Abstract
Skeletal muscle is one of the leading frameworks of the musculo-skeletal system, which works in synergy with the bones. Long skeletal muscles provide stability and mobility to the human body and are primarily composed of proteins. Conversely, improper functioning of various skeletal muscles leads to diseases and disorders, namely, age-related muscle disorder called sarcopenia, a group of genetic muscle disorders such as muscular dystrophies, and severe muscle wasting in cancer known as cachexia. However, skeletal muscle has an excellent ability to undergo hypertrophy and enhanced functioning during sustained exercise over time. Indeed, these processes of skeletal muscle regeneration/hypertrophy, as well as degeneration and atrophy, involve an interplay of various signaling pathways. Myostatin is one such chemokine/myokine with a significant contribution to muscle regeneration or atrophy in multiple conditions. In this review, we try to put together the role and regulation of myostatin as a function of muscle regeneration extrapolated to multiple aspects of its molecular functions.
Keywords: Muscle, Myostatin, Atrophy, Muscle degeneration
Graphical abstract
1. Introduction
The most abundant tissue in the human body is the skeletal muscle [1]. It is a key site for metabolic regulation and maintaining the homeostasis of the body. The dynamicity of the muscle mass is maintained by a fine balance between protein synthesis and degradation. Loss of muscle mass is especially observed after musculoskeletal trauma, damages imparted from neuromuscular disorders which cause denervation [2], chemical toxicity such as fluorosis [3], and also as a catabolic effect in conditions like diabetes [4] and cancer [5]. This loss of muscle mass is governed primarily by the Akt/mTOR pathway, which controls protein synthesis and degradation (Fig. 1) as a function of muscle metabolic activities [6].
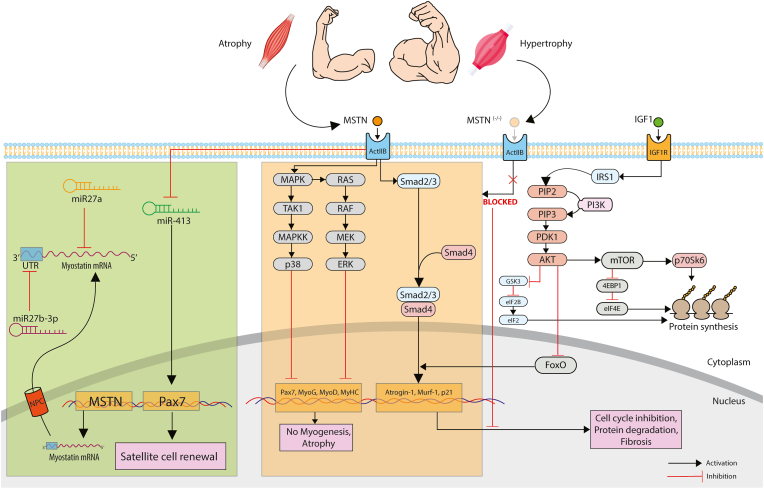
Fig. 1.
The overall role of myostatin in protein degradation and synthesis in skeletal muscle. Myostatin signalling is one of the fundamental signalling transduction pathways in skeletal muscle, which dictates the balance between protein synthesis and degradation thereby controlling conditions of atrophy and hypertrophy. Myostatin activates the Smad-mediated signalling which triggers the expression of various atrophy related E3-Ubiquitin ligases like Atrogin1 and MuRF1. On the other hand, myostatin blocks the transcription of genes related to myogenesis i.e., Pax7, MyoD, MyoG and MyHC. FoxO is a family of transcription factor which triggers the Smad-mediated expression of atrophy genes, but this signalling is blocked when AKT inhibits FoxO and simultaneously activates mTOR to trigger the protein synthesis pathway. MSTN also has significant interactions with miRNA of various families which results in upregulation and downregulation of myogenesis. (MSTN: Myostatin; ActIIB: ActivinIIB; MAPK: Mitogen Activated Protein Kinase; TAK1: Transforming growth factor-β (TGF-β)-activated kinase 1; MAPKK: Mitogen Activated Protein Kinase Kinase; ERK: extracellular signal-regulated kinases; PIP2: phosphatidylinositol bisphosphate; PIP3: phosphatidylinositol trisphosphate; PDK1: 3-phosphoinositide-dependent protein kinase-1; PI3K: Phosphoinositide 3-kinases; Akt: Protein kinase B; mTOR: mammalian target of rapamycin; GSK3: Glycogen synthase kinase-3; eIF2: Eukaryotic Initiation Factor 2; IGF1: Insulin-like growth factor 1; 4EBP1: Eukaryotic translation initiation factor 4E-binding protein 1; eIF4E: Eukaryotic Initiation Factor 4E; FoxO: Forkhead box; p70S6K: Ribosomal protein S6 kinase beta-1; MyHC: Myosin Heavy chain; miR: microRNA).
Over the years, myostatin, a member of the TGF-β (transforming growth factor–β) superfamily, has gained notice as a key regulator for maintaining muscle mass. An overexpression of this protein leads to muscle atrophy, whereas knockout studies have reported muscle overgrowth. The crosstalk between myostatin and the intracellular AKT signaling pathway validates and extends the contributions of myostatin to protein degradation and synthesis. Considering the profound biological significance of myostatin, several studies have investigated the role of myostatin in regulation of cellular response.
2. Role of myostatin in cachexia
Cachexia refers to a multifactorial syndrome that accounts for weight loss due to accelerated loss of skeletal muscle mass. The quality of life can be prognosed by these very indicators, i.e., muscle atrophy and weight loss. Moreover, cachexia is exhibited by more than 50% of patients in the advanced stages of cancer [7]. Since myostatin is central to muscle mass regulation, researchers have explored this particular myokine as a probable therapeutic intervention. Takayama, Kentaro et al. [8] identified a pro-domain peptide, peptide-2 of myostatin precursor, which inhibits mature myostatin. This peptide-2, when injected intramuscularly, prolongs survival in the cancer cachexia mice model [9]. Natural compounds have also shown inhibitory effects on myostatin. Magnolol, a natural compound obtained from Magnolia officinalis, containing anti-inflammatory and anti-oxidant activity, has been identified to target myostatin and its transducing pathway. It works by increasing the phosphorylation of FOXO3a, thereby downregulating the action of MuRF-1 and Atrogin-1 [10]. Hence, magnolol indirectly reduces the 26S proteasomal protein degradation in cachexia. Myostatin is associated with inflammation of the joints as well. Researchers have identified a relationship of elevated serum myostatin concentration with increased joint inflammation and reduced muscle mass in rheumatoid arthritis (RA) patients [11]. According to Gonzalez-Ponce et al. [12], concentration of serum myostatin >17 ng/ml is associated with both myopenia and rheumatoid cachexia.
Therefore, myostatin is a potential clinical biomarker for diagnosing rheumatoid cachexia in RA patients. Additionally, further studies are required in the light of using the peptide-2 as a therapeutic approach for patients with cancer cachexia.
3. Association of myostatin with bone inflammation
Muscles and bones are anatomically adjacent to one another and emerge from a common mesenchymal progenitor during embryogenesis. Furthermore, clinical data has revealed that muscle is the main factor driving the healing of traumatic bone injuries. The muscle-derived myokines play a crucial role in this process. For instance, during the early stages of fracture healing, there is an enhanced expression of myostatin receptors in chondrocytes in the fracture area, including the type IIB Activin receptor [13]. In addition to activating the SMAD pathway, myostatin also has been observed to inhibit the Wnt/β-catenin pathway to modulate bone formation and regeneration [14].
Rheumatoid arthritis, an autoimmune disease, is characterized by the profound infiltration of several proinflammatory cytokines into the synovial fluid. This leads to joint destruction and a loss in the adjacent muscle mass [15]. According to qPCR and ELISA assays on human MH7A cells, there is a dose-dependent increase in the IL-1β expression with myostatin [16]. This indicates a potential role of myostatin in inflammatory pathways, which can lead to inflammation-induced loss of muscle mass. Apart from IL-1β, TNF-α also plays a role in the inflammation process initiated by myostatin. Su, Chen-Ming et al. [17] elucidate the version of the PI3-Akt pathway which leads to the expression of TNF-α via the activation of AP-1 and the activation of c-Jun transcription factor resulting in the inflammation in the synovium of RA patients. TNF-α is a crucial cytokine responsible for bone destruction. Cumulatively, the number of the bone-forming osteoblasts decrease, while those of the bone-resorbing osteoclasts increases (Fig. 2) [18]. Another secreted inflammatory cytokine is IL-17A. This is secreted by Th17 cells and is associated with the pathogenesis of various immune-mediated inflammatory diseases like RA and multiple sclerosis. A study by Fennen, Michelle et al. [19] showed a deficiency of myostatin in arthritic mice reduced the process of Th17 recruitment to the joint sites, thereby diminishing the inflammation effect. Mechanistically, myostatin deficiency leads to reduced expression of the chemokine CCL20 which reduces the Th17 recruitment to the inflamed site in RA. Finally, alleviating inflammation in RA as a response to myostatin signaling has also been studied using fenofibrate, a PPARα agonist [20]. Fenofibrate suppresses myostatin expression at mRNA and protein levels along with 2 prominent skeletal muscle-specific E3-ubiquitin ligase Atrogin-1 and MuRF-1 to prevent muscle atrophy.
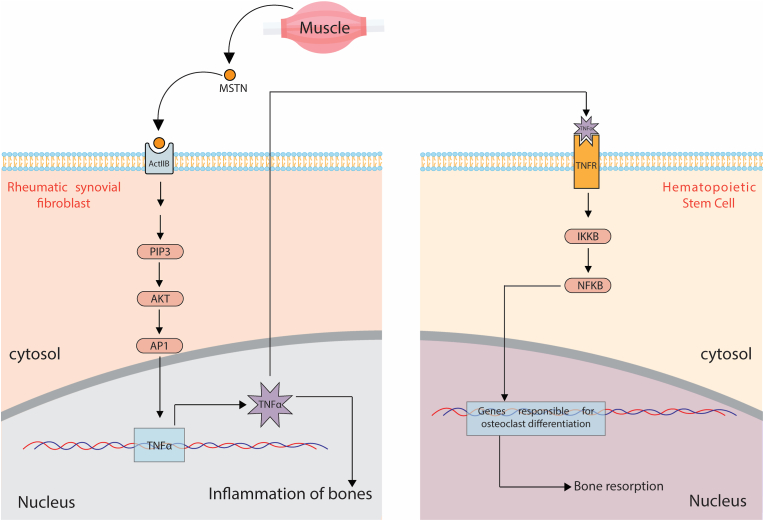
Fig. 2.
Myostatin acts as a crucial myokine in the TNF-α mediated inflammation of the bones in Rheumatoid Arthritis. Myostatin secreted from skeletal muscle acts as a paracrine factor on rheumatic synovial fibroblast, triggering the production of TNF-α. TNF-α, in turn, activates the canonical NF-kβ pathway and causes the resorption of bone and activation of genes responsible for osteoclast differentiation. (MSTN: Myostatin; ActIIB: Activin IIB; PIP3: phosphatidylinositol trisphosphate; AKT: Protein kinase B; AP1: Activator protein 1; TNFα: Tumour Necrosis Factor alpha; TNFR: Tumour Necrosis Factor alpha receptor; IKKB: inhibitor of nuclear factor kappa-B kinase subunit beta; NFKB: nuclear factor kappa-B).
Myostatin also exhibits significant effects on bone-marrow-derived mesenchymal stem cells (BMSCs). BMSCs from myostatin-null mice show better osteogenic differentiation than wild-type mice [21]. On the other hand, myostatin strongly activates receptor-associated nuclear factor κB ligand (RANKL), potentiating osteoclast formation in-vitro. Hence, it can be again concluded the resorption is more than that of the differentiation [18].
Taking everything into account, inflammation of bones has a close relationship with the signaling repertoire of the muscles as they are adjacently located, and myostatin becomes a key player in this crosstalk. Therefore, the development of myostatin-targeted therapeutic approaches can be beneficial for both muscle and bone regenerative strategies.
4. Regulation of myogenesis by miRNA-MSTN crosstalk
MicroRNAs (miRNAs) are small non-coding RNAs that are evolutionarily conserved [22]. From the discovery of the first miRNA (lin-4) in C. elegans, researchers have put substantial effort into studying the various miRNAs, which eventually indicated a broader phenomenon. More than 1900 miRNAs are identified in the human genome [23], which target mammalian mRNA by negatively regulating post-transcriptional mechanisms.
MicroRNAs (miRNAs) have emerged as critical regulators in many cellular processes, including proliferation and differentiation, cellular apoptosis, and determination of cell fate. Studies have also shown that miRNA plays a significant role in muscle stem cell (satellite cells) homeostasis [24] and also modulates the development of skeletal muscle [25]. It has been well established that TGF-β plays a role in regulating miRNA gene expression in skeletal muscles [26]. Hence, it is conceivable that myostatin being a member of the TGF-β superfamily, may regulate skeletal muscle metabolism by fine-tuning the expressions of various miRNAs. According to Wu et al. [27], miR-431, which is predominantly expressed in skeletal muscles, is particularly downregulated by myostatin. This aligns with the observation that miR-431 directly targets Pax7 and promotes self-renewal of the satellite cells [28]. MiR27a is a member of the miR27 family. Chen, Xiaoling et al. [29] demonstrated that mature miR-27a gets upregulated in differentiated C2C12 cells compared to undifferentiated ones. Along with this, myostatin is observed to have reduced expression [30]. This is in accordance with the fact that myostatin is a bonafide target of miR27a. Another microRNA, miR-27b-3p, which again belongs to the miR-27 family, has been identified as a potential interactor for myostatin [31]. Zhang, Genxi et al. [32], found that the seed sequence of miR-27b-3p can interact with the 3′UTR region of the myostatin gene. This was confirmed by reduced protein and mRNA expressions of myostatin in overexpressed miR27b-3p cells [33]. Therefore, considering that miR27b-3p negatively regulates myostatin, it is also to be considered that miR27b-3p over-expression can lead to an increase in bone strength thereby reducing the effect of RA discussed in the previous section.
The role of miRNAs has been substantially studied for various processes, including their role in muscle maintenance and homeostasis considering the regulation of myostatin as a critical factor. Since, myostatin heavily determines the fine balance between the loss and maintenance of muscle mass through its downstream signal transduction, the miRNA-myostatin interactions can pave way to therapeutic approaches, which are apparent in this significant crosstalk.
5. Involvement of myostatin in muscular dystrophy
Muscular dystrophies are a group of disorders which cause progressive muscle mass loss or atrophy [34]. Since myostatin is a myokine that triggers reduction of protein synthesis, it is a potent factor that works in the progressive atrophy. As the muscle mass loss is triggered due to injuries or dystrophy-associated-stress, as a protective measure, the satellite cells/muscle stem cells are activated. To bring about regeneration of the muscle tissue, it has been observed that IRE1α RNase is activated which degrades the myostatin mRNA and as a result, the growth and differentiation signalling are activated [35].
Becker muscular dystrophy (BMD) is an X-linked recessive disorder with a prevalence of 0.1–0.2 in Asia, 0.01 in South Africa, and 0.1 to 0.7 in European countries [36]. This genetic disorder is caused due to mutations in the DMD gene which codes for the muscle-specific dystrophin protein. In a clinical study it was observed that the levels of myostatin in steroid-treated BMD patients was low compared to other BDM patients. It was also observed that the levels of follistatin and myostatin were compromised although the patients presented advanced muscle atrophy [37]. Furthermore, in myotonic dystrophy type I (DM1) which is an autosomal dominant progressive muscle loss disorder, myostatin and myomiRNAs have been observed to be reduced after physical rehabilitation indicating the functional role of myostatin in dystrophic condition [38].
Finally, it is subjected to evaluation whether myostatin inhibition can alleviate the effects of the muscular dystrophy phenomenon. Although, in some cases it may have a beneficial effect, but alternatively, in other cases it might show no effect. This is evident from various clinical trials as well [39]. Hence, this area can be further investigated for better knowledge.
6. Myostatin inhibitors and activators in muscle disorders
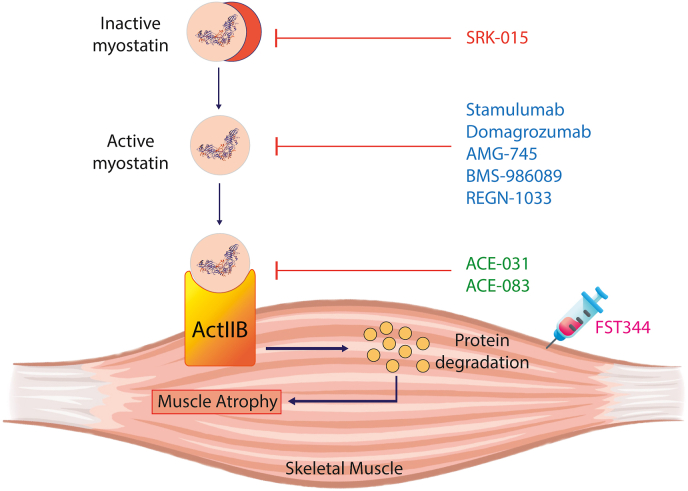
Over the years, multiple strategies have been implemented to mitigate the myostatin effects in muscle diseases (Table 1). These include the administration of activin receptor blockers, myostatin-neutralizing antibodies and pro-peptide, and gene transfer [[40], [41], [42]]. However, these strategies are unequal, and the extent of myostatin inhibition varies across strategies. Among these, neutralizing antibodies and pro-peptide demonstrate high specificity for myostatin inhibition, but other strategies are less discerning. However, the off-target effects of myostatin inhibition are generally positive and include the attenuation of age-associated alterations in several tissues [40]. For example, deletion or inactivation of myostatin gene improves bone mineral density and cardiac function in sarcopenic mice [43]. Additionally, these mice demonstrate resistance to diet-induced obesity, hyperlipidemia, and inflammatory infiltrates in body tissues [[43], [44], [45]]. However, the literature is inconsistent on this notion, and adverse effects of myostatin inhibition have also been reported in clinical trials [46,47]. In the following section, we will discuss the clinical trials investigating the effects of myostatin blockage in different muscle-wasting conditions (Fig. 3).
Table 1.
An overview of the clinical trials of various antibodies targeting myostatin. (SMA; spinal muscular atrophy, BMD; Becker muscular dystrophy, FSHD; Faciocapulohumeral muscular dystrophy, LGMD; limb-girdle muscular dystrophy, DMD; Duchenne muscular dystrophy, CMT; Charcot-Marie- Tooth disease, sIBM; sporadic inclusion body myositis).
| Name of the drug | Cellular target | Experimental condition | Remarks and references |
|---|---|---|---|
| SRK-015 | Inactive myostatin | Mice with sarcopenia, SMA, and dexamethasone. Clinical trials in patients with SMA |
High specificity and reduced cross-reactivity with other TGF-ß ligands. Phase 2 clinical trials are ongoing [68]. |
| Stamulumab | Myostatin | Cynomolgus monkeys, mdx mice, phase 1 (healthy) and 2 (BMD, FSHD, and LGMD) clinical trials | Discontinued due to lack of efficacy in clinical trials (69). |
| Domagrozumab | Myostatin | Cynomolgus monkeys, phase 1 (healthy) and 2 (DMD and LGMD) clinical trials | Trials terminated in phase 2 due to lack of efficacy (69). |
| AMG-745 | Myostatin | Phase 1 and 2 (kidney failure and protein energy malnutrition) clinical trials | Further clinical trials discontinued due to lack of efficacy (70). |
| BMS-986089 | Myostatin | Phase 1 (healthy) and 2 (DMD) clinical trials | Further clinical trials discontinued due to lack of efficacy [40]. |
| REGN-1033 | Myostatin | Phase 1 (healthy) and 2 (sarcopenia) clinical trials | No cross-reactivity with other TGF-ß ligands. Evaluation of its efficacy is ongoing (71). |
| ACE-031 | Myostatin, Activin-2 receptors, GDF-11 | Phase 1 (healthy) and 2 (DMD) clinical trials | Phase 2 trials were discontinued due to serious side effects, including bleeding disorders (72). |
| ACE-083 | Myostatin, Activin-2 receptors, GDF-11 | Phase 1 (healthy) and 2 (FSHD, CMT) clinical trials | Further clinical trials discontinued due to lack of efficacy (73). |
| FST344 | Myostatin, Activin-2 receptors, GDF-11 | Phase 1 (BMD, sIBM) and 2 (DMD) | Evaluation of its efficacy is ongoing. (74). |
Fig. 3.
The clinical interventions pertaining to activators and inhibitors of myostatin. The figure illustrates different therapeutic interventions, which are currently being worked upon to regulate activation and inhibition of myostatin and finetune sarcomeric protein synthesis and degradation.
Stamulumab and Domagrozumab were among the earliest myostatin inhibitors tested in clinical trials. Both antibodies showed encouraging results in preclinical trials in monkeys and mdx mice and showed excellent safety profiles in humans [[48], [49], [50]]. However, these antibodies failed to show significant improvement in human patients with muscular dystrophies and were subsequently removed from further clinical trials [51].
AMG-745 is a myostatin-neutralizing peptide that boosted muscle mass in mouse models of stroke and chronic renal diseases. The clinical trials involved patients with sarcopenia, chronic kidney disease, and protein-energy malnutrition [52,53]. However, the intravenous injections of AMG-745 failed to increase lean body mass in these patients, leading to study termination [40].
BMS-986089 is a myostatin-neutralizing adenectin fused with Fc domain of human IgG. Preliminary clinical trials demonstrated an adequate safety profile of this molecule in healthy humans. However, the trial on DMD patients was terminated after the study failed to reach its primary endpoint [40].
REGN-1033 and SRK-015 are monoclonal anti-myostatin antibodies demonstrating a high specificity to myostatin. Therefore, they do not cross-react with GDF11, activins, BMPs, and other members of TGF-ß family. Both antibodies demonstrated high efficacy in preventing muscle loss due to ageing, dexamethasone therapy, or spinal muscular atrophy in mice models [54,55]. Clinical trials for REGN-1033 in sarcopenia were completed, but the evaluation of the results is still ongoing. SRK-015 is currently being investigated in phase 2 and phase 3 clinical trials in patients with spinal muscular atrophy.
Blocking myostatin signalling by antagonizing its receptor is another therapeutic strategy to boost muscle mass and force. Several molecules are currently under investigation to enhance muscle mass by blocking myostatin receptors. For instance, ACE-031 and ACE-083 are fusion proteins that target ACVR2B receptors. ACE-031 was first developed and showed encouraging results in mice by increasing oxidative and glycolytic fibre sizes. It also enhanced thigh muscle area and systemic lean muscle mass in post-menopausal women [56,57]. However, it failed to replicate its safety profile in ambulant boys with DMD, and the trials were terminated following bleeding disorders [46]. ACE-083 is a modified form of ACE-031 and is designed to act locally to prevent systemic side effects [58]. In Phase 1 trials, it improved local muscle mass but not the strength in post-menopausal women [57]. However, the desired results were not obtained in the subsequent phase 2 trials involving patients with facioscapulohumeral muscular dystrophy and Charcot-Marie-Tooth disease, leading to study termination.
BYM-338 is a competitive blocker of activin type 2 receptors and was approved by FDA for the treatment of sporadic inclusion body myositis (sIBM) [59]. Unfortunately, despite encouraging results in earlier trials, subsequent studies failed to replicate the improvement in grip strength and walking speed leading to the discontinuation of BYM-338 for treating sIBM [60]. The antibody also showed encouraging efficacy in sarcopenic patients by enhancing gait speed, thigh muscles volume, and walking distance [61]. However, higher doses of the antibody resulted in unwanted side effects, including the deaths of two patients [61]. Conversely, the molecule has shown significant efficacy and safety profile in young patients with cast immobilization [62] and the elderly with pulmonary cachexia [63], which warrants further clinical trials.
Intramuscular gene transfer of follistatin 344 (FST344) is a novel strategy to block myostatin in muscle-wasting conditions. FST344 is delivered via a viral vector and is cleaved to produce an active FSH315 in circulation. Pre-clinical trials of FST344 in cynomolgus monkeys demonstrated an improvement in muscle mass and strength [64]. Likewise, the patients with Becker muscular dystrophy and sIBM improved walking speed following the FST344 gene transfer [65,66]. However, further trials are underway, and the results are under evaluation.
Altogether, the targeted therapies to suppress myostatin and/or its activity demonstrate various degrees of efficacy and toxicity. It may be imperative to clearly distinguish the molecular targets to improve the treatment efficacy and reduce the unwanted toxic effects of various therapies. In this context, the use of nanoparticles may be helpful in ensuring the targeted delivery of therapeutic molecules [67]. Indeed, the nanoparticles have improved the treatment efficacy and reduced the drug toxicity of therapeutic agents in sarcopenia and muscular dystrophies. Further research is required to improve myostatin specificity and targeted delivery of therapeutic agents for treating muscle-wasting diseases.
7. Conclusions and future aspects
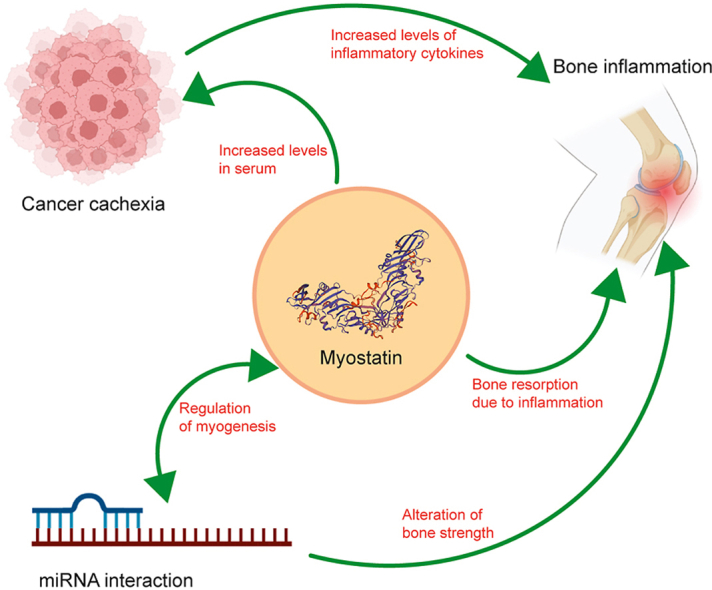
The role of myostatin is crucial in determining muscle homeostasis, as talked about throughout the article. It is an essential signalling molecule/myokine that dictates the fine balance between protein degradation and synthesis in skeletal muscles. Furthermore, the role of myostatin is not only restricted to muscles but can also be extrapolated to the bones as well. Hence, developing therapeutic interventions to target myostatin can be useful for tackling both muscle wastage disorders as well as inflammatory diseases of the bones. This would reduce the incidence of falls and fractures globally, especially in old age, and pave the way to improve the health of patients suffering from autoimmune diseases such as RA or sarcopenia.
CRediT author statement
Akash Mitra: Conceptualization, data collection, data curation, Writing – original draft. Writing-review & editing. Rizwan Qaisar: Data curation, Writing – original draft, Writing-review & editing. Bipasha Bose: Conceptualization, Writing-review & editing. Sudheer Shenoy P: Conceptualization, data collection, data curation, Writing-review & editing.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
The authors would also like to thank Yenepoya Research Centre, Yenepoya Deemed to be University, for providing the online library resources for writing this review article. This research was funded by the Department of Biotechnology (BT/PR39858/MED/30/2247/2020) Government of India and awarded to one of the corresponding authors. ORCID Akash Mitra: 0000-0002-1894-6668. Rizwan Qaisar: 0000-0001-8485-7172. Bipasha Bose: 0000-0002-2312-9734. Sudheer Shenoy P: 0000-0003-1347-8508.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
Contributor Information
Bipasha Bose, Email: Bipasha.bose@yenepoya.edu.in.
Shenoy P Sudheer, Email: shenoy@yenepoya.edu.in.
References
- 1.Frontera W.R., Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 2.Yang X., Xue P., Chen H., Yuan M., Kang Y., Duscher D., et al. Denervation drives skeletal muscle atrophy and induces mitochondrial dysfunction, mitophagy and apoptosis via miR-142a-5p/MFN1 axis. Theranostics. 2020;10 doi: 10.7150/thno.40857. 1415-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagendra A.H., Ray A., Chaudhury D., Mitra A., Ranade A.V., Bose B., et al. Sodium fluoride induces skeletal muscle atrophy via changes in mitochondrial and sarcomeric proteomes. PLoS One. 2022;17 doi: 10.1371/journal.pone.0279261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chazaud B., Mounier R. Diabetes-induced skeletal muscle fibrosis: fibro-adipogenic precursors at work. Cell Metabol. 2021;33:2095–2096. doi: 10.1016/j.cmet.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Dolly A., Dumas J.F., Servais S. Cancer cachexia and skeletal muscle atrophy in clinical studies: what do we really know? J Cachexia Sarcopenia Muscle. 2020;11:1413–1428. doi: 10.1002/jcsm.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L., Chen L., Wan L., Huo Y., Huang J., Li J., et al. Matrine improves skeletal muscle atrophy by inhibiting E3 ubiquitin ligases and activating the Akt/mTOR/FoxO3alpha signaling pathway in C2C12 myotubes and mice. Oncol Rep. 2019;42:479–494. doi: 10.3892/or.2019.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadeghi M., Keshavarz-Fathi M., Baracos V., Arends J., Mahmoudi M., Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit Rev Oncol Hematol. 2018;127:91–104. doi: 10.1016/j.critrevonc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Takayama K., Noguchi Y., Aoki S., Takayama S., Yoshida M., Asari T., et al. Identification of the minimum peptide from mouse myostatin prodomain for human myostatin inhibition. J Med Chem. 2015;58:1544–1549. doi: 10.1021/jm501170d. [DOI] [PubMed] [Google Scholar]
- 9.Ojima C., Noguchi Y., Miyamoto T., Saito Y., Orihashi H., Yoshimatsu Y., et al. Peptide-2 from mouse myostatin precursor protein alleviates muscle wasting in cancer-associated cachexia. Cancer Sci. 2020;111:2954–2964. doi: 10.1111/cas.14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge Z., Liu D., Shang Y., Li Y., Chen S.Z. Magnolol inhibits myotube atrophy induced by cancer cachexia through myostatin signaling pathway in vitro. J Nat Med. 2020;74:741–749. doi: 10.1007/s11418-020-01428-3. [DOI] [PubMed] [Google Scholar]
- 11.Murillo-Saich J.D., Vazquez-Villegas M.L., Ramirez-Villafana M., Saldana-Cruz A.M., Aceves-Aceves J.A., Gonzalez-Lopez L., et al. Association of myostatin, a cytokine released by muscle, with inflammation in rheumatoid arthritis: a cross-sectional study. Medicine (Baltimore. 2021;100 doi: 10.1097/MD.0000000000024186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Ponce F., Gamez-Nava J.I., Gomez-Ramirez E.E., Ramirez-Villafana M., Jacobo-Cuevas H., Rodriguez-Jimenez N.A., et al. Myostatin levels and the risk of myopenia and rheumatoid cachexia in women with rheumatoid arthritis. J Immunol Res. 2022;2022 doi: 10.1155/2022/7258152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puolakkainen T., Rummukainen P., Lehto J., Ritvos O., Hiltunen A., Saamanen A.M., et al. Soluble activin type IIB receptor improves fracture healing in a closed tibial fracture mouse model. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Y., Yi Q., Sun W., Huang D., Zhang H., Duan L., et al. Molecular basis and therapeutic potential of myostatin on bone formation and metabolism in orthopedic disease. Biofactors. 2023;49:21–31. doi: 10.1002/biof.1675. [DOI] [PubMed] [Google Scholar]
- 15.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 16.Hu S.L., Chang A.C., Huang C.C., Tsai C.H., Lin C.C., Tang C.H. Myostatin promotes interleukin-1beta expression in rheumatoid arthritis synovial fibroblasts through inhibition of miR-21-5p. Front Immunol. 2017;8:1747. doi: 10.3389/fimmu.2017.01747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su C.M., Hu S.L., Sun Y., Zhao J., Dai C., Wang L., et al. Myostatin induces tumor necrosis factor-alpha expression in rheumatoid arthritis synovial fibroblasts through the PI3K-Akt signaling pathway. J Cell Physiol. 2019;234:9793–9801. doi: 10.1002/jcp.27665. [DOI] [PubMed] [Google Scholar]
- 18.Dankbar B., Fennen M., Brunert D., Hayer S., Frank S., Wehmeyer C., et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. 2015;21:1085–1090. doi: 10.1038/nm.3917. [DOI] [PubMed] [Google Scholar]
- 19.Fennen M., Weinhage T., Kracke V., Intemann J., Varga G., Wehmeyer C., et al. A myostatin-CCL20-CCR6 axis regulates Th17 cell recruitment to inflamed joints in experimental arthritis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-93599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castillero E., Nieto-Bona M.P., Fernandez-Galaz C., Martin A.I., Lopez-Menduina M., Granado M., et al. Fenofibrate, a PPARalpha agonist, decreases atrogenes and myostatin expression and improves arthritis-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab. 2011;300:E790–E799. doi: 10.1152/ajpendo.00590.2010. [DOI] [PubMed] [Google Scholar]
- 21.Hamrick M.W., Shi X., Zhang W., Pennington C., Thakore H., Haque M., et al. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;40:1544–1553. doi: 10.1016/j.bone.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X.H. MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care. 2013;16:258–266. doi: 10.1097/MCO.0b013e32835f81b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goljanek-Whysall K., Sweetman D., Munsterberg A.E. microRNAs in skeletal muscle differentiation and disease. Clin Sci. 2012;123:611–625. doi: 10.1042/CS20110634. [DOI] [PubMed] [Google Scholar]
- 24.Cheung T.H., Quach N.L., Charville G.W., Liu L., Park L., Edalati A., et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Rourke J.R., Georges S.A., Seay H.R., Tapscott S.J., McManus M.T., Goldhamer D.J., et al. Essential role for Dicer during skeletal muscle development. Dev Biol. 2007;311:359–368. doi: 10.1016/j.ydbio.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L., Wang L., Lu L., Jiang P., Sun H., Wang H. Inhibition of miR-29 by TGF-beta-Smad3 signaling through dual mechanisms promotes transdifferentiation of mouse myoblasts into myofibroblasts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu R., Li H., Li T., Zhang Y., Zhu D. Myostatin regulates miR-431 expression via the Ras-Mek-Erk signaling pathway. Biochem Biophys Res Commun. 2015;461:224–229. doi: 10.1016/j.bbrc.2015.03.150. [DOI] [PubMed] [Google Scholar]
- 28.Wu R., Li H., Zhai L., Zou X., Meng J., Zhong R., et al. MicroRNA-431 accelerates muscle regeneration and ameliorates muscular dystrophy by targeting Pax7 in mice. Nat Commun. 2015;6:7713. doi: 10.1038/ncomms8713. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Huang Z., Chen D., Yang T., Liu G. Role of microRNA-27a in myoblast differentiation. Cell Biol Int. 2014;38:266–271. doi: 10.1002/cbin.10192. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z., Chen X., Yu B., He J., Chen D. MicroRNA-27a promotes myoblast proliferation by targeting myostatin. Biochem Biophys Res Commun. 2012;423:265–269. doi: 10.1016/j.bbrc.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 31.Allen D.L., Loh A.S. Posttranscriptional mechanisms involving microRNA-27a and b contribute to fast-specific and glucocorticoid-mediated myostatin expression in skeletal muscle. Am J Physiol Cell Physiol. 2011;300:C124–C137. doi: 10.1152/ajpcell.00142.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang G., He M., Wu P., Zhang X., Zhou K., Li T., et al. MicroRNA-27b-3p targets the myostatin gene to regulate myoblast proliferation and is involved in myoblast differentiation. Cells. 2021:10. doi: 10.3390/cells10020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaji H. Effects of myokines on bone. BoneKEy Rep. 2016;5:826. doi: 10.1038/bonekey.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey S.N., Kesari A., Yokota T., Pandey G.S. Muscular dystrophy: disease mechanisms and therapies. BioMed Res Int. 2015;2015 doi: 10.1155/2015/456348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He S., Fu T., Yu Y., Liang Q., Li L., Liu J., et al. IRE1alpha regulates skeletal muscle regeneration through Myostatin mRNA decay. J Clin Invest. 2021;131(17) doi: 10.1172/JCI143737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romitti P.A., Zhu Y., Puzhankara S., James K.A., Nabukera S.K., Zamba G.K., et al. Prevalence of Duchenne and becker muscular dystrophies in the United States. Pediatrics. 2015;135:513–521. doi: 10.1542/peds.2014-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marozzo R., Pegoraro V., Angelini C. MiRNAs, Myostatin, and muscle MRI imaging as biomarkers of clinical features in Becker muscular dystrophy. Diagnostics. 2020;10(9) doi: 10.3390/diagnostics10090713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pegoraro V., Cudia P., Baba A., Angelini C. MyomiRNAs and myostatin as physical rehabilitation biomarkers for myotonic dystrophy. Neurol Sci. 2020;41(10):2953–2960. doi: 10.1007/s10072-020-04409-2. [DOI] [PubMed] [Google Scholar]
- 39.Wagner K.R. The elusive promise of myostatin inhibition for muscular dystrophy. Curr Opin Neurol. 2020;33:621–628. doi: 10.1097/WCO.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 40.Suh J., Lee Y.S. Myostatin Inhibitors: panacea or predicament for musculoskeletal disorders? J Bone Metab. 2020;27:151–165. doi: 10.11005/jbm.2020.27.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White T.A., LeBrasseur N.K. Myostatin and sarcopenia: opportunities and challenges - a mini-review. Gerontology. 2014;60:289–293. doi: 10.1159/000356740. [DOI] [PubMed] [Google Scholar]
- 42.Grossmann M. Myostatin inhibition: a new treatment for androgen deprivation-induced sarcopenia? J Clin Endocrinol Metab. 2014;99:3625–3628. doi: 10.1210/jc.2014-3290. [DOI] [PubMed] [Google Scholar]
- 43.Morissette M.R., Stricker J.C., Rosenberg M.A., Buranasombati C., Levitan E.B., Mittleman M.A., et al. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8(5):573–583. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu P., Bhasin S., Hruz P.W., Herbst K.L., Castellani L.W., Hua N., et al. Genetic disruption of myostatin reduces the development of proatherogenic dyslipidemia and atherogenic lesions in Ldlr null mice. Diabetes. 2009;58:1739–1748. doi: 10.2337/db09-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkes J.J., Lloyd D.J., Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes. 2009;58:1133–1143. doi: 10.2337/db08-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell C., McMillan H.J., Mah J.K., Tarnopolsky M., Selby K., McClure T., et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2017;55:458–464. doi: 10.1002/mus.25268. [DOI] [PubMed] [Google Scholar]
- 47.Suh J., Kim N.K., Lee S.H., Eom J.H., Lee Y., Park J.C., et al. GDF11 promotes osteogenesis as opposed to MSTN, and follistatin, a MSTN/GDF11 inhibitor, increases muscle mass but weakens bone. Proc Natl Acad Sci U S A. 2020;117:4910–4920. doi: 10.1073/pnas.1916034117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogdanovich S., Krag T.O., Barton E.R., Morris L.D., Whittemore L.A., Ahima R.S., et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 49.St Andre M., Johnson M., Bansal P.N., Wellen J., Robertson A., Opsahl A., et al. A mouse anti-myostatin antibody increases muscle mass and improves muscle strength and contractility in the mdx mouse model of Duchenne muscular dystrophy and its humanized equivalent, domagrozumab (PF-06252616), increases muscle volume in cynomolgus monkeys. Skeletal Muscle. 2017;7:25. doi: 10.1186/s13395-017-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhattacharya I., Pawlak S., Marraffino S., Christensen J., Sherlock S.P., Alvey C., et al. Safety, Tolerability, pharmacokinetics, and pharmacodynamics of domagrozumab (pf-06252616), an antimyostatin monoclonal antibody, in healthy subjects. Clin Pharmacol Drug Dev. 2018;7:484–497. doi: 10.1002/cpdd.386. [DOI] [PubMed] [Google Scholar]
- 51.Walpurgis K., Thomas A., Thevis M. Detection of the myostatin-neutralizing antibody Domagrozumab in serum by means of Western blotting and LC-HRMS. Drug Test Anal. 2019;11:1714–1723. doi: 10.1002/dta.2729. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L., Rajan V., Lin E., Hu Z., Han H.Q., Zhou X., et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. Faseb J. 2011;25:1653–1663. doi: 10.1096/fj.10-176917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desgeorges M.M., Devillard X., Toutain J., Castells J., Divoux D., Arnould D.F., et al. Pharmacological inhibition of myostatin improves skeletal muscle mass and function in a mouse model of stroke. Sci Rep. 2017;7 doi: 10.1038/s41598-017-13912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Latres E., Pangilinan J., Miloscio L., Bauerlein R., Na E., Potocky T.B., et al. Myostatin blockade with a fully human monoclonal antibody induces muscle hypertrophy and reverses muscle atrophy in young and aged mice. Skeletal Muscle. 2015;5:34. doi: 10.1186/s13395-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long K.K., O'Shea K.M., Khairallah R.J., Howell K., Paushkin S., Chen K.S., et al. Specific inhibition of myostatin activation is beneficial in mouse models of SMA therapy. Hum Mol Genet. 2019;28:1076–1089. doi: 10.1093/hmg/ddy382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Attie K.M., Borgstein N.G., Yang Y., Condon C.H., Wilson D.M., Pearsall A.E., et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve. 2013;47:416–423. doi: 10.1002/mus.23539. [DOI] [PubMed] [Google Scholar]
- 57.Glasser C.E., Gartner M.R., Wilson D., Miller B., Sherman M.L., Attie K.M. Locally acting ACE-083 increases muscle volume in healthy volunteers. Muscle Nerve. 2018;57:921–926. doi: 10.1002/mus.26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearsall R.S., Davies M.V., Cannell M., Li J., Widrick J., Mulivor A.W., et al. Follistatin-based ligand trap ACE-083 induces localized hypertrophy of skeletal muscle with functional improvement in models of neuromuscular disease. Sci Rep. 2019;9 doi: 10.1038/s41598-019-47818-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amato A.A., Sivakumar K., Goyal N., David W.S., Salajegheh M., Praestgaard J., et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83:2239–2246. doi: 10.1212/WNL.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanna M.G., Badrising U.A., Benveniste O., Lloyd T.E., Needham M., Chinoy H., et al. Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019;18:834–844. doi: 10.1016/S1474-4422(19)30200-5. [DOI] [PubMed] [Google Scholar]
- 61.Rooks D., Praestgaard J., Hariry S., Laurent D., Petricoul O., Perry R.G., et al. Treatment of sarcopenia with bimagrumab: results from a phase ii, randomized, controlled, proof-of-concept study. J Am Geriatr Soc. 2017;65:1988–1995. doi: 10.1111/jgs.14927. [DOI] [PubMed] [Google Scholar]
- 62.Rooks D.S., Laurent D., Praestgaard J., Rasmussen S., Bartlett M., Tanko L.B. Effect of bimagrumab on thigh muscle volume and composition in men with casting-induced atrophy. J Cachexia Sarcopenia Muscle. 2017;8:727–734. doi: 10.1002/jcsm.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polkey M.I., Praestgaard J., Berwick A., Franssen F.M.E., Singh D., Steiner M.C., et al. Activin type ii receptor blockade for treatment of muscle depletion in chronic obstructive pulmonary disease. A randomized trial. Am J Respir Crit Care Med. 2019;199:313–320. doi: 10.1164/rccm.201802-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kota J., Handy C.R., Haidet A.M., Montgomery C.L., Eagle A., Rodino-Klapac L.R., et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009;1:6ra15. doi: 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendell J.R., Sahenk Z., Malik V., Gomez A.M., Flanigan K.M., Lowes L.P., et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol Ther. 2015;23:192–201. doi: 10.1038/mt.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendell J.R., Sahenk Z., Al-Zaidy S., Rodino-Klapac L.R., Lowes L.P., Alfano L.N., et al. Follistatin gene therapy for sporadic inclusion body myositis improves functional outcomes. Mol Ther. 2017;25:870–879. doi: 10.1016/j.ymthe.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed Z., Qaisar R. Nanomedicine for treating muscle dystrophies: opportunities, challenges, and future perspectives. Int J Mol Sci. 2022;23(19) doi: 10.3390/ijms231912039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pirruccello-Straub M., Jackson J., Wawersik S., Webster M.T., Salta L., Long K., et al. Blocking extracellular activation of myostatin as a strategy for treating muscle wasting. Sci Rep. 2018;8:2292. doi: 10.1038/s41598-018-20524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]