Abstract
The current study investigates whether prepregnancy maternal posttraumatic stress disorder (PTSD) symptoms, depressive symptoms, and stress predict children’s cortisol diurnal slopes and cortisol awakening responses (CARs) adjusting for relevant variables. Mothers were enrolled after delivering a baby and followed through their subsequent pregnancy with 5 years of longitudinal data on their subsequent child. This prospective design allowed assessment of PTSD symptoms, depressive symptoms, and perceived stress prior to pregnancy. Children provided three saliva samples per day on three consecutive days at two timepoints in early childhood (M age = 3.7 years, SD = 0.38; M age = 5.04 years, SD = 0.43). Mothers’ PTSD symptoms prior to pregnancy were significantly associated with flatter child diurnal cortisol slopes at 4 and 5 years, but not with child CAR. Findings at the age of 4 years, but not 5 years, remained statistically significant after adjustment for maternal socioeconomic status, race/ethnicity, child age, and other covariates. In contrast, maternal prepregnancy depressive symptoms and perceived stress did not significantly predict cortisol slopes or CAR. Results suggest that maternal prepregnancy PTSD symptoms may contribute to variation in early childhood physiology. This study extends earlier work demonstrating risk of adverse outcomes among children whose mothers experienced trauma but associations cannot be disentangled from effects of prenatal mental health of mothers on children’s early childhood.
Keywords: depression, HPA axis, preconception, PTSD, stress
1 |. INTRODUCTION
Developmental origins of disease and fetal programming models propose that the prenatal environment shapes fetal development and affects offspring health over the life course (D. J. P. Barker, 1998; Gluckman & Hanson, 2004). A growing body of research indicates that maternal experiences prior to conception may also shape the subsequent development and health of offspring (Fleming et al., 2018; Stephenson et al., 2018). Dysregulation ofthe hypothalamic–pituitary–adrenal (HPA) axis is a potential mechanism underlying the intergenerational transmission of maternal stress and mental health (Adam et al., 2017; Howland et al., 2017). The goal of the present study was to examine whether maternal mental health and perceived stress reported prior to conception relate to patterns of diurnal cortisol, a noninvasive measure of HPA axis functioning, in preschool-aged children.
Life-course models and empirical findings on maternal–child health suggest that parent health and experiences before conception shape pregnancy outcomes and child development (Lu & Halfon, 2003; Misra et al., 2003; Ramey et al., 2015). Maternal preconception stress increases risk of adverse birth outcomes, after controlling for pregnancy stress levels (e.g., Class et al., 2013; Class et al., 2015; Harville et al., 2010; Mahrer et al., 2021; Witt et al., 2012). Studies using animal models have experimentally elevated preconception maternal stress exposure, while controlling for further stress during pregnancy, and detected adverse neurodevelopmental outcomes in offspring (see Keenan et al., 2018 for a review). In humans, preconception maternal stress and mental health symptoms have been associated with adverse outcomes in infancy and childhood, including higher incidence or risk of sleep disturbances (Baird et al., 2009), emotional reactivity (Spry et al., 2020), negative affectivity (Swales et al., 2022), behavior problems (Letcher et al., 2020), and attention-deficit/hyperactivity disorder in males (J. Li et al., 2010). Studies of maternal mental health and stress prior to conception can provide insights about the long-term effects on offspring, with potential implications for screening and treatment in individuals prior to and during pregnancy.
These findings warrant further study of intergenerational effects of stress exposure on underlying physiological mechanisms, such as HPA axis activity. Preconception research examining child outcomes in humans is largely based on data from national registries without detailed physiological measures (e.g., J. Li et al., 2010) or retrospective maternal report of preconception mental health and stress (e.g., Witt et al., 2012, 2014), suggesting a need for prospective investigations.
Prenatal programming of the HPA axis is a demonstrated pathway through which fetal exposures affect health outcomes across the lifespan (Brand et al., 2010; Yehuda & Bierer, 2007; Yehuda et al., 2000, 2005). HPA axis activity can be measured through cortisol, a hormone that modulates activity of varied physiological systems (e.g., immune, cardiometabolic) that are important for health (DeMorrow, 2018). Cortisol is secreted throughout the day in a diurnal pattern characterized by high levels on morning awakening, an increase over the first 30–40 min after waking known as the cortisol awakening response (CAR), and gradual decreases across the day to reach low levels by bedtime, known as the diurnal slope (Kirschbaum & Hellhammer, 2000). This diurnal rhythm develops during infancy and early childhood to be consistent with the pattern in adults (Gunnar & Quevedo, 2007).
Dysregulation of the diurnal rhythm of the HPA axis is associated with adverse physical and mental health outcomes across the lifespan. During childhood, flatter diurnal slopes predict greater externalizing symptoms (Ruttle et al., 2011), mental health symptom severity (Shirtcliff & Essex, 2008), and risk of upper respiratory infections (Turner-Cobb et al., 2011). The magnitude of the CAR is also associated with higher externalizing symptoms in early childhood (McGinnis et al., 2016) and risk of internalizing disorders during adolescence and adulthood (Adam et al., 2010, 2014; Harris et al., 2000; Rao et al., 2010; Vrshek-Schallhorn et al., 2013). Overall, evidence indicates that dysregulation of diurnal cortisol has downstream effects on multiple aspects of biology, behavior, and health. Only a few studies have considered whether maternal stress and mental health symptoms prior to conception shape the developing HPA axis in humans. Slopen et al. (2018) found that maternal lifetime history of trauma exposure related to higher levels of hair cortisol in offspring at 3–4 years of age. Others reported that preconception trauma experienced by mothers shapes HPA outcomes in offspring, extending into adulthood (Lehrner et al., 2014; Yehuda et al., 2000; Yehuda et al., 2007; Yehuda et al., 2002). Such evidence indicates that maternal experiences even before pregnancy may influence the HPA axis in offspring. Notably, these studies assessed prepregnancy variables retrospectively and, to our knowledge, no prior study prospectively examined multiple prepregnancy predictors of offspring cortisol patterns.
2 |. CURRENT STUDY
Using a prospective cohort study in a socioeconomically, racially, and ethnically diverse sample of maternal–child pairs, we examined how maternal mental health symptoms and perceived stress prior to conception relate to diurnal cortisol slopes and CARs in preschool-aged children. We assessed mothers’ posttraumatic stress disorder (PTSD) symptoms, depressive symptoms, and perceptions of stress after one birth and prior to a subsequent pregnancy. Children provided cortisol samples via saliva at two time points, when they were approximately 4 and 5 years of age. We hypothesized that higher levels of maternal PTSD symptoms, depressive symptoms, and perceived stress prior to conception predict smaller CAR and flatter diurnal slopes in children.
3 |. METHOD
3.1 |. Participants
Participants were 85 mother–child dyads. Mothers were recruited by the Community Child Health Network (CCHN) following the birth of a child. Women completed study visits after that birth and during and after a subsequent pregnancy and livebirth.1
Participants then enrolled in a follow-up study and completed additional home visits during their subsequent child’s early childhood. Mothers provided ratings of prepregnancy stress and mental health and subsequent children provided saliva samples at the age of 4 years (n = 81 at Time 1). Of these children, 66 (77.6%) provided saliva samples again at the age of 5 years, and four additional children provided samples at the age of 5 years but not age 4 years (n = 70 at Time 2). Participation did not differ as a function of mothers’ education, race/ethnicity, age, family income, depressive symptoms, perceived stress, and post-traumatic symptoms, or by child’s race or sex, all ps > .1. Further detail on CCHN and the follow-up study is reported elsewhere (Dunkel Schetter et al., 2013; Mahrer et al., 2021; Morgan et al., 2020; Ramey et al., 2015). Table 1 presents description of the analytic sample.2
TABLE 1.
Characteristics of the study sample and descriptive statistics for study variables (n = 85)
| Variable | N | %/M | SD |
|---|---|---|---|
| Demographic variables | |||
| Household income as %FPL | 85 | 300.98 | 310.16 |
| Maternal education (years completed) | 84 | 12.98 | 3.32 |
| Child sex (n/% female) | 47 | 55.29% | |
| Child age at first early childhood visit (years) | 76 | 3.77 | 0.38 |
| Child age at second early childhood visit (years) | 65 | 5.04 | 0.43 |
| Child race/ethnicity | |||
| African American or Black | 10 | 11.76% | |
| White or Caucasian | 25 | 29.41% | |
| Latino or Hispanic | 42 | 49.41% | |
| Multiracial | 8 | 9.41% | |
| Study site | |||
| Lake County, IL | 67 | 78.82% | |
| North Carolina | 8 | 9.41% | |
| Washington, DC | 10 | 11.76% | |
| Maternal prepregnancy stress and mental health variables | |||
| PTSD symptoms | 64 | 24.59 | 9.02 |
| PSS Score | 85 | 12.48 | 5.15 |
| EPDS Score | 85 | 4.84 | 4.33 |
| Cortisol variables—Averages for 3 sampling days after first study visit | |||
| Sleep duration | 202 | 9.75 | 1.37 |
| Wake time | 203 | 8.20 | 1.39 |
| Cortisol: awakening (nmol/L) | 207 | 8.82 | 9.73 |
| Cortisol: 30 min after awakening (nmol/L) | 202 | 9.65 | 10.34 |
| Cortisol: bedtime (nmol/L) | 198 | 3.65 | 10.68 |
| Cortisol awakening response | 202 | 0.04 | 0.27 |
| Diurnal slope | 193 | −0.41 | 0.60 |
| Cortisol variables—Averages for 3 sampling days after second study visit | |||
| Sleep duration | 174 | 9.93 | 1.40 |
| Wake time | 173 | 7.59 | 1.19 |
| Cortisol: awakening (nmol/L) | 185 | 6.62 | 4.74 |
| Cortisol: 30 min after awakening (nmol/L) | 178 | 7.54 | 6.29 |
| Cortisol: bedtime (nmol/L) | 178 | 1.79 | 3.69 |
| Cortisol awakening response | 178 | 0.03 | 0.18 |
| Diurnal slope | 169 | −0.38 | 0.36 |
Abbreviations: PTSD, posttraumatic stress disorder; PSS, Perceived Stress Scale; EPDS, Edinburgh Postnatal Depression Scale. Descriptive statistics for cortisol variables at first and second study visit are provided for individual sampling days. 81 children provided up to three days of samples at the first study visit, and 70 children provided up to three days samples at the second study visit.
3.2 |. Procedures
Mothers completed structured in-home interviews at 6-month intervals after the earlier birth in English or Spanish. Mother–child pairs in the follow-up study completed additional home visits during early childhood when the subsequent child was approximately 4 years old (M = 3.77, SD = 0.38, range = 3.5–4.7) and approximately a year later (M = 5.04, SD = 0.43, range = 4.3–6.1). Parents received gift cards for completing the study visits and the child received a small gift at each visit. The Institutional Review Boards at each site approved the study, and mothers provided written informed consent for themselves and their child.
3.3 |. Measures
3.3.1 |. Sociodemographic and medical variables
Mothers reported children’s biological sex at birth and race/ethnicity as well as their own race/ethnicity, household income, and years of education completed. Timing of prepregnancy visits relative to conception was estimated based on child due date and birthdate.
3.3.2 |. Maternal PTSD symptoms
Mothers reported PTSD symptoms prior to pregnancy using the PTSD Checklist—Civilian Version (PCL-C; Blanchard et al., 1996) indicating how much they were bothered by symptoms in the past month, on a 5-point Likert scale from 1 (not at all) to 5 (extremely). Item ratings were summed, with higher scores indicating more PTSD symptoms (see Table 1). The PCL-C is a reliable, well-validated measure of PTSD symptoms (Ruggiero et al., 2003), previously used to assess PTSD symptoms in women during the perinatal period (e.g., Huth-Bocks et al., 2013). Prior work in the larger CCHN cohort provided further validation of this measure by documenting an association between PCL-C scores and past-year trauma burden (Thomas et al., 2021). Consistent with prior research, a cut-off score of 30 was used to indicate clinically elevated symptoms and a probable diagnosis of PTSD (Swales et al., 2022), and 13 participants (20.31%) met criteria for clinically elevated symptoms. The PCL-C demonstrated excellent internal consistency in the present sample (α = .91). On average, participants completed the PCL-C 56.4 weeks before conception (SD = 50.0, median = 37 weeks).
The sample for analyses including PTSD symptoms is smaller (n = 64) because eight women did not complete this assessment and 13 women were pregnant by 6 months after delivery of the first child (when this measure was completed), thus preventing a preconception measure for them. Of the children who completed cortisol samples at each time point, 56 children provided saliva samples at the age of 4 years and had preconception reports of their mother’s PTSD symptoms (69.1% of children who provided cortisol at the age of 4 years) and 51 children provided saliva samples at the age of 5 years and had preconception reports of their mother’s PTSD symptoms (72.9%).
3.3.3 |. Perceived stress
Perceived stress was measured using the 10-item brief version of the Perceived Stress Scale (PSS; Cohen et al., 1983) at an average of 25.3 weeks before conception of the study child (SD = 27.4). The PSS was administered during study visits scheduled at 6-month intervals after the previous birth, and the score from the visit most proximal to the study’s child date of conception was used in analyses. Participants responded on a scale ranging from 1 (never) to5 (almost always). Item responses were summed after four positively worded items were reverse-coded. Scores had a possible range of 10–50, with higher scores indicating higher perceived stress. Cronbach’s alpha indicated good reliability for this measure (𝛼 = .80).
3.3.4 |. Depressive symptoms
Participants completed the 10-item Edinburgh Postnatal Depression Scale (EPDS; Cox et al., 1987). The EPDS was administered 1, 6, and 12 months after the earlier birth. The score from the prepregnancy visit most proximal to the study child’s estimated date of conception was used in analyses (M = 38.0 weeks before conception, SD = 41.5). A composite was created using the sum of the 10 items. Scores can range from 0 to 30, with higher scores indicating more severe depressive symptoms. Cronbach’s alpha indicated good reliability for this measure (𝛼 = .83). In the present sample, nine mothers (10.59%) had EPDS scores >12, indicating probable postpartum depression.
3.3.5 |. Child cortisol
Parents collected saliva samples from their children three times per day for three days following each early childhood home visit. They were instructed to take samples immediately after the child woke up, 30 min. after awakening, and at bedtime.
Saliva was collected using absorbent Weck-Cel Spears (Beaver-Visitec International, Waltham, MA, USA). Instructions noted that children should not eat, drink, or brush teeth before completing a saliva sample. After collection, parents were asked to write the date and time of collection on a label, refrigerate the sample, and then return all collected samples by mail at the end of the 3-day collection period. Samples were centrifuged at 3000 RPM for 15 min to extract absorbed saliva and stored in an −80°C ultralow freezer. They were shipped on dry ice to the Technische Universität Dresden (Dresden, Germany) where they were assayed in singlet for salivary cortisol by immunoassay with chemoluminescence detection (CLIA) from IBL-Tecan (Hamburg, Germany), catalogue number R62111. Out-of-range observations were rerun. Samples were assayed in three batches over the course of the four-year study period. Average intra- and inter-assay coefficients of variation were less than 7 and 9%, respectively.
Parents also completed diaries documenting the child’s wake time, bedtime, sample collection times, and medication intake. MEMS track caps (MWV Switzerland Ltd.) on bottles containing eye spears electronically logged the time that each sample was collected for a subset of participants (n = 19) at the age of 4 years visit to check if timing of cortisol collection was reported accurately. Comparisons of self-reported collection times with data recorded using MEMS caps showed an average discrepancy of 2.34 min (SD = 25.95) for samples collected upon awakening, 2.98 min (SD = 25.95) for samples collected 30 min after waking, and 5.62 min (SD = 24.64) for bedtime samples.
Cortisol slope was calculated by subtracting waking cortisol from evening cortisol and dividing by the amount of time in hours between the two measurements. CAR was calculated by subtracting waking cortisol from waking +30 cortisol. Most waking +30 samples were provided within 20–40 min of the waking sample (86.99% at the age of 4 years; 89.89% at the age of 5 years). Demographic factors and maternal mental health were not related to odds of timely completion at the age of 4 years, although older children (B = −0.21, SE = 0.07, p < .001), children whose mothers had higher preconception PTSD symptoms (B = −0.11, SE = 0.05, p = .011), and children whose mothers were higher in depressive symptoms (B = −0.01, SE = 0.007, p = .0136) were less likely to provide samples on time at the age of 5 years. Models were tested with all values of CAR and after limiting values to those including waking +30 samples collected 20–40 min after waking samples.
3.4 |. Analytic plan
Prior to analysis, child cortisol was examined for outliers (>3 standard deviations from sample mean). All values of CAR and diurnal slope were within three standard deviations of the mean at the age of 5 years. At the age of 4 years, there was one extreme value for CAR and one extreme value for diurnal slope that exceeded three standard deviations above the mean; each of these values was winsorized to three standard deviations.
3.4.1 |. Unadjusted models
Hierarchical multilevel models tested the variance in cortisol slope and CAR predicted by mental health variables. Multilevel models with days (Level 1) nested within children (Level 2) were used. Separate models tested whether maternal PTSD symptoms, perceived stress, and depressive symptoms were related to children’s cortisol, with separate models for CAR and diurnal slope. Models were tested for children’s cortisol at ages of 4 and 5 years. All models covaried for children’s use of corticosteroid medication, given potential associations with cortisol output (n = 4, 7.1% of sample at the age of 4 years; n = 6, 11.8% at the age of 5 years; dummy-coded as no use = 0, use = 1).3
3.4.2 |. Adjusted models
Covariates were selected based on theory and previous research showing that they are associated with cortisol slopes and/or the CAR (DeSantis et al., 2007; Jessop & Turner-Cobb, 2008; Martin et al., 2012). All models were repeated adjusting for mother’s reports of child wake time and sleep duration from the previous night at Level 1, and child sex, child race/ethnicity, child age, mother’s years of education, household income relative to the poverty line, and current symptoms at Level 2. Models with depressive symptoms and perceived stress as the predictor variables adjusted for maternal depressive symptoms or perceived stress, respectively, at the time when child cortisol was collected in early childhood. While maternal PTSD symptoms were not assessed at the early childhood visits, we tested models controlling for levels of perceived stress and depressive symptoms at the time of the study visit to account for current mental health status. Maternal anxiety symptoms were measured only at the study visit when children were aged 5 years using the Overall Anxiety Severity and Impairment Scale (Campbell-Sills et al., 2009) and included in models predicting age 5 years diurnal cortisol parameters from PTSD to further adjust for current mental health symptoms. Continuous covariates were mean centered. We also repeated the final adjusted models using Bayesian statistics to estimate credible intervals, given the relatively small sample size.
4 |. RESULTS
4.1 |. Preliminary analyses
Paired samples t-tests across all observations indicated that cortisol significantly increased from wake to 30 min postwake at the age of 4 years, t(201) = 2.13, p = .034, D = 0.16, and at the age of 5 years, t(177) = 2.20, p = .029, D = 0.17. Consistent with prior work in toddlers and young children showing negative CAR in some participants (Bäumler et al., 2013), 43.56% of CAR observations collected at the age of 4 years were negative, with 10.14% of participants having negative CAR observations for all 3 days, and 47.75% of CAR observations collected at the age of 5 years were negative, with 13.59% of participants having negative CAR observations for all 3 days. Cortisol significantly declined from wake to bedtime at the age of 4 years, t(192) = 8.79, p < .001, D = 0.63, and at the age of 5 years, t(168) = 12.81, p < .001, D = 1.03. CAR and slope were significantly inversely associated at the ages of 4 and 5 years, such that children with a greater CAR showed a steeper decline in cortisol from morning to evening. Table S1 presents bivariate Pearson’s correlation coefficients of study variables.
4.2 |. Primary analyses
4.2.1 |. PTSD symptoms
First, models examined associations between maternal preconception PTSD symptoms and child cortisol slope. The unadjusted model (covarying only steroid medication use) showed that more PTSD symptoms were associated with flatter cortisol slope at the age of 4 years (Table 2). In the model adjusted for all covariates, the association of PTSD symptoms with slope remained significant (Table 3). At the age of 5 years, PTSD symptoms were also associated with flatter cortisol slopes in the unadjusted model (Table 2) but not in the adjusted model (Table 3). Figure 1 presents results.
TABLE 2.
Multilevel models predicting child cortisol patterns at the ages of 4 and 5 years from maternal PTSD symptoms
| Age 4 years (n = 56) |
Age 5 years (n = 51) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | b | SE | 95% CI | Variable | b | SE | 95% CI |
| Dependent variable: cortisol slope | |||||||
| PTSD symptoms | 0.014** | 0.005 | [0.004,0.023] | PTSD symptoms | 0.007* | 0.004 | [0.000,0.014] |
| Steroid medication use | 0.163 | 0.182 | [−0.193,0.519] | Steroid medication use | −0.021 | 0.119 | [−0.254,0.212] |
| Constant | −0.427 | 0.048 | [−0.522,−0.332] | Constant | −0.388 | 0.040 | [−0.466,−0.309] |
| Dependent variable: cortisol awakening response | |||||||
| PTSD symptoms | −0.001 | 0.002 | [−0.006,0.004] | PTSD symptoms | −0.002 | 0.001 | [−0.005,0.000] |
| Steroid medication use | 0.031 | 0.084 | [−0.134,0.195] | Steroid medication use | −0.034 | 0.050 | [−0.132,0.063] |
| Constant | 0.035 | 0.024 | [−0.013,0.082] | Constant | 0.031 | 0.016 | [−0.001,0.063] |
Abbreviation: PTSD, posttraumatic stress disorder. Separate multilevel models were run for each maternal stress/mental health predictor variable at each time point.
p < .05
p < .01.
TABLE 3.
Multilevel models predicting child cortisol patterns at the ages of 4 and 5 years from maternal prepregnancy PTSD symptoms
| Age 4 years (n = 56) |
Age 5 years (n = 51) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | b | SE | 95% CI | Variable | b | SE | 95% CI |
| Dependent variable: cortisol slope | |||||||
| PTSD symptoms | 0.02* | 0.01 | [0.001,0.04] | PTSD symptoms | 0.01 | 0.01 | [−0.002,0.02] |
| Steroid medication | 0.33 | 0.30 | [−0.26,0.92] | Steroid medication | 0.01 | 0.13 | [−0.24,0.26] |
| Child sex (female) | 0.17 | 0.17 | [−0.16,0.49] | Child sex (female) | 0.00 | 0.09 | [−0.16,0.17] |
| Household Income | −0.02 | 0.03 | [−0.07, 0.03] | Household Income | −0.03 | 0.02 | [−0.07, 0.00] |
| Education | −0.14 | 0.09 | [−0.33,0.04] | Education | 0.00 | 0.01 | [−0.03,0.02] |
| Race/ethnicity | Race/ethnicity | ||||||
| Black | 0.02 | 0.33 | [−0.63,0.67] | Black | 0.12 | 0.16 | [−0.20,0.44] |
| White | 0.28 | 0.22 | [−0.16,0.72] | White | 0.17 | 0.11 | [−0.05,0.38] |
| Multiracial | 0.40 | 0.29 | [−0.17,0.97] | Multiracial | 0.05 | 0.15 | [−0.25,0.36] |
| Age | 0.40 | 0.27 | [−0.13,0.92] | Age | −0.12 | 0.12 | [−0.36,0.12] |
| Time to conception | 0.00 | 0.00 | [−0.005,0.002] | Time to conception | 0.00 | 0.00 | [−0.001,0.003] |
| Sleep duration | 0.02 | 0.06 | [−0.10,0.14] | Sleep duration | −0.02 | 0.07 | [−0.15,0.12] |
| Wake time | −0.07 | 0.08 | [−0.23,0.09] | Wake time | −0.04 | 0.07 | [−0.17,0.10] |
| Current depressive symptoms | −0.03 | 0.02 | [−0.07,0.02] | Current depressive symptoms | 0.02 | 0.01 | [−0.01,0.05] |
| Current perceived stress | 0.00 | 0.02 | [−0.03,0.04] | Current perceived stress | 0.00 | 0.02 | [−0.04,0.03] |
| Current anxiety symptoms | −0.02 | 0.01 | [−0.04,0.002] | ||||
| Constant | −0.38 | 0.27 | [−0.92,0.16] | Constant | −0.50*** | 0.09 | [−0.68,−0.31] |
| Dependent variable: cortisol awakening response | |||||||
| PTSD symptoms | 0.00 | 0.00 | [−0.01,0.003] | PTSD symptoms | 0.00 | 0.00 | [−0.01,0.003] |
| Steroid medication | −0.10 | 0.10 | [−0.29,0.09] | Steroid medication | −0.01 | 0.05 | [−0.11,0.09] |
| Child sex (female) | −0.01 | 0.05 | [−0.12,0.09] | Child sex (female) | 0.02 | 0.03 | [−0.04,0.09] |
| Household Income | 0.01 | 0.01 | [−0.01,0.02] | Household Income | −0.01 | 0.01 | [−0.02, 0.01] |
| Education | 0.01 | 0.03 | [−0.05,0.07] | Education | −0.01 | 0.01 | [−0.02,0.003] |
| Race/ethnicity | Race/ethnicity | ||||||
| Black | 0.18 | 0.10 | [−0.02,0.38] | Black | 0.01 | 0.06 | [−0.12,0.13] |
| White | −0.08 | 0.07 | [−0.22,0.07] | White | 0.08 | 0.05 | [−0.005,0.17] |
| Multiracial | −0.11 | 0.10 | [−0.30,0.08] | Multiracial | 0.03 | 0.06 | [−0.10,0.15] |
| Age | −0.09 | 0.09 | [−0.27,0.08] | Age | 0.02 | 0.05 | [−0.07,0.12] |
| Time to conception | 0.00 | 0.00 | [−0.006,0.002] | Time to conception | 0.00 | 0.00 | [−0.0004,0.001] |
| Sleep duration | 0.00 | 0.02 | [−0.04,0.04] | Sleep duration | 0.00 | 0.04 | [−0.08,0.08] |
| Wake time | −0.02 | 0.03 | [−0.08,0.03] | Wake time | −0.03 | 0.04 | [−0.12,0.05] |
| Current depressive symptoms | 0.00 | 0.01 | [−0.01,0.02] | Current depressive symptoms | 0.01 | 0.01 | [−0.003,0.02] |
| Current perceived stress | 0.00 | 0.01 | [−0.01,0.01] | Current perceived stress | 0.00 | 0.01 | [−0.02,0.01] |
| Current anxiety symptoms | −0.01* | 0.00 | [−0.02,−0.0005] | ||||
| Constant | 0.04 | 0.09 | [−0.14, 0.21] | Constant | −0.02 | 0.04 | [−0.10,0.05] |
Abbreviation: PTSD, posttraumatic stress disorder. Household income percentage of the federal poverty line was divided by 100 so that the coefficient could be more easily interpreted (i.e., coefficients represent expected change in outcome for earning an additional 100% of the federal poverty line).
p < .05.
p < .01
p < .001
FIGURE 1.
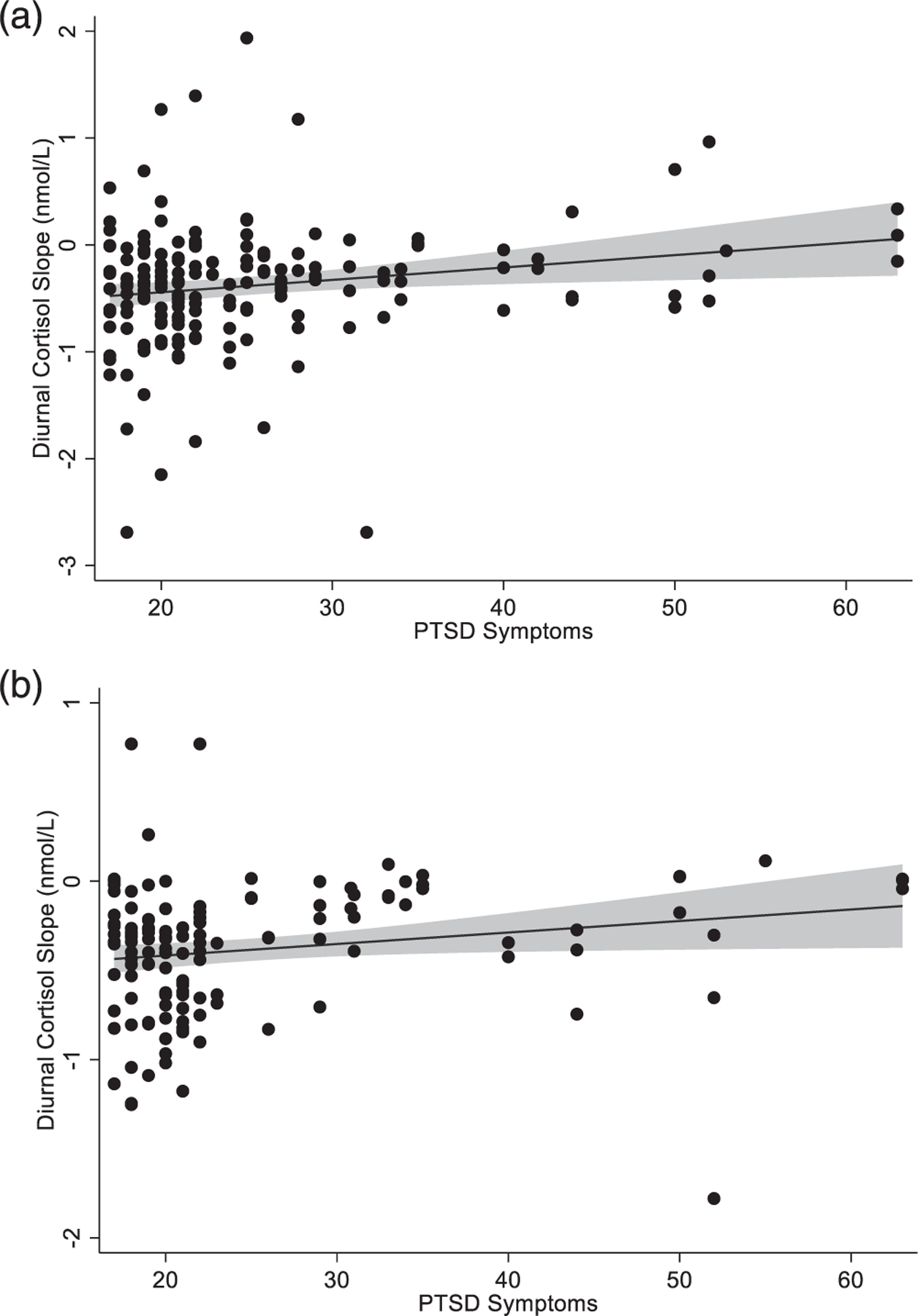
Scatterplots showing associations of maternal preconception posttraumatic stress disorder (PTSD) symptoms with child diurnal cortisol slope. Panel (a) displays data for slope at the age of 4 years (n = 56) and panel (b) displays data for slope at the age of 5 years (n = 51). Abbreviation: PTSD, Posttraumatic Stress Disorder
A parallel set of models tested associations of PTSD symptoms with CAR at the ages of 4 and 5 years. PTSD symptoms were not associated with CAR in adjusted or unadjusted models at the ages of 4 and 5 years (see Tables 2 and 3), both when testing all CAR values and values from samples collected 20 – 40 min after waking.
4.2.2. |. Depressive symptoms and perceived stress
Scores on the EPDS were not significantly associated with child cortisol slope or CAR in unadjusted models at the ages of 4 or 5 years (see Table S2) or adjusted models (see Table S3). Similarly, maternal preconception perceived stress was not significantly associated with child cortisol slope or CAR in unadjusted models at the ages of 4 or 5 years (see Table S4) or adjusted models (see Table S5). Again, results for CAR did not differ when testing all values and values from timely samples. We also tested models adjusting for maternal depressive symptoms and perceived stress assessed at study visits proximal to cortisol collection at the ages of 4 and 5 years. Associations between maternal prepregnancy depressive symptoms and current depressive symptoms were moderate at the age of 4 years, r(66) = .32, p = .006, and at the age of 5 years, r(60) = .44, p < .001. Similarly, associations between prepregnancy perceived stress and concurrent perceived stress were also moderate at the age of 4 years, r(66) = .48, p < .001, and at the age of 5 years, r(60) = .47, p = .004. Associations of perceived stress with CAR and diurnal slope remained nonsignificant when accounting for maternal perceived stress at the time of the visit. Similarly, associations of depressive symptoms with CAR and diurnal slope remained nonsignificant when accounting for mother’s reports of depressive symptoms at the time of the visit. Finally, we repeated all models with Bayesian estimates due to the relatively low sample size and the pattern of results was identical.
5 |. DISCUSSION
In a prospective study of mothers and their children, we found that maternal mental health symptoms prior to pregnancy were associated with diurnal cortisol slopes in early childhood. Symptoms of PTSD in mothers assessed before conception were associated with flatter diurnal cortisol slopes at the ages of 4 (n = 56) and 5 (n = 51) years, and at the age of 4 years, these associations were independent of child sex, race/ethnicity, and socioeconomic status. These findings are notable because flatter cortisol slopes have been associated with adverse outcomes across the lifespan (Adam et al., 2017; Bernard et al., 2015). However, maternal symptoms of depression and perceived stress prior to conception were not associated with diurnal cortisol slopes or CAR.
Prior work shows that prepregnancy, prenatal, or early postnatal adversity alter offspring HPA axis functioning (Bernard et al., 2010; Bruce et al., 2009; Gunnar & Vazquez, 2001; Yehuda et al., 2002, 2007). However, to our knowledge, this is the first prospective study showing that maternal posttraumatic stress symptoms reported prior to pregnancy are related to offspring cortisol patterns. PTSD symptoms prior to conception may influence diurnal slopes through multiple mechanisms. Traumatic stress may result in biological changes that impact gene expression, gametes, the intrauterine environment, or early postnatal care, thus altering the developing neuroendocrine system (Bowers & Yehuda, 2015). Epigenetic modifications can occur following exposure to traumatic stress and alter functional expression of genes implicated in HPA axis regulation (Yehuda & Bierer, 2009). For example, reviews of the literature indicate that individuals with PTSD have altered methylation of glucocorticoid receptor genes, lower daily cortisol output, and flattened cortisol slopes in comparison to those not exposed to trauma (Bowers & Yehuda, 2015; Morris et al., 2012; Speer et al., 2019). During pregnancy, maternal glucocorticoids are transported across the placenta, enter fetal circulation, and alter fetal stress architecture (Weinstock, 2005). Thus, epigenetic modifications resulting from trauma exposure and alterations in fetal exposure to maternal glucocorticoids during gestation may play a role in shaping diurnal cortisol patterns in offspring (Davis et al., 2011; Gutteling et al., 2005; Gutteling et al., 2004; Irwin et al., 2021; O’Connor et al., 2013).
These findings also complement prior work showing that traumatic stress exposure in parents is a risk factor for adverse outcomes in offspring, including heightened risk of psychopathology (Chemtob et al., 2010; Samuelson & Cashman, 2008; Weems & Scheeringa, 2013). For example, children of parents with PTSD are at higher risk of PTSD (Leen-Feldner et al., 2013; Scheeringa & Zeanah, 2001; Yehuda & Bierer, 2007). Concurrent work in this cohort reports that prepregnancy maternal PTSD symptoms are associated with greater child negative affectivity in early childhood (Swales et al., 2022). Blunted diurnal cortisol may be indicative of psychological differences in mental health risk or may serve as a mechanism underlying the intergenerational effects of traumatic experiences. Dysregulation of diurnal cortisol may also have implications for physical health outcomes across the lifespan. Diurnal variation in cortisol serves as a signal of central circadian rhythms to multiple peripheral biological systems, and disruption of these patterns has pervasive effects throughout the brain and body, including modulation of immune, metabolic, and cardiovascular activity. For example, dysregulated diurnal cortisol predicts include shorter sleep duration (Saridjan et al., 2017) and increased risk ofupper respiratory infections in children (Turner-Cobb, Rixon & Jessop, 2011) as well as poorer immune function, cancer progression, fatigue, and depression in adults (Adam et al., 2017).
The two other predictor variables, depressive symptoms and perceived stress, were not significantly associated with diurnal cortisol slope or CAR in children. To our knowledge, no prior studies have examined maternal depressive symptoms or perceived stress prior to conception as predictors of diurnal cortisol. One possible explanation for the differential effects of PTSD symptoms as compared to perceived stress and depressive symptoms is that more mild variations in distress prior to conception have weaker effects on maternal physiology as compared to traumatic stress. In addition, greater PTSD symptoms were associated with flatter cortisol slopes at the second study visit (aged 5 years) but the strength of the association was somewhat weaker and not robust to adjustment for covariates. Differences across timepoints suggest that the effects of maternal PTSD symptoms are strongest at certain sensitive developmental timepoints, but other factors may also contribute. For example, the transition to kindergarten typically occurs around the age of 5 years and may lead to changes in daily routine that affect wake time, morning routine, levels of stress, and diurnal cortisol patterns in children (Bruce et al., 2002; Quas et al., 2002). Taken together, the pattern of findings suggests the importance of considering timing, chronicity, and acuity of exposures and the developmental context of outcome assessment in shaping offspring diurnal cortisol outcomes. Furthermore, results must be replicated in larger cohorts with greater power to detect smaller effects of depressive symptoms and perceived stress.
5.1 |. Strengths and limitations
The present study included low-income Black/African American, Latinx, White, and multiracial mother/child pairs who are historically underrepresented in maternal–child health research. Strengths of the present study also include prospective assessment of maternal mental health and stress prior to conception. Notably, we also assessed maternal depressive symptoms and perceived stress at study visits proximal to cortisol collection at the ages of 4 and 5 years. Childhood assessments shared approximately 10–20% of the variance with preconception mental health measures. The variability in perceived stress and maternal mental health symptoms over the course of the study period suggests that the prospective data collected prior to pregnancy account for additional variance in maternal mental health and are not merely capturing a stable phenomenon.
The present study did not assess PTSD symptoms during pregnancy, the postpartum period, or early childhood. It is beyond the scope of these findings to conclude that PTSD symptoms prior to pregnancy influence child cortisol patterns independent of prenatal, postnatal, and early childhood influences. Rather, these results suggest that symptoms detected before conception may relate to offspring physiology and complement research demonstrating that maternal mental health across the lifespan has implications for mothers, children, and families. For example, prior work has shown that maternal posttraumatic stress predicts adverse maternal outcomes including permissive parenting behaviors and negative emotion during childhood (Franz et al., 2022); therefore, the observed effects could be due to the continuation of PTSD symptoms beyond the period prior to conception and into early childhood, contributing to differences in parenting behaviors, which in turn influence child diurnal cortisol patterns. It is also possible that the association between prepregnancy PTSD symptoms and child cortisol slopes is due to genetics. However, experimental work in animal models indicates that prepregnancy maternal stress affects offspring neuroendocrine function independently of genetic influences (e.g., Huang et al., 2010; H. Li et al., 2010; Zaidan & Gaisler-Salomon, 2015).
Though only waking samples collected within typical awakening times (4 AM and 1 PM) and wake +30 samples collected within 20–40 min of the first sample were included in analyses, compliance issues may have influenced our findings with respect to CAR because collecting samples immediately upon awakening and 30 min later may have been challenging, particularly among families with young children. Child awakening times were also reported by parents in daily diaries and were not verified using an objective method (e.g., actigraphy) as recommended in expert consensus guidelines for the assessment of CAR (Stalder et al., 2016). Future studies should ideally incorporate objective recording of wake and sampling times to accurately estimate CAR profiles in children.
5.2 |. Future directions and implications
Study results highlight the importance of screening and intervention to improve maternal mental health prior to the start of pregnancy. Current practice guidelines suggest screening women for symptoms of depression and anxiety early in pregnancy (Milgrom & Gemmill, 2014), but it may be important to screen for symptoms of PTSD as well. For women who have given birth, screening between pregnancies or prior to conception of subsequent children would be ideal, as sensitive developmental windows occur early in pregnancy before parents typically initiate prenatal care. For example, much of the structural development of the HPA axis in the fetus occurs early in the first trimester of pregnancy (Howland, Sandman & Glynn, 2017). Preconception mental health also predicts maternal mental health during and after pregnancy (Kee et al., 2021; Witt et al., 2011). Thus, the interpregnancy interval may be a window of opportunity to ameliorate conditions during a subsequent perinatal period and is therefore ideal for screening and intervention especially in women with a history of mental health problems. To date, prepregnancy interventions to address adverse birth outcomes have primarily focused on maternal nutrition and health behaviors (M. Barker et al., 2018). The life-course framework that has been applied to effective nutrition and behavioral interventions before conception, during pregnancy, and throughout childhood could also be applied to maternal mental health, with the potential to impact development and long-term health of both mothers and offspring. Screening during encounters with the healthcare system that occur before, during, and after pregnancy may be the most effective way to enhance detection of symptoms and provide opportunities for intervention, provided that routine screenings are coupled with referral services and support systems for providers and patients (Gjerdincjen et al., 2009; Gordon et al., 2006; Scholle et al., 2003; Seehusen et al., 2005). Early identification of women with elevated levels of PTSD symptoms would also allow obstetric providers to be aware that they are caring for individuals with mental health concerns, facilitating the provision of trauma-informed care. Overall, women’s mental health before, during, and after pregnancy plays an important role in maternal and child health outcomes and treating symptoms of PTSD may ameliorate adverse outcomes across the life course.
Future work may explore potential pathways underlying the association between maternal preconception mental health and child HPA axis activity, which could include alterations in maternal physiology or behavior during pregnancy, early parenting practices, or other mechanisms. Though prior work suggests that diurnal cortisol patterns predict important physical and mental health outcomes across the lifespan, additional work is needed to explore the implications of diurnal cortisol patterns in children for subsequent risk of psychopathology or other adverse outcomes. Future studies could also examine paternal factors before or during pregnancy to better understand transmission of risk from parent to child.
6 |. CONCLUSIONS
Our findings in a sample of predominantly low-income, preschool-aged children suggested that maternal posttraumatic stress symptoms prior to conception predict diurnal cortisol slopes in offspring. Higher symptoms of PTSD prior to conception predicted a flatter slope in offspring during early childhood. While preconception effects of PTSD cannot be disentangled completely from ongoing effects on mothers during pregnancy and children’s early childhood, these findings emphasize the importance of preconception maternal mental health and are relevant to interventions that aim to apply a life course perspective to improving maternal and child health outcomes.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD072021; Dunkel Schetter, PI) and earlier work by the Child Community Health Network (CCHN), supported through cooperative agreements with the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; U HD44207, U HD44219, U HD44226, U HD44245, U HD44253, U HD54791, U HD54019, U HD44226–05S1, U HD44245–06S1, R03 HD59584) and the National Institute for Nursing Research (U NR008929).
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: RO1HD072021; Earlier work by the Child Community Health Network (CCHN), supported through cooperative agreements with the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: UHD44207, UHD44219, UHD44226, UHD44245, UHD44253, UHD54791, UHD54019, UHD44226–05S1, UHD44245–06S1, R03HD59584; National Institute of Nursing Research, Grant/Award Number: UNR008929
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST
The authors do not have conflicts of interest to declare.
Participants in the CCHN cohort were followed for up to 2 years after the first birth, and there were 447 subsequent pregnancies identified by study staff. To determine whether mental health after the first child’s birth predicted who went on to have another child in this sample, we compared these 447 participants to the 1068 women who remained in contact with the study through the end of the follow-up period but did report a subsequent pregnancy. There were no differences between the two groups in PTSD symptoms at 6 months postpartum or perceived stress at 1 month, 6 months, or 12 months postpartum, or in depressive symptoms at 1 month, 6 months, or 12 months postpartum (all ps > .10). Thus, it appears unlikely that mental health after the first child predicted who went on to have another child in this sample.
The analytic sample of 85 mother–child dyads included in analyses did not differ significantly from the full cohort (n = 129) with respect to study site, length of interpregnancy interval, child sex, household income, mother’s education, mother’s relationship status, or maternal prepregnancy perceived stress, depressive symptoms, and PTSD symptoms. Children in the analytic sample were slightly younger at the time of the first study visit (M = 3.77, SD = 0.47) than those in the full cohort (M = 3.85, SD = 0.42), t(125) = 2.33, p = .02); there were no significant age differences at the second study visit. Mothers in this sample were less likely to identify as African American/Black (13% vs. 21% in the full cohort), and children were also less likely to be Black (11% vs. 19% in the full cohort).
In sensitivity analyses, we repeated analyses excluding participants with reported use of corticosteroid medication. Results were similar and the pattern of statistical significance was identical. Therefore, we present final models including all participants and covarying for corticosteroid medication.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, & Griffith JW (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35(6), 921–931. 10.1016/j.psyneuen.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, & Gilbert KE (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Vrshek-Schallhorn S, Kendall AD, Mineka S, Zinbarg RE, & Craske MG (2014). Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up. Psychoneuroendocrinology, 44, 47–59. 10.1016/j.psyneuen.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J, Hill CM, Kendrick T, Inskip HM, & Group, t. S. S. (2009). Infant sleep disturbance is associated with preconceptional psychological distress: Findings from the Southampton Women’s Survey. Sleep, 32(4), 566–568. 10.5665/sleep/32.4.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP (1998). In utero programming of chronic disease. Clinical Science, 95(2), 115–128. 10.1042/cs0950115 [DOI] [PubMed] [Google Scholar]
- Barker M, Dombrowski SU, Colbourn T, Fall CHD, Kriznik NM, Lawrence WT, …, & Stephenson J (2018). Intervention strategies to improve nutrition and health behaviours before conception. The Lancet, Vol., 391, pp. 1853–1864. 10.1016/S0140-6736(18)30313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler D, Kirschbaum C, Kliegel M, Alexander N, & Stalder T (2013). The cortisol awakening response in toddlers and young children. Psychoneuroendocrinology, 38(11), 2485–2492. 10.1016/J.PSYNEUEN.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Bernard K, Butzin-Dozier Z, Rittenhouse J, & Dozier M (2010). Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of child protective services. Archives of Pediatrics and Adolescent Medicine, 164(5), 438–443. 10.1001/archpediatrics.2010.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Zwerling J, & Dozier M (2015). Effects of early adversity on young children’s diurnal cortisol rhythms and externalizing behavior. Developmental Psychobiology, 57(8), 935–947. 10.1002/dev.21324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, & Forneris CA (1996). Psychometric properties of the PTSD checklist (PCL). Behaviour Research and Therapy, 34(8), 669–673. 10.1016/0005-7967(96)00033-2 [DOI] [PubMed] [Google Scholar]
- Bowers ME, & Yehuda R (2015). Intergenerational transmission of stress in humans. Neuropsychopharmacology 2016 41:1, 41(1), 232–244. 10.1038/npp.2015.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, & Stowe ZN (2010). The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology, 35(5), 686–693. 10.1016/j.psyneuen.2009.10.009 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Davis EP, & Gunnar MR (2002). Individual differences in children’s cortisol response to the beginning of a new school year. Psychoneuroendocrinology, 27, 635–650. Retrieved from www.elsevier.com/locate/psyneuen [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, & Levine S (2009). Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology, 51(1), 14–23. 10.1002/dev.20333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, …, & Stein MB (2009). Validation of a brief measure of anxiety-related severity and impairment: The Overall Anxiety Severity and Impairment Scale (OASIS). Journal of Affective Disorders, 112(1–3), 92. 10.1016/J.JAD.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemtob CM, Nomura Y, Rajendran K, Yehuda R, Schwartz D, & Abramovitz R (2010). Impact of maternal posttraumatic stress disorder and depression following exposure to the September 11 attacks on preschool children’s behavior. Child Development, 81(4), 1129–1141. 10.1111/j.1467-8624.2010.01458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Khashan AS, Lichtenstein P, Långström N, & D’Onofrio BM (2013). Maternal stress and infant mortality: The importance of the preconception period. Psychological Science, 24(7), 1309–1316. 10.1177/0956797612468010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Mortensen PB, Henriksen TB, Dalman C, D’Onofrio BM, & Khashan AS (2015). Preconception maternal bereavement and infant and childhood mortality. Psychosomatic Medicine, 77(8), 863–869. 10.1097/psy.0000000000000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression scale. British Journal of Psychiatry, 150, (JUNE), 782–786. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, & Sandman CA (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 52(2), 119–129. 10.1111/j.1469-7610.2010.02314.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMorrow S (March 26). (2018). Role of the hypothalamic–pituitary–adrenal axis in health and disease. International Journal of Molecular Sciences, Vol., 19, p. 986. 10.3390/ijms19040986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, & Craske MG (2007). Racial/Ethnic Differences in Cortisol Diurnal Rhythms in a Community Sample of Adolescents. Journal of Adolescent Health, 41(1), 3–13. 10.1016/j.jadohealth.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Schetter D, C, S., P, L., G, R., Clark-Kauffman E, Raju TNK, & Hillemeier MM (2013). Shedding light on the mechanisms underlying health disparities through community participatory methods: The stress pathway. Perspectives on Psychological Science, 8(6), 613–633. 10.1177/1745691613506016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, …, & Godfrey KM (2018). Origins of lifetime health around the time of conception: Causes and consequences. The Lancet, 391(10132), 1842–1852. 10.1016/S0140-6736(18)30312-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz MR, Kumar SA, Brock RL, Calvi JL, & DiLillo D (2022). Parenting behaviors of mothers with posttraumatic stress: The roles of cortisol reactivity and negative emotion. Journal of Family Psychology, 36(1), 130–139. 10.1037/FAM0000865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerdincjen D, Crow S, McGovern P, Miner M, & Center B (2009). Postpartum depression screening at well-child visits: Validity of a 2-question screen and the PHQ-9. The Annals of Family Medicine, 7(1), 63–70. 10.1370/AFM.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, & Hanson MA (2004). Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatric Research, 56(3), 311–317. [DOI] [PubMed] [Google Scholar]
- Gordon TEJ, Cardone IA, Kim JJ, Gordon SM, & Silver RK (2006). Universal perinatal depression screening in an academic medical center. Obstetrics and Gynecology, 107(2), 342–347. 10.1097/01.AOG.0000194080.18261.92 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez DM (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13(3), 515–538. 10.1017/S0954579401003066 [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, & Buitelaar JK (2005). Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology, 30(6), 541–549. https://S0306-45300500017-X[pii] [DOI] [PubMed] [Google Scholar]
- Gutteling BM, De Weerth C, & Buitelaar JK (2004). Maternal prenatal stress and 4–6 year old children’s salivary cortisol concentrations pre- and post-vaccination. Stress (Amsterdam, Netherlands), 7(4), 257–260. 10.1080/10253890500044521 [DOI] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari S, Stanford K, Cleary SE, Shiers HM, …, & Herbert J (2000). Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. British Journal of Psychiatry, 177, (DEC.), 505–510. 10.1192/bjp.177.6.505 [DOI] [PubMed] [Google Scholar]
- Harville EW, Boynton-Jarrett R, Power C, & Hyppönen E (2010). Childhood hardship, maternal smoking, and birth outcomes: A prospective cohort study. Archives of Pediatrics and Adolescent Medicine, 164(6), 533–539. 10.1001/archpediatrics.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland MA, Sandman CA, & Glynn LM (2017). Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Review of Endocrinology and Metabolism, 12(5), 321–339. 10.1080/17446651.2017.1356222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Shi X, Xu H, Yang H, Chen T, Chen S, & Chen X (2010). Chronic unpredictable stress before pregnancy reduce the expression of brain-derived neurotrophic factor and N-methyl-D-aspartate receptor in hippocampus of offspring rats associated with impairment of memory. Neurochemical Research, 35(7), 1038–1049. 10.1007/s11064-010-0152-0 [DOI] [PubMed] [Google Scholar]
- Huth-Bocks AC, Krause K, Ahlfs-Dunn S, Gallagher E, & Scott S (2013). Relational trauma and posttraumatic stress symptoms among pregnant women. Psychodynamic Psychiatry, 41(2), 277–302. 10.1521/pdps.2013.41.2.277 [DOI] [PubMed] [Google Scholar]
- Irwin JL, Meyering AL, Peterson G, Glynn LM, Sandman CA, Hicks LM, & Davis EP (2021). Maternal prenatal cortisol programs the infant hypothalamic–pituitary–adrenal axis. Psychoneuroendocrinology, 125, 105106. 10.1016/J.PSYNEUEN.2020.105106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop DS, & Turner-Cobb JM (2008). Measurement and meaning of salivary cortisol: A focus on health and disease in children. Stress (Amsterdam, Netherlands), 11(1), 1–14. 10.1080/10253890701365527 [DOI] [PubMed] [Google Scholar]
- Kee MZL, Ponmudi S, Phua DY, Rifkin-Graboi A, Chong YS, Tan KH, …, & Meaney MJ (2021). Preconception origins of perinatal maternal mental health. Archives of Women’s Mental Health, 24(4), 605–618. 10.1007/s00737-020-01096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Hipwell AE, Class QA, & Mbayiwa K (2018). Extending the developmental origins of disease model: Impact of preconception stress exposure on offspring neurodevelopment. Developmental Psychobiology, 60(7), 753–764. 10.1002/dev.21773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, & Hellhammer DH (2000). Salivary cortisol. In (Fink G Ed.), Encyclopedia of Stress (p. Vol., 3, pp. 379–383). 10.1016/B978-012373947-6.00334-2 [DOI] [Google Scholar]
- Leen-Feldner EW, Feldner MT, Knapp A, Bunaciu L, Blumenthal H, & Amstadter AB (2013). Offspring psychological and biological correlates of parental posttraumatic stress: Review of the literature and research agenda. Clinical Psychology Review, 33(8), 1106–1133. 10.1016/j.cpr.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Lehrner A, Bierer LM, Passarelli V, Pratchett LC, Flory JD, Bader H, …, & Yehuda R (2014). Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology, 40, 213–220. 10.1016/j.psyneuen.2013.11.019.Maternal [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher P, Greenwood CJ, Romaniuk H, Spry E, Macdonald JA, McAnally H, …, & Olsson CA (2020). Adolescent and young adult mental health problems and infant offspring behavior: Findings from a prospective intergenerational cohort study. Journal of Affective Disorders, 272, (March), 521–528. 10.1016/j.jad.2020.03.101 [DOI] [PubMed] [Google Scholar]
- Li H, Zhang L, Fang Z, Lin L, Wu C, & Huang Q (2010). Behavioral and neurobiological studies on the male progeny of maternal rats exposed to chronic unpredictable stress before pregnancy. Neuroscience Letters, 469(2), 278–282. 10.1016/J.NEULET.2009.12.017 [DOI] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, & Obel C (2010). Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark. European Child and Adolescent Psychiatry, 19(10), 747–753. 10.1007/s00787-010-0113-9 [DOI] [PubMed] [Google Scholar]
- Mahrer NE, Guardino CM, Hobel C, & Dunkel Schetter C (2021). Maternal stress before conception is associated with shorter gestation. Annals of Behavioral Medicine, 55(3), 242–252. 10.1093/ABM/KAAA047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CG, Bruce J, & Fisher PA (2012). Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: The role of parental psychosocial risk and monitoring. Hormones and Behavior, 61(5), 661–668. 10.1016/j.yhbeh.2012.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis EW, Lopez-Duran N, Martinez-Torteya C, Abelson JL, & Muzik M (2016). Cortisol awakening response and internalizing symptoms across childhood: Exploring the role of age and externalizing symptoms. International Journal of Behavioral Development, 40(4), 289–295. 10.1177/0165025415590185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom J, & Gemmill AW (2014). Screening for perinatal depression. Best Practice and Research: Clinical Obstetrics and Gynaecology, 28(1), 13–23. 10.1016/j.bpobgyn.2013.08.014 [DOI] [PubMed] [Google Scholar]
- Morgan JE, Lee SS, Mahrer NE, Guardino CM, Davis EP, Shalowitz MU, …, & Dunkel Schetter C (2020). Prenatal maternal C-reactive protein prospectively predicts child executive functioning at ages 4–6 years. Developmental Psychobiology, 62(8), 1111–1123. 10.1002/DEV.21982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Compas BE, & Garber J (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review, Vol., 32, pp. 301–315. 10.1016/j.cpr.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Winter MA, Hunn J, Carnahan J, Pressman EK, Glover V, …, & Caserta MT (2013). Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain, Behavior, and Immunity, 32, 21–28. 10.1016/j.bbi.2013.02.002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas JA, Murowchick E, Bensadoun J, & Boyce WT (2002). Predictors of children’s cortisol activation during the transition to kindergarten. Journal of Developmental and Behavioral Pediatrics, 23(5), 304–313. 10.1097/00004703-200210000-00002 [DOI] [PubMed] [Google Scholar]
- Ramey SL, Schafer P, Declerque JL, Lanzi RG, Hobel C, Shalowitz M, …, & Raju TNK (2015). The Preconception Stress and Resiliency Pathways model: A multi-level framework on maternal, paternal, and child health disparities derived by Community-Based Participatory Research. Maternal and Child Health Journal, 19(4), 707–719. 10.1007/s10995-014-1581-1 [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen CL, & Poland RE (2010). Longitudinal course of adolescent depression: Neuroendocrine and psychosocial predictors. Journal of the American Academy of Child & Adolescent Psychiatry, 49(2), 141–151. 10.1016/j.jaac.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero KJ, Del Ben K, Scotti JR, & Rabalais AE (2003). Psychometric properties of the PTSD Checklist - Civilian Version. Journal of Traumatic Stress, 16(5), 495–502. 10.1023/A:1025714729117 [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Fisher B-D, D, S., D, M., & Schwartzman AE (2011). Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior, 59(1), 123–132. 10.1016/j.yhbeh.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson KW, & Cashman C (2008). Effects of intimate partner violence and maternal posttraumatic stress symptoms on children’s emotional and behavioral functioning. Journal of Emotional Abuse, 8(1–2), 139–153. 10.1080/10926790801986007 [DOI] [Google Scholar]
- Saridjan NS, Kocevska D, Luijk MPCM, Jaddoe VWV, Verhulst FC, & Tiemeier H (2017). The prospective association of the diurnal cortisol rhythm with sleep duration and perceived sleeping problems in preschoolers: The generation R study. Psychosomatic Medicine, 79(5), 557–564. 10.1097/PSY.0000000000000440 [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, & Zeanah CH (2001). A relational perspective on PTSD in early childhood. Journal of Traumatic Stress, 14(4), 799–815. 10.1023/A:1013002507972 [DOI] [PubMed] [Google Scholar]
- Scholle SH, Haskett RF, Hanusa BH, Pincus HA, & Kupfer DJ (2003). Addressing depression in obstetrics/gynecology practice. General Hospital Psychiatry, 25(2), 83–90. 10.1016/S0163-8343(03)00006-9 [DOI] [PubMed] [Google Scholar]
- Seehusen DA, Baldwin LM, Runkle GP, & Clark G (2005). Are family physicians appropriately screening for postpartum depression? The Journal of the American Board of Family Practice, 18(2), 104–112. 10.3122/JABFM.18.2.104 [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, & Essex MJ (2008). Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology, 50(7), 690–703. 10.1002/dev.20336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Roberts AL, LeWinn KZ, Bush NR, Rovnaghi CR, Tylavsky F, & Anand KJS (2018). Maternal experiences of trauma and hair cortisol in early childhood in a prospective cohort. Psychoneuroendocrinology, 98, (April), 168–176. 10.1016/j.psyneuen.2018.08.027 [DOI] [PubMed] [Google Scholar]
- Speer KE, Semple S, Naumovski N, D’Cunha NM, & McKune AJ (2019). HPA axis function and diurnal cortisol in post-traumatic stress disorder: A systematic review. Neurobiology of Stress, 11, 100180. 10.1016/J.YNSTR.2019.100180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spry E, Moreno-Betancur M, Becker D, Romaniuk H, Carlin JB, Molyneaux E, …, & Patton GC (2020). Maternal mental health and infant emotional reactivity: A 20-year two-cohort study of preconception and perinatal exposures. Psychological Medicine, 50(5), 827–837. 10.1017/S0033291719000709 [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, …, & Clow A (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. 10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Stephenson J, Heslehurst N, Hall J, Schoenaker DAJM, Hutchinson J, Cade JE, …, & Mishra GD (2018). Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. The Lancet, 391(10132), 1830–1841. 10.1016/S0140-6736(18)30311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales DA, Poggi Davis E, Mahrer NE, Guardino CM, Shalowitz MU, Ramey SL, & Dunkel Schetter C (2022). Preconception maternal post-traumatic stress and child negative affectivity: Prospectively evaluating the intergenerational impact of trauma. Development and Psychopathology, 1–11. Retrieved from 10.1017/S0954579421001760 [DOI] [PMC free article] [PubMed]
- Thomas JL, Cleveland S, Pietrzak RH, Dunkel Schetter C, & Sumner JA (2021). Elucidating posttraumatic stress symptom dimensions and health correlates among postpartum women. Journal of Affective Disorders, 294, (July), 314–321. 10.1016/j.jad.2021.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Cobb JM, Rixon L, & Jessop DS (2011). Hypothalamic-pituitary-adrenal axis activity and upper respiratory tract infection in young children transitioning to primary school. Psychopharmacology, 214(1), 309–317. 10.1007/s00213-010-1965-x [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, & Adam EK (2013). The cortisol awakening response predicts major depression: Predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine, 43(3), 483–493. 10.1017/S0033291712001213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, & Scheeringa MS (2013). Maternal depression and treatment gains following a cognitive behavioral intervention for posttraumatic stress in preschool children. Journal of Anxiety Disorders, 27(1), 140–146. 10.1016/j.janxdis.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M (2005). The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behavior, and Immunity, 19(4), 296–308. 10.1016/J.BBI.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Witt WP, Cheng ER, Wisk LE, Litzelman K, Chatterjee D, Mandell K, & Wakeel F (2014). Maternal stressful life events prior to conception and the impact on infant birth weight in the United States. American Journal of Public Health, 104(1), 81–89. 10.2105/AJPH.2013.301544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt WP, Wisk LE, Cheng ER, Hampton JM, Creswell PD, Hagen EW, …, & DeLeire T(2011). Poor prepregnancy and antepartum mental health predicts postpartum mental health problems among US Women: A nationally representative population-based study. Women’s Health Issues, 21(4), 304–313. 10.1016/j.whi.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt WP, Wisk LE, Cheng ER, Hampton JM, & Hagen EW (2012). Preconception mental health predicts pregnancy complications and adverse birth outcomes: A national population-based study. Maternal and Child Health Journal, 16(7), 1525–1541. 10.1007/s10995-011-0916-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, & Bierer LM (2007). Transgenerational transmission of cortisol and PTSD risk. In Progress in Brain Research, (Vol., 167, pp). 121–135. 10.1016/S0079-6123(07)67009-5 [DOI] [PubMed] [Google Scholar]
- Yehuda R, & Bierer LM (2009). The relevance of epigenetics to PTSD: Implications for the DSM-V. Journal of Traumatic Stress, 22(5), 427–434. 10.1002/JTS.20448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, Breslau I, & Dolan S (2000). Low cortisol and risk for PTSD in adult offspring of Holocaust survivors. American Journal of Psychiatry, 157(8), 1252–1259. 10.1176/appi.ajp.157.8.1252 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Blair W, Labinsky E, & Bierer LM (2007). Effects of parental PTSD on the cortisol response to dexamethasone administration in their adult offspring. American Journal of Psychiatry, 164(1), 163–166. 10.1176/ajp.2007.164.1.163 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, & Berkowitz GS (2005). Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. Journal of Clinical Endocrinology and Metabolism, 90(7), 4115–4118. 10.1210/jc.2005-0550 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, & Bierer LM (2002). Cortisol levels in adult offspring of Holocaust survivors: Relation to PTSD symptom severity in the parent and child. Psychoneuroendocrinology, 27(1–2), 171–180. 10.1016/S0306-4530(01)00043-9 [DOI] [PubMed] [Google Scholar]
- Zaidan H, & Gaisler-Salomon I (2015). Prereproductive stress in adolescent female rats affects behavior and corticosterone levels in second-generation offspring. Psychoneuroendocrinology, 58, 120–129. 10.1016/J.PSYNEUEN.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Lu MC, & Halfon N (2003). Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal, 7(1), 13–30. 10.1023/A:1022537516969 [DOI] [PubMed] [Google Scholar]
- Misra DP, Guyer B, & Allston A (2003). Integrated perinatal health framework. American Journal of Preventive Medicine, 25(1), 65–75. 10.1016/s0749-3797(03)00090-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


