Abstract
Aims
Although body mass index (BMI) is the most commonly used anthropometric measure, newer indices such as the waist-to-height ratio, better reflect the location and amount of ectopic fat, as well as the weight of the skeleton, and may be more useful.
Methods and results
The prognostic value of several newer anthropometric indices was compared with that of BMI in patients with heart failure (HF) and reduced ejection fraction (HFrEF) enrolled in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure. The primary outcome was HF hospitalization or cardiovascular death. The association between anthropometric indices and outcomes were comprehensively adjusted for other prognostic variables, including natriuretic peptides. An ‘obesity-survival paradox’ related to lower mortality risk in those with BMI ≥25 kg/m2 (compared with normal weight) was identified but this was eliminated by adjustment for other prognostic variables. This paradox was less evident for waist-to-height ratio (as an exemplar of indices not incorporating weight) and eliminated by adjustment: the adjusted hazard ratio (aHR) for all-cause mortality, for quintile 5 vs. quintile 1, was 1.10 [95% confidence interval (CI) 0.87–1.39]. However, both BMI and waist-to-height ratio showed that greater adiposity was associated with a higher risk of the primary outcome and HF hospitalization; this was more evident for waist-to-height ratio and persisted after adjustment e.g. the aHR for HF hospitalization for quintile 5 vs. quintile 1 of waist-to-height ratio was 1.39 (95% CI 1.06–1.81).
Conclusion
In patients with HFrEF, alternative anthropometric measurements showed no evidence for an ‘obesity-survival paradox’. Newer indices that do not incorporate weight showed that greater adiposity was clearly associated with a higher risk of HF hospitalization.
Keywords: Heart failure with reduced ejection fraction, Obesity, Body mass index, Angiotensin receptor-neprilysin inhibitor, Clinical trial
Structured Graphical Abstract
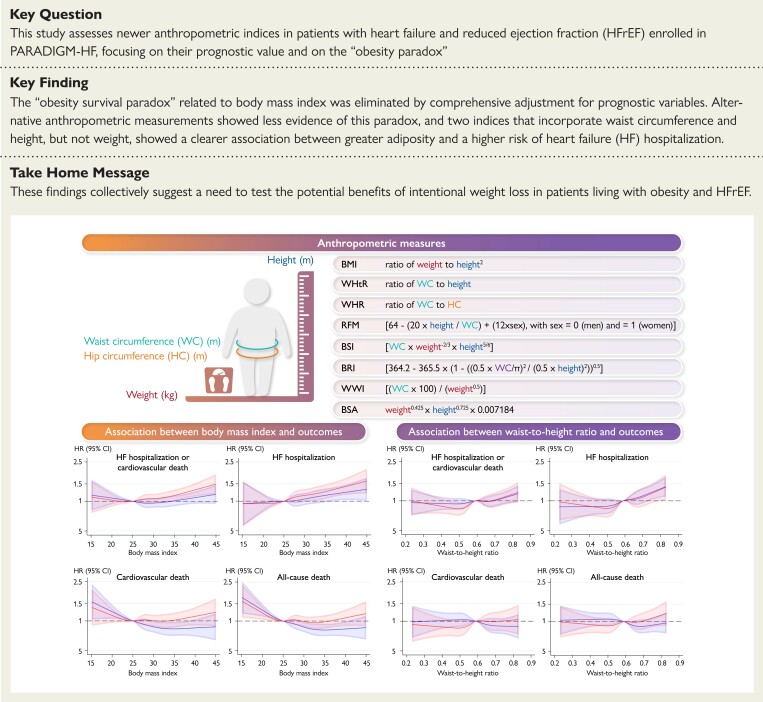
Structured Graphical Abstract.
The upper part of the figure describes the calculation of each of the anthropometric measures. The lower part of the figure shows the risk of outcomes according to continuous body mass index (left panel) and waist-to-height ratio (right panel). The solid line represents the hazard ratio and the shaded area the 95% confidence interval. The blue spline is adjusted for treatment and region. The red spline is adjusted for treatment, age, sex, region, systolic blood pressure, heart rate, estimated glomerular filtration rate, left ventricular ejection fraction, log of n-terminal pro-B-type natriuretic peptide, body mass index (only in the waist-to-height ratio analyses), New York Heart Association functional class, heart failure aetiology, duration of heart failure, prior heart failure hospitalization, a history of diabetes, and atrial fibrillation. BMI, body mass index; BRI, body roundness index; BSA, body surface area; BSI, body shape index; CI, confidence interval; HF, heart failure; HR, hazard ratio; RFM, relative fat mass; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio; WWI, weight-adjusted-waist index.
See the editorial comment for this article ‘Revisiting the obesity paradox in heart failure: what is the best anthropometric index to gauge obesity?’, by R. Sato and S. von Haehling, https://doi.org10.1093/eurheartj/ehad079.
Introduction
Although obesity has been repeatedly shown to be an independent risk factor for the development of heart failure (HF),1–3 its prognostic importance in established HF, especially HF with reduced ejection fraction (HFrEF), is less clear and an ‘obesity-survival paradox’ has been described in patients with HFrEF.4–8 However, the associations between obesity and outcomes in HFrEF have generally been based on body mass index (BMI), calculated as weight in kilograms divided as height in meters squared, which has many limitations as a measure of adiposity. BMI does not take into account the location of body fat or its amount, relative to muscle, or the weight of the skeleton, which may differ according to sex, age, and race.9–12 In HF specifically, there is also the contribution of retained fluid to body weight. Consequently, alternative anthropometric indices have been proposed such as waist circumference, waist-to-hip ratio, and weight-adjusted-weight index, which may better reflect intra-abdominal fat (‘central obesity’) and body shape index, body roundness index, and relative fat mass, which have been suggested to better reflect the distribution of body fat and total fat mass.13–17 Recently, some of these were found to be better predictors of incident HF in the general population than BMI,18–20 but they are not commonly measured in clinical practice.
Waist-to-height ratio is of particular interest as it should, to some extent, take account of sex- and race-based differences in stature and the distribution of body fat, and the National Institute for Health and Care Excellence in the United Kingdom has recently suggested that waist-to-height ratio should replace BMI in the evaluation of adiposity.21,22
To complicate matters further, the association between BMI (and potentially any other anthropometric index) and outcome in patients with HFrEF is also confounded by the relationship between adiposity and natriuretic peptide levels. Higher BMI is associated with lower natriuretic peptide levels, possibly due to increased clearance or potentially because patients with obesity present with symptoms at an earlier stage in the development of HF.23,24 Although natriuretic peptide level is one of the most powerful prognostic variables in HFrEF,25 few analyses of the association between BMI (or other anthropometric indices) and outcome have accounted for this.4–8,26–34 Therefore, we have examined the newer anthropometric indices described above in patients with HFrEF, focusing on their prognostic value and whether an ‘obesity-survival paradox’ is observed, as has been reported for BMI. We have also adjusted all analyses for natriuretic peptide levels. We carried out these analyses in a global population enrolled in the prospective comparison of angiotensin receptor-neprilysin inhibitor with angiotensin-converting enzyme inhibitor (ACE-i) to determine impact on global mortality and morbidity in heart failure trial (prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure [PARADIGM-HF]) which included 1832 women and 6567 men with HFrEF enrolled in 47 countries on six continents.35
Methods
PARADIGM-HF was a randomized, double-blind, placebo-controlled trial in patients with chronic HFrEF, evaluating the efficacy and safety of the angiotensin receptor-neprilysin inhibitor sacubitril/valsartan compared with enalapril, added to standard care. The design and primary results of PARADIGM-HF have been reported previously.35,36 The institutional review boards of all participating institutions approved the protocol, and all patients gave written informed consent.
Patients and study procedures
Key inclusion criteria were age ≥18 years, New York Heart Association (NYHA) functional class II-IV, left ventricular ejection fraction (LVEF) of ≤35% (changed from ≤40% by a protocol amendment), elevated natriuretic peptide levels, and treatment with a stable dose of an ACE-i or angiotensin receptor blocker (ARB) equivalent to enalapril 10 mg/day for at least 4 weeks before the screening visit. Key exclusion criteria were symptomatic hypotension or systolic blood pressure <95 mmHg at randomization (100 mmHg at screening), estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 at randomization (or screening), potassium >5.4 mmol/L at randomization (>5.2 mmol/L at screening), a history of angioedema, and intolerance to ACE-i or ARB.36
On trial entry, ongoing therapy with ACE-i or ARB was stopped, and patients received enalapril 10 mg twice daily for 2 weeks followed by sacubitril/valsartan, up-titrated from 100 mg twice daily to 200 mg twice daily, for additional 4–6 weeks. Patients tolerating both drugs at the target doses were then randomly assigned to double-blind therapy with sacubitril/valsartan or enalapril in a 1:1 ratio.36
Anthropometric measures
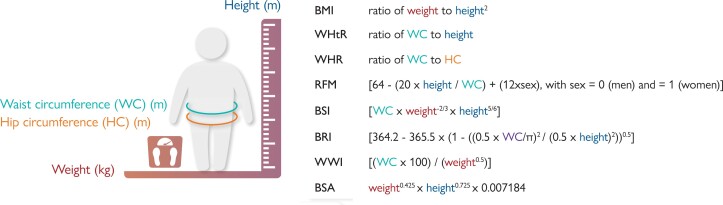
Data on anthropometric measures were obtained at the randomization visit. The calculation of each of these measures is described in Figure 1.
Figure 1.
Calculation of anthropometric measures. This figure describes the calculation of each of the anthropometric measures. BMI, body mass index; BRI, body roundness index; BSA, body surface area; BSI, body shape index; RFM, relative fat mass; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio; WWI, weight-adjusted-waist index.
In the analyses using BMI, patients were divided according to the World Health Organization (WHO) BMI categories, i.e. underweight (<18.5 kg/m2); normal weight (18.5–24.9 kg/m2); overweight (25.0–29.9 kg/m2); obesity class I (30.0–34.9 kg/m2); obesity class II (35.0–39.9 kg/m2) and obesity class III (≥40 kg/m2). The choice of waist-to-height ratio as the exemplar anthropometric index that does not include weight was prespecified as it is easiest to calculate. We analyzed this index by quintile as the lowest quintile identified patients with a waist-to-height ratio in the range 0.150–0.520, the upper end of which approximates the <0.5 regarded as healthy.
Outcomes
The primary outcome in PARADIGM-HF was the composite of HF hospitalization or cardiovascular death. In the present analysis, we also examined each of the components of the primary outcome, non-cardiovascular death, and death from any cause.
Statistical analyses
Baseline characteristics were summarized as frequencies with percentages, means with standard deviation (SD), or medians with interquartile ranges. Differences in baseline characteristics were tested using the Cochran–Armitage trend test for binary variables, the Cochran–Mantel–Haenszel test for categorical variables, and the Jonckheere–Terpstra test and linear regression for non-normal and normally distributed continuous variables, respectively.
Time-to-event data, regardless of treatment allocation, were evaluated using Cox proportional-hazards models, adjusted for treatment-group assignment and geographic region, and hazard ratios (HR) with 95% confidence intervals (CIs) were reported. In addition, HRs adjusted for treatment-group assignment, age, sex, race, geographic region, systolic blood pressure, heart rate, eGFR, LVEF, BMI (not included when analyzing outcomes according to BMI), log of n-terminal pro-B-type natriuretic peptide (NT-proBNP), NYHA functional class, HF aetiology, duration of HF, prior HF hospitalization, and a history of diabetes and atrial fibrillation were reported. The relationship between anthropometric measurements as continuous variables and the risk of outcomes was also examined in restricted cubic spline analyses (with the median value as reference).
To compare the effects of sacubitril/valsartan vs. enalapril, time-to-event data were evaluated with Cox proportional-hazards models, with geographical region and treatment-group assignment as fixed-effect factors.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and STATA version 17.0 (College Station, TX). A P-value of .05 was considered statistically significant.
Results
Of the 8399 patients randomized in PARADIGM-HF, data on BMI were available in 99.9% (as were data on body surface area), and data on waist-to-height ratio were available in 98.6% (as were data on waist circumference, relative fat mass, body roundness index, body shape index, and weight-adjusted-weight index although our focus was on waist-to-height ratio). Waist-to-hip ratio was available in 97.7% of randomized patients. Median duration of follow-up was 27 months (25th-75th percentile, 19–36 months).
Patient characteristics
Body mass index
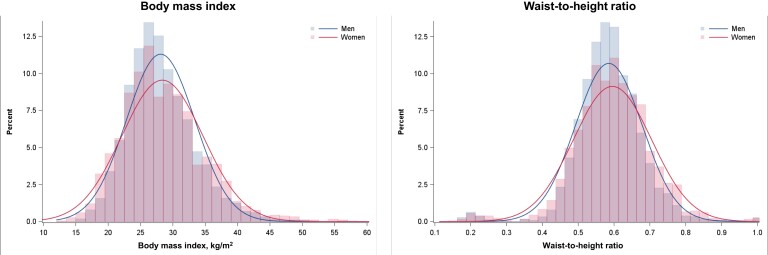
Median BMI was 27.5 kg/m2 (25th-75th percentile, 24.5–31.0 kg/m2) and 27.6 kg/m2 (25th-75th percentile, 24.0–32.0 kg/m2) in men and women, respectively (Figure 2A). In total, 153 patients had a BMI <18.5 kg/m2; 2268 patients between 18.5–24.9 kg/m2; 3249 patients between 25–29.9 kg/m2; 1810 patients between 30–34.9 kg/m2; and 909 patients ≥35 kg/m2. Detailed baseline characteristics according to these BMI categories are presented in Table 1a. Compared with patients with normal weight, those with higher BMI were younger, more often female, and white. Patients with higher BMI had more comorbidity, longer duration HF, more prior hospitalization for HF, and worse NYHA functional class, and Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. However, they had higher LVEF and were more likely to have a non-ischemic aetiology (although patients with a BMI <18.5 kg/m2 were more likely than the other BMI categories to have a non-ischemic aetiology). Patients with higher BMI had lower natriuretic peptide levels (irrespective of the presence of atrial fibrillation) but a higher urinary cGMP/BNP ratio (as a marker of tissue responsiveness to natriuretic peptides). Patients with higher BMI had higher aldosterone, hemoglobin, uric acid, and blood urea nitrogen, levels, as well as neutrophil/lymphocyte ratio, compared with patients with normal weight, but a lower eGFR (Table 1a).
Figure 2.
Distribution of body mass index and waist-to-height-ratio according to sex. This figure shows the frequency distribution curves of body mass index and waist-to-height ratio, respectively, according to sex. The red line and bars represent women, and the blue line and bars represent men.
Table 1a.
Baseline characteristics according to body mass index
| Underweight | Normal weight | Overweight | Obesity class I | Obesity class II/III | P-value | |
|---|---|---|---|---|---|---|
| BMI <18.5 | BMI 18.5–24.9 | BMI 25–29.9 | BMI 30–34.9 | BMI ≥35 | ||
| n = 153 | n = 2268 | n = 3249 | n = 1810 | n = 909 | ||
| Age (years), mean (SD) | 60.9 ± 13.9 | 64.5 ± 12.2 | 64.6 ± 11.1 | 63.4 ± 10.7 | 60.3 ± 10.4 | <.001 |
| Male sex, n (%) | 89 (58.2) | 1776 (78.3) | 2635 (81.1) | 1417 (78.3) | 643 (70.7) | .04 |
| Geographic region, n (%) | <.001 | |||||
| ȃNorth America | 3 (2.0) | 98 (4.3) | 208 (6.4) | 158 (8.7) | 135 (14.9) | |
| ȃLatin America | 24 (15.7) | 420 (18.5) | 601 (18.5) | 295 (16.3) | 91 (10.0) | |
| ȃWestern Europe and other | 16 (10.5) | 427 (18.8) | 875 (26.9) | 489 (27.0) | 238 (26.2) | |
| ȃCentral Europe | 12 (7.8) | 541 (23.9) | 1090 (33.5) | 755 (41.7) | 428 (47.1) | |
| ȃAsia-Pacific | 98 (64.1) | 782 (34.5) | 475 (14.6) | 113 (6.2) | 17 (1.9) | |
| Race, n (%) | <.001 | |||||
| ȃWhite | 30 (19.6) | 1092 (48.1) | 2238 (68.9) | 1419 (78.4) | 758 (83.4) | |
| ȃBlack | 8 (5.2) | 134 (5.9) | 127 (3.9) | 83 (4.6) | 76 (8.4) | |
| ȃAsian | 99 (64.7) | 785 (34.6) | 488 (15.0) | 115 (6.4) | 20 (2.2) | |
| ȃOther | 16 (10.5) | 257 (11.3) | 396 (12.2) | 193 (10.7) | 55 (6.1) | |
| Physiological measures, median (IQR) or mean (SD) | ||||||
| ȃSystolic blood pressure (mmHg) | 118.4 ± 15.5 | 118.7 ± 14.8 | 121.4 ± 15.0 | 123.4 ± 15.6 | 124.7 ± 15.8 | <.001 |
| ȃHeart rate (bpm) | 76.1 ± 11.3 | 71.8 ± 11.6 | 71.8 ± 11.9 | 72.8 ± 12.4 | 74.4 ± 12.6 | <.001 |
| ȃBody mass index | 17.5 (16.9–18.0) | 23.0 (21.5–24.1) | 27.2 (26.1–28.5) | 31.9 (30.8–33.1) | 37.7 (36.1–40.4) | <.001 |
| ȃWaist-to-height ratio | 0.46 (0.42–0.49) | 0.52 (0.48–0.55) | 0.58 (0.55–0.61) | 0.64 (0.61–0.67) | 0.71 (0.67–0.76) | <.001 |
| ȃWaist circumference | 75 (68–81) | 87 (81–93) | 99 (92–104) | 109 (103–115) | 121 (113–130) | <.001 |
| ȃWaist-to-hip ratio | 0.91 (0.84–0.94) | 0.93 (0.88–0.98) | 0.96 (0.92–1.02) | 0.99 (0.94–1.05) | 1.00 (0.94–1.06) | <.001 |
| ȃRelative fat mass | 23.8 (18.9–31.4) | 26.7 (23.6–30.0) | 30.3 (28.1–33.1) | 33.4 (31.7–36.1) | 37.2 (35.0–44.7) | <.001 |
| ȃWeight-adjusted-waist index | 11.0 (10.1–12.0) | 11.0 (10.3–11.5) | 11.1 (10.6–11.7) | 11.3 (10.8–11.9) | 11.5 (10.9–12.1) | <.001 |
| ȃBody shape index | 0.088 (0.080–0.095) | 0.084 (0.080–0.088) | 0.084 (0.080–0.087) | 0.083 (0.079–0.087) | 0.082 (0.078–0.086) | <.001 |
| ȃBody roundness index | 2.6 (1.9–3.3) | 3.8 (3.1–4.4) | 5.0 (4.3–5.7) | 6.4 (5.7–7.2) | 8.2 (7.1–9.5) | <.001 |
| Current smoker, n (%) | 26 (17.0) | 380 (16.8) | 455 (14.0) | 231 (12.8) | 116 (12.8) | <.001 |
| Ischemic cause of HF, n (%) | 69 (45.1) | 1371 (60.4) | 2006 (61.7) | 1079 (59.6) | 504 (55.4) | .22 |
| Duration of HF, n (%) | <.001 | |||||
| ȃ<=1 year | 87 (56.9) | 814 (35.9) | 929 (28.6) | 475 (26.2) | 215 (23.7) | |
| ȃ1–5 years | 53 (34.6) | 878 (38.7) | 1270 (39.1) | 667 (36.9) | 361 (39.7) | |
| ȃ> 5 years | 13 (8.5) | 576 (25.4) | 1050 (32.3) | 668 (36.9) | 333 (36.6) | |
| LVEF, mean (SD) | 26.5 ± 6.4 | 28.8 ± 6.3 | 29.5 ± 6.1 | 30.0 ± 6.1 | 30.5 ± 6.1 | <.001 |
| NYHA class at randomization, n (%) | <.001 | |||||
| ȃI | 11 (7.2) | 130 (5.7) | 150 (4.6) | 72 (4.0) | 25 (2.8) | |
| ȃII | 122 (79.7) | 1667 (73.6) | 2325 (71.7) | 1256 (69.5) | 541 (59.6) | |
| ȃIII | 20 (13.1) | 455 (20.1) | 745 (23.0) | 462 (25.6) | 335 (36.9) | |
| ȃIV | 0 (0.0) | 14 (0.6) | 24 (0.7) | 16 (0.9) | 6 (0.7) | |
| KCCQ-OSS, mean (SD) | 74.7 ± 18.6 | 75.0 ± 18.5 | 74.6 ± 18.6 | 71.2 ± 20.3 | 65.5 ± 21.1 | <.001 |
| KCCQ-CSS, mean (SD) | 79.6 ± 17.1 | 78.9 ± 18.1 | 77.5 ± 18.4 | 73.8 ± 20.2 | 67.8 ± 20.8 | <.001 |
| Medical history, n (%) | ||||||
| ȃHospitalization for HF | 85 (55.6) | 1343 (59.2) | 2053 (63.2) | 1165 (64.4) | 623 (68.5) | <.001 |
| ȃHypertension | 65 (42.5) | 1360 (60.0) | 2290 (70.5) | 1427 (78.8) | 792 (87.1) | <.001 |
| ȃDiabetes | 23 (15.0) | 579 (25.5) | 1083 (33.3) | 747 (41.3) | 471 (51.8) | <.001 |
| ȃHistory of atrial fibrillation | 17 (11.1) | 640 (28.2) | 1191 (36.7) | 779 (43.0) | 459 (50.5) | <.001 |
| ȃAtrial fibrillation on electrocardiogram | 10 (6.6) | 398 (17.9) | 784 (24.6) | 524 (29.2) | 318 (35.5) | <.001 |
| ȃPrevious myocardial infarction | 30 (19.6) | 934 (41.2) | 1525 (46.9) | 785 (43.4) | 355 (39.1) | .32 |
| ȃPrevious stroke | 8 (5.2) | 189 (8.3) | 279 (8.6) | 154 (8.5) | 95 (10.5) | .06 |
| ȃChronic obstructive pulmonary disease | 22 (14.4) | 275 (12.1) | 389 (12.0) | 240 (13.3) | 154 (16.9) | .002 |
| ȃCancer | 6 (3.9) | 91 (4.0) | 175 (5.4) | 91 (5.0) | 49 (5.4) | .08 |
| Treatment, n (%) | ||||||
| ȃBeta-blocker | 134 (87.6) | 2076 (91.5) | 3006 (92.5) | 1720 (95.0) | 865 (95.2) | <.001 |
| ȃMineralocorticoid-receptor antagonist | 84 (54.9) | 1244 (54.9) | 1786 (55.0) | 1030 (56.9) | 522 (57.4) | .09 |
| ȃDiuretic | 123 (80.4) | 1720 (75.8) | 2564 (78.9) | 1518 (83.9) | 804 (88.4) | <.001 |
| ȃDigitalis | 62 (40.5) | 817 (36.0) | 906 (27.9) | 476 (26.3) | 274 (30.1) | <.001 |
| ȃAntiplatelet | 87 (56.9) | 1323 (58.3) | 1902 (58.5) | 961 (53.1) | 459 (50.5) | <.001 |
| ȃOral anticoagulant | 11 (7.2) | 536 (23.6) | 1028 (31.6) | 706 (39.0) | 400 (44.0) | <.001 |
| ȃStatin | 67 (43.8) | 1142 (50.4) | 1907 (58.7) | 1079 (59.6) | 525 (57.8) | <.001 |
| ȃImplantable cardioverter-defibrillator | 5 (3.3) | 225 (9.9) | 519 (16.0) | 329 (18.2) | 163 (17.9) | <.001 |
| ȃCardiac resynchronization therapy | 1 (0.7) | 129 (5.7) | 245 (7.5) | 122 (6.7) | 75 (8.3) | .002 |
| Biomarkers, median (IQR) or mean (SD) a | ||||||
| NT-proBNP (pg/mL) | ||||||
| ȃNo atrial fibrillation on electrocardiogram | 2510 (1225–6807) | 1964 (991–4371) | 1450 (831–2836) | 1229 (728–2196) | 1072 (672–1938) | <.001 |
| ȃAtrial fibrillation on electrocardiogram | 3515 (1605–10835) | 2893 (1632–5715) | 2102 (1203–4053) | 1748 (1113–3231) | 1508 (876–2468) | <.001 |
| BNP (pg/mL) | ||||||
| ȃNo atrial fibrillation on electrocardiogram | 428 (159–763) | 309 (167–633) | 254 (151–448) | 223 (141–391) | 208 (132–346) | <.001 |
| ȃAtrial fibrillation on electrocardiogram | 578 (215–1167) | 334 (195–664) | 264 (173–471) | 221 (146–396) | 194 (143–290) | <.001 |
| ucGMP/BNP ratio (nmol/pg) | ||||||
| ȃNo atrial fibrillation on electrocardiogram | 1.5 (1.0–3.5) | 3.2 (1.6–6.7) | 4.6 (2.6–7.9) | 4.8 (2.6–7.6) | 5.2 (2.7–10.2) | <.001 |
| ȃAtrial fibrillation on electrocardiogram | 2.8 (0.9–3.4) | 5.1 (2.9–8.4) | 5.1 (2.6–10.0) | 5.7 (3.6–9.0) | 7.5 (4.1–11.1) | .001 |
| Aldosterone (pmol/L) | ||||||
| ȃNo mineralocorticoid-receptor antagonist | 246 (179–566) | 180 (126–269) | 212 (138–289) | 221 (136–327) | 260 (165–355) | <.001 |
| ȃMineralocorticoid-receptor antagonist | 322 (98–430) | 262 (155–394) | 261 (158–466) | 327 (190–535) | 325 (196–597) | .001 |
| Troponin T (ng/L) | 19 (8–26) | 15 (10–24) | 15 (10–23) | 15 (10–23) | 14 (9–22) | .59 |
| ST2 (ng/mL) | 22 (18–37) | 33 (26–43) | 32 (25–41) | 33 (27–42) | 32 (25–40) | .90 |
| MMP-9 (ng/mL) | 119 (53–173) | 58 (35–121) | 63 (38–119) | 64 (41–126) | 78 (44–161) | .005 |
| MMP-2 (ng/mL) | 147 (133–173) | 133 (114–157) | 134 (115–154) | 135 (118–159) | 137 (119–158) | .13 |
| TIMP1 (ng/mL) | 158 (137–199) | 127 (104–158) | 122 (104–149) | 123 (105–148) | 129 (106–153) | .96 |
| Galectin 3 (ng/mL) | 24 (21–30) | 17 (14–23) | 17 (14–21) | 17 (14–21) | 18 (15–22) | .11 |
| GDF-15 (ng/L) | 2238 (1983–4798) | 1805 (1176–2536) | 1590 (1153–2298) | 1695 (1219–2466) | 1598 (1074–2404) | .20 |
| Hemoglobin (g/L) | 128 (119–139) | 135 (125–146) | 140 (130–151) | 143 (132–153) | 143 (132–154) | <.001 |
| Hemoglobin A1c (%) | 6.0 (5.7–6.4) | 6.1 (5.7–6.6) | 6.2 (5.8–6.8) | 6.3 (5.9–7.0) | 6.5 (6.0–7.5) | <.001 |
| White blood cell count (109/L) | 6.4 (5.6–8.1) | 6.5 (5.4–7.8) | 6.7 (5.7–8.0) | 6.8 (5.8–8.1) | 7.2 (6.0–8.6) | <.001 |
| Neutrophil count (109/L) | 3.9 (3.1–5.1) | 4.0 (3.1–5.0) | 4.2 (3.3–5.2) | 4.3 (3.5–5.3) | 4.5 (3.6–5.6) | <.001 |
| Neutrophil/lymphocyte ratio | 2.1 (1.6–3.1) | 2.3 (1.7–3.1) | 2.4 (1.7–3.2) | 2.4 (1.8–3.2) | 2.4 (1.8–3.4) | <.001 |
| Monocyte count (109/L) | 0.4 (0.3–0.5) | 0.4 (0.3–0.6) | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | <.001 |
| Lymphocyte count (109/L) | 1.8 (1.4–2.2) | 1.7 (1.3–2.2) | 1.8 (1.4–2.2) | 1.8 (1.4–2.2) | 1.8 (1.5–2.3) | <.001 |
| eGFR (mL/min/1.73 m2) | 69 (56–86) | 68 (55–82) | 66 (54–79) | 65 (53–78) | 66 (53–79) | <.001 |
| Creatinine (μmol/L) | 87 (74–101) | 93 (80–111) | 96 (82–114) | 96 (82–116) | 96 (80–114) | <.001 |
| Blood urea nitrogen (mmol/L) | 5.4 (3.9–8.0) | 6.4 (5.0–8.6) | 6.8 (5.4–8.9) | 6.8 (5.7–8.6) | 6.8 (5.4–8.9) | <.001 |
| Cystatin (mg/L) | 1.4 (1.2–1.7) | 1.2 (1.0–1.5) | 1.2 (1.0–1.4) | 1.1 (1.0–1.4) | 1.2 (1.0–1.4) | .40 |
| Urine albumin-creatinine ratio (mg/mmol) | ||||||
| ȃNo diabetes | 3.9 (0.9–8.1) | 0.8 (0.2–2.3) | 0.9 (0.3–2.6) | 0.8 (0.2–2.8) | 1.1 (0.3–3.2) | .22 |
| ȃDiabetes | 4.3 (2.7–5.8) | 1.5 (0.5–6.1) | 1.7 (0.6–7.1) | 2.1 (0.7–6.3) | 1.9 (0.7–8.1) | .17 |
| KIM-1 (pg/mL) | 260 (165–318) | 124 (84–185) | 120 (81–179) | 134 (92–201) | 149 (99–220) | <.001 |
| Uric acid (μmol/L) | 357 (297–434) | 381 (321–464) | 399 (327–482) | 404 (339–488) | 428 (351–512) | <.001 |
| ALT (IU/L) | 16 (11–21) | 17 (13–22) | 18 (14–24) | 19 (14–25) | 19 (15–25) | <.001 |
| AST (IU/L) | 23 (19–27) | 21 (18–26) | 21 (17–25) | 20 (17–25) | 20 (17–24) | <.001 |
| Alkaline phosphatase (IU/L) | 79 (65–97) | 74 (60–92) | 71 (58–88) | 70 (57–87) | 71 (58–88) | <.001 |
| Total bilirubin (μmol/L) | 9 (7–14) | 9 (7–14) | 9 (7–14) | 9 (7–12) | 9 (7–12) | <.001 |
| Albumin (g/L) | 42 (39–44) | 43 (41–45) | 43 (41–45) | 43 (41–45) | 42 (40–44) | .44 |
BNP, B-type natriuretic peptide; CSS, clinical summary score; eGFR, estimated glomerular filtration rate; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; NT-proBNP, n-terminal pro-B-type natriuretic peptide; OSS, overall summary score; SD, standard deviation; ucGMP, urinary cyclic guanosine monophosphate.
Randomization visit, except ST2, MMP2, MMP9, TIMP1, GDF-15, and KIM-1, which were collected at the screening visit. Number of patients with missing biomarkers are: NT-proBNP, n = 14; BNP, n = 59; ucGMP/BNP ratio, n = 6375; aldosterone, n = 6381; troponin T, n = 6345; ST2, n = 6803; MMP-9, n = 6999; MMP-2, n = 6998; TIMP1, n = 6983; galectin 3, n = 6354; GDF-15, n = 6850; Hemoglobin, n = 274; hemoglobin A1c, n = 180; white blood cell count, n = 294; neutrophil count, n = 421; neutrophil/lymphocyte ratio, n = 421; monocyte count, n = 421; lymphocyte count, n = 421; eGFR, n = 0; creatinine, n = 0; blood urea nitrogen, n = 161; Cystatin, n = 6343; urine albumin-creatinine ratio, n = 6416; KIM-1, n = 6846; uric acid, n = 186; ALT, n = 219; AST, n = 236; alkaline phosphatase, n = 199; total bilirubin, n = 199; albumin, n = 179.
Waist-to-height ratio
Median waist-to-height ratio was 0.58 (25th-75th percentile, 0.54–0.64) and 0.59 (25th-75th percentile, 0.53–0.66) in men and women, respectively (Figure 2B). Baseline characteristics of patients according to quintiles of waist-to-height ratio are presented in Table 1b. Overall, the differences were similar to those described above and in Table 1a.
Table 1b.
Baseline characteristics according to quintile of waist-to-height ratio
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-value | |
|---|---|---|---|---|---|---|
| n = 1635 | n = 1659 | n = 1658 | n = 1655 | n = 1674 | ||
| Age (years), mean (SD) | 62.3 ± 12.9 | 64.1 ± 11.3 | 64.3 ± 11.2 | 64.9 ± 10.7 | 63.4 ± 10.7 | .001 |
| Male sex, n (%) | 1250 (76.5) | 1346 (81.1) | 1370 (82.6) | 1314 (79.4) | 1193 (71.3) | <.001 |
| Geographic region, n (%) | <.001 | |||||
| ȃNorth America | 103 (6.3) | 94 (5.7) | 115 (6.9) | 119 (7.2) | 161 (9.6) | |
| ȃLatin America | 239 (14.6) | 285 (17.2) | 304 (18.3) | 305 (18.4) | 275 (16.4) | |
| ȃWestern Europe and other | 243 (14.9) | 386 (23.3) | 415 (25.0) | 458 (27.7) | 479 (28.6) | |
| ȃCentral Europe | 446 (27.3) | 507 (30.6) | 572 (34.5) | 616 (37.2) | 679 (40.6) | |
| ȃAsia-Pacific | 604 (36.9) | 387 (23.3) | 252 (15.2) | 157 (9.5) | 80 (4.8) | |
| Race, n (%) | <.001 | |||||
| ȃWhite | 769 (47.0) | 1006 (60.6) | 1108 (66.8) | 1232 (74.4) | 1332 (79.6) | |
| ȃBlack | 118 (7.2) | 87 (5.2) | 64 (3.9) | 70 (4.2) | 82 (4.9) | |
| ȃAsian | 606 (37.1) | 389 (23.4) | 261 (15.7) | 161 (9.7) | 85 (5.1) | |
| ȃOther | 142 (8.7) | 177 (10.7) | 225 (13.6) | 192 (11.6) | 175 (10.5) | |
| Physiological measures, median (IQR) or mean (SD) | ||||||
| ȃSystolic blood pressure (mmHg) | 118.7 ± 14.5 | 119.5 ± 14.8 | 121.8 ± 15.2 | 122.5 ± 15.2 | 124.3 ± 16.0 | <.001 |
| ȃHeart rate (bpm) | 72.3 ± 11.6 | 71.8 ± 12.1 | 71.9 ± 11.6 | 72.1 ± 12.3 | 73.8 ± 12.3 | <.001 |
| ȃBody mass index | 22.7 (20.6–24.7) | 25.2 (23.6–26.9) | 27.4 (25.6–29.4) | 30.0 (27.9–32.1) | 34.4 (31.2–37.7) | <.001 |
| ȃWaist-to-height ratio | 0.48 (0.46–0.51) | 0.55 (0.53–0.56) | 0.59 (0.58–0.59) | 0.63 (0.62–0.64) | 0.70 (0.67–0.74) | <.001 |
| ȃWaist circumference | 81 (75–86) | 92 (89–96) | 99 (96–103) | 107 (102–110) | 118 (112–125) | <.001 |
| ȃWaist-to-hip ratio | 0.90 (0.85–0.95) | 0.94 (0.90–0.98) | 0.97 (0.93–1.01) | 0.99 (0.95–1.04) | 1.02 (0.97–1.08) | <.001 |
| ȃRelative fat mass | 23.8 (21.1–25.4) | 27.7 (26.7–28.5) | 30.0 (29.4–30.7) | 32.5 (31.7–33.3) | 36.4 (34.6–46.0) | <.001 |
| ȃWeight-adjusted-waist index | 10.1 (9.6–10.6) | 10.8 (10.5–11.2) | 11.2 (10.8–11.6) | 11.5 (11.1–11.9) | 12.0 (11.6–12.5) | <.001 |
| ȃBody shape index | 0.078 (0.073–0.083) | 0.083 (0.079–0.086) | 0.084 (0.081–0.087) | 0.085 (0.081–0.088) | 0.086 (0.083–0.090) | <.001 |
| ȃBody roundness index | 3.1 (2.6–3.5) | 4.3 (4.0–4.5) | 5.1 (4.9–5.3) | 6.1 (5.8–6.4) | 7.8 (7.2–8.9) | <.001 |
| Current smoker, n (%) | 292 (17.9) | 254 (15.3) | 240 (14.5) | 196 (11.8) | 208 (12.4) | <.001 |
| Ischemic cause of HF, n (%) | 906 (55.4) | 1000 (60.3) | 1036 (62.5) | 1039 (62.8) | 984 (58.8) | .02 |
| Duration of HF, n (%) | <.001 | |||||
| ȃ<=1 year | 624 (38.2) | 528 (31.8) | 470 (28.3) | 430 (26.0) | 436 (26.0) | |
| ȃ1–5 years | 613 (37.5) | 656 (39.5) | 647 (39.0) | 656 (39.6) | 623 (37.2) | |
| ȃ> 5 years | 398 (24.3) | 475 (28.6) | 541 (32.6) | 569 (34.4) | 615 (36.7) | |
| LVEF, mean (SD) | 28.4 ± 6.2 | 29.2 ± 6.4 | 29.5 ± 6.2 | 30.0 ± 6.1 | 30.4 ± 6.1 | <.001 |
| NYHA class at randomization, n (%) | <.001 | |||||
| ȃI | 85 (5.2) | 104 (6.3) | 70 (4.2) | 63 (3.8) | 55 (3.3) | |
| ȃII | 1172 (71.8) | 1188 (71.7) | 1185 (71.5) | 1210 (73.3) | 1080 (64.6) | |
| ȃIII | 366 (22.4) | 356 (21.5) | 396 (23.9) | 364 (22.0) | 517 (30.9) | |
| ȃIV | 10 (0.6) | 10 (0.6) | 6 (0.4) | 14 (0.8) | 20 (1.2) | |
| KCCQ-OSS, mean (SD) | 74.3 ± 18.6 | 75.2 ± 18.0 | 74.6 ± 18.9 | 73.4 ± 19.1 | 67.4 ± 21.1 | <.001 |
| KCCQ-CSS, mean (SD) | 78.5 ± 18.3 | 78.5 ± 17.9 | 77.6 ± 18.4 | 75.9 ± 19.1 | 69.7 ± 21.0 | <.001 |
| Medical history, n (%) | ||||||
| ȃHospitalization for HF | 991 (60.6) | 995 (60.0) | 1054 (63.6) | 1045 (63.1) | 1121 (67.0) | <.001 |
| ȃHypertension | 935 (57.2) | 1087 (65.5) | 1164 (70.2) | 1297 (78.4) | 1382 (82.6) | <.001 |
| ȃDiabetes | 362 (22.1) | 498 (30.0) | 551 (33.2) | 640 (38.7) | 815 (48.7) | <.001 |
| ȃHistory of atrial fibrillation | 472 (28.9) | 561 (33.8) | 591 (35.6) | 668 (40.4) | 761 (45.5) | <.001 |
| ȃAtrial fibrillation on electrocardiogram | 304 (19.0) | 385 (23.5) | 370 (22.8) | 440 (26.8) | 513 (31.0) | <.001 |
| ȃPrevious myocardial infarction | 625 (38.2) | 715 (43.1) | 763 (46.0) | 785 (47.4) | 698 (41.7) | .004 |
| ȃPrevious stroke | 143 (8.7) | 141 (8.5) | 148 (8.9) | 123 (7.4) | 166 (9.9) | .55 |
| ȃChronic obstructive pulmonary disease | 193 (11.8) | 180 (10.8) | 209 (12.6) | 221 (13.4) | 264 (15.8) | <.001 |
| ȃCancer | 64 (3.9) | 82 (4.9) | 82 (4.9) | 87 (5.3) | 91 (5.4) | .046 |
| Treatment, n (%) | ||||||
| ȃBeta-blocker | 1497 (91.6) | 1526 (92.0) | 1559 (94.0) | 1539 (93.0) | 1586 (94.7) | <.001 |
| ȃMineralocorticoid-receptor antagonist | 938 (57.4) | 914 (55.1) | 911 (54.9) | 924 (55.8) | 929 (55.5) | .44 |
| ȃDiuretic | 1247 (76.3) | 1291 (77.8) | 1304 (78.6) | 1349 (81.5) | 1458 (87.1) | <.001 |
| ȃDigitalis | 635 (38.8) | 561 (33.8) | 502 (30.3) | 409 (24.7) | 407 (24.3) | <.001 |
| ȃAntiplatelet | 906 (55.4) | 933 (56.2) | 995 (60.0) | 933 (56.4) | 908 (54.2) | .55 |
| ȃOral anticoagulant | 380 (23.2) | 511 (30.8) | 509 (30.7) | 588 (35.5) | 651 (38.9) | <.001 |
| ȃStatin | 740 (45.3) | 903 (54.4) | 1019 (61.5) | 1031 (62.3) | 966 (57.7) | <.001 |
| ȃImplantable cardioverter-defibrillator | 160 (9.8) | 237 (14.3) | 241 (14.5) | 285 (17.2) | 286 (17.1) | <.001 |
| ȃCardiac resynchronization therapy | 99 (6.1) | 107 (6.4) | 109 (6.6) | 107 (6.5) | 136 (8.1) | .03 |
| Biomarkers, median (IQR) or mean (SD)a | ||||||
| NT-proBNP (pg/mL) | ||||||
| ȃNo atrial fibrillation on electrocardiogram | 1934 (972–4360) | 1644 (898–3546) | 1481 (838–3003) | 1306 (744–2425) | 1213 (727–2212) | <.001 |
| ȃAtrial fibrillation on electrocardiogram | 2883 (1480–5583) | 2109 (1331–4093) | 2212 (1189–4145) | 1803 (1107–3346) | 1672 (1034–2859) | <.001 |
| BNP (pg/mL) | ||||||
| ȃNo atrial fibrillation on electrocardiogram | 299 (160–637) | 287 (167–548) | 251 (155–465) | 230 (143–392) | 219 (136–390) | <.001 |
| ȃAtrial fibrillation on electrocardiogram | 329 (186–665) | 277 (172–488) | 262 (170–446) | 230 (156–412) | 215 (143–354) | <.001 |
| ucGMP/BNP ratio (nmol/pg) | ||||||
| ȃNo atrial fibrillation on electrocardiogram | 3.4 (1.6–7.1) | 4.2 (2.5–8.0) | 4.7 (2.5–7.9) | 4.6 (2.4–8.2) | 4.6 (2.5–7.7) | .02 |
| ȃAtrial fibrillation on electrocardiogram | 3.8 (1.5–6.6) | 6.7 (3.2–10.2) | 5.6 (2.8–10.0) | 5.9 (2.8–9.8) | 6.3 (3.8–10.5) | .02 |
| Aldosterone (pmol/L) | ||||||
| ȃNo mineralocorticoid-receptor antagonist | 208 (125–327) | 199 (135–283) | 190 (131–275) | 228 (141–322) | 231 (161–347) | .003 |
| ȃMineralocorticoid-receptor antagonist | 305 (171–522) | 279 (156–458) | 250 (178–483) | 294 (164–469) | 330 (208–558) | .04 |
| Troponin T (ng/L) | 16 (9–25) | 13 (9–22) | 15 (10–23) | 14 (10–22) | 15 (10–25) | .32 |
| ST2 (ng/mL) | 32 (24–43) | 32 (27–42) | 32 (25–41) | 32 (26–42) | 32 (25–41) | .72 |
| MMP-9 (ng/mL) | 56 (35–107) | 62 (39–123) | 63 (39–117) | 59 (38–132) | 75 (42–139) | .008 |
| MMP-2 (ng/mL) | 137 (115–162) | 132 (113–151) | 134 (117–152) | 133 (115–157) | 136 (118–159) | .22 |
| TIMP1 (ng/mL) | 130 (104–161) | 122 (102–149) | 122 (103–146) | 122 (104–150) | 128 (107–151) | .27 |
| Galectin 3 (ng/mL) | 17 (14–22) | 17 (14–22) | 17 (14–21) | 17 (14–21) | 18 (15–22) | .007 |
| GDF-15 (ng/L) | 1780 (1215–2434) | 1620 (1130–2373) | 1517 (1104–2246) | 1622 (1200–2304) | 1763 (1222–2573) | .14 |
| Hemoglobin (g/L) | 135 (125–147) | 139 (129–150) | 141 (130–151) | 141 (130–152) | 141 (130–152) | <.001 |
| Hemoglobin A1c (%) | 6.0 (5.7–6.5) | 6.1 (5.7–6.7) | 6.2 (5.8–6.8) | 6.2 (5.8–6.9) | 6.4 (5.9–7.4) | <.001 |
| White blood cell count (10^9/L) | 6.5 (5.3–7.8) | 6.6 (5.4–7.8) | 6.8 (5.7–8.1) | 6.8 (5.7–8.1) | 7.1 (6.0–8.4) | <.001 |
| Neutrophil count (10^9/L) | 3.9 (3.1–5.0) | 4.1 (3.2–5.0) | 4.2 (3.4–5.2) | 4.3 (3.4–5.3) | 4.5 (3.6–5.5) | <.001 |
| Neutrophil/lymphocyte ratio | 2.2 (1.6–3.1) | 2.4 (1.7–3.3) | 2.4 (1.8–3.1) | 2.4 (1.8–3.2) | 2.5 (1.8–3.4) | <.001 |
| Monocyte count (10^9/L) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | <.001 |
| Lymphocyte count (10^9/L) | 1.7 (1.4–2.2) | 1.7 (1.3–2.2) | 1.8 (1.4–2.2) | 1.8 (1.4–2.3) | 1.8 (1.4–2.3) | <.001 |
| eGFR (mL/min/1.73m^2) | 69 (56–83) | 67 (54–79) | 65 (53–78) | 65 (53–79) | 65 (52–78) | <.001 |
| Creatinine (μmol/L) | 92 (79–109) | 95 (81–113) | 96 (83–114) | 95 (81–115) | 95 (80–116) | <.001 |
| Blood urea nitrogen (mmol/L) | 6.4 (5.0–8.2) | 6.8 (5.4–8.6) | 6.8 (5.4–8.6) | 6.8 (5.7–8.6) | 7.1 (5.7–9.3) | <.001 |
| Cystatin (mg/L) | 1.2 (1.0–1.4) | 1.1 (1.0–1.4) | 1.1 (1.0–1.4) | 1.2 (1.0–1.4) | 1.2 (1.0–1.5) | .01 |
| Urine albumin-creatinine ratio (mg/mmol) | ||||||
| ȃNo diabetes | 0.9 (0.0–2.8) | 0.8 (0.2–2.1) | 1.0 (0.3–2.8) | 0.8 (0.3–2.9) | 1.1 (0.3–2.9) | .19 |
| ȃDiabetes | 1.6 (0.3–9.6) | 1.5 (0.7–4.9) | 1.8 (0.8–7.1) | 1.7 (0.6–7.3) | 2.1 (0.7–7.8) | .08 |
| KIM-1 (pg/mL) | 126 (82–192) | 115 (78–176) | 123 (80–181) | 125 (88–197) | 151 (99–213) | <.001 |
| Uric acid (μmol/L) | 375 (315–458) | 393 (327–476) | 404 (333–482) | 404 (339–488) | 410 (333–500) | <.001 |
| ALT (IU/L) | 17 (13–23) | 18 (14–24) | 18 (14–24) | 18 (14–24) | 18 (14–24) | <.001 |
| AST (IU/L) | 22 (18–27) | 21 (18–26) | 21 (17–25) | 20 (17–25) | 20 (17–24) | <.001 |
| Alkaline phosphatase (IU/L) | 73 (59–91) | 71 (58–89) | 72 (57–90) | 70 (57–86) | 73 (59–90) | <.001 |
| Total bilirubin (μmol/L) | 9 (7–14) | 10 (7–14) | 9 (7–12) | 9 (7–12) | 9 (7–12) | <.001 |
| Albumin (g/L) | 43 (41–45) | 43 (41–45) | 43 (41–45) | 43 (41–45) | 43 (41–44) | <.001 |
BNP, B-type natriuretic peptide; CSS, clinical summary score; eGFR, estimated glomerular filtration rate; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; NT-proBNP, n-terminal pro-B-type natriuretic peptide; OSS, overall summary score; SD, standard deviation; ucGMP, urinary cyclic guanosine monophosphate.
Randomization visit, except ST2, MMP2, MMP9, TIMP1, GDF-15, and KIM-1, which were collected at the screening visit. Number of patients with missing biomarkers are: NT-proBNP, n = 14; BNP, n = 59; ucGMP/BNP ratio, n = 6375; Aldosterone, n = 6381; troponin T, n = 6345; ST2, n = 6803; MMP-9, n = 6999; MMP-2, n = 6998; TIMP1, n = 6983; galectin 3, n = 6354; GDF-15, n = 6850; Hemoglobin, n = 274; hemoglobin A1c, n = 180; White blood cell count, n = 294; Neutrophil count, n = 421; neutrophil/lymphocyte ratio, n = 421; monocyte count, n = 421; lymphocyte count, n = 421; eGFR, n = 0; creatinine, n = 0; blood urea nitrogen, n = 161; cystatin, n = 6343; urine albumin-creatinine ratio, n = 6416; KIM-1, n = 6846; uric acid, n = 186; ALT, n = 219; AST, n = 236; alkaline phosphatase, n = 199; total bilirubin, n = 199; albumin, n = 179.
Overlap between body mass index categories and waist-to-height ratio quintiles
The overlap between BMI categories and waist-to-height ratio quintiles is shown in Table 2. Of the 2397 patients not overweight or obese, according to BMI, only 1253 (52%) were in waist-to-height ratio quintile 1 (covering the range of waist-to-height ratio thought to be healthy). The remaining 1144 participants (48%) not overweight or obese, according to BMI, were in waist-to-height ratio quintiles 2–5 (mainly quintiles 2 and 3).
Table 2.
Correlation between body mass index categories and waist-to-height ratio quintiles
| Underweight | Normal weight | Overweight | Obesity class I | Obesity II/III | |
|---|---|---|---|---|---|
| BMI <18.5 | BMI 18.5–24.9 | BMI 25–29.9 | BMI 30–34.9 | BMI ≥35 | |
| n = 151 | n = 2246 | n = 3203 | n = 1779 | n = 901 | |
| WHtR quintile 1 | 129 | 1124 | 347 | 30 | 5 |
| WHtR quintile 2 | 14 | 746 | 819 | 69 | 11 |
| WHtR quintile 3 | 4 | 282 | 1055 | 295 | 21 |
| WHtR quintile 4 | 2 | 71 | 745 | 715 | 122 |
| WHtR quintile 5 | 2 | 23 | 237 | 670 | 742 |
Spearman correlation coefficient: 0.74.
There was less reclassification of patients classified as obese by BMI; of the 2680 patients categorized as in obesity class I or class II/III, 2249 (84%) were in the fourth or fifth quintile of waist-to-height ratio.
Outcomes according to anthropometric measures
Body mass index
In the analyses of BMI by WHO category, the risk of the primary outcome did not differ significantly between patients with obesity and those with normal weight in the analysis adjusted for only region and treatment. However, the risk of death (whether due to cardiovascular or all causes), was significantly lower in overweight and obese patients; conversely, the risk of heart failure hospitalization was higher among individuals in obesity class II/III (Table 3a). After adjustment for prognostic variables, including NT-proBNP, the association between higher BMI and lower risk of death was eliminated and the association between higher BMI and higher risk of HF hospitalization was accentuated (Table 3a).
Table 3a.
Outcomes according to body mass index
| Underweight | Normal weight | Overweight | Obesity class I | Obesity class II/III | |
|---|---|---|---|---|---|
| BMI <18.5 | BMI 18.5–24.9 | BMI 25–29.9 | BMI 30–34.9 | BMI ≥35 | |
| n = 153 | n = 2268 | n = 3249 | n = 1810 | n = 909 | |
| HF hospitalization or cardiovascular death | |||||
| ȃn (%) | 38 (24.8) | 556 (24.5) | 764 (23.5) | 432 (23.9) | 239 (26.3) |
| ȃEvent rate per 100 person-years (95% CI) | 12.5 (9.1–17.1) | 12.1 (11.1–13.1) | 11.2 (10.4–12.0) | 11.3 (10.3–12.4) | 12.6 (11.1–14.3) |
| ȃHR (95% CI)a | 1.02 (0.73–1.42) | Reference | 0.94 (0.84–1.06) | 0.94 (0.83–1.08) | 1.04 (0.88–1.21) |
| ȃHR (95% CI)b | 1.20 (0.86–1.67) | Reference | 0.90 (0.81–1.01) | 0.86 (0.75–0.99) | 0.92 (0.78–1.09) |
| ȃHR (95% CI)c | 1.06 (0.76–1.47) | Reference | 1.03 (0.92–1.16) | 1.09 (0.95–1.25) | 1.24 (1.05–1.48) |
| HF hospitalization | |||||
| ȃn (%) | 18 (11.8) | 289 (12.7) | 451 (13.9) | 273 (15.1) | 163 (17.9) |
| ȃEvent rate per 100 person-years (95% CI) | 5.9 (3.7–9.4) | 6.3 (5.6–7.0) | 6.6 (6.0–7.3) | 7.1 (6.3–8.0) | 8.6 (7.4–10.0) |
| ȃHR (95% CI)a | 0.96 (0.60–1.55) | Reference | 1.04 (0.90–1.22) | 1.10 (0.93–1.31) | 1.26 (1.03–1.54) |
| ȃHR (95% CI)b | 1.16 (0.72–1.88) | Reference | 0.98 (0.84–1.14) | 0.96 (0.81–1.15) | 1.06 (0.86–1.32) |
| ȃHR (95% CI)c | 1.03 (0.63–1.67) | Reference | 1.12 (0.96–1.31) | 1.22 (1.01–1.46) | 1.43 (1.15–1.78) |
| Cardiovascular death | |||||
| ȃn (%) | 30 (19.6) | 387 (17.1) | 474 (14.6) | 242 (13.4) | 117 (12.9) |
| ȃEvent rate per 100 person-years (95% CI) | 9.3 (6.5–13.3) | 7.9 (7.1–8.7) | 6.5 (5.9–7.1) | 5.8 (5.1–6.6) | 5.6 (4.6–6.7) |
| ȃHR (95% CI)a | 1.14 (0.78–1.65) | Reference | 0.85 (0.74–0.98) | 0.77 (0.65–0.91) | 0.75 (0.60–0.93) |
| ȃHR (95% CI)b | 1.27 (0.87–1.86) | Reference | 0.83 (0.73–0.96) | 0.75 (0.63–0.89) | 0.72 (0.58–0.91) |
| ȃHR (95% CI)c | 1.11 (0.76–1.63) | Reference | 0.96 (0.83–1.11) | 0.96 (0.80–1.15) | 1.00 (0.79–1.26) |
| Non-cardiovascular death | |||||
| ȃn (%) | 6 (3.9) | 79 (3.5) | 119 (3.7) | 65 (3.6) | 24 (2.6) |
| ȃEvent rate per 100 person-years (95% CI) | 1.9 (0.8–4.1) | 1.6 (1.3–2.0) | 1.6 (1.4–1.9) | 1.6 (1.2–2.0) | 1.1 (0.8–1.7) |
| ȃHR (95% CI)a | 1.47 (0.64–3.39) | Reference | 0.88 (0.66–1.17) | 0.81 (0.58–1.13) | 0.57 (0.36–0.91) |
| ȃHR (95% CI)b | 1.75 (0.75–4.08) | Reference | 0.87 (0.65–1.16) | 0.83 (0.59–1.18) | 0.64 (0.39–1.06) |
| ȃHR (95% CI)c | 1.70 (0.73–3.98) | Reference | 0.91 (0.68–1.23) | 0.91 (0.64–1.30) | 0.72 (0.44–1.19) |
| All-cause death | |||||
| ȃn (%) | 36 (23.5) | 466 (20.5) | 593 (18.3) | 307 (17.0) | 141 (15.5) |
| ȃEvent rate per 100 person-years (95% CI) | 11.2 (8.1–15.5) | 9.5 (8.7–10.4) | 8.1 (7.5–8.8) | 7.4 (6.6–8.3) | 6.7 (5.7–7.9) |
| ȃHR (95% CI)a | 1.18 (0.84–1.67) | Reference | 0.86 (0.75–0.97) | 0.78 (0.67–0.90) | 0.71 (0.58–0.86) |
| ȃHR (95% CI)b | 1.33 (0.94–1.88) | Reference | 0.84 (0.74–0.95) | 0.76 (0.65–0.89) | 0.71 (0.57–0.87) |
| ȃHR (95% CI)c | 1.19 (0.84–1.69) | Reference | 0.95 (0.84–1.08) | 0.95 (0.81–1.11) | 0.94 (0.76–1.16) |
BMI, body mass index; CI, confidence interval; HF, heart failure; HR, hazard ratio.
Adjusted for treatment and region.
Adjusted for treatment, age, sex, region, systolic blood pressure, heart rate, estimated glomerular filtration rate, left ventricular ejection fraction, NYHA functional class, HF etiology, duration of HF, prior HF hospitalization, a history of diabetes, and atrial fibrillation.
Adjusted for the above-mentioned variables and log of NT-proBNP.
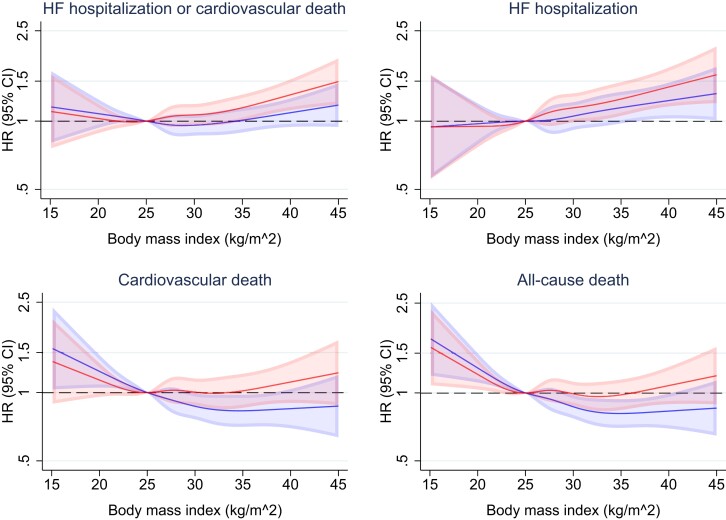
When BMI was examined as a continuous variable, a similar picture was evident although the small ‘underweight’ BMI category appeared to be associated with a higher risk of death from non-cardiovascular causes (Figure 3).
Figure 3.
Outcomes according to body mass index. This figure shows the risk of heart failure hospitalization or cardiovascular death, its components, and all-cause death, according to continuous body mass index. The solid line represents the hazard ratio and the shaded area the 95% CI. The reference is a body mass index of 25 kg/m2. The blue spline is adjusted for treatment and region. The red spline is adjusted for treatment, age, sex, region, systolic blood pressure, heart rate, estimated glomerular filtration rate, left ventricular ejection fraction, log of n-terminal pro-B-type natriuretic peptide, New York Heart Association functional class, heart failure aetiology, duration of heart failure, prior heart failure hospitalization, a history of diabetes, and atrial fibrillation. CI, confidence interval; HF, heart failure; HR, hazard ratio.
Waist-to-height ratio
In the analyses of waist-to-height ratio by quintile, the risk of the primary outcome did not differ significantly between patients in quintile 5, compared to those in quintile 1, in the analysis adjusted for only region and treatment (Table 3b). However, the risk of death (whether due to cardiovascular or all causes), tended to be lower in patients in quintile 5, although this was not as clear as for BMI. Conversely, the risk of HF hospitalization was significantly higher in individuals in quintile 5, compared with quintile 1. After adjustment for prognostic variables, the association between higher waist-to-height ratio and lower risk of death was eliminated and the association between higher waist-to-height ratio and higher risk of HF hospitalization persisted (Table 3).
Table 3b.
Outcomes according to quintile of waist-to-height ratio
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
|---|---|---|---|---|---|
| n = 1635 | n = 1659 | n = 1658 | n = 1655 | n = 1674 | |
| HF hospitalization or cardiovascular death | |||||
| ȃn (%) | 378 (23.1) | 378 (22.8) | 433 (26.1) | 390 (23.6) | 425 (25.4) |
| ȃEvent rate per 100 person-years (95% CI) | 11.4 (10.3–12.6) | 10.9 (9.8–12.0) | 12.4 (11.3–13.6) | 11.2 (10.1–12.4) | 12.3 (11.1–13.5) |
| ȃHR (95% CI)a | Reference | 0.98 (0.85–1.13) | 1.13 (0.98–1.30) | 1.02 (0.88–1.18) | 1.12 (0.97–1.29) |
| ȃHR (95% CI)b | Reference | 1.00 (0.86–1.16) | 1.18 (1.01–1.39) | 1.12 (0.93–1.33) | 1.18 (0.96–1.44) |
| ȃHR (95% CI)c | Reference | 1.02 (0.88–1.19) | 1.24 (1.06–1.46) | 1.18 (0.99–1.41) | 1.27 (1.03–1.55) |
| HF hospitalization | |||||
| ȃn (%) | 198 (12.1) | 208 (12.5) | 257 (15.5) | 230 (13.9) | 284 (17.0) |
| ȃEvent rate per 100 person-years (95% CI) | 6.0 (5.2–6.9) | 6.0 (5.2–6.9) | 7.4 (6.5–8.3) | 6.6 (5.8–7.5) | 8.2 (7.3–9.2) |
| ȃHR (95% CI)a | Reference | 1.02 (0.84–1.24) | 1.26 (1.04–1.52) | 1.12 (0.92–1.37) | 1.37 (1.13–1.65) |
| ȃHR (95% CI)b | Reference | 1.01 (0.83–1.24) | 1.24 (1.00–1.54) | 1.13 (0.89–1.43) | 1.30 (1.00–1.69) |
| ȃHR (95% CI)c | Reference | 1.04 (0.85–1.28) | 1.31 (1.05–1.62) | 1.19 (0.93–1.51) | 1.39 (1.06–1.81) |
| Cardiovascular death | |||||
| ȃn (%) | 263 (16.1) | 248 (14.9) | 263 (15.9) | 240 (14.5) | 224 (13.4) |
| ȃEvent rate per 100 person-years (95% CI) | 7.5 (6.6–8.4) | 6.7 (5.9–7.6) | 7.0 (6.2–7.9) | 6.4 (5.6–7.3) | 5.9 (5.2–6.7) |
| ȃHR (95% CI)a | Reference | 0.93 (0.78–1.10) | 0.98 (0.82–1.17) | 0.91 (0.76–1.09) | 0.85 (0.70–1.02) |
| ȃHR (95% CI)b | Reference | 0.98 (0.82–1.18) | 1.09 (0.90–1.33) | 1.11 (0.89–1.39) | 1.06 (0.82–1.38) |
| ȃHR (95% CI)c | Reference | 1.01 (0.84–1.21) | 1.16 (0.95–1.41) | 1.19 (0.95–1.49) | 1.15 (0.88–1.49) |
| Non-cardiovascular death | |||||
| ȃn (%) | 58 (3.5) | 55 (3.3) | 58 (3.5) | 60 (3.6) | 55 (3.3) |
| ȃEvent rate per 100 person-years (95% CI) | 1.6 (1.3–2.1) | 1.5 (1.1–1.9) | 1.5 (1.2–2.0) | 1.6 (1.2–2.1) | 1.4 (1.1–1.9) |
| ȃHR (95% CI)a | Reference | 0.82 (0.56–1.19) | 0.80 (0.55–1.16) | 0.82 (0.56–1.18) | 0.71 (0.49–1.04) |
| ȃHR (95% CI)b | Reference | 0.82 (0.56–1.21) | 0.81 (0.53–1.23) | 0.86 (0.55–1.36) | 0.87 (0.52–1.48) |
| ȃHR (95% CI)c | Reference | 0.83 (0.57–1.22) | 0.82 (0.54–1.25) | 0.88 (0.56–1.39) | 0.90 (0.53–1.52) |
| All-cause death | |||||
| ȃn (%) | 321 (19.6) | 303 (18.3) | 321 (19.4) | 300 (18.1) | 279 (16.7) |
| ȃEvent rate per 100 person-years (95% CI) | 9.1 (8.2–10.2) | 8.2 (7.3–9.2) | 8.5 (7.6–9.5) | 8.0 (7.2–9.0) | 7.3 (6.5–8.2) |
| ȃHR (95% CI)a | Reference | 0.91 (0.77–1.06) | 0.94 (0.81–1.11) | 0.89 (0.76–1.05) | 0.82 (0.69–0.97) |
| ȃHR (95% CI)b | Reference | 0.95 (0.81–1.12) | 1.04 (0.87–1.24) | 1.06 (0.87–1.30) | 1.03 (0.81–1.30) |
| ȃHR (95% CI)c | Reference | 0.97 (0.83–1.15) | 1.09 (0.91–1.31) | 1.13 (0.92–1.38) | 1.10 (0.87–1.39) |
CI, confidence interval; HF, heart failure; HR, hazard ratio.
Adjusted for treatment and region.
Adjusted for treatment, age, sex, region, systolic blood pressure, heart rate, estimated glomerular filtration rate, left ventricular ejection fraction, body mass index, NYHA functional class, HF etiology, duration of HF, prior HF hospitalization, a history of diabetes, and atrial fibrillation.
Adjusted for the above-mentioned variables and log of NT-proBNP.
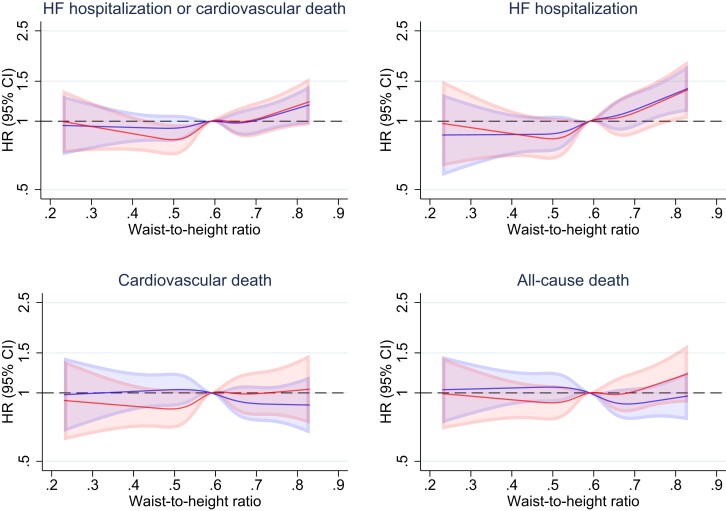
When waist-to-height ratio was examined as a continuous variable, a similar picture was evident although a low waist-to-height ratio did not appear to be associated with a higher risk of death from non-cardiovascular causes (Figure 4).
Figure 4.
Outcomes according to waist-to-height ratio. This figure shows the risk of heart failure hospitalization or cardiovascular death, its components, and all-cause death, according to continuous waist-to-height ratio. The solid line represents the hazard ratio and the shaded area the 95% CI. The reference is the median waist-to-height ratio (0.58). The blue spline is adjusted for treatment and region. The red spline is adjusted for treatment, age, sex, region, systolic blood pressure, heart rate, estimated glomerular filtration rate, left ventricular ejection fraction, log of n-terminal pro-B-type natriuretic peptide, body mass index, New York Heart Association functional class, heart failure aetiology, duration of heart failure, prior heart failure hospitalization, a history of diabetes, and atrial fibrillation. CI, confidence interval; HF, heart failure; HR, hazard ratio.
Other anthropometric measures
The association between the other anthropometric measures examined as continuous variables and outcomes are shown in Supplementary material online, Figures S1–S7. The association between waist-to-hip ratio examined as a continuous variable and outcomes in men and women, respectively, are shown in Supplementary material online, Figures S8 and S9. After adjustment for prognostic variables, greater adiposity, as assessed by body roundness index and relative fat mass, was associated with a higher risk of HF hospitalization. None of the anthropometric measures (i.e. waist circumference, waist-to-hip ratio, body shape index, weight-adjusted-waist index, and body surface area) was associated with cardiovascular death.
Effects of sacubitril/valsartan according to anthropometric measures
Body mass index
Compared with enalapril, sacubitril/valsartan reduced the risk of HF hospitalization or cardiovascular death across BMI categories (P for interaction = 0.50) (Table 4). The beneficial effect of sacubitril/valsartan was consistent across BMI categories for HF hospitalization (P for interaction = .41), cardiovascular death (P for interaction = .81), and death from any cause (P for interaction = .97) (Table 4).
Table 4.
Effects of sacubitril/valsartan compared with enalapril according to body mass index
| Underweight | Normal weight | Overweight | Obesity class I | Obesity class II/III | P-value for interaction | |
|---|---|---|---|---|---|---|
| BMI <18.5 | BMI 18.5–24.9 | BMI 25–29.9 | BMI 30–34.9 | BMI ≥35 | ||
| n = 153 | n = 2268 | n = 3249 | n = 1810 | n = 909 | ||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| HF hospitalization or cardiovascular death | 0.80 (0.42–1.53) | 0.91 (0.77–1.07) | 0.79 (0.68–0.91) | 0.75 (0.62–0.90) | 0.70 (0.54–0.91) | .50 |
| HF hospitalization | 0.93 (0.37–2.38) | 0.97 (0.77–1.22) | 0.75 (0.62–0.90) | 0.74 (0.59–0.95) | 0.71 (0.52–0.97) | .41 |
| Cardiovascular death | 0.79 (0.38–1.66) | 0.88 (0.72–1.07) | 0.80 (0.67–0.96) | 0.72 (0.55–0.92) | 0.73 (0.50–1.06) | .81 |
| All-cause death | 0.81 (0.41–1.60) | 0.90 (0.75–1.08) | 0.82 (0.70–0.96) | 0.83 (0.66–1.04) | 0.81 (0.58–1.13) | .97 |
BMI, body mass index; CI, confidence interval; HF, heart failure; HR hazard ratio.
Models adjusted for region.
Waist-to-height ratio
The beneficial effect of sacubitril/valsartan was also consistent for the primary and secondary endpoints according to quintiles of waist-to-height ratio (see Supplementary material online, Table S1).
Discussion
Among the patients with HFrEF in PARADIGM-HF, there was no longer evidence of a BMI-related ‘obesity-survival paradox’ after comprehensive adjustment for other prognostic variables. Moreover, the counterintuitive epidemiologic observation of a lower risk of death in patients with greater adiposity was less apparent with the newer anthropometric indices. All anthropometric indices examined showed that greater adiposity was associated with a higher risk of HF hospitalization as this was more evident with the newer indices (Structured Graphical Abstract).
In the minimally adjusted analyses (adjusted for only region and treatment assignment), overweight and obesity, defined using conventional BMI categories, were associated with a lower risk of death from any cause, and cardiovascular causes, compared with normal weight, as has been reported previously.4–8,37–40 However, most prior studies did not adjust for other prognostic variables which vary greatly across BMI categories, particularly the most powerful of these, i.e. natriuretic peptides.4–8,26–34,37–40 We were able to adjust for a broad range of prognostic variables, including NT-proBNP, and thereby minimize the impact of any potential residual confounding. After this adjustment, the ‘survival paradox’ related to high BMI was eliminated. Furthermore, none of the newer anthropometric measures showed the same association between greater adiposity and a lower risk of cardiovascular and all-cause death as was seen with BMI when adjusted for conventional risk variables (but not NT-proBNP); additional adjustment for NT-proBNP eliminated any suggestion of an ‘obesity-survival paradox’ with BMI. Indeed, when each of these indices was analyzed as a continuous variable their relationship with death was entirely flat. It is not certain why the newer indices showed a weaker relationship between adiposity and fatal outcomes, compared with BMI. However, an inspection of patient characteristics and biomarkers suggested a somewhat steeper gradient in age, atrial fibrillation, and NYHA class, as well as natriuretic peptide, aldosterone, and hemoglobin levels across BMI categories (normal to obesity class II/III) compared with the other indices (lowest to highest quintile). This may also explain why adjustment for other prognostic variables changed the association between higher adiposity and risk of death more for BMI than the other anthropometric indices.
Importantly, three of the newer anthropometric indices (waist-to-height ratio, relative fat mass, and body roundness index) demonstrated a significantly higher risk of HF hospitalization (and the composite of HF hospitalization or death from cardiovascular causes) in patients with greater adiposity, an association that was less apparent with BMI. These newer indices incorporate waist circumference and height, with the former better reflecting intra-abdominal fat (‘central obesity’) and the latter accounting for sex- and race-based differences in stature and skeletal weight. Notably, neither newer index included measured overall weight, the interpretation of which in patients with HF may be confounded by fluid retention or unintentional weight loss due to other illness. Interestingly, despite the steeper gradient in prognostic variables across BMI categories, compared to waist-to-height ratio quintiles, described above, the association between higher BMI and HF hospitalization was not as strong as that between higher waist-to-height ratio and HF hospitalization. This suggests that these new anthropometric indices identify pathophysiologic processes not reflected by conventional prognostic variables e.g. related to the distribution of body fat.
Different associations with outcomes were seen at the lower end of the range of adiposity and these also varied between the anthropometric indices. When considered as a continuous variable, a very low BMI was associated with a higher risk of death, which has been shown previously. However, we demonstrated that this was explained by a significant excess of non-cardiovascular death rather than cardiovascular death. The excess risk persisted after adjustment for other prognostic variables. This relationship was not as clearly evident with waist-to-height ratio (or the other newer anthropometric indices).
The relationship between adiposity and health-related quality of life (measured with the Kansas City Cardiomyopathy Questionnaire) was consistent between BMI and waist-to-height ratio. Both showed a steep decline in health-related quality of life with increasing adiposity, a relationship which was consistent with the relationship between waist-to-height ratio and HF hospitalization but opposite to that for BMI and mortality (in an unadjusted analysis).
Collectively these data show that greater adiposity in HFrEF is associated with a higher symptom burden, worse quality of life, and a greater risk of HF hospitalization. Other studies have shown that obese patients also have a higher risk of developing diabetes, atrial fibrillation, sleep apnea, and other comorbid conditions, compared with non-obese patients.26,34,41–44 Also, BMI > 35 kg/m2 is a contraindication to heart transplantation.45 Therefore, there is a strong rationale for promoting weight loss in obese patients especially as the ‘obesity-survival paradox’ seems to be an artifact of unadjusted analyses of BMI. Unfortunately, few randomized controlled trials using dietary and exercise intervention, bariatric surgery, or novel pharmacological therapies have been conducted in patients with HFrEF, although the latter are being investigated in individuals with HFpEF.46–52 The 2021 European Society of Cardiology and 2022 American College of Cardiology/American Heart Association guidelines do not provide any recommendation regarding weight management in HFrEF.45,53 Efforts to find effective and safe approaches to reducing weight in patients with HFrEF are therefore warranted.
Effect of sacubitril/valsartan according to anthropometric measures
The effect of HF therapies according to anthropometric measures is also of interest because of the hypothesis that the ‘obesity-survival paradox’ could, to some extent, reflect a greater effect of treatment in obese patients. Support for this was provided by a post hoc analysis of the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) trial, in which the benefit of the mineralocorticoid receptor antagonist, eplerenone, appeared to be greater in patients with larger waist circumference.54 However, such a relationship has not been established for other effective therapies in HFrEF, and a recent analysis of the dapagliflozin and prevention of adverse outcomes in heart failure (DAPA-HF) trial demonstrated that the benefit of dapagliflozin was consistent across the spectrum of BMI.34 In the present analysis, the beneficial effect of sacubitril/valsartan, compared with enalapril, was consistent for all outcomes across the spectrum of BMI and waist-to-height ratio. Specifically, there was no evidence of a diminished benefit in underweight patients or a larger benefit in obese patients.
Study limitations
This study has some limitations. Due to the observational nature of this study, the possibility of unmeasured confounding, despite adjustment for known prognostic variables, remains. Abdominal anthropometric measurements, such as waist circumference, are associated with higher measurement error than BMI, especially when these measurements are performed by different individuals.55 In addition, the analyses on the association between anthropometric measures at randomization and clinical adverse outcomes did not account for any change in e.g. weight or waist circumference during follow-up. Previous studies have suggested that the ‘obesity-survival paradox’ in HF may be affected by the level of cardiorespiratory fitness.56–59 Although it would have been interesting to examine the association between anthropometric measures and outcomes according to cardiorespiratory fitness levels, these data were not available. Finally, only 153 patients had a BMI <18.5 kg/m2 (the ‘underweight’ BMI category) and 171 patients a waist-to-height ratio <0.40, and our findings clearly cannot be extrapolated to patients with a low BMI or waist-to-height ratio.
Conclusion
In a large cohort of patients with HFrEF, the ‘obesity-survival paradox’ related to BMI was eliminated by comprehensive adjustment for prognostic variables. Importantly, alternative anthropometric measurements showed less evidence of this paradox, and two indices that incorporate waist circumference and height, but not weight, showed a clearer association between greater adiposity and a higher risk of HF hospitalization. Greater adiposity was associated with worse symptoms and health-related quality of life, irrespective of the anthropometric index used. These findings collectively suggest a need to test the potential benefits of intentional weight loss in patients living with obesity and HFrEF.
Supplementary Material
Acknowledgements
None.
Contributor Information
Jawad H Butt, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK; Department of Cardiology, Copenhagen University Hospital—Rigshospitalet, Copenhagen, Denmark.
Mark C Petrie, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Pardeep S Jhund, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Naveed Sattar, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Akshay S Desai, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Lars Køber, Department of Cardiology, Copenhagen University Hospital—Rigshospitalet, Copenhagen, Denmark.
Jean L Rouleau, Institut de Cardiologie de Montréal, Université de Montréal, Montréal, QC, Canada.
Karl Swedberg, Department of Molecular and Clinical Medicine, University of Gothenburg, Gothenburg, Sweden.
Michael R Zile, Department of Medicine, Medical University of South Carolina and Ralph H. Johnson Veterans Administration Medical Center, Charleston, South Carolina, USA.
Scott D Solomon, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Milton Packer, Baylor Heart and Vascular Institute, Baylor University Medical Center, Dallas, TX, USA.
John J V McMurray, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Author contributions
Michael Zile, MD (Writing – review & editing: Equal), Karl Swedberg, MD (Writing – review & editing: Equal), Jean L Rouleau, MD (Writing – review & editing: Equal), John J.J.V. Mcmurray, MD, Milton Packer, MD (Writing – review & editing: Equal), Scott D. Solomon, MD (Writing – review & editing: Equal), Pardeep S. Jhund (Writing – review & editing: Equal), Mark C. Petrie, MBChB (Writing – review & editing: Equal), Jawad H. Butt, MD (Writing – original draft: Lead), Lars Kober, MD (Writing – review & editing: Equal), Akshay S Desai, MD (Writing – review & editing: Equal), and Naveed Sattar, MD (Writing – review & editing: Equal).
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
Trial data will be made available by the sponsor, Novartis, in accordance with their data sharing policy.
Funding
The PARADIGM-HF trial was funded by Novartis. Profs. McMurray and Jhund are supported by British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
References
- 1. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation 2016;133:639–649. 10.1161/CIRCULATIONAHA.115.016801 [DOI] [PubMed] [Google Scholar]
- 2. Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313. 10.1056/NEJMoa020245 [DOI] [PubMed] [Google Scholar]
- 3. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, et al. Relationship between physical activity, body mass Index, and risk of heart failure. J Am Coll Cardiol 2017;69:1129–1142. 10.1016/j.jacc.2016.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J, Begley A, Jackson R, Harrison M, Pellicori P, Clark AL, et al. Body mass index and all-cause mortality in heart failure patients with normal and reduced ventricular ejection fraction: a dose–response meta-analysis. Clin Res Cardiol 2019;108:119–132. 10.1007/s00392-018-1302-7 [DOI] [PubMed] [Google Scholar]
- 5. Padwal R, Mcalister FA, Mcmurray JJV, Cowie MR, Rich M, Pocock S, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obes 2014;38:1110–1114. 10.1038/ijo.2013.203 [DOI] [PubMed] [Google Scholar]
- 6. Oga EA, Eseyin OR. The obesity paradox and heart failure: a systematic review of a decade of evidence. J Obes 2016;2016:1–9. 10.1155/2016/9040248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahajan R, Stokes M, Elliott A, Munawar DA, Khokhar KB, Thiyagarajah A, et al. Complex interaction of obesity, intentional weight loss and heart failure: a systematic review and meta-analysis. Heart 2020;106:58–68. 10.1136/heartjnl-2019-314770 [DOI] [PubMed] [Google Scholar]
- 8. Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis 2018;61:151–156. 10.1016/j.pcad.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 9. Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev 2016;17:262–275. 10.1111/obr.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes 2010;34:791–799. 10.1038/ijo.2010.5 [DOI] [PubMed] [Google Scholar]
- 11. Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis 2018;61:103–113. 10.1016/j.pcad.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 12. Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes 2008;32:S56–S59. 10.1038/ijo.2008.87 [DOI] [PubMed] [Google Scholar]
- 13. Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One 2012;7:e39504. 10.1371/journal.pone.0039504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woolcott OO, Bergman RN. Relative fat mass (RFM) as a new estimator of whole-body fat percentage ─ A cross-sectional study in American adult individuals. Sci Rep 2018;8:10980. 10.1038/s41598-018-29362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep 2018;8:16753. 10.1038/s41598-018-35073-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013;21:2264–2271. 10.1002/oby.20408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang C, Rexrode KM, Van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008;117:1658–1667. 10.1161/CIRCULATIONAHA.107.739714 [DOI] [PubMed] [Google Scholar]
- 18. Loehr LR, Rosamond WD, Poole C, Mcneill AM, Chang PP, Folsom AR, et al. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the atherosclerosis risk in communities study. Circ Hear Fail 2009;2:18–24. 10.1161/CIRCHEARTFAILURE.108.813782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gelber RP, Gaziano JM, Orav EJ, Manson JAE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol 2008;52:605–615. 10.1016/j.jacc.2008.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suthahar N, Meems LMG, Withaar C, Gorter TM, Kieneker LM, Gansevoort RT, et al. Relative fat mass, a new index of adiposity, is strongly associated with incident heart failure: data from PREVEND. Sci Rep 2022;12:147. 10.1038/s41598-021-02409-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashwell M, Gibson S. Waist-to-height ratio as an indicator of early health risk: simpler and more predictive than using a matrix based on BMI and waist circumference. BMJ Open 2016;6:e010159. 10.1136/bmjopen-2015-010159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://www.nice.org.uk/guidance/cg189/resources/obesity-identification-assessment-and-management-pdf-35109821097925 National Institute for Health and Care Excellence. Obesity: identification, assessment and management. (11 December 2022) [PubMed]
- 23. Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol 2006;47:85–90. 10.1016/j.jacc.2005.08.050 [DOI] [PubMed] [Google Scholar]
- 24. Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol 2004;43:1590–1595. 10.1016/j.jacc.2003.10.066 [DOI] [PubMed] [Google Scholar]
- 25. Savarese G, Orsini N, Hage C, Vedin O, Cosentino F, Rosano GMC, et al. Utilizing NT-proBNP for eligibility and enrichment in trials in HFpEF, HFmrEF, and HFrEF. JACC Heart Fail 2018;6:246–256. 10.1016/j.jchf.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 26. Streng KW, Voors AA, Hillege HL, Anker SD, Cleland JG, Dickstein K, et al. Waist-to-hip ratio and mortality in heart failure. Eur J Heart Fail 2018;20:1269–1277. 10.1002/ejhf.1244 [DOI] [PubMed] [Google Scholar]
- 27. Piepoli MF, Corrà U, Veglia F, Bonomi A, Salvioni E, Cattadori G, et al. Exercise tolerance can explain the obesity paradox in patients with systolic heart failure: data from the MECKI score research group. Eur J Heart Fail 2016;18:545–553. 10.1002/ejhf.534 [DOI] [PubMed] [Google Scholar]
- 28. Chandramouli C, Tay WT, Bamadhaj NS, Tromp J, Teng THK, Yap JJL, et al. Association of obesity with heart failure outcomes in 11 Asian regions: a cohort study. PLoS Med 2019;16:e1002916. 10.1371/journal.pmed.1002916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Zhang Y, Shi Y, Dong W, Mu Y, Wang J, et al. Influence of Waist-to-hip ratio on the prognosis of heart failure patients with revascularized coronary heart disease. Front Cardiovasc Med 2021;8:732200. 10.3389/fcvm.2021.732200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davos CH, Doehner W, Rauchhaus M, Cicoira M, Francis DP, Coats AJS, et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail 2003;9:29–35. 10.1054/jcaf.2003.4 [DOI] [PubMed] [Google Scholar]
- 31. Gustafsson F, Kragelund CB, Torp-Pedersen C, Seibæk M, Burchardt H, Akkan D, et al. Effect of obesity and being overweight on long-term mortality in congestive heart failure: influence of left ventricular systolic function. Eur Heart J 2005;26:58–64. 10.1093/eurheartj/ehi022 [DOI] [PubMed] [Google Scholar]
- 32. Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation 2005;112:1756–1762. 10.1161/CIRCULATIONAHA.104.530972 [DOI] [PubMed] [Google Scholar]
- 33. Krishnamoorthy A, Greiner MA, Bertoni AG, Eapen ZJ, O’Brien EC, Curtis LH, et al. The obesity and heart failure epidemics among African Americans: insights from the Jackson heart study. J Card Fail 2016;22:589–597. 10.1016/j.cardfail.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adamson C, Jhund PS, Docherty KF, Bělohlávek J, Chiang CE, Diez M, et al. Efficacy of dapagliflozin in heart failure with reduced ejection fraction according to body mass index. Eur J Heart Fail 2021;23:1662–1672. 10.1002/ejhf.2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 36. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the prospective comparison of ARNI with ACEI to determine impact. Eur J Heart Fail 2013;15:1062–1073. 10.1093/eurjhf/hft052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J 2008;156:13–22. 10.1016/j.ahj.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 38. Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015;115:1428–1434. 10.1016/j.amjcard.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 39. Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol 2003;91:891–894. 10.1016/S0002-9149(03)00031-6 [DOI] [PubMed] [Google Scholar]
- 40. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol 2001;38:789–795. 10.1016/S0735-1097(01)01448-6 [DOI] [PubMed] [Google Scholar]
- 41. Cornier M-A, Després J-P, Davis N, Grossniklaus DA, Klein S, Lamarche B, et al. Assessing adiposity: a scientific statement from the American heart association. Circulation 2011;124:1996–2019. 10.1161/CIR.0b013e318233bc6a [DOI] [PubMed] [Google Scholar]
- 42. Parcha V, Patel N, Kalra R, Suri SS, Arora G, Wang TJ, et al. Obesity and serial nt-probnp levels in guided medical therapy for heart failure with reduced ejection fraction: insights from the guide-it trial. J Am Heart Assoc 2021;10:e018689. 10.1161/JAHA.120.018689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes Mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American heart association. Circulation 2016;134:e535–e578. 10.1161/CIR.0000000000000450 [DOI] [PubMed] [Google Scholar]
- 44. Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation 2021;143:E984–E1010. 10.1161/CIR.0000000000000973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 46. McCloskey CA, Ramani GV, Mathier MA, Schauer PR, Eid GM, Mattar SG, et al. Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surg Obes Relat Dis 2007;3:503–507. 10.1016/j.soard.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 47. Evangelista LS, Heber D, Li Z, Bowerman S, Hamilton MA, Fonarow GC. Reduced body weight and adiposity with a high-protein diet improves functional Status, lipid profiles, glycemic control, and quality of life in patients with heart failure: a feasibility study. J Cardiovasc Nurs 2009;24:207–215. 10.1097/JCN.0b013e31819846b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pritchett AM, Deswal A, Aguilar D. Lifestyle modification with diet and exercise in obese patients with heart failure—a pilot study. J Obes Weight Loss Ther 2012;02:1–8. 10.4172/2165-7904.1000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Horwich TB, Broderick S, Chen L, McCullough PA, Strzelczyk T, Kitzman DW, et al. Relation among body mass index, exercise training, and outcomes in chronic systolic heart failure. Am J Cardiol 2011;108:1754–1759. 10.1016/j.amjcard.2011.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure HF-ACTION randomized controlled trial. JAMA 2009;301:1451–1459. 10.1001/jama.2009.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity (STEP-HFpEF). https://clinicaltrials.gov/ct2/show/NCT04788511 .
- 52. A Study of Tirzepatide (LY3298176) in Participants With Heart Failure With Preserved Ejection Fraction and Obesity (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT04847557 .
- 53. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation;2022:e895–e1032. [DOI] [PubMed] [Google Scholar]
- 54. Olivier A, Pitt B, Girerd N, Lamiral Z, Machu JL, McMurray JJV, et al. Effect of eplerenone in patients with heart failure and reduced ejection fraction: potential effect modification by abdominal obesity. Insight from the EMPHASIS-HF trial. Eur J Heart Fail 2017;19:1186–1197. 10.1002/ejhf.792 [DOI] [PubMed] [Google Scholar]
- 55. Verweij LM, Terwee CB, Proper KI, Hulshof CTJ, van Mechelen W. Measurement error of waist circumference: gaps in knowledge. Public Health Nutr 2013;16:281–288. 10.1017/S1368980012002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McAuley PA, Keteyian SJ, Brawner CA, Dardari ZA, Al Rifai M, Ehrman JK, et al. Exercise capacity and the obesity paradox in heart failure: the FIT (henry ford exercise testing) project. Mayo Clin Proc 2018;93:701–708. 10.1016/j.mayocp.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 57. Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc 2013;88:251–258. 10.1016/j.mayocp.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Clark AL, Fonarow GC, Horwich TB. Impact of cardiorespiratory fitness on the obesity paradox in patients with systolic heart failure. Am J Cardiol 2015;115:209–213. 10.1016/j.amjcard.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 59. Elagizi A, Carbone S, Lavie CJ, Mehra MR, Ventura HO. Implications of obesity across the heart failure continuum. Prog Cardiovasc Dis 2020;63:561–569. 10.1016/j.pcad.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Trial data will be made available by the sponsor, Novartis, in accordance with their data sharing policy.