Abstract
Background
Population screening for genetic risk of adult-onset preventable conditions has been proposed as an attractive public health intervention. Screening unselected individuals can identify many individuals who will not be identified through current genetic testing guidelines.
Methods
We sought to evaluate enrollment in and diagnostic yield of population genetic screening in a resource-limited setting among a diverse population. We developed a low-cost, short-read next-generation sequencing panel of 25 genes that had 98.4% sensitivity and 99.98% specificity compared to diagnostic panels. We used email invitations to recruit a diverse cohort of patients in the University of Washington Medical Center system unselected for personal or family history of hereditary disease. Participants were sent a saliva collection kit in the mail with instructions on kit use and return. Results were returned using a secure online portal. Enrollment and diagnostic yield were assessed overall and across race and ethnicity groups.
Results
Overall, 40,857 people were invited and 2889 (7.1%) enrolled. Enrollment varied across race and ethnicity groups, with the lowest enrollment among African American individuals (3.3%) and the highest among Multiracial or Other Race individuals (13.0%). Of 2864 enrollees who received screening results, 106 actionable variants were identified in 103 individuals (3.6%). Of those who screened positive, 30.1% already knew about their results from prior genetic testing. The diagnostic yield was 74 new, actionable genetic findings (2.6%). The addition of more recently identified cancer risk genes increased the diagnostic yield of screening.
Conclusions
Population screening can identify additional individuals that could benefit from prevention, but challenges in recruitment and sample collection will reduce actual enrollment and yield. These challenges should not be overlooked in intervention planning or in cost and benefit analysis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13073-023-01174-7.
Keywords: Population genetic screening, Hereditary cancer, Hereditary hypercholesterolemia, Diverse communities
Background
Between 1 and 2% of the population has a genetic variant conferring a high lifetime risk of cancer or cardiovascular disease that can be mitigated by early application of screening, lifestyle changes, or medical intervention [1–4]. Despite the availability of clinical testing and useful prevention methods, over 85% of people who are detected to have hereditary cancer risk have already developed cancer [5–7]. Similarly, the majority of people with hereditary hypercholesterolemia find out about their disease predisposition later in adulthood [8]. Identifying at-risk individuals early on is a challenge. Many at-risk individuals do not have a family history of the disease, most physicians and health systems do not have a process to routinely screen family history for genetic testing criteria, and limited access to genetic counseling may prevent individuals with family history from seeking testing [1, 9, 10]. Cascade screening starting with individuals who have already been diagnosed could theoretically identify all at-risk individuals [11], but there are many barriers to effective family outreach [12, 13].
Expanding genetic screening to unselected members of the population has been proposed as a strategy to identify individuals who would benefit from timely medical intervention [14, 15]. The potential of population screening has been illustrated by studies that have implemented population screening using biobank samples in cohorts recruited for genomic research where as many as 75% of positive results were in individuals who would not have been identified through current genetic testing guidelines [1–4]. However, the effectiveness of population screening among those who are not already enrolled in genetic research has not been thoroughly studied. One Israeli study of BRCA1 and BRCA2 screening among Ashkenazi Jewish individuals found that healthcare recruiters achieved between 55 and 92% enrollment depending on the study site and recruitment strategy [16]. The DNA10K population genetic screening program in the NorthShore Health System in Illinois found 20% enrollment [17].
The diagnostic yield of invitation-based screening for genetic risk of preventable disease using a panel testing approach has also not been reported. Here, we define the diagnostic yield of population genetic screening as the number of individuals with newly-identified actionable findings. Many factors can influence diagnostic yield including the size, sensitivity, and specificity of the screening panel and the pretest characteristics of the target population specified in the design of the intervention. Including more genes in a screening panel is expected to increase yield. The Israeli screening study included three BRCA1 and BRCA2 variants common in the Ashkenazi Jewish population, BRCA1 NM_007294.3:c.68_69delAG, BRCA1 NM_007294.3:c.5266dupC, and BRCA2 NM_000059.3:c.5946delT [16]. Geisinger’s MyCode research project, the Healthy Nevada Project, and several ongoing screening studies have opted to use broader genetic screening panels [1, 3, 4, 17, 18]. Recruitment strategy can also influence yield. Provider-referral to genetic screening has been associated with higher participation than self-referral; however, self-referral may have higher diagnostic yield due to a higher likelihood of a personal and family history of the condition screened among self-referred [4, 16]. Redundant testing, which may or may not be avoidable, reduces meaningful yield; MyCode and the Healthy Nevada Project screening trials reported that positive results were already available to 18% and 12% of screened participants, respectively [1, 2].
We sought to conduct population genetic screening for common, preventable inherited diseases among a group of the adult University of Washington Medicine (UWM) patients. The mandate of the study funders was to develop an effective, low-cost screening panel of 20 to 30 genes related to hereditary cancer and hyperlipidemia; then recruit and screen over 2500 individuals enriched for social and ethnic minorities in 2 years. Time and financial constraints precluded extensive community outreach or multi-arm trials. Thus, a feature of this study is that it may reflect the exigencies of public health programs more accurately than prior studies of population screening.
Understanding the feasibility and effectiveness of population genetic screening in a diverse population is dependent on both the enrollment and yield among those who may be less likely to receive genetic testing with the current barriers to healthcare [19]. Our goals were to identify the baseline enrollment and yield of genetic screening among a diverse group in a limited resource setting, which contrasts with previously published population genetic screening studies performed in the context of prior biobank recruitment and consent. Study enrollment and dropout were assessed overall and across race and ethnicity. Results may guide future public health population genetic screening efforts and inform appropriate cost-effectiveness evaluations.
Methods
Participants
Participants for genetic screening were identified through a UWM medical record search. Potential participants were limited to adults 25 to 60 years old who had visited UWM hospitals or clinics at least two times in the last 5 years (2015–2020) to increase the chances that participants would have access to follow-up care. We focused on this age range to maximize the possibility that participants would benefit from any recommended preventive interventions based on genetic screening results. UWM includes a tertiary/quaternary care academic hospital, two community hospitals, a safety-net hospital providing much of the uncompensated care in the region, and a network of primary care clinics throughout Western Washington.
Patients who self-identified as racial and ethnic minorities in the electronic health record (EHR) were preferentially selected for recruitment to assess the feasibility of population genetic screening among a diverse population (see Fig. 1 and study protocol in Additional File 1: Figure S1). Exact enrollment goals for self-identified racial and ethnic minorities were defined by our partners to meet the needs of a separate project and were 10% African American, 46% Asian American, 10% Hispanic, 6% Native American or Pacific Islander, 20% White and 8% identifying as Other or Multiracial. A total of 40,857 people were invited to enroll in the population genetic screening research study conducted at UWM. In order to ensure input from sexual and gender minorities 1,000 of these invitations were sent to individuals self-identifying as LGBTQ + according to EHR records. Demographic details of enrolled participants are listed in Table S1: Detailed demographics of enrollees (Additional File 1).
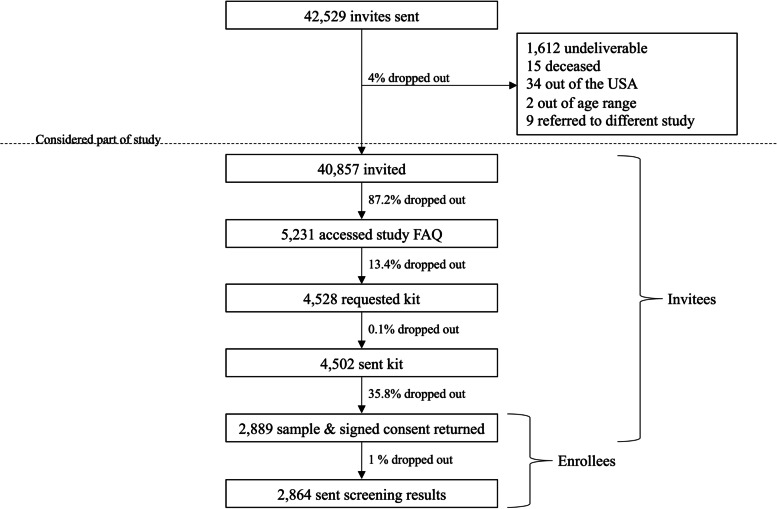
Fig. 1.
Study participation at different stages of population genetic screening. Flow diagram showing the enrollment and dropout of study participants. “Dropout” at the last stage may have been due to technical failures or failure to return results to participants
Individuals were excluded if their coded EHR information indicated previous orders for hereditary cancer panel testing ordered through the University of Washington. However, no detailed medical record review was conducted to exclude individuals who may have received genetic testing prior to entry into the University of Washington health system or testing not recorded formally in the EHR. No other specific health profiles were selected for inclusion or exclusion. Study invitations were sent via email and enrollment forms were only available online and in English. The study was approved by the University of Washington IRB (00009032).
Protocol
Recruitment email invitations
Study recruitment, data collection, and enrollment steps are depicted in Figure S1 (Additional File 1). Study invitations and subsequent surveys were sent from June 2020 to July 2021 using REDCap. Email invitations (see Additional File 1: Supplemental Methods, Initial recruitment email) appeared to come from the study email, a University of Washington email address, were signed by the study PI (BHS), and contained an introductory description to the study and a link to additional study information. The link led to study information and frequently asked questions (FAQ) (see Additional File 1: Supplemental Methods, Study information), which included details about genetic screening and study consent. Individuals who did not enroll in screening were sent up to two reminder emails about study participation.
Baseline data collection (T0), DNA kit request, and enrollment
At the end of the FAQ, interested persons were asked if they were willing to continue to answer an initial survey (T0) containing 24 close-ended questions related to personal and family medical history, factors influencing decision-making about genetic screening, and intent to share results (Additional File 1: Supplemental Methods, T0 Survey). After the survey, people were asked if they would like to receive an at-home DNA sample collection kit. People who declined were asked if they would voluntarily share their reason(s) at several points of the online enrollment process.
Those who requested DNA screening were sent an Oragene OGR 500 saliva collection kit, written and emailed electronic study consent forms, and a stamped return envelope. If completed kits and consent forms were not returned within three weeks, up to three reminder emails were sent to encourage completion. In some instances, a phone call from the study coordinator replaced the final reminder email.
Study enrollment was complete once both the DNA kit with a collected sample and signed consent forms were returned. Invitees include all people who were sent study invites while enrollees include those who provided DNA samples and signed consent forms.
Panel and sequencing
Once DNA kits were received in the laboratory, the samples were sequenced. Genes were selected for panel sequencing if National Comprehensive Cancer Network (NCCN) detection, prevention, and risk reduction guidelines contained gene-specific mentions or if related to CDC Tier 1 applications (see Table 1 for a list of genes sequenced) [20, 21]. There was a trade-off between size and cost with no clear cutoff about which genes to include and which to exclude. With small capture and multiplexing, the cost of capture and sequencing reagents was under $100 per person. Sequencing was performed using Illumina sequencing on MiSeq or Next Seq sequencers after Lotus capture. The bioinformatics pipeline for next-generation sequence analysis was a minimally-modified version of the clinical UW ColoSeq assay pipeline [22, 23]. The assay was able to detect single nucleotide changes, indels, and larger copy number variants with 98.4% sensitivity and 99.98% across all variant types. Testing was done within the University of Washington, Genetics and Solid Tumors Laboratory, which is CAP accredited and licensed to perform clinical testing in the State of Washington (see Supplemental Methods in Additional File 1 for assay information and sequencing details).
Table 1.
Full list of genes screened
| Gene | Associated condition(s) |
|---|---|
| APC | Colon polyps, colon cancer |
| APOB | High cholesterol, coronary artery disease |
| ATM | Breast cancer |
| BMPR1A | Colon polyps, colon cancer |
| BRCA1 | Breast, ovarian, and pancreatic cancer |
| BRCA2 | Breast, ovarian, and pancreatic cancer |
| BRIP1 | Breast and ovarian cancer |
| CDH1 | Breast and stomach cancer |
| CHEK2 | Breast and colon cancer |
| EPCAM | Colon, endometrial, and ovarian cancer |
| HOXB13 | Prostate cancer |
| LDLR | High cholesterol, coronary artery disease |
| MLH1 | Colon, endometrial, and ovarian cancer |
| MSH2 | Colon, endometrial, and ovarian cancer |
| MSH6 | Colon, endometrial, and ovarian cancer |
| MUTYH | Colon polyps, colon cancer |
| NTHL1 | Colon polyps, colon cancer |
| PALB2 | Breast and ovarian cancer |
| PMS2 | Colon, endometrial, and ovarian cancer |
| PTEN | Colon polyps, colon cancer |
| RAD51C | Ovarian cancer |
| RAD51D | Ovarian cancer |
| SMAD4 | Colon polyps, colon cancer |
| STK11 | Colon polyps, colon cancer |
| TP53 | Breast and many other cancers |
Post DNA kit return data collection (T1)
Enrollees were notified of kit receipt at the lab via email and asked to complete a second online survey (T1), which asked more detailed questions about personal and family medical history, demographics, intent to share results, and knowledge about genetics (Additional File 1: Supplemental Methods, T1 Survey). The T1 survey also included questions about prior genetic testing, bone marrow transplants, and others in their family who had received genetic testing results. Up to two reminder emails were sent to encourage completion.
Results return
Enrollees whose test indicated no increased risk for the screened conditions were notified that their results were available via email and were able to access their result letter for viewing and printing on a secure and private website using two identifiers: a code uniquely assigned to them and their birthdate. This result letter (Additional File 1: Supplemental methods, Example uninformative results letter) indicated that no actionable DNA changes were detected in the screened genes (even if a likely benign, benign, or variant of uncertain significance was detected in their sample, details of such variants were not provided). The purpose of the letter was to point out that the enrollee’s risk was not known to be increased compared to the general population. Importantly, the letter further emphasized that enrollees should consult with their physician for personal prevention recommendations given their lifestyle and personal or family health history.
Only variants classified as pathogenic and likely pathogenic were reported. Lower penetrance variants were only reported if there were multiple meta-analyses supporting increased risk. A study genetic counselor contacted enrollees whose test indicated increased risk to schedule a phone conversation. The enrollee’s result letter (Additional File 1: Supplemental Methods, Example positive results letter) was posted on the secure website described previously at the time of genetic counseling. Genetic testing results were not placed in the EHR, and enrollees were encouraged to share their results with their medical provider and obtain confirmatory clinical testing. Both the consent form and result letter stressed that medical management and/or follow-up would not occur within the research study. Study genetic counselor and faculty were available for additional consultations with the enrollee or their provider, if requested.
Post result return data collection (T2)
Enrollees received an email request to complete an optional post-results survey (T2) after they were sent their genetic screening results. The T2 survey consisted of both close- and open-ended items and asked about plans to share screening results, future health plans, and feelings about results and genetic screening (Additional File 1: Supplemental Methods, T2 Survey). Up to two reminder emails were sent to encourage completion.
Data analysis
We used descriptive statistics to evaluate enrollment, yield, and result return. We used logistic regression to examine the association between race and ethnicity and enrollment. Model A included age, gender, and race and ethnicity with “Asian” used as a reference. Model B added a gender by race and ethnicity interaction term. We also conducted an exploratory analysis to assess the relationship of sexual orientation with study enrollment by adding sexual orientation and a sexual orientation by race and ethnicity interaction term to Model A. We used self-reported personal and family history, including history of prior genetic testing to determine if enrollees were likely to have qualified for genetic testing under NCCN guidelines for testing cancer-risk genes or if they would have qualified for familial hypercholesterolemia testing under the American College of Cardiology guidelines [24, 25]. Because personal and family history were self-reported and the study did not have access to complete medical records, it was only possible to evaluate a few of the guideline criteria. Comparisons of self-report of testing between groups receiving positive and uninformative results were performed using Fisher’s exact test. We applied the RE-AIM framework to briefly summarize the study results [26]. Additional details about data coding and analysis are provided in a Supplemental Methods section of Additional File 1.
Results
Study demographics
Of the 40,857 people invited to enroll in the population genetic screening research study, 2889 (7%) enrolled. Demographics of invitees and enrollees are shown in Table 2. While 54% of invitees were female, 60% of enrollees were female. Within enrollees, 12% were African American, 57% Asian, 6.2% Native American, 0.8% Multiracial or Other Race, and 23% White. Detailed information about enrollees, including health history, education, and income, is listed in Table S1 (Additional File 1). Among enrollees, 53% of people had a family history of a cancer diagnosis, 61% reported having a college or advanced degree, and 38% reported a household income greater than $100,000.
Table 2.
Electronic health record demographics of study invitees and enrollees
| Invitees | Enrollees | |
|---|---|---|
| N | 40,857 | 2889 |
| Agea | 39.6 (10.2) | 40.3 (10.1) |
| Genderb | ||
| Female | 22,207 (54.3) | 1720 (59.5) |
| Male | 18,537 (45.4) | 1099 (38.1) |
| Other | 81 (0.2) | 50 (1.7) |
| Prefer not to answer | 32 (0.1) | 20 (0.7) |
| Raceb | ||
| African American | 10,639 (26) | 354 (12.3) |
| Asian | 22,043 (54) | 1646 (57.0) |
| Multiracial/other | 177 (0.4) | 23 (0.8) |
| Native American | 2543 (6.2) | 182 (6.2) |
| White | 5437 (13.3) | 671 (23.2) |
| Missing | 18 (0.1) | 13 (0.5) |
| Ethnicityb | ||
| Hispanic | 4099 (10) | 369 (12.8) |
| Non-Hispanic | 36,739 (89.9) | 2507 (86.8) |
| Missing | 19 (0.1) | 13 (0.4) |
aMean (SD)
bN (%)
Screening enrollment and dropout
Email invitations for screening were undeliverable in 1612 (3.8%) instances, 87.2% of invitees did not click on the link in the study invitation, and 13.4% did not request a DNA kit for sample collection after reading more about the screening study (Fig. 1). Dropout was also seen after kits for sample collection were sent, with 35.8% not completing collection or signing consent forms. In total, 2864 individuals received screening results (7% of those invited and 99% of those that returned kits and consent). A study genetic counselor was able to speak with 102/103 (99%) individuals receiving positive screening results. For individuals receiving uninformative screening results, 2399/2761 (87%) accessed their result letter online.
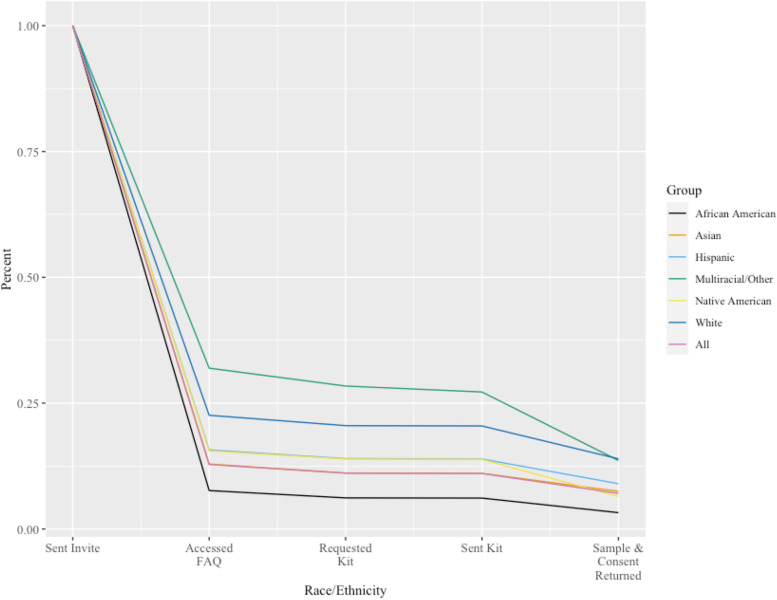
Trends in study involvement and dropout for all invitees and across race and ethnicity groups are shown in Fig. 2 and are detailed in Table S2 (Additional File 1). African American individuals had the lowest rate of accepting the invitation to learn about the study compared to other groups, and a higher percentage of the small Multiracial or Other identifying individuals group continued to access the study FAQ compared to other groups. Between when DNA sample collection kits and consent forms were sent out and returned, the greatest dropout was seen for Multiracial or Other Race individuals and Native American individuals.
Fig. 2.
Study dropout by race and ethnicity. Lines trace the proportion of each group remaining in the study cohort at five stages of the study. The entire group is represented by the red line
After adjusting for age and gender, results from logistic regression Model A (Additional File 1: Table S3) showed that African American individuals had lower odds of enrollment compared to Asian individuals (OR: 0.41, 95% CI: [0.36, 0.46]). White, Multiracial or Other Race, and Hispanic individuals had higher odds of enrollment compared to Asian individuals (OR: 1.81, 95% CI: [1.61, 2.04]; OR: 1.75, 95% CI: [1.1, 2.79]; OR: 1.21, 95% CI: [1.07, 1.36], respectively). Enrollment odds did not significantly differ between Native American and Asian individuals. An exploratory analysis adding sexual orientation and a sexual orientation by race and ethnicity interaction term to this model showed no independent association between sexual orientation and enrollment.
Results from Model B showed a significant interaction with male gender and African American race (p = 0.004), such that the odds of enrollment were lower for African American men compared to African American women (Additional File 1: Table S4). Similarly, a significant interaction was also seen with male gender and Hispanic ethnicity (p = 0.008), such that the odds of enrollment were lower for Hispanic men compared to Hispanic women.
Screening yield
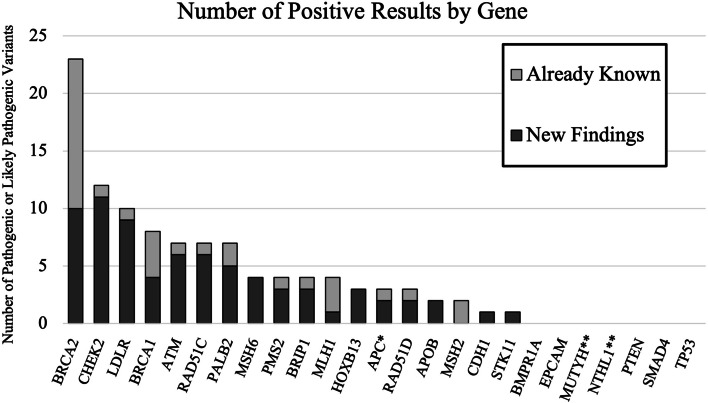
Of the 2864 enrollees who received results, 103 screened positive for at least one actionable variant (3.6%) (see Additional File 1: Table S5 for a complete list of variants identified). Positive results were reported for 17 unique genes (Fig. 3). Three individuals had pathogenic variants in two genes (ATM and BRIP1, BRCA2 and CHEK2, BRCA2 and LDLR). The test panel identified 57 pathogenic or likely pathogenic variants in 9 genes (BRCA1, BRCA2, MLH1, MSH2, MSH6, PMS2, EPCAM, APOB, and LDLR) associated with CDC Tier-1 priority syndromes for genetic disease prevention (Hereditary breast and ovarian cancer (HBOC), Lynch syndrome, Familial hypercholesterolemia (FH)) [20] giving a yield of 2.0% for these genes. The panel identified 48 pathogenic or likely pathogenic variants in 10 other genes, most of which cause a relatively lower lifetime risk of disease than Tier-1 syndrome-associated genes, giving a 1.6% carrier rate for this group of genes. No positive results were identified in the BMPR1A, EPCAM, MUTYH, NTHL1, PTEN, SMAD4, or TP53 genes. The most frequently observed variant was reported in five individuals (CHEK2 NM_007194.3:c.470 T > C), four variants were reported in three individuals (CHEK2 NM_007194.3:c.1100del, HOXB13 NM_006361.6:c.251G > A, LDLR NM_000527.4:c.1747C > T, and RAD51C NM_058216.3:c.394dup), and two variants reported in two individuals (APC NM_000038.5:c.3920 T > A and APOB NM_000384.3:c.10580G > A). Many of these variants are common, lower-penetrance variants without clear actionability in the population screening setting.
Fig. 3.
Screening results. Number of positive results by gene. Light gray bars show results that were already known by participants. Dark gray bars show new findings. *New findings were for APC p.Ile1307Lys, already know finding was for APC c.221-2A > G. **Study design was that only homozygous or compound heterozygous MUTYH and NTHL1 individuals were to be reported as positive
Thirty-one of the 103 individuals (30.1%) who screened positive reported knowing about their results from prior genetic testing in the T1 survey (Table 3), although two of the three individuals who had two findings only knew about one of these (Additional File 1: Table S5). Of the 2761 enrollees with uninformative results, 56 (2.0%) reported receiving prior sequencing for at least one of the genes on the screening panel, but none of the enrollees with uninformative results reported previous knowledge of having pathogenic or likely pathogenic variants in the genes tested. The frequency of reporting prior testing for genes in the panel was significantly different between those receiving positive and uninformative results (p < 0.0001). For the 9 genes associated with Tier-1 syndromes, 42% (24/57) of pathogenic variants were already known reducing the true diagnostic yield from 2.0% to 1.2% for these genes. For other genes, 17% (8/48) of variants were already known, reducing diagnostic yield from 1.6% to 1.4% for this group of genes. Previously known variants were most likely to be associated with Tier-1 syndromes (p = 0.006). The overall diagnostic yield was 2.6%, with 74 new pathogenic or likely pathogenic variants identified in the 2864 samples sequenced.
Table 3.
Enrollee personal and family history of disease
| N | Aware of risk variant prior to screen | Known close relativea with relevant variant | Personal diagnosis of disease (any age) | Close relativea diagnosed with disease (any age) | Met any guidelines for testing | |
|---|---|---|---|---|---|---|
| Enrollees with positive screening (n = 103b) | ||||||
| BOCc | 73 | 23 | 16 | 12 | 33 | 16 |
| Lynch, CRC, polyposisd | 30 | 7 | 4 | 5 | 7 | 5 |
| FHe | 13 | 1 | 0 | NA | NA | 0 |
| Enrollees with uninformative screening (n = 2761) | ||||||
| BOC | NA | NA | 18 | 99 | 717 | 45 |
| Lynch, CRC, polyposis | NA | NA | 6 | 30 | 345 | 59 |
| FH | NA | NA | 0 | NA | NA | 12 |
aParent, sibling, child
bOne enrollee received positive screening results for both BOC (breast or ovarian cancer) and FH. CHEK2 was considered to increase risk for both breast and colorectal cancer (CRC). HOXB13 was not considered in any of these three groups
cGenes considered to be associated with BOC: ATM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, PALB2, RAD51C, RAD51D
dGenes with results considered to be associated with Lynch syndrome, CRC, or polyposis: APC, CHEK2, MLH1, MSH2, MSH6, PMS2, STK11
eGenes considered to be associated with FH: APOB, LDLR
Personal and family history of cancer and guidelines
Twenty individuals with positive results were aware of a pathogenic or likely pathogenic variant in a first-degree relative before enrolling in the study (19.4%) (Table 3). Of these individuals who knew about a close relative with their variant, genetic screening results were already known to 17 and new to three of the individuals (2.9% of all enrollees with positive results); one of these three received clinical testing after enrolling in the study and before receiving screening results. Only 24 people with uninformative screening results (0.9%) reported knowledge of a pathogenic variant in a first degree relative in study surveys. Of the 61 individuals who were likely to have met HBOC screening criteria, 16 had variants associated with an increased risk of breast or ovarian cancer. Of the 64 individuals who were likely to have met Lynch syndrome or polyposis screening criteria, five had variants associated with increased colon cancer risk or polyposis. Of the 12 screened who were likely to have met personal and family history criteria for hypercholesterolemia screening, none had variants associated with familial hypercholesterolemia. Overall, 21 of 103 (20.5%) people with positive screening results and 116 of 2761 (4.2%) people with uninformative screening results were likely to have met guidelines for diagnostic testing based on self-reported information from study surveys.
RE-AIM results summary [26]
Reach
The target population was a diverse group of patients receiving medical care at UWM, unselected for personal or family history of hereditary disease. The average age of invitees and enrollees was approximately 40 years old. While 54% of invitees were female, about 60% of enrollees were female. Among invitees, 26% were African American, 54% Asian, 0.4% Multiracial or Other Race, 6.2% Native American, and 13.3% White. Within enrollees, 12.3% were African American, 57% Asian, 0.8% Multiracial or Other Race, 6.2% Native American, and 23.2% White.
Effectiveness
The largest amount of study dropout was seen after email invitations were sent for screening (87.2%). Larger dropout also occurred after DNA kits were sent out for sample collection, with 35.8% of people receiving kits not returning DNA samples and signed consent forms. New findings in genes related to CDC Tier 1 syndromes were identified in 1.2% of enrollees. New findings in other genes with possible actionability were identified in 1.4% of enrollees.
Adoption
Not evaluated outside custom study protocols.
Implementation
The study was designed with a minimal resource setting in mind. Invitations, surveys, and uninformative results reports were automated. All sample and sequencing supplies and reagents cost less than $100 per person. Telehealth genetic counseling was provided to individuals with positive results.
Maintenance/sustainment
Not evaluated as part of this study.
Discussion
The methods used in this screening study for assay design; ascertaining, consenting, and enrolling participants; collecting samples; and disseminating genetic results may be representative of what a population genetic screening program might look like if implemented as a public health initiative by a resource-limited healthcare or government organization. Theoretically, every trial will be tailored to both the resources available and the needs of the community. Importantly, our strategy differed from prior trials of population genetic screening which were designed to determine the yield of screening in an ideal situation using samples already collected, consented, and quality-controlled in a biorepository [1–4].
Enrollment in our genetic screening trial was lower than that reported by previous population genetic screening studies [3, 17, 27]. Among new participants of the BioMe Biobank, 93% indicated that they wished to receive genetic results and more than 85% of people in the Geisinger MyCode biobank consented to participate in screening [3, 27]. It is possible participants in both biobank studies were already amenable to research participation and genetic testing leading to higher screening enrollment. Integration of screening into existing healthcare practices may also increase enrollment. DNA10K, a population genetic screening program mediated by primary care providers, reported that 78% of patients had a genetic screening order placed by their provider, but this was after only 28% of those contacted had expressed interest. Additionally, the majority of people invited for DNA10K screening were White [17]. Results from our study may more closely reflect enrollment outcomes if screening were implemented among an unselected population as part of a stand-alone program and suggest that long-term institutional buy-in and integrating programs with existing healthcare may be very important.
Implementing population screening is a challenging multi-step process. This study demonstrates that several issues still need to be addressed before participation is considered a viable option for many. Our results replicate historical patterns of differential use of genetic services [28–34] and highlight the need to recognize geographic, socioeconomic, education, gender, sexual orientation, and racial and ethnic diversity. The Healthy Nevada Project and Alabama Genomic Health Initiative (AGHI) have engaged in a variety of outreach efforts using mass media, public events, multiple site enrollment, and community partnerships and have seen success in recruiting individuals from different racial and ethnic backgrounds [35, 36]. On-going and long-term efforts to build trust through stakeholder engagement can also aid recruitment efforts and may be necessary for equitable enrollment [37]. Dropout between when kits were sent and returned indicates that in-person collection or additional support may be necessary to mitigate difficulties with sample collection. Dropout during this period may also signal that screening is not a high priority for some individuals, despite initial interest. Our results indicate that screening programs which include recruitment and sample collection may be very different than screening programs that take advantage of already collected, high-quality DNA. Efforts to use recruiters, recruitment sites, or physicians to engage the community are likely to yield higher enrollment and also substantially increase the overall costs of screening [16, 17, 38].
This study found that a large proportion of individuals with hereditary disease risk are missed under optimal guideline-based clinical testing, which is consistent with prior studies [1, 2, 39, 40]. Although we are not able to make direct comparisons, our data are consistent with findings that individuals with increased disease risk due to personal or family history may be more likely to self-refer to screening [16]. In addition, we found that individuals who have already received prior, potentially redundant or overlapping, genetic testing may also be more likely to seek additional testing through screening interventions. This may be due to an increased interest and comfort level with learning genetic information due to prior testing, a desire to confirm previous genetic results, and in the case of research projects such as ours, a stronger proclivity for research participation. It can be challenging to comprehensively determine who has and has not met prior genetic testing exclusion criteria with current EHRs [41, 42], which may also limit selective screening in health systems. If not implemented thoughtfully and systematically, the reality may be that genetic screening will be like other screening tests that are repeated many times as technology improves and patients move between health systems. Moving forward, recruitment procedures that more stringently assess prior genetic screening through a pre-test questionnaire could assist with guiding future screening decisions and limit potentially redundant testing.
The true diagnostic yield of 2.6% of this study was substantially lower than the 3.6% carrier rate or total percentage of individuals who received positive results. We were surprised at the high rate of individuals who already knew about their genetic risk, as it was greater than what has been reported in other screening programs such as MyCode (30% compared to approximately 13%) [43].This reduced the overall diagnostic yield substantially in comparison with the carrier rate. The diagnostic yield and carrier rate were both higher than those observed in the most similar prior study [1]. The higher yield of our study was apparently due to several additional genes on the panel. Selection of genes was challenging and inconsistencies in panel design will limit comparisons as long as guidelines change and understanding of representation missing in existing databases grows [24, 44–46]. If the comparison is limited to the 8 genes sequenced in both studies (APOB, BRCA1, BRCA2, LDLR, MLH1, MSH2, MSH6, and PMS2) the carrier rate of our study was higher (2.0%), but the final diagnostic yield of 1.2% was nearly identical. The similarity in yields between the two studies, despite demographic and recruitment differences, suggests that rare pathogenic variants may be represented at similar proportions in many populations.
There are many limitations to this study. Recruitment using only email is known to be less effective than other forms of recruiting. Our study only invited people for genetic screening who already had access to care in the UWM system. While this system includes a safety-net hospital, it may still underrepresent individuals without access to healthcare. Our enrichment for minorities may have produced results that are not representative of the general population. In addition, many of our analyses depend on EHR classifications of race and ethnicity; while these identifications are thought to be based on self-report, they may not be complete. We did not place screening results in the EHR, which may be a barrier to follow-up. As noted above, many limitations were judged to be acceptable in this pilot study as results would be more likely to mirror limitations of strategies that a resource-limited government or healthcare organization might undertake to implement population screening. The study is now following patients to monitor post-screening perceptions, barriers to follow-up care, and outcomes.
Conclusions
Enrollment in population genetic screening in our diverse community-ascertained cohort for variants in 25 genes associated with preventable adult-onset disease was 7%, with a 2.6% diagnostic yield for individuals screened. While population screening has the potential to identify additional individuals that could benefit from prevention, implementation challenges remain, particularly during recruitment and sample collection. These challenges to widespread adoption of population screening reduce actual enrollment and diagnostic yield and should not be overlooked in intervention planning or in cost and benefit analysis.
Supplementary Information
Additional file 1: Figure S1. Study protocol for population genetic screening. Table S1. Detailed demographics of enrollees. Table S2. Study participation at different stages of population genetic screening by race and ethnicity. Table S3. Association of race and ethnicity with enrollment. Table S4. Probability of screening enrollment for men and women aged 40 in different race and ethnicity groups. Table S5. Variants detected in population genetic screening study. Supplemental Methods. (1) Screening assay design and validation, (2) Data analysis, (3) Initial recruitment email, (4) Study information and FAQ, (5) Summary of survey instruments and measures, (6) T0 survey, (7) T1 survey, (8) T2 survey, (9) Example uninformative results letter, (10) Example positive results letter.
Acknowledgements
Not applicable.
Abbreviations
- AGHI
Alabama Genomic Health Initiative
- EHR
Electronic health record
- FAQ
Frequently asked questions
- UWM
University of Washington Medicine
Authors’ contributions
Conceptualization: A.T.C., B.H.S., N.D.R., S.M.F.; resources: J.K.; data curation: B.H.S., J.K., N.D.R.; formal analysis: B.H.S., N.D.R.; funding acquisition: B.H.S.; investigation: E.M., D.T., J.H., J.K, S.C., S.H.; methodology: A.T.C., B.H.S., K.K., N.D.R., S.M.F.; software: N.D.R.; writing-original draft: B.H.S., N.D.R.; writing-review and editing: all authors. All authors read and approved the final manuscript.
Funding
This study was funded by the Brotman Baty Institute for Precision Medicine.
Availability of data and materials
Datasets generated and/or analyzed during the current study are not publicly available due to privacy concerns as they include identifiable data and data from the University of Washington EHR. Consent forms did not include broad data sharing of genomic information, as genomic data was not considered to be deidentifiable in this context. Deidentified datasets can be made available from the corresponding author (shirtsb@uw.edu) within 4 months of a reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the University of Washington IRB (00009032) and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants receiving genetic screening, as required by the IRB.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grzymski JJ, Elhanan G, Morales Rosado JA, et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat Med. 2020;26(8):1235–1239. doi: 10.1038/s41591-020-0982-5. [DOI] [PubMed] [Google Scholar]
- 2.Manickam K, Buchanan AH, Schwartz MLB, et al. Exome sequencing-based screening for BRCA1/2 expected pathogenic variants among adult biobank participants. JAMA Netw Open. 2018;1(5):e182140. doi: 10.1001/jamanetworkopen.2018.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abul-Husn NS, Soper ER, Braganza GT, et al. Implementing genomic screening in diverse populations. Genome Med. 2021;13(1):17. doi: 10.1186/s13073-021-00832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.East KM, Kelley WV, Cannon A, et al. A state-based approach to genomics for rare disease and population screening. Genet Med. 2021;23(4):777–781. doi: 10.1038/s41436-020-01034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellcross CA, Leadbetter S, Alford SH, Peipins LA. Prevalence and healthcare actions of women in a large health system with a family history meeting the 2005 USPSTF recommendation for BRCA genetic counseling referral. Cancer Epidemiol Biomarkers Prev. 2013;22(4):728–735. doi: 10.1158/1055-9965.EPI-12-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh H, Schiesser R, Anand G, Richardson PA, El-Serag HB. Underdiagnosis of Lynch syndrome involves more than family history criteria. Clin Gastroenterol Hepatol. 2010;8(6):523–529. doi: 10.1016/j.cgh.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer. J Clin Oncol. 2017;35(34):3800–3806. doi: 10.1200/JCO.2017.73.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duell PB, Gidding SS, Andersen RL, et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: the CASCADE FH registry. Atherosclerosis. 2019;289:85–93. doi: 10.1016/j.atherosclerosis.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Grant P, Langlois S, Lynd LD, GenCOUNSEL Study. Austin JC, Elliott AM. Out-of-pocket and private pay in clinical genetic testing: a scoping review. Clin Genet. 2021;100(5):504–521. doi: 10.1111/cge.14006. [DOI] [PubMed] [Google Scholar]
- 10.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299(11):1320–1334. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- 11.Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38(13):1398–1408. doi: 10.1200/JCO.19.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts MC, Dotson WD, DeVore CS, et al. delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood) 2018;37(5):801–808. doi: 10.1377/hlthaff.2017.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan S, Won NY, Dotson WD, Wright ST, Roberts MC. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28(12):1631–1644. doi: 10.1038/s41431-020-00725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312(11):1091–1092. doi: 10.1001/jama.2014.12483. [DOI] [PubMed] [Google Scholar]
- 15.Manchanda R, Lieberman S, Gaba F, Lahad A, Levy-Lahad E. Population screening for inherited predisposition to breast and ovarian cancer. Annu Rev Genomics Hum Genet. 2020;21:373–412. doi: 10.1146/annurev-genom-083118-015253. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman S, Tomer A, Ben-Chetrit A, et al. Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: proactive recruitment compared with self-referral. Genet Med. 2017;19(7):754–762. doi: 10.1038/gim.2016.182. [DOI] [PubMed] [Google Scholar]
- 17.David SP, Dunnenberger HM, Ali R, et al. Implementing primary care mediated population genetic screening within an integrated health system. J Am Board Fam Med. 2021;34(4):861–865. doi: 10.3122/jabfm.2021.04.200381. [DOI] [PubMed] [Google Scholar]
- 18.Kelly MA, Leader JB, Wain KE, et al. Leveraging population-based exome screening to impact clinical care: the evolution of variant assessment in the Geisinger MyCode research project. Am J Med Genet C Semin Med Genet. 2021;187(1):83–94. doi: 10.1002/ajmg.c.31887. [DOI] [PubMed] [Google Scholar]
- 19.Angelo F, Veenstra D, Knerr S, Devine B. Prevalence and prediction of medical distrust in a diverse medical genomic research sample. Genet Med. 2022;24(7):1459–1467. doi: 10.1016/j.gim.2022.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Tier 1 Genomics Applications and their Importance to Public Health CDC. Published October 24, 2022. Accessed 20 Sept, 2021. https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm.
- 21.Taylor A, Brady AF, Frayling IM, et al. Consensus for genes to be included on cancer panel tests offered by UK genetics services: guidelines of the UK Cancer Genetics Group. J Med Genet. 2018;55(6):372–377. doi: 10.1136/jmedgenet-2017-105188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritchard CC, Smith C, Salipante SJ, et al. ColoSeq provides comprehensive lynch and polyposis syndrome mutational analysis using massively parallel sequencing. J Mol Diagn. 2012;14(4):357–366. doi: 10.1016/j.jmoldx.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirts BH, Casadei S, Jacobson AL, et al. Improving performance of multigene panels for genomic analysis of cancer predisposition. Genet Med. 2016;18(10):974–81. 10.1038/gim.2015.212. [DOI] [PubMed]
- 24.Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(1):77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 25.Sturm AC, Knowles JW, Gidding SS, et al. Clinical genetic testing for familial hypercholesterolemia: JACC scientific expert panel. J Am Coll Cardiol. 2018;72(6):662–680. doi: 10.1016/j.jacc.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 26.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/AJPH.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey DJ, Fetterolf SN, Davis FD, et al. The Geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genet Med. 2016;18(9):906–913. doi: 10.1038/gim.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canedo JR, Miller ST, Myers HF, Sanderson M. Racial and ethnic differences in knowledge and attitudes about genetic testing in the US: systematic review. J Genet Couns. 2019;28(3):587–601. doi: 10.1002/jgc4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy AM, Bristol M, Domchek SM, et al. Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. JCO. 2016;34(22):2610–2618. doi: 10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones T, McCarthy AM, Kim Y, Armstrong K. Predictors of BRCA1/2 genetic testing among Black women with breast cancer: a population-based study. Cancer Med. 2017;6(7):1787–1798. doi: 10.1002/cam4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams CD, Bullard AJ, O’Leary M, Thomas R, Redding TS, Goldstein K. Racial/Ethnic Disparities in BRCA Counseling and Testing: a Narrative Review. J Racial Ethn Health Disparities. 2019;6(3):570–583. doi: 10.1007/s40615-018-00556-7. [DOI] [PubMed] [Google Scholar]
- 32.Hall MJ, Olopade OI. Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006;24(14):2197–2203. doi: 10.1200/JCO.2006.05.5889. [DOI] [PubMed] [Google Scholar]
- 33.Suther S, Kiros GE. Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med. 2009;11(9):655–662. doi: 10.1097/GIM.0b013e3181ab22aa. [DOI] [PubMed] [Google Scholar]
- 34.Roberts MC, Mensah GA, Khoury MJ. Leveraging implementation science to address health disparities in genomic medicine: examples from the field. Ethn Dis. 2019;29(Suppl 1):187–192. doi: 10.18865/ed.29.S1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grzymski JJ, Coppes MJ, Metcalf J, et al. The Healthy Nevada Project: rapid recruitment for population health study. Published online January 19, 2018:250274. 10.1101/250274
- 36.May T, Cannon A, Moss IP, et al. Recruiting diversity where it exists: the alabama genomic health initiative. J Genet Couns. 2020;29(3):471–478. doi: 10.1002/jgc4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horowitz CR, Sabin T, Ramos M, et al. Successful recruitment and retention of diverse participants in a genomics clinical trial: a good invitation to a great party. Genet Med. 2019;21(10):2364–2370. doi: 10.1038/s41436-019-0498-x. [DOI] [PubMed] [Google Scholar]
- 38.The All of Us Research Program Investigators. The “All of Us” Research Program. N Engl J Med. 2019;381(7):668–76. 10.1056/NEJMsr1809937. [DOI] [PMC free article] [PubMed]
- 39.Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111(39):14205–14210. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abul-Husn NS, Soper ER, Odgis JA, et al. Exome sequencing reveals a high prevalence of BRCA1 and BRCA2 founder variants in a diverse population-based biobank. Genome Med. 2019;12(1):2. doi: 10.1186/s13073-019-0691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirts BH, Salama JS, Aronson SJ, et al. CSER and eMERGE: current and potential state of the display of genetic information in the electronic health record. J Am Med Inform Assoc. 2015;22(6):1231–1242. doi: 10.1093/jamia/ocv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elhanan G, Kiser D, Neveux I, et al. Incomplete penetrance of population-based genetic screening results in electronic health record. Front Genet. 2022;13:866169. doi: 10.3389/fgene.2022.866169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchanan AH, Kirchner HL, Schwartz MLB, et al. Clinical outcomes of a genomic screening program for actionable genetic conditions. Genet Med. 2020;22(11):1874–1882. doi: 10.1038/s41436-020-0876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller DT, Lee K, Abul-Husn NS, et al. ACMG SF v3.1 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2022;24(7):1407–1414. doi: 10.1016/j.gim.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Webber EM, Hunter JE, Biesecker LG, et al. Evidence-based assessments of clinical actionability in the context of secondary findings: updates from ClinGen’s Actionability Working Group. Hum Mutat. 2018;39(11):1677–1685. doi: 10.1002/humu.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao ND, Shirts BH. Using species richness calculations to model the global profile of unsampled pathogenic variants: Examples from BRCA1 and BRCA2. PLoS One. 2023;18(2):e0278010. doi: 10.1371/journal.pone.0278010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Study protocol for population genetic screening. Table S1. Detailed demographics of enrollees. Table S2. Study participation at different stages of population genetic screening by race and ethnicity. Table S3. Association of race and ethnicity with enrollment. Table S4. Probability of screening enrollment for men and women aged 40 in different race and ethnicity groups. Table S5. Variants detected in population genetic screening study. Supplemental Methods. (1) Screening assay design and validation, (2) Data analysis, (3) Initial recruitment email, (4) Study information and FAQ, (5) Summary of survey instruments and measures, (6) T0 survey, (7) T1 survey, (8) T2 survey, (9) Example uninformative results letter, (10) Example positive results letter.
Data Availability Statement
Datasets generated and/or analyzed during the current study are not publicly available due to privacy concerns as they include identifiable data and data from the University of Washington EHR. Consent forms did not include broad data sharing of genomic information, as genomic data was not considered to be deidentifiable in this context. Deidentified datasets can be made available from the corresponding author (shirtsb@uw.edu) within 4 months of a reasonable request.