Abstract
Alzheimer’s disease (AD) is an immense and growing public health crisis. Despite over 100 years of investigation, the etiology remains elusive and therapy ineffective. Despite current gaps in knowledge, recent studies have identified dysfunction or loss-of-function of Pin1, a unique cis-trans peptidyl prolyl isomerase, as an important step in AD pathogenesis. Here I review the functionality of Pin1 and its role in neurodegeneration.
Introduction
Alzheimer’s disease remains among the fastest growing, most debilitating and costly diseases in the western world. The search for etiology has consumed scientists and physicians since its description by Alois Alzheimer in 1907-1911. He identified a constellation of pathology consisting of neuronal loss, brain atrophy, amyloid plaques and intracellular tangles that was distinct from syphilitic brain syndromes prevalent at the time. In 1984, plaques were purified [1] and the major component identified as a 39-44 amino acid peptide [2, 3]. The predicted amyloid precursor consisted of 695 amino acids and showed characteristics of a cell-surface, glycosylated receptor. The so-called amyloid precursor protein (APP) was ubiquitously expressed in multiple, alternatively spliced mRNAs and translated as isoforms of 564, 695, 717 and 770 amino acids, depending on the tissue [4, 5]. Ultimately 2 paralogs denoted amyloid precursor like protein 1 and 2 (APLP1 and 2) were also identified [6]. APP’s transcriptional and post-transcriptional responses were sensitive to outside-in cytokine driven signaling and mimicked typical acute phase responses [7]. Over time, Aβ42 and related species accumulate, especially in aged individuals or those homozygous for APO E4 [8], carrying germline, APP mutations at or near the Aβ42 cleavage sites [3], trisomy 21 (Down’s Syndrome)[9], presenilin 1/2 mutations [10], or traumatic brain injuries [11]. Soluble, oligomerized Aβ42 reaches toxic levels and damages neurons and synaptic connections, inhibits plasticity, enhances the accumulation of intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein, and activates microglia and astrocytes which release cytokines, chemokines, reactive oxygen species and nitric oxide. Over years, this toxic environment causes synaptic loss, mitochondrial and metabolic failure with deposition of hyperphosphorylated tau, neuronal cell death, chronic gliosis and ultimately brain atrophy. Reduced blood flow due to classic atherosclerosis or intravascular amyloid deposition commonly amplifies and accelerates these events.
As AD evolves, many proteins show changes in expression, distribution or function. The vast majority are associated rather than causal events and have not been shown to drive neuronal dysfunction or death. The breadth of change in afflicted neurons reflects many dysregulated signaling pathways that are evoked. Given that reality, protein(s) with involvement in multiple pathways, especially signaling cascades should be carefully considered as etiologic drivers of AD. Pin1 is one such molecule.
Pin1
Pin1 is a ubiquitously expressed, 18KD cis-trans prolyl isomerase. It was identified in 1996 with a yeast-2 hybrid screen for Never in Mitosis Associated (NIMA-1) kinase interactors [13]. Pin1 deletion pevented cell-cycle progression and is lethal in yeast [13, 14]. It is very highly conserved eukaryotic homolog to bacterial parvulin, yeast Ess1 and drosophila DoDo. Pin1, Ess1 and Dodo share a bipartite structure with a 40 amino acid, N-terminal, type IV WW domain connected via a flexible linker to a ~120-125 amino acid isomerase domain [12]. These homologs are functionally interchangeable as Pin1 or dodo complements deleted Ess1 in yeast [13, 14]. The WW-domain contains 2 essential and conserved tryptophans which mediate binding to Ser/Thr-Pro or pSer/pThr-Pro dipeptides [15-17] and positions the isomerase domain for the conversion of the WW binding target from cis to trans or the reverse. Pin1 mediated isomerization is ~1000 fold faster if the Ser/Thr are phosphorylated [18]. Notably bacterial parvulin lacks the WW targeting domain as does a Pin1 mammalian ortholog Par14/17. Pin1 along with FKBP and cyclophilin constitute the so-called immunophilin superfamily and share cis-trans prolyl isomerase activity. However, FKBP and cyclophilin target X-Pro dipeptide bonds rather than only pSer/Thr-Pro. Much controversy remains concerning the functions of FKBP and cyclophilins in yeast but the latest data suggests their deletion greatly reduces growth and viability [19]. Nevertheless, Pin1 is the only known enzyme with pSer/Thr-Pro selectivity which probably accounts for its very high conservation over hundreds of millions of years of evolution.
The Ser/Thr-Pro dipeptide in cis is the target for the so-called proline-directed kinases (PDKs) which includes MAPKs (p38, Erk and JNK), CK1/2, GSK3 and CDKs among others. The cis-trans conversion mediated by Pin1 is possible because the planar Pro can exist either cis or trans to the N-terminal Ser/Thr. The conformation around this peptide bond has relevance as PDKs and opposing phosphatases show selective activity towards either the cis or trans isomer [18, 20]. For example, cis but not trans tau is progressively phosphorylated by GSK3 at Thr231-Pro232 as AD evolves [21] which is opposed by PP2A which favors the trans form. The final conformation of the bond plays a key role in a variety of other protein functions including susceptibility to degradation, localization, activity and protein-protein interactions [22]. Not surprisingly, total pS/T modifications are very prevalent and constitute approximately 96% of the entire AD brain phosphoproteome [23, 24] while 2/3rds of identified pS/pT sites in axon growth cones precede a Pro [25]. Once phosphorylated, Pin1 catalysis increases the cis-trans conversion rate by 103 to 106 fold from spontaneous [26]. Trans pSer/Thr-Pro can be attacked by PP2A and other phosphatases that typically prefer pSer/Thr-Pro this isoform [27]. Because the rate of spontaneous, nonenzymatic interconversion is so slow, once isomerized and dephosphorylated, protein targets are essentially locked for their lifespan into the trans conformation. Conversely, in the absence of Pin1, cis pSer/Thr-Pro tau cannot be converted to trans and efficiently attacked by PP2A, leading to accumulation, microtubule toxicity, mitochondrial and axonal catastrophe and eventual neurodegeneration [28, 29]. Therefore, Pin1 activity is absolutely critical for transmitting PDK initiated signaling and maintaining cellular phospho-proteome homeostasis at Ser and Thr sites. Dysregulation of the phosphoproteome is increasingly seen as a driver of AD pathology [30].
Pin1 is ubiquitously expressed throughout all tissues but shows highest levels in reproductive organs and brain (https://www.proteinatlas.org/ENSG00000127445-PIN1/tissue). However, expression alone can be deceptive as Pin1 activity varies dramatically between cells and tissues. Activity is very low in quiescent peripheral immune cells such as lymphocytes and eosinophils despite ample expression [31] but constitutively active in neurons [32, 33] where it can be found in the axon growth cone, dendritic spines, soma and nucleus. In the immune system, isomerase activity is very rapidly (seconds to minutes) upregulated by lectin, cytokine and chemokine driven, outside-in signaling which alters Pin1 phosphorylation (see below). Conversely, at synapses, isomerase activity can be transiently suppressed by glutamatergic [33] or Aβ42 signaling [34]. These data demonstrate that Pin1 has both generic as well as cell-specific and possibly organelle-specific functions. Pin1 is often highly expressed in epithelial, CNS and hematopoietic cancers, likely facilitating cell-cycle progression and growth which is often associated with poor prognosis [35]. Conversely, Pin1 inhibition can disrupt tumor cell proliferation and enhances apoptosis suggesting it may be an attractive target for combination anti-cancer therapy [36-38].
Not surprisingly, the regulation of Pin1 is complex but mostly focused on post-translational events. Pin1 is the target of multiple kinases and at least 1 phosphatase which alter Pin1 activity, localization and half-life. PKA [39] and Aurora A [40] phosphorylation at Ser 16 disrupts WW domain-target interactions, essentially silencing Pin1 activity and blocking cell-cycle progression while COT and RSK phosphorylation at the same site do not reduce proliferation [41]. These divergent conclusions likely reflect differences in analytical endpoints rather than solely based on the effects of these kinases on Pin1-WW mediated target binding. DAPK1 phosphorylation of Ser71 [42] inhibits activity, possibly by interfering with the phosphate binding domain in the isomerase domain [43]. As such DAPK1 activators have shown some promise as anti-cancer agents [44]. Ser 111 is phosphorylated in healthy neurons [23][24] and required for activity but dephosphorylated by calcineurin after Aβ42 outside-in signaling [34]. C113 can be oxidized, blocking isomerase activity and increasing Pin1 cytoplasmic localization at the expense of nuclear [45]. Ser 108, 115, 147 and 154 moieties have been reported as phosphorylation sites although the biological role of these modifications have not been elucidated. Finally, Pin1 can also be acetylated [46], sumoylated [47], ubiquitinated [48] and methylated [49], again with uncertain significance.
Pin1 and Alzheimer’s disease
Pin1 was first implicated in AD pathogenesis in 1999 [50]. The Lu laboratory reasoned that hyperphosphorylated tau, the main component of paired helical filaments (PHF) and neurofibrillary tangles (NFTs), a common and established feature of evolving AD, would be regulated by Pin1 due to its repetitive pS/T-P motifs. Further support for this hypothesis was the observation that tau normally undergoes cycles of phosphorylation/dephosphorylation during cell-cycle progression and can be detected with MPM-2, a phospho-specific mAb that identifies a subset of mitotic phospho-proteins [51] previously shown to bind to Pin1. Accumulation of hyperphosphorylated tau, PHFs and NFTs would therefore be predicted if there was a Pin1 loss-of-function in evolving AD. The Lu lab was able to show by in vitro binding and antibody competition assays that Pin1 interacted only with pT231, one of several possible Pin1 recognition sequences in ptau. Other groups have subsequently implicated T212 as a second Pin1 binding site [52]. Biological relevance was established by demonstrating Pin1 bound to PHFs derived from AD brain but did not bind to tau from normal brain [50]. Immunohistochemistry showed that Pin1 was largely colocalized with PHFs in the cytoplasm in AD brain tissue [50]. While total Pin1 was not significantly different in AD brain, the available, soluble fraction was reduced by ~5 fold with a corresponding increase of Pin1 in the insoluble fraction.
Interestingly the maintenance of phosphorylation at T212 and T231 promoted phosphorylation at multiple additional sites including S199, S396, S400 and S404 in neurons treated with Aβ42 or okadaic acid, a potent PP2A inhibitor [53]. These results suggested that failure of Pin1 to isomerize ptau and enable PP2A mediated dephosphorylation of T231 and possibly T212, leads to a cascade of additional and toxic phosphorylation events that further promote PHF formation. Wild type neurons treated with the Pin1 inhibitor juglone, or Pin1 knockout neurons both showed increased pT231 tau content after exposure to Aβ42 or oxidation [54]. The logical inference from these studies is that Aβ42 or oxidation induces tau phosphorylation at T231 or T212. Pin1 binds with high affinity to these sites and mediates a cis-trans conversion of the intervening peptide bond. Presentation of the phosphate group now in trans allows PP2A to remove the group, prevent tau hyperphosphorylation and maintain tau functionality. Failure to do so creates a pro-phosphorylation milieu that predisposes to tau toxicity both directly as PHFs and NFTs and as a sink to remove Pin1 from the soluble fraction.
These data point to the critical role of Pin1 expression but more precisely, to the maintenance of Pin1 isomerase activity to prevent AD related, tau phenotypes. pTau mediated neurodegeneration is not seen uniformly across the entire brain, however. The entorhinal cortex, hippocampus and neocortical pyramidal neurons are particularly affected while cerebellum and other brain regions are typically spared [55, 56]. Even within highly susceptible regions such as the hippocampus, involvement is often nonuniform. Selective loss of Pin1 activity could explain the regional or subregional vulnerability of distinct brain regions to excess ptau or Aβ42 mediated signaling. Indeed, Pin1 expression was inversely correlated to NFT formation in the hippocampus with the most affected regions showing the least expression [57]. Similarly, the Allen Brain Atlas ISH data shows that Pin1 mRNA levels are the lowest in the most vulnerable regions of the brain including hippocampus and entorhinal cortex but approximately double in the cerebellum and cerebellar cortex. The biological or physiological underpinnings for these differences are not known. Thus, low Pin1 expression strongly correlates with the presence of tau pathology.
Critical in vivo evidence for Pin1’s role in tau pathology and AD came from analysis of germline Pin1 KO mice [58]. These animals were developed by Uchida’s group in Japan in a mixed 129/Sv and C57L/B6 background through the insertion of a silencing neo-TK cassette into the Pin1 gene [59]. The mice developed normally but post-starvation cultures of Pin1−/− MEFs were unable to reenter the cell cycle despite refeeding. Drosophila lacking the Pin1 homolog dodo also developed normally [14]. These data suggest the presence of proteins capable of complementing at least some of Pin1’s functionality (see discussion of Immunophilins above). Nevertheless, neuroanatomic and biochemical analysis by Lu’s laboratory of the Pin1 KO mice demonstrated age dependent and regional accumulation of ptau, PHFs and eventual neurodegeneration that closely resembled that seen in tau or tau mutant overexpressing mice [60, 61]. Motor and behavioral defects were also observed at 9-10 months of age while western blotting and immunohistochemistry showed dramatically increased MPM2, AT8, AT180 and Alz50 staining, indicative of hyperphosphorylated tau. Murine Aβ42, while normal at 6 months of age was increased ~30% at 15 months of age. Therefore, the 2 major proteins pathologically implicated in AD evolution – ptau and Aβ42 – were significantly elevated in Pin1 null mice. The only potential caveat to these results is the mixed 129/Sv and C57L/B6 background of the Pin1 KO mice [59]. Once in a stable, pure C57L/B6 background, the phenotypes of the germline KO changed with loss of germ cells and profound infertility making breeding very difficult [62, 63]. Despite breeding fertile, heterozygous pairs, viable Pin1−/− offspring were rare, consistent with profound defects in cell-cycle progression and mitosis seen in vitro in Pin1 null MEFs [55]. Notably, there has not been a published reassessment of CNS phenotypes in the pure bred, germ-line Pin1 knockout mouse.
Nevertheless, the impact of Pin1 on AD pathogenesis was further clarified by evaluation of the phenotypes of progeny from germline, mixed background Pin1 KO mice and an AD model, Tg2576. The latter were among the first AD mouse models which was engineered to produce high levels of mutant (double mutation (K670N/M671L)), human APP695 and equally robust (~14 fold compared to human AD patients) Aβ42 [64]. Tg2576 develop normally but begin to show cognitive impairment at approximately 6 months of age which progressively worsens. By 11-13 months of age, animals show numerous Aβ42 plaques, modest vascular amyloid and diffuse oxidative damage but no evidence of tau pathology (e.g. NFTs) or neurodegeneration [65]. Dendritic spine loss occurs by 4.5 months in the CA1 [66] with reduced LTP compared to wild-type controls [67]. Insoluble Aβ42 levels in the compound mice (Pin1−/− x Tg2576) [68] were unchanged in young animals but rose significantly higher by 6 months of age compared with Tg2576 littermates. Soluble Aβ40 and Aβ42 were also unchanged as was neurodegeneration. The latter was unexpected as Pin1 null mice independently show tauopathy and neurodegeneration [58]. These data suggest the 50% C57/Bl6 genotype contributed by Tg2576 sufficiently altered the progeny such that tauopathy was modulated.
How Pin1 loss enhanced insoluble Aβ42 production in the compound mice was first explored in vitro in N18 neuroblastoma cells. Thr668-Pro669 of the APP intracellular domain is phosphorylated by cdc2 kinase during the cell cycle [69], although additional kinases and multiple other sites in APP have also been identified [70]. Binding studies and NMR spectroscopy revealed phosphorylation dependent binding to and cis to trans isomerization of this site by Pin1. Once in trans, APP processing favored so-called α-cleavage which occurs on the cell surface and prevents Aβ42 production. In the absence of Pin1, APP processing was more endosomal [71-73] and became more amyloidogenic with greater production of Aβ42. In vitro studies confirmed that APP processing was heavily influenced by Pin1 which drove α-cleavage when present and β−γ cleavage when absent [68]. However, the conclusion that isomerization of APP at Thr668-Pro669 was responsible for these observations has been controversial as knock-in mice with Ala substituted for Thr668 did not show changes in APP processing [74, 75] compared to wild type. As the intracellular domain of APP (AICD) interacts with Fe65, Mint/X-11 and other trans factors which are transported to the nucleus after cleavage [76, 77], the effects ascribed to Thr668 phosphorylation and subsequent Pin1 isomerization may be incorrect. Alternatively, the effects of Pin1 may be indirect through the activation/suppression of other proteins which then influence APP processing or Aβ42 production and subsequent oligomerization. For example, a variety of kinases including typical PKC, CamKII [34, 78], DAPK1 [79, 80] and GSK3β [81] all phosphorylate the AICD and all interact with Pin1 [82, 83]. PKC [32], DAPK1 [42] and CamKII [82] also regulate Pin1 through phosphorylation (see above discussion). Therefore, data suggests bidirectional regulation of Pin1 by/to kinases which influence Pin1 activity and the number of phosphorylated, Pin1 targets based on kinase activity. This complexity must be clarified to better establish Pin1’s role in the regulation of APP processing and Aβ42 production in the brain of normal individuals or AD patients.
The appearance of hyperphosphorylated tau is typically late in human AD and in the absence of tau mutation, follows years of CNS exposure to gradually rising levels of soluble, multimeric Aβ42 [84, 85]. A consistent early result of Aβ42 exposure is a progressive loss of synapses and dendritic spines which presumably underlies the clinical appearance of mild cognitive impairment (MCI) in patients or reductions in LTP and elevations in LTD in slices from AD model mice or WT slices exposed to exogenous Aβ42 [86, 87]. In addition to dysregulated Ca2+ fluxes [88, 89] Aβ42 induces oxidative stress [89, 90], possibly through the induction of catalase or the attack by metalloproteins [91]. Pin1 contains a critical active site sulfhydryl (C113) which is oxidized in the brain of both MCI and AD patients [92, 93]. The finding of extensive oxidation early in disease is consistent with mouse data and suggests a pathognomonic role for Pin1 in AD development. While changes in Pin1 function were not experimentally demonstrated in these studies, it is likely isomerase activity was negatively affected by C113 oxidation. These results also show that Pin1 is sensitive to outside-in Aβ42 signaling, suggesting blockade of this pathway might preserve Pin1 activity and attenuate AD pathology [94].
Pin1 is also subject to a variety of other post-translational modifications that affect its activity, stability and location. These include phosphorylation, sumoylation, acetylation and ubiquitination. In the context of AD, dysregulation of these regulatory cascades, induced by Aβ42 or ptau can have substantial downstream effects on Pin1 functionality. The WW-domain can be phosphorylated at Ser16 by a variety of kinases including PKA [39], Aurora A [40], Ribosomal S6 kinase 2 (RSK2) [95] and COT/Tpl2 [96]. Consistent with an in vivo role, Ser16 phosphorylation is very elevated in AD brain or in vitro in cells overexpressing tau [97]. As Ser16 is located nearly in the center of the WW domain, the introduction of a large, negatively charged phosphate would be expected to interfere with ligand recognition and binding. Indeed, this has been clearly demonstrated for PKA mediated phosphorylation at this site [39]. However, subsequent studies have argued that Ser16 phosphorylation mediated by RSK2 or COT had a positive, not negative effect on Pin1 binding to targets [95] [96] [41]. These studies focused on the downstream biological outcome coincident with Ser16 modification without explicitly demonstrating how these modifications directly affected Pin1 binding to partners or isomerase activity. We and others [39] have found that Ser16Glu substitution blocks WW domain binding and thus prevents Pin1 mediated isomerization of targets. This was well illustrated by the profound blockade of endogenous Pin1 function and the induction of apoptosis by the expression of dominant-negative WW domain fragments containing Ser16Ala but not by Ser16Glu [39].
Other Pin1 posttranslational modifications have been identified including phosphorylation at Ser65 by Plk1 [98], Ser71 by DAPK1 [42] and Ser138 by MLK3 [99]. Plk1 [100] and DAPK1 are overexpressed in AD neurons [42] while MLK3 [101] is activated by Aβ42, making the action of all three kinases relevant to AD pathobiology. Phosphorylation at Ser65 prevents sumoylation while modification at Ser71 suppresses Pin1 activity. Ser138 phosphorylation has the opposite effect and is associated with increased Pin1 activity and nuclear localization. Using proteomics, we recently [34] identified a number of novel Pin1 PTMs induced by multimeric Aβ42 signaling. Intact and translationally functional synaptoneurosomes were isolated by percoll gradient centrifugation from mouse brain and exposed briefly (10 min) to physiologic concentrations (100 nM) of multimeric Aβ42. This brief exposure time and concentration were sufficient to completely block Pin1 activity [34] suggesting signaling triggered inhibitory, post-translational modifications. We did not establish the lowest, effective Aβ42 concentration for this effect. Pin1 immunoprecipitants were analyzed for differences in protein interactors as well as PTMs. Particularly notable changes after Aβ42 treatment included the dephosphorylation of Pin1 at Ser111, Ser147 and Ser154 as well as a strong association of Pin1 with calcineurin (CaN, PP2B). Of note, recent phosphoproteomics has confirmed these data in MCI and AD brain [23, 24]. CaN is among the most abundant phosphatases in brain and plays a critical role in plasticity and memory [102, 103]. Aβ42 signaling is known to upregulate CaN activity in cells [104, 105] and CaN expression in patients and AD animal models is elevated [105, 106]. Conversely, CaN normalization restores synaptic plasticity [107], dendritic spine density [108] and learning and memory [109, 110]. However, complete knock-down of CaN inhibited working memory [111] and synaptic plasticity [112] demonstrating that normal brain function requires CaN expression and activity within a tightly controlled range.
The above data from other labs and our co-immunoprecipitation results suggested Pin1 activity could be regulated by CaN and Aβ42 signaling which would further establish and enlarge the pathobiological relevance of Pin1 in AD. We investigated that possibility by generating single and multiple Ser to Glu and Ser to Ala mutants in Pin1 at position 111, 147 and 154 and introducing them into neurons, synaptoneurosomes or tumor cell lines. While most experimental work has utilized Ser111 mutants due to its proximity to the Cys 113 active site, we have observed similar data with multiple mutants as well. After transduction or transfection, Ser111Glu mutants were entirely resistant to inhibitory Aβ42 signaling and retained full activity while Ser111Ala mutants were isomerase dead [34]. Treatment of cells or synaptoneurosomes with FK-506, a potent and specific CaN inhibitor fully rescued wild type Pin1 activity after Aβ42 treatment but had no effect on Ser111Glu activity nor did it rescue isomerase null Ser111Ala mutants. To minimize off target effects, FK506 was employed at its IC50 (~5 nM) and experiments were repeated with cyclosporin and yielded similar results. Therefore, we concluded that Aβ42 signaling induced dephosphorylation by CaN at Ser111 of Pin1 leading to loss of isomerase activity. This is the first report of Pin1 regulation by a phosphatase but as DAPK1, PKA, MLK3, Plk1 and PKMζ [32], all influence Pin1 function, stability or location, phosphatases must also play an opposing role in the control of Pin1. Whether CaN is the only phosphatase opposing these kinases is unknown. Based on the cell and tissue specific regulation of Pin1, I suspect different phosphatases will be important in different cell types. Second, the effects were quite rapid with activity blockade within minutes. This suggest that in early, evolving AD as the concentration of soluble, multimeric Aβ42 rises to an inhibitory level, Pin1 will be less active. Phosphoproteomics have revealed ~50% reductions in pSer111 content in the cortex of MCI patients although there was partial rebound in many full-blown AD cases [23, 24] As discussed above, the loss of Pin1 will directly contribute to the accumulation of hyperphosphorylated tau and the initiation of neurodegeneration (Fig 2). Thus Pin1 loss-of-function can be viewed as a bridge between Aβ42 signaling and tau hyperphosphorylation.
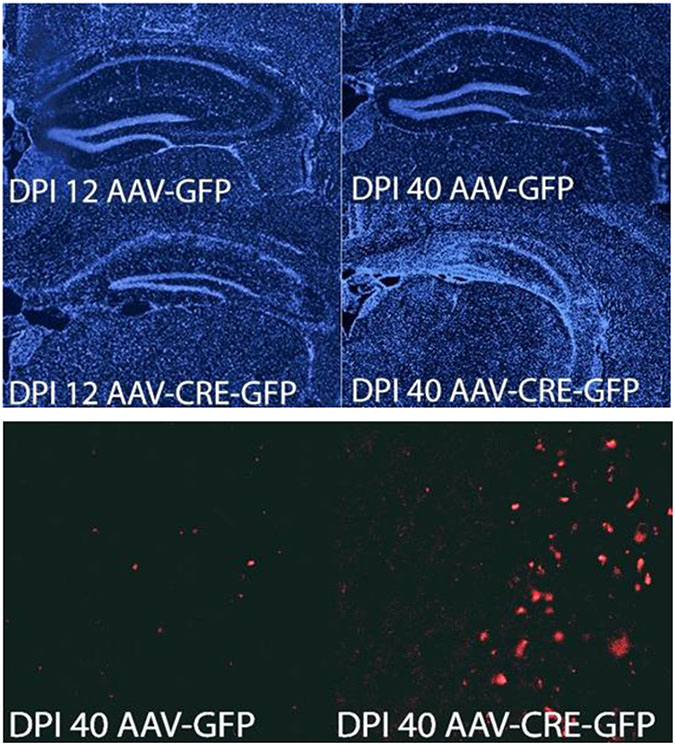
Fig 2.
Loss of Pin1 causes neuronal cell death and AT8 immunoreactivity in the hippocampus. Pin1fl/fl mice were injected in the hippocampus with AAV-GFP-Cre or AAV-GFP, prior to sacrifice after 12 or 40 days. Top 4 images stained with DAPI (blue), bottom 2 images stained with anti-AT8 (red, 40X).
In order to establish other pathophysiologic effects of Pin1 suppression by Aβ42 and CaN signaling, we assessed dendritic spine density. Spines are lost early in AD progression and correlate with memory and learning reductions [113, 114] [86]. Spine counts are significantly reduced in patients with early AD (MCI) [115, 116], correlate well with cognitive losses, appear before tauopathy and are recapitulated in AD mouse models that overexpress Aβ42 [66]. We hypothesized genetic ablation of Pin1 would mirror Pin1 suppression seen in AD or mouse models induced by Aβ42 and help establish if Pin1 was essential for spine maintenance in AD. Indeed, dendritic spine counts in cultured DIV21 neurons were reduced by ~35% either after Pin1 KO or after 1 h of Aβ42 treatment of wild-type cells [34]. In both cases, spines were rescued after delivery of exogenous Pin1. Consistent with the synaptoneurosome data above, pretreatment with FK506 completely blocked Aβ42-mediated spine loss but only in neurons with Pin1 expression [34]. These data established that Pin1 was a critical target for Aβ42-CaN signaling in spines. When WT Pin1 was replaced with Pin1 Ser111Glu or Ser111Ala mutants, cells with Ser111Glu were also fully resistant to Aβ42-mediated spine loss irrespective of CaN blockade with FK506. As expected, Ser111Ala mutants failed to rescue Aβ42-mediated spine loss irrespective of CaN blockade. These data collectively demonstrated that Pin1 activity was required to maintain dendritic spines, that Pin1 suppression by CaN-Aβ42 signaling, driven by Ser111 dephosphorylation was a necessary and sufficient step in initiating spine loss and that FK506 could potentially be repurposed to normalize CaN activity, blunt Aβ42 signaling and preserve Pin1 function in early AD.
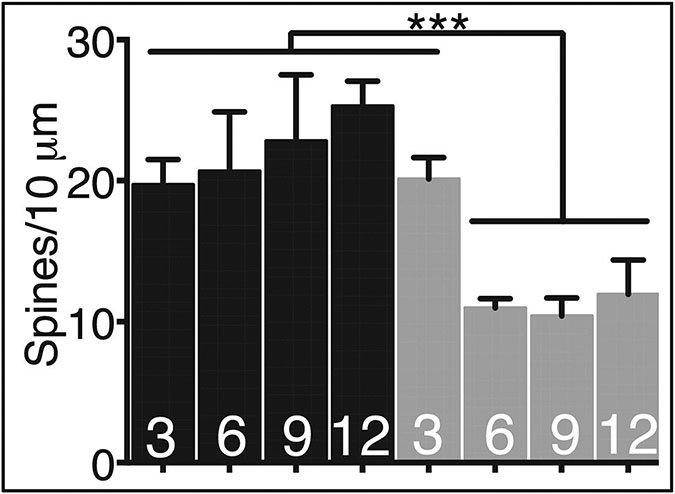
We also evaluated the effects of acute Pin1 KO in adult mice. Floxed mice were injected with AAV-Cre-GFP or control AAV-GFP into hippocampus or entorhinal cortex. Mice were harvested at various times thereafter which revealed KO neurons in the injected regions exhibited ~ 40% loss of synapses by 6 DPI which remained constant but reduced over time [34] (Fig 1). Both entorhinal cortex (EC) and hippocampus showed significant architectural dysruption, cell death and astro-glial proliferation by 12 DPI which progressively worsened, leaving both the hippocampus and EC entirely defaced by 40 DPI (Fig 2). As observed in the Pin1 germ-line KO mice, ptau was readily detected with anti-AT8 antibody after Pin1 silencing (Fig 2) but unlike the prior observations where ptau elevation appeared at 9-10 months of age, ptau appeared within 40 DPI after conditional KO. These data suggest that KO of Pin1 in adult mice has different and possibly accelerated phenotypes than observed after germ-line KO. The very rapid cell death is notable and suggests loss of Pin1 triggered acute events through an unknown mechanism along with slower, ptau dependent neurodegeneration observed at 40 DPI. As Pin1 has been implicated in intrinsic and extrinsic apoptosis pathways [117-119] as well as proinflammatory cytokine production [17] in the peripheral immune system, it is tempting to speculate activation of these pro-death pathways in neurons and/or astrocytes-glia after Pin1 KO. Other plausible causes include axonal [33] or dendritic dysfunction [34][116, 120] secondary to Pin1 loss with subsequent retrograde cell death. Regardless, the mechanism of cell death elicited by Pin1 KO is likely to be one relevent to and seen in AD neurons.
Fig 1.
Pin1 is required for spine maintenance in vivo. 2 months old Pin1fl/fl mice were injected in the hippocampus with AAV-GFP (black bars) or AAV-GFP-Cre (grey bars) at equal titer and synaptophysin positive spines counted 3–12 days thereafter, denoted along X axis. ***P < 0.001 between groups.
We recently evaluated if CaN inhibition with the FDA approved, transplant antirejection drug, FK506 could reduce/block AD pathologies (Stallings, N et al. submitted, 2022). Short-term, generally high dose FK506 has restored plasticity [108] and reduced Aβ42 burden in AD model mice [121]. Interestingly, solid organ recipients who received FK506 showed significantly reduced AD incidence than age-matched recipients treated with mTOR inhibitors or an age-matched, nontransplanted population [106]. We hypothesized that low-dose FK506 capable of normalizing CaN activity in the brain would prevent Pin1 inhibition by Aβ42 signaling while leaving peripheral immunity intact. To test this, FK506 time release pellets were implanted subcutaneously into 3 month-old APP/PS1 mice. Pellets were designed to release ~1 mg FK506/kg body weight to maintain drug at <10 nM plasma for 3 months. Mass spectroscopy analysis showed mice had somewhat higher levels for the first month which dropped and remained at target for the last 2 months of treatment. Behavioral testing at 6 months showed treated mice performed as well as untreated controls in the Morris Water Maze. Neuropathology revealed increased synaptic connections and dendritic spines, and reductions in microglial activation and Aβ42 plaque content. CaN and Pin1 activity was normalized in treated APP/PS1 mice while splenocytes activated with phorbol ester and ionophore showed normal IL-2 mRNA expression. An open label, human trial in MCI patients (NCT04263519) was recently initiated but has been delayed due to COVID.
Plasticity and Pin1
As AD evolves, synaptic plasticity declines [86, 122, 123]. This has been ascribed to synaptic dysfunction and later synaptic loss due to escalating soluble, multimeric Aβ42 levels and likely accounts for early, clinically detectable reductions in learning and memory that occur in MCI patients [124]. While Pin1 had been localized to the cytoplasm and nucleus, we showed by western blotting, Pin1 activity assay, immunofluorescence and immuno-EM that Pin1 was also highly expressed and highly active in post-synaptic dendritic spines [32]. Pin1 was likely translated in situ as glutamate treatment of intact neurons increased spine localized Pin1 by 3 fold in ~30 minutes. As most forms of long-term memory and learning require glutamate-induced, de novo, dendritic spine protein synthesis [125], we evaluated if Pin1 KO affected this process. Translationally active SN from WT and Pin1 KO mice were pulsed with S-35 methionine before glutamate/glycine treatment [32]. WT preparations showed a consistent, transient 40-60% increase in overall dendritic protein synthesis which was suppressed entirely by anisomycin. Pin1 KO SN however showed constitutively elevated protein synthesis which was unaffected by glutamate but quantitatively similar to that seen in WT SN after glutamate. Similar observations have been observed in FMR−/− SN after mGluR5 activation [126, 127]. These data suggested that glutamaturgic signaling transiently inhibited Pin1 activity, permitting a temporary upregulation of dendritic protein sythesis. Isomerase assays confirmed this and revealed complete and very rapid suppression of Pin1 activity due to Ser16 phosphorylation by PKCζ or PKMζ [32]. We also showed that under basal conditions, Pin1 interacted with hyperphosphorylated eIF4E, 4E-BP1 and 4E-BP-2, providing a molecular link to translation initiation and regulation. While it was not determined at the time, involvement of CaN or another phosphatase would presumably reactivate Pin1 while the protein degradation machinery would reduce Pin1 content on the post-synaptic side to basal levels.
Paradoxically, while Pin1 loss/inhibition is clearly associated with evolving AD and diminishing cognition and reduced expression in spines [128], hippocampal slices from young, germ-line Pin1 KO mice [58][63] show normal E-LTP but elevated, anisomycin sensitive, L-LTP when elicited with a train of four, high frequency burst stimuli [32]. A similar phenotype was seen in FKBP KO mice [129] which showed elevated phosphorylation of mTOR and S6 Kinase which are upstream of 4E-BP1 and eIF4B, respectively, suggesting FKBP also participates in dendritic protein synthesis. Conversely, cyclophilinD KO mice showed impaired short-term memory which could be replicated in WT mice by cyclosporine infusion [130, 131]. However, cyclophilinD KO in the context of an AD model improved cognitive function [131]. In aggregate these data suggest that the entire class of immunophilins/prolyl isomerases participate in the positive and negative regulation of synaptic plasticity via control of activation dependent, de novo protein synthesis. Whether enhanced L-LTP or cognition are maintained as Pin1 or FKBP KO mice age has not been formally assessed but given elevated ptau and cell death, adult germ-line KO mice must show loss of plasticity.
Stimulation capable of inducing L-LTP increases the numbers and maturity of dendritic spines [132, 133], presumably reflecting alterations in the spine proteome. Pin1 contributes to cognition and memory by playing a role in the maintenance and structure of dendritic spines. Adult WT mice treated with Pin1 inhibitors or Pin1 germline KO mice showed an increased density of mature, mushroom-shaped, dendritic spines [134]. Such a phenotype would be consistent with elevated L-LTP discussed above. Loss of Pin1 derepressed PSD95, increasing synaptic NMDA-R content and currents. Additional recent reports have confirmed the Pin1-PSD95 interaction on the post-synaptic side and downstream effects of isomerization on synaptic NMDA-R and AMPA-R expression and currents [135]. However, Pin1 likely interacts with multiple sites on PSD95 with opposing effects on the latter’s abundance, turnover and function. Interestingly, overexpression of Pin1 reduced spine numbers, demonstrating that deviations in Pin1 expression and presumably function was detrimental to normal physiology. Nevertheless, if valid, these observations suggest Pin1 loss should mitigate, not worsen cognitive decline in evolving AD.
These unexpected results encouraged us to generate conditional mice with floxed Pin1 alleles [34]. Cre-mediated loss of Pin1 in P1, DIV21-24 neurons or the hippocampus of adult, floxed mice caused significant reductions in dendritic spines within 6 days and eventual cell death in vivo [34](Fig 1). Neuronal cell death was associated with the accumulation of hyperphosphorylated tau (Fig 1), as seen in late-stage AD. Thus, these results resemble those observed in Tg2576 AD mice lacking Pin1 [68]. Why Pin1 germ-line or chemical KO enhances spines but Cre-mediated excision has the opposite effect remains unclear. Pin1 inhibitors and possibly siRNAs are never fully specific and observations after their use could be partially attributable to off-target effects. The germ-line Pin1 KO mice have extremely poor fertility due to cell-cycle arrest. Those that are born must have compensated for Pin1 loss, possibly by overexpression of another prolyl isomerase, again creating nonphysiologic phenotypes.
Pin1 and neuroinflammation
Cross-talk between neurons and supporting cells, especially astrocytes and microglia, is critical for normal brain function and homeostasis. Microglia are CNS-resident myeloid cells whose function includes responses to infection and support of neuronal plasticity. Neurons and microglia produce IL-33 which activates microglia to phagocytize dendritic spines, intact neurons and extracellular matrix [136]. Loss of detectable IL-33 accompanies AD evolution [137] while exogenous IL-33 antagonized AD pathology and cognitive defects [138]. In response to Aβ42, microglial TLRs [139] and NLRs [140] become activated with the production of proinflammatory cytokines [141, 142], NO, chemokines [143] and ROS capable of interfering with neuronal function and viability [144-146]. In animal models or patients with AD, IL-1, TNFα, IFNα/β, IL-6, GM-CSF, IL-12 and IL-23 are detectable or upregulated [147].
Innate and humoral cytokine production in the peripheral immune system is heavily reliant on both transcriptional and post-transcriptional control systems [148-150]. These include TLR/IRAK/IRF7 dependent transcription [150], reduced mRNA decay and increased translation. Pin1 binds to IRAK1 and is required for the elaboration of type 1 IFN in dendritic cells after TLR7/9 activation by IL-1 receptor signaling [151]. Post-transcriptional regulation is often mediated by sequence or conformation specific mRNA binding proteins which interact with highly conserved cis elements such as AUUUA repeats [152, 153] or stable stem-loop structures [154]. Pin1 has been implicated in post-transcriptional control of cytokine production by activated T cells [155]), eosinophils [31, 156-158] and neutrophils [159]. Pin1 also mediates NF-kB activation after cytokine or LPS [160, 161]. Usually outside-in signaling simultaneously activates quiescent Pin1 and triggers phosphorylation of Ser/Thr-Pro sites of cognate mRNA binding protein enabling their isomerization and change of function [17]. Macrophages employ many of the same regulatory schema suggesting that microglia would as well. However, one notable difference is that the component cells of the brain (neurons, astrocytes and microglia) express active Pin1 under basal or homeostatic conditions while cells in the periphery require outside-in signaling. It is tempting to speculate that upon neuronal or potentially microglial/astrocytic activation induced by Aβ42 [34], glutamate [31], ptau (Stallings, O’Neal and Malter, unpublished observations) or hypoxia transiently inhibit Pin1, leading to stabilization of proinflammatory cytokine mRNAs and their translation. This suggests that in chronic disease such as AD, there would be a smoldering but gradually increasing proinflammatory environment due to the relentless accumulation of Aβ42 and ptau along with autocrine glial activation by previously released cytokines such as TNF and IL-1 [162].
Pin1 and Neuronal Cell Death
Apoptosis is one of the main cell death pathways active in advanced AD although autophagic and necrotic cell death also occur [163, 164]. Extrinsically triggered apoptosis involves death receptor and caspase 8 cleavage while intrinsic pathways increase mitochondrial membrane permeability through BCL-2 proapoptotic family member activation [165]. Both extrinsic and intrinsic apoptotic pathways are activated in evolving AD and genes associated with death are increased in expression at the expense of pro-survival pathways [164]. Pin1 has been reported to interact with FADD and caspase 8 of the extrinsic pathway [117] and Bax and cIAP [118] of the intrinsic pathway. In both scenarios, active Pin1 was pro-survival and prevented the initiation of caspase cleavage and apoptosis. Pin1 can also act upstream on critical pro-survival regulators including p53 and p73 [166], MAPK [167], survivin [168], Fbw7 [169], ataxia telangiectasia and Rad3-related (ATR) [170]. Therefore, loss of Pin1 can directly accelerate apoptotic cell death through multiple mechanisms. Pin1 has also been implicated in autophagic [171] , necroptopic [172] and necrotic [173, 174] neuronal cell death.
It is well known that AD progressively involves distinct brain regions which likely accounts for the characteristic and sequential clinical manifestations. The disease likely starts in the EC, next affecting the adjacent hippocampus and eventually spreading to the temporal cortex [175]. How and why AD starts and spreads along this consistent path is poorly understood. The EC accumulates ptau as NFTs early in AD with corresponding neuronal loss [176]. While detailed analysis of Pin1 protein levels and activity in different brain regions during AD evolution is yet to be done, EC Pin1 mRNA expression is among the lowest in the brain, closely followed by hippocampus and neocortex [Allen Brain Atlas, https://human.brain-map.org/]. These data suggest that regional AD vulnerability correlates with Pin1 expression.
Summary and Perspectives
A variety of in vitro and in vivo biochemical, molecular, neuroanatomic, behavioral and physiologic evidence implicates Pin1 loss, a cis-trans prolyl isomerase, as a key determinant in the erosion of higher cognitive function and eventual neuronal cell death characteristic of Alzheimer’s disease. Pin1’s involvement in multiple pro-survival and homeostatic signaling cascades places it uniquely in many patho-biologically relevant pathways that are dysfunctional in AD. The identification of Aβ42-CaN as a rapid, potent, inhibitory signal clarifies how Pin1 activity, along with potential contributions from germ-line SNPs [177, 178] or somatic mutations [179] can be gradually lost and lead to early synaptic disease manifesting as MCI as well as later tau pathology, neurodegeneration and full-blown AD.
Clearly preservation of Pin1 activity could antagonize AD progression [88]. Since AD, as is cancer, typically a disease of the aged, Pin1 activation could inadvertently accelerate tumor development or progression [35], a undesirable outcome. This suggests Pin1 targeted therapy should ideally be focused on the CNS and be titratable with the goal of activity normalization. Such an approach would require lipophilic therapeutics capable of penetrating the blood-brain barrier and concentrating in the brain. At present, there are no direct Pin1 agonists that meet these criteria. However, interference with known, inhibitor modulators of Pin1 such as DAPK1 [38, 44] or the Aβ42-CaN signaling cascade [88][110][100] are viable options. The former phosphorylates Ser71 while the latter dephosphorylates Ser111 and possibly Ser147 and Ser 154. Low nM DAPK1 inhibitors [180] have been developed and used to attenuate PTZ-induced epilepsy [181] and proposed for AD as well [182]. FDA approved agents FK506 and cyclosporine target CaN. The latter are lipophilic, penetrate the BBB, accumulate in the brain, show excellent target selectivity and potency and have a well-documented, long-term safety profile. Other options include RAGE receptor inhibitors which have shown safety and efficacy in preclinical models of ARDS [183]. Antibodies which target tau have also failed in phase II clinical trials sponsored by Roche, Lilly and AC Immune [184]. Anti-cis-ptau and a few other anti-tau antibodies are entering early phase clinical trials. Interestingly, anti-Aβ42 antibodies which fit some of the above criteria, while often effective for clearing fibrillar and plaque Aβ42 in animal models and some patients, have almost uniformly failed to significantly alter AD evolution [185]. Of note, these agents have usually not significantly altered the total brain burden of Aβ42 [171]. This suggests that antibody treatment may have left enough residual Aβ42 to continue CaN activation and suppress Pin1. Despite our improved understanding of AD pathogenesis, substantially more investment and research are needed to identify effective therapeutics for this crippling disease.
Acknowledgements:
The author would like to thank the past and present members of the laboratory for their technical skills, hard work, ideas and creativity. This work has been and continues to be supported by NIH RFAG055400 and R56AG071310 to J.S.M. The author has no financial conflicts to disclose.
Bibliography:
- 1.Glenner GG and Wong CW, Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun, 1984. 122(3): p. 1131–5. [DOI] [PubMed] [Google Scholar]
- 2.Citron M, et al. , Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature, 1992. 360(6405): p. 672–4. [DOI] [PubMed] [Google Scholar]
- 3.Kang J, et al. , The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature, 1987. 325(6106): p. 733–6. [DOI] [PubMed] [Google Scholar]
- 4.de Sauvage F and Octave JN, A novel mRNA of the A4 amyloid precursor gene coding for a possibly secreted protein. Science, 1989. 245(4918): p. 651–3. [DOI] [PubMed] [Google Scholar]
- 5.Tanzi RE, et al. , Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature, 1988. 331(6156): p. 528–30. [DOI] [PubMed] [Google Scholar]
- 6.Walsh DM, et al. , The APP family of proteins: similarities and differences. Biochem Soc Trans, 2007. 35(Pt 2): p. 416–20. [DOI] [PubMed] [Google Scholar]
- 7.Banati RB, et al. , Amyloid precursor protein (APP) as a microglial acute phase protein. Neuropathol Appl Neurobiol, 1994. 20(2): p. 194–5. [PubMed] [Google Scholar]
- 8.Liu CC, et al. , Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol, 2013. 9(2): p. 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rumble B, et al. , Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N Engl J Med, 1989. 320(22): p. 1446–52. [DOI] [PubMed] [Google Scholar]
- 10.Citron M, et al. , Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med, 1997. 3(1): p. 67–72. [DOI] [PubMed] [Google Scholar]
- 11.Molgaard CA, et al. , Epidemiology of head trauma and neurocognitive impairment in a multi-ethnic population. Neuroepidemiology, 1990. 9(5): p. 233–42. [DOI] [PubMed] [Google Scholar]
- 12.Hanes SD, Prolyl isomerases in gene transcription. Biochim Biophys Acta, 2015. 1850(10): p. 2017–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu KP, Hanes SD, and Hunter T, A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature, 1996. 380(6574): p. 544–7. [DOI] [PubMed] [Google Scholar]
- 14.Maleszka R, et al. , The Drosophila melanogaster dodo (dod) gene, conserved in humans, is functionally interchangeable with the ESS1 cell division gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A, 1996. 93(1): p. 447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng CA, et al. , Structural characterisation of PinA WW domain and a comparison with other group IV WW domains, Pin1 and Ess1. Biochim Biophys Acta, 2008. 1784(9): p. 1208–14. [DOI] [PubMed] [Google Scholar]
- 16.Lu PJ, et al. , Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science, 1999. 283(5406): p. 1325–8. [DOI] [PubMed] [Google Scholar]
- 17.Shen ZJ, Esnault S, and Malter JS, The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils. Nat Immunol, 2005. 6(12): p. 1280–7. [DOI] [PubMed] [Google Scholar]
- 18.Lu KP and Zhou XZ, The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol, 2007. 8(11): p. 904–16. [DOI] [PubMed] [Google Scholar]
- 19.Kasahara K, Physiological function of FKBP12, a primary target of rapamycin/FK506: a newly identified role in transcription of ribosomal protein genes in yeast. Curr Genet, 2021. 67(3): p. 383–388. [DOI] [PubMed] [Google Scholar]
- 20.Ubersax JA and Ferrell JE Jr., Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol, 2007. 8(7): p. 530–41. [DOI] [PubMed] [Google Scholar]
- 21.Hooper C, Killick R, and Lovestone S, The GSK3 hypothesis of Alzheimer's disease. J Neurochem, 2008. 104(6): p. 1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulf G, et al. , Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol, 2005. 7(5): p. 435–41. [DOI] [PubMed] [Google Scholar]
- 23.Ping L, et al. , Global quantitative analysis of the human brain proteome and phosphoproteome in Alzheimer's disease. Sci Data, 2020. 7(1): p. 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai B, et al. , Deep Multilayer Brain Proteomics Identifies Molecular Networks in Alzheimer's Disease Progression. Neuron, 2020. 105(6): p. 975–991 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igarashi M and Okuda S, Evolutionary analysis of proline-directed phosphorylation sites in the mammalian growth cone identified using phosphoproteomics. Mol Brain, 2019. 12(1): p. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiene-Fischer C, Aumuller T, and Fischer G, Peptide bond cis/trans isomerases: a biocatalysis perspective of conformational dynamics in proteins. Top Curr Chem, 2013. 328: p. 35–67. [DOI] [PubMed] [Google Scholar]
- 27.Zhou XZ, et al. , Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell, 2000. 6(4): p. 873–83. [DOI] [PubMed] [Google Scholar]
- 28.Cho JH and Johnson GV, Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau's ability to bind and stabilize microtubules. J Neurochem, 2004. 88(2): p. 349–58. [DOI] [PubMed] [Google Scholar]
- 29.Kondo A, et al. , Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature, 2015. 523(7561): p. 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karikari TK, et al. , Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol, 2022. [DOI] [PubMed] [Google Scholar]
- 31.Esnault S, Shen ZJ, and Malter JS, Pinning down signaling in the immune system: the role of the peptidyl-prolyl isomerase Pin1 in immune cell function. Crit Rev Immunol, 2008. 28(1): p. 45–60. [DOI] [PubMed] [Google Scholar]
- 32.Westmark PR, et al. , Pin1 and PKMzeta sequentially control dendritic protein synthesis. Sci Signal, 2010. 3(112): p. ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sosa LJ, et al. , Protein interacting with NIMA (never in mitosis A)-1 regulates axonal growth cone adhesion and spreading through myristoylated alanine-rich C kinase substrate isomerization. J Neurochem, 2016. 137(5): p. 744–55. [DOI] [PubMed] [Google Scholar]
- 34.Stallings NR, et al. , Pin1 mediates Abeta42-induced dendritic spine loss. Sci Signal, 2018. 11(522). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Z and Hunter T, Prolyl isomerase Pin1 in cancer. Cell Res, 2014. 24(9): p. 1033–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wulf G, et al. , The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res, 2003. 5(2): p. 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryo A, et al. , Prolyl isomerase Pin1: a catalyst for oncogenesis and a potential therapeutic target in cancer. J Cell Sci, 2003. 116(Pt 5): p. 773–83. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, et al. , Prolyl isomerase Pin1: a promoter of cancer and a target for therapy. Cell Death Dis, 2018. 9(9): p. 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu PJ, et al. , Critical role of WW domain phosphorylation in regulating phosphoserine binding activity and Pin1 function. J Biol Chem, 2002. 277(4): p. 2381–4. [DOI] [PubMed] [Google Scholar]
- 40.Lee YC, et al. , Pin1 acts as a negative regulator of the G2/M transition by interacting with the Aurora-A-Bora complex. J Cell Sci, 2013. 126(Pt 21): p. 4862–72. [DOI] [PubMed] [Google Scholar]
- 41.Chen D, Wang L, and Lee TH, Post-translational Modifications of the Peptidyl-Prolyl Isomerase Pin1. Front Cell Dev Biol, 2020. 8: p. 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee TH, et al. , Death-associated protein kinase 1 phosphorylates Pin1 and inhibits its prolyl isomerase activity and cellular function. Mol Cell, 2011. 42(2): p. 147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu N, et al. , The C113D mutation in human Pin1 causes allosteric structural changes in the phosphate binding pocket of the PPIase domain through the tug of war in the dual-histidine motif. Biochemistry, 2014. 53(34): p. 5568–78. [DOI] [PubMed] [Google Scholar]
- 44.Chen D, Zhou XZ, and Lee TH, Death-Associated Protein Kinase 1 as a Promising Drug Target in Cancer and Alzheimer's Disease. Recent Pat Anticancer Drug Discov, 2019. 14(2): p. 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CH, et al. , Pin1 cysteine-113 oxidation inhibits its catalytic activity and cellular function in Alzheimer's disease. Neurobiol Dis, 2015. 76: p. 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choudhary C, et al. , Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science, 2009. 325(5942): p. 834–40. [DOI] [PubMed] [Google Scholar]
- 47.Chen CH, et al. , SENP1 deSUMOylates and regulates Pin1 protein activity and cellular function. Cancer Res, 2013. 73(13): p. 3951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akimov V, et al. , UbiSite approach for comprehensive mapping of lysine and N-terminal ubiquitination sites. Nat Struct Mol Biol, 2018. 25(7): p. 631–640. [DOI] [PubMed] [Google Scholar]
- 49.Larsen SC, et al. , Proteome-wide analysis of arginine monomethylation reveals widespread occurrence in human cells. Sci Signal, 2016. 9(443): p. rs9. [DOI] [PubMed] [Google Scholar]
- 50.Lu PJ, et al. , The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature, 1999. 399(6738): p. 784–8. [DOI] [PubMed] [Google Scholar]
- 51.Illenberger S, et al. , The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer's disease. Mol Biol Cell, 1998. 9(6): p. 1495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smet C, et al. , The peptidyl prolyl cis/trans-isomerase Pin1 recognizes the phospho-Thr212-Pro213 site on Tau. Biochemistry, 2004. 43(7): p. 2032–40. [DOI] [PubMed] [Google Scholar]
- 53.Bulbarelli A, et al. , Pin1 affects Tau phosphorylation in response to Abeta oligomers. Mol Cell Neurosci, 2009. 42(1): p. 75–80. [DOI] [PubMed] [Google Scholar]
- 54.Galas MC, et al. , The peptidylprolyl cis/trans-isomerase Pin1 modulates stress-induced dephosphorylation of Tau in neurons. Implication in a pathological mechanism related to Alzheimer disease. J Biol Chem, 2006. 281(28): p. 19296–304. [DOI] [PubMed] [Google Scholar]
- 55.Arendt T, et al. , Cortical distribution of neurofibrillary tangles in Alzheimer's disease matches the pattern of neurons that retain their capacity of plastic remodelling in the adult brain. Neuroscience, 1998. 83(4): p. 991–1002. [DOI] [PubMed] [Google Scholar]
- 56.Holzer M, et al. , Abnormally phosphorylated tau protein in Alzheimer's disease: heterogeneity of individual regional distribution and relationship to clinical severity. Neuroscience, 1994. 63(2): p. 499–516. [DOI] [PubMed] [Google Scholar]
- 57.Holzer M, et al. , Inverse association of Pin1 and tau accumulation in Alzheimer's disease hippocampus. Acta Neuropathol, 2002. 104(5): p. 471–81. [DOI] [PubMed] [Google Scholar]
- 58.Liou YC, et al. , Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature, 2003. 424(6948): p. 556–61. [DOI] [PubMed] [Google Scholar]
- 59.Fujimori F, et al. , Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G(0) arrest. Biochem Biophys Res Commun, 1999. 265(3): p. 658–63. [DOI] [PubMed] [Google Scholar]
- 60.Ishihara T, et al. , Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron, 1999. 24(3): p. 751–62. [DOI] [PubMed] [Google Scholar]
- 61.Lewis J, et al. , Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet, 2000. 25(4): p. 402–5. [DOI] [PubMed] [Google Scholar]
- 62.Atchison FW and Means AR, Spermatogonial depletion in adult Pin1-deficient mice. Biol Reprod, 2003. 69(6): p. 1989–97. [DOI] [PubMed] [Google Scholar]
- 63.Atchison FW, Capel B, and Means AR, Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development, 2003. 130(15): p. 3579–86. [DOI] [PubMed] [Google Scholar]
- 64.Hsiao K, et al. , Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science, 1996. 274(5284): p. 99–102. [DOI] [PubMed] [Google Scholar]
- 65.Irizarry MC, et al. , APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol, 1997. 56(9): p. 965–73. [DOI] [PubMed] [Google Scholar]
- 66.Lanz TA, Carter DB, and Merchant KM, Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol Dis, 2003. 13(3): p. 246–53. [DOI] [PubMed] [Google Scholar]
- 67.Jacobsen JS, et al. , Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A, 2006. 103(13): p. 5161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pastorino L, et al. , The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature, 2006. 440(7083): p. 528–34. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki T, et al. , Cell cycle-dependent regulation of the phosphorylation and metabolism of the Alzheimer amyloid precursor protein. EMBO J, 1994. 13(5): p. 1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oliveira J, et al. , Protein Phosphorylation is a Key Mechanism in Alzheimer's Disease. J Alzheimers Dis, 2017. 58(4): p. 953–978. [DOI] [PubMed] [Google Scholar]
- 71.Haass C, et al. , Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med, 2012. 2(5): p. a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pastorino L, et al. , Alzheimer's disease-related loss of Pin1 function influences the intracellular localization and the processing of AbetaPP. J Alzheimers Dis, 2012. 30(2): p. 277–97. [DOI] [PubMed] [Google Scholar]
- 73.Fisher CL, et al. , Cyclic cis-Locked Phospho-Dipeptides Reduce Entry of AbetaPP into Amyloidogenic Processing Pathway. J Alzheimers Dis, 2017. 55(1): p. 391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barbagallo AP, et al. , Tyr(682) in the intracellular domain of APP regulates amyloidogenic APP processing in vivo. PLoS One, 2010. 5(11): p. e15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sano Y, et al. , Physiological mouse brain Abeta levels are not related to the phosphorylation state of threonine-668 of Alzheimer's APP. PLoS One, 2006. 1: p. e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ando K, et al. , Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem, 2001. 276(43): p. 40353–61. [DOI] [PubMed] [Google Scholar]
- 77.Nakaya T and Suzuki T, Role of APP phosphorylation in FE65-dependent gene transactivation mediated by AICD. Genes Cells, 2006. 11(6): p. 633–45. [DOI] [PubMed] [Google Scholar]
- 78.Gandy S, Czernik AJ, and Greengard P, Phosphorylation of Alzheimer disease amyloid precursor peptide by protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A, 1988. 85(16): p. 6218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim BM, et al. , Inhibition of death-associated protein kinase 1 attenuates the phosphorylation and amyloidogenic processing of amyloid precursor protein. Hum Mol Genet, 2016. 25(12): p. 2498–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buxbaum JD, et al. , Processing of Alzheimer beta/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation. Proc Natl Acad Sci U S A, 1990. 87(15): p. 6003–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma SL, et al. , Prolyl isomerase Pin1 promotes amyloid precursor protein (APP) turnover by inhibiting glycogen synthase kinase-3beta (GSK3beta) activity: novel mechanism for Pin1 to protect against Alzheimer disease. J Biol Chem, 2012. 287(10): p. 6969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimizu T, et al. , Prolyl Isomerase Pin1 Directly Regulates Calcium/Calmodulin-Dependent Protein Kinase II Activity in Mouse Brains. Front Pharmacol, 2018. 9: p. 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abrahamsen H, et al. , Peptidyl-prolyl isomerase Pin1 controls down-regulation of conventional protein kinase C isozymes. J Biol Chem, 2012. 287(16): p. 13262–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selkoe DJ, Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci, 2000. 924: p. 17–25. [DOI] [PubMed] [Google Scholar]
- 85.Hardy J and Selkoe DJ, The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science, 2002. 297(5580): p. 353–6. [DOI] [PubMed] [Google Scholar]
- 86.Scheff SW, et al. , Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology, 2007. 68(18): p. 1501–8. [DOI] [PubMed] [Google Scholar]
- 87.Shankar GM, et al. , Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med, 2008. 14(8): p. 837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mattson MP, et al. , beta-Amyloid precursor protein metabolites and loss of neuronal Ca2+ homeostasis in Alzheimer's disease. Trends Neurosci, 1993. 16(10): p. 409–14. [DOI] [PubMed] [Google Scholar]
- 89.Behl C, et al. , Hydrogen peroxide mediates amyloid beta protein toxicity. Cell, 1994. 77(6): p. 817–27. [DOI] [PubMed] [Google Scholar]
- 90.Harris ME, et al. , Direct evidence of oxidative injury produced by the Alzheimer's beta-amyloid peptide (1-40) in cultured hippocampal neurons. Exp Neurol, 1995. 131(2): p. 193–202. [DOI] [PubMed] [Google Scholar]
- 91.Cheignon C, et al. , Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol, 2018. 14: p. 450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sultana R, et al. , Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging, 2006. 27(11): p. 1564–76. [DOI] [PubMed] [Google Scholar]
- 93.Butterfield DA, et al. , Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiol Dis, 2006. 22(2): p. 223–32. [DOI] [PubMed] [Google Scholar]
- 94.O'Neal MA, Stallings NR, and Malter JS, Alzheimer's Disease, Dendritic Spines, and Calcineurin Inhibitors: A New Approach? ACS Chem Neurosci, 2018. 9(6): p. 1233–1234. [DOI] [PubMed] [Google Scholar]
- 95.Cho YS, et al. , TPA-induced cell transformation provokes a complex formation between Pin1 and 90 kDa ribosomal protein S6 kinase 2. Mol Cell Biochem, 2012. 367(1-2): p. 85–92. [DOI] [PubMed] [Google Scholar]
- 96.Kim G, et al. , COT phosphorylates prolyl-isomerase Pin1 to promote tumorigenesis in breast cancer. Mol Carcinog, 2015. 54(6): p. 440–8. [DOI] [PubMed] [Google Scholar]
- 97.Ando K, et al. , Tau pathology modulates Pin1 post-translational modifications and may be relevant as biomarker. Neurobiol Aging, 2013. 34(3): p. 757–69. [DOI] [PubMed] [Google Scholar]
- 98.Eckerdt F, et al. , Polo-like kinase 1-mediated phosphorylation stabilizes Pin1 by inhibiting its ubiquitination in human cells. J Biol Chem, 2005. 280(44): p. 36575–83. [DOI] [PubMed] [Google Scholar]
- 99.Rangasamy V, et al. , Mixed-lineage kinase 3 phosphorylates prolyl-isomerase Pin1 to regulate its nuclear translocation and cellular function. Proc Natl Acad Sci U S A, 2012. 109(21): p. 8149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song B, et al. , Inhibition of Polo-like kinase 1 reduces beta-amyloid-induced neuronal cell death in Alzheimer's disease. Aging (Albany NY), 2011. 3(9): p. 846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou F, Xu Y, and Hou XY, MLK3-MKK3/6-P38MAPK cascades following N-methyl-D-aspartate receptor activation contributes to amyloid-beta peptide-induced apoptosis in SH-SY5Y cells. J Neurosci Res, 2014. 92(6): p. 808–17. [DOI] [PubMed] [Google Scholar]
- 102.Malleret G, et al. , Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell, 2001. 104(5): p. 675–86. [DOI] [PubMed] [Google Scholar]
- 103.Mansuy IM, et al. , Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell, 1998. 92(1): p. 39–49. [DOI] [PubMed] [Google Scholar]
- 104.Reese LC, et al. , Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell, 2008. 7(6): p. 824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reese LC, et al. , Dysregulated phosphorylation of Ca(2+) /calmodulin-dependent protein kinase II-alpha in the hippocampus of subjects with mild cognitive impairment and Alzheimer's disease. J Neurochem, 2011. 119(4): p. 791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taglialatela G, Rastellini C, and Cicalese L, Reduced Incidence of Dementia in Solid Organ Transplant Patients Treated with Calcineurin Inhibitors. J Alzheimers Dis, 2015. 47(2): p. 329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mansuy IM, Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun, 2003. 311(4): p. 1195–208. [DOI] [PubMed] [Google Scholar]
- 108.Rozkalne A, Hyman BT, and Spires-Jones TL, Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis, 2011. 41(3): p. 650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dineley KT, et al. , Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem, 2007. 88(2): p. 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cavallucci V, et al. , Calcineurin inhibition rescues early synaptic plasticity deficits in a mouse model of Alzheimer's disease. Neuromolecular Med, 2013. 15(3): p. 541–8. [DOI] [PubMed] [Google Scholar]
- 111.Cottrell JR, et al. , Working memory impairment in calcineurin knock-out mice is associated with alterations in synaptic vesicle cycling and disruption of high-frequency synaptic and network activity in prefrontal cortex. J Neurosci, 2013. 33(27): p. 10938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeng H, et al. , Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell, 2001. 107(5): p. 617–29. [DOI] [PubMed] [Google Scholar]
- 113.Mi Z, et al. , Loss of precuneus dendritic spines immunopositive for spinophilin is related to cognitive impairment in early Alzheimer's disease. Neurobiol Aging, 2017. 55: p. 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Counts SE, et al. , Hippocampal drebrin loss in mild cognitive impairment. Neurodegener Dis, 2012. 10(1-4): p. 216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DeKosky ST and Scheff SW, Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol, 1990. 27(5): p. 457–64. [DOI] [PubMed] [Google Scholar]
- 116.Terry RD, et al. , Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol, 1991. 30(4): p. 572–80. [DOI] [PubMed] [Google Scholar]
- 117.Oh J and Malter JS, Pin1-FADD interactions regulate Fas-mediated apoptosis in activated eosinophils. J Immunol, 2013. 190(10): p. 4937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shen ZJ, et al. , The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation. Nat Immunol, 2009. 10(3): p. 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dourlen P, et al. , The peptidyl prolyl cis/trans isomerase Pin1 downregulates the Inhibitor of Apoptosis Protein Survivin. Biochim Biophys Acta, 2007. 1773(9): p. 1428–37. [DOI] [PubMed] [Google Scholar]
- 120.Terry RD, Cell death or synaptic loss in Alzheimer disease. J Neuropathol Exp Neurol, 2000. 59(12): p. 1118–9. [DOI] [PubMed] [Google Scholar]
- 121.Hong HS, et al. , FK506 reduces amyloid plaque burden and induces MMP-9 in AbetaPP/PS1 double transgenic mice. J Alzheimers Dis, 2010. 22(1): p. 97–105. [DOI] [PubMed] [Google Scholar]
- 122.Selkoe DJ, Alzheimer's disease is a synaptic failure. Science, 2002. 298(5594): p. 789–91. [DOI] [PubMed] [Google Scholar]
- 123.Babri S, et al. , Effect of Aggregated beta-Amyloid (1-42) on Synaptic Plasticity of Hippocampal Dentate Gyrus Granule Cells in Vivo. Bioimpacts, 2012. 2(4): p. 189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Selkoe DJ, Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res, 2008. 192(1): p. 106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kelleher RJ 3rd, Govindarajan A, and Tonegawa S, Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron, 2004. 44(1): p. 59–73. [DOI] [PubMed] [Google Scholar]
- 126.Westmark CJ and Malter JS, FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol, 2007. 5(3): p. e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Todd PK, Mack KJ, and Malter JS, The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A, 2003. 100(24): p. 14374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu L, et al. , Pathological Role of Peptidyl-Prolyl Isomerase Pin1 in the Disruption of Synaptic Plasticity in Alzheimer's Disease. Neural Plast, 2017. 2017: p. 3270725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoeffer CA, et al. , Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron, 2008. 60(5): p. 832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mouri A, et al. , The role of cyclophilin D in learning and memory. Hippocampus, 2010. 20(2): p. 293–304. [DOI] [PubMed] [Google Scholar]
- 131.Du H, et al. , Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging, 2011. 32(3): p. 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Govindarajan A, et al. , The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron, 2011. 69(1): p. 132–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao G and Harris KM, Developmental regulation of the late phase of long-term potentiation (L-LTP) and metaplasticity in hippocampal area CA1 of the rat. J Neurophysiol, 2012. 107(3): p. 902–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Antonelli R, et al. , Pin1 Modulates the Synaptic Content of NMDA Receptors via Prolyl-Isomerization of PSD-95. J Neurosci, 2016. 36(20): p. 5437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Delgado JY, Nall D, and Selvin PR, Pin1 Binding to Phosphorylated PSD-95 Regulates the Number of Functional Excitatory Synapses. Front Mol Neurosci, 2020. 13: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He D, et al. , Disruption of the IL-33-ST2-AKT signaling axis impairs neurodevelopment by inhibiting microglial metabolic adaptation and phagocytic function. Immunity, 2022. 55(1): p. 159–173 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liang CS, et al. , The role of interleukin-33 in patients with mild cognitive impairment and Alzheimer's disease. Alzheimers Res Ther, 2020. 12(1): p. 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fu AK, et al. , IL-33 ameliorates Alzheimer's disease-like pathology and cognitive decline. Proc Natl Acad Sci U S A, 2016. 113(19): p. E2705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stewart CR, et al. , CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol, 2010. 11(2): p. 155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sheedy FJ, et al. , CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol, 2013. 14(8): p. 812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Griffin WS, et al. , Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A, 1989. 86(19): p. 7611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fillit H, et al. , Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci Lett, 1991. 129(2): p. 318–20. [DOI] [PubMed] [Google Scholar]
- 143.Bamberger ME, et al. , A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci, 2003. 23(7): p. 2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barger SW and Basile AS, Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem, 2001. 76(3): p. 846–54. [DOI] [PubMed] [Google Scholar]
- 145.Bachiller S, et al. , Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front Cell Neurosci, 2018. 12: p. 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heppner FL, Ransohoff RM, and Becher B, Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci, 2015. 16(6): p. 358–72. [DOI] [PubMed] [Google Scholar]
- 147.Morimoto K, et al. , Expression profiles of cytokines in the brains of Alzheimer's disease (AD) patients compared to the brains of non-demented patients with and without increasing AD pathology. J Alzheimers Dis, 2011. 25(1): p. 59–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ivanov P and Anderson P, Post-transcriptional regulatory networks in immunity. Immunol Rev, 2013. 253(1): p. 253–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pullmann R Jr., et al. , Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol, 2007. 27(18): p. 6265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nechama M, et al. , The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in IL-33-induced allergic airway inflammation. Nat Commun, 2018. 9(1): p. 1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tun-Kyi A, et al. , Essential role for the prolyl isomerase Pin1 in Toll-like receptor signaling and type I interferon-mediated immunity. Nat Immunol, 2011. 12(8): p. 733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Caput D, et al. , Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A, 1986. 83(6): p. 1670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shaw G and Kamen R, A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell, 1986. 46(5): p. 659–67. [DOI] [PubMed] [Google Scholar]
- 154.Hentze MW, et al. , Cloning, characterization, expression, and chromosomal localization of a human ferritin heavy-chain gene. Proc Natl Acad Sci U S A, 1986. 83(19): p. 7226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Esnault S, et al. , Pin1 modulates the type 1 immune response. PLoS One, 2007. 2(2): p. e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shen ZJ and Malter JS, Regulation of AU-Rich Element RNA Binding Proteins by Phosphorylation and the Prolyl Isomerase Pin1. Biomolecules, 2015. 5(2): p. 412–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shen ZJ, et al. , TLR-7 Stress Signaling in Differentiating and Mature Eosinophils Is Mediated by the Prolyl Isomerase Pin1. J Immunol, 2018. 201(12): p. 3503–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shen ZJ and Malter JS, Eosinophils, Pin1 and the Response to Respiratory Viral Infection and Allergic Stimuli. Crit Rev Immunol, 2019. 39(2): p. 135–149. [DOI] [PubMed] [Google Scholar]
- 159.Boussetta T, et al. , The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-alpha-induced priming of the NADPH oxidase in human neutrophils. Blood, 2010. 116(26): p. 5795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ryo A, et al. , Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell, 2003. 12(6): p. 1413–26. [DOI] [PubMed] [Google Scholar]
- 161.Atkinson GP, et al. , The prolyl isomerase Pin1 regulates the NF-kappaB signaling pathway and interleukin-8 expression in glioblastoma. Oncogene, 2009. 28(42): p. 3735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lau SF, Fu AKY, and Ip NY, Cytokine signaling convergence regulates the microglial state transition in Alzheimer's disease. Cell Mol Life Sci, 2021. 78(10): p. 4703–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Su JH, et al. , Activated caspase-3 expression in Alzheimer's and aged control brain: correlation with Alzheimer pathology. Brain Res, 2001. 898(2): p. 350–7. [DOI] [PubMed] [Google Scholar]
- 164.Brokaw DL, et al. , Cell death and survival pathways in Alzheimer's disease: an integrative hypothesis testing approach utilizing -omic data sets. Neurobiol Aging, 2020. 95: p. 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Galluzzi L, et al. , Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ, 2018. 25(3): p. 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wahl GM and Carr AM, The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat Cell Biol, 2001. 3(12): p. E277–86. [DOI] [PubMed] [Google Scholar]
- 167.Dougherty MK, et al. , Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell, 2005. 17(2): p. 215–24. [DOI] [PubMed] [Google Scholar]
- 168.Cheng CW, et al. , PIN1 inhibits apoptosis in hepatocellular carcinoma through modulation of the antiapoptotic function of survivin. Am J Pathol, 2013. 182(3): p. 765–75. [DOI] [PubMed] [Google Scholar]
- 169.Min SH, et al. , Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Mol Cell, 2012. 46(6): p. 771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Makinwa Y, Musich PR, and Zou Y, Phosphorylation-Dependent Pin1 Isomerization of ATR: Its Role in Regulating ATR's Anti-apoptotic Function at Mitochondria, and the Implications in Cancer. Front Cell Dev Biol, 2020. 8: p. 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang SC, Hu XM, and Xiong K, The regulatory role of Pin1 in neuronal death. Neural Regen Res, 2023. 18(1): p. 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dong Y, et al. , Hyperphosphorylated tau mediates neuronal death by inducing necroptosis and inflammation in Alzheimer's disease. J Neuroinflammation, 2022. 19(1): p. 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Del Rosario JS, et al. , Death Associated Protein Kinase (DAPK) -mediated neurodegenerative mechanisms in nematode excitotoxicity. BMC Neurosci, 2015. 16: p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang S, et al. , Pin1 Promotes Regulated Necrosis Induced by Glutamate in Rat Retinal Neurons via CAST/Calpain2 Pathway. Front Cell Neurosci, 2017. 11: p. 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Braak H, Braak E, and Bohl J, Staging of Alzheimer-related cortical destruction. Eur Neurol, 1993. 33(6): p. 403–8. [DOI] [PubMed] [Google Scholar]
- 176.Braak H and Braak E, Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol, 1991. 82(4): p. 239–59. [DOI] [PubMed] [Google Scholar]
- 177.Segat L, et al. , PIN1 promoter polymorphisms are associated with Alzheimer's disease. Neurobiol Aging, 2007. 28(1): p. 69–74. [DOI] [PubMed] [Google Scholar]
- 178.Ma SL, et al. , A PIN1 polymorphism that prevents its suppression by AP4 associates with delayed onset of Alzheimer's disease. Neurobiol Aging, 2012. 33(4): p. 804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Park JS, et al. , Brain somatic mutations observed in Alzheimer's disease associated with aging and dysregulation of tau phosphorylation. Nat Commun, 2019. 10(1): p. 3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Okamoto M, et al. , Identification of death-associated protein kinases inhibitors using structure-based virtual screening. J Med Chem, 2009. 52(22): p. 7323–7. [DOI] [PubMed] [Google Scholar]
- 181.Gan CL, et al. , Inhibition of Death-associated Protein Kinase 1 protects against Epileptic Seizures in mice. Int J Biol Sci, 2021. 17(9): p. 2356–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu LZ, Li BQ, and Jia JP, DAPK1: a Novel Pathology and Treatment Target for Alzheimer's Disease. Mol Neurobiol, 2019. 56(4): p. 2838–2844. [DOI] [PubMed] [Google Scholar]
- 183.Audard J, et al. , Inhibition of the Receptor for Advanced Glycation End-Products in Acute Respiratory Distress Syndrome: A Randomised Laboratory Trial in Piglets. Sci Rep, 2019. 9(1): p. 9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mullard A, Anti-tau antibody failures stack up. Nat Rev Drug Discov, 2021. 20(12): p. 888. [DOI] [PubMed] [Google Scholar]
- 185.van Dyck CH, Anti-Amyloid-beta Monoclonal Antibodies for Alzheimer's Disease: Pitfalls and Promise. Biol Psychiatry, 2018. 83(4): p. 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]