Abstract
The main aimed of this study was to provide information on microplastics present in the freshwater of fish farm ponds. In addition, the study showes a relationship between the seasonal, spatial distribution and the amount of microplastics found. This study was conducted in 35 fish farms located in the Rondônia state, Brazil, the sample collects were carried out in the two Amazonian hydrological seasons (dry and rainy). The study was developed in a completely randomized factorial scheme 35 × 3 x 3 (35 fish farms, 3 ponds and 3 repetitions per ponds). Microplastic sampling was performed following a modified method based on National Oceanic and Atmospheric Administration (NOAA). Samples of 250 mL freshwater collected, which were deionized and pre-filtered through 6.0 mm mesh granulometric sieves. The average abundances of the different hydrological seasons were compared by Student's t-test, with differences statistically significant at p < 0.05. The microplastics were morphological categorized into fibers and colors blue, red or transparent. Microplastic contamination was confirmed in freshwater of 9 fish farming, with greater abundance of blue fibers and greater quantification in the rainy season. Fish farms P3, P4 and P6 had the highest quantifications of blue fiber in the two seasons (6 and 43, 19 and 56, 11 and 88 items mL−1, respectively). Almost all fish farms had a higher abundance of microplastics in the rainy season. It is important to highlight the prominence of microplastics in the blue fiber rainy season (286 items mL−1) compared to the dry season (58 items mL−1). Fish farms P3, P4 and P6 showed a strong positive correlation between the factors distance from the nearest urban area (r = 0.94, 0.79 and 0.97, respectively) and seasonality (r = 0.98, 0.77 and 0.96, respectively). Rainfall variations influenced the abundance of microplastics, especially of blue fibers. Fish farms are supplied with fresh water by rivers or streams, so it is possible that microplastics originate outside the fish farm, perhaps they were introduced due to high soil occupation, although surface runoff (of water contaminated by sewage) caused by heavy rains the most important factor. Therefore, one factor must be considered, surface runoff and groundwater contaminated by urban, agricultural and urban effluents may have contaminated rivers and streams and then contaminated the water in the fish farm ponds.
Keywords: Fish farming, Freshwater pollution, Microplastic contamination, Brazilian Western Amazon
1. Introduction
Plastics are versatile, flexible, resistant and inexpensive materials, making them widely used in modern society. According to Plastic Europe [1], 359 million tons of plastic were produced in year 2018 and less than 10% was recycled. The increase in plastic production is attributed to the exponential growth of the world's population, and it is estimated that between 4.8 and 12.7 million tons of plastic enter the oceans every year, mostly in the form of plastic debris dumped by rivers and lakes during extreme flooding [2]. In addition, these water bodies can act as sinks for these plastic materials and, once in these water bodies, plastic removal and recovery becomes difficult and, in the case of small fragments and colored fibers, completely impossible. Representing a greater proportion of plastic debris are microplastics, defined as plastic particles with diameters in the range of 0.001–5.0 mm, and their accumulation in bodies of water is a topic of particular concern [3].
The source of these microplastics can primary, such as microbeads, pellets, personal care products and cosmetics, or secondary, such as those formed as a result of the wear and tear of larger plastics [4]. These particles are considered environmental hazards in water bodies since they can affect aquatic life directly or indirectly when ingested and transferred to the food chain [5,6]. For example, Qiang and Cheng [7] observed that the presence of microplastics significantly decreased the swimming competence of Danio rerio larvae. Furthermore, as a consequence of the high stability, mobility in water and hydrophobic nature of microplastics, they can absorb substances and serve as vehicles in the transport of contaminants in aquatic systems, enabling their transfer and accumulation at trophic levels [8]. In view of these facts, there is a demand for investigations of the abundance and distribution of microplastics in different environments and regions, especially tropical ones where studies on this topic are scarce.
Population growth, together with the population's lifestyle, is causing an increase in the consumption of non-biodegradable materials, especially those of plastic origin, generating a significant amount of solid waste in water resources [9]. In Brazil, in year 2019 alone, WWF estimates that 11.3 million tons of plastic waste were produced. With this, Brazil corresponds to the 4th position of the largest generators of plastic waste in the world [10]. According to Huang et al. [11], undoubtedly, as a consequence of the inefficient management of plastic waste, they are discarded without any treatment. It is estimated that between 6 and 26% are recycled, while 94% are deposited in landfills or directly into the environment [9].
In the sanitation ranking of 200 countries, Brazil occupies the 112nd position, behind some countries in North Africa and the Middle East with lower per capita income than Brazil [12]. In addition, basic sanitation in Brazil is precarious, where only about 55% of Brazilian municipalities have some type of sewage service network, and when referring to the North of Brazil, these data are much worse, more than 90% of municipalities do not have any sewage system [13]. Although the sewage treatment process does not guarantee the complete removal of microplastic particles from wastewater, all these factors certainly contribute to the presence of microplastics in water, making it difficult to develop studies aimed at their identification, quantification and urgent removal.
Microplastic contamination can derive from many sources and reach high densities widely distributed in a watershed. This water pollution can reach beaches, sea water column, sediments, soil, atmosphere, freshwater from rivers, lakes and even aquacultural systems such as fish farms [14]. The latter is a very serious case, for example, the Rondônia state is the third largest producer of fishes in Brazil and the largest producer of native fishes in the Amazon, corresponding to a production of 59.6 thousand tons of fish produced in year 2021 [15]. Microplastic contamination can cause damage to the water quality of fish farm ponds, as well as an impediment to the final product quality.
Until now, in Brazil, research on microplastic contamination has been mainly focused on ocean coastal environments. Lima et al. [16] found that the density of microplastics represents half of the total density of freshwater fish in tropical regions. In addition, they also observed a positive relationship between the rainy season and the presence of microplastics. Faria et al. [17] investigated the contamination of the South American Pantanal, where they found an average of 9.6 ± 8.3 items m−3, with fiber being the most dominant form. An important aspect to consider is that, although Brazil is an underdeveloped country, plastic consumption patterns in some very urbanized regions are similar to those observed in developing and developed countries. In year 2018, Brazil manufactured 8.3 million tons of plastic materials, which represents 57% of the amount produced in South America [18], and of the plastic waste generated, Brazil recycles only about 1.3%, shown the potential for environmental contamination by these residues.
There are several studies in the literature that showes the presence of microplastic contamination in the oceans. However, in freshwater these studies are scarce, and in reference to Amazonian fish farming, studies on this topic are even rarer. Therefore, in view of these aspects, the main aimed of this study was to provide information on microplastics present in the freshwater of fish farm ponds. In addition, the study presents a relationship between the seasonal, spatial distribution and the amount of microplastics found.
2. Material and methods
2.1. Study area
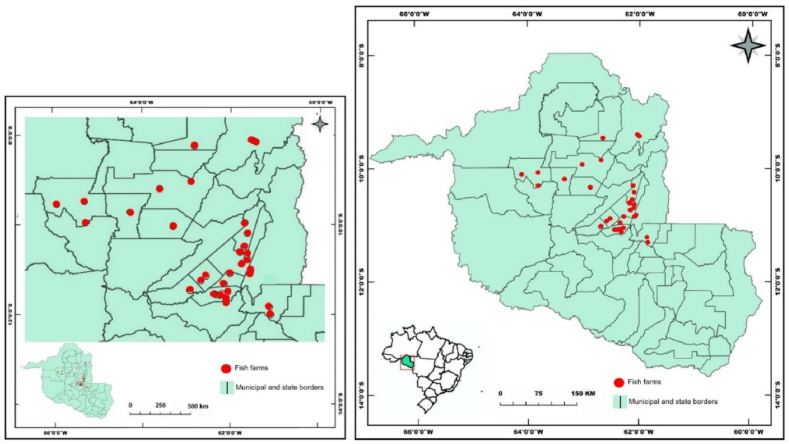
The Rondônia state stands out in the production of Colossoma macropomum (Cuvier, 1818), the Central-East microregion has 879 registered fish farms. Also, the Vale do Jamari microregion has 707 registered fish farms [19]. These two microregions were chosen for this study because they better represent fish production practices in Rondônia state, Brazil. There were 35 fish farms located in the municipalities of Ji-Paraná, Ouro Preto do Oeste, Mirante da Serra, Nova União and Vale do Paraíso, belonging to the Center-East microregion. And also, in the municipalities of Ariquemes, Alto Paraíso, Buritis, Cacaulândia, Campo Novo de Rondônia, Cujubim, Monte Negro and Rio Crespo, belonging to the microregion of Vale do Jamari (Fig. 1).
Fig. 1.
Geographic location of sampling points in fish farms located in municipalities from Rondônia state, Brazil.
On average, the fish farms adopted a semi-intensive production system (a maximum of 6.0 kg m−2 per year with an annual cycle), covered up to 5 ha of water depth, distributed in ponds (excavated tanks so they are called in the Amazon), with an average depth of 1.60 m, which are intended for the fattening of C. macropomum.
The Rondônia state climate is classified in the Köppen system as predominant type Am - Rainy Tropical Climate [20]. Since the climatological average air temperature during the coldest month is greater than 18 °C (megathermal) and a well-defined dry season, when there is a moderate water deficit with rainfall indices lower than 50 mm month−1. The average annual rainfall ranges between 1400 and 2600 mm year−1, while the monthly average air temperature ranges 24–26 °C [21]. Regarding meteorological data, the months of October/2021 (200.00 mm), December/2021 (189.50 mm), January/2022 (336.05 mm) and February/2022 (359.66 mm), showed the highest precipitation averages. While the months of August/2021 and September/2021 had the highest average temperatures, >35 °C, respectively. There were no significant variations in the other months.
The rainfall data during the development in the current study were obtained at the Instituto Nacional de Pesquisas Espaciais (INPE), at Centro de Previsão do Tempo e Estudos Climáticos (CPTEC), in Ouro Preto do Oeste city, Rondônia state, Brazil.
2.2. Sampling, quantification and qualification of microplastics
The study was developed in a completely randomized factorial scheme 35 × 3 x 3 (35 fish farms, 3 ponds and 3 repetitions per ponds). The 35 fish farms defined for data collect, based on the availability of the Agência de Assistência Técnica e Extensão Rural (EMATER) Agency of Rondônia state and fish farms are commercially active. Sample collections were carried out in the two Amazonian hydrological seasons, dry (May to August/2022) and rainy (September/2021 to April/2022). Three samples of freshwater were obtained from three different points in the fish farm ponds. That is, the collect freshwater points considered were, in the supply channel, in the drainage pipe and in the tanks water column [9].
Microplastic sampling was performed following a modified method based on National Oceanic and Atmospheric Administration (NOAA) [22]. To obtain the microplastics, horizontal and vertical hauls were carried out with a zooplankton net. Samples of 250 mL of fresh water were collected, which were pumped and filtered through 6.0 mm mesh granulometric sieves [3]. Then, the sieve was rinsed with sterile and pre-filtered freshwater and the retained microplastics were transferred to a vial containing saline solution (0.85%) [3], stored and transported for laboratory analysis.
Residual solids were transferred to a clean beaker using 200 mL of distilled water. The quantification and qualification of microplastics was performed through a Neubauer chamber with the aid of a micropipette, after ensuring it was sanitized with distilled water and disinfected with alcohol 54°. It is important to detail that the slides (with 0.05 mL of freshwater) were analyzed 9 times [18].
These analyzes were performed with the aid of a Trinocular stereoscopic microscope (Sigma, USA) with 10× magnification and equipped with a digital camera. From here, photomicrographs of the images obtained were created using a professional photographic camera (Canon EOS Rebel T8i EF-S 18–55 mm). To contribute to the interpretation, the photomicrographs were analyzed using image analysis software, Olympus Stream.
The microplastics were morphological categorized into fragments, fibers and microspheres and into colors (blue, red or transparent) according to the methodologies of Bertoldi et al. [3]. On the laboratory bench, when quantifying each sample point, petri dishes with 50 mL of distilled water were placed to carry out the laboratory blank count.
To avoid potential microplastic contamination in the laboratory, all instruments and materials were thoroughly washed with deionized and pre-filtered water three times before use, the number of people working in the same space was reduced, and all researchers wore cotton coats, masks and gloves of nitrile during the process. Materials and samples were covered to prevent air contamination. For more details on the methodology for detecting and quantifying solid waste, see Bertoldi et al. [3].
2.3. Statistical analysis
To determine the variation in observation of microplastics in the stereomicroscope, the counts were performed twice, in different hydrological seasons (dry and rainy), by the same individual observer. For observation, the same microplastic characteristics were adopted in both counts. The abundance of microplastics (dry and rainy) was demonstrated in terms of average counts for each location, with the standard deviation between the two counts. The averages of the different hydrological seasons were contrasted by Student's t-test, with differences considered statistically significant at p < 0.05. All results were considered statistically significant at p < 0.05. Finally, Pearson's correlation was applied to the abundance averages with the factors distance from the nearest urban area and seasonality variations.
Regarding the distance to the nearest urban area, the distance was calculated from the measurements of distances in the ArcGis 2021 Q4 Release software. All statistical analysis were performed using RStudio Development Core Team, version 3.5.3.
3. Results
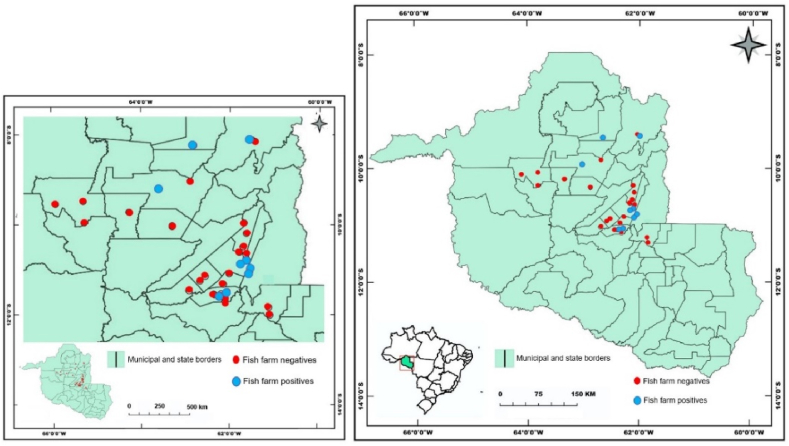
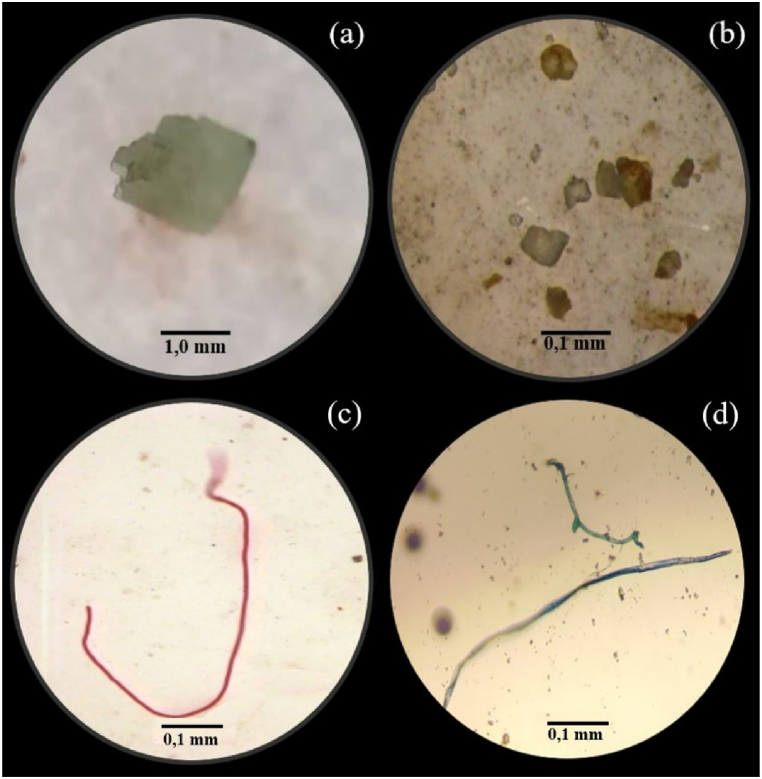
In the current study was carried out in 10 fish farms in the Vale do Jamari microregion and in 25 fish farms in the Central-East microregion, both microregions in interior of the Rondônia state, Brazil. Of the 35 fish farms visited and sampled, microplastic contamination was detected in 9 fish farms (Fig. 2). Were found 3 morphological categories of microplastics, transparent fiber (Fig. 3 a), several transparent fibers in the same sample (Fig. 3 b), red fiber (Fig. 3 c) and blue fibers (Fig. 3 d). However, microplastics of the fragment and microsphere categories were not found.
Fig. 2.
Evidence of microplastic contamination (negative and positive) in fish farms in Rondônia state, Brazil.
Fig. 3.
Microplastics found in freshwater from excavated tanks fish farm in Rondônia state, Brazil. (a) Transparent fiber, (b) several transparent fibers in the same sample, (c) red fiber and (d) blue fibers. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1 showes the quantifications of microplastics (item mL−1) in the freshwater of fish farm ponds at the hydrological seasons (dry and rainy). Microplastics were morphological categorized into transparent fiber, red fiber and blue fiber. Blue fiber was prominent among the microplastic categories. The P7 fish farm had the highest quantification of transparent fibers in the two seasons (10 and 9 items mL−1), while the P6 fish farm had the highest quantification of red fiber in the two seasons (7 and 22 items mL−1). In addition, fish farms P3, P4 and P6 had the highest quantifications of blue fiber in the two seasons (6 and 43, 19 and 56, 11 and 88 items mL−1, respectively).
Table 1.
Quantification of microplastics in freshwater from excavated tanks in fish farms in the state of Rondônia, Brazil.
| Fish farms | Morphological category | Quantification (item 250 mL−1) |
|
|---|---|---|---|
| Dry | Rainy | ||
| P1 | Transparent fiber | 2 | 2 |
| Red fiber | 1 | 5 | |
| Blue fiber | 2 | 8 | |
| P2 | Transparent fiber | 1 | 1 |
| Red fiber | 1 | 2 | |
| Blue fiber | 3 | 6 | |
| P3 | Transparent fiber | 1 | 1 |
| Red fiber | 2 | 3 | |
| Blue fiber | 6 | 43 | |
| P4 | Transparent fiber | 3 | 3 |
| Red fiber | 5 | 11 | |
| Blue fiber | 19 | 56 | |
| P5 | Transparent fiber | 2 | 3 |
| Red fiber | 3 | 5 | |
| Blue fiber | 4 | 32 | |
| P6 | Transparent fiber | 4 | 9 |
| Red fiber | 7 | 22 | |
| Blue fiber | 11 | 88 | |
| P7 | Transparent fiber | 10 | 9 |
| Red fiber | 1 | 5 | |
| Blue fiber | 3 | 7 | |
| P8 | Transparent fiber | 3 | 1 |
| Red fiber | 2 | 5 | |
| Blue fiber | 3 | 16 | |
| P9 | Transparent fiber | 1 | 5 |
| Red fiber | 6 | 6 | |
| Blue fiber | 6 | 30 | |
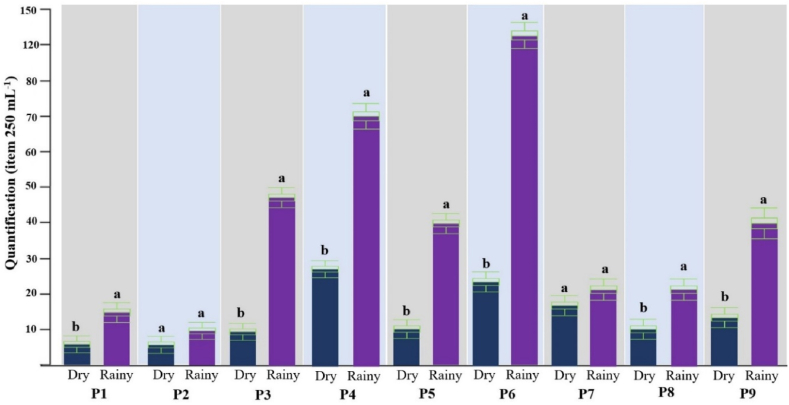
The abundance of microplastics is shown in Fig. 4, with total values for the dry and rainy hydrological seasons per fish farm. Almost all fish farms had a higher abundance of microplastics in the rainy season (p < 0.05), with the exception of fish farms P2 and P7 (p > 0.05). It is important to highlight the prominence of microplastics in the rainy season in fish farms P3, P4 and P6 (47, 70 and 119 items 250 mL−1, respectively) in relation to the dry season (9, 27 and 22 items 250 mL−1, respectively).
Fig. 4.
Abundance of microplastics in freshwater from fish farms in different hydrological seasons (dry and rainy), different letters (a,b) because there is statistical difference (p < 0.05) by Student's t-test.
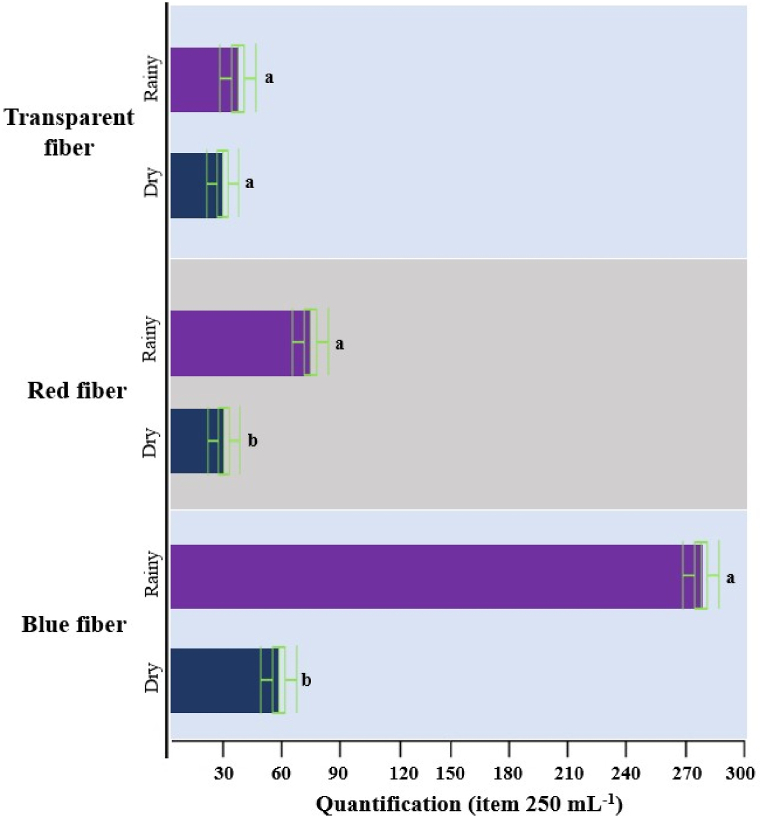
Concerning the abundance of microplastic categories is shown in Fig. 5, with total values for the dry and rainy hydrological seasons. The blue fiber and red fiber categories were more abundant in the rainy season (p < 0.05), with the exception of the transparent fiber category, there was no statistical difference (p > 0.05). It is important to highlight the prominence of microplastics in the blue fiber rainy season (286 items mL−1) compared to the dry season (58 items mL−1).
Fig. 5.
Abundance of microplastics categories in different hydrological seasons (dry and rainy), different letters (a,b) because there is statistical difference (p < 0.05) by Student's t-test.
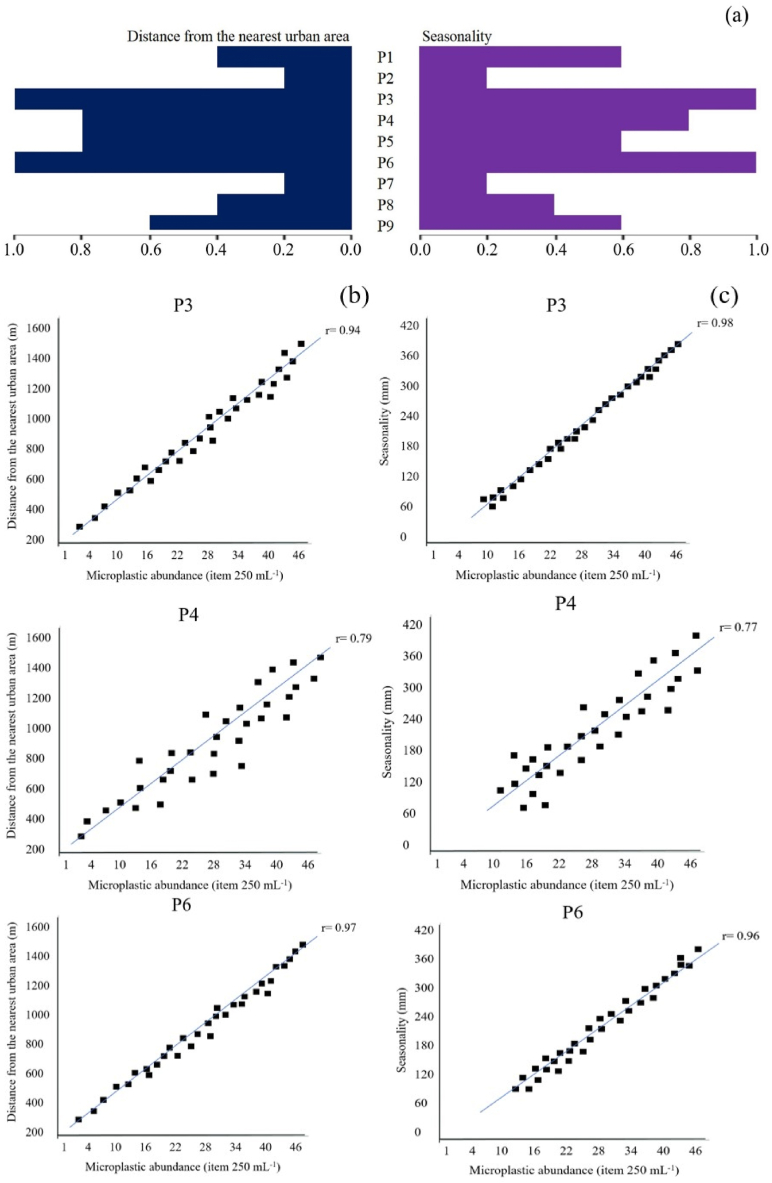
It's important to highlight the Pearson's correlations results of the abundance values of microplastics between distance from the nearest urban area and seasonality (Fig. 6 a). Fish farms P3, P4 and P6 showed a strong positive correlation between the factors distance from the nearest urban area (r = 0.94, 0.79 and 0.97, respectively) (Fig. 6 b) and seasonality (r = 0.98, 0.77 and 0.96, respectively) (Fig. 6 c). While the other fish farms showed weak positive correlations for these two factors.
Fig. 6.
Pearson's correlation microplastic abundance between distance from the nearest urban area and seasonality (a) and r values of the correlations between the factors distance from the nearest urban area (b) and seasonality (c) of P3, P4 and P6 fish farms.
4. Discussion
Governmental environmental biomonitoring agencies around the world still do not have quantification limit legislation because researchers have not yet reached a consensus to recommend standardization of methods for sampling microplastics in lentic freshwater bodies in water renewal flow (for examle fish farm ponds) [3]. The differences in the microplastic abundance of the showed studies may due to the sampling procedures fresh water sampling from fish farm ponds, such as the mesh net chosen, since it has been reported that a mesh size of 333 μm might lead to an underestimation of the microplastic abundance, particularly for particles that are <333 μm [23,24]. However, in the current study, the mesh opening was 6.0 mm, allowing the verification of several fragments, not sampling only the elements present in the microplankton.
Some researchers recommend microplastic sampling with mesh nets smaller than 100 μm, because according to them [24,25] they better represent microplastic contamination in lentic aquatic environments. However, fish farms ponds are bodies of water with continuous water renewal, are not lentic bodies of water like lakes and dams where water is moved almost exclusively by rain and wind. This fact is confirmed in the study by Fernandes et al. [26] performed sampling with different mesh sizes (64 and 200 μm) and observed that the abundance of microplastic was significantly higher in samples collected with a 64 μm mesh, while samples collected with a 200 μm mesh were underestimated by ∼1.5–6.8 times.
The study by Garcia et al. [23] suggested that microplastic sampling should be performed with a larger mesh opening than the plankton net. These authors sampled microplastics with mesh sizes of 120 and 300 μm and found that sampling with the larger mesh showed seven times higher density of microplastic than 300 μm mesh. All these aspects suggest that the net mesh can influence the abundance of microplastics, demonstrating the importance of standardising the microplastic extraction protocol. In addition, the reported data showed widespread contamination with microplastics, even in remote places. In the current study, we observed that the hydrological processes of the fish farms and population density are the main factors that determine the distribution and abundance of microplastics.
Previous research suggests that the distribution and transport of microplastics within aquatic environments are mainly affected by anthropogenic factors [27], physical and chemical characteristics of the microplastics [28], hydrological characteristics [29,30], and meteorological conditions [31]. Therefore, these characteristics should considered to better understand the distribution of microplastics in the environment [32,33].
Rainfall variations influenced (r > 0.90) the abundance of microplastics, especially of blue fibers. So much so that fish farms closer to urban areas P3, P4 and P6 had the highest quantifications of microplastic, especially with greater abundance of blue fibers. The fish farms are supplied with freshwater by river springs or streams, so we can understand that microplastics originate outside the fish farm, so we understand that they were introduced due to the high occupation of the soil, although mainly by rainy in the freshwater in the ponds (excavated tanks). Rainfall through surface runoff and groundwater contaminated by urban, agricultural and urban effluents contaminated the water from fish farms.
The authors of the corrent study did not find a study on the detection of microplastics in freshwater from fish farms in Brazil and not in other countries. Research was found in aquifers, reservoirs, artesian wells, rivers and streams. Matos et al. [34] in research on wells in Cuiabá city, Brazil, found that the closer the wells are to urban areas, the greater the soil occupation, the greater the quantification of microplastics. In summary, they detected the presence of microplastics in 81.8% of freshwater samples. More specifically, Matos et al. [34] analyzed 869 artesian wells in the urban area and 200 wells in the rural area, in the urban area 88% of the artesian wells were confirmed with microplastic contamination and only 25% of the wells in the rural area confirmed with microplastic contamination.
Mintenig et al. [35] comments in his research, although plastic is a durable and resistant material, one of the likely hypotheses for plastic microparticles in freshwater is through the abrasion of sewer pipes and supply channels made of asbestos. Furthermore, Re [36] makes an analogy on the hypothesis of insertion and transport of synthetic microfibers through soil and rock into aquifers and water reservoirs. In the Melbourne state, Australia, Samandra et al. [37], monitored 7 shallow wells, 1 of which of them in a residential area and 1 in an industrial area and obtained respectively 97 and 27 items L−1, one of the theories is that microplastics could enter groundwater through means of soil infiltration or by isolated contamination introduced through the well. According to Bi et al. [38] large-scale urbanization often results in water contamination underground around the world. Cao et al. [39] mention that intensive industrialization and rapid urbanization are one of the factors responsible for the deterioration of groundwater and surface water quality.
It is important to highlight that microplastics may not come from the fish farming area, although they can introduced by leaching, for example, microplastic fibers are carried by heavy rains. The authors of this study recommend continuing research on this subject, for example carrying out an intensive sampling of all possible sources of water intake, surface runoff and commercial fish feeding. In view of this, this information may expose environmental pollution in springs, lakes and reservoirs that provide fresh water for fish farms, as well as commercial artificial feeding and artisanal fish feeding. Another factor to consider, these water supply resources have been polluted by domestic and industrial sewage. Therefore, microplastic fibers can be transported from the supply lakes to the fish ponds by heavy rains or by water abstraction. Therefore, the identification of microplastics from fish tanks can an indicator that water from fish tanks has had contact with sewage.
Concerning the potential sources of microplastic, these plastic fibers enter freshwater systems primarily from secondary microplastics, which are generated by the breakdown of larger fibers of plastic [31,32,40]. As such, the existence of microplastics in freshwater can attributed to many sources, including the discharge of untreated wastewater, road runoff, infiltration of landfill leachate, effluent from wastewater treatment plants, and inadequate waste disposal [41,42]. The discussion of the microplastics origins is a significant challenge since their mechanism of diffusion and transport is extremely complex. Based on our microplastic micrographics and data, we assume that the microplastics in fish farms mainly originated from secondary sources resulting from the degradation or fragmentation of larger debris within the freshwater in excavated tanks. In excavated tanks, plastics tend to stay in the water for long periods of time, and they can fragmented into microplastic by the action of sunlight or other weathering factors [43]. This can corroboratedby the high carbonyl index estimated for 58% [44]. Other potential sources of microplastic contamination include domestic effluent. Thus, as most sites are located proximal to residential areas, household sewage, transported by small rivulets, may an important carrier of primary microplastics into the excavated tanks. It has been reported in literature that small plastic debris can represent a potential threat to aquatic biota, since they can mistakenly ingested by aquatic organisms and transported through the food chain [41,44].
Regarding the morphological categories in microplastics in the form of fiber (into colors blue, red or transparent) were numerically dominant at all sampling sites. These results are in agreement with data from the literature, where colored fibers were the most abundant in this morphological category as well [45,46]. The high abundance of colored microplastics may imply that many sources release plastics into ponds (excavated tanks). Furthermore, the high frequency of transparent microplastics might explained by the fading of colored plastics in the environment due to exposure to UV light and other stressors. Wong et al. [32] found a greater amount of colourless microplastics and observed that many particles appeared to white or pale in colour on the outside. However, when these particles were fragmented, the inside was colored. Thus, the data confirm that the microplastic may become colourless or fade in the environment. On the other hand, the high proportion of transparent microplastics indicates the use of plastic bags, food boxes, and/or plastic bowls [40,41]. Additionally, the black fibers may have originated from the natural wear of tires from vehicles, which are easily washed from the road during rainy events and can enter the aquatic body by the rivulets and drain systems of the city [47]. The fact that all the collected samples contained colored microplastics provide strong evidence of anthropogenic influx as a source of synthetic materials, although it also showes a wide range of sources [48]. The high number of colored microplastics might be derived from the plastic products used in food packing, which represents 20.3% of all Brazilian plastic production [49].
In addition, the high proportion of the white-transparent colour in the current study is consistent with other results in the literature and may also demonstrate that the microplastics remained in the environment for a certain period of time, and due to environmental weathering, they may have faded [30,50]. Therefore, it is urgent to continue studies to quantify microplastics in rivers, streams, lakes, water reservoirs, artesian wells, hydrographic subbasins, water column and sediments from fish farm ponds and even in the muscle tissue of farmed fish. Therefore, further studies on groundwater in Brazil are needed to fill in the gaps on the possible sources and transport of microplastics to these compartments and, furthermore, strengthen Brazilian laws.
Additionally, while many studies have been done to demonstrate the impact of microplastic contamination on the environment, social policies need to be endorsed to raise awareness of the use and disposal of plastic waste. As previously mentioned, Brazil has a lower rate of basic sanitation, and, therefore, it is essential to carry out research in Brazil on water contamination by microplastics since the country occupies a prominent place worldwide in terms of the production and use of plastic.
5. Conclusion
In this study, microplastic contamination in fish farm ponds was investigated for the first time in the Amazon. Microplastic contamination was confirmed in freshwater of 9 fish farms in Rondônia state, Brazil, with greater abundance of blue fibers and greater quantification in the rainy season. It is important to highlight the abundance of microplastics between distance from the nearest urban area and seasonality. Fish farms P3, P4 and P6 showed a strong positive correlation between distance from the nearest urban area and seasonality. Rainfall variations influenced the abundance of microplastics, especially of blue fibers. So much so that fish farms closer to urban areas P3, P4 and P6 had the highest quantifications of microplastic, especially with greater abundance of blue fibers.
Fish farms are supplied with fresh water by rivers or streams, so it is possible that microplastics originate outside the fish farm, perhaps they were introduced due to high soil occupation, although surface runoff (of water contaminated by sewage) caused by heavy rains the most important factor. Therefore, one factor must be considered, surface runoff and groundwater contaminated by urban, agricultural and urban effluents may have contaminated rivers and streams and then contaminated the water in the fish farm ponds. Given this scenario, it is recommended also that this study be continued, with intensive sampling of water supply sources, through a topographic survey to better understand the end of surface runoff in fish farms, as well as to develop research on microplastic contamination in rivers and streams that supply fish farms in the Rondônia state.
Author contribution statement
Jerônimo Vieira Dantas Filho, PhD.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Vinícius Perez Pedroti, MSc.; Bruna Lucieny Temponi Santos, MSc; Maria Mirtes de Lima Pinheiro, MSc; Átila Bezerra de Mira, MSc: Performed the experiments.
Francisco Carlos da Silva, PhD.: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Emerson Carlos Soares e Silva, PhD.; Elica Amara Cecilia Guedes, PhD; Jucilene Cavali, PhD: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Sandro de Vargas Schons, PhD.: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
The research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through by Fundação Rondônia de Amparo ao Desenvolvimento das Ações Científicas e Tecnológicas e à Pesquisa do Estado de Rondônia (FAPERO), awarded a postdoctoral scholarship to Jerônimo Vieira Dantas Filho [167879/2022-7].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.PlasticsEurope . 2019. Plastics – the Facts 2019. [Google Scholar]
- 2.Bläsing M., Amelung W. Plastics in soil: analytical methods and possible sources. Sci. Total Environ. 2018 doi: 10.1016/j.scitotenv.2017.08.086. [DOI] [PubMed] [Google Scholar]
- 3.Bertoldi C., Lara L.Z., Mizushuma F.A.L., Martins F.C.G., Battisti M.A., Hinrichs R., Fernandes A.N. First evidence of microplastic contamination in the freshwater of Lake Guaíba, Porto Alegre, Brazil. Sci. Total Environ. 2021;179 doi: 10.1016/j.scitotenv.2020.143503. [DOI] [PubMed] [Google Scholar]
- 4.Wagner M., Lambert S. Freshwater microplastics - the handbook of environmental? Mar. Microplastics. 2018 doi: 10.1007/978-3-319-616155. [DOI] [Google Scholar]
- 5.Crooks N., Parker H., Pernetta A.P. Brain food? Trophic transfer and tissue retention of microplastics by the velvet swimming crab (Necora puber) J. Exp. Mar. Biol. Ecol. 2019;519 doi: 10.1016/j.jembe.2019.151187. [DOI] [Google Scholar]
- 6.Zhang F., Wang X., Xu J., Zhu L., Peng G., Xu P., Li D. Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea. Mar. Pollut. Bull. 2019;146:173–182. doi: 10.1016/j.marpolbul.2019.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Qiang L., Cheng J. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio) Ecotoxicol. Environ. Saf. 2019;176:226–233. doi: 10.1016/j.ecoenv.2019.03.088. [DOI] [PubMed] [Google Scholar]
- 8.Pegado T.S.S., Schmid K., Winemiller K.O., Chelazzi D., Cincinelli A., Dei L., Giarrizzo T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018;133:814–821. doi: 10.1016/j.marpolbul.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Matos L.F.D., Caixeta D.S., Golin R., Deluque D.L., Morais E.B., Silva J.B. Evidence of microplastics in groundwater in the municipality of Cuiabá-MT/Brazil. Revista Ibero-Americana de Ciências Ambientais. 2022;13:177–185. doi: 10.6008/CBPC2179-6858.2022.002.0016. [DOI] [Google Scholar]
- 10.Lindeque P.K., Cole M., Coppock R.L., Lewis C.N., Miller R.Z., Watts A.J.R., Galloway T.S., et al. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ. Pollut. 2020;265 doi: 10.1016/j.envpol.2020.114721. [DOI] [PubMed] [Google Scholar]
- 11.Huang W., Song B., Liang J., Niu Q., Zeng G., Shen M., Zhang Y., et al. Microplastics and associated contaminants in the aquatic environment: a review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard Mater. 2020 doi: 10.1016/j.jhazmat.2020.124187. [DOI] [PubMed] [Google Scholar]
- 12.CEBDS. Trata Brasil . CEBDS; Brasilia: 2020. Economic Benefits of Expanding the Brazilian Sanitation: Quality of Life, Productivity, Education and Environmental Appreciation. [Google Scholar]
- 13.ANA . ANA; Brasilia: 2017. Agência Nacional de Águas (ANA) - Atlas Esgotos - Despoluição de bacias hidrográficas. [Google Scholar]
- 14.Li C., Busquets R., Campos L.C. Assessment of microplastics in freshwater systems: a review. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135578. [DOI] [PubMed] [Google Scholar]
- 15.Peixe BR . Peixe BR; Pinheiros, SP: 2022. Associação Brasileira da Piscicultura. Anuário da Peixe BR de 2022. [Google Scholar]
- 16.Lima A.R.A., Barletta M., Costa M.F., Ramos J.A.A., Dantas D.V., Melo P.A.M.C., Justino A.K.S., Ferreira G.V.B. Changes in the composition of ichthyoplankton assemblage and plastic debris in mangrove creeks relative to moon phases. J. Fish. Biol. 2016;89:619–640. doi: 10.1111/jfb.12838. [DOI] [PubMed] [Google Scholar]
- 17.Faria E., Girard P., Nardes C., Moreschi A., Christo S., Ferreira Junior A.L., Costa M. Microplastics pollution in the South American Pantanal. Case Stud. Chem. Environ. Eng. 2019 doi: 10.7287/peerj.preprints.27754v1. [DOI] [Google Scholar]
- 18.ABIPLAST . Abiplast; 2019. Profile 2019; p. 45. [Google Scholar]
- 19.RONDÔNIA . SEDAM; Porto Velho: 2022. Secretaria de Estado do desenvolvimento Ambiental. Números da Piscicultura de Rondônia. [Google Scholar]
- 20.Alvares C.A., Stape J.L., Sentelhas P.C., Gonçalves J.L.M., Sparovek G. Koppen's climate classification map for Brazil. Meteorologische Zeitschrisft. 2013;22:711–728. doi: 10.1127/0941-2948/2013/0507. [DOI] [Google Scholar]
- 21.INPE. Instituto Nacional de Pesquisas Espaciais . CPTEC; 2022. Centro de Previsão de Tempo e Estudos Climáticos (CPTEC). Estação meteorológica de Ouro Preto do Oeste – RO. [Google Scholar]
- 22.Masura J., Baker J., Foster G., Courtney A. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: recommendations for quantifying synthetic particles in waters and sediments. NOAA Tech. Memo. 2015:39. NOS-OR&R-4. [Google Scholar]
- 23.Garcia T.M., Campos C.C., Mota E.M.T., Santos N.M.O., de Santana Campelo R.P., Prado L.C.G., Melo Junior M., de Oliveira Soares M. Microplastics in subsurface waters of the western equatorial Atlantic (Brazil) Mar. Pollut. Bull. 2020;150 doi: 10.1016/j.marpolbul.2019.110705. [DOI] [PubMed] [Google Scholar]
- 24.Lindeque P.K., Cole M., Coppock R.L., Lewis C.N., Miller R.Z., Watts A.J.R., Wilson- McNeal A., Wright S.L., Galloway T.S. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Mar. Pollut. Bull. 2020;265 doi: 10.1016/j.envpol.2020.114721. [DOI] [PubMed] [Google Scholar]
- 25.Hanvey J.S., Lewis P.J., Lavers J.L., Crosbie N.D., Pozo K., Clarke B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods. 2017;9:1369–1383. doi: 10.1039/c6ay02707e. [DOI] [Google Scholar]
- 26.Fernandes A.N., Bertoldi C., Lara L.Z., Stival J., Alves N.M., Cabrera P.M., Grassi M.T. Microplastics in Latin America ecosystems: a critical review of the current stage and research needs. J. Braz. Chem. Soc. 2020;33(4):303–326. doi: 10.21577/0103-5053.20220018. [DOI] [Google Scholar]
- 27.Wang W., Ndungu A.W., Li Z., Wang J. Microplastics pollution in inland freshwaters of China: a case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017;575:1369–1374. doi: 10.1016/j.scitotenv.2016.09.213. [DOI] [PubMed] [Google Scholar]
- 28.Cole M., Lindeque P., Fileman E., Halsband C., Goodhead R., Moger J., Galloway T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013;47:6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- 29.Jiang C., Yin L., Li Z., Wen X., Luo X., Hu S., Yang H., Long Y., Deng B., Huang L., Liu Y. Microplastic pollution in the rivers of the Tibet Plateau. Environ. Pollut. 2019;249:91–98. doi: 10.1016/j.envpol.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Yuan W., Liu X., Wang W., Di M., Wang J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019;170:180–187. doi: 10.1016/j.ecoenv.2018.11.126. [DOI] [PubMed] [Google Scholar]
- 31.Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- 32.Wong G., Löwemark L., Kunz A. Microplastic pollution of the Tamsui River and its tributaries in Northern Taiwan: spatial heterogeneity and correlation with precipitation. Environ. Pollut. 2020;260 doi: 10.1016/j.envpol.2020.113935. [DOI] [PubMed] [Google Scholar]
- 33.Xu S., Ma J., Ji R., Pan K., Miao A.J. Microplastics in aquatic environments: occurrence, accumulation, and biological effects. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134699. [DOI] [PubMed] [Google Scholar]
- 34.Matos L.F.D., Caixeta D.S., Golin R., Deluque A.L., Morais E.B., Silva J.B. Evidence of microplastics in groundwater in the municipality of Cuiabá-MT/Brazil. Revista Ibero-Americana de Ciências Ambientais. 2022;13:177–185. doi: 10.6008/CBPC2179-6858.2022.002.0016. [DOI] [Google Scholar]
- 35.Mintenig S.M., Loder M.G., Primpke S., Gerdts G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019;648:631–635. doi: 10.1016/j.scitotenv.2018.08.178. [DOI] [PubMed] [Google Scholar]
- 36.Re V. Shedding light on the invisible: addressing the potential for groundwater contamination by plastic microfibers. Hydrogeol. J. 2019;27:2719–2727. doi: 10.1007/s10040-019-01998-x. [DOI] [Google Scholar]
- 37.Samandra S., Johnston J.M., Jaeger J.E., Symons B., Xie S., Curriell M., Ellis A.V., Clarke B.O. Microplastic contamination of an unconfined groundwater aquifer in Victoria, Australia. Sci. Total Environ. 2022:802. doi: 10.1016/j.scitotenv.2021.149727. [DOI] [PubMed] [Google Scholar]
- 38.Bi P., Huang G., Liu C., Li L. Geochemical factors controlling natural background levels of phosphate in various groundwater units in a large-scale urbanized area. J. Hydrol. 2022;608 doi: 10.1016/J.JHYDROL.2022.127594. [DOI] [Google Scholar]
- 39.Cao X., Lu Y., Wang C., Zhang M., Yuan J., Zhang A., Song S., Baninla Y., Khan K., Wang Y. Hydrogeochemistry and quality of surface water and groundwater in the drinking water source area of an urbanizing region. Ecotoxicol. Environ. Saf. 2019;186 doi: 10.1016/J.ECOENV.2019.109628. [DOI] [PubMed] [Google Scholar]
- 40.Xiong X., Zhang K., Chen X., Shi H., Luo Z., Wu C. Sources and distribution of microplastics in China's largest inland lake – Qinghai Lake. Environ. Pollut. 2018;235:899–906. doi: 10.1016/j.envpol.2017.12.081. [DOI] [PubMed] [Google Scholar]
- 41.Li J., Liu H., Paul Chen J. Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018 doi: 10.1016/j.watres.2017.12.056. [DOI] [PubMed] [Google Scholar]
- 42.Waldschläger K., Lechthaler S., Stauch G., Schüttrumpf H. The way of microplastic through the environment – application of the source-pathway-receptor model (review) Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.136584. [DOI] [PubMed] [Google Scholar]
- 43.Enfrin M., Dumée L.F., Lee J. Nano/microplastics in water and wastewater treatment processes – origin, impact and potential solutions. Water Res. 2019 doi: 10.1016/j.watres.2019.06.049. [DOI] [PubMed] [Google Scholar]
- 44.de Souza e Silva Pegado T., Schmid K., Winemiller K.O., Chelazzi D., Cincinelli A., Dei L., Giarrizzo T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018;133:814–821. doi: 10.1016/j.marpolbul.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Koelmans A.A., Mohamed Nor N.H., Hermsen E., Kooi M., Mintenig S.M., De France J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019 doi: 10.1016/j.watres.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan Z., Sun Y., Liu Q., Lin C., Sun X., He Q., Zhou K., Lin H. Riverine microplastic pollution matters: a case study in the Zhangjiang River of Southeastern China. Mar. Pollut. Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111516. [DOI] [PubMed] [Google Scholar]
- 47.Ballent A., Corcoran P.L., Madden O., Helm P.A., Longstaffe F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016;110:383–395. doi: 10.1016/j.marpolbul.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 48.Gallagher A., Rees A., Rowe R., Stevens J., Wright P. Microplastics in the Solent estuarine complex, UK: an initial assessment. Mar. Pollut. Bull. 2016;102:243–249. doi: 10.1016/j.marpolbul.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Kadac-Czapska K., Knez E., Gierszewska M., Olewnik-Kruszkowska E., Grembebecka M. Microplastics derived from food packaging waste—their origin and health risks. Materials. 2023;16:674. doi: 10.3390/ma16020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gela S.M.G., Aragaw T.A. Abundance and characterization of microplastics in main urban ditches across the Bahir Dar city, Ethiopia. Front. Environ. Sci. 2022 doi: 10.3389/fenvs.2022.831417. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.