Abstract
Rationale
Bedside biomarkers that allow early identification of infants with bronchopulmonary dysplasia-associated pulmonary hypertension (BPD-PH) are critically important, given the higher risk of death in these infants.
Objectives
We hypothesized that infants with BPD-PH have patterns of intermittent hypoxemia (IH) that differ from infants with BPD without PH.
Methods
We conducted a matched case-control study of extremely preterm infants from 22 weeks 0 days to 28 weeks 6 days born between 2018 and 2020 at the University of Alabama at Birmingham. BPD-PH status was determined using echocardiographic data performed after postnatal Day 28. Physiologic data were compared between infants with BPD-PH (cases) and BPD alone (control subjects). Receiver operating characteristic (ROC) analysis estimated the predictive ability of cumulative hypoxemia, desaturation frequency, and duration of intermittent hypoxemic events in the week preceding echocardiography to discriminate between cases and control subjects.
Measurements and Main Results
Forty infants with BPD-PH were compared with 40 infants with BPD alone. Infants with and without PH had a similar frequency of IH events, but infants with PH had more prolonged hypoxemic events for desaturations below 80% (7 s vs. 6 s; P = 0.03) and 70% (105 s vs. 58 s; P = 0.008). Among infants with BPD-PH, infants who died had longer hypoxemic events below 70% (145 s vs. 72 s; P = 0.01). Using the duration of hypoxemic events below 70%, the areas under the ROC curves for diagnosis of BPD-PH and death in BPD-PH infants were 0.71 and 0.77, respectively.
Conclusions
Longer duration of intermittent hypoxemic events was associated both with a diagnosis of BPD-PH and with death among infants with BPD-PH.
Keywords: intermittent hypoxemia, bronchopulmonary dysplasia, pulmonary hypertension, preterm infant
At a Glance Commentary
Scientific Knowledge on the Subject
Intermittent hypoxemic events have been associated with pulmonary hypertension development in other pulmonary disease processes, including sleep apnea and chronic obstructive pulmonary disease.
What This Study Adds to the Field
In this investigation, we detail characteristics of intermittent hypoxemia that may help care providers continuously and noninvasively detect the development of pulmonary hypertension in preterm infants.
Bronchopulmonary dysplasia (BPD) is one of the most common morbidities of very preterm infants (1) and may result in long-term sequelae (2). Of infants with BPD, BPD-associated pulmonary hypertension (BPD-PH) complicates disease in approximately 40% of extremely preterm infants and is associated with a substantially higher risk for mortality (3). As currently recommended, screening methods (4), including serial echocardiography, have limited sensitivity in detecting both disease and disease severity (5). Thus, additional methods for early diagnosis of BPD-PH are necessary.
Immature control of breathing in the preterm infant results in frequent episodes of intermittent hypoxemia (IH). Recent evidence from a randomized controlled trial of oxygen saturation targeting in extremely preterm infants suggests that the frequency and duration of IH may provide clinical use for BPD prediction (6). Moreover, observational data from other patient populations with obstructive sleep apnea and chronic obstructive pulmonary disease suggests that patients who develop pulmonary hypertension have a lower average nocturnal arterial oxygen saturation (∼88%) compared with patients without pulmonary hypertension (∼94%) (7) and spend a longer percentage of time with oxygen saturations below 90% (8, 9). As IH may predict which infants develop BPD and prior data suggest that IH may be associated with pulmonary hypertension development, comparing IH between infants with BPD-PH and infants with BPD but without pulmonary hypertension may provide a continuous biomarker for BPD-PH.
We performed a single-center study contrasting IH events in infants with BPD by pulmonary hypertension status. In comparing the frequency, duration, and severity of IH events, we hypothesized that IH events in infants who developed BPD-PH would be more frequent and longer in duration compared with infants with BPD but without pulmonary hypertension.
Methods
Study Design
We conducted a retrospective, observational case-control study of prospectively collected data in extremely preterm infants admitted at the UAB (University of Alabama at Birmingham) between 2018 and 2020. The included infants were a subset of the infants enrolled at UAB as part of the multicenter cohort of the PreVENT (Prematurity-related Ventilatory Control) study (10). Approval from the institutional review board was obtained for physiological and clinical data collection with a waiver of consent. At UAB, infants remaining on respiratory support after 28 postnatal days are systematically screened every month for pulmonary hypertension by echocardiogram. Infants were included if born at less than 29 weeks gestation, remained on respiratory support on postnatal Day 28, had an echocardiogram performed to screen for BPD-PH, and had oxygen saturation data available in the week preceding echocardiographic diagnosis. Infants with major congenital anomalies or genetic syndromes were excluded.
Infants diagnosed with BPD-PH at any time point during hospitalization were defined as cases, whereas infants with BPD but without echocardiographic evidence of pulmonary hypertension throughout hospitalization were defined as control subjects. The diagnosis of BPD-PH was on the basis of echocardiographic findings, as previously reported (11). The cardiologists reporting on echocardiograms were masked to the clinical status of infants. Two forms of matching were performed: 1) cases and control subjects were matched on the basis of gestational age at birth (±1 wk); and 2) IH comparisons in the week preceding diagnosis in cases, and, in control subjects, IH events preceding the postmenstrual age (PMA) of matched case echocardiograms. Given known covariates associated with risk for BPD-PH, demographic characteristics compared between cases and control subjects included gestational age, birth weight, small for gestational age, patent ductus arteriosus at or beyond 28 postnatal days, early-onset sepsis, late-onset sepsis, and respiratory support at postnatal Day 28 and 36 weeks postmenstrual age.
Characterization of IH
Bedside cardiorespiratory monitoring data was obtained using Philips IntelliVue MP70 or MP50 monitors using Nellcor pulse oximetry sensors, an averaging time of 8 seconds, and recorded using the BedMaster system (Excel Medical Electronics) at 125 Hz with data analyzed using numeric data at 1 Hz, which were collected as .STP files, converted to HDF5 files, and analyzed using MATLAB. In our neonatal intensive care unit, oxygen saturation alarm limits are 88–95% for the first postnatal month, after which alarm limits are increased to 90–95% with bedside use of oxygen saturation histograms to iteratively inform care provider response to desaturations as previously described (12). As IH events commonly occur in extremely preterm infants with changes in frequency by postnatal age (13), IH in the week preceding BPD-PH diagnosis was analyzed to better isolate potential associations between IH and the presence of BPD-PH.
Recorded data included the lowest oxygen saturation achieved at the time of IH (defined as less than 80%) and the duration of the IH. Therefore, IH events were compared between cases and control subjects using: 1) the cumulative duration of hypoxemia per day; 2) the median daily frequency of IH; and 3) the median daily duration of IH events. These parameters were analyzed with a desaturation threshold below 80% and below 70%, with data in the below 80% threshold inclusive of data used within the below 70% threshold. Both thresholds were considered to determine whether more extreme events differed between groups. Though there is no standard definition for a desaturation event, prior studies have investigated a range of desaturation thresholds contributory to pulmonary hypertension development (14, 15) with lower accuracy notable at lower saturations (16, 17).
The total number of desaturation events (defined as oxygen saturation as measured by pulse oximetry [SpO2] below 80%) with a minimal duration of at least Δt (Δt = 1,2,…,100 s) was computed separately for control subjects and cases groups and divided by a total recording length of a group (in h) to get the mean number of events per hour with a duration of at least Δt.
Statistical Analysis
There have been no prior investigations relating the duration of IH to the presence of BPD-PH limiting the ability for power estimation. In a prior study analyzing associations between the duration of IH and the development of severe BPD (6), the median proportion of time per day with a SpO2 below 80% was approximately 8% compared with approximately 3% in infants without BPD during the eighth postnatal week across a population with a median gestational age of 25.5 weeks, which approximates the postnatal age at which BPD-PH is most commonly diagnosed (3). In addition, the adjusted risk ratio for developing severe BPD increased by approximately 1% in the proportion of time per day with SpO2 below 80% (e.g., the adjusted risk ratios with 2% vs. 3% per day below 80% were three and four, respectively). An additional consideration in this population was that approximately 40% of infants with severe BPD would be anticipated to also have associated pulmonary hypertension on the basis of prior epidemiologic studies (3). In the present study, including a broader population of infants with BPD (using the mild BPD definition per National Institute of Child Health and Human Development (NICHD) criteria [18]), we anticipated cases to spend 7% per day with a SpO2 below 80%. Using a confidence level of 95%, a power of 0.8, and an assumed odds ratio of six, we anticipated needing 38 infants per group for adequate power for the proposed analysis.
To evaluate the diagnostic accuracy of IH events for BPD-PH classification, two analytical strategies were employed: 1) receiver operating characteristics (ROC) analysis of IH characteristics to more precisely estimate predictive thresholds; and 2) logistic regression to identify the predictive use of IH characteristics compared with baseline differences between cases and control subjects. For ROC analyses, all continuous IH characteristics that differed between cases and control subjects (P < 0.10) for the binary outcome of BPD-PH were used to generate independent areas under the curve. ROC analysis was also performed within cases for the outcome of death. To appraise the classification accuracy of IH characteristics in relation to other known covariates associated with BPD-PH, logistic regression included demographic, clinical, and successively included IH characteristics that differed between cases and control subjects (P < 0.10). For regression analyses of cases for the outcome of death, a forward stepwise likelihood ratio is used, given the anticipated lower sample size between groups. Demographics, clinical characteristics, and IH characteristics were compared between cases and control subjects using a t test or Mann-Whitney test for continuous measurements and a Fisher exact test for categorical variables. All statistical analyses were performed using SPSS version 28.
Results
Patient Population
Over the course of the study, data from 265 infants were available. From this population, 40 infants developed BPD-PH at a median postmenstrual age of 37.8 (interquartile range [IQR], 28–42) weeks, and 40 infants with BPD but without pulmonary hypertension were available for matched comparisons with a median postnatal age at echo of 37 weeks (IQR, 33–40). The median difference in the PMA at echocardiographic matching between cases and control subjects was 1.2 weeks (IQR, 0.6–1.6). Of the infants with BPD-PH, 13 infants died with a median duration of time between BPD-PH diagnosis and death of 127 days (IQR, 87–271). At the time of BPD-PH diagnosis, four infants had severe disease on the basis of estimated PA pressures by echocardiogram. As infants were matched using gestational age, gestational age did not differ between cases and control subjects (24.4; IQR, 23–26 vs. 24.6; IQR, 23–26). However, cases were born at a lower birth weight (589; IQR, 528–660 vs. 648; IQR, 581–851; P = 0.004) and were more often small for gestational age (12/40 vs. 3/40; P = 0.01). Cases were more often diagnosed with a patent ductus arteriosus (PDA) (22/40 vs. 11/40; P = 0.01) and had a trend toward higher rates of late-onset sepsis (12/40 vs. 5/40; P = 0.06). Infants with BPD-PH who died tended to have a higher FiO2 on postnatal Day 28 (0.70 vs. 0.50; P = 0.06) and more often had a PDA (10/13 vs. 12/27; P = 0.05) compared with infants that survived. Additional characteristics are presented in Table 1.
Table 1.
Demographic and Clinical Characteristics by Pulmonary Hypertension and Survival Status
| BPD (n = 40) |
BPD-PH (n = 40) |
P Value | BPD-PH |
|||
|---|---|---|---|---|---|---|
| Survivors (n = 27) |
Death (n = 13) |
P Value | ||||
| Demographic characteristics | ||||||
| Gestational age (wk), median (IQR) | 24.6 (23–26) | 24.4 (23–26) | 0.65 | 24.6 (23–26) | 23.7 (23–26) | 0.18 |
| Birth weight (g), median (IQR) | 648 (581–851) | 589 (528–660) | 0.004 | 600 (542–715) | 565 (475–610) | 0.15 |
| Multiple gestation, n (%) | 11 (28) | 9 (23) | 0.61 | 6 (22) | 3 (23) | 0.95 |
| Male sex, n (%) | 22 (55) | 21 (53) | 0.82 | 13 (48) | 8 (62) | 0.43 |
| White race, n (%) | 17 (43) | 11 (28) | 0.16 | 7 (26) | 4 (31) | 0.75 |
| Antenatal corticosteroids, n (%) | 36 (90) | 38 (95) | 0.40 | 26 (96) | 12 (92) | 0.59 |
| Cesarean section, n (%) | 28 (70) | 24 (60) | 0.35 | 17 (63) | 7 (54) | 0.58 |
| Histologic chorioamnionitis, n (%) | 23 (58) | 17 (43) | 0.18 | 11 (41) | 6 (46) | 0.75 |
| Small for gestational age, n (%) | 3 (8) | 12 (30) | 0.01 | 6 (22) | 12 (30) | 0.12 |
| Prolonged rupture of membranes, n (%) | 16 (42) | 8 (20) | 0.05 | 7 (26) | 1 (8) | 0.17 |
| Clinical characteristics | ||||||
| Inhaled nitric oxide, n (%) | 4 (10) | 19 (48) | <0.001 | 10 (37) | 9 (69) | 0.06 |
| Corticosteroids for BPD, n (%) | 21 (53) | 31 (78) | 0.02 | 21 (78) | 10 (77) | 0.95 |
| Pharmacologic PDA treatment, n (%) | 6 (15) | 21 (53) | <0.001 | 12 (44) | 9 (69) | 0.14 |
| B-type natriuretic peptide, n (%) | — | — | — | 116 (66–218) | 196 (73–321) | 0.57 |
| 28-d respiratory support | ||||||
| Max FiO2, median (IQR) | 45 (32–65) | 52 (45–75) | 0.08 | 50 (45–70) | 70 (50–94) | 0.06 |
| Nasal cannula, n (%) | 3 (8) | 3 (8) | 1.00 | 3 (11) | 0 (0) | 0.21 |
| Noninvasive ventilation, n (%) | 20 (50) | 16 (40) | 0.37 | 12 (44) | 4 (31) | 0.41 |
| Invasive ventilation, n (%) | 17 (43) | 21 (53) | 0.37 | 12 (44) | 9 (69) | 0.14 |
| 36-wk respiratory support | ||||||
| Room air, n (%) | 0 (0) | 3 (8) | 0.08 | 2 (7) | 1 (8) | 0.97 |
| Nasal cannula, n (%) | 24 (60) | 15 (38) | 0.04 | 14 (52) | 1 (8) | 0.007 |
| Noninvasive ventilation, n (%) | 10 (25) | 11 (28) | 0.80 | 8 (30) | 3 (23) | 0.66 |
| Invasive ventilation, n (%) | 6 (15) | 9 (23) | 0.39 | 3 (11) | 6 (46) | 0.01 |
| Other outcomes | ||||||
| Patent ductus arteriosus, n (%) | 11 (28) | 22 (55) | 0.01 | 12 (44) | 10 (77) | 0.05 |
| Early-onset sepsis, n (%) | 1 (3) | 1 (3) | 1.00 | 1 (4) | 0 (0) | 0.48 |
| Late-onset sepsis, n (%) | 5 (13) | 12 (30) | 0.06 | 7 (26) | 5 (39) | 0.42 |
| NEC ⩾ 2, n (%) | 6 (15) | 4 (10) | 0.50 | 2 (7) | 2 (15) | 0.43 |
| Surgical NEC, n (%) | 1 (3) | 1 (3) | 1.00 | 1 (4) | 0 (0) | 0.48 |
| Death, n (%) | 3 (8) | 13 (33) | 0.005 | — | — | — |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; BPD-PH = BPD-associated pulmonary hypertension; IQR = interquartile range; NEC = necrotizing enterocolitis stage; PDA = patent ductus arteriosus.
IH Characteristic Comparisons
With regard to IH characteristics, cases and control subjects had a similar frequency (P = 0.21) and cumulative duration of IH below 80% (P = 0.98). Similarly, the frequency (P = 0.09) and cumulative duration (P = 0.15) of IH did not differ among cases between survivors and infants that died (Figure 1). In analyzing the median duration of each IH event, cases had IH events of longer duration compared with control subjects whether analyzed by a threshold below 80% (6; IQR, 5–8 vs. 7; IQR, 6–8; P = 0.03) or below 70% (58; IQR, 41–89 vs. 105; IQR, 54–150; P = 0.008) (Table 2 and Figure 2). In analyzing the IH characteristics within infants with BPD-PH, survivors had no difference in the cumulative daily duration and frequency of IH compared with infants that died (Figure 1). Infants who later died had a longer duration of more severe events below 70% (72; IQR, 40–112 vs. 145; IQR, 116–201; P = 0.01) (Figure 3 and Table 2).
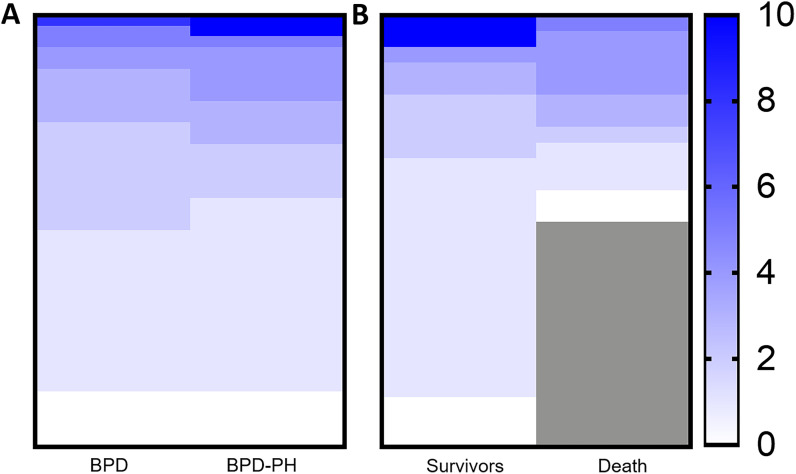
Figure 1.
Cumulative hypoxemia by pulmonary hypertension and survival. Heat map detailing the average cumulative daily proportion (%) of the day infants were hypoxemic below a saturation of 80% between infants with (A) bronchopulmonary dysplasia (BPD) and infants with BPD-associated pulmonary hypertension (BPD-PH) and (B) within infants with BPD-PH by survival status. Each row represents an individual patient. The cumulative proportion did not differ between any group.
Table 2.
Characteristics of Intermittent Hypoxemic Events by Pulmonary Hypertension and Survival Status
| BPD (n = 40) |
BPD-PH (n = 40) |
P Value | BPD-PH |
P Value | ||
|---|---|---|---|---|---|---|
| Survivors (n = 27) |
Death (n = 13) |
|||||
| Cumulative daily duration, s | ||||||
| <80 | 1,345 (598–2,196) | 1,072 (533–2,365) | 0.98 | 1,003 (507–1,824) | 2,343 (863–3,257) | 0.11 |
| <70* | 420 (188–781) | 425 (217–1,189) | 0.54 | 409 (190–917) | 954 (272–1,394) | 0.21 |
| Frequency per intermittent hypoxemic events/d | ||||||
| <80 | 87 (61–203) | 72 (45–148) | 0.21 | 59 (41–128) | 108 (65–202) | 0.06 |
| <70* | 8 (5–22) | 7 (3–14) | 0.16 | 7 (2–13) | 6 (3–25) | 0.26 |
| Duration per intermittent hypoxemic event/d, s | ||||||
| <80 | 6 (5–8) | 7 (6–8) | 0.03 | 7 (6–8) | 8 (7–9) | 0.25 |
| <70* | 58 (41–89) | 105 (54–150) | 0.008 | 72 (40–112) | 145 (116–201) | 0.01 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; BPD-PH = BPD-associated pulmonary hypertension.
Data using a threshold under 70% are not exclusive to data using a threshold under 80%.
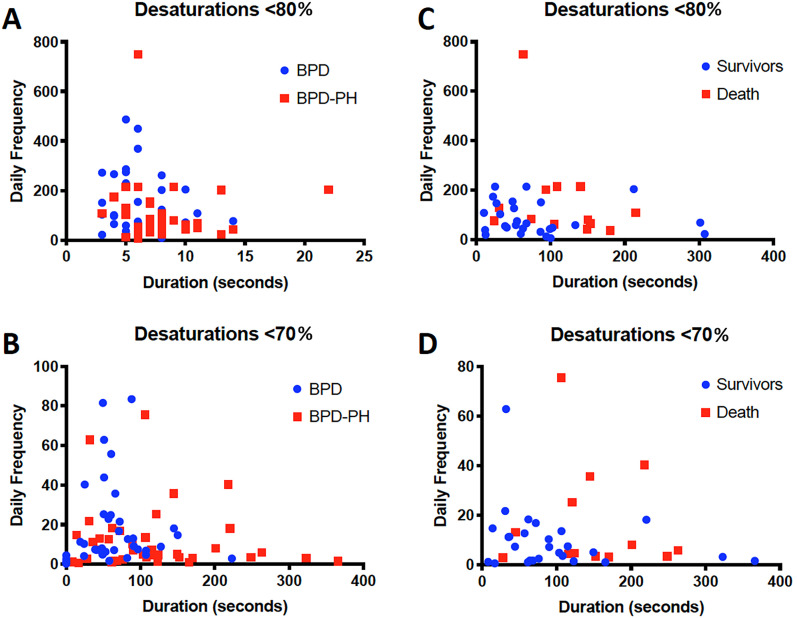
Figure 2.
Intermittent hypoxemic events by pulmonary hypertension (PH) and survival. (A and B) Characterization of intermittent hypoxemic events in the week preceding the initial echocardiographic diagnosis of bronchopulmonary dysplasia associated PH (BPD-PH; n = 40) compared with events in infants with BPD without PH (n = 40). (C and D) Comparisons within infants with BPD-PH between survivors (n = 27) and infants that died (n = 13). Events are stratified by thresholds of intermittent hypoxemia events ([A and C] <80%; [B and D] <70%) with further classification by median frequency and median duration of observed events. Data using a threshold below 70% are not exclusive to data using a threshold below 80%. The duration of events differed between cases and control subjects for desaturations (<80%: P = 0.03; <70%: P = 0.008) and between survivors and infants that died with events less than 70% (P = 0.01). The frequency of events did not differ.
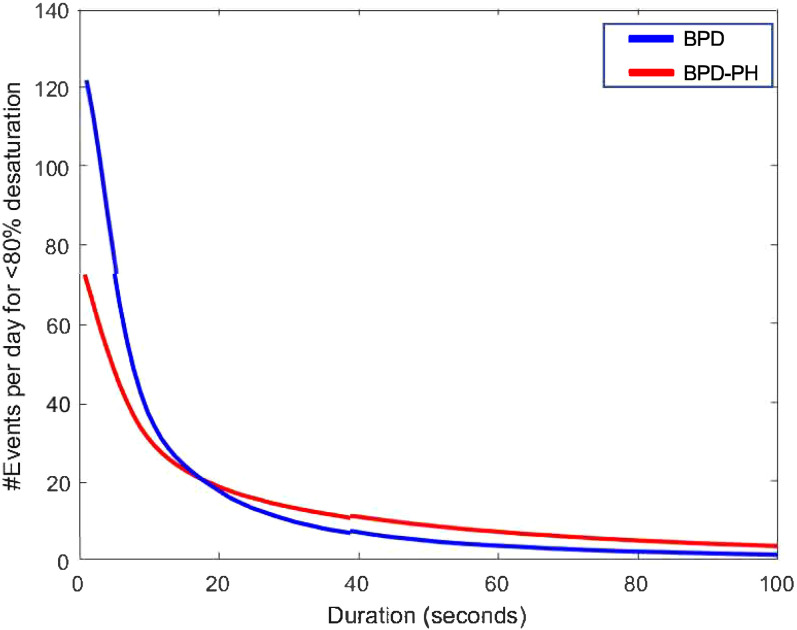
Figure 3.
The total number of intermittent hypoxemic events per day by pulmonary hypertension (PH) status. Infants with bronchopulmonary dysplasia (BPD)-associated PH were more likely to have intermittent hypoxemic events longer than 18 seconds and more than twice as likely to have events longer than 1 minute when compared with infants with BPD alone.
The mean number of desaturations as a function of their minimal duration is shown in Figure 3. On average, cases are more likely to have intermittent hypoxemic events longer than 18 seconds and more than twice as likely to have events longer than 1 minute when compared with control subjects. There were, on average, seven events per day longer than 1 minute among cases compared with 3.5 events per day in the control subject group.
IH and BPD-PH Detection
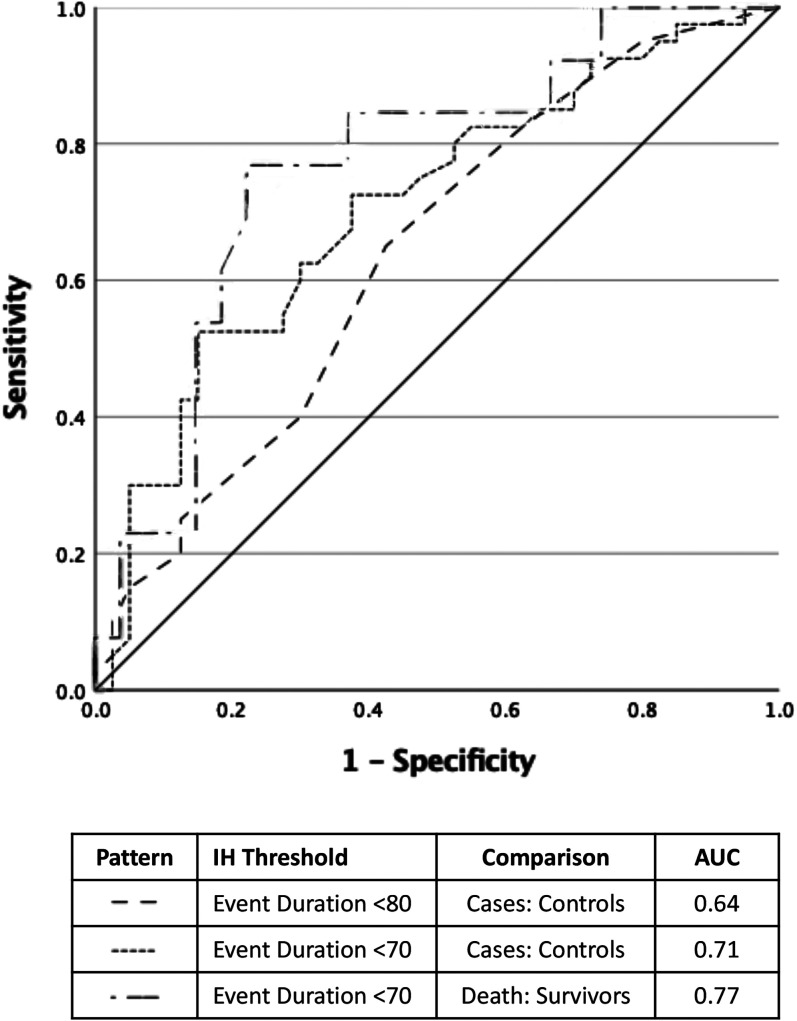
Using IH severity strata, ROC analysis resulted in the following areas under the curve between cases and control subjects: 0.64 and 0.71, whether event duration was analyzed by a threshold below 80% or below 70%, respectively (Figure 4). The area under the curve from ROC analysis for survival in infants with BPD-PH using a threshold below 70% was 0.77 (Figure 4). In integrating clinical and IH characteristics into regression models, the model adjusted for the following characteristics between cases and control subjects: birth weight, PDA, late-onset sepsis, and FiO2. Neither the adjusted odds ratio (OR) for the duration of IH below 80% (1.09; 95% confidence interval [CI], 0.87–1.35) nor for IH duration below 70% (1.00; 95% CI, 0.96–1.03) were significant following adjustment between cases and control subjects (Table 3). Within cases, the duration of IH events below 70% differed by survival status (adjusted OR, 1.22; 95% CI, 1.00–1.47; P = 0.04).
Figure 4.
Prediction of pulmonary hypertension (PH) status by IH duration. Receiver operating characteristic curve for the diagnosis of bronchopulmonary dysplasia (BPD)-associated PH by case:control comparisons using the duration of hypoxemic events and for survival within infants with BPD-PH. AUC = area under the curve; IH = intermittent hypoxemia.
Table 3.
Odds Ratios of Bronchopulmonary Dysplasia-associated Pulmonary Hypertension and Survival by Clinical Covariates
| Variable | Crude OR (95% CI) | Adjusted OR* | 95% CI |
P Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Analysis between cases and control subjects* | |||||
| Birth weight† | 0.62 (0.44–0.87) | 1.00 | 0.48 | 0.94 | 0.02 |
| Patent ductus arteriosus | 3.22 (1.27–8.19) | 3.18 | 1.13 | 8.95 | 0.03 |
| Late-onset sepsis | 3.00 (0.95–9.53) | 2.39 | 0.66 | 8.64 | 0.18 |
| FiO2 at postnatal Day 28‡ | 1.20 (0.99–1.45) | 1.01 | 0.94 | 1.44 | 0.17 |
| IH event duration <80%§ | 1.22 (1.01–1.47) | 1.09 | 0.87 | 1.35 | 0.46 |
| IH event duration <70%§ | 1.00 (1.00–1.00) | 1.00 | 0.96 | 1.03 | 0.76 |
| Analysis by survivalǁ | |||||
| Patent ductus arteriosus | 4.17 (0.93–18.62) | 6.81 | 1.09 | 42.42 | 0.04 |
| FiO2 at postnatal Day 28‡ | 1.03 (1.00–1.06) | NS | — | — | — |
| IH event duration <70%§ | 1.03 (1.00–1.07) | 1.22 | 1.00 | 1.47 | 0.04 |
Definition of abbreviations: CI = confidence interval; IH = intermittent hypoxemia; OR = odds ratio; NS = not significant.
Analyses by logistic regression between cases and control subjects.
For every 100 g change in birth weight.
For every 10% change in FiO2.
For every second duration of an intermittent hypoxemic event. Separate regression models performed for each IH characteristic.
Analyses by forward stepwise likelihood ratio within cases by outcome of survival.
Discussion
In this observational case-control investigation in extremely preterm infants with lung disease, IH differed between infants with BPD-PH and infants with BPD but without pulmonary hypertension in the week before PH was diagnosed in those with BPD-PH. More specifically, the average duration of individual IH events but not the total duration of IH below 80% differed between groups. However, the predictive use of IH characteristics was only retained within cases for the outcome of mortality following adjustment for baseline differences, suggesting that IH parameters may have greater use in predicting outcomes in infants with established BPD-PH rather than establishing the presence of BPD-PH. Moreover, in infants with BPD-PH, characteristics of IH events could discriminate between survivors and infants that later died, with a median interim of 127 days between BPD-PH diagnosis and death.
Previous evidence has related hypoxemia to increases in pulmonary vascular resistance and evaluated the impact of both intermittent and sustained hypoxia on pulmonary vascular remodeling. Chronic hypoxia is one of the most common animal model exposures used to induce pulmonary vascular remodeling. In a neonatal rat model, rats exposed to 14 days of hyperoxia and subsequently recovered in IH had increased arterial wall thickness and increased pulmonary vascular resistance compared with exposure to control subjects later exposed to IH (19). In an adult rat model, hypobaric hypoxia exhibits a doubling in the thickness of hilar pulmonary arteries from smooth muscle cell hypertrophy and changes in the extracellular matrix (20). In a lamb model for persistent pulmonary hypertension of the newborn, targeting oxygen saturations of 85–89% results in reduced pulmonary perfusion and higher pulmonary vascular resistance compared with higher saturation targets (14). Whereas the cumulative exposure to hypoxemia below 80% did not differ between cases and control subjects in our study, the duration of IH events and the duration by IH severity strata differed. Given that shorter durations of IH exposure may not result in increased right ventricular systolic pressures (21) compared with more prolonged IH exposure (22) in mice, extremely preterm infants’ risk for BPD-PH may be similarly impacted by the duration of IH events.
The association between IH duration and preterm outcomes has previously been reported in randomized trials of extremely preterm infants exposed to different oxygen saturation targets. In the Canadian Oxygenation Trial, a secondary analysis associated the outcomes of death and neurodevelopmental impairment by the duration of IH. By using stepwise logistic models, varying lengths of IH duration were related to adverse outcomes, with the central finding that episodes lasting more than 1 minute were more commonly associated with late death or disability (OR, 3.40; 95% CI, 1.95–5.93; P < 0.001) compared with shorter episodes (23). Similarly, in analyzing episodes from the Surfactant Positive Airway Pressure and Pulse Oximetry Trial, investigators reported that a higher frequency of both short IH events and long IH events was associated with an increased risk for mortality (24). In the present study, infants with BPD-PH were twice as likely to have an event lasting longer than 1 minute. Given that infants with BPD-PH have a high risk for mortality (3) and other adverse long-term outcomes (25), the development of BPD-PH may be a plausible mechanism for these observations noted from these secondary analyses.
Few biomarkers accurately predict extremely preterm infants’ risk for developing BPD-PH. We and others have previously reported baseline and clinical differences associated with the development of BPD-PH in extremely preterm infants that were also observed in this cohort, including birth weight, small for gestational age, FiO2, and severe BPD status (11, 26). In addition to these variates, previously considered predictors of later BPD-PH development have included metabolomic signature differences from umbilical blood (27), BNP (B-type natriuretic peptide) (28, 29), and various markers of angiogenesis (30). Although each of these considerations may identify infants at higher risk, none of these predictors confer a continuous, noninvasive metric through which BPD-PH may be identified in real time. Moreover, the current recommendations from the American Heart Association regarding screening for BPD-PH are limited to a diagnostic echocardiogram in infants with moderate to severe BPD at a postnatal age of 36 weeks postmenstrual age (4). However, a BPD-PH diagnosis may have been missed in 50% of infants diagnosed after 36 weeks PMA in this cohort, and in 20% of infants, disease may have been detected more than 1 week earlier using the identified characteristics of IH.
As BPD-PH may increase infants’ risk for mortality fivefold, investigators have identified risk factors for death in infants with BPD-PH, including BNP and echocardiographic characteristics. Contrary to our group’s previously reported association between the highest BNP during hospitalization and mortality (28), BNP did not confer predictive use in regression models for death. However, the present study only included BNP measurements at the time of BPD-PH diagnosis. In a recent cohort study of extremely preterm infants with BPD-PH, suprasystemic pulmonary artery pressures were associated with worse survival (hazard ratio, 6.46; 95% CI, 1.86–22.44; P = 0.003) when compared with infants with subsystemic or systemic level pulmonary artery pressure (26). Prior investigations, further classifying BPD-PH severity with cardiac catheterization, have similarly related the severity of BPD-PH to the probability of survival (31). However, echocardiography has limited ability to characterize the severity of pulmonary hypertension compared with cardiac catheterization (5). Of the infants who died only in the present cohort at the time of BPD-PH diagnosis, only one was diagnosed with severe disease (8%), and 10 infants (77%) had either mild disease or severity that could not be estimated. Further validation studies may support the use of IH characteristics as a continuous, noninvasive biomarker for death in infants with BPD-PH.
Timely detection of BPD-PH using IH characteristics may confer therapeutic opportunities in this high-risk population. Although 33% of cases died in the present study, 44% of infants had BPD-PH resolution suggesting that BPD-PH trajectory, though severe in some patients, may also be modifiable. Prior animal investigations have demonstrated that chronic IH exposure followed by normoxic recovery may reduce pulmonary vascular remodeling (32). This is currently supported by recommendations from the American Heart Association to maintain oxygen saturations of 92–95% in infants with BPD-PH (4). If IH characteristics confer real-time detection of BPD-PH, monitoring for attenuation of these same IH metrics may similarly provide clinicians with a novel measure of therapeutic use.
There is currently limited evidence for therapeutic strategies that either prevent BPD-PH or improve outcomes in infants with established BPD-PH. Moreover, meta-analyses of pulmonary vasodilators, including oxygen saturation targeting and inhaled nitric oxide, have not specifically assessed the impact of these therapies on BPD-PH (33, 34). The use of sildenafil is associated with improvement in BPD-PH but does not improve survival (35). Although the present analysis suggests that prolonged hypoxemic events may predict BPD-PH, the impact of event reduction on outcomes remains to be studied. Regarding other plausible therapeutic opportunities to prevent BPD-PH development, the proportion of infants with a PDA differed between infants with and without BPD-PH and by survival status among infants with BPD-PH. Should evidence support a PDA as mechanistically contributory to BPD-PH pathogenesis, the clinical decision for PDA closure should first consider temporary occlusion to determine the hemodynamic implications of permanent closure (4).
Strengths of this study include a relatively large cohort of extremely preterm infants with established BPD-PH for which high-fidelity physiologic measurements were available. This not only enabled case:control comparisons but also regression modeling to account for anticipated differences in baseline characteristics. Although data were prospectively collected, it was retrospectively applied to infants with established BPD-PH. Whether prospective, continuous application of IH characteristics to infants at risk for BPD-PH can provide earlier detection remains to be studied. In addition, the interaction between IH characteristics and BPD-PH is limited to association. Whether IH physiologically contributes to BPD-PH pathogenesis or is a result of existing disease could not be evaluated. The accuracy of pulse oximetry may also be limited at the lower saturation strata used in this investigation, as reported previously (36). Lastly, validation of these findings by other centers will be needed before broader diagnostic application.
Conclusions
In this case-control study comparing extremely preterm infants with BPD-PH to infants with BPD but without pulmonary hypertension, the average duration of each event but not the frequency or cumulative duration of IH events differed between cases and control subjects. However, the discriminating use of these findings was not significant after adjustment between cases and control subjects and only differed by survival status within cases. Given the limited ability of clinicians to predict disease severity and monitor the impact of therapeutic strategies in limiting BPD-PH severity, defining IH characteristics in individual infants may permit a range of applications for the detection and management of PH in this high-risk population on further validation in an independent cohort.
Footnotes
Supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (K23HL157618, LDCC U01 HL133708, U01 HL133536, and U01 HL133536-05S1).
Author Contributions: S.J.G.: helped with manuscript conceptualization, data curation, formal analysis, original draft preparation, review and editing, and provided final approval. C.P.T.: contributed to original draft preparation, review and editing, and provided final approval. A.N.: contributed to interpretation of data for the work, statistical analysis, manuscript review and editing, and provided final approval. P.I.: contributed to manuscript review and editing and provided final approval of the version to be published. W.A.C.: contributed to conceptualization, interpretation of data for the work, original draft preparation, review and editing, and provided final approval. N.A.: contributed to conceptualization, interpretation of data for the work, original draft preparation, review and editing, and provided final approval of the version to be published.
Originally Published in Press as DOI: 10.1164/rccm.202203-0580OC on November 30, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics . 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med . 2019;200:751–759. doi: 10.1164/rccm.201812-2348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arjaans S, Zwart EAH, Ploegstra MJ, Bos AF, Kooi EMW, Hillege HL, et al. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: a systematic review and meta-analysis. Paediatr Perinat Epidemiol . 2018;32:258–267. doi: 10.1111/ppe.12444. [DOI] [PubMed] [Google Scholar]
- 4. Abman SH, Ivy DD, Archer SL, Wilson K, AHA/ATS Joint Guidelines for Pediatric Pulmonary Hypertension Committee Executive summary of the American Heart Association and American Thoracic Society joint guidelines for pediatric pulmonary hypertension. Am J Respir Crit Care Med . 2016;194:898–906. doi: 10.1164/rccm.201606-1183ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics . 2008;121:317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jensen EA, Whyte RK, Schmidt B, Bassler D, Vain NE, Roberts RS, Canadian Oxygen Trial Investigators Association between intermittent hypoxemia and severe bronchopulmonary dysplasia in preterm infants. Am J Respir Crit Care Med . 2021;204:1192–1199. doi: 10.1164/rccm.202105-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest . 1996;109:380–386. doi: 10.1378/chest.109.2.380. [DOI] [PubMed] [Google Scholar]
- 8. Samhouri B, Venkatasaburamini M, Paz Y Mar H, Li M, Mehra R, Chaisson NF. Pulmonary artery hemodynamics are associated with duration of nocturnal desaturation but not apnea-hypopnea index. J Clin Sleep Med . 2020;16:1231–1239. doi: 10.5664/jcsm.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toraldo DM, Minelli M, De Nuccio F, Nicolardi G. Chronic obstructive pulmonary disease phenotype desaturator with hypoxic vascular remodelling and pulmonary hypertension obtained by cluster analysis. Multidiscip Respir Med . 2012;7:39. doi: 10.1186/2049-6958-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dennery PA, Di Fiore JM, Ambalavanan N, Bancalari E, Carroll JL, Claure N, et al. Pre-Vent: the prematurity-related ventilatory control study. Pediatr Res . 2019;85:769–776. doi: 10.1038/s41390-019-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics . 2012;129:e682–e689. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gentle S, El-Ferzli G, Winter L, Salas AA, Philips Iii JB. Oxygen saturation histogram monitoring to reduce death or retinopathy of prematurity: a quality improvement initiative. J Perinatol . 2020;40:163–169. doi: 10.1038/s41372-019-0486-7. [DOI] [PubMed] [Google Scholar]
- 13. Di Fiore JM, MacFarlane PM, Martin RJ. Intermittent hypoxemia in preterm infants. Clin Perinatol . 2019;46:553–565. doi: 10.1016/j.clp.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rawat M, Chandrasekharan P, Gugino SF, Koenigsknecht C, Nielsen L, Wedgwood S, et al. Optimal oxygen targets in term lambs with meconium aspiration syndrome and pulmonary hypertension. Am J Respir Cell Mol Biol . 2020;63:510–518. doi: 10.1165/rcmb.2019-0449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res . 2009;66:539–544. doi: 10.1203/PDR.0b013e3181bab0c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosychuk RJ, Hudson-Mason A, Eklund D, Lacaze-Masmonteil T. Discrepancies between arterial oxygen saturation and functional oxygen saturation measured with pulse oximetry in very preterm infants. Neonatology . 2012;101:14–19. doi: 10.1159/000326797. [DOI] [PubMed] [Google Scholar]
- 17. Dawson JA, Bastrenta P, Cavigioli F, Thio M, Ong T, Siew ML, et al. The precision and accuracy of Nellcor and Masimo oximeters at low oxygen saturations (70%) in newborn lambs. Arch Dis Child Fetal Neonatal Ed . 2014;99:F278–F281. doi: 10.1136/archdischild-2013-305091. [DOI] [PubMed] [Google Scholar]
- 18. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med . 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 19. Mankouski A, Kantores C, Wong MJ, Ivanovska J, Jain A, Benner EJ, et al. Intermittent hypoxia during recovery from neonatal hyperoxic lung injury causes long-term impairment of alveolar development: a new rat model of BPD. Am J Physiol Lung Cell Mol Physiol . 2017;312:L208–L216. doi: 10.1152/ajplung.00463.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyrick B, Reid L. Hypoxia-induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am J Pathol . 1980;100:151–178. [PMC free article] [PubMed] [Google Scholar]
- 21. Zhen X, Moya EA, Gautane M, Zhao H, Lawrence ES, Gu W, et al. Combined intermittent and sustained hypoxia is a novel and deleterious cardio-metabolic phenotype. Sleep (Basel) . 2021;45:zsab290. doi: 10.1093/sleep/zsab290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, et al. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol . 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, et al. Canadian Oxygen Trial Investigators Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA . 2015;314:595–603. doi: 10.1001/jama.2015.8841. [DOI] [PubMed] [Google Scholar]
- 24. Di Fiore JM, Martin RJ, Li H, Morris N, Carlo WA, Finer N, et al. SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health; Human Development Neonatal Research Network Patterns of oxygenation, mortality, and growth status in the surfactant positive pressure and oxygen trial cohort. J Pediatr . 2017;186:49–56.e1. doi: 10.1016/j.jpeds.2017.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakanishi H, Uchiyama A, Kusuda S. Impact of pulmonary hypertension on neurodevelopmental outcome in preterm infants with bronchopulmonary dysplasia: a cohort study. J Perinatol . 2016;36:890–896. doi: 10.1038/jp.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arjaans S, Haarman MG, Roofthooft MTR, Fries MWF, Kooi EMW, Bos AF, et al. Fate of pulmonary hypertension associated with bronchopulmonary dysplasia beyond 36 weeks postmenstrual age. Arch Dis Child Fetal Neonatal Ed . 2021;106:45–50. doi: 10.1136/archdischild-2019-318531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. La Frano MR, Fahrmann JF, Grapov D, Pedersen TL, Newman JW, Fiehn O, et al. Umbilical cord blood metabolomics reveal distinct signatures of dyslipidemia prior to bronchopulmonary dysplasia and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol . 2018;315:L870–L881. doi: 10.1152/ajplung.00283.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cuna A, Kandasamy J, Sims B. B-type natriuretic peptide and mortality in extremely low birth weight infants with pulmonary hypertension: a retrospective cohort analysis. BMC Pediatr . 2014;14:68. doi: 10.1186/1471-2431-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Montgomery AM, Bazzy-Asaad A, Asnes JD, Bizzarro MJ, Ehrenkranz RA, Weismann CG. Biochemical screening for pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Neonatology . 2016;109:190–194. doi: 10.1159/000442043. [DOI] [PubMed] [Google Scholar]
- 30. Kim DH, Kim HS. Serial changes of serum endostatin and angiopoietin-1 levels in preterm infants with severe bronchopulmonary dysplasia and subsequent pulmonary artery hypertension. Neonatology . 2014;106:55–61. doi: 10.1159/000358374. [DOI] [PubMed] [Google Scholar]
- 31. Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics . 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 32. Umeda A, Miyagawa K, Mochida A, Takeda H, Takeda K, Okada Y, et al. Effects of normoxic recovery on intima-media thickness of aorta and pulmonary artery following intermittent hypoxia in mice. Front Physiol . 2020;11:583735. doi: 10.3389/fphys.2020.583735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Neonatal Oxygenation Prospective Meta-analysis (NeOProM) Collaboration Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA . 2018;319:2190–2201. doi: 10.1001/jama.2018.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev . 2017;1:CD000509. doi: 10.1002/14651858.CD000509.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Graaf M, Rojer LA, Helbing W, Reiss I, Etnel JRG, Bartelds B. EXPRESS: sildenafil for bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis. Pulm Circ . 2019;9:2045894019837875. doi: 10.1177/2045894019837875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wackernagel D, Blennow M, Hellström A. Accuracy of pulse oximetry in preterm and term infants is insufficient to determine arterial oxygen saturation and tension. Acta Paediatr . 2020;109:2251–2257. doi: 10.1111/apa.15225. [DOI] [PubMed] [Google Scholar]