencies for Gαq-calcium signaling at 5-HT2 receptor subtypes as well as the potencies to induce HTR in mice, have been reported by another group for most of the tested compounds.15 Other studies have examined the binding affinities as well as the potencies and efficacies of a large set of tryptamine psychedelics at 5-HT2A, 5-HT2C, and 5-HT1A receptors.ref24,ref28 However, the functional activities for other signaling pathways linked to 5-HT2A and at other 5-HT receptors and targets are still largely unknown. This study fills an important gap in knowledge by providing a comprehensive evaluation of the potential non-5-HT2A targets for psychedelic tryptamines, and complements the existing data regarding functional activities for compounds at 5-HT2 receptors.15 Future studies should determine the intrinsic efficacies of these tryptamine psychedelics at identified 5-HT and non-5-HT sites of action.
Importantly, ligand induced receptor signaling does not always correlate with receptor binding at 5-HT2 receptors.26,27 At the present time, however, it is unclear which 5-HT2A-linked functional assay(s) are capable of predicting psychedelic-like or potential therapeutic effects of psychedelic compounds. One study reported a positive correlation between potencies for psychedelic induced Gαq-calcium mobilization at rat 5-HT2Ain vitro and potencies for producing HTR in mice,28 while another study found that potencies for β-arrestin 2 recruitment at 5-HT2Ain vitro predicts bioactive doses of phenethylamine psychedelics in humans.29 However, potencies of psychedelics for in vitro phosphoinositide hydrolysis linked to 5-HT2A receptor function do not correlate with their potencies for drug discrimination in rats.30 Rodent studies assessing the role of various signaling pathways in mediating behavioral effects of psychedelics have revealed modest effects of some 5-HT2A receptor-mediated signaling cascades, but the summed findings suggest that multiple signaling pathways could be involved.31−36 In contrast to the enigmatic role of specific signaling pathways, receptor binding data from multiple laboratories show that affinity for [3H]ketanserin-labeled 5-HT2A receptors predicts psychedelic-like effects of drugs in rodents and subjective psychedelic effects in humans.23,37−41 One study found no correlation between HTR potencies of N,N-diallyltryptamines and 5-HT2A binding affinity alone, but found a significant correlation in a multiple regression analysis when also including 5-HT1A affinity.25 Therefore, understanding the complex pharmacology of tryptamine psychedelics will require resolving these discrepancies and clarifying the relationships between in vitro affinity, in vitro functional potency/efficacy, and in vivo effects.
Effects of 4-PrO-DMT in Mice
The recreational use of 4-PrO-DMT has been reported in humans and there is interest in developing related psilocybin analogues as novel medications. However, no previous studies of the pharmacological effects of 4-PrO-DMT in animal models have been conducted. Based on the reported subjective experience in humans, as well as the receptor binding profile and 5-HT2A agonist activity of 4-PrO-DMT shown here, we surmised that the drug would produce psychedelic-like effects akin to psilocybin in vivo. To test this hypothesis, we conducted mouse studies to measure acute dose-related (0.03–30 mg/kg s.c.) effects of 4-PrO-DMT on HTR, temperature change, and locomotor activity over a 30 min testing period.
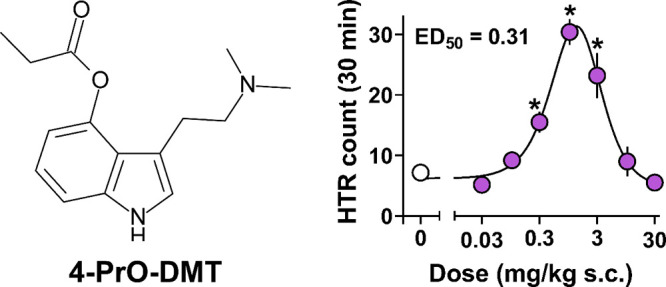
Mice treated with 4-PrO-DMT exhibited increases in HTR frequency at doses from 0.3–3 mg/kg s.c. that were statistically higher than HTR in vehicle controls (Figure 2A; Table S2). 4-PrO-DMT induced a typical inverted U shape dose–response curve for HTR, with an ascending limb potency to produce HTR of 0.31 mg/kg s.c., similar to previous studies examining the effects of psilocin, psilocybin, and psilacetin in mice.15,22,42 The time-course of HTR effects for 4-PrO-DMT was similar to the time-course effects of other related psilocybin analogues with the N,N-dimethyl moiety. Time-course effects across the session are shown in Figure 2B and reveal that the HTR counts peaked from 5–10 min post injection and waned to control levels by the end of the session. At the highest doses tested (10 and 30 mg/kg s.c.), total HTR count was reduced to vehicle control levels, and these mice only displayed HTR activity for the first 5 min of the session.
Figure 2.
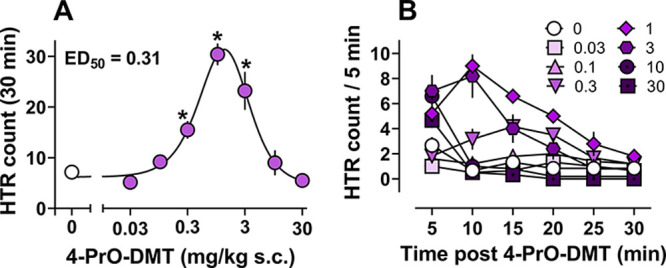
Dose–response of 4-PrO-DMT (0.03–30 mg/kg s.c.) to produce HTR. (A) Dose–response curve and potency for 4-PrO-DMT ) to produce HTR. * = statistically significant values (p < 0.05) compared to vehicle controls (0 mg/kg). (B) Time-course of dose-related HTR produced by 4-PrO-DMT over the 30 min session. Data represent mean ± SEM HTR count for n = 5–6/dose.
The descending limb of the inverted U shaped HTR curve coincided with significant decreases in locomotor activity and body temperature (3–30 mg/kg s.c.) relative to vehicle controls. The dose–response curves for temperature and locomotor effects of 4-PrO-DMT are depicted in Figure 3 with additional information in Table S2. Potencies for temperature change and total distance traveled across the session were 11.7 and 4.8 mg/kg s.c. respectively. Notably, these effects were exhibited at doses 3–10-fold higher than those that producing reliable HTR. Thus, these 5-HT syndrome-like effects are probably most relevant to overdose situations. These results are also consistent with other studies of psilocybin and related analogues showing hypolocomotion and hypothermia at high doses.22,43 Importantly, combinations of drug-induced symptoms like hypothermia and hypolocomotion in rodents have been shown to predict compounds that will produce 5-HT syndrome in humans.44
Figure 3.
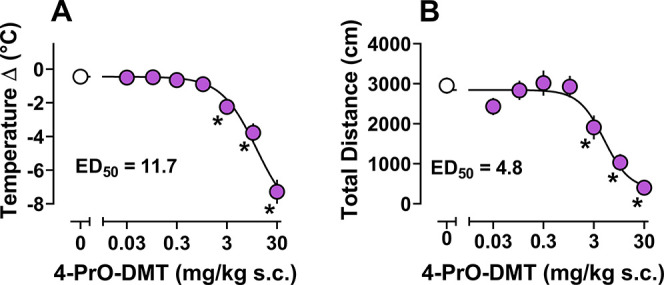
Dose–response of 4-PrO-DMT (0.03–30 mg/kg s.c.) for effects on body temperature and locomotor activity. (A) Dose–response curve and potency for 4-PrO-DMT to change body temperature across the 30 min pre to post session. (B) Dose–response curve and potency for 4-PrO-DMT to decrease distance traveled. * = statistically significant values (p < 0.05) compared to vehicle controls (0 mg/kg). Data represent mean ± SEM for n = 5–6/dose.
Receptor Contributions to Effects of 4-PrO-DMT in Mice
We also sought to determine the receptors mediating HTR, locomotor suppression, and hypothermic effects of 4-PrO-DMT in mice. To do this, we utilized antagonist pretreatment experiments to assess receptor contributions to effects of 4-PrO-DMT. Based on previous studies with psilocybin and related analogues, we surmised that HTR induced by 4-PrO-DMT would be blocked by pretreatment with the 5-HT2A antagonist, MDL100907 (M100907), while pretreatment with the 5-HT1A antagonist, WAY100635, would block locomotor and temperature related effects of the drug.
The effects of 0.01 mg/kg s.c. M100907 pretreatment on HTR induced by 0.6 mg/kg s.c. 4-PrO-DMT given 30 min later are shown in Figure 4A and further summarized in Tables S3 and S4. Results show that the vehicle/4-PrO-DMT condition produced significantly higher HTR counts compared to all other groups, including saline vehicle controls. Blockade of 5-HT2A receptors in the M100907/4-PrO-DMT group prevented the increase in HTR produced by 4-PrO-DMT, demonstrating involvement of this receptor in the HTR. These results are consistent with many studies implicating the 5-HT2A receptor as a critical target in psychedelic-like effects in rodents and psychedelic subjective effects in humans.45−49 M100907 pretreatment did not produce any changes in temperature or locomotor activity over the session (Figure S2A,B).
Figure 4.
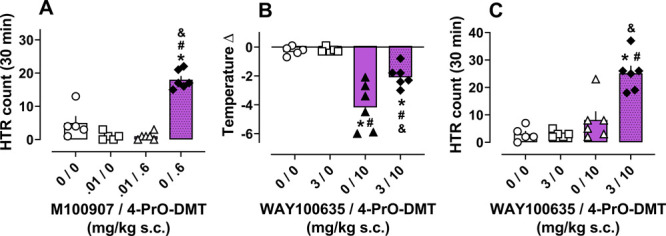
Effects of 5-HT2A and 5-HT1A antagonist pretreatment on HTR and hypothermia induced by 4-PrO-DMT. (A) M100907 pretreatment blocked HTR induced by 4-PrO-DMT. (B - C) WAY100635 pretreatment partially blocked hypothermia produced by 4-PrO-DMT (B) and revealed HTR (C). *, #, or & = statistically significant values (p < 0.05) compared to 0/0, antagonist/0, or 0/4-PrO-DMT conditions, respectively. Data represent mean ± SEM for n = 5–6/dose.
The effects of 3 mg/kg s.c. WAY100635 pretreatment on temperature change, HTR count, and locomotor activity are shown in Figure 4B,C and further summarized in Figure S2C,D, Table S5, and Table S6. Temperature decreases induced by the vehicle/4-PrO-DMT condition were significantly lower than vehicle/vehicle and WAY100635/vehicle conditions. The hypothermic effect was partially, but not fully blocked in the WAY100635/4-PrO-DMT vs vehicle/4-PrO-DMT condition, suggesting a partial role for 5-HT1A receptors in effects of 10 mg/kg s.c. 4-PrO-DMT. This result supports findings from multiple laboratories showing a role of 5-HT1A in hypothermic responses to psilocybin and related analogues at 1–3 mg/kg.22,43 WAY100635 may have only partially reduced hypothermic responses in the present studies due to the high dose of 4-PrO-DMT used (10 mg/kg s.c.). At higher doses, the hypothermic effects of 4-PrO-DMT and other psilocybin analogues may involve non-5-HT1A receptor interactions, such as those observed in the radioligand binding assays shown here and elsewhere.21,22,49 Additionally, vehicle/4-PrO-DMT treatment reduced distance traveled (Figure 4D), but this effect was not statistically significant. The lack of a significant effect could in part be due to the extra habituation to the chambers during the 30 min after WAY100635, but before agonist administration. Habituation to the testing chamber has been previously shown to impact locomotor effects of serotonergic drugs and psychedelics.50,51 Pretreatment with 3 mg/kg s.c. WAY100635 prior to agonist administration appeared to rescue the reduced distance traveled seen in the vehicle/4-PrO-DMT group (Figure S2C), but this effect was not statistically significant, also likely due to habituation differences between dose–response and antagonist study designs.
WAY100635 pretreatment also modulated the HTR activity induced by high dose 4-PrO-DMT (10 mg/kg s.c.) (Figure 4C). Specifically, the vehicle/4-PrO-DMT condition did not produce a HTR that was significant compared to vehicle controls, but the WAY100635/4-PrO-DMT condition displayed robust HTR (∼25 HTR counts/30 min). This intriguing finding suggests that agonist activity of 4-PrO-DMT at 5-HT1A at high doses (3–30 mg/kg s.c.) might be causally related to the descending limb of the HTR dose–response curve. Further, this finding supports an established role of the 5-HT1A receptor in modulating 5-HT2A receptor activity of psychedelics.25,52−54 In particular, previous studies found that 5-HT1A agonists reduce, while antagonists enhance, 5-HT2A mediated effects of psychedelics. It has been shown that HTR produced by psilocybin and related analogues is unchanged or slightly increased after the same WAY100635 pretreatment used here, but these studies used lower doses of WAY100635 and lower doses of test agonists which may account for the discrepancy. One prior study found that the obscure lysergamide, lysergic acid morpholide (LSM-775), only produced the HTR in mice when animals were pretreated with WAY100635.52 Another previous study showed that HTR potencies of N,N-diallyl tryptamines in mice are negatively related to 5-HT1A and positively related to 5-HT2A affinities in a multiple regression analysis.25 These previous findings in combination with the present results further support the regulatory relationship between 5-HT1A and 5-HT2A receptors in psychedelic drug action of tryptamines.
One other potential limitation regarding the WAY100635 antagonist experiments, is the finding that this drug and its major metabolite are only mildly selective at 5-HT1A vs D4 receptors, where they act as agonists rather than as antagonists.55 4-PrO-DMT did not display any affinity for D4 receptors in primary target screening, but it cannot be fully ruled out that agonist activity of WAY100635 at D4 did not contribute to the observed blockade of hypothermic effects of 4-PrO-DMT at high doses. On the other hand, evidence in the literature suggests that D4 agonist activity does not play a major role in body temperature regulation in other rodents,56 suggesting that D4 agonist effects did not play a role in the results from the present WAY100635 experiments.
Conclusion
The present data reveal that 4-acetoxy and 4-propionoxy compounds display similar, albeit weaker, binding affinities across 5-HT receptors compared to their 4-hydroxy analogues having the same N,N-dialkyl groups. In addition to 5-HT receptor affinities, structure-related differences in inhibition constants for non-5-HT receptors and targets (histamine, dopamine, adrenergic, and the serotonin transporter) were observed. 4-PrO-DMT and several 4-hydroxy tryptamine psychedelics also displayed agonist activities in vitro at 5-HT2 receptors. In mice, 4-PrO-DMT produced a dose-dependent increase in HTR, which was similar to previous reports for psilocybin, and consistent with its reported subjective effects as an NPS in human users. Additionally, at higher doses, 4-PrO-DMT produced hypolocomotion and hypothermia corresponding with the descending limb of the HTR dose–response curve. The HTR produced by 4-PrO-DMT was blocked by antagonist pretreatment with the 5-HT2A antagonist M100907, indicating involvement of this receptor. Hypothermic and hypolocomotive effects observed upon administering high doses of 4-PrO-DMT were partially to fully blocked in mice pretreated with 5-HT1A antagonist WAY100635, suggesting a role for this receptor in these effects. WAY100635 pretreatment significantly increased the total number of HTR seen after administering a high dose of 4-PrO-DMT, suggesting that 5-HT1A effects can counteract 5-HT2A mediated HTR under certain conditions. Overall, these results highlight pharmacological target profile differences between tryptamine psychedelics and provide data that will be useful in the continued monitoring of emerging NPS and informed development of psychedelic-assisted therapies.
Methods
Drugs
Commercial samples of 4-AcO-MET hydrofumarate, 4-HO-MET hydrofumarate, 4-AcO-EPT hydrofumarate, 4-HO-EPT hydrofumarate and 4-AcO-MALT hydrofumarate (The Indole Shop, Canada) as well as 4-PrO-DMT hydrofumarate, 4-AcO-MPT hydrofumarate, 4-HO-MPT hydrofumarate, 4-HO-MALT hydrofumarate, 4-AcO-DALT hydrofumarate and 4-HO-DiPT hydrofumarate (ChemLogix, Canada) were purified via recrystallization.57 4-HO-DALT hydrofumarate was synthesized via hydrolysis of 4-AcO-DALT hydrofumarate. Synthetic details are provided in the Supporting Information. (+)M100907 was generously provided by Kenner Rice, Ph.D. and Agnieszka Sulima, Ph.D. (NIDA). WAY100635 maleate was purchased from Cayman Chemical (Item No. 14599). For in vitro studies, compounds were initially dissolved in 100% DMSO at 10 mM and subsequently diluted in assay buffer for experiments. For in vivo studies, drug doses represent the weight of the salt. Drugs were administered to mice at 0.01 mL/g body weight via s.c. injection on the back. Saline was used as the vehicle control for all drugs except M100907, which utilized a 1% DMSO 99% saline vehicle.
Comprehensive receptor binding screen
A comprehensive binding screen was provided by the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH PDSP). The initial primary screening phase tested 10 μM concentrations of test ligand against radioligands for each of the targets listed below:
5-HT1A, 5-HT1B, 5-HT1D, 5-ht1e, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT5A, 5-HT6, 5-HT7a, Alpha1A, Alpha1B, Alpha1D, Alpha2A, Alpha2B, Alpha2C, Beta1, Beta2, Beta3, BZP Rat Brain Site, D1, D2, D3, D4, D5, DAT, DOR, GABAA, H1, H2, H3, H4, KOR, M1, M2, M3, M4, M5, MOR, NET, PBR, SERT, Sigma 1, Sigma 2, AMPA, Kainate (Rat Brain), NMDA, NR2B
In all instances where radioligand binding was reduced by 50% or greater in primary screening, secondary screening was initiated to construct full concentration-effect curves for inhibition of radioligand binding and determine inhibition constants at each target identified.
In addition to the comprehensive binding screen, NIMH PDSP also constructed concentration–response curves for agonist activity of several of the test compounds at 5-HT2 receptors using calcium mobilization assays with results relative to the standard ligand 5-HT.
All details of the competition binding assays and 5-HT2 receptor functional assays run by PDSP can be found online in the assay protocol book.58
Mouse Studies
Details and Design
A single cohort of 12 male C57BL/6J mice (The Jackson Laboratory # 000664) was purchased for in vivo studies and group housed 3 per cage for acclimation to the facility. Mice were single housed after temperature transponder implantation. Mice had ad libitum access to food and water and were housed in a 12/12 light-dark cycle with lights on at 0700 local time. All experiments were approved by the NIDA IRP Animal Care and Use Committee and conducted in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care in Baltimore, MD, USA.
Mice were tested every 1–2 weeks with a minimum of 7 days between treatments to mitigate any influence of tolerance on behavioral study measures.59−61 Dose–response studies for 4-PrO-DMT (0.03–30 mg/kg s.c.) were conducted first, followed by antagonist reversal studies with 0.01 mg/kg s.c. M100907 and 3 mg/kg s.c. WAY100635 pretreatments. Experiments were conducted between 0900–1700 local time during the light phase. Prior to each experiment, all mice were acclimated to the testing room in their home cage for at least 1 h prior to testing.
Temperature Transponder Implantation
All mice were implanted subcutaneously with temperature transponders (14 × 2 mm, model IPTT-300, Bio Medic Data Systems, Inc., Seaford, DE, USA) at least 1 week prior to any experimental sessions as previously described .62,63 The implanted temperature transponders allowed body temperature (° C) of each mouse to be measured non-invasively using a hand-held receiver designed to read signal from the transponders.
Behavioral Testing
Behavioral testing to assess the dose-related effects of 4-PrO-DMT on HTR, temperature change, and locomotor activity was conducted as previously described.22 Briefly, mouse body weight and baseline temperature were recorded, followed by a brief 5 min acclimation to the testing chamber. After brief acclimation, mouse body temperature was again recorded for baseline measurement, mice were injected with various doses of 4-PrO-DMT, and returned to the testing chambers for 30 min. During the session, locomotor activity was measured with photobeam tracking of distance traveled in cm in the horizontal plane. HTR events were recorded simultaneously using an overhead GoPro Hero 7 Black camera (960p resolution at 120 frames per sec) and analyzed after each experiment using a computer software-based scoring platform (Clever Sys Inc. TopScan) validated in our laboratory.64 Open field chambers used for the studies (Coulbourn Instruments, Holliston, MA, USA) were modified with cylindrical inserts and custom flooring useful in aiding software-based detection of HTR events. After each session, mice were returned to their home cage after recording post experiment temperature, and temperature data represent the change from baseline to the end of the session.
Antagonist Studies
Antagonist studies involved s.c. administration of either receptor antagonists or solvent vehicle after a brief 5 min acclimation period to the test chamber. Before returning mice to the testing chamber, baseline temperature was recorded to allow later assessment of any change in temperature induced by receptor antagonists. Locomotor activity during the 30 min after antagonist pretreatment was recorded to detect any potential adverse effects of antagonist treatments. Thirty min after antagonist pretreatment or vehicle, mice received s.c. 4-PrO-DMT or its vehicle, were subjected to temperature testing, then returned to the testing chamber for another 30 min of recording (video and photobeam). The remainder of the antagonist experiments were analogous to the dose–response study conditions.
Data Analyses
GraphPad Prism 9 (La Jolla, CA, USA) was used to conduct all statistical analyses and graphically represent data. Non-linear regression analyses were used to determine the receptor inhibition constants/affinities (Ki) and thepotencies for calcium mobilization (EC50) as well as effects in mice (ED50). HTR potency data was determined using the rising phase of the inverted U dose–response curve. In mouse studies, one-way ANOVA with either Tukey’s or Dunnett’s post hoc tests were used to compare groups to controls. The mean HTR count over the 30 min testing period, temperature change from baseline, and distance traveled in the horizontal plane (cm) were used for the statistical comparisons conducted. For all analyses, the alpha level was set at 0.05. Group sizes, exact p-values for post hoc comparisons, and descriptive statistics for each data set can be found in either the figure legends or the Supporting Information.
Crystallographic Characterization of 4-PrO-DMT Hydrofumarate
Single crystals of 4-PrO-DMT hydrofumarate were obtained from the slow evaporation of an aqueous solution, and data was collected on a Bruker D8 Venture diffractometer. Crystallographic details are provided in the Supporting Information.
Acknowledgments
Our team gratefully acknowledges the NIMH PDSP and the laboratory of Bryan L. Roth, M.D., Ph.D. for providing receptor and other target binding profiles, affinity data, and 5-HT2 receptor functional data (Contract # HHSN-271-2018-00023-C; Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA). Dr. Roth directs the NIMH PDSP at the University of North Carolina at Chapel Hill. We also thank Kenner Rice, Ph.D., and Agnieszka Sulima, Ph.D. (NIDA) for providing M100907 for the present studies.
Glossary
Abbreviations
- 5-HT
Serotonin
- 5-HT1A
Serotonin 1A receptor
- 5-HT1B
Serotonin 1B receptor
- 5-HT1D
Serotonin 1D receptor
- 5-ht1e
Serotonin 1e receptor
- 5-HT2A
Serotonin 2A receptor
- 5-HT2B
Serotonin 2B receptor
- 5-HT2C
Serotonin 2C receptor
- 5-HT3,
Serotonin 3 receptor
- 5-HT5A
Serotonin 5A receptor
- 5-HT6
Serotonin 6 receptor
- 5-HT7a
Serotonin 7a receptor
- Alpha2A
Alpha 2A receptor
- Alpha2B
Alpha 2A receptor
- Alpha2C
Alpha 2C receptor
- DAT
Dopamine transporter
- SERT
Serotonin transporter
- H1
Histamine H1 receptor
- H2
Histamine H1 receptor
- M4
Muscarinic M4 receptor
- D2
Dopamine D2 receptor
- D3
Dopamine D3 receptor
- D4
Dopamine D4 receptor
- D5
Dopamine D5 receptor
- KOR
Kappa opioid receptor
- psilocybin
4-phosphoryloxy-N,N-dimethyltryptamine
- psilocin
4-hydroxy-N,N-dimethyltryptamine
- psilacetin or 4-AcO-DMT
4-acetoxy-N,N-dimethyltryptamine
- 4-PrO-DMT
4-propionoxy-N,N-dimethyltryptamine
- 4-AcO-MET
4-acetoxy-N-methyl-N-ethyltryptamine
- 4-HO-MET
4-hydroxy-N-methyl-N-ethyltryptamine
- 4-AcO-DET
4-acetoxy-N,N-diethyltryptamine
- 4-HO-DET
4-hydroxy-N,N-diethyltryptamine
- 4-AcO-MPT
4-acetoxy-N-methyl-N-propyltryptamine
- 4-HO-MPT
4-hydroxy-N-methyl-N-propyltryptamine
- 4-AcO-EPT
4-acetoxy-N-ethyl-N-propyltryptamine
- 4-HO-EPT
4-hydroxy-N-ethyl-N-propyltryptamine
- 4-AcO-MALT
4-acetoxy-N-methyl-N-allyltryptamine
- 4-HO-MALT
4-hydroxy-N-methyl-N-allyltryptamine
- 4-AcO-DALT
4-acetoxy-N,N-diallyltryptamine
- 4-HO-DALT
4-hydroxy-N,N-diallyltryptamine
- 4-HO-DiPT
4-hydroxy-N,N-diisopropyltryptamine
- M100907
(+)MDL 100907
- WAY100635
WAY-100653
- NIMH PDSP
National Institute of Mental Health Psychoactive Drug Screening Program
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00222.
Figure S1, concentration–response curves for 5-HT2 functional activity; Table S1, potency and efficacy values for 5-HT2 functional activity; Table S2, statistical info for 4-PrO-DMT dose–response mouse experiments; Table S3, descriptive statistics for 4-PrO-DMT and M100907 antagonist experiments; Table S4, ANOVA post test results for 4-PrO-DMT and M100907 antagonist experiments; Table S5, descriptive statistics for 4-PrO-DMT and WAY100635 antagonist experiments; Table S6, ANOVA post test results for 4-PrO-DMT and WAY100635 antagonist experiments; Figure S2, control measures for antagonist studies in mice; Figure S3, crystal structure of 4-PrO-DMT hydrofumarate; Tables S7–S11, analytical characterization of 4-PrO-DMT hydrofumarate (PDF)
Author Contributions
Study design: G.C.G., M.H.B., D.R.M. Chemical synthesis and analysis: D.R.M., J.A.G., D.N.K.P., M.N. Mouse experiments: G.C.G. Manuscript was drafted by G.C.G., critically reviewed by M.H.B., A.R.C., D.R.M., and final version approved by all authors.
The current research was supported in part by collaborative research funding allocated by CaaMTech to University of Massachusetts Dartmouth (D.R.M.). Crystallographic and NMR data reported in Supporting Information were collected on instruments funded by the NSF (CHE-1229339, CHE-1429086). This work was also supported by grant number DA-000522-13 from NIDA Intramural Research Program (IRP) as well as by a collaborative research and development agreement between CaaMTech and NIDA IRP (M.H.B.).
The authors declare the following competing financial interest(s): A.R.C. has an ownership stake in CaaMTech, Inc., owning patent applications concerning new tryptamine compounds, their compositions, formulations, method of use, and their syntheses. No other authors report any competing financial interests related to the data in this publication.
Supplementary Material
References
- Nichols D. E. Psilocybin: from ancient magic to modern medicine. Journal of Antibiotics 2020, 73, 679–686. 10.1038/s41429-020-0311-8. [DOI] [PubMed] [Google Scholar]
- Nichols D. E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H. A.; Wurst M. G.; Daniels R. N. DARK Classics in Chemical Neuroscience: Psilocybin. ACS Chem. Neurosci. 2018, 9, 2438–2447. 10.1021/acschemneuro.8b00186. [DOI] [PubMed] [Google Scholar]
- Carbonaro T. M.; Bradstreet M. P.; Barrett F. S.; MacLean K. A.; Jesse R.; Johnson M. W.; Griffiths R. R. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J. Psychopharmacol. 2016, 30, 1268–1278. 10.1177/0269881116662634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.; Richards W.; Griffiths R. Human hallucinogen research: guidelines for safety. J. Psychopharmacol. 2008, 22, 603–620. 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E.; Johnson M. W.; Nichols C. D. Psychedelics as Medicines: An Emerging New Paradigm. Clin. Pharmacol. Ther. 2017, 101, 209–219. 10.1002/cpt.557. [DOI] [PubMed] [Google Scholar]
- Schindler E. A. D. Psychedelics as preventive treatment in headache and chronic pain disorders. Neuropharmacology 2022, 215, 109166. 10.1016/j.neuropharm.2022.109166. [DOI] [PubMed] [Google Scholar]
- Nutt D.; Carhart-Harris R. The Current Status of Psychedelics in Psychiatry. JAMA Psychiatry 2021, 78, 121–122. 10.1001/jamapsychiatry.2020.2171. [DOI] [PubMed] [Google Scholar]
- Johnson M. W. Classic Psychedelics in Addiction Treatment: The Case for Psilocybin in Tobacco Smoking Cessation. Curr. Top. Behav. Neurosci. 2022, 56, 213. 10.1007/7854_2022_327. [DOI] [PubMed] [Google Scholar]
- Palamar J. J.; Barratt M. J.; Ferris J. A.; Winstock A. R. Correlates of new psychoactive substance use among a self-selected sample of nightclub attendees in the United States. Am. J. Addict. 2016, 25, 400–407. 10.1111/ajad.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani V.; Corkery J. M.; Guirguis A.; Napoletano F.; Arillotta D.; Zangani C.; Vento A.; Schifano F. Psychonauts’ psychedelics: A systematic, multilingual, web-crawling exercise. Eur. Neuropsychopharmacol. 2021, 49, 69–92. 10.1016/j.euroneuro.2021.03.006. [DOI] [PubMed] [Google Scholar]
- Mallaroni P.; Mason N. L.; Vinckenbosch F. R. J.; Ramaekers J. G. The use patterns of novel psychedelics: experiential fingerprints of substituted phenethylamines, tryptamines and lysergamides. Psychopharmacology (Berlin) 2022, 239, 1783–1796. 10.1007/s00213-022-06142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar J. J.; Acosta P. A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines. Hum. Psychopharmacol. 2020, 35, e2719 10.1002/hup.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin A. T.; Shulgin A.. TiHKAL: The Continuation; Transform Press, 1997. [Google Scholar]
- Klein A. K.; Chatha M.; Laskowski L. J.; Anderson E. I.; Brandt S. D.; Chapman S. J.; McCorvy J. D.; Halberstadt A. L. Investigation of the Structure-Activity Relationships of Psilocybin Analogues. ACS Pharmacol. Transl. Sci. 2021, 4, 533–542. 10.1021/acsptsci.0c00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M. B.; Dolan S. B.; Forster M. J. Locomotor and discriminative stimulus effects of four novel hallucinogens in rodents. Behavioural Pharmacol. 2017, 28, 375. 10.1097/FBP.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M. B.; Hoch A.; Carbonaro T. M. Discriminative Stimulus Effects of Substituted Tryptamines in Rats. ACS Pharmacol. Transl. Sci. 2021, 4, 467–471. 10.1021/acsptsci.0c00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction . New psychoactive substances: global markets, glocal threats and the COVID-19 pandemic: an update from the EU early warning system, December 2020. 10.2810/921262 [DOI]
- The Small & Handy 4-Pro-DMT Thread, June 2019. www.bluelight.org/xf/threads/the-small-handy-4-pro-dmt-thread.875366/.
- Calm and Relaxing Trip: An Experience with 4-PrO-DMT & Cannabis, March 2021. www.erowid.org/exp/115276.
- Roth B. L.National Institute of Mental Health Psychoactive Drug Screening Program (NIMH PDSP): Assay Protocol Book Version III, March 2018. https://pdsp.unc.edu/pdspweb/content/UNC-CH%20Protocol%20Book.pdf.
- Halberstadt A. L.; Geyer M. A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatfelter G. C.; Pottie E.; Partilla J. S.; Sherwood A. M.; Kaylo K.; Pham D. N. K.; Naeem M.; Sammeta V. R.; DeBoer S.; Golen J. A.; Hulley E. B.; Stove C. P.; Chadeayne A. R.; Manke D. R.; Baumann M. H. Structure–Activity Relationships for Psilocybin, Baeocystin, Aeruginascin, and Related Analogues to Produce Pharmacological Effects in Mice. ACS Pharmacol. Translational Sci. 2022, 5, 1181. 10.1021/acsptsci.2c00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozell L. B; Eshleman A. J.; Swanson T. L; Bloom S. H; Wolfrum K. M; Schmachtenberg J. L; Olson R. J; Janowsky A. J.; Abbas A. I Pharmacologic activity of substituted tryptamines at 5-HT(2A)R, 5-HT(2C)R, 5-HT(1A)R, and SERT. J. Pharmacol. Exp. Ther. 2023, 10.1124/jpet.122.001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A.; Moning O. D.; Hoener M. C.; Liechti M. E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. European Neuropsychopharmacology 2016, 26, 1327–1337. 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Hernández-Alvarado R. B.; Madariaga-Mazón A.; Ortega A.; Martinez-Mayorga K. DARK Classics in Chemical Neuroscience: Salvinorin A. ACS Chem. Neurosci. 2020, 11, 3979–3992. 10.1021/acschemneuro.0c00608. [DOI] [PubMed] [Google Scholar]
- Klein L. M.; Cozzi N. V.; Daley P. F.; Brandt S. D.; Halberstadt A. L. Receptor binding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamine analogs. Neuropharmacology 2018, 142, 231–239. 10.1016/j.neuropharm.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough B. E.; Landavazo A.; Decker A. M.; Partilla J. S.; Baumann M. H.; Rothman R. B. Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology 2014, 231, 4135–4144. 10.1007/s00213-014-3557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B. L.; Choudhary M. S.; Khan N.; Uluer A. Z. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J. Pharmacol. Exp. Ther. 1997, 280, 576–583. [PubMed] [Google Scholar]
- Egan C.; Grinde E.; Dupre A.; Roth B. L.; Hake M.; Teitler M.; Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse 2000, 35, 144–150. . [DOI] [PubMed] [Google Scholar]
- Nichols D. E.; Sassano M. F.; Halberstadt A. L.; Klein L. M.; Brandt S. D.; Elliott S. P.; Fiedler W. J. N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem. Neurosci. 2015, 6, 1165–1175. 10.1021/cn500292d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie E.; Cannaert A.; Stove C. P. In vitro structure-activity relationship determination of 30 psychedelic new psychoactive substances by means of β-arrestin 2 recruitment to the serotonin 2A receptor. Arch. Toxicol. 2020, 94, 3449–3460. 10.1007/s00204-020-02836-w. [DOI] [PubMed] [Google Scholar]
- Rabin R. A.; Regina M.; Doat M.; Winter J. C. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol., Biochem. Behav. 2002, 72, 29–37. 10.1016/S0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Schmid C. L.; Raehal K. M.; Bohn L. M. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 1079–1084. 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. L.; Bohn L. M. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ß-arrestin2/Src/Akt signaling complex in vivo. J. Neurosci. 2010, 30, 13513–13524. 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz R. M.; Nadkarni V.; Means C. R.; Pogorelov V. M.; Chiu Y.-T.; Roth B. L.; Wetsel W. C. LSD-stimulated behaviors in mice require β-arrestin 2 but not β-arrestin 1. Sci. Rep. 2021, 11, 17690. 10.1038/s41598-021-96736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Zhu H.; Gao H.; Tian X.; Tan B.; Su R. Gs signaling pathway distinguishes hallucinogenic and nonhallucinogenic 5-HT2AR agonists induced head twitch response in mice. Biochem. Biophys. Res. Commun. 2022, 598, 20–25. 10.1016/j.bbrc.2022.01.113. [DOI] [PubMed] [Google Scholar]
- López-Giménez J. F.; González-Maeso J. Hallucinogens and Serotonin 5-HT(2A) Receptor-Mediated Signaling Pathways. Curr. Top. Behav. Neurosci. 2017, 36, 45–73. 10.1007/7854_2017_478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C. E. Serotonergic Psychedelics: Experimental Approaches for Assessing Mechanisms of Action. Handb. Exp. Pharmacol. 2018, 252, 227–260. 10.1007/164_2018_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titeler M.; Lyon R. A.; Glennon R. A. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology 1988, 94, 213–216. 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Luethi D.; Liechti M. E. Monoamine Transporter and Receptor Interaction Profiles in Vitro Predict Reported Human Doses of Novel Psychoactive Stimulants and Psychedelics. Int. J. Neuropsychopharmacol. 2018, 21, 926–931. 10.1093/ijnp/pyy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore J. S.; Decker A. M.; Sulima A.; Rice K. C.; Partilla J. S.; Blough B. E.; Baumann M. H. Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats. Neuropharmacology 2018, 142, 240–250. 10.1016/j.neuropharm.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon R. A.; Titeler M.; McKenney J. D. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984, 35, 2505–2511. 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Glennon R. A.; Titeler M.; Young R. Structure-activity relationships and mechanism of action of hallucinogenic agents based on drug discrimination and radioligand binding studies. Psychopharmacol. Bull. 1986, 22, 953–958. [PubMed] [Google Scholar]
- Sherwood A. M.; Halberstadt A. L.; Klein A. K.; McCorvy J. D.; Kaylo K. W.; Kargbo R. B.; Meisenheimer P. Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin. J. Nat. Prod. 2020, 83, 461–467. 10.1021/acs.jnatprod.9b01061. [DOI] [PubMed] [Google Scholar]
- Erkizia-Santamaría I.; Alles-Pascual R.; Horrillo I.; Meana J. J.; Ortega J. E. Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed. Pharmacother. 2022, 154, 113612. 10.1016/j.biopha.2022.113612. [DOI] [PubMed] [Google Scholar]
- Haberzettl R.; Bert B.; Fink H.; Fox M. A. Animal models of the serotonin syndrome: A systematic review. Behavioural Brain Res. 2013, 256, 328–345. 10.1016/j.bbr.2013.08.045. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Vollenweider-Scherpenhuyzen M. F.; Bäbler A.; Vogel H.; Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Kometer M.; Schmidt A.; Jäncke L.; Vollenweider F. X. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J. Neurosci. 2013, 33, 10544–10551. 10.1523/JNEUROSCI.3007-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen M. K.; Fisher P. M.; Burmester D.; Dyssegaard A.; Stenbæk D. S.; Kristiansen S.; Johansen S. S.; Lehel S.; Linnet K.; Svarer C.; Erritzoe D.; Ozenne B.; Knudsen G. M. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 2019, 44, 1328–1334. 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J.; Weisstaub N. V.; Zhou M.; Chan P.; Ivic L.; Ang R.; Lira A.; Bradley-Moore M.; Ge Y.; Zhou Q.; Sealfon S. C.; Gingrich J. A. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Koedood L.; Powell S. B.; Geyer M. A. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 2011, 25, 1548–1561. 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Buell M. R.; Price D. L.; Geyer M. A. Differences in the locomotor-activating effects of indirect serotonin agonists in habituated and non-habituated rats. Pharmacol., Biochem. Behav. 2012, 102, 88–94. 10.1016/j.pbb.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. Effect of Hallucinogens on Unconditioned Behavior. Curr. Top. Behav. Neurosci. 2016, 36, 159–199. 10.1007/7854_2016_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. D.; Kavanagh P. V.; Twamley B.; Westphal F.; Elliott S. P.; Wallach J.; Stratford A.; Klein L. M.; McCorvy J. D.; Nichols D. E.; Halberstadt A. L. Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test Anal. 2018, 10, 310–322. 10.1002/dta.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny T.; Preller K. H.; Kraehenmann R.; Vollenweider F. X. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur. Neuropsychopharmacol. 2016, 26, 756–766. 10.1016/j.euroneuro.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Darmani N. A.; Martin B. R.; Pandey U.; Glennon R. A. Do functional relationships exist between 5-HT1A and 5-HT2 receptors?. Pharmacol., Biochem. Behav. 1990, 36, 901–906. 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Chemel B. R.; Roth B. L.; Armbruster B.; Watts V. J.; Nichols D. E. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology 2006, 188, 244–251. 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Chaperon F.; Tricklebank M. D.; Unger L.; Neijt H. C. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology 2003, 44, 1047–1053. 10.1016/S0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Pham D. N. K.; Chadeayne A. R.; Golen J. A.; Manke D. R. Psilacetin derivatives: fumarate salts of the meth-yl-ethyl, meth-yl-allyl and diallyl variants of the psilocin prodrug. Acta Crystallogr. E Crystallogr. Commun. 2021, 77, 101–106. 10.1107/S2056989021000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C. E.; Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012, 4, 556–576. 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani N. A.; Martin B. R.; Glennon R. A. Withdrawal from chronic treatment with (±)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur. J. Pharmacol. 1990, 186, 115–118. 10.1016/0014-2999(90)94066-7. [DOI] [PubMed] [Google Scholar]
- de la Fuente Revenga M.; Jaster A. M.; McGinn J.; Silva G.; Saha S.; González-Maeso J. Tolerance and Cross-Tolerance among Psychedelic and Nonpsychedelic 5-HT(2A) Receptor Agonists in Mice. ACS Chem. Neurosci. 2022, 13, 2436–2448. 10.1021/acschemneuro.2c00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin D.; McCorvy J. D.; Glatfelter G. C.; Luethi D.; Szöllősi D.; Ljubišić T.; Kavanagh P. V.; Dowling G.; Holy M.; Jaentsch K.; Walther D.; Brandt S. D.; Stockner T.; Baumann M. H.; Halberstadt A. L.; Sitte H. H. (2-Aminopropyl)benzo[β]thiophenes (APBTs) are novel monoamine transporter ligands that lack stimulant effects but display psychedelic-like activity in mice. Neuropsychopharmacology 2022, 47, 914–923. 10.1038/s41386-021-01221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatfelter G. C.; Partilla J. S.; Baumann M. H. Structure-activity relationships for 5F-MDMB-PICA and its 5F-pentylindole analogs to induce cannabinoid-like effects in mice. Neuropsychopharmacology 2022, 47, 924–932. 10.1038/s41386-021-01227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatfelter G. C.; Chojnacki M. R.; McGriff S. A.; Wang T.; Baumann M. H. Automated Computer Software Assessment of 5-Hydroxytryptamine 2A Receptor-Mediated Head Twitch Responses from Video Recordings of Mice. ACS Pharmacol. Translational Sci. 2022, 5, 321. 10.1021/acsptsci.1c00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.