Abstract
Objectives
Sacral insufficiency fracture (SIF) is not an uncommon osteoporosis fracture among the elderly. Aside from traditional treatments, sacroplasty and teriparatide (TPTD) injection have been introduced. This report aims to compare the effects of sacroplasty and teriparatide on clinical outcomes of SIF.
Methods
Thirty-one elderly patients with SIF were enrolled in this retrospective observational study. Four male patients were excluded. Fourteen patients who received TPTD for 6 months were classified into the TPTD group (TT), and 13 who underwent sacroplasty were classified into the sacroplasty group (SS). All patients in both groups were instructed to take calcium and vitamin D supplements daily. Their symptoms and signs, visual analog score (VAS), Oswestry disability index (ODI), and radiographic studies were retrospectively reviewed.
Results
The TT group showed significantly lower VAS than SS group after 3 (P < 0.001) and 6 months of treatment (P < 0.001). The TT group also has significant lower ODI than SS group after 1 (P = 0.010), 3 (P = 0.005) and 6 months (P < 0.001) of treatment. Upon generalized estimating equations (GEE) analysis, the TT group showed significantly more reduction in both VAS and ODI compared to the SS group at 1 month (P = 0.022, P = 0.001), 3 months (P < 0.001, P < 0.001), and 6 months (P < 0.001, P < 0.001) post-treatment.
Conclusions
Postmenoposal woman with SIF who received TPTD healed better than those who underwent sacroplasty after 1 month treatment.
Keywords: Sacral insufficiency fracture, Sacroplasty, Teriparatide, Low back pain, Generalized estimating equation
1. Introduction
Sacral insufficiency fracture (SIF) is not an uncommon osteoporosis fracture in the elderly population, especially among postmenopausal woman [1]. SIFs are difficult to detect on plain radiographs, and the sacrum is rarely included in routine lumbar magnetic resonance imaging (MRI). Because SIF is an unexpected cause of low back pain, the time to diagnosis is significantly longer than that in patients with other types of osteoporotic fractures. The most reliable method of diagnosing SIF is MRI [2,3]. Patients with SIFs usually complain of low back and groin pain and coxalgia, all of which affect their quality of life considerably. SIF is associated with 9.8% and 17.5% mortality rates, respectively, at 6 months and 1 year after occurrence [4]. Treatments for SIFs were limited to conservative methods such as bed rest and oral analgesia [5] until the introduction of sacroplasty. Sacroplasty is considered a safe and effective method for stabilizing the sacrum by injecting bone cement into lesions [2,[5], [6], [7], [8], [9], [10]]. Sacroplasty helps to improve patients' visual analog scale (VAS) pain scores [[5], [6], [7]]. Teriparatide (TPTD) has been used to treat osteoporosis to improve bone mineral density (BMD) and reduce fracture risks [8,9]. Recently, TPTD has been shown to improve pain relief and functional outcomes in patients with SIF [11,12]. However, there are no studies that report the outcome of SIFs treated by sacroplasty as compared to TPTD. We noted anecdotally that some patients who underwent sacroplasty still complained of buttock or low back pain in the first 1 or 2 months after surgery, whereas those who received TPTD treatment rarely had such pain. This retrospective report aims to analyze patients with SIFs who received either TPTD treatment or sacroplasty and to compare the effects of the 2 treatments on improvement of the VAS and Oswestry disability index (ODI) scores, as well as assess the fracture healing process. SIF patients’ medical records at our institution collected during the 2011–2021 period were collected andanalyzed. All SIF patients at the hospital during this time period received either TPTD injection treatment or sacroplasty surgery. No SIF patients in the time frame recieved conservative treatment (bed rest and/or analgesia) or other SIF treatment.
2. Methods
This study was approved by the institutional review board (approval number B11004008), passed on November 5th, 2021 by the research ethics committee of the Buddhist Dalin Tzu Chi Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Patients in this medical university-affiliated hospital who were diagnosed with SIF between 2011 and 2021 were eligible for this study. Patients either underwent sacroplasty or received TPTD injection. A total of 32 patients were diagnosed with SIF, and one of them was lost to follow-up. Sixteen patients who received TPTD injection were included in the TT group, while the other 15 patients who underwent sacroplasty were included in the SS group. The patients were followed-up for 6 months.
In the TT group, all patients received percutaneous TPTD injection, given in 20 μg once a day, for 6 months. In the SS group, patients underwent sacroplasty after SIF was diagnosed. Sacroplasty was performed by an experienced surgeon in an operating theater. The patient was placed prone and injected with local anesthesia after aseptic draping. Under fluoroscopic guidance, cement was injected into the lesions using the procedure needles with the long-axis approach [13]. The wounds were covered with gauze dressings, and no sutures were made. All patients did not receive osteoporotic treatment prior to their SIF treatment. They were prescribed 1000 mg calcium, 400 iu vitamin D supplements after SIF diagnosis. Compliance was insured at each follow-up by the doctor. Denosumab were prescribed to both group of patients after bone reunion after 6 months of SIF treatment.
All eligible patients underwent MRI, bone scintigraphy, and plain radiography prior to treatment. They were also assessed for associated injuries, and symptoms and signs shown prior to treatment (Table 1). Demographic data on the patients’ age, sex, body mass index (BMI), symptoms, and total hip bone mineral density (BMD) were collected. Data on the ODI and VAS scores before treatment and at 2 weeks, 1 month, 3 months, and 6 months after the initial treatment were obtained. Two independent, experienced radiologist assessed fracture healing using pelvis radiography (anteroposterior (AP) and sacral lateral views). The presentation of callus formation and sclerosis at the fracture sites at 3 months was reviewed using radiographs.
Table 1.
Demographic characteristics of patients with sacral insufficiency fracture.
| TT group (N = 14) | SS group (N = 13) | P-value | |
|---|---|---|---|
| Age, yrs | 76.7 ± 6.1 | 77.8 ± 9.7 | 0.727 |
| BMI, kg/ | 23.1 ± 4.3 | 23.9 ± 4.2 | 0.641 |
| Height, cm | 152.3 ± 5.9 | 151.0 ± 6.7 | 0.651 |
| Weight, kg | 51.1 ± 11.3 | 56.5 ± 10.6 | 0.269 |
| Total hip BMD, g/cm2 | 0.505 ± 0.141 | 0.448 ± 0.110 | 0.278 |
| Status of osteoporosis, Total hip T-score | −3.1 ± 0.8 | −3.3 ± 1.0 | 0.560 |
| Time from pain to diagnosis, week | 5.0 ± 4.3 | 8.7 ± 7.1 | 0.195 |
| Associate injury | |||
| Compression fracture | 1 | 2 | 0.521 |
| Pubic ramus fracture | 3 | 6 | 0.187 |
| Symptoms and signs | |||
| Low back pain | 14 | 12 | 0.481 |
| Buttock pain | 13 | 12 | 0.741 |
| Groin pain | 3 | 7 | 0.293 |
| Sciatica | 5 | 7 | 0.573 |
| VAS | |||
| Pre-treatment | 8.0 ± 1.0 | 7.7 ± 0.8 | 0.404 |
| Post treatment week 2 | 5.0 ± 0.8 | 4.7 ± 1.3 | 0.629 |
| Post treatment month 1 | 3.8 ± 1.1 | 4.6 ± 1.2 | 0.106 |
| Post treatment month 3 | 1.8 ± 0.6 | 3.8 ± 1.5 | < 0.001∗ |
| Post treatment month 6 | 0.6 ± 0.8 | 2.7 ± 1.4 | < 0.001∗ |
| ODI (%) | |||
| Pre-treatment | 82.7 ± 9.7 | 82.6 ± 9.1 | 0.989 |
| Post treatment week 2 | 64.6 ± 8.2 | 68.3 ± 3.5 | 0.169 |
| Post treatment month 1 | 48.8 ± 8.0 | 56.9 ± 4.1 | 0.010∗ |
| Post treatment month 3 | 22.6 ± 9.4 | 32.4 ± 4.8 | 0.005∗ |
| Post treatment month 6 | 11.2 ± 3.5 | 20.7 ± 4.9 | < 0.001∗ |
| Radiographic findings at 3 months | |||
| Callus formation | 6/14 | 6/13 | 0.870 |
| Sclerosis at fracture site | 6/14 | 1/13 | 0.038∗ |
TT, teriparatide treatment; SS, sacroplasty surgery; BMD,bone mineral density.
BMI, body mass index; ODI, Oswestry disability index; VAS, visual analogue scale.
∗P < 0.05.
Continuous data are expressed as mean ± standard deviation. The Mann–Whitney U test was used to compare differences between the 2 groups. Categorical variables were tested using the chi-squared test. Repeated measurements in the 2 groups at each observation interval were compared using generalized estimating equations (GEE). Variables collected (age, BMI, total hip BMD, time from pain to diagnosis, and associated injury) were explored in the models. Comparison of the improvement in VAS and ODI scores in the 2 groups was then made by GEE analysis after adjusting for the significant associated variable (associated injury). Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA). A P-value < 0.05 was considered statistically significant.
3. Results
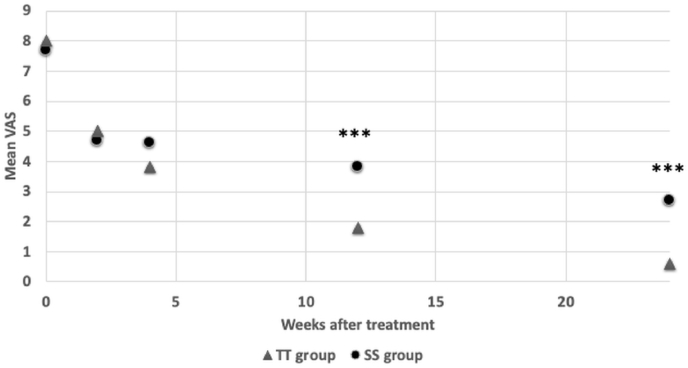
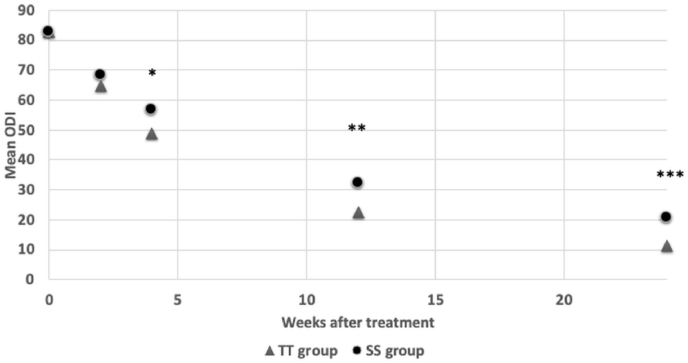
The clinical features of the patients are summarized in Table 1. Thirty-one patients were enrolled in this study. Only 4 (12%) of the patients were men, to avoid interference of the sex factor, they were excluded and only women were involved. Of the 27 remaining patients, the 2 groups do not differ for age, BMI, height, body weight, or associated pubic ramus fractures. They were all classified as osteoporotic with a mean T-score of −3.1. There were no significant differences in the VAS and ODI scores in both groups before treatment. Upon independent sample t-test, the TT group and SS group does not have significant different in neither VAS nor ODI for the first 2 weeks of treatment. However, the TT group showed significantly lower VAS than SS group after 3 months of treatment (P < 0.001) and 6 months of treatment (P < 0.001). The TT group also has significant lower ODI than SS group after 1 month (P = 0.010), 3 months (P = 0.005), and 6 months (P < 0.001) of treatment (Fig. 1, Fig. 2). Upon GEE analysis for associated factors, associated injuries were significant factors that influenced the patients’ VAS scores during treatment (Table 2). These factors were adjusted for when running GEE analysis for improvement of VAS and ODI in the 2 treatments (Table 3). There were no significant changes in VAS or ODI scores in the first 2 weeks; however, the TT group showed a significant reduction in both VAS and ODI scores at 1 month (P = 0.048, P = 0.003), 3 months (P = 0.001, P < 0.001), and 6 months (P < 0.001, P < 0.001) post-treatment. Callus bridging was identified in 6 patients from both groups at 3 months. Sclerosis changes at the fracture site were more common in the TPTD group (43% vs 8%, P = 0.038) (Fig. 3, Fig. 4). No specific complication related symptoms were noticed in both groups.
Fig. 1.
The mean visual analgue score (VAS) change of teriparatide (TT) and sacroplaty (SS) group are shown. At 3 months and 6 months after treatment, TT group showed significantly lower VAS than SS group. ∗P < 0.05 ∗∗P < 0.01 ∗∗∗P < 0.001.
Fig. 2.
The mean Oswestry disability index (ODI) change of teriparatide (TT) and sarcoplasty (SS) group were shown. At 1 month, 3 months, and 6 months after treatment, TT group showed significantly lower ODI than SS group. ∗P < 0.05 ∗∗P < 0.01 ∗∗∗P < 0.001.
Table 2.
GEE analysis for factors associated with VAS and ODI during treatment.
| Factors | Coefficient | VAS 95% CI |
P- value | Coefficient | ODI 95% CI |
P-value |
|---|---|---|---|---|---|---|
| Age, yrs | −0.01 | −0.07, 0.03 | 0.751 | 0.08 | −0.24, 0.41 | 0.609 |
| BMI, kg/ | 0.02 | −0.01, 0.06 | 0.216 | 0.07 | −0.36, 0.50 | 0.756 |
| Hip BMD, g/ | 0.07 | −2.72, 0.49 | 0.170 | −4.32 | −23.18, 14.54 | 0.653 |
| Status of osteoporosis (T-score) | −0.03 | −0.48, −0.05 | 0.016∗ | −0.22 | −2.60, 2.16 | 0.856 |
| Time from pain to diagnosis | 0.02 | −0.06, 0.05 | 0.792 | −0.26 | 1.73, 11.22 | 0.178 |
| Associate injury | 3.67 | −0.04, 0.95 | 0.071 | 4.32 | 0.85, 7.78 | 0.015∗ |
BMD, bone mineral density; BMI, body mass index; ODI, Oswestry disability index; VAS, visual analogue scale.
∗P < 0.05.
Table 3.
GEE analysis for improvement of VAS and ODI in two different treatments.
| Factors | Coefficient | VAS 95% CI |
P-value | Coefficient | ODI 95% CI |
P-value |
|---|---|---|---|---|---|---|
| Intercept | 6.86 | 6.07, 7.64 | <0.001∗ | 4.32 | 0.85, 7.78 | 0.015∗ |
| Treatment method by Time | ||||||
| Post treatment week 2 | −0.23 | −1.13, 0.66 | 0.612 | −5.05 | −12.53, 2.43 | 0.186 |
| Post treatment month 1 | −1.22 | −2.26, −0.17 | 0.022∗ | −10.39 | −16.25, −4.52 | 0.001∗ |
| Post treatment month 3 | −2.31 | −3.50, −1.12 | < 0.001∗ | −19.75 | −28.75, −10.74 | < 0.001∗ |
| Post treatment month 6 | −2.19 | −3.35, −1.03 | < 0.001∗ | −20.38 | −28.45, −12.31 | < 0.001∗ |
ODI, Oswestry disability index; VAS, visual analogue scale.
Interaction for VAS and ODI with time and treatment method has been adjusted by T-score and associate injury.
∗P < 0.05.
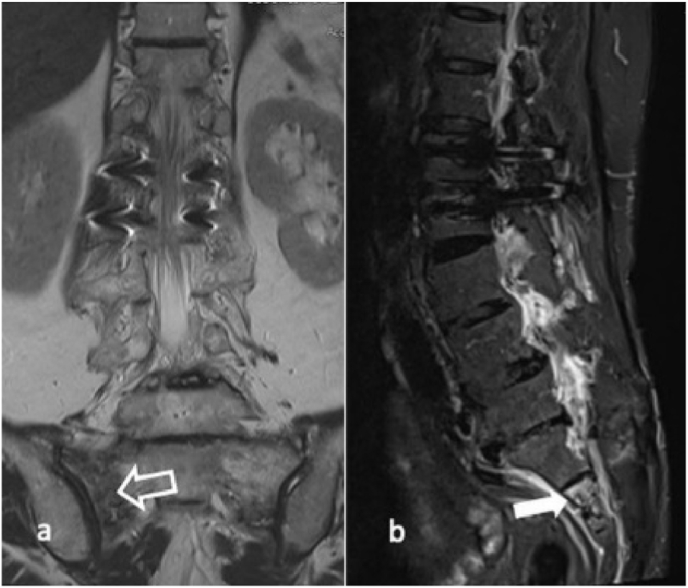
Fig. 3.
A 70-year-old female sustained SIF and her (a) MRI scan showing sacral fracture near the sacral-iliac joint (open arrow) by coronal view and (b) S2 level (white arrow) sagittal view.
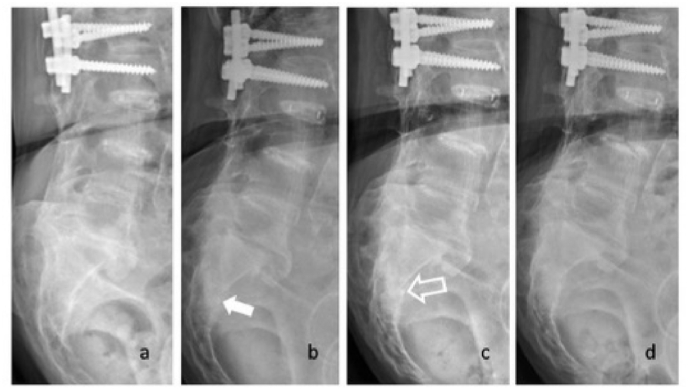
Fig. 4.
(a) Sacral fracture was nearly invisible on lateral view of sacral X-ray at pre-treatment. (b) Some callus formation (white arrow) was identified at one month follow-up. (c) Sclerosis change (open arrow) of fracture site was identified at 2 months follow-up. (d) Complete heal of bone was noted on 6 months follow-up.
4. Discussion
There were several treatment methods reported for SIF, including conservative treatment [5], transiliac-transsacral screw stabilization [2], sacroplasty, and TPTD. This report focuses on the treatment effect of sacroplasty and TPTD injection. The results of this study showed that the VAS and ODI scores improved over time in both groups. Of the 2 groups, TT group showed significantly more improvement than SS group in the VAS and ODI scores for SIF at 3 months after initial treatment. In a prospective case series study, sacroplasty showed a significant reduction in the VAS and ODI scores after surgery and an improvement in activities of daily living (ADL) scores at 3 months postoperatively [14]. In a prospective cohort study, Frey et al [6] showed that sacroplasty could alleviate pain in SIFs [6]. In some case series, TPTD was also effective in alleviating the symptoms caused by SIFs after 1 month of treatment [6,12,[15], [16], [17]]. A retrospective case-control study by Yoo et al [12] showed that elderly patients with SIF who received TPTD for a minimum of 3 months reported significant pain reduction at 1 month and earlier mobilization at 1.2 weeks. Both sacroplasty and TPTD therapy demonstrated clinical effects on SIF, regardless of pain relief and functional improvement. The results of our study were similar to those of previous studies. Most studies on the treatment of sacroplasty and TPTD for SIF showed significant alleviation of functional pain instability from 1 to 3 months post-treatment.
Sacroplasty was originally used for the treatment of painful sacral fractures resulting from tumor metastasis or osteoporosis and has been considered a safe procedure [7,17]. Case reports and prospective case-series studies has also showed that sacroplasty is a safe and effective treatment for SIFs, with better effect than bed rest and oral analgesic medication for SIFs [[5], [6], [7],14,15,[18], [19], [20]]. Urits et al [2] reviewed some studies and concluded that sacroplasty was an effective treatment for the alleviation of pain and improvement of mobilization in SIFs. Elderly individuals are vulnerable to long-term opioid treatment; therefore, it is rational to minimize the use of opioids. A study by Frey et al [6] showed that patients who underwent sacroplasty reported less analgesic medication use at 3 months. Choi et al [15] reported an average ODI score improvement from 59% preoperatively to 15.5% at 1 month postoperatively. The results of our study showed that the average ODI score improved from 81.1% preoperatively to 56.7% at 1 month postoperatively. Biomechanical analysis showed that the injected cement in sacral fracture decreased local strains by 40–60%, while overall stiffness only increased by 1–4% [21,22]. In a cadaveric study, sacroplasty did not restore sacral strength or stiffness, and the authors recommended the use of modest amounts (≤ 3 mL) of cement to reduce the risk of extravasation [22]. Cement extravasation is a risk factor for neurovascular injury and hinders fracture healing.
TPTD is an anabolic agent that increases the BMD of the spine and hip in postmenopausal patients with osteoporosis. It has been used in a phase 2 randomized-controlled trial to treat pelvic fracture and showned improved physical performance not significant in the placebo group [23]. In a prospective cohort study, Cho et al [24] observed earlier posterior lumbar interbody fusion in their TPTD group. Its anabolic effects increase bone turnover, which stimulates bone remodeling at both the cancellous and endosteal bone [25]. Wu et al [26] reported a 73-year-old woman with sacral and pubic insufficiency fractures that recovered well 3 months after TPTD treatment. A small case series by Kasukawa et al [16] reported that TPTD enhanced bone healing and reduced pain scales. Peichi et al [27] observed that TPTD accelerated healing in geriatric pelvic fractures in a prospective randomized controlled study. Another retrospective study also reported faster pain relief with TPTD treatment [12]. The prospective and retrospective studies both demonstrated that TPTD treatment improved functional outcomes in both the timed “Up and Go” test and ADL scores. Our patients who received TPTD had greater reduction in pain scales and greater improvement in functional disability after a fixed time frame of 3 and 6 months after treatment. The proportion of patients with callus formation at 3 months was similar in both groups. The presence of sclerotic changes was observed more often in the TT group. Our findings on callus formation and the presence of sclerotic changes are similar to those of a case series by Kasukawa et al [16], in which all SIFs healed with sclerotic changes at a fracture site following TPTD treatment. Sclerotic changes at fracture sites and whether the association between sclerotic changes and pain relief is due to the healing potential of TPTD, require further study.
Sacroplasty and TPTD therapy have shown good results in terms of pain relief and functional outcomes in SIFs. The results of our study showed that TPTD performed better at alleviating pain and improving functional disability. Sacroplasty may provide immediate support with limited stiffness [2], whereas TPTD stabilizes the fractures by increasing bone turnover, which enhances healing through the fracture gap [16]. It has also been noted from our results that the TT group showed more sclerotic change during follow up at the end of 3 months period. The cement hindering fracture healing is a concern; however, further studies are needed to confirm this. We speculate that patients with SIF benefited from the anabolic effects of TPTD on fracture healing compared to short-term support by sacroplasty.
Some authors have reported complications associated with sacroplasty. The incidence of periprocedural cement extravasation is 7.4%–12.5% [6,15]. Most of these complications are clinically silent; however, cement leakage into the fracture gap during sacroplasty predisposes to a 5th lumbar root injury [28]. Other complications such as infection, pulmonary embolism, and intravasal leakage reportedly cause serious comorbidities [29]. Elderly people, especially those with high risk, often have insufficient cardiopulmonary reservoirs and sometimes cannot tolerate anesthesia. Therefore, TPTD therapy is a alternative treatment for SIFs free of sacroplasty induced complications. It should be emphasized that the side effects of hypercalcemia in TPTD require medical attention during treatment. Although no complication related symptoms were reported in the TT or SS group of our paper.
This study has some limitations. First, it had a small sample size, which might have affected the significance of the research content, since limited data was used. The fact that SIFs are commonly underdiagnosed [30] and the number of SIFs is higher than expected should be considered. Second, there was selection bias. The treatment patient recieved was determined by the surgeon's preference. The cases were collected by 4 various surgeons at our institution and retrospectively reviewed, with the cases of both groups evenly distributed over the decade. The time from pain to diagnosis in both groups, though not significant, exists a difference. It is unclear how this may affect the data collected, but it may mean the SS group is more advanced in time for fracture healing. In addition, the patients in this study were treated by different physicians, who may have different experience in surgical performance that affected the outcomes. A randomized controlled prospective study is recommended in the future to explore the long-term effects of sacroplasty and TPTD therapy in SIFs.
5. Conclusions
Postmenoposal woman with SIF who received TPTD injection healed better than those who underwent sacroplasty after 1 month, 3 months, and 6 months of treatment.
CRediT author statement
Yao-Chun Yang: Conceptualization, Formal analysis, Writing - Original Draft. Min-Hong Hsieh: Methodology, Validation, Formal analysis, Data Curation, Writing - Review & Editing. Jui-Teng Chien: Investigation, Resources, Writing - Review & Editing. Keng-Chang Liu: Investigation, Resources, Writing - Review & Editing. Chang-Chen Yang: Conceptualization, Formal analysis, Writing - Review & Editing, Data Curation.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
The authors would like to thank Yu Hao Chang for his enthusiastic help in GEE analysis assistance. ORCID Yao-Chun Yang: 0000-0002-8954-7090. Min-Hong Hsieh: 0000-0002-7141-9568. Jui-Teng Chien: 0000-0002-9009-6303. Keng-Chang Liu: 0000-0002-6702-7166. Chang-Chen Yang: 0000-0002-4812-1202.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Tamaki Y., Nagamachi A., Inoue K., Takeuchi M., Sugiura K., Omichi Y., et al. Incidence and clinical features of sacral insufficiency fracture in the emergency department. Am J Emerg Med. 2017;35:1314–1316. doi: 10.1016/j.ajem.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Urits I., Orhurhu V., Callan J., Maganty N.V., Pousti S., Simopoulos T., et al. Sacral insufficiency fractures: a review of risk factors, clinical presentation, and management. Curr Pain Headache Rep. 2020;24:10. doi: 10.1007/s11916-020-0848-z. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita H., Miyakoshi N., Kobayashi T., Abe T., Kikuchi K., Shimada Y. Comparison of patients with diagnosed and suspected sacral insufficiency fractures. J Orthop Sci. 2019;24:702–707. doi: 10.1016/j.jos.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Park J.W., Park S.M., Lee H.J., Lee C.K., Chang B.S., Kim H. Mortality following benign sacral insufficiency fracture and associated risk factors. Arch Osteoporos. 2017;12:100. doi: 10.1007/s11657-017-0395-3. [DOI] [PubMed] [Google Scholar]
- 5.Mahmood B., Pasternack J., Razi A., Saleh A. Safety and efficacy of percutaneous sacroplasty for treatment of sacral insufficiency fractures: a systematic review. J Spine Surg. 2019;5:365–371. doi: 10.21037/jss.2019.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey M.E., Warner C., Thomas S.M., Johar K., Singh H., Mohammad M.S., et al. Sacroplasty: a ten-year analysis of prospective patients treated with percutaneous sacroplasty: literature review and technical considerations. Pain Physician. 2017;20 [PubMed] [Google Scholar]
- 7.Gibbs W.N., Doshi A. Sacral fractures and sacroplasty. Neuroimaging Clin N Am. 2019;29:515–527. doi: 10.1016/j.nic.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Johnston C.B., Dagar M. Osteoporosis in older adults. Med Clin North Am. 2020;104:873–884. doi: 10.1016/j.mcna.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi M. The Importance of teriparatide in the treatment of osteoporosis. Clin Calcium. 2016;26:905–913. [PubMed] [Google Scholar]
- 10.Ortiz A.O., Brook A.L. Sacroplasty Tech Vasc Interv Radiol. 2009;12:51–63. doi: 10.1053/j.tvir.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Hohenberger G., Schwarz A., Hartwig E., Bücking B. Teriparatide as a therapy approach in sacral insufficiency fractures] Unfallchirurg. 2017;120:1000–1003. doi: 10.1007/s00113-017-0400-8. [DOI] [PubMed] [Google Scholar]
- 12.Yoo J.I., Ha Y.C., Ryu H.J., Chang G.W., Lee Y.K., Yoo M.J., et al. Teriparatide treatment in elderly patients with sacral insufficiency fracture. J Clin Endocrinol Metab. 2017;102:560–565. doi: 10.1210/jc.2016-3582. [DOI] [PubMed] [Google Scholar]
- 13.Smith D.K., Dix J.E. Percutaneous sacroplasty: long-axis injection technique. Am J Roentgenol. 2006;186:1252–1255. doi: 10.2214/AJR.05.0823. [DOI] [PubMed] [Google Scholar]
- 14.Lyders E.M., Whitlow C.T., Baker M.D., Morris P.P. Imaging and treatment of sacral insufficiency fractures. Am J Neuroradiol. 2010;31:201–210. doi: 10.3174/ajnr.A1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi K.C., Shin S.H., Lee D.C., Shim H.K., Park C.K. Effects of percutaneous sacroplasty on pain and mobility in sacral insufficiency fracture. J Korean Neurosurg Soc. 2017;60:60–66. doi: 10.3340/jkns.2016.0505.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasukawa Y., Miyakoshi N., Ebina T., Hongo M., Ishikawa Y., Kudo D., et al. Enhanced bone healing and decreased pain in sacral insufficiency fractures after teriparatide treatment: retrospective clinical-based observational study. Clin Cases Miner Bone Metab. 2017;14:140–145. doi: 10.11138/ccmbm/2017.14.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho C.H., Mathis J.M., Ortiz O. Sacral fractures and sacroplasty. Neuroimaging Clin N Am. 2010;20:179–186. doi: 10.1016/j.nic.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Simon J.I., Surrey D.E., Kitei P., Sonagere M., Gehret J., Young G., et al. Successful repeat sacroplasty in a patient with a recurrent sacral insufficiency fracture: a case presentation. Pm R. 2017;9:1171–1174. doi: 10.1016/j.pmrj.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Frey M.E., Depalma M.J., Cifu D.X., Bhagia S.M., Carne W., Daitch J.S. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: a prospective, multicenter, observational pilot study. Spine J. 2008;8:367–373. doi: 10.1016/j.spinee.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Frey M.E., DePalma M.J., Cifu D.X., Bhagia S.M., Daitch J.S. Efficacy and safety of percutaneous sacroplasty for painful osteoporotic sacral insufficiency fractures: a prospective, multicenter trial. Spine. 2007;32:1635–1640. doi: 10.1097/BRS.0b013e318074d4e1. [DOI] [PubMed] [Google Scholar]
- 21.Anderson D.E., Cotton J.R. Mechanical analysis of percutaneous sacroplasty using CT image based finite element models. Med Eng Phys. 2007;29:316–325. doi: 10.1016/j.medengphy.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Richards A.M., Mears S.C., Knight T.A., Dinah A.F., Belkoff S.M. Biomechanical analysis of sacroplasty: does volume or location of cement matter? Am J Neuroradiol. 2009;30:315–317. doi: 10.3174/ajnr.A1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieves J.W., Cosman F., McMahon D., Redko M., Hentschel I., Bartolotta R., et al. Teriparatide and pelvic fracture healing: a phase 2 randomized controlled trial. Osteoporos Int. 2022;33:239–250. doi: 10.1007/s00198-021-06065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho P.G., Ji G.Y., Shin D.A., Ha Y., Yoon D.H., Kim K.N. An effect comparison of teriparatide and bisphosphonate on posterior lumbar interbody fusion in patients with osteoporosis: a prospective cohort study and preliminary data. Eur Spine J. 2017;26:691–697. doi: 10.1007/s00586-015-4342-y. [DOI] [PubMed] [Google Scholar]
- 25.Arlot M., Meunier P.J., Boivin G., Haddock L., Tamayo J., Correa-Rotter R., et al. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res. 2005;20:1244–1253. doi: 10.1359/JBMR.050309. [DOI] [PubMed] [Google Scholar]
- 26.Wu C.C., Wei J.C., Hsieh C.P., Yu C.T. Enhanced healing of sacral and pubic insufficiency fractures by teriparatide. J Rheumatol. 2012;39:1306–1307. doi: 10.3899/jrheum.111458. [DOI] [PubMed] [Google Scholar]
- 27.Peichl P., Holzer L.A., Maier R., Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 28.Bastian J.D., Keel M.J., Heini P.F., Seidel U., Benneker L.M. Complications related to cement leakage in sacroplasty. Acta Orthop Belg. 2012;78:100–105. [PubMed] [Google Scholar]
- 29.Gjertsen O., Schellhorn T., Nakstad P.H. Fluoroscopy-guided sacroplasty: special focus on preoperative planning from three-dimensional computed tomography. Acta Radiol. 2008;49:1042–1048. doi: 10.1080/02841850802350659. [DOI] [PubMed] [Google Scholar]
- 30.Kao F.C., Hsu Y.C., Liu P.H., Yeh L.R., Wang J.T., Tu Y.K. Osteoporotic sacral insufficiency fracture: an easily neglected disease in elderly patients. Medicine. 2017;96 doi: 10.1097/MD.0000000000009100. [DOI] [PMC free article] [PubMed] [Google Scholar]