Introduction
Prostate cancer is the second most common malignancy in men, with multiple treatment options ranging from active surveillance, radiation, and surgery depending on comorbidities and cancer staging.1,2 Brachytherapy can be an attractive treatment option for prostate cancer, given shortened treatment time and equivalent outcomes to surgery, particularly in the early-stage localized setting.3,4 However, brachytherapy is characterized by more acute urinary irritation compared with other therapies in the initial 6 months after treatment, although the symptoms steadily improve and generally resolve within a year.5,6
Focal prostate cancer treatment options based on nanoparticles are under investigation, intending to target lesions focally at a cellular scale to potentially reduce toxicity on adjacent tissue and, in turn, risks of urinary, bowel, and erectile dysfunction.7,8 One emerging technology exploits the ability of intravenously infused gold nanoshells (AuroShell; Nanospectra, Houston, TX) to accumulate passively in tumor tissue via the enhanced permeability and retention effect. The particles do not accumulate in healthy tissue as they cannot access normal vasculature and instead are cleared from the bloodstream by the reticuloendothelial system. AuroShell particles comprise a thin gold shell, 10- to 20-nm thick, deposited on a solid silica (silicon dioxide) core. To prevent aggregation of the particles in a saline environment and to provide steric hindrance in vivo, a 5000 molecular weight methoxy polyethylene glycol chain is attached through a thiol (sulfur) bond. The polyethylene glycol coating improves the stability of the AuroShell particles in an isotonic aqueous solution and may also enhance the circulating half-life on administration. When illuminated with a near-infrared light source, these accumulated nanoparticles absorb and convert the light into heat, causing selective hyperthermic cell death through thermal ablation without affecting nontumorous tissue. Following treatment, the particles are cleared through the liver or sequester in the liver and spleen with no known side effects.9 Ablation of tumors using AuroShells was effectively demonstrated in cell studies and animal models as well as a clinical pilot study treating men with prostate cancer. It is the only inorganic material that is approved by the United States Food and Drug Administration for photothermal therapy.7,10, 11, 12 We report on the toxicity and short-term efficacy for a single patient who had gold nanoshells accumulated in the prostate gland and afterward was treated with low-dose-rate (LDR) brachytherapy using palladium 103 (103Pd). The patient consented to the administration of gold nanoshells during a clinical trial (ClinicalTrials.gov identifier: NCT04240639). Although the trial's intent was to excite the infused nanoshells with the interstitial placement of a near-infrared light source in the prostate gland, technical difficulties prevented the placement of the specialized trial catheters required for light excitation. The patient elected to proceed with a standard-of-care prostate LDR brachytherapy procedure instead. Posttreatment follow-up ≤1 year revealed neither biochemical recurrence nor toxicity or health-related quality of life different than what was expected for brachytherapy.
Case Presentation
A 57-year-old White male patient was referred for urologic consultation after a routine screening. His prostate-specific antigen (PSA) value was 4.34 ng/mL in February 2018. The patient had a multiparametric pelvis magnetic resonance image in March 2018, which was negative for suspicious prostatic foci. He reported moderate obstructive symptoms with weak force of stream, nocturia of 1 to 2 times, and a sense of incomplete bladder emptying. His digital rectal exam indicated a symmetrical prostate of 40 g with no nodules. The patient deferred biopsy and continued to follow up with his urologist. In October 2019, the patient's PSA rose to 5.58 ng/mL, with no change in his voiding symptoms and no irritative complaints. He elected to undergo a transrectal ultrasound-guided prostate biopsy in December 2019. His PSA doubling time using the log slope calculation at the time of biopsy was 68 months. The biopsy revealed a prostate volume of 33.5 cc (height 29 mm, width 46 mm, length 47 mm), PSA density of 0.17 with no nodules or calculi, normal seminal vesicles, and a small middle lobe. The biopsy reported 3 of 12 cores positive with 5% involvement of core biopsy material with Gleason grade 3+3 prostatic adenocarcinoma in the right mid-apex, 30% involvement in the left middle base, and 5% to 10% involvement in the left mid-apex of the prostate. No angiolymphatic or perineural invasion, significant inflammation, or evidence of atrophy was noted. The patient was stratified as having National Comprehensive Cancer Network low-risk Gleason 3+3 clinical-stage T1c prostate cancer and elected for active surveillance.
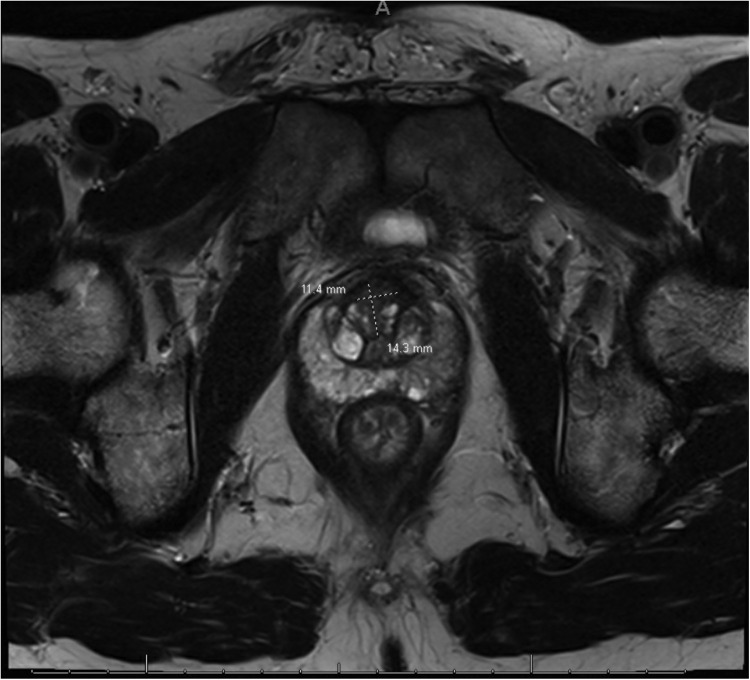
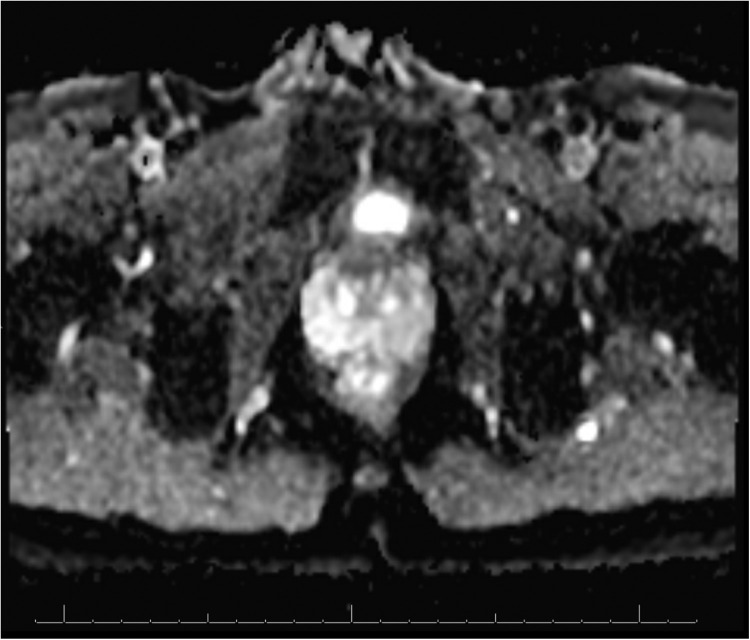
A follow-up magnetic resonance image on December 16, 2020, showed a 1.4- × 1.1-cm T2 hypointense lesion (Fig. 1), demonstrating restricted diffusion (Fig. 2) and early postcontrast enhancement in the anterior apical gland adjacent to the fibromuscular stroma suspicious for prostate cancer (Fig. 1). This was characterized as a PI-RADS 4 lesion. The patient underwent a targeted transperineal prostate biopsy using the UroNav System (Phillips) in early 2021, revealing all 3 of the targeted cores as Gleason 3+4. In contrast, the remaining cores from the conventional 12-core standard biopsy were benign. The patient was interested in pursuing a nanoparticle focal therapy-based clinical trial. He signed informed consent and enrolled in an open-label, multicenter, single-dose study of AuroLase therapy for the focal ablation of prostate tissue via AuroShell nanoparticle-directed thermal ablation.
Figure 1.
T2-weighted small field-of-view magnetic resonance image revealing anterior lesion.
Figure 2.
Diffusion restriction image showing corroboration of lesion seen on T2 sequences in the anterior gland.
In May 2021, the patient received an intravenous infusion of up to 7.5 mL/kg of AuroShell particles concentrated to 100 optical density (approximately 2.77 × 1011 particles/mL or 36 mg particles/kg of patient weight) with a plan for transperineal focal therapy the next day. The planned interstitial focal treatment had to be aborted due to unforeseen equipment limitations, which disabled the appropriate placement of the light-source catheters in the pattern required by the study. After consultation with both urology and radiation oncology, the patient weighed alternative options, which included but were not limited to radical prostatectomy or various forms of radiation therapy. The patient was not interested in pursuing radical prostatectomy but was interested in radiation therapy options. The patient was informed that the combination of AuroShell gold nanoparticles and salvage radiation had not been attempted previously and that there could be unknown risks to the procedure. The treating physicians judged that, despite the unknown risks, it was improbable that pursuing immediate radiation therapy would result in toxicity beyond what was expected for the brachytherapy procedure. After informed consent was obtained, the patient agreed to proceed with a standard-of-care LDR brachytherapy implant using 103Pd sources prescribed at 125 Gy to the periphery of the prostate gland.
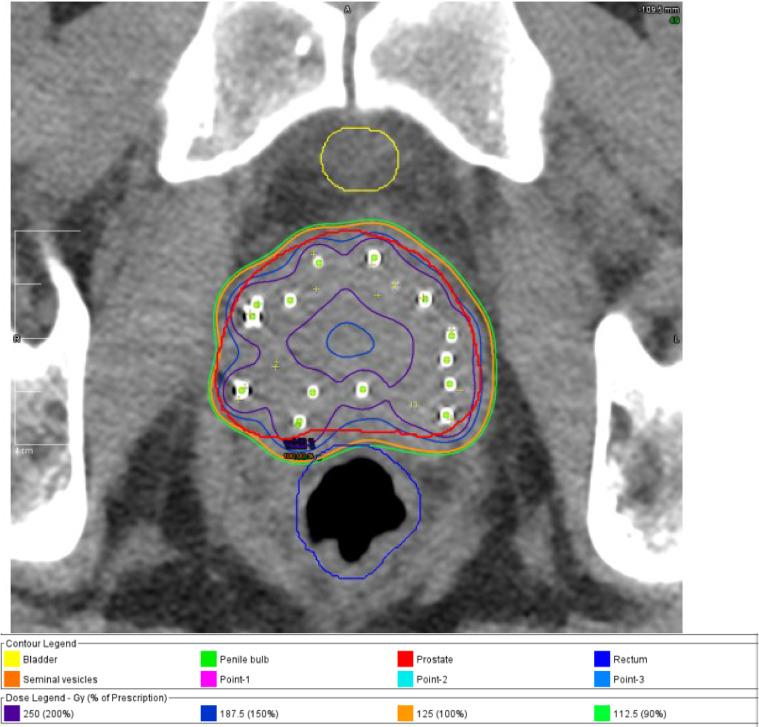
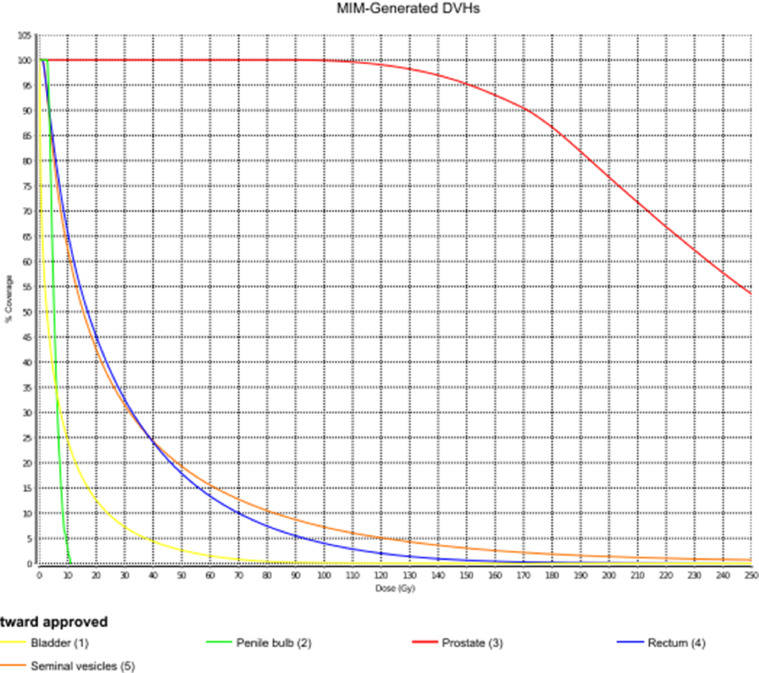
A PSA value drawn before the procedure was 9.0 ng/mL. The brachytherapy procedure began approximately 19 hours after his infusion of gold nanoshells and was completed in an additional 2 hours without incident. The brachytherapy procedure used 25 needles to deliver 81 sources of 2.62 U/seed of 103Pd. The total implanted radioactivity was 212.22 U. Postimplant dosimetry was performed on postoperative day 1, revealing a target V100% = 98.66%, D90% = 137.08%, and a rectal V100% = 1.67% (D1cc = 79.86%) (Figure 3, Figure 4, Figure 5).
Figure 3.
Representative cross-section showing seed placement and isodose lines after prostate brachytherapy.
Figure 4.
Dose-volume histogram parameters at day 1 postimplant dosimetry.
Figure 5.
Anterior-posterior projection of 103Pd brachytherapy seeds in the region of the prostate gland.
The patient responded well to treatment with no adverse effects and was started on 0.4 mg tamsulosin orally twice a day after the procedure to reduce lower urinary tract symptoms anticipated after the implant. The patient had a PSA nadir of 0.21 at 4 months after therapy. His most recent PSA was 0.33 at 11 months posttreatment. The patient had a pretreatment total testosterone value of 857, and this value decreased to 736 at 11 months posttreatment.
At his 6-month follow-up visit, the patient reported no change in urinary frequency, a weaker stream, and occasional mild urinary incontinence, which did not require using pads.
At 1 year postbrachytherapy, the patient subjectively reported an overall high quality of life. He stated that his perception of orgasm strength was weaker compared with pretreatment, while his nocturia of 1 to 2 times and urinary bother were similar to the pretreatment baseline. Overall, the patient has no trend toward biochemical recurrence or persisting side effects that significantly affect his quality of life. Table 1 summarizes the patient's pre- and posttreatment health-related quality of life surveys including American Urological Association symptom index, sexual health inventory for men, and Merrick scores for urinary, sexual, and bowel function.13, 14, 15 Table 1 also includes Expanded Prostate Cancer Index Composite for Clinical Practice scores quantifying incontinence, irritative, rectal, sexual, and hormonal symptoms.16 All of these scores are stable from the patient's pretreatment values. He does continue to take 0.4 mg tamsulosin orally twice a day.
Table 1.
Health-related quality of life indices
| Period | Date | AUA | SHIM | RFAS | EPIC-CP incontinence |
EPIC-CP irritative |
EPIC-CP rectal |
EPIC-CP sexual |
EPIC-CP hormonal |
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | 12/26/2020 | 19 | 25 | N/A | |||||
| 4/28/2021 | 17 | 25 | N/A | ||||||
| 6 mo posttreatment | 11/11/2021 | 15 | 25 | 4 | 2 | 4 | 1 | 0 | 0 |
| 1 y posttreatment | 5/26/2022 | 18 | 25 | 3 | 0 | 4 | 0 | 0 | 0 |
Abbreviations: AUA = American Urological Association symptom index; EPIC-CP = Expanded Prostate Cancer Index Composite for Clinical Practice; N/A = not applicable; RFAS = rectal function assessment scale; SHIM = sexual health inventory for men.
Function scores. Treated in May 2021.
Discussion
There are several definitive treatment options for localized prostate cancer, including radical prostatectomy, various forms of brachytherapy, and focal therapy,1 but they have a risk of heightened urinary, bowel, and sexual dysfunction.5 Human clinical trials currently exist that use focally directed light on tumors to thermally ablate them with millimeter precision. The light excites in vivo gold nanoparticles that are located near the tumors.7,10,11 Nanoparticles are generally defined as having a diameter between 1 and 100 nm, a size that sits between atomic and molecular diameters, which accounts for their unusual properties.
The use of intratumorally injected radioactive nanoparticles, termed nanobrachytherapy, is an area of current research interest and has been performed in animal models.10,11 Furthermore, there is a well-studied history of using intratumorally injected, radioactive, nanoscale, colloidal gold (198Au) in men with prostate cancer by directly injecting the gold into the prostate gland.17 In 1950, Dr Rubin Flocks at the University of Iowa had originally intended to perform a gold-encapsulated radon seed implant on an 80-year-old patient with prostate cancer. When it was discovered that the radon seeds were unavailable midprocedure, he substituted 60 mCi 198Au colloid into the tumor. He noted tumor regression on follow-up and, after that, performed and published on the outcomes of >1500 patients with this technique.17,18 Although intratumorally injected, radioactive colloidal gold had proven therapeutic benefits, it fell out of favor as modern external beam and brachytherapy procedures emerged after the 1970s. The light absorption properties of gold nanoparticles are being leveraged in AuroLase Nanospectra Therapy, an ongoing multisite clinical trial developing ultrafocal tissue ablation therapy for prostate tumors. Nanoparticles, composed of a gold metal shell and a nonconducting silica core, are delivered intravenously and accumulate in the tumor. An interstitial fiber optic probe emits near-infrared laser energy to the nanoparticles, which convert the light into heat that thermally ablates the tumors. By passing ionizing radiation over the nanoparticle density structures to activate the removal of secondary electrons, it may be possible to amplify the effect of radiation and create a high dose adjacent to the nanoparticles. Clinical trial results showed successful focal ablation of low- to intermediate-grade prostate tumors in 15 patients using laser-excited gold-silica nanoshells in combination with magnetic resonance–ultrasound fusion imaging. Eighty-seven and a half percent of lesions in the ablation zone were negative for tumor at 12 months posttreatment.7
Through selective ablation of the tumor and surrounding blood vessels, AuroLase therapy hopes to reduce toxicity and systemic side effects that might otherwise occur through more conventional approaches.7,8 The initial trials of this emerging technology are being investigated in the early stage, focal ablation setting. Aside from the goal of ablating cancer, it is hoped that this approach would preserve the ability of practitioners to safely perform a salvage procedure with surgery or radiation if persistence or recurrence of disease were detected.
We report on the first patient to receive salvage radiation therapy after the infusion of AuroShell nanoparticles. Due to technical difficulties, the patient could not receive the light-excitation treatment component and proceeded to a conventional whole-gland 103Pd brachytherapy implant. Although nanotherapy and brachytherapy are known to be safe and effective therapies separately, there was a risk that the gold nanoparticles could have acted as a radiosensitizer, leading to increased toxicity when combined with seed implants. However, considering the lack of complications in the treated patient, this combinatorial therapy of nanoparticles and brachytherapy radiation has the potential to be safe and effective at eradicating cancer.
Conclusion
This case report documents the potentially safe and effective use of salvage ionizing radiation via brachytherapy after infusion of gold nanoparticles, resulting in a biochemical response without sacrificing quality of life. We detail the safety of salvage brachytherapy after gold nanoshell infusion. Persons who undergo nanoshell infusion may consider brachytherapy as a salvage option if they cannot complete the light stimulation aspect of the therapy or have persistent cancer after completing AuroLase therapy. A clinical trial is required to fully determine the safety and efficacy of combining nanoparticles with salvage brachytherapy, and further investigation is warranted, given the lack of serious complications or deleterious effects on genitourinary, bowel, and sexual function in this patient.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Tward reports that he received consulting fees and/or grants from Bayer, Myriad, Myovant, and Boston Scientific.
This study uses the Huntsman Cancer Institute cancer registry data. The authors do not own the data and, thus, are only permitted to share them in aggregate form (ie, publications) and not in original form.
References
- 1.Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(suppl 1):22–29. doi: 10.1111/j.1464-410X.2011.10827.x. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Skowronek J. Current status of brachytherapy in cancer treatment–Short overview. J Contemp Brachytherapy. 2017;9:581–589. doi: 10.5114/jcb.2017.72607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams VM, Kahn JM, Thaker NG, et al. The case for brachytherapy: Why it deserves a renaissance. Adv Radiat Oncol. 2021;6 doi: 10.1016/j.adro.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman KE, Penson DF, Zhao Z, et al. Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2020;323:149–163. doi: 10.1001/jama.2019.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Rastinehad AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci USA. 2019;116 doi: 10.1073/pnas.1906929116. 18,590-18,596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern JM, Kibanov Solomonov VV, Sazykina E, Schwartz JA, Gad SC, Goodrich GP. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int J Toxicol. 2016;35:38–46. doi: 10.1177/1091581815600170. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. [Google Scholar]
- 10.Khan MK, Minc LD, Nigavekar SS, et al. Fabrication of {198Au0} radioactive composite nanodevices and their use for nanobrachytherapy. Nanomedicine. 2008;4:57–69. doi: 10.1016/j.nano.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seniwal B, Thipe VC, Singh S, Fonseca TCF. Freitas de Freitas L. Recent advances in brachytherapy using radioactive nanoparticles: An alternative to seed-based brachytherapy. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.766407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Luo L, Zeng L, et al. Porous gold nanoshells on functional NH2-MOFs: Facile synthesis and designable platforms for cancer multiple therapy. Small. 2018;14 doi: 10.1002/smll.201801851. [DOI] [PubMed] [Google Scholar]
- 13.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 14.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 15.Merrick GS, Butler WM, Wallner KE, Hines AL, Allen Z. Late rectal function after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;57:42–48. doi: 10.1016/s0360-3016(03)00501-7. [DOI] [PubMed] [Google Scholar]
- 16.Chang P, Szymanski KM, Dunn RL, et al. Expanded prostate cancer index composite for clinical practice: Development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J Urol. 2011;186:865–872. doi: 10.1016/j.juro.2011.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosevear HM, Lightfoot AJ, O'Donnell MA, Platz CE, Loening SA, Hawtrey CE, Rubin H. Flocks and colloidal gold treatments for prostate cancer. Sci World J. 2011;11:1560–1567. doi: 10.1100/tsw.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flocks RH, Kerr HD, Elkins HB, Culp DA. The treatment of carcinoma of the prostate by interstitial radiation with radioactive gold (Au198): A follow-up report. J Urol. 1954;71:628–633. doi: 10.1016/S0022-5347(17)67835-2. [DOI] [PubMed] [Google Scholar]