ABSTRACT
Recurrent urinary tract infections (rUTI) are common in kidney transplant recipients (KTR) and are associated with multidrug resistance and increased morbidity/mortality. Novel antibiotic alternatives to reduce UTI recurrence are critically needed. We describe a case of rUTI due to extended spectrum beta lactamase (ESBL) Klebsiella pneumoniae in a KTR that was treated successfully with 4 weeks of adjunctive intravenous bacteriophage therapy alone, without concomitant antibiotics, and with no recurrence in a year of follow-up.
KEYWORDS: phage, rUTI, kidney transplant, bacteriophage, ESBL
INTRODUCTION
Urinary tract infections (UTIs) occur frequently in kidney transplant recipients (KTR), adversely impact allograft function, are associated with multidrug resistance (MDR), and cause significant morbidity (1–5). Recurrent UTI (rUTI) is common in KTRs and are related to persistent nidus and/or gut and urinary biome persistence (6, 7). Antibiotic treatment of asymptomatic bacteriuria does not prevent future recurrence (2), and alternatives are critically needed.
There is increasing interest in using lytic bacteriophages (phage) for the treatment of MDR infections, though they are generally used with concomitant antibiotics (8–12). Two recent KTR cases suggest efficacy in the eradication of MDR pathogens, although effect of the phage was unclear, as it was used with concomitant antibiotics (13, 14). In a third case, the patient underwent native nephrectomy that was thought to be the infection reservoir (15). For the first time, we describe the clinical course of a KTR with a complicated rUTI that was treated with adjunctive phage alone, without concomitant antibiotics.
CASE PRESENTATION
The patient was a 70-year-old female with combined liver/kidney transplantation in March of 2020 for nonalcoholic fatty liver disease and diabetes-related kidney disease. She developed recurrent transplant pyelonephritis, bacteremia, and cystitis, mainly due to K. pneumoniae (including from extended spectrum beta lactamase [ESBL] producing isolates) and she required multiple hospital admissions and multiple courses of antibiotics over the next 1.5 years. She did not have a chronic indwelling urinary catheter or a ureteral stent, and she did not require intermittent self-catheterization. She was postmenopausal and maintained on vaginal estrogen. Imaging was negative for renal calculi, indwelling hardware, or cysts. The patient suffered several episodes of acute kidney injury when admitted with sepsis and bacteremia and eventually developed chronic kidney disease. Her initial immunosuppression consisted of steroid induction that was followed by maintenance with tacrolimus, mycophenolate mofetil, and prednisone. The mycophenolate was changed to sirolimus in April of 2020, and the overall tacrolimus trough goal was reduced from 8 to 10 ng/mL to approximately 5 ng/mL from May of 2020, onwards. The patient had several ESBL K. pneumoniae isolates that were variably resistant to ciprofloxacin, fosfomycin, nitrofurantoin, and trimethoprim-sulfamethoxazole, as noted in Table S2.
CHALLENGE QUESTION
How can we reduce the risk of future UTI, infection-associated hospitalization, and further kidney damage in this patient?
Long term antibiotic suppression with IV ertapenem (the patient had previously broken through oral suppression with nitrofurantoin and fosfomycin with the development of resistance)
Fecal microbiota transplant
Chemical prophylaxis with methionine
Phage therapy
STRATEGY AND OUTCOME
We treated her with phage in an effort to eradicate ESBL colonization and prevent further infections. As we aimed to eradicate colonization and not treat an acute infection, we used adjunctive phage alone, without concomitant antibiotics (all previously published phage experience in transplant recipients has been with concomitant antibiotics).
A 4-week course of phage was initiated in September of 2021 with a three-phage cocktail that was administered intravenously (IV) twice daily, with regulatory approval from the FDA (single patient eIND 27811) and the local institutional review board (UCSD IRB number 20-0163) as well as signed informed consent.
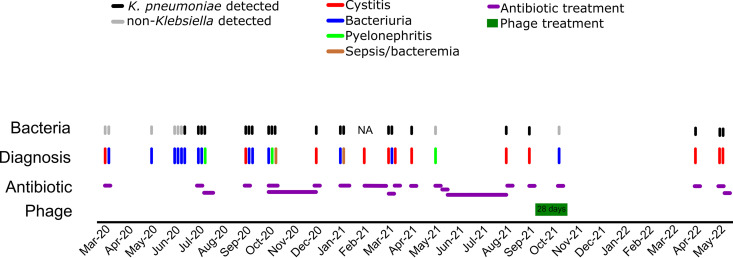
The clinical course, microbiological results, and treatment (antibiotics or phage) are detailed in Fig. 1 and Table S1. The patient had four symptomatic K. pneumoniae UTIs in the 6 months prior to phage initiation and none in the following 6 months. The first K. pneumoniae UTI after the phage treatment occurred 206 days after the phage initiation but with no recurrence of ESBL strains by a one-year follow-up. Postphage K. pneumoniae infections were susceptible to oral antibiotics, and the patient has not required IV antibiotics since.
FIG 1.
Clinical course and treatment administration. Clinical episode diagnoses from March of 2020 to May of 2022 are represented as colored lines. The microbiology analysis of the urine culture at each clinical episode is indicated as either black (K. pneumoniae was identified) or gray (non-Klebsiella was identified). The administered antibiotics are shown as orange bars, with the smallest bar width representing a five-day treatment course. The phage treatment lasted for 28 days and is indicated by a green box.
PHAGE SELECTION
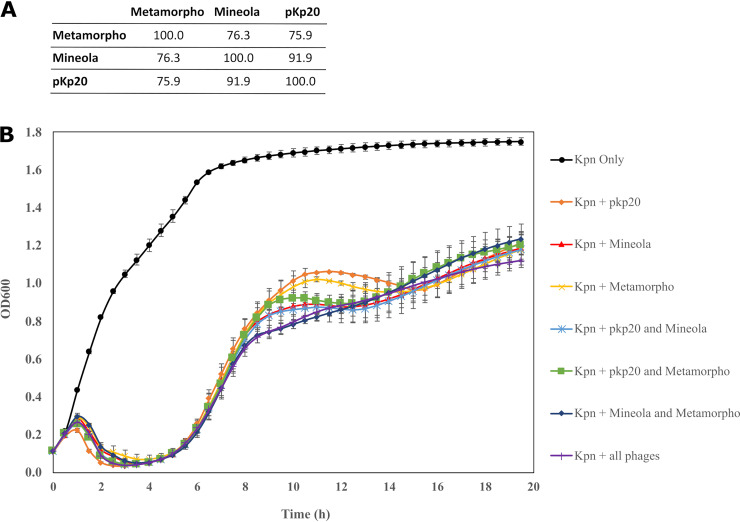
Descriptions of materials, methods, and production are provided in the supplementary appendix. After screening 111 Klebsiella phages against 4 ESBL K. pneumoniae isolates, 3 lytic phages (Table 1) that demonstrated the effective inhibition of host isolates (Fig. 2) were selected.
TABLE 1.
Characteristics of phages included in the treatment cocktail
| Phage name | Source | Isolation host | Morphology | Accession number | Genome size (bp) | Total protein-coding genes | ICTV taxonomic subfamily, genus, speciesa |
|---|---|---|---|---|---|---|---|
| Metamorpho | Texas A&M University | K. aerogenes ATCC 13048 | Myophage | MT701588 | 171,475 | 287 | Tevenvirinae; Jiaodavirus |
| Mineola | Texas A&M University | K. pneumoniae clinical isolate (ST258) | Myophage | MH333064 | 166,130 | 276 | Tevenvirinae; Jiaodavirus; Jiaodavirus JD18 |
| pKp20 | Monash University | FADDI-KP057 | Myophage | OP331213 | 165,762 | 271 | Tevenvirinae; Jiaodavirus; Klebsiella virus JD18 |
ICTV taxonomy placement is based on the nucleotide similarity that was determined via BLASTn against the NCBI nt database.
FIG 2.
Characterization of individual phages and phage cocktails. (A) DNA sequence relatedness of the three phages, showing the pairwise percent DNA sequence identities, as determined by ProgressiveMauve. (B) Inhibition activity of individual and phage cocktails (of two phages and of three phages) against the patient isolate (from April of 2021) at a multiplicity of inhibition of 0.01 over a 20 h period. The error bars represent the standard deviation (SD) from two independently repeated experiments.
PHARMACOKINETICS
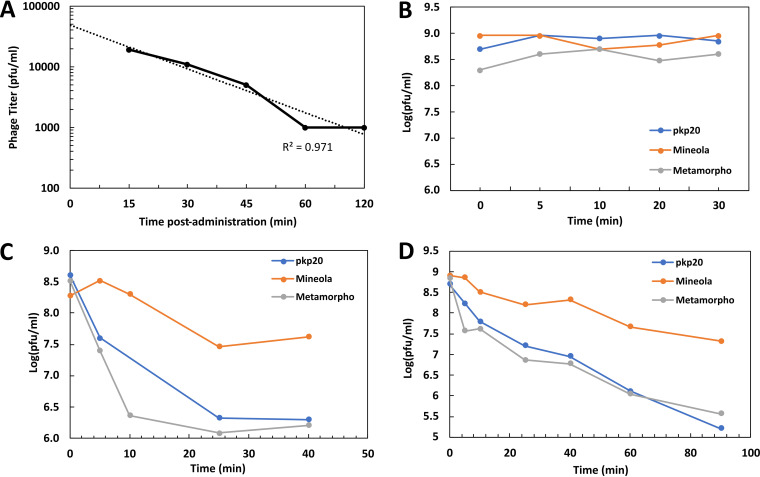
Following the first dose, infective phage particles were detectable in the patient’s serum collected at 15 min postinjection, indicating their rapid distribution into the systemic circulation. A gradual decrease in phage titers was observed with a calculated half-life of 0.4 h (Fig. 3A). We did not detect infective phage particles in urine; however, urine collection was not timed to phage administration. We spiked live phage into urine samples and determined the titers of recoverable phages, and the results indicated that urine did not inactivate the phage.
FIG 3.
The phage pharmacokinetics on day 1 and the serum neutralization during treatment. Following the IV phage injection (at 5 × 109 PFU/dose) on day 1, the patient serum samples were collected at different time points (15, 30, 45, 60, and 120 min), and the infective phage titers were determined (A). Serum samples were also collected on (B) day 1, (C) day 8, and (D) day 15 to test their neutralization effects on phages by spiking phages to serum to approximately 109 PFU/mL and then determining the titers after incubation to assess the viable phages.
SERUM NEUTRALIZATION
The patient developed a partial serum neutralization response to phages from day 8, onwards (Fig. 3B–D). Serum neutralization was variable toward different phages, with the weakest neutralization being observed against the phage Mineola (half-life of 0.18 h on day 8 and 0.29 h on day 15) and the greatest neutralization being observed against the phage pKp20 (half-life of 0.09 h on day 8 and 0.16 h on day 15). All three phages demonstrated an extended half-life in day 15 serum samples, compared to those observed a week earlier.
DIVERSITY OF K. PNEUMONIAE IN URINE
Among 20 K. pneumoniae isolates that were collected between 06/2020 and 05/2022, 15 were ESBL (Table S2). Genome sequencing revealed 5 distinct Klebsiella sequence types (which correlate with 5 different phylogenetic clades clustered based on genome-wide comparison), encoding 4 identifiable capsule locus types and a variety of AMR genes (Table 2; Table S3; Fig. S1). Genome sequencing and antibiograms suggested that the infections were independent occurrences rather than a chronic infection caused by a single organism.
TABLE 2.
Phage susceptibility and genome sequence characteristics of selected Klebsiella isolatesa
| Isolate ID | Date of isolation (month-year) | Sensitivity to phage |
Best matched locus | Sequence type | Phylogenetic cladeb | AMR genes in genome | ESBL | Number of antibiotics resistant toc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Metamorpho | Mineola | pKp20 | ||||||||
| B8142334 | Sep-20 | Z | R | R | KL102 | 307 | A | 18 | Yes | 9 |
| B9236220 | Oct-20 | R | R | R | KL102 | 307 | A | 18 | Yes | 11 |
| B9236223 | Oct-20 | Z | R | R | KL102 | 307 | A | 18 | Yes | 11 |
| C2042673 | Jan-21 | R | R | R | KL30 | 3647 | E | 5 | No | 2 |
| C4023081d | Mar-21 | S | S | S | KL21 | Novel | C | 21 | Yes | 12 |
| C5012365 | Apr-21 | S | S | S | KL21 | Novel | C | 19/19 | Yes | 13 |
| 20210909B1e | Sep-21 | Z | Z | Z | KL17 | 1015 | B | 6 | No | 1 |
| September to October of 2021: Klebsiella directed phage treatment | ||||||||||
| D7091294 | Apr-22 | Z | Z | S | KL7 | 6 | D | 3 | No | 1 |
| D8232378 | May-22 | R | R | R | NA | NA | NA | NA | No | 0 |
Z, clear zone was observed at high phage concentrations (>108 PFU/mL), but there was no plaque formation at lower dilutions; R, resistance (no observable clearing at any phage concentration); S, sensitive with EOP comparable to that of the propagation host; ESBL, extended-spectrum beta-lactamase; NA, not available.
Determined via whole-genome comparison. See supplementary data.
Out of a total of 19 types of antibiotics tested.
Phage propagation host.
Isolated 7 days prior to phage treatment.
URINE MICROBIOTA DURING PHAGE TREATMENT
The total microorganism counts in the urine ranged between 103 and 104 CFU/mL. 16S gene identification and metagenomic analyses revealed a bacterial population dominated by species of Streptococcus, Corynebacterium, Enterococcus, and Anaerococcus (Tables S4 and S5). Klebsiella spp. were not identifiable in day 8 samples but were identified in samples from day 15, 22, and 29 at extremely low levels (0.16%, 0.19%, and 0.39%, respectively, of the total population). Metagenomic data could not be obtained for the day 1 sample due to DNA extraction failures. After the phage treatment was completed, monthly surveillance urine samples did not test positive for Klebsiella for a period of 6 months until April of 2022.
DISCUSSION
We report a case of intravenous phage that was used alone for the targeted eradication of ESBL K. pneumoniae colonization in an immunosuppressed patient with recurrent infections. We demonstrate the safety and potential utility of this approach as a means of addressing the common problem of rUTI.
The cycle of rUTI was resolved for almost 6 months following phage initiation, and targeted ESBL strains have not been observed to date (>1 year out). The patient developed UTIs from day 206 onwards, but these were due to new K. pneumoniae isolates that were antibiotic-sensitive, as was revealed via sequencing and antibiotic susceptibility testing (isolates from April and May of 2022) (Table 2), and they were easily treated with oral antibiotics, leading to a significantly improved quality of life.
While ESBL K. pneumoniae isolates commonly caused pre-phage UTI, whole-genome sequencing revealed that these were successive infections by at least four different bacterial strains over the preceding year; this phenomenon was noted by others, as well (16). The isolation of multiple strains complicated attempts at phage therapy, as phages tend to be strain-specific; genetic engineering and the development of broad host-range phages may overcome this issue. Metagenomic analyses of urine samples indicated that Klebsiella was absent or present at minimal levels during the phage therapy. Post-phage K. pneumoniae isolates represented a new infection and not a recurrence of previous isolates. We hypothesize that the phage targeted a gut/urinary reservoir of the novel ESBL clones that were identified in previous UTI episodes; unfortunately, we were not able to collect stool specimens to test this hypothesis. A recent study demonstrated that the relative gut abundance of Escherichia and Enterococcus species were independent risk factors for bacteriuria and UTI in KTR; a strain analysis established a close alignment between gut and urinary species in the same subjects (17).
The host immune response, as evidenced by partial phage inactivation by the patient’s serum, was observed 1 week after phage administration, which is consistent with the results of previous reports, despite the patient’s immunosuppression regimen (18–20). In contrast to one study, in which complete inactivation of the phage was observed from week 2, onwards (19), our data showed lower rates of phage inactivation in the serum from day 15 versus day 8, and the reason for this is unclear. Clinical success, despite in vitro serum neutralization, suggests that shorter courses of phage therapy may be effective. At this point, it is unclear from the literature whether or not serum neutralization effectively limits further treatment. We have previously reported several successful outcomes with repeat courses of phage therapy, including one case with serum neutralization present at day 0 of phage therapy (10, 18, 19). Alternatively, a case with clinical deterioration that was coincident with the antibody response to phage was also published (21).
In conclusion, we demonstrate that standalone phage therapy without concomitant antibiotics in the setting of rUTI in a KTR was associated with a reduction in UTI frequency and a lack of recurrence of the targeted ESBL K. pneumoniae isolate. This strategy will need to be assessed in prospective clinical trials.
Data availability.
The complete genome sequences of the K. pneumoniae phages Mineola, Metamorpho, and pkp20 were deposited in GenBank under the accession numbers MH333064, MT701588, and OP331213, respectively. Genome sequences of the bacterial strains are deposited under Bioproject PRJNA941389.
ACKNOWLEDGMENTS
We thank Cara Fiore and Nikunj Sharma at the Food and Drug Administration.
The work conducted at the Center for Phage Technology was supported by funding from Texas A&M University, Texas AgriLife Research. The work performed at the University of California, San Diego (UCSD), was supported by a grant from the UC San Diego Chancellor’s Innovation Fund and the Monash-UCSD Seed Fund. The work performed at Monash University was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R21 AI156766) and the Monash-UCSD Seed Fund. J.L. is a National Health and Medical Research Council (NHMRC) Principal Research Fellow (APP1157909).
J.J.G. is a member of the Scientific Advisory Board for Deerland Enzymes, Inc., and Janssen. S.A. is a consultant for BioMx and Phico, a member of Medical Advisory Board for Pherecydes Pharma, and received research funding from the Contrafect Corporation, Armata Pharmaceuticals, Cystic Fibrosis Foundation, and National Institutes of Health (U19 AI157981, UL1 TR001442). No other author has a conflict of interest to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Mei Liu, Email: mei.liu@ag.tamu.edu.
Saima Aslam, Email: saslam@health.ucsd.edu, @DocSaimaAslam.
REFERENCES
- 1.Jackson KR, Motter JD, Bae S, Kernodle A, Long JJ, Werbel W, Avery R, Durand C, Massie AB, Desai N, Garonzik-Wang J, Segev DL. 2021. Characterizing the landscape and impact of infections following kidney transplantation. Am J Transplant 21:198–207. 10.1111/ajt.16106. [DOI] [PubMed] [Google Scholar]
- 2.Coussement J, Kamar N, Matignon M, Weekers L, Scemla A, Giral M, Racapé J, Alamartine É, Mesnard L, Kianda M, Ghisdal L, Catalano C, Broeders EN, Denis O, Wissing KM, Hazzan M, Abramowicz D, Bacteriuria in Renal Transplantation (BiRT) study group . 2021. Antibiotics versus no therapy in kidney transplant recipients with asymptomatic bacteriuria (BiRT): a pragmatic, multicentre, randomized, controlled trial. Clin Microbiol Infect 27:398–405. 10.1016/j.cmi.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Pesce F, Martino M, Fiorentino M, Rollo T, Simone S, Gallo P, Stallone G, Grandaliano G, Schena A, Margiotta M, Mininni D, Palieri R, Lucarelli G, Battaglia M, Gesualdo L, Castellano G. 2019. Recurrent urinary tract infections in kidney transplant recipients during the first-year influence long-term graft function: a single-center retrospective cohort study. J Nephrol 32:661–668. 10.1007/s40620-019-00591-5. [DOI] [PubMed] [Google Scholar]
- 4.Olenski S, Scuderi C, Choo A, Bhagat Singh AK, Way M, Jeyaseelan L, John G. 2019. Urinary tract infections in renal transplant recipients at a quaternary care centre in Australia. BMC Nephrol 20:479. 10.1186/s12882-019-1666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rostkowska OM, Kuthan R, Burban A, et al. 2020. Analysis of susceptibility to selected antibiotics in Klebsiella pneumoniae, Escherichia coli, Enterococcus faecalis and Enterococcus faecium causing urinary tract infections in kidney transplant recipients over 8 years: single-center study. Antibiotics (Basel) 9:E284. 10.3390/antibiotics9060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gozdowska J, Czerwińska M, Chabros Ł, Młynarczyk G, Kwiatkowski A, Chmura A, Durlik M. 2016. Urinary tract infections in kidney transplant recipients hospitalized at a transplantation and nephrology ward: 1-year follow-up. Transplant Proc 48:1580–1589. 10.1016/j.transproceed.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 7.Korayem GB, Zangeneh TT, Matthias KR. 2018. Recurrence of urinary tract infections and development of urinary-specific antibiogram for kidney transplant recipients. J Glob Antimicrob Resist 12:119–123. 10.1016/j.jgar.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Young R, Gill JJ. 2015. Microbiology. Phage therapy redux–what is to be done? Science 350:1163–1164. 10.1126/science.aad6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordillo Altamirano FL, Barr JJ. 2019. Phage therapy in the postantibiotic era. Clin Microbiol Rev 32:e00066-18. 10.1128/CMR.00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslam S, Lampley E, Wooten D, et al. 2020. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect Dis 7:ofaa389. 10.1093/ofid/ofaa389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uyttebroek S, Chen B, Onsea J, Ruythooren F, Debaveye Y, Devolder D, Spriet I, Depypere M, Wagemans J, Lavigne R, Pirnay J-P, Merabishvili M, De Munter P, Peetermans WE, Dupont L, Van Gerven L, Metsemakers W-J. 2022. Safety and efficacy of phage therapy in difficult-to-treat infections: a systematic review. Lancet Infect Dis 22:e208–20–e220. 10.1016/S1473-3099(21)00612-5. [DOI] [PubMed] [Google Scholar]
- 12.Hatfull GF, Dedrick RM, Schooley RT. 2022. Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med 73:197–211. 10.1146/annurev-med-080219-122208. [DOI] [PubMed] [Google Scholar]
- 13.Kuipers S, Ruth MM, Mientjes M, de Sévaux RGL, van Ingen J. 2019. A Dutch case report of successful treatment of chronic relapsing urinary tract infection with bacteriophages in a renal transplant patient. Antimicrob Agents Chemother 64:e01281-19. 10.1128/AAC.01281-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao J, Wu N, Zeng Y, Chen L, Li L, Yang L, Zhang Y, Guo M, Li L, Li J, Tan D, Cheng M, Gu J, Qin J, Liu J, Li S, Pan G, Jin X, Yao B, Guo X, Zhu T, Le S. 2020. Non-active antibiotic and bacteriophage synergism to successfully treat recurrent urinary tract infection caused by extensively drug-resistant Klebsiella pneumoniae. Emerg Microbes Infect 9:771–774. 10.1080/22221751.2020.1747950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rostkowska OM, Międzybrodzki R, Miszewska-Szyszkowska D, Górski A, Durlik M. 2021. Treatment of recurrent urinary tract infections in a 60-year-old kidney transplant recipient. The use of phage therapy. Transpl Infect Dis 23:e13391. 10.1111/tid.13391. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, Ma Y, Zhao Q, Wang L, Guo L, Ye L, Zhang Y, Yang J. 2012. Similarity and divergence of phylogenies, antimicrobial susceptibilities, and virulence factor profiles of Escherichia coli isolates causing recurrent urinary tract infections that persist or result from reinfection. J Clin Microbiol 50:4002–4007. 10.1128/JCM.02086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magruder M, Sholi AN, Gong C, Zhang L, Edusei E, Huang J, Albakry S, Satlin MJ, Westblade LF, Crawford C, Dadhania DM, Lubetzky M, Taur Y, Littman E, Ling L, Burnham P, De Vlaminck I, Pamer E, Suthanthiran M, Lee JR. 2019. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat Commun 10:5521. 10.1038/s41467-019-13467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez-Sanchez C, Gonzales F, Buckley M, et al. 2021. Successful treatment of Staphylococcus aureus prosthetic joint infection with bacteriophage therapy. Viruses 13:1182. 10.3390/v13061182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terwilliger A, Clark J, Karris M, et al. 2021. Phage therapy related microbial succession associated with successful clinical outcome for a recurrent urinary tract infection. Viruses 13:2049. 10.3390/v13102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Łusiak-Szelachowska M, Zaczek M, Weber-Dąbrowska B, Międzybrodzki R, Kłak M, Fortuna W, Letkiewicz S, Rogóż P, Szufnarowski K, Jończyk-Matysiak E, Owczarek B, Górski A. 2014. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol 27:295–304. 10.1089/vim.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dedrick RM, Freeman KG, Nguyen JA, Bahadirli-Talbott A, Smith BE, Wu AE, Ong AS, Lin CT, Ruppel LC, Parrish NM, Hatfull GF, Cohen KA. 2021. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat Med 27:1357–1361. 10.1038/s41591-021-01403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.00037-23-s0001.pdf, PDF file, 0.4 MB (372.7KB, pdf)
Supplemental material. Download aac.00037-23-s0002.xlsx, XLSX file, 0.03 MB (32.4KB, xlsx)
Data Availability Statement
The complete genome sequences of the K. pneumoniae phages Mineola, Metamorpho, and pkp20 were deposited in GenBank under the accession numbers MH333064, MT701588, and OP331213, respectively. Genome sequences of the bacterial strains are deposited under Bioproject PRJNA941389.