Abstract
Background:
Retriage is the emergency transfer of severely injured patients from nontrauma and lower-level trauma centers to higher-level trauma centers. We identified the barriers to retriage at sending centers in a single health system.
Methods:
We conducted a failure modes effects and criticality analysis at 4 nontrauma centers and 5 lower-level trauma centers in a single health system. Clinicians from each center described the steps in the trauma assessment and retriage process to create a process map. We used standardized scoring to characterize each failure based on frequency, impact on retriage, and prevention safeguards. We ranked each failure using the scores to calculate a risk priority number.
Results:
We identified 26 steps and 93 failures. The highest-risk failure was refusal by higher-level trauma centers (receiving hospitals) to accept a patient. The most critical failures in the retriage process based on total risk, frequency, and safeguard scores were (1) refusal from a receiving higher-level trauma center to accept a patient (risk priority number = 191), (2) delay in a sending center's consultant examination of a patient in the emergency department (risk priority number = 177), and (3) delay in receiving hospital's consultant calling back (risk priority number = 177).
Conclusion:
We identified (1) addressing obstacles to determining clinical indications for retriage and (2) identifying receiving level I trauma centers who would accept the patient as opportunities to increase timely retriage. Establishing clear clinical indications for retriage that sending and receiving hospitals agree on represents an opportunity for intervention that could improve the retriage of injured patients.
Introduction
Despite decades of research informing field triage algorithms used by emergency medical services (EMS) personnel in the field, 17% to 34% of seriously injured patients are undertriaged to nontrauma centers (NTCs) or level II trauma centers.1,2 Field undertriage of injured patients persists because, in part, of the underestimations of the injury severity in the field and geographic mismatch between where the trauma occurs and the location of the trauma centers.3 The emergency transfer of trauma patients, field-triaged to an NTC or lower-level trauma center (LLTC) to a higher-level trauma center, is called retriage. Although the Centers for Disease Control and Prevention and the American College of Surgeons Committee on Trauma developed guidelines for triage of severely injured patients, adherence at the state and regional levels varies widely. Further, although many high-level trauma centers encourage transfers, factors like the request process, insurance, having a diagnosis, identifying the admitting physician, and bed availability can prevent them from accepting patients in a timely fashion. Ideally, retriage can be accomplished in an hour, but, in reality, the process takes >4 hours on average.4-6 Lengthy diagnostic evaluations and treatments4,7 could delay definitive care and increase mortality in patients who are eventually retriaged.6,8-10 When retriage is expeditious, survival is similar compared with patients who are taken directly to higher-level trauma centers.6 Despite higher mortality in NTCs, 30% of severely injured patients are never retriaged.2,11,12 However, to date, most retriage studies have been secondary analyses of administrative data13 that, although providing important descriptive statistics, offer little information about the retriage process and its underlying failures.
The objective of this study was to identify the barriers and/or failures in the process that contributes to the failure to retriage, or the delay in the retriage of trauma patients >120 minutes from emergency department (ED) arrival at sending NTCs and LLTCs.14
Methods
We conducted a failure modes effects and criticality analysis (FMECA)15,16 of the trauma patient retriage process at 4 NTCs and 5 LLTCs in a single academic health system. The FMECA is a robust prospective, systems engineering approach that has been adapted from other high-risk industries, such as automotive and nuclear power, to proactively, systematically, and comprehensively identify and characterize systematic procedural risks and vulnerabilities.17,18 The approach has been used frequently to assess complex health processes, including the discharge of high-risk patients with diabetes, intravenous drug administration, stroke patient transfers, and transplantation procedures.19-25 The approach provides a robust assessment and can yield valuable insights into significant quality and process improvement opportunities.
Clinicians and staff involved in the care of the injured patients from 4 NTCs and 5 Illinois Department of Public Health-designated level II trauma centers in a single academic health system participated in the FMECA. Illinois does not have level III or IV trauma centers, and classification as a level I or II center is based on coverage and specialist availability.26 The Institutional Review Board at Northwestern University approved the study. The study had 4 phases: I, recruitment of clinicians and staff; II, description of steps in the retriage process; III, identification of barriers and/or failures of each step and the underlying causes of the barriers or failures; and IV, characterization of each failure’s frequency (F), impact or harm to a trauma patient (I), and any existing safeguards to mitigate the failure (S) using standardized scores. These scores were used to calculate a risk prediction number (RPN = F x I x S) for each failure.25 The RPN was used to order each failure from highest to lowest risk, with higher scores indicating more critical failures in the process.
Phase I: recruitment of clinicians and staff
Representative clinicians and staff involved in trauma care, including surgeons, emergency medicine physicians, trauma coordinators, and trauma-trained ED nurses, were recruited at each of the system’s NTC and LLTC. We used snowball sampling, initially recruiting and obtaining informed consent of trauma coordinators at each of the participating NTCs and LLTCs. The coordinators were then asked to identify nurses and physicians who were the most knowledgeable about the retriage process at their site. After an introductory meeting with initial participants, we asked them to identify additional clinicians who would have insight into the trauma evaluation and retriage process (Table I).
Table I.
Failure modes effects and criticality analysis participant list
| Trauma center level(s) | Role | Number of participants |
|---|---|---|
| Level II | Emergency medicine physician | 8 |
| Level II and nontrauma | Emergency medicine physician | 3 |
| Nontrauma | Emergency medicine physician | 1 |
| Level II | General surgeon | 2 |
| Level II | Associate manager, emergency department | 1 |
| Level II | Emergency department nurse | 5 |
| Level II | Trauma coordinator | 4 |
| Level II and nontrauma | Trauma coordinator | 1 |
| Nontrauma | Outcomes manager | 1 |
Phase II: description of steps in the retriage process
In a 60-minute virtual session, participants were asked to describe, in their own words and from their own perspectives, the steps in the systems and processes of care leading to a decision to retriage a trauma patient and communicate that decision to a receiving higher-level trauma center. The session was recorded and field notes were taken by the research team. Each identified step, task, or communication was placed in its appropriate sequence in the retriage process, as described by the participants, and used to create a preliminary process map. The map was then sent to all participants for revisions and feedback before finalization.
Phase III: identification of the failures in each step and the underlying causes
In a second virtual session, the participants were asked, collectively, to proceed through the identified steps of the process map and identify potential failures of each step, and then, to describe the underlying causes of each failure.
Phase IV: characterization of the identified failures
Participants were asked to characterize each failure based on their frequency, impact on the retriage process, and existing safeguards to mitigate or prevent them using a standardized 10-point scale (Table II). Given that this was the first application of the FMECA methodology in trauma care, definitions for the values describing frequency, impact, and safeguards were assigned by study participants. For frequency, a score of “1” indicates a failure that almost never occurs (1/10,000 retriage cases), whereas a high score of “10” denotes failures that occur in almost every retriage case (1/10+). Similarly, an impact score of 1 was assigned to failures that did not present any clear disruption to the process, whereas a score of 10 indicates failures that are likely to result in severe negative outcomes for a patient. A safeguard score of 1 is defined as a failure where detection is almost certain, whereas a score of 10 identifies failures without known methods of prevention.
Table II.
Standardized scores for characterizing failures
| Score | Effect of failure (severity) | Frequency | Existing safeguard to mitigate failure (detection) |
|---|---|---|---|
| 1 | None: no reason to expect failure | None: 1/10,000 | Almost certain: current control(s) almost certain to detect failure mode |
| 2 | Very low: minor disruption to process; no process delay | Very low: 1/5,000 | Very high: very high likelihood current control(s) will detect failure mode |
| 3 | Low: minor process delay (~1–4 min) | Low: 1/2,000 | High: semiautomatic mean of detection with warning that does not prevent the process from continuing |
| 4 | Low to moderate: minor to moderate process delay (~5–9 min) | Low to moderate: 1/1,000 | Moderately high: semiautomatic mean of detection that does not prevent the process from continuing |
| 5 | Moderate: moderate process delay (~10–19 min) | Moderate: 1/500 | Moderate: double human review with a checklist or standard aid, or triple human review without checklist or standard aid |
| 6 | Moderate to high: moderate disruption to process (~20–29 min) | Moderate to high: 1/200 | Low: single human review with a checklist, standard aid, or double human review without checklist or standard aid |
| 7 | High: high disruption to process; significant process delay (≥30 min) | High: 1/100 | Very low: very low likelihood current control(s) will detect failure mode |
| 8 | Very high: significant process delay | Very high: 1/50 | Remote: remote likelihood current control(s) will detect failure mode |
| 9 | Hazard: potential health, safety, or environmental issue | Very high: 1/20 | Very remote: no human review performed |
| 10 | Hazard: potential safety, health or environmental issue | Very high: 1/10+ | Almost impossible: no known control(s) available to detect failure mode |
Once each failure was assigned scores from the 10-point scale for frequency, impact, and safeguards, each of a given failure’s indicators was multiplied to produce its RPN.
Validation of findings by participants
A review of the FMECA’s 4 phases with participants in the final virtual session resulted in minor clarifications of the map and scoring of the failures. These clarifications were related to individual differences in the centers and slight disparities in frequency due to variations in geography, staffing, and proximity to higher-level trauma centers. However, they did not result in any modification of the rank ordering of the failures. Furthermore, participants confirmed that the highest-risk failures were major obstacles to appropriate and timely retriage of trauma patients.
Selection of initial targets for solution design
In a final virtual session, the process map, results of the risk table, and failures ranked by RPN were presented to the participants. Participants at each NTC and LLTC confirmed whether the highest-ranked items reflected critical failures in their process that affect appropriate and timely retriage of trauma patients. Participants were asked to suggest initial targets for solution design and practical strategies to prevent or mitigate the highest-ranked failures.
Results
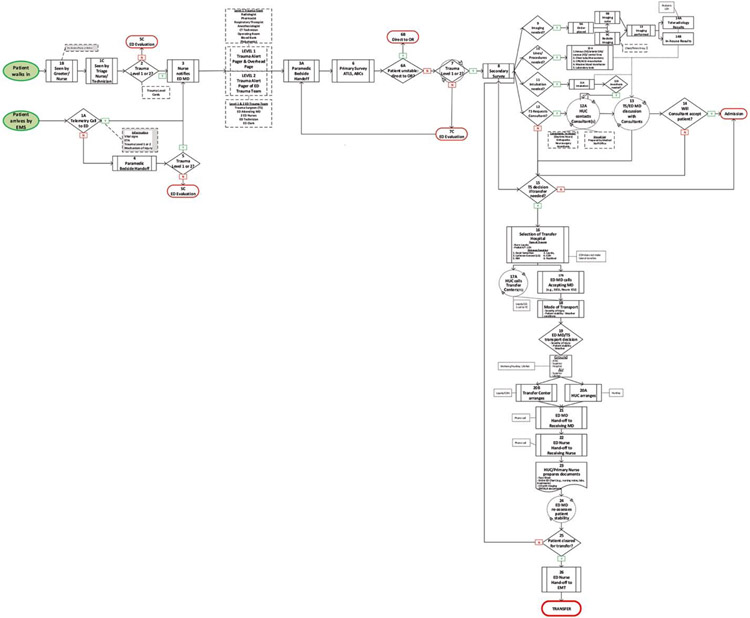
A total of 27 clinicians from the 4 NTCs and 5 LLTCs were recruited for the FMECA (Table I). Participants identified 26 (n = 26) steps in the retriage process, spanning from the arrival of an injured patient in the ED to the end, with a patient’s transportation to the receiving higher-level trauma center (Figure 1). Ninety-three (n = 93) specific failures in the 26-step retriage process were identified.
Figure 1.
Low-level trauma center process map
The 3 highest-risk failures based on RPN were (1) a receiving higher-level trauma center refusing to accept a patient, (2) delay in a sending center’s consultant examination of a patient in the ED, and (3) delay in a receiving higher-level trauma center’s consultant/physician returning a call back to a sending center that requested retriage (Table III).
Table III.
Top 10 overall failures in retriage process at nontrauma, lower-level, and high-level trauma centers
| Identification of receiving high-level trauma center | ||||
|---|---|---|---|---|
| Step | Step description | Potential failures | Potential effects | RPN |
| 16 | Selection of high-level trauma center | Refusal to accept patient | Multiple attempts to identify high-level trauma center to take trauma case | 191 |
| 16 | Selection of high-level trauma center | Delay in receiving consultant calling back | Delay high-level trauma center accepting patient | 177 |
| 17B | Sending ED MD calls receiving MD | Inpatient and ICU bed availability extremely limited | Delays selection of receiving center | 169 |
| 16 | Selection of high-level trauma center | Refusal of consultant to accept case at receiving center | Delays care for patient | 159 |
| 17A | HUC calls high-level trauma center | Receiving center does not call back | Delays retriage process | 159 |
| Determination of clinical indications for retriage | ||||
|---|---|---|---|---|
| Step | Step description | Potential failures | Potential effects | RPN |
| 13 | TS/ED MD discussion with consultants | Difficult to get consultant to see patient quickly | Delay determining whether patient needs retriage | 177 |
| 14A | Teleradiology results | Trauma studies not prioritized | Delays reading | 176 |
| 13 | TS/ED MD discussion with consultants | Lack of on-call consultant for certain specialties | Retriage obligated because of consultant availability | 171 |
| 12 | Consultant needed | No consultant available/on call for needed specialty | Delay determining whether patient needs retriage | 155 |
| 9A | Imaging orders placed | Challenges transmitting imaging to TS and consultants because of high number of patients | Delay determining whether patient needs retriage | 154 |
ED MD, emergency department physician; HUC, health unit coordinator; ICU, intensive care unit; RPN, risk priority number; TS, trauma surgeon.
Among all identified failures, 64 (62%) were characterized as resulting in moderate to high delay in the retriage process (impact score ≥6; delay ~20–29 min). Failures that can lead to retriage times >120 minutes include (1) sudden clinical decompensation after initial trauma evaluation, (2) a difficult airway requiring multiple attempts to intubate, and (3) sending center attempts to stabilize a patient in the ED rather than sending to the operating room.
The most frequent failures were (1) interruptions during a paramedic’s bedside patient handoff upon ED arrival to the ED trauma team; (2) sending center consultant being unable to evaluate a patient in the ED, thereby delaying retriage determination; and (3) lack of an on-call specialty consultant at a sending center.
Failures with the fewest current safeguards for prevention or detection were (1) lack of availability of sending center specialty consultants, (2) inadequate trauma assessments in the ED waiting room for walk-in trauma patients, and (3) inadequate details provided by the patient or family about the traumatic event.
Determination of clinical indications for retriage
The NTCs and/or LLTCs reported needing to provide a clinical justification for retriage, as in steps 9 and 12 (before step 15). Two forms of clinical justification were identified: radiological confirmation of a suspected injury diagnosis and consultant evaluation at the sending center. Radiological confirmation of a suspected injury diagnosis added additional steps (steps 9–14A/B) to the trauma evaluation. The highest-ranked failures of these steps were trauma imaging studies not being prioritized (RPN = 176), as shown in Table III. The next highest-ranked failures were challenges in transmitting images to sending center radiologists (RPN = 154), difficulty sending radiology results to the sending center (RPN = 114), and difficulty sharing imaging/radiology results with the receiving center (RPN = 65).
Consultants at sending centers were identified as being involved in steps 12 through 14. Most high-risk failures that occurred at the sending centers were related to these steps (Table IV). Failures included the delay or failure of a sending center consultant to evaluate a trauma patient (RPN = 177), lack of specific on-call consultants (RPN = 171), failure or delay of a consultant to respond to a request for consultation (RPN = 153), and failure of a consultant to accept a patient to their service (RPN = 149).
Table IV.
Top internal process failures in nontrauma and lower-level trauma centers–Determination of clinical indication for retriage and consultant capabilities
| Step | Step description | Potential failures | Potential effects | RPN |
|---|---|---|---|---|
| 13 | TS/ED MD discussion with consultants | Difficult to get consultant to see patient quickly | Delay determining whether patient needs retriage | 177 |
| 14A | Teleradiology results | Trauma study reads not prioritized | Delays reading | 176 |
| 13 | TS/ED MD discussion with consultants | Lack of on-call consultant for certain specialties | Retriage because of consultant availability | 171 |
| 12 | Consultant needed | No consultant listed on call for required specialty | Delay determining whether patient needs retriage | 155 |
| 9a | Imaging orders placed | Challenges transmitting imaging orders because of high of patients | Delay determining whether patient needs retriage | 154 |
| 12A | HUC contact consultant(s) | Consultant delayed calling back | Delay determining whether patient needs retriage | 153 |
| 14 | Will consultant accept the patient? | Consultant does not feel comfortable accepting | Delay in delivery of necessary care for patient | 150 |
ED MD, emergency department physician; HUC, health unit coordinator; RPN, risk priority number; TS, trauma surgeon.
Identification and acceptance of patient retriage to a receiving higher-level trauma center
Difficulty in securing an acceptance from the receiving higher-level trauma center was a major barrier. As depicted in the process map, selecting a receiving higher-level trauma center (step 16) for retriage of a trauma patient is a complicated process. Multiple patient characteristics are considered, such as patient age (eg, pediatric) and type of injury (eg, burn), as well as higher-level trauma center characteristics, such as proximity, Illinois emergency medical services region, and extracorporeal membrane oxygenation capability. Step 17A was reported as occasionally needing to be executed multiple times before a sending center either received a call back from a receiving center or acceptance of a retriage. Additional failures included needing to contact multiple receiving centers (RPN = 191) and lack of availability of ED, inpatient, and intensive care unit beds at the receiving center even after acceptance (RPN = 111). There was also a lack of available critical care transportation (RPN = 141), which further contributed to delays. Of the 12 failures with the fewest safeguards, 5 (40%) occurred during or after the retriage decision, suggesting that solutions and interventions to reduce retriage times are also needed at the receiving higher-level trauma centers.
Discussion
The retriage process of seriously injured patients from sending NTCs and LLTCs to receiving higher-level trauma centers involves many steps. Each step is susceptible to one or many failures that could delay retriage and timely delivery of definitive trauma care. The prevention or mitigation of some failures at the sending center is potentially feasible because they are part of a single health system. However, the design and implementation of solutions/interventions at the sending centers alone will be insufficient. Many high-risk failures are related to identifying a receiving center and securing acceptance of retriage. Designing and implementing receiving center interventions are considerably more challenging because most of the centers are not part of the single health care system.
The FMECA’s success analyzing complex processes outside of trauma care highlights how its use in this context for the first time is a strength of this study. The approach has been widely used in the military,17, 27 food service,28 power industry,29-31 and, more recently, health care.18, 19, 25, 32-35 Studies addressing patient handoffs between the operating room and intensive care unit have found that process improvements focused on critical failures had a measurable impact on information transfer between care teams, the timeliness of care delivered, and the duration of the process as a whole.36, 37 McElroy et al37 used the FMECA to develop a series of recommendations for advance notification by the transferring team, establishing roles and responsibilities of each participating team member and anticipatory guidance for the receiving team to improve the patient handoff process after liver transplantation. Standardized transfer criteria effectively mitigate information gaps and improve the continuity of care during handoffs between ambulance and ED teams,38 as well as between receiving centers and transferring centers.39, 40
Further design work is needed to formulate the requirements and specifications of any novel standardized processes for an entire health system.
The findings from this study pointed to specific targets for the design of solutions and interventions to optimize the retriage processes at both the sending and receiving centers. In the interest of developing a system-wide intervention, we examined the steps that had high-risk failures across the centers. Accurate determination of clinical indications for retriage during the initial ED evaluation and early identification at receiving higher-level trauma center could reduce delays in the process. Additionally, establishing system-wide guidelines about specialty care for severely injured patients that (1) define which cases can be safely treated at each center, (2) which cases must be retriaged, and (3) which receiving centers will always receive retriage from a given center would expedite the retriage processes at both sending and receiving centers.
There were several limitations to this work. The FMECA methodology, due to its reliance on the perceptions and recollections of participants, may be subject to bias. Bias is an important limitation, as individuals often describe the subjective views of what they believe occurs rather than what actually occurs. However, this method is a unique way to inductively generate hypotheses about why retriage fails to occur from the experience of critical stakeholders in the process. We are currently quantifying requests to retriage timing and reasons, as identified by the FMECA. The method is also prone to sampling bias, given our reliance on a convenient sample. However, we attempted to mitigate this risk by conducting snowball sampling to seek a representative sample of roles, experience, sex, and ethnicity. Furthermore, participants may not recognize and report all the failures that occur, particularly system-level failures. All sessions had to be held virtually because of the COVID-19 pandemic. Typically, direct observation of a process, and of the clinicians and staff involved in a process, can overcome these limitations but were not allowed. This analysis relied on insight from teams at LLTCs and NTCs in the health system. This was partly because the study team was from the system’s high-level trauma center and had intimate knowledge of defects and failures at their center. Nonetheless, the FMECA process was replicated with the system’s high-level trauma center. The retriage failures were quite complex, thus beyond the scope of this article. These findings should be generalized with caution, given that all participants are from centers affiliated with a single academic health system. However, it is reasonable to assume that most health systems will have similar challenges. In conclusion, The FMECA of the retriage process revealed a complex process (N = 26 steps) with multiple specific failures (N = 93), many of which can be addressed to ensure more rapid retriage of trauma patients. Although this study focused on processes of sending centers within a single health system, the FMECA methodology can be easily applied to other processes. The findings of this study suggested obstacles to determination of clinical indications for retriage heavily relied on consultant and identification of receiving level I trauma center that would accept a patient, representing opportunities to improve timely retriage. Establishing clear clinical indications for retriage that sending and receiving centers agree on represents an opportunity for intervention that could improve the retriage of injured patients.
Funding/Support
This work was funded by the American Association for the Surgery of Trauma, the American College of Surgeons, and the National Institutes of Health/National Heart, Lung, and Blood Institute (K23HL157832–01).
Footnotes
Conflict of interest/Disclosure
The authors have no conflicts of interests or disclosures to report.
References
- 1.Newgard CD, Zive D, Holmes JF, et al. A multisite assessment of the American College of Surgeons Committee on Trauma field triage decision scheme for identifying seriously injured children and adults. J Am Coll Surg. 2011;213: 709e721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiang H, Wheeler KK, Groner JI, Shi J, Haley KJ. Undertriage of major trauma patients in the US emergency departments. Am J Emerg Med. 2014;32: 997e1004. [DOI] [PubMed] [Google Scholar]
- 3.Newgard CD, Nelson MJ, Kampp M, et al. Out-of-hospital decision making and factors influencing the regional distribution of injured patients in a trauma system. J Trauma. 2011;70:1345e1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deane SA, Gaudry PL, Woods WP, Read CM, McNeil RJ. Interhospital transfer in the management of acute trauma. Aust N Z J Surg. 1990;60:441e446. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen E, Skaggs DL, Harris LR, Andras LM. Transfer time after acceptance to a level I trauma center. J Am Acad Orthop Surg Glob Res Rev. 2018;2:e081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Valle JM, Newton C, Kline RA, Spain DA, Pirrotta E, Wang NE. Rapid retriage of critically injured trauma patients. JAMA Surg. 2017;152:981e983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez D, Haas B, de Mestral C, et al. Institutional and provider factors impeding access to trauma center care: an analysis of transfer practices in a regional trauma system. J Trauma Acute Care Surg. 2012;73:1288e1293. [DOI] [PubMed] [Google Scholar]
- 8.Harrington DT, Connolly M, Biffl WL, Majercik SD, Cioffi WG. Transfer times to definitive care facilities are too long: a consequence of an immature trauma system. Ann Surg. 2005;241:961e966;discussion: 966e968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chipko J, Davis D, Cha J, Herron T, Ciesla D. Should hypotensive trauma patients ever be transferred to a trauma center? Am Surg. 2019;85:1010e1012. [PubMed] [Google Scholar]
- 10.Newgard CD, McConnell KJ, Hedges JR, Mullins RJ. The benefit of higher level of care transfer of injured patients from nontertiary hospital emergency departments. J Trauma. 2007;63:965e971. [DOI] [PubMed] [Google Scholar]
- 11.Vassar MJ, Holcroft JJ, Knudson MM, Kizer KW. Fractures in access to and assessment of trauma systems. J Am Coll Surg. 2003;197:717e725. [DOI] [PubMed] [Google Scholar]
- 12.Nathens AB, Jurkovich GJ, MacKenzie EJ, Rivara FP. A resource-based assessment of trauma care in the United States. J Trauma. 2004;56:173e178. ; discussion: 178. [DOI] [PubMed] [Google Scholar]
- 13.Stevens DP, Bowen JL, Johnson JK, et al. A multi-institutional quality improvement initiative to transform education for chronic illness care in resident continuity practices. J Gen Intern Med. 2010;25(Suppl 4):S574eS580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holcomb JB. Transport time and preoperating room hemostatic interventions are important: improving outcomes ofter oevere truncal injury. Crit Care Med. 2018;46:447e453. [DOI] [PubMed] [Google Scholar]
- 15.Hollnagel E. Cognitive Reliability and Error Analysis Method: CREAM. New York (NY): Elsevier; 1998. [Google Scholar]
- 16.Croskerry P. Patient Safety in Emergency Medicine. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 17.Latino RJ, Flood A. Optimizing FMEA and RCA efforts in health care. J Healthc Risk Manag. 2004;24:21e28. [DOI] [PubMed] [Google Scholar]
- 18.Khare RK, Nannicelli AP, Powell ES, Seivert NP, Adams JG, Holl JL. Use of risk assessment analysis by failure mode, effects, and criticality to reduce door-to-balloon time. Ann Emerg Med. 2013;62:388e398. [DOI] [PubMed] [Google Scholar]
- 19.Pollack TA, Illuri V, Khorzad R, et al. Risk assessment of the hospital discharge process of high-risk patients with diabetes. BMJ Open Qual. 2018;7: e000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apkon M, Leonard J, Probst L, DeLizio L, Vitale R. Design of a safer approach to intravenous drug infusions: failure mode effects analysis. Qual Saf Health Care. 2004;13:265e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmail R, Cummings C, Dersch D, et al. Using healthcare failure mode and effect analysis tool to review the process of ordering and administrating potassium chloride and potassium phosphate. Healthc Q. 2005;8(Spec No): 73e80. [DOI] [PubMed] [Google Scholar]
- 22.Steinberger DM, Douglas SV, Kirschbaum MS. Use of failure mode and effects analysis for proactive identification of communication and handoff failures from organ procurement to transplantation. Prog Transplant. 2009;19: 208e215. [DOI] [PubMed] [Google Scholar]
- 23.Holl JL, Khorzad R, Zobel R, et al. Risk assessment of the door-in-door-out process at primary stroke centers for patients with acute stroke requiring transfer to comprehensive stroke centers. J Am Heart Assoc. 2021;10: e021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgmeier J. Failure mode and effect analysis: an application in reducing risk in blood transfusion. Jt Comm J Qual Improv. 2002;28:331e339. [DOI] [PubMed] [Google Scholar]
- 25.McElroy LM, Khorzad R, Nannicelli AP, Brown AR, Ladner DP, Holl JL. Failure mode and effects analysis: a comparison of two common risk prioritisation methods. BMJ Qual Saf. 2016;25:329e336. [DOI] [PubMed] [Google Scholar]
- 26.Williams C, Weston R, Feinglass J, Crandall M. Pediatric bicycle helmet legislation and crash-related traumatic brain injury in Illinois, 1999-2009. J Surg Res. 2018;222:231e237. [DOI] [PubMed] [Google Scholar]
- 27.DeRosier J, Stalhandske E, Bagian JP, Nudell T. Using health care failure mode and effect analysis: the VA National Center for Patient Safety's prospective risk analysis system. Jt Comm J Qual Improv. 2002;28:248e289. [DOI] [PubMed] [Google Scholar]
- 28.Bertolini M, Bevilacqua M, Roberto M. FMECA approach to product traceability in the food industry. Food Control. 2006;17:137e145. [Google Scholar]
- 29.Lifar AS, Brom AE. FMECA Use for the equipment reliability analysis in hydropower engineering. IOP Conf Ser: Earth Environ Science. 2019;272:e032192. [Google Scholar]
- 30.Adhikary DD, Bose GK, Bose D, Mitra S. Multi criteria FMECA for coal-fired thermal power plants using COPRAS-G. Int J Qual Reliab Manag. 2014;31: 601e614. [Google Scholar]
- 31.Bevilacqua M, Braglia M, Gabbrielli R. Monte Carlo simulation approach for a modified FMECA in a power plant. Qual Reliab Eng Int. 2000;16:313e324. [Google Scholar]
- 32.Petrovic MA, Aboumatar H, Baumgartner WA, et al. Pilot implementation of a perioperative protocol to guide operating room-to-intensive care unit patient handoffs. J Cardiothorac Vasc Anesth. 2012;26:11e16. [DOI] [PubMed] [Google Scholar]
- 33.Thornton VL, Holl JL, Cline DM, Freiermuth CE, Sullivan DT, Tanabe P. Application of a proactive risk analysis to emergency department sickle cell care. West J Emerg Med. 2014;15:446e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilliam ML, Mistretta SQ, Martins SL, Holl JL. A systematic approach to improving intrauterine device services in family planning clinics. Contraception. 2014;90:542e547. [DOI] [PubMed] [Google Scholar]
- 35.Powell ES, O’Connor LM, Nannicelli AP, et al. Failure mode effects and criticality analysis: innovative risk assessment to identify critical areas for improvement in emergency department sepsis resuscitation. Diagnosis. 2014;1:173e181. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmnan J, Twite M, Barrett C, et al. A handoff protocol from the cardiovascular operating room to cardiac ICU is associated with improvements in care beyond the immediate postoperative period. Jt Comm J Qual Patient Saf. 2013;39:306e311. [DOI] [PubMed] [Google Scholar]
- 37.McElroy LM, Collins KM, Koller FL, et al. Operating room to intensive care unit handoffs and the risks of patient harm. Surgery. 2015;158:588e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brost N, Crilly J, Wallis M, Patterson E, Chaboyer W. Clinical handover of patients arriving by ambulance to the emergency department, a literature review. Int Emerg Nurs. 2020;18:210e220. [DOI] [PubMed] [Google Scholar]
- 39.Cwinn MA, Forster AJ, Cwinn AA, Hebert G, Calder L, Stiell IG. Prevalence of information gaps for seniors transferred from nursing homes to the emergency department. CJEM. 2009;11:462e471. [DOI] [PubMed] [Google Scholar]
- 40.Jones JS, Dwyer PR, White LJ, Firman R. Patient transfer from nursing home to emergency department: outcomes and policy implications. Acad Emerg Med. 1997;4:908e915. [DOI] [PubMed] [Google Scholar]