Abstract
Background
Colorectal cancer (CRC), has a link between obesity, especially visceral fat. The body roundness index (BRI) can more accurately assess body fat and visceral fat levels. It is, however, unknown whether BRI is associated with CRC risk.
Methods
53,766 participants were enrolled from the National Health and Nutrition Examination Survey (NHANES). Analysing the corelation between BRI and CRC risk was performed using logistic regression. Stratified analyses revealed the association based on the population type. Receiver operating characteristic curve (ROC) was performed for predicting CRC risk using different anthropometric indices.
Results
The risk of CRC mounting apparently with elevated BRI for participants with CRC compared to normal participants (P-trend < 0.001). The association persisted even after adjusting for all covariates (P-trend = 0.017). In stratified analyses, CRC risk increased with increasing BRI, especially among those who were inactive (OR (95% CI): Q3 3.761 (2.139, 6.610), P < 0.05, Q4 5.972 (3.347, 8.470), P < 0.01), overweight (OR (95% CI): Q3 2.573 (1.012, 7.431), P < 0.05, Q4 3.318 (1.221, 9.020), P < 0.05) or obese (OR (95% CI): Q3 3.889 (1.829, 8.266), P < 0.001, Q4 4.920 (2.349, 10.308), P < 0.001). ROC curve showed that BRI had a better ability in forecasting the risk of CRC than other anthropometric indices such as body weight etc. (all P < 0.05).
Conclusions
CRC risk and BRI have a positive and significant relationship, particularly in inactive participants with BMI ≥ 25 kg/m2. It is hoped that these results will raise awareness of the importance of reducing visceral fat deposition.
Keywords: Body roundness index, Abdominal obesity, Visceral fat, Colorectal cancer, Anthropometric index
Introduction
Colorectal cancer (CRC), whose prevalence ranked third and mortality ranked second among all cancers [1]. Many countries, including China, are experiencing a rise in CRC incidence, and new cases are occurring at an increasingly young age [2, 3]. The occurrence and development of CRC is a long-term, multistage process, and the environment, dietary habits, lifestyle, and genetics are all influential factors in the development of CRC [4]. This means that early detection and exclusion of CRC risk factors and early preventive colonoscopy can effectively reduce the incidence of CRC.
A significant risk factor for CRC is being obese, particularly abdominal obesity (visceral fat). There is a higher risk of CRC in obese individuals [5]. Excess adipose tissue, especially visceral fat in obese individuals, induces several pathophysiological processes associated with CRC, such as insulin resistance, chronic tissue inflammation, and oxidative stress [6]. Adipose tissue also secretes several cytokines and adipokines that affect intestinal immune system homeostasis, directly contributing to CRC progression and development [7, 8]. Computed tomography (CT) and magnetic resonance imaging (MRI) are effective for measuring accurate visceral fat, but the time consumption, high cost, and limited accessibility limit their application in everyday life. Bioelectrical impedance analysis is an easy-to-use, cost-effective, non-invasive technique to measure the body adiposity content [9]. Anthropometric indices, since they are non-invasive, cheap, and convenient [10], can be considered for population health screening and early risk finding. Therefore, anthropometric indices are commonly used to assess body fat in most studies.
Body roundness index (BRI) is a tool for measuring body fat and visceral fat, first introduced in 2013 [11]. As a result of its ability to reflect height-related body roundness (abdominal obesity) and provide an estimate of %body fat and %visceral fat that is more accurate, the BRI may be more useful than other anthropometric indices [11]. There is evidence that BRI is closely related to many human diseases, cardiovascular diseases [12–14], diabetes [15], and metabolic syndrome [16], for example. However, BRI and cancer have been little studied. There have only been a few studies linking BRI to liver and endometrial cancers [17, 18].
Based on 53,766 individuals’ data from the National Health and Nutrition Examination Survey (NHANES), the purpose of this study is to examine the correlation between BRI and CRC risk and provide ideas and strategies for early detection of CRC.
Materials and methods
Study participants and research design
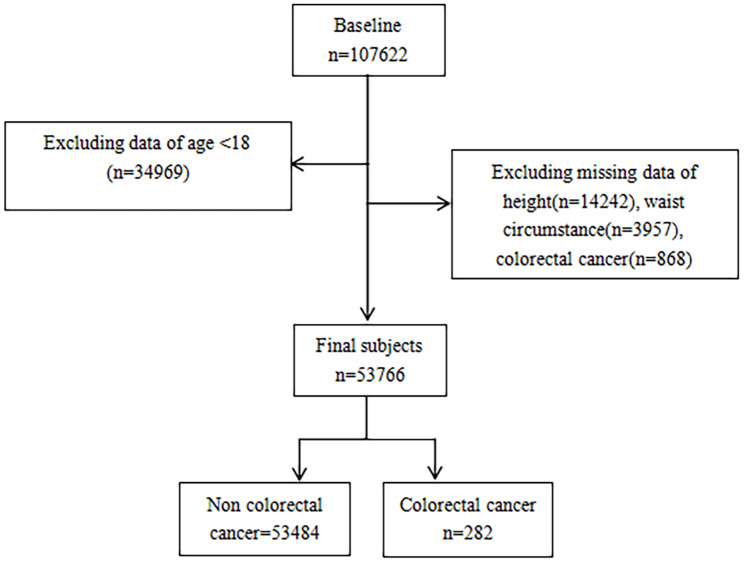
An annual study of the health of the American population, the NHANES is conducted in the United States, and all data are directly available through an online website. All participants provided written informed consent. For this analysis, the inclusion criteria were all participants from 1999 to 2020, and participants whose age < 18 years old and lacking important clinical data were excluded. 53,766 participants were ultimately enrolled (Fig. 1).
Fig. 1.
Flow chart of the study
Data collection and measurements
Data were obtained from the NHANES database website. The database contains demographic data, including race, gender, age, etc.; dietary data; anthropometric data containing waist circumference (WC), weight, body mass index (BMI), etc.; laboratory data including albumin, glycated haemoglobin (HbA1c), etc.; and questionnaire data, including drinking habits, smoking habits, medical conditions, etc. A team of trained researchers collected basic information about participants. Regularly trained health technicians gathered blood and urine samples and anthropometric measurements at the mobile examination centre (MEC) and sent them to designated testing centres for uniform testing.
Definition of variables
The presence or absence of CRC was collected by self-reports. The NHANES questionnaire contained information on age, gender, race, smoking, alcohol consumption, and exercise. Participants were classified according to their alcohol intake habits in the past 12 months: non-drinkers, occasional drinkers, and regular drinkers. There were three categories of smokers, namely non-smokers, occasional smokers, and regular smokers. We defined exercisers as those who engaged in moderate-intensity exercise, fitness, or recreational activities during the week. Taking weight (kg) and height (m) squared and dividing them together gives BMI. WC (cm) divided by height (cm) results in waist-to-height ratio (WHtR). BRI was computed by 364.2-365.5*(1-[WC(m)/2π]2/[0.5*height(m)]2) ½ [11].
Statistical analysis
Univariate analysis and comparison of individual baseline characteristics between participants were performed first. Percentages (%) were conducted to express categorical variables, and differences were analysed by chi-square tests. For continuous variables, Kolmogorov-Smirnov test was performed, and reported values were represented by mean and standard deviation. The skewness distribution variable was expressed as median and quartile spacing, comparing groups using Mann-Whitney U tests. An analysis of multivariable logistic regression models was conducted, for exploring the relationship between BRI quartiles with cancer risk. Throughout the analysis, the lowest quartile was regarded as a reference. The ultimate model was adjusted for race, gender, age, fasting blood glucose (FBG), exercise, total cholesterol (TC), smoking habits, drinking habits, triglycerides (TG), albumin, and low-density lipoprotein cholesterol (LDL-C). Odds ratios (OR) and 95% confidence intervals (CI) conducted to present the final results. Stratified analysis was conducted for gender, exercise, and BMI, to further explore the correlation between BRI quartiles with CRC risk consequently. Finally, after adjustment for covariates, receiver operating characteristic (ROC) curve was analysed. Moreover, the area under the receiver operating characteristic curve (AUC) of BRI with weight, BMI, WC, and WHtR were compared. IBM SPSS Statistics Version 26.0 and MedCalc Statistical Software Version 19.7.4 were used to analyse the data. There was statistical significance if the P value was less than 0.05 on a two-sided basis.
Results
Characteristics of study participants at the baseline
At the baseline level of the study, 53,766 individuals were enrolled, 26,106 males and 27,760 females. Demographic and clinical data about the study participants were displayed in Table 1, which were based on the diagnosis of CRC. Different from the non-CRC group, the CRC group was generally older, exercised less, and had significantly higher levels of TG (1.61 vs. 1.41), LDL-C (2.90 vs. 2.85), and FBG (6.22 vs. 5.61) (all P ≤ 0.001). BMI (28.65 vs. 27.80), WC (101.95 vs. 97.20), weight (77.65 vs. 67.70), WHtR (0.57 vs.0.53) and BRI (5.67 vs. 4.13), all of these are a higher level for the CRC group (all P < 0.001).
Table 1.
Baseline characteristics
| Variables | Total | Non colorectal cancer | Colorectal cancer | P value |
|---|---|---|---|---|
| Number | 53,766 | 53,484 | 282 | |
| Age, years | 47.00 (31.00) | 47.00 (31.00) | 72.00 (19.00) | < 0.001 |
| Men, n (%) | 26,106 (48.6) | 25,960 (48.5) | 146 (51.8) | 0.001 |
| Race, n (%) | < 0.001 | |||
| Mexican American | 9430 (17.5) | 9399 (17.6) | 31 (11.0) | |
| Other Hispanic | 4553 (8.5) | 4537 (8.5) | 16 (5.7) | |
| Non-Hispanic White | 22,996 (42.8) | 22,840 (42.7) | 156 (55.3) | |
| Non-Hispanic Black | 11,713 (21.8) | 11,651 (21.8) | 62 (22.0) | |
| Other | 5074 (9.4) | 5057 (9.5) | 17 (6.0) | |
| Smoking habits, n (%) | < 0.001 | |||
| No | 12,699(53.8) | 12,578 (53.7) | 121(75.6) | |
| Occasional | 2067 (8.8) | 2061 (8.8) | 6 (3.8) | |
| Regular | 8822 (37.4) | 8789 (37.5) | 33 (20.6) | |
| Drinking habits, n (%) | < 0.001 | |||
| No | 9289 (22.0) | 9189 (21.9) | 100 (41.7) | |
| Occasional | 17,874 (42.4) | 17,801 (42.4) | 73 (30.4) | |
| Regular | 15,036 (35.6) | 14,969 (35.7) | 67 (27.9) | |
| Exercise, n (%) | < 0.001 | |||
| Yes | 22,535 (43.7) | 22,426 (43.8) | 109 (38.9) | |
| No | 28,984 (56.3) | 28,813 (56.2) | 171 (61.1) | |
| TC, mmol/L | 4.89 (1.50) | 4.89 (1.50) | 4.81 (1.81) | < 0.001 |
| TG, mmol/L | 1.41 (1.84) | 1.41 (1.84) | 1.61 (1.875) | 0.001 |
| HDL-C, mmol/L | 1.29 (0.51) | 1.31 (0.51) | 1.29 (0.54) | < 0.001 |
| LDL-C, mmol/L | 2.85 (1.21) | 2.85 (1.21) | 2.90 (1.32) | < 0.001 |
| FBG, mmol/L | 5.65 (1.39) | 5.61 (1.34) | 6.22 (2.17) | < 0.001 |
| HbA1c, (%) | 5.50 (0.60) | 5.50 (0.60) | 5.70 (0.90) | 0.001 |
| Albumin, g/dL | 41.00 (17.00) | 41.00 (16.00) | 40.00 (38.60) | < 0.001 |
| BMI, kg/m2 | 27.80 (8.15) | 27.80 (8.15) | 28.65 (7.93) | < 0.001 |
| WC, cm | 97.20 (21.30) | 97.20 (21.38) | 101.95 (22.45) | < 0.001 |
| Height, cm | 166.80 (14.60) | 166.80 (14.60) | 166.65 (14.90) | 0.004 |
| WHtR | 0.53(0.08) | 0.53(0.07) | 0.57(0.08) | < 0.001 |
| BRI | 4.14 (2.92) | 4.13 (2.89) | 5.67 (2.77) | < 0.001 |
Notes: Data are expressed as median (IQR) for skewed variables and percentage (%) for categorical variables
Abbreviations: BMI: body mass index; BRI: body roundness index; FBG: fasting blood glucose; HbA1c: glycosylated haemoglobin; HDL-C: high-density lipoprotein cholesterol; IQR: interquartile range; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride; WC: waist circumstance; WHtR: waist-to-height ratio
Associations between BRI and the risk of CRC
Followed by a correlation analysis between BRI and CRC risk using progressively adjusted multivariate regression, the first quartile interval of BRI as a control (Table 2). In general, BRI was positively correlated with CRC risk in Model 1, and CRC risk increased with increasing BRI (P-trend < 0.001). In Model 2, BRI was statistically significant only when it was at high values (OR (95% CI): Q3 1.757 (1.153, 2.678), Q4 1.891 (1.247, 2.867), P-trend < 0.001). Then, all confounders were adjusted for Model 3, BRI remained correlated with the risk of CRC (OR (95% CI): Q3 2.663 (1.002, 7.076), Q4 2.771 (1.025, 7.171), P-trend = 0.017). This suggests an association between BRI and CRC risk.
Table 2.
Association between BRI quartiles and the risk of CRC in participants
| Variables | BRI Quartiles | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| Model 1 | |||||
| OR (95% CI) | 1 | 1.669(1.061, 2.626) | 3.250(2.157, 4.898) | 3.519(2.344, 5.284) | |
| P value | 0.027 | < 0.001 | < 0.001 | < 0.001 | |
| Model 2 | |||||
| OR (95% CI) | 1 | 1.065(0.673, 1.647) | 1.757(1.153, 2.678) | 1.891(1.247, 2.867) | |
| P value | 0.789 | 0.003 | 0.009 | < 0.001 | |
| Model 3 | |||||
| OR (95% CI) | 1 | 1.881(0.681, 5.195) | 2.663(1.002, 7.076) | 2.771(1.025, 7.171) | |
| P value | 0.223 | 0.045 | 0.048 | 0.017 | |
Model 1: unadjusted; Model 2: adjusted for age, race, sex; Model 3: further adjusted for exercise, drinking habits, smoking habits, albumin, FBG, TG, TC and LDL-C based on Model 2
Abbreviations: BMI: body mass index; BRI: body roundness index; CI, confidence interval; FBG: fasting blood glucose; LDL-C: low-density lipoprotein cholesterol; OR, odds ratio; TC: total cholesterol; TG: triglyceride
Stratification analysis based on gender, exercise, and BMI
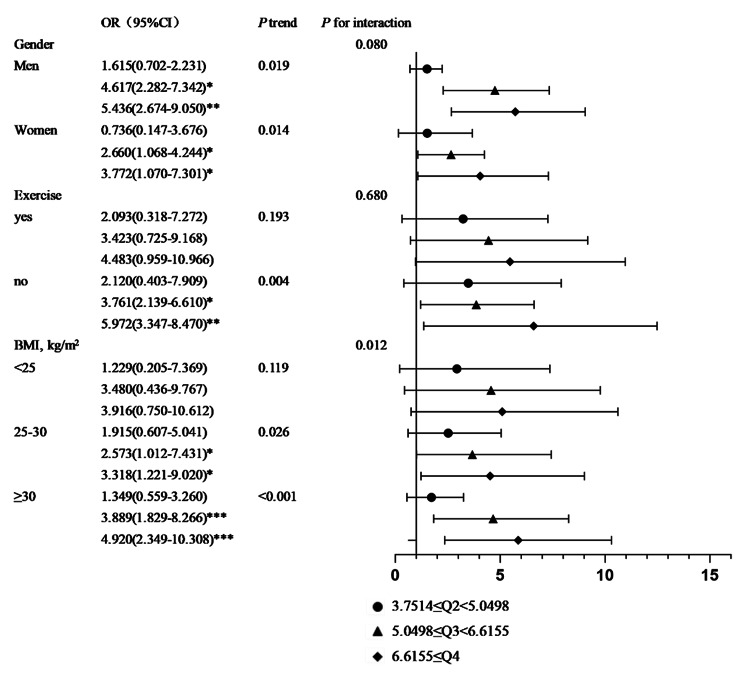
By using a stratified analysis in conjunction with Model 3, the stability of the correlation between BRI and CRC risk was further confirmed in different populations (Fig. 2). As a result of stratification by gender, BRI was significantly correlated with CRC risk in the third and fourth quartile intervals in men (OR (95% CI): Q3 4.617 (2.282, 7.342), Q4 5.436 (2.674, 9.050), P-trend = 0.019). The same was true among women (OR (95% CI): Q3 2.660 (1.068, 4.244), Q4 3.772 (1.070, 7.301), P-trend = 0.014). In stratified analyses by whether or not they exercised, a significant mounting in association between CRC with BRI in those who did not exercise (OR (95% CI): Q3 3.761 (2.139, 6.610), Q4 5.972 (3.347, 8.470), P-trend = 0.004)). Stratified by BMI (< 25 kg/m2; 25–30 kg/m2; ≥30 kg/m2), the most common anthropometric index in daily life, the risk of CRC elevated with mounting BRI, especially in participants with a BMI of 25–30 kg/m2 (OR (95% CI): Q3 2.573 (1.012, 7.431), Q4 3.318 (1.221, 9.020), P-trend = 0.026) and BMI greater than 30 kg/m2 (OR (95% CI): Q3 3.889 (1.829, 8.266), Q4 4.920 (2.349, 10.308), P-trend < 0.001).
Fig. 2.
Association between BRI and CRC risk in different participants. Notes: *, P value < 0.05; **, P value < 0.01; ***, P value < 0.001
BRI can better predict CRC risk
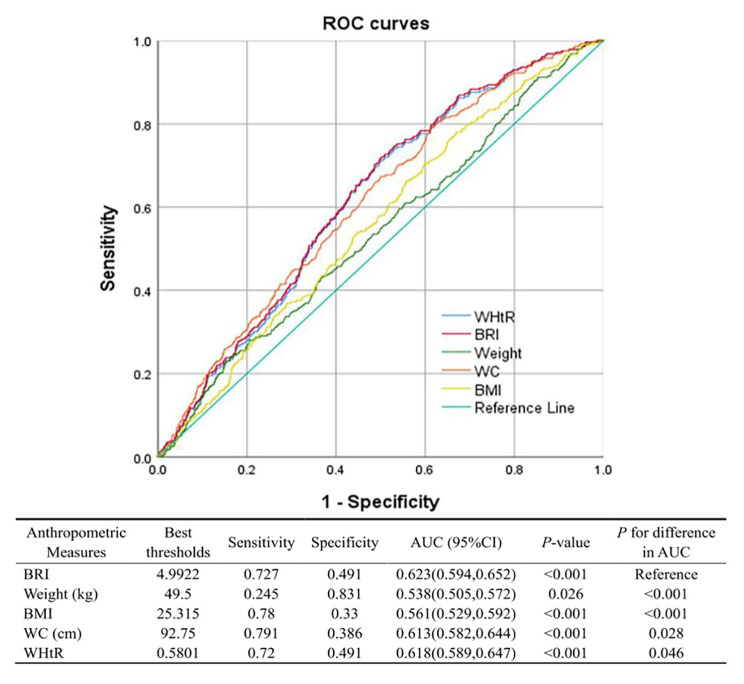
BMI and weight are the most extensively used anthropometric indices, while commonly used indices reflecting central obesity are WC and WHtR. Therefore, AUC was calculated to contrast the accuracy of BRI and different anthropometric indices in forecasting CRC risk (Fig. 3). In our study, BRI performed better than all five anthropometric indices (AUC (95% CI): 0.623 (0.594–0.652), all P < 0.05), The optimal cut-off value for BRI was 4.9922.
Fig. 3.
ROC curves of different anthropometric indices for the prediction of CRC risk
Discussion
The BRI level was link to elevated risk for CRC in this study. The correlation existed persistently after full adjustment for confounders such as age, gender, exercise, smoking, alcohol consumption, albumin, FBG, TC, TG, and LDL-C, suggesting that CRC might be influenced by BRI. In addition, individuals with a higher BRI showed a higher risk of CRC, according to the stratified analysis, particularly among those who were inactive, overweight, and obese. Next, sensitivity analysis revealed that BRI had a better ability to predict CRC risk than weight, BMI, WC, and WHtR. BRI has rarely been linked to cancer, and this relationship is examined specifically for the first time in this study. Detecting CRC at an early stage is crucial to treating it. Exercise and weight control to reduce fat, especially visceral fat, can help prevent the disease and reduce its risk, benefiting the population even more.
Obesity, as a chronic inflammatory condition, is closely related to cardiovascular diseases, diabetes, cancer, and metabolic syndrome in humans [19–21]. Increased abnormal adipose tissue, especially visceral adipose tissue, can cause an imbalance in body homeostasis by secreting inflammatory factors and lipid factors, leading to pathological processes such as oxidative stress, insulin resistance, and altered cell proliferation [22–25]. The traditional anthropometric indices used to measure the degree of obesity are weight, BMI, WC, WHtR, etc. Weight and BMI are the most widely used but have limitations that cannot assess the specific distribution of body fat. WC and WHtR are mostly used to assess central obesity but cannot distinguish visceral fat from subcutaneous fat, which is more harmful. Therefore, new anthropometric indices have been continuously developed and applied. The standard for measuring visceral fat distribution is MRI and CT. However, they are time-consuming, expensive, and very inconvenient to use daily. BRI, as a promising index which reflects height-related body roundness and can be devoted to estimate visceral fat levels more accurately than traditional anthropometric indices. BRI has been reported to be an effective predictor of diabetes (including prediabetes), cardiometabolic diseases, and metabolic syndrome [13–16, 26]. Increased BRI is significantly related to cardiovascular disease outcomes and even all-cause mortality [12]. In addition, studies predicting cancer risk by anthropometric indices have also been reported [27–29]. In conclusion, as a marker reflecting body fat and visceral fat levels, BRI is expected to be an emerging health screening indicator and disease risk predictor.
Currently, cancer-related deaths from CRC rank second worldwide, and epidemiological studies prove that obesity links with cancer [30–32]. A total of 1.347 million new cases of CRC worldwide in 2012 were attributed to obesity in 5.8% of men and 7.0% of women [33]. Similarly, a study has inspected a summary of more than 1,000 epidemiological research programs and discovered that subjects with a BMI > 25 kg/m2 had a 1.2-1.5-fold elevated risk of CRC and that those with a BMI > 30 kg/m2 had a 1.5-1.8-fold elevated risk of CRC [30]. In addition, it was also shown that obesity was strongly connected with early-onset CRC with an approximate relative risk (RR) of 1.54 (1.01–2.35) [34]. Being overweight and obese in childhood and adolescence can lead to an increased incidence of early CRC [35, 36]. CRC usually develops from adenoma or polyp carcinoma, and a recently published meta-analysis indicated that excessive BMI was an isolated risk of adenomas and polyps and reported that the risk for colorectal adenoma (CRA) consistently increased by 42-44% in the overweight population and obesity population using controls with BMI < 25 kg/m2 [37]. Therefore, early and regular CRC screening is necessary for the obese population. Despite this, studies have shown that CRC screening compliance is significantly lower in obese populations than in normal populations. Research shows that obesity affects the likelihood of CRC screening discontinuation [38]. A Dutch survey also found that obesity was related to an obvious reduction in screening for CRC (OR: 0.8, 0.66–0.97) [39, 40]. This is unclear for why obese people have a lower adherence to CRC screening recommendations. Therefore, anthropometric indices to predict the risk of disease are a convenient, simple, quick, and more acceptable means.
BRI is investigated in the study as a potential risk factor for CRC and additional evidence is provided to validate the corelation between visceral fat with CRC risk as evaluated by BRI. BRI has better predictive ability than traditional anthropometric indices. Compared with screening methods such as colonoscopy, MRI, and CT, it saves time and effort. Simultaneously, this study should cause broader concern in clinical practice about visceral adipose distribution instead of just concentrating on reducing disease risk by losing weight. This study encourages the experimentation with BRI as an indicator to assess CRC risk in daily clinical practice.
Comparisons with other studies and what does the current work add to the existing knowledge
The harm of visceral fat has attracted increasing attention, and in line with this study, most previous research on the visceral fat and CRC have confirmed the apparent correlation between them. Research has shown that CRC risk increases apparently with mounting WC and WHtR and shows a dose-dependent response [41, 42]. Compared with BMI, WC can better predict the occurrence of advanced CRC and more accurately reflect visceral fat levels with stronger carcinogenic effects [43]. Additionally, Dong et al. revealed that higher WHtR was related to elevated CRC risk (RR 95% CI: 1.39 (1.25, 1.53)) [44]. A study by Abal L., et al. also found that CRC was positively correlated with WC as well as between CRC and CRA [45]. However, some studies have also found no association between visceral fat assessed by WC and CRC [46], possibly as the result of an insufficient samples and single sample source. A link between visceral fat index, BRI, and CRC is explored in this study, filling the gap between BRI and CRC research currently available. Simultaneously, in this study, BRI showed superior predictive power to anthropometric measures including weight, BMI, WC, and WHtR. A significant link was observed between BRI and CRC risk even after correcting for confounding factors, indicating that BRI has the potential to be a predictor of CRC risk.
Study strengths and limitations
Until now, no study has explored the relationship between BRI with CRC risk in the general population, and the large sample size is another advantage of this study. However, a few limitations also exist. Firstly, because the cross-sectional nature limited its ability to elucidate cause and effect, it is not sufficient to suggest that BRI are causally related to CRC risks. Secondly, the sample data were obtained from the NHANES database in the United States, and the regional and ethnic differences that exist limit the generalization and applicability of the findings to some extent. Thirdly, this study only analysed whether BRI correlates with overall CRC risk and did not evaluate the corelation among BRI to the progress, treatment, and prognosis of CRC. Finally, because the classification of the presence or absence of colorectal cancer was based on self-report, some recall bias should be considered. Future more detailed and in-depth studies are needed to further validate the relationship in follow-up.
Conclusion
This study indicates that increased BRI is significantly linked to CRC risk, especially in inactive, overweight, and obese individuals. Compared with traditional anthropometric indicators, for example body weight, BMI, WC, and WHtR, BRI is more powerful in predicting the risk of CRC, and it is more convenient and efficient than the measurement of visceral fat by MRI or CT. This study aims to raise public awareness of the corelation between visceral fat represented by BRI and cancer and to encourage people to actively reduce body weight through diet control, regular exercise and other methods, and especially to reduce visceral fat deposition.
Acknowledgements
We greatly appreciate all of the study participants.
List of abbreviations
- AUC
area under the curve
- BMI
body mass index
- BRI
body roundness index
- CIs
confidence intervals
- CRA
colorectal adenoma
- CRC
colorectal cancer
- CT
computed tomography
- FBG
fasting blood glucose
- HbA1c
glycated haemoglobin
- IQR
interquartile range
- LDL-C
low-density lipoprotein cholesterol
- MEC
mobile examination centre
- MRI
magnetic resonance imaging
- NHANES
the National Health and Nutrition Examination Survey
- ORs
odds ratios
- ROC
receiver operating characteristic
- TG
triglycerides
- TC
total cholesterol
- WC
waist circumference
- WHtR
waist-to-height ratio.
Author contributions
GD and CP designed and supervised the study. WG, LJ and DL undertook the interpretation of the data, performed statistical analysis, and wrote the manuscript. YZ, WZ, YZ, JG, and LZ made substantial contributions to the data reduction, design, and intellectual content of the studies. All authors read and approved the final version of the manuscript. WG, LJ and DL are equally contributing first authors.
Funding
No.
Data availability
The data are publicly available on the NHANES website: https://www.cdc.gov/nchs/nhanes/Index.htm.
Declarations
Ethics approval and consent to participate
This investigation was approved by the National Center for Health Statistics Ethics Review Board. Informed consent was obtained from all subjects involved in the NHANES.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
The original online version of this article was revised: the authors identified an error in figure 1 caption and would like to remove the supplementary material.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenxing Gao, Lujia Jin, and Dingchang Li should be considered joint first authors.
Change history
5/8/2023
A Correction to this paper has been published: 10.1186/s12944-023-01824-0
Contributor Information
Peng Chen, Email: cp_phd_301@163.com.
Guanglong Dong, Email: dongguanglong@301hospital.com.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lui RN, Tsoi KKF, Ho JMW, Lo CM, Chan FCH, Kyaw MH, et al. Global increasing incidence of Young-Onset Colorectal Cancer Across 5 continents: a Joinpoint Regression Analysis of 1,922,167 cases. Cancer Epidemiol Biomarkers Prev. 2019;28:1275–82. doi: 10.1158/1055-9965.EPI-18-1111. [DOI] [PubMed] [Google Scholar]
- 3.Chambers AC, Dixon SW, White P, Williams AC, Thomas MG, Messenger DE. Demographic trends in the incidence of young-onset colorectal cancer: a population-based study. Br J Surg. 2020;107:595–605. doi: 10.1002/bjs.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett-Hartman AN, Lee JK, Demb J, Gupta S. An update on the epidemiology, molecular characterization, diagnosis, and screening strategies for early-onset Colorectal Cancer. Gastroenterology. 2021;160:1041–9. doi: 10.1053/j.gastro.2020.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yehuda-Shnaidman E, Schwartz B. Mechanisms linking obesity, inflammation and altered metabolism to colon carcinogenesis. Obes Rev. 2012;13:1083–95. doi: 10.1111/j.1467-789X.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 6.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarasiuk A, Mosinska P, Fichna J. The mechanisms linking obesity to colon cancer: an overview. Obes Res Clin Pract. 2018;12:251–9. doi: 10.1016/j.orcp.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Khan S, Luck H, Winer S, Winer DA. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat Commun. 2021;12:2598. doi: 10.1038/s41467-021-22727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amano K, Bruera E, Hui D. Diagnostic and prognostic utility of phase angle in patients with cancer.Rev Endocr Metab Disord. 2022. [DOI] [PubMed]
- 10.de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64:650–8. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obes (Silver Spring) 2013;21:2264–71. doi: 10.1002/oby.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M, Yu X, Xu L, Wu S, Tian Y. Associations of longitudinal trajectories in body roundness index with mortality and cardiovascular outcomes: a cohort study. Am J Clin Nutr. 2022;115:671–8. doi: 10.1093/ajcn/nqab412. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Wu M, Wu S, Tian Y. Relationship between body roundness index and the risk of heart failure in chinese adults: the Kailuan cohort study. ESC Heart Fail. 2022;9:1328–37. doi: 10.1002/ehf2.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, He Y, Yang L, Liu Q, Li C, Wang Y, et al. Body roundness Index and Waist-Hip ratio result in Better Cardiovascular Disease Risk Stratification: results from a large chinese cross-sectional study. Front Nutr. 2022;9:801582. doi: 10.3389/fnut.2022.801582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Q, Zhang K, Li Y, Zhen Q, Shi J, Yu Y, et al. Capacity of a body shape index and body roundness index to identify diabetes mellitus in Han chinese people in Northeast China: a cross-sectional study. Diabet Med. 2018;35:1580–7. doi: 10.1111/dme.13787. [DOI] [PubMed] [Google Scholar]
- 16.Rico-Martin S, Calderon-Garcia JF, Sanchez-Rey P, Franco-Antonio C, Martinez Alvarez M, Sanchez Munoz-Torrero JF. Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta-analysis. Obes Rev. 2020;21:e13023. doi: 10.1111/obr.13023. [DOI] [PubMed] [Google Scholar]
- 17.Li ZY, Tan YT, Wang J, Fang J, Liu DK, Li HL, et al. Dose-response relationship between fat distribution and liver cancer incidence: a prospective cohort study in chinese men. Cancer Epidemiol. 2022;76:102091. doi: 10.1016/j.canep.2021.102091. [DOI] [PubMed] [Google Scholar]
- 18.Bo LL, Wang YQ, Liu YY, Li XD, Zhou R, Wang JL. [Analyze of obesity indicators and effect of fertility preservation treatment in patients with endometrial atypical hyperplasia and early endometrial cancer] Zhonghua fu chan ke za zhi. 2022;57:767–74. doi: 10.3760/cma.j.cn112141-20220727-00487. [DOI] [PubMed] [Google Scholar]
- 19.Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and Cardiovascular Diseases. Circ Res. 2020;126:1477–500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 20.Caballero B. Humans against obesity: who Will Win? Adv Nutr. 2019;10:4–S9. doi: 10.1093/advances/nmy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher EJ, LeRoith D. Obesity and cancer. Cancer Metastasis Rev. 2022;41:463–4. doi: 10.1007/s10555-022-10049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Cifarelli V, Appak-Baskoy S, Peche VS, Kluzak A, Shew T, Narendran R, et al. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat Commun. 2021;12:3350. doi: 10.1038/s41467-021-23808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Tang H, Huang P, Wang J, Deng P, Li Y, et al. Assessment of causal effects of visceral adipose tissue on risk of cancers: a mendelian randomization study. Int J Epidemiol. 2022;51:1204–18. doi: 10.1093/ije/dyac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JM, Chung E, Cho ES, Lee JH, Shin SJ, Lee HS, et al. Impact of subcutaneous and visceral fat adiposity in patients with colorectal cancer. Clin Nutr. 2021;40:5631–8. doi: 10.1016/j.clnu.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Calderon-Garcia JF, Roncero-Martin R, Rico-Martin S, De Nicolas-Jimenez JM, Lopez-Espuela F, Santano-Mogena E et al. Effectiveness of Body Roundness Index (BRI) and a Body Shape Index (ABSI) in Predicting Hypertension: A Systematic Review and Meta-Analysis of Observational Studies.Int J Environ Res Public Health. 2021;18. [DOI] [PMC free article] [PubMed]
- 27.Andreasson A, Hagstrom H, Skoldberg F, Onnerhag K, Carlsson AC, Schmidt PT, et al. The prediction of colorectal cancer using anthropometric measures: a swedish population-based cohort study with 22 years of follow-up. United Eur Gastroenterol J. 2019;7:1250–60. doi: 10.1177/2050640619854278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parra-Soto S, Malcomson FC, Ho FK, Pell JP, Sharp L, Mathers JC, et al. Associations of a body shape index (ABSI) with Cancer incidence, All-Cause, and at 23 Sites-Findings from the UK Biobank prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2022;31:315–24. doi: 10.1158/1055-9965.EPI-21-0591. [DOI] [PubMed] [Google Scholar]
- 29.Jochems SHJ, Wood AM, Haggstrom C, Orho-Melander M, Stattin P, Stocks T. Waist circumference and a body shape index and prostate cancer risk and mortality. Cancer Med. 2021;10:2885–96. doi: 10.1002/cam4.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and Cancer–viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold M, Touillaud M, Dossus L, Freisling H, Bray F, Margaritis I, et al. Cancers in France in 2015 attributable to high body mass index. Cancer Epidemiol. 2018;52:15–9. doi: 10.1016/j.canep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6:e6–e15. doi: 10.1016/S2213-8587(18)30150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N et al. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:1229-40.e5. [DOI] [PubMed]
- 35.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820–6. doi: 10.1136/gutjnl-2018-317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong MC, Chan CH, Cheung W, Fung DH, Liang M, Huang JL, et al. Association between investigator-measured body-mass index and colorectal adenoma: a systematic review and meta-analysis of 168,201 subjects. Eur J Epidemiol. 2018;33:15–26. doi: 10.1007/s10654-017-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knudsen MD, Berstad P, Hjartaker A, Gulichsen EH, Hoff G, de Lange T, et al. Lifestyle predictors for non-participation and outcome in the second round of faecal immunochemical test in colorectal cancer screening. Br J Cancer. 2017;117:461–9. doi: 10.1038/bjc.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unanue-Arza S, Solis-Ibinagagoitia M, Diaz-Seoane M, Mosquera-Metcalfe I, Idigoras I, Bilbao I, et al. Inequalities and risk factors related to non-participation in colorectal cancer screening programmes: a systematic review. Eur J Public Health. 2021;31:346–55. doi: 10.1093/eurpub/ckaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen JB, Berg-Beckhoff G, Leppin A. To do or not to do - a survey study on factors associated with participating in the danish screening program for colorectal cancer. BMC Health Serv Res. 2021;21:43. doi: 10.1186/s12913-020-06023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS ONE. 2013;8:e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11:19–30. doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 43.Gathirua-Mwangi WG, Monahan P, Song Y, Zollinger TW, Champion VL, Stump TE, et al. Changes in adult BMI and Waist Circumference are Associated with increased risk of Advanced Colorectal Neoplasia. Dig Dis Sci. 2017;62:3177–85. doi: 10.1007/s10620-017-4778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong Y, Zhou J, Zhu Y, Luo L, He T, Hu H et al. Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies.Biosci Rep. 2017;37. [DOI] [PMC free article] [PubMed]
- 45.Abar L, Vieira AR, Aune D, Sobiecki JG, Vingeliene S, Polemiti E, et al. Height and body fatness and colorectal cancer risk: an update of the WCRF-AICR systematic review of published prospective studies. Eur J Nutr. 2018;57:1701–20. doi: 10.1007/s00394-017-1557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh TH, Byeon JS, Myung SJ, Yang SK, Choi KS, Chung JW, et al. Visceral obesity as a risk factor for colorectal neoplasm. J Gastroenterol Hepatol. 2008;23:411–7. doi: 10.1111/j.1440-1746.2007.05125.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are publicly available on the NHANES website: https://www.cdc.gov/nchs/nhanes/Index.htm.