Nearly half to two-thirds of extremely preterm infants (<28 weeks of gestation) have moderate to large patent ductus arteriosus (PDA) shunts, which may lead to sustained exposure to excessive pulmonary blood flow (Qp) for days to weeks (1). Natural history studies of extremely preterm infants report a median time of PDA closure of 72 days (2). The association of PDA and bronchopulmonary dysplasia (BPD) is well documented; recent evidence highlights the increased likelihood of the most severe forms of BPD in patients with both prolonged mechanical ventilation and exposure to a large PDA (3) (Figure 1). The relationship of prolonged PDA exposure to pulmonary vascular maldevelopment, however, and its most severe form, pulmonary arterial hypertension (PAH), although biologically plausible, has received little scientific investigation. Almost 70 years ago, Hultgren and colleagues first reported the development of PAH, on either cardiac catheterization or autopsy, in eight patients after prolonged PDA exposure (4). Randomized clinical trials to date have focused exclusively on modulation of BPD, without any consideration of the impact on the immature pulmonary vascular bed or risk of PAH. Therefore, the direct effect of a prolonged PDA shunt, indirect effects through BPD development, and modulator effects of PDA treatment on pulmonary vascular remodeling and PAH remains unknown.
Figure 1.
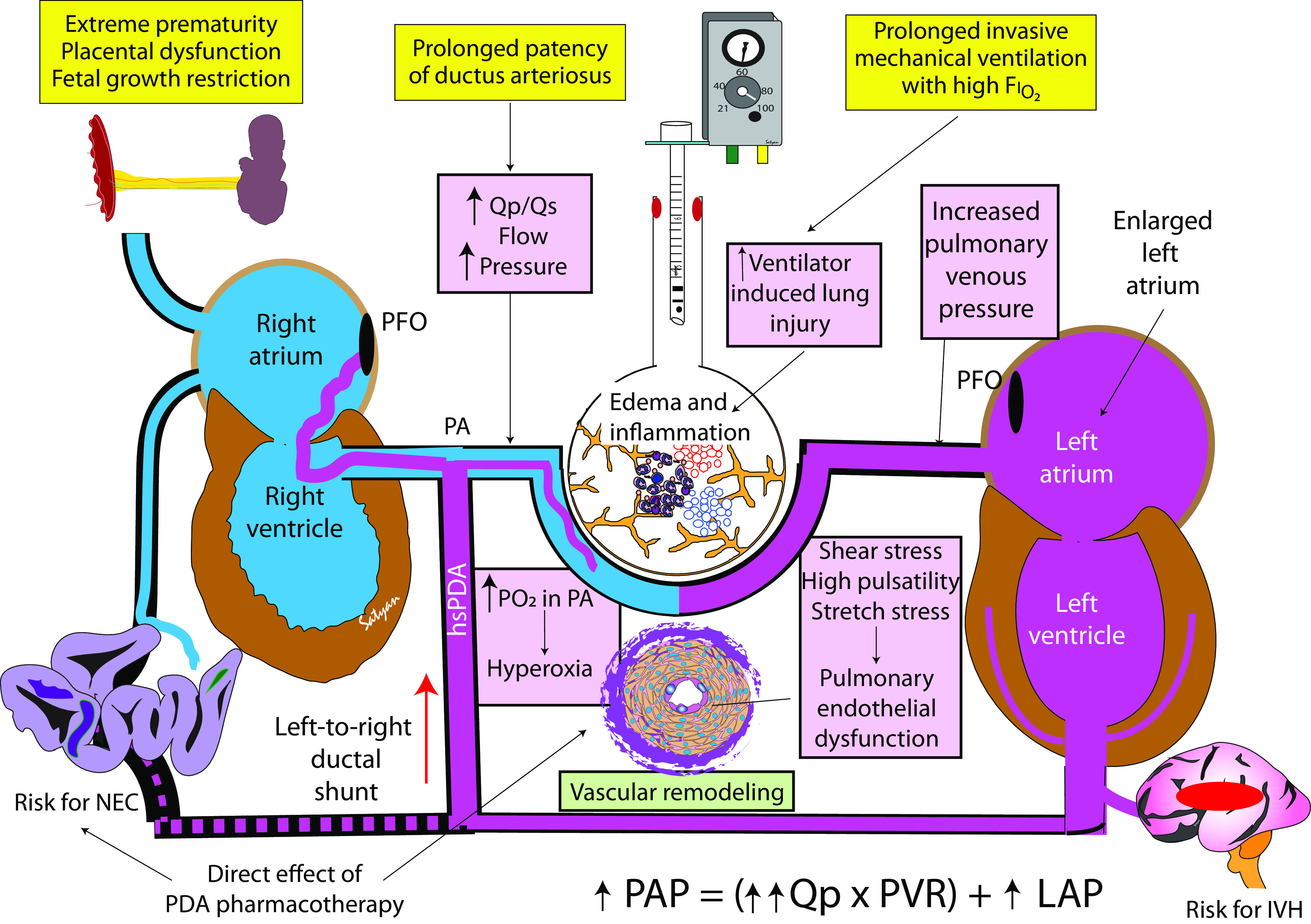
Prolonged patency of ductus arteriosus and association with pulmonary vascular disease in extremely preterm infants. Primary inciting events (in yellow boxes) include extreme prematurity, patency of a hsPDA, and prolonged duration of invasive mechanical ventilation. Various pathophysiologic contributors to vascular modeling are shown in pink boxes. hsPDA = hemodynamically significant patent ductus arteriosus; IVH = intraventricular hemorrhage; LAP = left atrial pressure; NEC = necrotizing enterocolitis; PA = pulmonary artery; PAP = pulmonary arterial pressure; PDA = patent ductus arteriosus; PFO = patent foramen ovale; PVR = pulmonary vascular resistance; Qp = pulmonary blood flow; Qs = systemic blood flow. Image courtesy of Satyan Lakshminrusimha.
In this issue of the Journal, Gentle and colleagues (pp. 921–928) report a possible association between prolonged PDA exposure and PAH in a cohort of preterm infants, born at less than 28 weeks’ gestational age, who remained on assisted respiratory support on Postnatal Day 28 (5). After adjustment for relevant covariates (birth weight, FiO2 on Day 28), both PDA (adjusted odds ratio [aOR], 4.29; 95% confidence interval [CI], 1.89–9.77) and moderate to large PDA (aOR, 4.15; 95% CI, 1.78–9.64) were strongly associated with BPD–PAH at discharge. In addition, by probit analysis, each additional month of PDA exposure was associated with increased probability for the primary outcome of death or BPD–PAH at discharge. In an era when an increased number of clinicians have adopted a conservative approach to PDA, the implications at first glance may appear alarming. Although the findings are biologically plausible, several pathophysiologic factors need consideration before causality may be implied.
First, the interplay among pulmonary arterial pressure (PAP), Qp, and pulmonary vascular resistance (PVR) in neonatal PAH is explained by the equation PAP = PVR × Qp + left atrial pressure. Therefore, in the setting of prolonged PDA exposure, PAH may relate to developmental (pulmonary vascular remodeling and elevated PVR) or physiologic (excessive Qp and/or left atrial hypertension) factors (Figure 1). Although the latter is potentially modifiable with elimination of the ductal shunt, the former, which forms the basis of the authors’ supposition, may be less amenable to pharmacological modulation. The relationship among oxygen exposure, target oxygen saturation, and ambient PaO2 is an additional, and poorly investigated, consideration in the setting of prolonged PDA shunt exposure. Intermittent or sustained periods of hypoxia, oftentimes followed by hyperoxia, may contribute to both pulmonary vascular injury and ongoing ductal patency (6). In addition, in the setting of a high-volume PDA shunt, oxygen saturation, which is a surrogate for hyperoxia exposure, is not a reliable determinant of oxygen free radical–mediated lung endothelial injury. Pulmonary overcirculation from left-to-right shunts, as observed with a large, unrestricted ventricular septal defect (VSD), is associated with excessive shear stress, leading to high risk of increased PVR and PAH (7). In addition, pulmonary arterial oxygen saturation (SpaO2) is increased in the setting of either PDA or VSD, thereby reducing the denominator in the shunt calculation: Qp/systemic blood flow = (SaO2 − mixed venous oxygen saturation)/(pulmonary venous oxygen saturation − SpaO2). Such high SpaO2 may potentially enhance Qp and contribute to oxygen toxicity and endothelial dysfunction, resulting in PAH (7). It is therefore possible that high SpaO2 and high pressure in infants with large PDAs might promote pulmonary vascular disease and PAH. Of importance, pressure- or stretch-induced vasoconstriction, known as the myogenic (or Bayliss) response (8), plays an important physiologic role in the maintenance of high PVR and time-dependent autoregulation of Qp in the normal fetus. Experimental models of intrauterine chronic pulmonary hypertension, induced by PDA closure, lead to abolition of flow-induced vasodilation and increased myogenic response, thereby contributing to abnormal vasoreactivity (9). It is therefore plausible that pulsatile flow–induced endothelial injury, because of prolonged PDA shunt exposure, mediates an enhanced myogenic response and hypertensive remodeling independent of oxygen tension. It is not possible to determine the relative contribution of flow-driven (modifiable with PDA closure) versus resistance-driven (candidate for pulmonary vasodilator therapy) within the present study design. For example, the presence of right-to-left transductal flow is not universally pathognomonic of resistance-driven PAH and may be seen in patients with excessive Qp. Advanced hemodynamic evaluation using cardiac catheterization may provide additional insights regarding these two distinct phenotypes.
Second, the independent effects of severity of BPD and duration of PDA exposure are difficult to tease out in this study design. Although PDA and BPD are often biological cotravelers, because of the increased likelihood of prolonged oxygen and exposure and duration of mechanical ventilation, the direct effects of BPD on pulmonary arterial remodeling and impaired angiogenesis are important mechanistic considerations. Systematic reviews suggest that 20% (95% CI, 14–25%) of preterm infants with BPD develop PAH (10). In addition, the severity of BPD correlates well with the incidence of PAH. A recent observational study demonstrated that both severity of BPD and exposure to PDA for >28 days were independently associated with increased risk of PAH (11).
Several animal experimental models and recent human observations, however, provide compelling evidence of PDA-attributable pulmonary vascular disease. Increased Qp and PAP, secondary to endothelial injury, have been demonstrated in neonatal lambs after in utero placement of a shunt between the aorta and the main pulmonary artery (12). Pulmonary vascular remodeling is also evident by 4 weeks, with increased medial thickness of pulmonary arteries of <200 μm in diameter. Endothelial injury secondary to increased Qp and shear stress alters the production of vasoactive substances such as nitric oxide, further promoting increased risk of PAH development (13). Invasive pulmonary hemodynamic evaluation in infants undergoing interventional PDA closure revealed that infants with prolonged PDA exposure (>8 wk postnatally) had evidence of elevated PVR (14).
In summary, although causality should not be assumed, the observations of Gentle and colleagues (5) highlight the importance of enhanced surveillance for PAH in high-risk preterm infants with prolonged PDA exposure and lay the foundation for future mechanistic investigation. The risk of future PAH from prolonged ductal patency must be taken into consideration when contemplating definitive (catheterization-based or surgical closure) therapy for PDA. In addition, there is an urgent need to integrate PAH as an important outcome in clinical trials of PDA management.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202211-2146ED on December 5, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics . 2006;117:1113–1121. doi: 10.1542/peds.2005-1528. [DOI] [PubMed] [Google Scholar]
- 2. Semberova J, Sirc J, Miletin J, Kucera J, Berka I, Sebkova S, et al. Spontaneous closure of patent ductus arteriosus in infants ⩽ 1500 g. Pediatrics . 2017;140:e2014258. doi: 10.1542/peds.2016-4258. [DOI] [PubMed] [Google Scholar]
- 3. Clyman RI, Hills NK, Liebowitz M, Johng S. Relationship between duration of infant exposure to a moderate-to-large patent ductus arteriosus shunt and the risk of developing bronchopulmonary dysplasia or death before 36 weeks. Am J Perinatol . 2020;37:216–223. doi: 10.1055/s-0039-1697672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hultgren H, Selzer A, Purdy A, Holman E, Gerbode F. The syndrome of patent ductus arteriosus with pulmonary hypertension. Circulation . 1953;8:15–35. doi: 10.1161/01.cir.8.1.15. [DOI] [PubMed] [Google Scholar]
- 5. Gentle SJ, Travers CP, Clark M, Carlo WA, Ambalavanan N. Patent ductus arteriosus and development of bronchopulmonary dysplasia with pulmonary hypertension. Am J Respir Crit Care Med . 2023;207:921–928. doi: 10.1164/rccm.202203-0570OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van der Sterren S, Agren P, Zoer B, Kessels L, Blanco CE, Villamor E. Morphological and functional alterations of the ductus arteriosus in a chicken model of hypoxia-induced fetal growth retardation. Pediatr Res . 2009;65:279–284. doi: 10.1203/PDR.0b013e318194fa8f. [DOI] [PubMed] [Google Scholar]
- 7. Kulik TJ. Pulmonary blood flow and pulmonary hypertension: is the pulmonary circulation flowophobic or flowophilic? Pulm Circ . 2012;2:327–339. doi: 10.4103/2045-8932.101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol . 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Storme L, Parker TA, Kinsella JP, Rairigh RL, Abman SH. Chronic hypertension impairs flow-induced vasodilation and augments the myogenic response in fetal lung. Am J Physiol Lung Cell Mol Physiol . 2002;282:L56–L66. doi: 10.1152/ajplung.2002.282.1.L56. [DOI] [PubMed] [Google Scholar]
- 10. Arjaans S, Zwart EAH, Ploegstra MJ, Bos AF, Kooi EMW, Hillege HL, et al. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: a systematic review and meta-analysis. Paediatr Perinat Epidemiol . 2018;32:258–267. doi: 10.1111/ppe.12444. [DOI] [PubMed] [Google Scholar]
- 11. Wang C, Ma X, Xu Y, Chen Z, Shi L, Du L. A prediction model of pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Front Pediatr . 2022;10:925312. doi: 10.3389/fped.2022.925312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, et al. In utero placement of aortopulmonary shunts: a model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation . 1995;92:606–613. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- 13. Black SM, Bekker JM, McMullan DM, Parry AJ, Ovadia B, Reinhartz O, et al. Alterations in nitric oxide production in 8-week-old lambs with increased pulmonary blood flow. Pediatr Res . 2002;52:233–244. doi: 10.1203/00006450-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 14. Philip R, Waller BR, Chilakala S, Graham B, Stecchi N, Apalodimas L, et al. Hemodynamic and clinical consequences of early versus delayed closure of patent ductus arteriosus in extremely low birth weight infants. J Perinatol . 2021;41:100–108. doi: 10.1038/s41372-020-00772-2. [DOI] [PubMed] [Google Scholar]


