Abstract
The field of research in bladder cancer has seen significant advances in recent years. Next-generation sequencing has identified the genes most mutated in bladder cancer. This wealth of information allowed the definition of driver mutations, and identification of actionable therapeutic targets, as well as a clearer picture of patient prognosis and therapeutic direction. In a similar vein, our understanding of the cellular aspects of bladder cancer has grown. The identification of the cellular geography and the populations of different cell types and quantifications of normal and abnormal cell types in tumours provide a better prediction of therapeutic response. Non-invasive methods of diagnosis, including liquid biopsies, have seen major advances as well. These methods will likely find considerable utility in assessing minimal residual disease following treatment and for early-stage diagnosis. A significant therapeutic impact on patients with bladder cancer is found in the use of immune checkpoint inhibitor therapeutics. These therapeutics have been shown to cure some patients with bladder cancer and significantly decrease adverse events. These developments provide patients with better monitoring opportunities, unique therapeutic options and greater hope for prolonged survival.
Bladder cancer is among the most prevalent cancers worldwide, with 549,393 new cases reported in 2018 (REF.1). This disease can present as non-muscle-invasive bladder cancer (NMIBC), muscle-invasive bladder cancer (MIBC) or as a metastatic form of the disease, with each having different molecular drivers. While there were few advances in clinical management of bladder cancer over the past three decades, this trend has been considerably altered over the past several years. Advances in our understanding of bladder cancer biology, coupled with large-scale gene expression and sequencing efforts, have led to more clinically favourable targeted treatments and effective immunotherapies. These advances have also provided clinicians with the ability to characterize bladder cancer into distinct molecular and histological subtypes (FIG. 1).
Fig. 1 |. Pathological and molecular features of human bladder cancer.
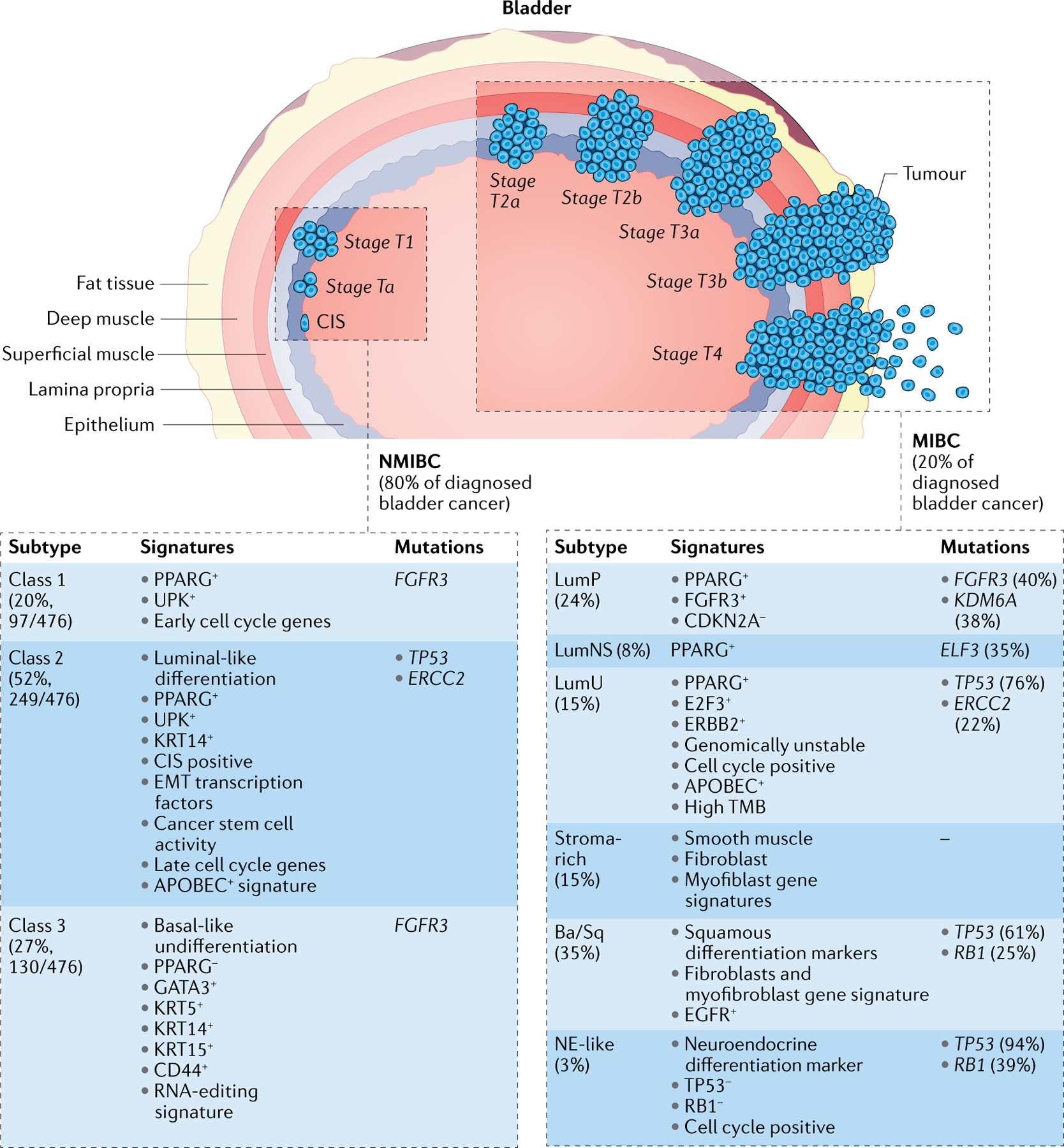
Urothelial carcinoma of the bladder is comprised of two major groups on the basis of clinical staging with different clinical outcomes and therapy options: non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). Staging of urothelial bladder carcinoma is shown according to the tumour, lymph node, metastasis (TNM240) system. The UROMOL study classified NMIBC into three classes: class 1, luminal-like signature; class 2, luminal-like, epithelial–mesenchymal transition (EMT) and cancer stem cell signatures; and class 3, basal-like signature. Six subgroups of MIBC are shown: luminal papillary (LumP), luminal non-specified (LumNS), luminal unstable (LumU), stroma-rich, basal/squamous (Ba/Sq) and neuroendocrine-like (NE-like). APOBEC, apolipoprotein B mRNA editing catalytic polypeptide-like family of proteins; FGFR3, fibroblast growth factor receptor 3; UPK, uroplakin; TMB tumour mutation burden.
Sequencing and gene expression studies have led to the discovery of numerous DNA, RNA and protein biomarkers of bladder cancer. This provides the opportunity for a tectonic shift in how bladder cancer is diagnosed and how recurrences are detected. The sequencing and gene expression studies have also shown that several distinct molecular signatures and subtypes exist, providing a more accurate prediction of disease progression and therapeutic response as will be discussed in this Review. Additionally, clinicians hope to supplant invasive cystoscopy with blood-based and urine-based diagnostics soon. Furthermore, recent successes in immunotherapeutic regimens and renewed interest in the tumour microenvironment (TME) have expanded the repertoire of potential targeted treatments2. These advances are shifting how we manage the patient with bladder cancer, with the expectation that increases in overall survival and improvement in quality of life will continue their upward trend3. In this Review we will examine recent developments in the molecular and translational aspects of bladder cancer biology and discuss their current or potential future clinical applications in the management of this disease. Prior reviews on animal models and molecular biology of bladder cancer will serve as background, and their contents not reiterated here.
Genetic alterations in bladder cancer
Next-generation sequencing helped provide the wealth of molecular information needed for molecular classification of tumours from patients with bladder cancer. Whole-transcriptome mRNA profiling of tumour tissue samples from patients allowed researchers to establish a molecular taxonomy for bladder cancer4–10, while still recognizing there is some variation across molecular subtype classification due to patient selection, methodology differences11–15 and pathological and clinical criteria16. Bladder cancer was found to be one of the most frequently mutated human cancers, following lung and skin cancer in mutation rates17,18. Chief among these mutations is mutations in the promoter of the gene encoding telomerase reverse transcriptase (TERT), occurring with a frequency of 70–80% in patients with bladder cancer19–22. The identification of frequent mutations has, in turn, promoted novel therapeutic approaches, as well as urine and blood surveillance for both early detection and disease monitoring following treatments.
Non-muscle-invasive molecular subtypes
In clinical practice, around 80% of patients with bladder cancer have a diagnosis of NMIBC, with good life expectancy (5-year survival rates greater than 85% (REF.23)) and a low chance of developing invasive diseases24,25. However, despite advancements in research and care, the 5-year survival rate for NMIBC has not markedly changed, and the frequent recurrence of NMIBC has put a heavy load on public health systems23,26,27. Multiple studies have characterized DNA and RNA alterations in NMIBC. Deletions in chromosome 9 (harbouring the CDKN2A gene) as well as mutations in genes encoding fibroblast growth factor receptor 3 (FGFR3) and PI3K and the promoter of the gene encoding TERT, were identified as early events in urothelial malignancy28–31. Hedegaard and colleagues performed a comprehensive transcriptional analysis including 460 patients with early-stage NMIBC (345 patients with stage Ta NMIBC, 112 patients with stage T1 NMIBC and 3 patients with carcinoma in situ) and 16 patients with MIBC and identified three major subclasses with basal-like and luminal-like characteristics and distinct clinical outcomes (UROMOL study)32. Class 1 tumours showed enrichment in early cell cycle genes and are associated with good prognosis. Class 2 tumours exhibited high expression of late cell cycle genes and are predominantly associated with high-risk NMIBC. Class 1 and class 2 tumours showed high expression of uroplakins, markers of bladder luminal/umbrella cells. Class 3 tumours were enriched in KRT5 and KRT15 expression, which are markers of undifferentiated or basal cells, as well as elevated expression levels of long non-coding RNA. Importantly, class 2 tumours are associated with lower rates of survival compared with class 1 or class 3 tumours. Furthermore, that 14 of 16 MIBCs were classified as class 2 tumours highlights the similarity between MIBC and high-risk NMIBC. Moreover, proteome analysis on tissue specimens from 117 patients (98 patients with NMIBC and 19 patients with MIBC) led to the identification of three NMIBC proteomic subtypes (NPS)33. NPS1 represented a small group (17%, 17/98) of mostly T1 grade 3 high-risk samples with overexpression of an immune and/or inflammatory profile, whereas NPS2 (43%, 42/98) showed a more immune infiltrated/mesenchymal phenotype. NPS3 (40%, 39/98) showed enrichment of luminal and differentiation markers KRT20, CDH1 and uroplakins. They also exhibit high expression of undifferentiation markers KRT5 and ITGA6/ITGB4, which resembles class 1 and class 3 tumours from the UROMOL study. In line with the clinicopathological feature, NPS3 patients were of the lowest risk group with better survival outcome. A major goal in the future will be to coalesce the genomic, transcriptomic and proteomic classifications into unified classifications and to increase the feasibility of gathering the needed data from each patient for clinicians to make the most informed choice.
Muscle-invasive molecular subtypes
Dissecting tumour molecular signatures and subtypes is also urgently needed for MIBC, in which the tumour has advanced beyond the epithelial layer and into the muscle (FIG. 1). To address this need, classification by molecular subtypes has been undertaken by several groups, published previously and summarized here. A great number of criteria are taken into consideration for these classifications (Supplementary Table 1). However, a consensus MIBC molecular classification was achieved with a single sample classifier integrating six non-overlapping patient subtypes (FIG. 1), which was also highly consistent with previous classification studies34. This consensus classification included luminal papillary (LumP), luminal non-specified (LumNS), luminal unstable (LumU), stroma-rich, basal/squamous (Ba/Sq) and neuroendocrine-like (NE-like) subtypes34. In the consensus classification, luminal tumours (LumP, LumNS and LumU) showed enriched urothelial differentiation, active PPARG and GATA3 regulons and FGFR3 genetic alterations (mutation, fusion or genomic amplification). The LumP subtype was the least aggressive subtype and was more often associated with lower stage (T2) and younger patients. This subtype was frequently associated with FGFR3 mutations and increased FGFR3 regulon activity, suggesting a potential benefit from FGFR3 inhibitors. LumP tumours also harboured more frequent mutations of KDM6A and deletion of CDKN2A, yet harboured wild-type TP53. LumNS tumours were enriched with micropapillary histological variants and were associated with carcinoma in situ, a rare non-muscle-invasive type with a greater chance to evolve to muscle-invasive disease. LumNS tumours showed PPARG amplification, upregulation of PPARG downstream target genes, and ELF3 mutations. LumU tumours exhibited a significant enrichment in genomic instability and copy number variations. They also exhibited increased mutations in the genes encoding the apolipoprotein B mRNA editing catalytic polypeptide-like family of proteins (APOBEC). Moreover, LumU tumours exhibited the highest levels of TP53 and ERCC2 mutations, the latter of which is associated with sensitivity to chemotherapeutic agents.
The stroma-rich subtype displayed intermediate levels of urothelial differentiation and is notable for prominent stromal cell infiltration and high levels of smooth muscle, endothelial, fibroblast and myofibroblast gene signatures. Stromal-rich tumours were enriched with T and B cell markers. Ba/Sq subtype tumours displayed high expression levels of basal cell marker genes KRT14, KRT5 and KRT6, as well as loss of luminal cell marker genes GATA3 and FOXA1. Tumours of the Ba/Sq group were aggressive with poor prognosis, and were often found in female patients and were of higher stage. In Ba/Sq tumours, the most frequently mutated genes were TP53 and RB1. Regulon analysis showed that Ba/Sq tumours are enriched with STAT3, HIF1A, and EGFR regulon activities. Like the stroma-rich subtype, Ba/Sq tumours contained high levels of non-tumour cells. The NE-like subtype is the most homogenous subtype, with 81% of tumours displaying neuroendocrine histological features and evidence of immune infiltration. Moreover, TP53 was ubiquitously mutated in NE-like tumours, co-occurring with RB1 mutations and deletion. The NE-like subtype was also the most aggressive subtype in patients, resulting in only 1-year median overall survival.
To date, molecular subtyping of MIBC has mainly focused on stratifying global mRNA expression, which comprises less than 2% of total transcription, due to the majority of transcribed genes being ribosomal RNAs and non-coding RNAs. Robertson and colleagues performed tumour clustering based on the expression levels of non-coding RNAs, including long non-coding RNAs and microRNAs7. Long non-coding RNA clustering identified a tumour group with high-purity, papillary histology and organ-confined cancer7, which was also observed in a follow-up study35. Patients with tumours of this subtype showed enhanced activity in pathways involving FGFR3, Sonic hedgehog (SHH) and wild-type p53 proteins7,35. As genomic regions encoding mRNA represent only 1.1% of the human genome, with intronic (24%) and intergenic (75%) DNA36 constituting the rest, genetic alterations in intronic and intergenic DNA regions are not uncommon. In bladder cancer, investigators have examined mutation patterns such as those associated with APOBEC editing featuring C>T and C>G mutations37,38. Among all cancer types, MIBC has the highest enrichment score of APOBEC-specific mutations38. The most famous APOBEC-specific mutations are in the TERT gene promoter, and bladder cancer has the highest frequency of mutations in this region39 (see TERT promoter alterations). Other non-coding mutation elements have been identified in bladder cancer, including mutations in other promoters (PLEKHS1, TBC1D12, WDR74, LEPROTL and PLXDC1), in 3′ untranslated regions (TBC1D12, WDR74 and LEPROTL1) and in an enhancer for ADGRG6 (REFs40,41). Molecular subtyping of bladder cancer is finding application in the clinical setting (BOX 1).
Box 1 | Clinical application of molecular subtypes in bladder cancer
Molecular classification of tumours to identify the many different subtypes of bladder cancer (Supplementary Table 1) is defining tumours beyond just the classical basal or luminal type. With use of genetic signatures to better define bladder cancer tumours, reminiscent of breast cancer subtypes, luminal tumours were found to respond to treatment better than basal tumours8. The high mutational profile of basal tumours is the likely reason behind this fact, with a high degree of mutations in progrowth genes such as the ErbB family, allowing treatment with drugs such as afatinib242. No progrowth gene in bladder cancer has more activating mutations or amplified expression than the members of the fibroblast growth factor receptor (FGFR) gene family, most notably FGFR3. Molecular stratification by FGFR genetic status is key for treating patients with FGFR inhibitors such as erdafitinib or pemigatinib (for example, FIGHT-201 (REF.243)). Subtyping tumours by inactivating mutations in DNA repair genes such as ATM, RB1 and FANCC is also clinically applicable as these tumours exhibit a positive response to platinum-based neoadjuvant chemotherapy, with a much higher resolution coming following the completion of the current RETAIN clinical trial244. Identifying changes to epigenetic modifiers is another important molecular characterization for the clinic. DNA methyltransferase inhibitors such as decitabine245 and guadecitabine246 are being investigated for their ability to enhance the efficacy of cisplatin and atezolizumab, respectively. Beyond mutational assessment is gene expression assessment, where specific molecular subtypes exhibit differential responses to neoadjuvant chemotherapy and variable overall survival11,34. For patients with tumours of the basal subtype, luminal tumours with high expression of PDL1 or with tumours with high levels of tumour-infiltrating lymphocytes, there is the clinical option of being treated with one of the six immune checkpoint inhibitors approved for treating bladder cancer. Improved molecular subtyping and clinical utility of that information is currently being used in trials such as the BISCAY trial247, which is allocating patients to one of seven modules, dependent on their genomic alterations. While clearly molecular subtyping has made its way into clinical decision-making, dramatic increases in molecular profiling of bladder cancer tumours need to be undertaken to the fullest extent possible, as even immature information can be assessed retrospectively to provide a significant benefit to future clinical applications.
Box 2 | The urinary microbiota
The human microbiota is the populations of bacteria, bacteriophages, fungi, protozoans and viruses that exist within and on the human body248. Only in the past decade have we begun to grasp the extent to which the microbiota facilitates carcinogenesis by modulating cell proliferation and/or death, immune function and metabolism of chemokines and pharmaceuticals249. Advances in high-throughput sequencing and culturing techniques have challenged previous dogma that urine is sterile and have established there also exists a urinary microbiota250. While few studies have explored the impact of the urinary microbiota on bladder cancer pathogenesis, it is theorized to be relevant to genitourinary cancers. Chronic genitourinary tract infections have been identified as a risk factor for bladder cancer due to their promotion of chronic inflammation251. The clearest example of this occurrence is the link between a history of schistosomiasis infections and bladder squamous carcinoma. Schistosoma haematobium parasitic blood flukes lay eggs in the urinary bladder, inducing chronic cystitis and fibrosis, which contribute to tumorigenesis252. Analysis of the urinary microbiota in those infected with S. haematobium or those who presented with abnormal bladder pathology concurrent with S. haematobium infection revealed taxa different from those in healthy persons. Of note, the phyla Proteobacteria and Firmicutes primarily compose the urinary microbiota, and five genera were differentially abundant in infected participants with and without induced bladder pathology: Facklamia, Veillonella, Fusobacterium, Bacteroides and Aerococcus253. Several studies have verified an altered microbiota in patients with bladder cancer, with increased abundance of genera such as Streptococcus, Pseudomonas, Anaerococcus, Fusobacterium, Acinetobacter and Sphingobacterium254–256. Herbaspirillum, Porphyrobacter and Bacteroides were enriched in patients with bladder cancer with high risk of recurrence and progression257. Unfortunately, studies to date have not been able to fully determine if these bacterial taxa are directly contributing to bladder disease or are opportunistically colonizing altered tissue. The gut microbiota field is far more developed, but few studies have included bladder cancer in their purview. Notably, antibiotic-induced imbalances in microbial communities (dysbiosis) was shown to affect the therapeutic efficacy of immune checkpoint inhibitors, with patients with bladder cancer having significantly shorter progression-free and overall survival258. On a positive therapeutic front, oral administration of a common probiotic, Lactobacillus casei, in conjunction with intravesical instillation of epirubicin following transurethral resection, increased 3-year recurrence-free survival from 60% to 75% in patients with non-muscle-invasive bladder cancer259.
TERT promoter alterations
TERT promoter mutations are found in 70–80% of patients with urothelial bladder cancer, while it has been reported that the frequency of mutations in normal urothelium is very low19–22,42–47. TERT, the catalytic subunit of the telomerase holoenzyme, is responsible for preventing telomere shortening by adding sequences to chromosomal ends. Telomerase activity is high in embryonic and normal stem cells but nearly undetectable in most somatic cells due primarily to transcriptional downregulation of TERT expression48. TERT gene promoter mutations in urothelial cancer cell lines lead to higher levels of TERT expression and enzymatic activity compared with wild-type cell lines49. Compared with low TERT expression, high TERT expression is associated with reduced disease-specific survival of patients with bladder cancer49. TERT re-expression in cancer cells commonly occurs because of increased promoter activity caused by two hot-spot point mutations in the core promoter region at −124 C>T (C228T) or −146 C>T (C250T) from the start codon ATG49. The C228T and C250T mutations are present in 50–70% and 8–15% of patients with urothelial bladder cancer, respectively19–22,42–47. Both mutations are mutually exclusive from each other50–52, and both NMIBC and MIBC have these mutations at similar frequency22,46,53. Among various histological subtypes of urothelial bladder cancer, TERT promoter mutations have been reported in plasmacytoid variant54, nested variant55, papillary urothelial carcinomas56 and glandular lesions57. Two studies examined the mutation status in inverted papillomas, with one reporting mutations in only 15% of cases44 while the other reported no mutations56. Besides urothelial bladder cancer, other bladder cancer types have TERT promoter mutations. In bladder squamous cell carcinoma 80% of tumours have mutations58. In 11 patients with small cell carcinoma of urinary bladder that were examined, all had the C228T mutation59. Finally, in bladder adenocarcinoma, TERT promoter mutations were detected in 13% (2/15) of cases60. Thus, TERT promoter mutations are ubiquitous in bladder cancer types and are limited to the two specific mutations mentioned above.
Epigenetic modifications
Bladder cancer also exhibits significant epigenetic dysregulation such as changes in DNA methylation61. The Cancer Genome Atlas (TCGA) analysis of MIBC samples from patients has identified clusters of DNA hypomethylation and hypermethylation7. DNA hypomethylation appears to be more common in stage 1 and 2 MIBC and NMIBC than in MIBC of higher stages, while DNA hypermethylation is linked to silencing of tumour suppressor genes (for example, TP53, RB1, CDKN2A and CDH1) and is associated with aggressive disease7. In line with this, classification of MIBC according to a DNA methylation signature may provide a benefit for identifying predictive biomarkers and also treatment therapies61.
Advances in tumour genomic analysis have also revealed that bladder cancer has a much higher mutational load than most other cancers, particularly in chromatin remodelling genes encoding proteins such as histone demethylase KDM6A, histone methyltransferases (KMT2C and KMT2D), histone acetylases (CREBBP and EP300) and the SWI/SNF chromatin remodelling complex (ARID1A)7 (FIG. 2). Most of the alterations are inactivating mutations or deletions7, indicating that loss of epigenetic regulation may be a major force in shaping bladder cancer progression. KDM6A is an X-linked histone demethylase specific for histone H3 Lys27 dimethylation and trimethylation62, and research showed that its functional loss is a strong driver of bladder cancer progression. Silencing of KDM6A with short hairpin RNA promoted bladder cancer cell proliferation and migration in vitro, and xenograft tumour growth in vivo compared with controls42. Furthermore, conditional knockout of Kdm6a in the urothelium increased the risk of bladder cancer progression in female mice63. Consistent with this, the presence of KDM6A mutations in female patients with cancer predicts a poorer prognosis, in line with other sex differences observed in patients and discussed in the section Biological sex-based features63. Mice with Kdm6a deficiency and Trp53 haploinsufficiency spontaneously developed carcinoma in situ in the urothelium and had accelerated disease when exposed to N-butyl-N-(4-hydroxybutyl) nitrosamine treatment64. However, genomic sequencing has also revealed that deletion of KDM6A in bladder cancer cells led to upregulation of PRC2-mediated and EZH2-mediated transcriptional repression, which rendered these cells more sensitive to EZH2 inhibitors65.
Fig. 2 |. Major genomic alterations in human bladder cancer.
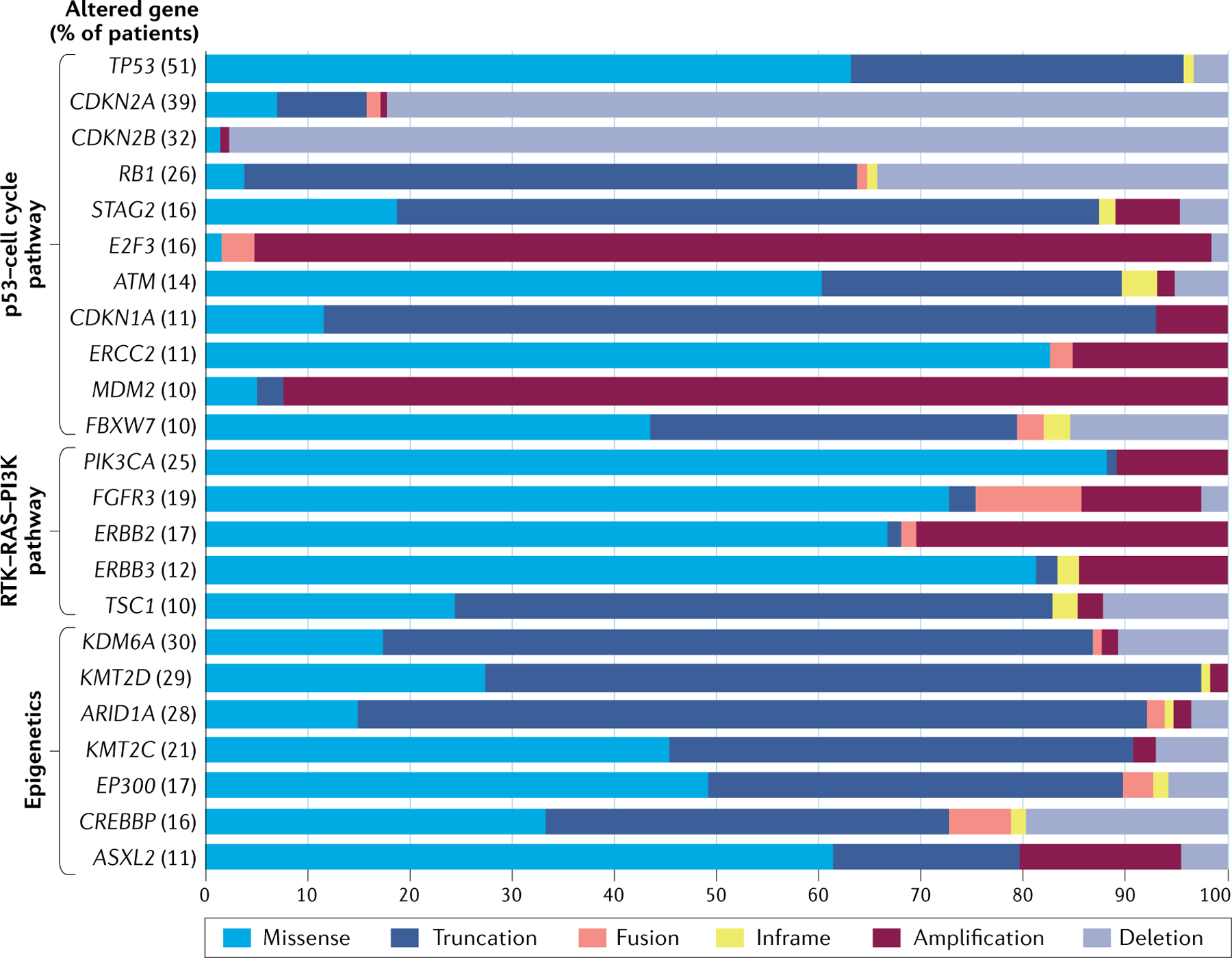
Whole-transcriptome mRNA profiling of patient samples identified frequently altered genes and pathways that drive bladder cancer (The Cancer Genome Atlas (TCGA)241). The data shown in this figure were retrieved from TCGA Pan Cancer Atlas in CBioPortal, and represent the frequency of altered genes in patient samples (n = 406) and the proportions of the different types of alterations per gene. Somatic alterations of genes are most common in pathways related to p53, the cell cycle, receptor tyrosine kinase (RTK)–RAS–PI3K and epigenetic modifications. Alterations in TP53 and cell cycle genes are common in muscle-invasive bladder cancer (MIBC), including CDKN2A, CDKN2B, RB1, STAG2, E2F3, ATM, CDKN1A, ERCC2, MDM2 and FBXW7. Common mutations for TP53 are missense and truncating mutations (frameshift, insertion/deletion and splice site mutation), which induce loss of funtion. Genes limiting cell cycle activity such as CDKN2A, CDKN2B, RB1 and CDKN1A are commonly inactivated by homologous deletion and truncating mutations. Progrowth signalling genes E2F3 and MDM2 commonly exhibit amplification. STAG2 encodes a subunit of the cohesin complex, which regulates sister chromatid separation during cell division and is frequently silenced by truncating mutations in MIBC. ATM encodes a DNA damage sensor protein and exhibits loss of function when mutated (mostly missense and truncating mutations) in 14% of patients with MIBC. The protein product of ERCC2 is involved in nucleotide excision repair, ERCC2 is mutated in 11% of patients with MIBC and missense mutations drive MBIC cisplatin sensitivity. FBXW7 encodes a protein that functions in cell cycle exit and stem cell maintenance and is often found mutated in MIBC. Activating mutations in FGFR3, PIK3CA, ERBB2 and ERBB3 enhance tumour growth. TSC1 negatively regulates mechanistic target of rapamycin complex 1 signalling and the gene is frequently inactivated by truncating mutations. Bladder cancer often has truncating and missense mutations in chromatin remodelling genes such as ARID1A, KDM6A, KMT2D, KMT2C, EP300, CREBBP and ASXL2.
Together with KDM6A, the histone methyltransferases KMT2D and KMT2C participate in scaffolding nuclear regulatory structures called ‘complex of proteins associated with set1’ (COMPASS)66. Silencing KMT2D using small interfering RNA promoted bladder cancer cell proliferation and invasion in an in vitro transwell assay, as well as xenograft tumour growth in vivo, suggesting the gene has a tumour suppressor role in bladder cancer. Silencing KMT2C decreased histone H3 Lys4 methylation at enhancer regions, leading to decreased expression of DNA damage response genes (for example, ATM, ATR, CHEK2 and BRCA1) and an increase in chromatin instability of bladder cancer cells.
CREBBP and EP300 are two closely related proteins whose histone acetyltransferase domain acetylate histones, thereby remodelling chromatin into different transcription states67. One-third of patients with bladder cancer harbour genetic alterations in the genes encoding EP300 and/or CREBBP, and it has been discovered that loss of histone acetyltransferase domain activity through mutations or deletions is associated with lower rates of survival of patients68. ARID1A is another frequently mutated gene in bladder cancer tumours7. ARID1A and the associated SWI/SNF complex are recruited to DNA double-strand breaks to facilitate the DNA damage repair69. Clinically, meta-analysis showed that higher levels of microsatellite instability and tumour mutation burden were found in patients with bladder cancer with genetic alterations of ARID1A, and this was associated with greater progression-free survival rates after immunotherapy70 — showing that in addition to genetic and epigenetic modifications in bladder cancer cells, cancer cell-extrinsic factors and the TME are important to consider.
Tumour microenvironment
The TME consists of malignant and non-malignant cells where cancer cells have co-opted non-malignant cells, including immune and stromal cells, into ‘helping’ them prosper (FIG. 3). It is increasingly evident that the interplay of these cellular compartments affects cancer progression and response to therapeutics. Therefore, current preclinical models incorporate these compartments and enable investigation of specific cell type-dependent contributions to cancer progression (TABLE 1).
Fig. 3 |. Non-tumour cell types implicated in bladder cancer progression.
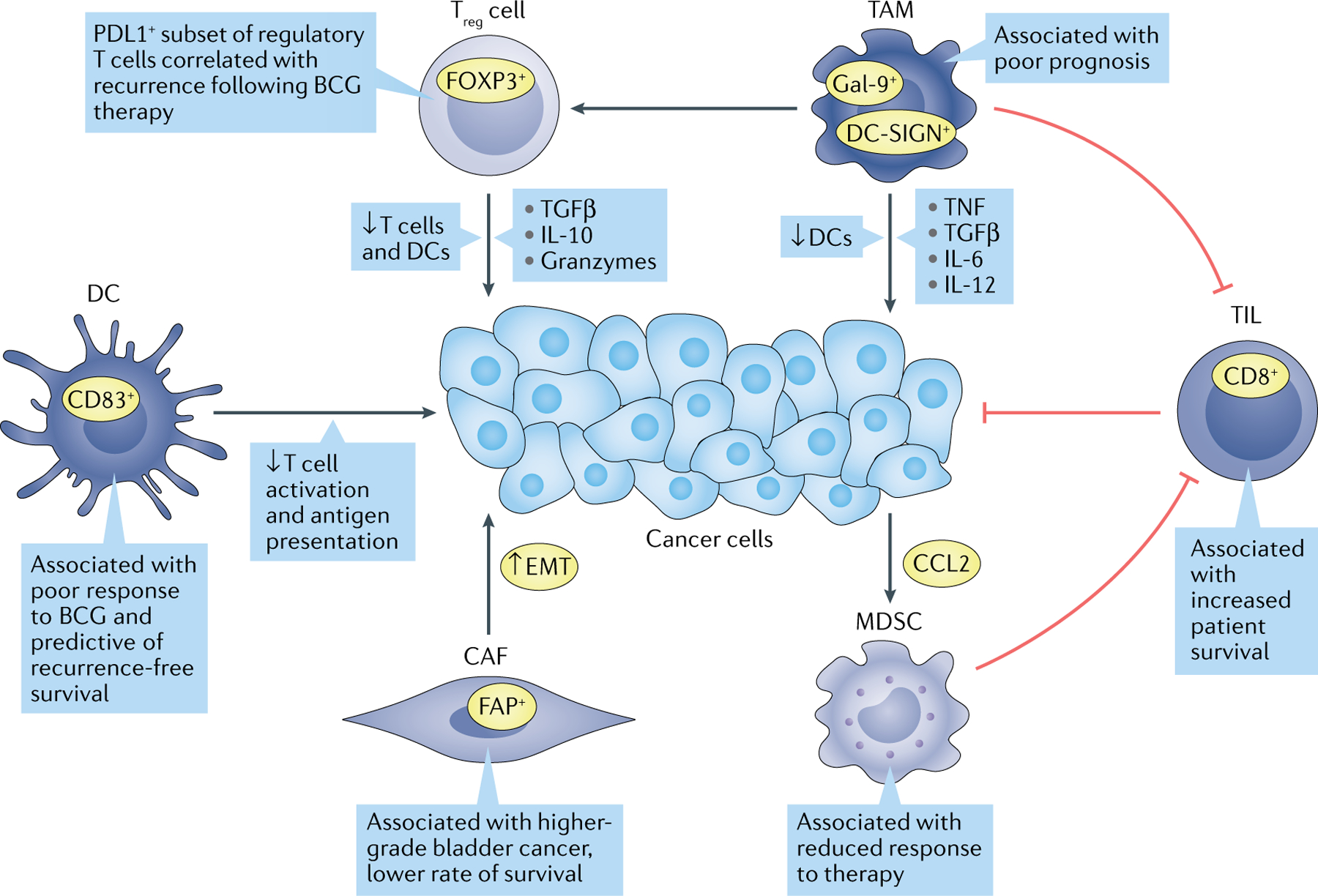
Signalling between cancer, stromal and immune cells modulates tumour growth and aggressiveness. Many of these cell types have been correlated with therapeutic resistance and lower survival outcome for patients. Green arrows indicate protumorigenic associations, while red bars indicate antitumour activity. CAF, cancer-associated fibroblast; CCL2, C-C motif chemokine ligand 2; DC, dendritic cell; DC-SIGN, dendritic cell-specific C-type lectin; EMT, epithelial–mesenchymal transition; FAP, fibroblast activation protein; FOXP3, forkhead box protein P3; Gal-9, galectin 9; MDSC, myeloid-derived suppressor cell; TAM, tumour-associated macrophage; TIL, tumour-infiltrating lymphocyte; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor; Treg cell, regulatory T cell.
Table 1 |.
Preclinical models used in discovery and translation
| Model | Description | Relevance | Limitations | Refs |
|---|---|---|---|---|
| Urinary tract induced pluripotent stem cells | Human induced pluripotent stem cells are generated from urinary tract tissue | Used to characterize mechanisms regulating bladder differentiation and model disease | Viruses used to induce embryonic genes may cause cancer. Methods have not been extensively validated | 226 |
| Patient-derived organoids | Maintenance of bladder cancer cells in 3D cultures | Cell–cell and cell–matrix interactions are more similar to tumours, allowing more accurate high-throughput drug screening | Models have not been extensively validated. Heterogeneity of cells can hinder reproducibility | 227,228 |
| Patient-derived xenograft | Patient tumour samples implanted into immunodeficient mice | Models specific patient samples and subtypes. NCI has developed a national repository. Humanized mice improve therapeutic opportunity | Cannot query immune mechanisms. Limited translation to patients | NCI Patient-Derived Models Repository (NCI at Frederick, Frederick National Laboratory for Cancer Research, Frederick, MD, USA) |
| Renal engraftment | Combination of bladder cancer cells with embryonic mesenchyme under the renal capsule of immunocompromised mice | Incorporates stromal–epithelial interactions in functional studies | Microenvironment does not recapitulate bladder tissue | 229 |
| Orthotopic engraftment | Human bladder cancer cells implanted into the bladder wall of immunodeficient mice | Microenvironment more closely models bladder disease | Tumours do not develop de novo. Cannot query immune mechanisms | 230 |
| Syngeneic engraftment | Murine bladder cancer cells implanted into an immunocompetent syngeneic host | Ease of analysis for immunotherapeutic targets | Microenvironment does not recapitulate bladder tissue | 225 |
| Carcinogen induced | Administration of carcinogens through drinking water or intravesical instillation. Common agents include N-butyl-N-(4-hydroxybutyl) nitrosamine and N-methyl-N-nitrosourea | Tumours develop de novo from a carcinogen known to be associated with human bladder cancer | Cannot probe specific molecular subtypes due to heterogeneity. Substantial investment of time for tumour development | 231 |
| Genetically engineered mouse | Expression of oncogenes and/or knockout of tumour suppressors in the urothelium/germ line | Models common mutations observed in human bladder disease | Substantial investment of time for model development. Limited targeting of genes rarely recapitulates complexity of disease | 232 |
A comprehensive review of murine and human bladder cancer cell lines has recently been published233 and is not reiterated here. NCI, US National Cancer Institute.
Cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs) are the predominant stromal cell type observed in the TME and have been implicated in the pathogenesis of multiple cancers71. While no defined marker profile has been established, these activated fibroblasts are often identified by expression of vimentin, α-smooth muscle actin, fibroblast activation protein (FAP), S100 calcium-binding protein A4 (S100A4) and platelet-derived growth factor receptor-β (PDGFRβ). While known for their role in remodelling of the extracellular matrix, CAFs have attracted attention as a potential therapeutic target due to their role in modulating the immune system and crosstalk with cancer cells. However, this concept is controversial as conflicting studies show that their function can be tumour promoting72 and/or tumour inhibiting even in the same cancer type (in this case, pancreatic cancer), dependent on the setting73. This can be partially explained by the heterogeneity of CAF populations observed in the TME74 and the lack of known mechanisms that regulate stromal differentiation during cancer progression. Immunohistochemical analyses of primary tumours from patients with bladder cancer have shown increased presence of CAFs compared with normal urinary bladder tissue75, and expression of CAF markers CD90, FAP and PDGFRβ positively correlated with the aggressiveness of bladder cancer76. Additionally, a hierarchical cluster analysis of 344 patients with bladder cancer revealed a patient cluster with dominant expression of FAP to have a lower 5-year survival outcome76. These findings were further substantiated by a study that found expression of FAP positively correlated with muscle invasiveness and negatively correlated with survival in patients with bladder cancer of the basal phenotype77. In addition, stromal expression of CAF markers has been strongly correlated with expression of epithelial–mesenchymal transition (EMT) markers in cancer cells, and stimulated fibroblasts can induce cancer cell EMT in vitro78. Moreover, human CAFs promote resistance to cisplatin by increasing the antiapoptotic gene BCL2 in cancer cells through IGF1-oestrogen receptor-α (ERα) signalling in vivo79. These studies indicate that CAFs play a significant role in bladder cancer progression and contribute to aggressive disease.
Tumour-infiltrating immune cells
The approval of immune checkpoint inhibitors (ICIs) for the treatment of metastatic bladder cancer (see Immunotherapies) has reinvigorated interest in the immune component of the TME for its prognostic and therapeutic potential. ICIs target negative regulating cell receptors on immune cells, predominantly T cells, and prevent those receptors from signalling, leading to reactivation of those cells and promotion of a durable antitumour response. Currently approved ICIs target cytotoxic T lymphocyte-associated protein 4 (CTLA4) and the PD1–PDL1 axis. However, there are significant research efforts focused on expanding the known repertoire of targetable co-stimulatory molecules80.
Tumour-infiltrating lymphocytes.
Tumour-infiltrating lymphocytes (TILs) are indicative of a cell-mediated host response to tumour cells. Cytotoxic CD8+ T cells are well characterized due to their direct role in restraining tumour growth. On recognition of foreign antigens, CD8+ T cells can induce apoptosis through release of cytotoxins or cell-surface expression of death ligands81. In MIBC, quantification of TILs that locate to the tumour-adjacent stroma has been shown to predict immune phenotype, patient survival and molecular tumour subtype82. Tumours have been often classified by the extent of TIL presence, resulting in three classifications: tumours that are uninflamed with low infiltration of TILs, tumours that are inflamed with low or high infiltration of TILs, and tumours with moderate TIL infiltration yet lack PDL1+ immune cells82. Inflamed tumour phenotypes were also characterized by a chemokine gene expression signature. High inflammation in tumours was shown to be correlated with increased survival of patients with nearly any type of cancer. In bladder cancer, total tumour gene expression analysis has shown that an EMT-related gene expression profile correlated with increased T cell infiltration83. However, Wang et al. found that stromal cells contributed more to EMT-related gene expression than cancer cells. Their findings support the notion that the high levels of T cells observed in tumours are actually present in the stroma and are not directly interacting with cancer cells, rendering those T cells ineffective in their duties as antitumour agents. Consistent with this, patients with high CD8+ T cell infiltration and a low stroma-related gene expression signature have longer overall survival, while a high stroma-related gene expression signature is associated with worse outcomes in response to immune checkpoint blockade83. In the immune exclusion phenotype, which is common in metastatic urothelial cancer, CD8+ T cells are observed only in the peritumoural stroma83, strongly supporting the notion that the advanced disease may have reduced T cell infiltration due to stroma-induced changes in the TME. These modulations include sequestration of T cells in stroma due to cytokine expression84 and physical exclusion of T cells from the tumour bulk due to increased deposition of fibronectin and collagen fibres in the extracellular matrix85. Studies exploring tumour oncogenic pathways have identified FGFR3 mutations primarily in non-inflamed tumours and β-catenin and peroxisome proliferator-activated receptor-γ as possible regulators of T cell exclusion86. From a therapeutic standpoint, reducing stromal cell influence by abrogation of transforming growth factor-β signalling, facilitated T cell infiltration and enhanced immune checkpoint therapy effectiveness84.
Tumour-associated macrophages.
Macrophages residing in the tumour tissue or those that are recruited from the bone marrow to intratumoural regions of hypoxia and necrosis can become tumour-associated macrophages (TAMs)87. These TAMs potentiate cancer growth88, metastasis89 and resistance to therapy90 through secretion of growth factors, cytokines and proteases. TAMs also promote chronic inflammation, a suppressing event for immune-related tumour cytotoxicity, through release of mediators such as tumour necrosis factor (TNF), transforming growth factor-β, IL-6 and IL-12 (REF.91). Although macrophages can be polarized across a continuum of phenotypes92, they are generally subtyped as ‘M1’ or ‘M2’. The M1 lineage promotes acute inflammation in response to pathogens and tumour cells, while the M2 lineage promotes chronic inflammation, which in turn leads to immunosuppression and protumour growth93. TAMs isolated from patients with bladder cancer are predominantly of the M2 type and are found in greater quantities in higher-grade disease compared with low-grade disease88.
In patients with bladder cancer in situ, the disease has not invaded physiological boundaries yet (FIG. 1) and can be treated via intravesical instillations of a therapeutic agent, most commonly bacillus Calmette–Guérin (BCG). How BCG exerts its effect on bladder cancer has not been fully elucidated, but it stimulates a local immune response in the lumen of the bladder, with a collateral outcome being an enhanced immune response against the bladder cancer cells. First given to patients in 1977, this is an early example of immunotherapy. When the disease recurs after BCG treatment, it was found that it was in instances of high TAM infiltration, 47.6% versus 4.8% (REF.94). In MIBC, the frequency of galectin 9-positive (Gal-9+) TAMs positively correlated with tumour stage and grade95. Patients with MIBC and higher percentages of Gal-9+ TAMs had strong correlations with poorer prognosis, reduced intratumoural presence of CD8+ T cells and dendritic cells, and increased presence of immunosuppressive regulatory T cells (Treg cells) and mast cells as compared with patients with lower percentages of Gal-9+ TAMs95. It was these patients who derived greater survival benefit following adjuvant chemotherapy95. Another subclassification of M2 macrophages, dendritic cell-specific C-type lectin (DC-SIGN) TAMs, have been observed in MIBC and are associated with poorer prognosis. Patients with MIBC and low levels of DC-SIGN TAM infiltration had more favourable survival outcomes after adjuvant chemotherapy than patients with high DC-SIGN TAM infiltration96. The latter group of patients exhibited increased levels of Treg cells as well as increased levels of intratumoural cytotoxic CD8+ T cells, but the cells had reduced levels of the cytotoxins perforin, granzyme B and interferon-γ (IFNγ), while also showing increased expression of co-inhibitory receptors TIGIT and LAG3 (REF.96), indicative of an overall immunosuppressive environment in patients with high DC-SIGN TAM infiltration.
Other immune cells.
Stromal cells can recruit M2 macro-phages to the TME and promote Treg cell infiltration97. Cancer cells themselves can recruit immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs), an umbrella term encompassing a wide range of immature myeloid cells, and defective dendritic cells that promote T cell tolerance of tumour antigens98. In normal tissues, myeloid cells promote a tolerogenic microenvironment to restrict excessive inflammation and prevent autoimmune disease99. In cancer, myeloid cells foster immune evasion, stimulate proliferation and promote invasion and metastasis100, ultimately hindering immunotherapy. Treg cells secrete inhibitory cytokines, granzyme A and granzyme B to suppress T cell activation and proliferation, as well as modulate dendritic cell maturation and function101. Urinalysis of patients with NMIBC revealed conventional Treg cells are not substantially present before BCG instillation, but the treatment can induce a subset of PDL1+ Treg cells that can persist after treatment102. With both subsets taken into account, high levels of urine Treg cells were correlated with rapid recurrence following BCG therapy102. These findings support combining use of an ICI with BCG instillation for the treatment of NMIBC (see “Immunotherapies”).
In patients with bladder cancer, monocytic MDSCs (M-MDSCs) are enriched in cancerous tissues and suppress CD8+ T cell activities. A preclinical model indicated that gemcitabine treatment can induce bladder cancer cells to secrete C-C motif chemokine ligand 2 (CCL2), promoting M-MDSC recruitment and limiting response to therapy103. Inhibiting the CCL2 chemokine receptor alone blocked metastasis in models104, and when combined with gemcitabine can reduce M-MDSC chemotaxis and increase overall survival in syngeneic mouse models of bladder cancer103. Additionally, targeting MDSCs with α-Ly6C and α-Gr1 antibodies in combination with immune checkpoint therapy was shown to reduce tumour volume and increase infiltration of CD8+ T cells in a murine xenograft model of bladder cancer105.
Dendritic cells are antigen-presenting cells that recognize pathogen-associated and danger-associated signals to prime naive T cells and contribute to the antitumour immune response. Their dysfunction in the TME results in ineffective antigen presentation and T cell activation100. In NMIBC, high levels of tumourinfiltrating dendritic cells are associated with a poor response to BCG instillation106. Consistent with these findings, the ratio of CD8+ T cells to various immunosuppressive cell types has emerged as a prognostic marker in bladder cancer, as in many other cancer types. In NMIBC, a CD8+ T cell to MDSC ratio of less than 1 is predictive of shorter recurrence-free survival107. In MIBC, the CD8+ T cell to Treg cell ratio is associated with response to neoadjuvant chemotherapy, with those having a ratio of less than 1 having no response108.
While the TME field is fairly nascent in bladder cancer, advances in imaging and single-cell analysis technologies can further elucidate the significance of stromal and infiltrating immune cell types in cancer progression and therapeutic interventions109. Application of these technologies to clinical trials is especially appealing and may reveal mechanisms of therapeutic sensitivity and resistance. More broadly, single-cell RNA sequencing can be applied to survey stromal and immune cell populations, in conjunction with tumour cells, to determine favourable courses of treatment110. Applied to specific populations, such as T cells, single-cell RNA sequencing can identify tumour-specific states of CD8+ T cells and produce a gene signature predictive of clinical response to therapies such as ICIs111. The incorporation of TME components in patient care has the potential to significantly improve the diagnoses and treatment of patients with bladder cancer. While much remains to be discovered, current research indicates the findings will be well worth the investment.
Population-based features
Ethnicity and socio-economic factors
While patient care has greatly improved over the decades, there still exists a noticeable racial disparity in overall survival of patients with bladder cancer. In an analysis of age at diagnosis, disease stage and other variables between 1973 and 2014 with use of the SEER (Surveillance, Epidemiology, and End Results) database, racial differences were noted in bladder cancer112. In the American military health system, which provides universal health care to all beneficiaries, Black patients were more likely to present with MIBC than white patients113. However, white and Black patients were not significantly different in overall and recurrence-free survival regardless of muscle invasion. This suggests the racial disparity observed in higher-grade bladder cancer may be partially due to societal factors affecting access to health care. Additionally, Black patients have lower overall survival than white patients following radical cystectomy for transitional cell carcinoma114. When considered in the context that Black patients are less likely to receive surgical treatment, and those that do receive cystectomies are in low-volume hospitals, the racial divide observed in bladder cancer is further deepened114,115. While biological factors contributing to advanced bladder cancer should not be discounted, socio-economic differences likely exist in the care that the two patient populations are receiving. Further studies are overdue to increase our understanding of this discrepancy.
Biological sex-based features
Patients with a female genitourinary system and hormonal environment are more likely to present with local advanced disease at the time of diagnosis even when significant covariates such as age and health-care access are considered116. Compared with patients with a male genitourinary system and hormonal environment, these patients may have a higher risk of progression, disease recurrence and death117 when all risk variables are considered, but this is still a matter of significant debate. In some studies, reduced survival in these patients is maintained when that data are adjusted for age, disease stage, co-morbidity burden, socio-demographic factors and clinical management118, driving hypotheses that differential exposure to environmental hazards, sex steroid hormone regulation and molecular variances may account for the discrepancies in incidence and mortality. Additional evidence for a role of sex steroid hormones in bladder cancer progression includes the observation that there is an increased risk of bladder cancer progression among postmenopausal patients119 and reduced risk among patients who have experienced hormone replacement therapy120. While few studies have explored the contribution of the urinary microbiota to bladder cancer pathogenesis (see BOX 2), the male and female genitourinary flora are dominated by different genera that may partially account for the sex disparity121,122.
In preclinical models, sex chromosomes and hormones have roles in bladder cancer development63. X-linked genes, such as KDM6A, can be sexually dimorphic and protect against bladder cancer. Androgen receptor (AR), ERα and ERβ are present in bladder tumours, albeit at low levels, and their expression patterns are similar between biologically male and female patients. However, specific percentages differ greatly between disease-type cohorts. In one comprehensive immunohistochemical study of 188 bladder tumours, 141 non-neoplastic bladders and 14 lymph node metastasis tissues, AR was found to be expressed moderately (42% of primary tumours and 71% of metastatic tumours), ERα was expressed infrequently (27% of primary tumours and 64% of metastatic tumours) and ERβ was expressed moderately (49% of primary tumours and 71% of metastatic tumours). All three receptors were more frequently observed to be expressed in lymph node metastases as compared with primary tumour, while expression of AR and ERα inversely correlated and expression of ERβ directly correlated with higher-grade disease123. Experiments in a carcinogen-induced mouse model have also demonstrated AR expression is crucial to bladder cancer initiation and progression124, whereas ERα expression restrains initiation and growth125 and ERβ expression supports growth and invasion126. These findings support the idea that ERβ is the prevalent ER observed to play a role in bladder cancer.
Retrospective studies of patients with prostate cancer treated with androgen deprivation therapy and patients with benign prostatic hyperplasia treated with 5α-reductase inhibitors revealed lower risk of development127 and recurrence of bladder cancer127–129 as compared with surgical treatment and radiotherapy or no ADT. The role of AR signalling in mechanisms pertinent to bladder cancer progression have recently been extensively detailed and will not be reiterated here130. Preclinical models have indicated AR-negative bladder cancer cells are more sensitive to ionizing radiation as compared with AR-positive cells131. Administration of the antiandrogen drug hydroxyflutamide enhanced radio-sensitivity, suggesting abolishing AR signalling may increase the efficacy of radiotherapy131. Taken together, the findings suggest AR is as a promising therapeutic target to reduce bladder cancer development and increase the efficacy of current treatments.
Molecular diagnosis
The current standard of care for bladder cancer diagnosis is cystoscopy and urine cytology132 (TABLE 2). The advantages and disadvantages of cystoscopy from a patient and health-care system perspective have been well described. Urine cytology provides a unique opportunity to assess eluent from an organ and is non-invasive. However, it has lacked adequate sensitivity for low-grade bladder cancer133,134. Among bladder cancer diagnoses, nearly 25% will be given the aggressively growing muscle-invasive or metastatic classification3. Thus, developing highly accurate, cost-effective, non-invasive tests for bladder cancer diagnosis and surveillance is an area of need in clinical diagnosis, particularly for early and low-grade urothelial tumours132.
Table 2 |.
Currently available non-invasive molecular diagnostics
| Studies | Materials | Targets | Number of patients per study | Refs |
|---|---|---|---|---|
| UroMuTERT | Urinary cellular DNA and cfDNA | TERT promoter | 93 patients with bladder cancer and 94 controls | 234 |
| 1 gene | cfDNA | TERT promoter | 53 patients with bladder cancer and 36 controls; 104 patients with bladder cancer | 146,235 |
| 2 gene | cfDNA | TERT promoter and FGFR3 | 153 patients with bladder cancer and controls | 236 |
| UroSEEK | Urinary cellular DNA | TERT promoter region (TERTSeqS) + 10 genes (FGFR3, PIK3CA, TP53, HRAS, KRAS, ERBB2, CDKN2A, MET, MLL and VHL) (UroSeqS) | 570 patients with bladder cancer; 527 patients with bladder cancer | 145 |
| Somatic mutation, 23 genes | Urinary cellular DNA and cfDNA | TERT promoter, FGFR3, PIK3CA, TP53, ERCC2, RHOB, ERBB2, HRAS, RXRA, ELF3, CDKN1A, KRAS, KDM6A, AKT1, FBXW7, ERBB3, SF3B1, CTNNB1, BRAF, C3orf70, CREBBP, CDKN2A and NRAS | 956 patients with bladder cancer | 237 |
| Stanford University uCAPP-Seq | Urinary cellular DNA and cfDNA | High-throughput targeted sequencing approach | 118 patients with bladder cancer and 67 controls | 238 |
| Hong Kong methylomic and copy number analysis | cfDNA | Shallow-depth paired-end genome-wide bisulfite sequencing | 46 patients with bladder cancer and 39 controls | 40 |
| 5 gene + 7 gene panel | Urinary cellular DNA and cf DNA | 5 genes for urine supernatant cfDNA (TERT, FGFR3, TP53, PIK3CA, and KRAS); 7 genes for urine sediment cellular DNA (TERT, FGFR3, TP53, HRAS, PIK3CA, KRAS and ERBB2) | 16 patients with bladder cancer | 239 |
cfDNA, cell-free DNA.
Evaluation of RNA and DNA in urine from patients has been investigated for decades135 as a diagnostic marker, yet has only recently been approved136. Such evaluations now include panels rather than one marker, along with various assessments such as measuring gene expression levels, sequence variations, histone modifications and DNA methylation. Recent advances in next-generation sequencing and isolation techniques have also shed light on the diagnostic value of cell-free DNA (cfDNA) detection and profiling137,138. A recent study showed importantly that, compared with urinary cellular DNA, urinary cfDNA more faithfully correlated with the genetic alterations in tumour tissue139. Of the mutations detected via circulating cfDNA in bladder cancer to date, TERT promoter mutations appear most commonly, making them perhaps the most useful for primary and secondary (recurrence) detection of bladder cancer22,43,140–147. FGFR3 is also highly mutated in bladder cancer, and its use has been shown to offer excellent tumour detection sensitivity22,43,140–146. Panels incorporating TERT promoter mutations enable the diagnosis of bladder cancer months to years before clinical manifestation or positive cytology findings141–143,145. One recent study reported detection of TERT promoter mutations in urine 10 years before the presence of bladder cancer symptoms148.
Like cfDNA in the urine, increased levels of cfDNA have also been detected in the plasma of patients when compared with levels from normal controls149,150. While most cfDNA is believed to come from normal haematopoietic breakdown151, there are significant levels that come from tumours150,152. Thus, plasma cfDNA for early detection of disease and disease relapse is being rigorously studied in human cancers, including bladder cancer153. Assaying plasma cfDNA has been shown to non-invasively detect bladder cancer up to 4 years before the patient presented with the disease154. For example, in patients with NMIBC compared with patients who have been declared disease-free of bladder cancer, higher levels of plasma and urine cfDNA are detected155. Furthermore, as expected, the levels of plasma and urine cfDNA significantly increase on metastatic relapse156, and analysis of serially collected plasma samples from patients led to identification of disease relapse earlier than by radiographic imaging after radical cystectomy156–158. Thus, the minimally invasive approach of isolating cfDNA from blood and urine is an extremely promising method of early detection and post-treatment monitoring of disease. The current limitations of identifying the cancer mutation signature in cfDNA above the normal background signal will diminish further with advances in next-generation sequencing and more detailed molecular subtyping.
Therapeutic advances
Non-muscle-invasive disease
Increasing intravesical dwell time and uptake of current therapeutic agents.
Relative to treating MIBC, treating NMIBC has a major advantage in that non-systemic chemotherapy can be applied into just the lumen of the bladder (intravesical chemotherapy), sparing the normal tissues in the rest of the body from the toxicity of the agents used. Furthermore, intravesical chemotherapy is quite effective, along with transurethral resection of the bladder, in reducing recurrence of disease159,160. Mitomycin C, epirubicin, thiotepa, gemcitabine and doxorubicin are the most commonly used agents for this purpose, and although they are effective, there is room for improvement. Thus, a number of clinical trials are ongoing to validate changes to the delivery of therapeutics (TABLE 3) in an intravesical setting. To increase the effectiveness, investigators have sought to increase the dwell time and thus contact time of therapeutics with regard to the tumour cells lining the bladder lumen through implantation of carriers that allow localized diffusion of the drug over time. This approach has been approved by the FDA for a number of eye treatments161. Several trials are using a gemcitabine-releasing intravesical system (TAR-200) in a 21-day dwell period in combination with nivolumab for treatment of NMIBC162 and in patients with MIBC who are unfit for radical cystectomy163. Mixing chemotherapy agents in hydrogels is another approach to achieving sustained release and increasing the dwell time. A phase II, dose-escalation study investigated the efficacy and disease recurrence rate in patients with NMIBC treated with mitomycin C in hydrogel before transurethral resection of the bladder164, with a follow-up phase IIb study165 currently active. This approach was so successful in combating upper urinary tract urothelial cancer that it recently gained FDA approval166.
Table 3 |.
Clinical trials discussed in this Review
| Interventions | Administration type | Phase | Start date | Completion date | Status | Recruiting? | Number of patients | Ref. |
|---|---|---|---|---|---|---|---|---|
| Non-muscle-invasive disease a | ||||||||
| TAR-200 drug delivery system (gemcitabine); nivolumab | Intravesical; systemic | I | Jan. 2019 | Sep. 2020 | Completed | No | 25 | 162 |
| TAR-200 drug delivery system (gemcitabine) | Intravesical | I | Jan. 2018 | Mar. 2020 | Completed | No | 35 | 163 |
| TC-3 gel (mitomycin C) | Intravesical | II | Dec. 2014 | Jun. 2017 | Completed | No | 14 | 164 |
| UGN-102 (gemcitabine) | Intravesical | II | Oct. 2018 | Nov. 2020 | Active | No | 63 | 165 |
| TLD1433 infusion and photodynamic therapy | Intravesical | I | Dec. 2016 | Aug. 2018 | Completed | No | 6 | 170 |
| TLD1433 infusion and photodynamic therapy | Intravesical | II | Aug. 2019 | May 2022 | Active | Yes | 125 | 172 |
| Pembrolizumab | Systemic | II | Feb. 2016 | Jul. 2023 | Active | Yes | 260 | 182 |
| Attenuated measles virus (MV-NIS) | Intravesical | I | Jul. 2018 | Apr. 2021 | Active | Yes | 16 | 183 |
| Toca 511 | Intravesical | I | Dec. 2019 | NA | Terminated | No | 0 | 184 |
| Nadofaragene firadenovec (adenovirus vector; interferon-α2b gene) | Intravesical | III | Sep. 2016 | Aug. 2022 | Active | No | 157 | 185 |
| Ty21a (typhoid vaccine) | Intravesical | I | Feb. 2018 | Mar. 2021 | Active | Yes | 25 | 186 |
| Recombinant EphB4–HSA fusion protein | Intravesical | I | May 2020 | May 2023 | Active | Not yet | 36 | 187 |
| ALT-803 (IL-15 receptor-α fusion); BCG | Intravesical | II | Jun. 2017 | Jan. 2023 | Active | Yes | 183 | 188 |
| BC-819 (plasmid expressing diphtheria toxin in malignant cells) | Intravesical | II | Dec. 2018 | Aug. 2020 | Terminated | No | 140 | 189 |
| Muscle-invasive disease b | ||||||||
| COXEN; 75 approved agents | Various | II | Mar. 2017 | Oct. 2019 | Completed | No | 8 | 200 |
| COXEN; chemotherapy | Various | II | Jul. 2014 | Oct. 2022 | Active | No | 237 | 201 |
| Enfortumab vedotin (antibody–drug conjugate targeted at nectin 4) | Systemic | II | Oct. 2017 | May 2025 | Active | No | 219 | 211 |
| Atezolizumab | Systemic | III | Jun. 2016 | Jun. 2021 | Active | No | 1,200 | 219 |
| Avelumab + gemcitabine/carboplatin | Systemic | II | May 2018 | Aug. 2020 | Active | No | 85 | 222 |
| Avelumab | Systemic | III | Apr. 2016 | Jun. 2023 | Active | No | 700 | 223 |
Clinical trials discussed in the text are listed here in order of discussion. BCG, bacillus Calmette–Guérin; COXEN, coexpression extrapolation; EphB4, ephrin type B receptor 4; HSA, human serum albumin; NA, not applicable.
For non-muscle-invasive bladder cancer, the most promising interventions are administered via intravesical instillation, except for immune blockade therapy, which is given systemically intravascularly.
For muscle-invasive bladder cancer, the administrations are all given systemically as the disease has spread beyond the bladder lumen.
There are also approaches to increase uptake of chemotherapy by cancer cells following intravesical administration. These include albumin-bound nanoparticles to deliver paclitaxel167 or rapamycin168 in bladder cancer. Preclinical studies showed that the delivery of paclitaxel wrapped in polymers reduced bladder weight and led to the paclitaxel concentration in bladder tumours being higher than that in cremaphor– paclitaxal-treated mice169. Another advance is the use of photodynamic therapy in clinical trials with promising results. In 2018, a phase Ib clinical trial of the photo-sensitizer TLD1433 was completed on six patients with bladder cancer170. The therapy was well tolerated, and patients exhibited no evidence of disease at 6 months171. A phase II trial is currently recruiting to further explore the therapeutic benefits of this photosensitive molecule172.
Immunotherapies.
The standard of care for patients with NMIBC with a high risk of recurrence is currently intravesical administration of BCG. This therapy reduces recurrence171 and progression173 and increases survival of high-risk patients174, and can also be given as a maintenance therapy for patients at intermediate risk and high risk of NMIBC175. However, management of the ‘BCG-refractory’ patient176 is the greatest challenge in the field as the lifestyle-altering radical cystectomy is currently the most common option. Therefore, combining BCG with other therapies is being evaluated, including combining BCG with IFNα177, recombinant adenovirus IFNα178, or a combination of IFNα, IL-2 and subcutaneous granulocyte–macrophage colony-stimulating factor (GM-CSF)179. The most exciting approach recently has been to investigate ICIs180. The FDA recently approved the ICI pembrolizumab, a PD1-blocking antibody, for administration to patients with BCG-refractory NMIBC or those unable or unwilling to undergo cystectomy181, and 46% of the responding patients had a complete response lasting at least 12 months182. Currently, there are 35 phase II and phase III clinical trials assessing BCG in bladder cancer, and 148 active clinical trials assessing ICIs in bladder cancer, with some using a variety of BCG courses.
There are other immune-related approaches to targeting NMIBC in clinical trials. Similar in concept to BCG, there are viral particles that are administered intravesically that selectively infect cancer cells to elicit an immune response or deliver a unique cargo. These include an attenuated version of the measles virus183, delivering an enzyme to locally activate chemotherapy184, a virus to promote expression of IFNα specifically in cancer cells185, the use of a coxsackievirus to selectively attack cancer cells and promote lysis, and an attenuated strain of Salmonella enterica subsp. enterica serovar Typhi (aetiological agent of typhoid)186. Additional therapeutic approaches for NMIBC and intravesical administration include a recombinant protein fusion187, an IL-15 superagonist188 and a recombinant plasmid that promotes diphtheria toxin expression preferentially in malignant cells189, although this latter study was ended due to poor efficacy. Taken together, the number and variety of immunotherapies illustrate the current focus towards harnessing a patient’s immune system to combat bladder cancer.
Muscle-invasive and metastatic disease
Molecular markers.
The main goals in the setting of MIBC are prevention of local and metastatic recurrence or treat such events successfully if they occur190. The current standard of care for muscle-invasive disease is neoadjuvant platinum-based chemotherapy followed by radical cystectomy3,191. One of the needs in this area has been finding predictors of chemotherapy sensitivity. Identifying patients who are chemosensitive would not only provide a benefit to them but would spare unnecessary toxicity and a possibly fatal delay in radical cystectomy for patients who are chemotherapy resistant192,193. A number of genes have been implicated in this process to various degrees. Copper transporter 1 (CTR1) transports cisplatin into bladder cancer cells194, and patients with high expression of CTR1 in their tumours have more favourable outcomes than patients with low or undetectable tumoural CTR1 expression195. Many chemotherapy agents generally function by binding to and damaging DNA. In bladder cancer, the decreased expression or loss of function of the DNA damage response genes ERCC1 and ERCC2 (REF.196) and BRCA1 (REF.197) is a strong indicator of cisplatin sensitivity and increased patient survival198,199. Recently, a phase II clinical trial200 has been testing a novel biomarker concept aimed at matching patient genomic profiles with a specific targeted therapy. This concept, coexpression extrapolation (COXEN), aims to predict patient tumour response on the basis of sensitivities of laboratory cell lines and their known genomic profiles, and has been the first such idea to be tested in a clinical trial201. The results of this phase II trial presented at ASCO 2019 annual meeting showed the COXEN Gemcitabine+Cisplatin score to be a significant predictor for downstaging when participants in the Gembitabine+Cisplatin and dose dense MVAC (Methotrexate+Vinblastine Sulfate+Doxyrubicin Hydrochloride (Adriamycin)+Cisplatin) arms were combined202.
Host genetics are also important in chemotherapy response. In an analysis of 80 SNPs implicated in developing bladder cancer or response to platinum chemotherapy, six SNPs affecting IL1B, CCND1, PARD6B, GALNTL4 and XPA have been associated with complete or partial response to neoadjuvant chemotherapy203. Of particular interest, gene expression of ERAP2 has been linked to the genotype of loci rs2927608 and is inversely correlated with overall survival of patients with bladder cancer of the luminal subtype receiving immune checkpoint therapy204. In addition, one of the most common targets in bladder cancer is members of the FGFR family. In bladder cancer, expression of one or more of these family members is often elevated or activated by virtue of amplifications, activating mutations and/or gene fusions205. FGFR inhibitors are becoming a significant line of therapy for patients with MIBC, owing to increased molecular profiling that leads to the identification of patients with FGFR genetic alterations. In 2019 the first FGFR kinase inhibitor was approved by the FDA, erdafitinib, as a treatment for patients with bladder cancer who have an FGFR genetic alteration206. There are currently three additional clinical trials recruiting particpants to determine additional indications and regimens for erdafitinib. Infigratinib (BGJ398) is another FGFR inhibitor that has shown success in patients with MIBC who have molecularly profiled genetic alterations in FGFR genes. This drug has shown success207 in an early-stage clinical trial208, and in early 2020 a phase III clinical trial209 was initiated to expand the investigation.
Another molecular directed approach involves the identification of tumours that express high levels of a specific cell membrane protein so that those tumours specifically can be treated with antibodies to those proteins. Linking a toxin to these antibodies has led to drugs called ‘antibody–drug conjugates’. In 2019 the FDA approved the antibody–drug conjugate enfortumab vedotin for breakthrough therapy designation on the basis of positive outcomes in a phase II trial210,211. This antibody–drug conjugate is administered intravenously and is approved for patients with locally advanced or metastatic bladder cancer who previously received an ICI and platinum-containing chemotherapy.
Immune checkpoint inhibitor regimens.
MIBC has one of the highest mutational profiles when compared with other cancers212–214, and cancers with high mutational burden such as melanoma215 and non-small-cell lung cancer216–218 respond very favourably to ICIs. Atezolizumab, a monoclonal antibody that binds PDL1, as opposed to the cognate receptor PD1 expressed on T cells, was approved for treating patients with MIBC. Recent findings from the IMvigor130 study219 revealed the continued success of this agent for advanced bladder cancer. However, the FDA no longer considered the benefit–risk profile favourable for all cisplatin-ineligible patients. Therefore, in June 2018, the indication for both atezolizumab and pembrolizumab was modified to include only patients with MIBC who are not eligible for cisplatin-containing chemotherapy and who have high expression of PDL1 or are not eligible for any platinum-containing chemotherapy regardless of the level of PDL1 expression220. Most recently, the anti-PDL1 agent avelumab was approved for breakthrough therapy designation for first-line maintenance therapy for patients with MIBC who successfully responded to chemotherapy. Avelumab is also approved by the FDA for patients whose MIBC has advanced after chemotherapy. For MIBC there is currently no formal maintenance therapy, but this is where ICIs are likely to shine3,221. Avelumab has already shown efficacy against advanced and metastatic bladder cancer in ongoing phase II (REF.222) and phase III (REF.223) clinical trials, and was very recently approved as maintenance therapy for locally advanced or metastatic urothelial bladder cancer224.
While the aforementioned approaches are showing great promise relative to past therapies, it must be recognized that response rates can still be increased. Two great challenges in this area include the development of robust companion biomarkers that can predict which patients will respond to specific ICI therapies, and assign the other patients to novel therapies being evaluated in clinical trials, and improvement on ICIs by developing novel combination therapies with targeted therapies, such as the FGFR inhibitors. Although a daunting challenge given the number of possible combinations with ICI, novel functional genomics approaches have the promise for high-throughput sampling of the combinatorial landscape to discover clinically tractable combinations. One such approach demonstrating the proof of principle of this concept has identified combining anti-PD1 treatment with the tyrosine kinase inhibitor dasatinib, as the therapies displayed synergy across several tumour types, including bladder cancer225. Further development of molecular marker analysis of patient response will allow future combination trials to select patients optimally to test these regimens rapidly and efficiently.
Conclusions and future directions
For decades there were few advancements in bladder cancer therapeutics. The last major advance was BCG administration, beginning in 1977. However, the past 7 years have seen unprecedented advances thanks mainly to patient tumour molecular profiling and check-point blockade immunotherapy. The former has led to a significant clinical utility in urine screening to assess bladder cancer recurrence, with several commercial clinical tests available. In addition to urine tests, molecular profiling can be used on blood samples, which would be useful for detecting recurrence. Checkpoint blockade immunotherapy has been a significant clinical advance in nearly all cancers. Most significantly, ICIs continue to displace previous treatment regimens as first-line and second-line therapies in bladder cancer, a sign of their significant potential and the more thorough understanding of their clinical applications. The challenges lying ahead include the development of companion biomarkers to assign patients to the therapies they are most responsive to, and more effective combination therapy with checkpoint blockade immunotherapies, small molecules and other biologically based therapies.
Supplementary Material
Cystoscopy
A process whereby an imaging device is inserted into a patient’s urethra for the purpose of imaging the urethra and bladder lining to look for abnormalities.
Uroplakins
Protein complexes normally expressed on the surface of luminal cells of the bladder and that are an indicator of terminal differentiation. Loss of uroplakin III expression is therefore indicative of disease progression and is correlated with poor prognosis.
Regulons
Groups of genes regulated as a unit and controlled by the same regulatory gene that expresses a protein acting as a repressor or activator.
Gene promoter
sequence of DNA to which RNA polymerase and transcription factors bind to initiate RNA synthesis of a gene.
Telomere
Cap made of a repetitive nucleotide sequence at the ends of a chromosome to protect it from deterioration.
Plasmacytoid variant
Infiltrating urothelial carcinoma that is characterized by tumour cells that have a striking morphologic resemblance to and immunohistochemical overlap with plasma cells, and that harbours a CDH1 mutation.
Nested variant
A rare neoplasm that is histologically characterized by large numbers of small, closely packed, haphazardly arranged nests of urothelial cells infiltrating the lamina propria and the muscularis propria.
Papillary urothelial carcinomas
Tumours with thin, finger-like growths that start in the bladder lining and extend into the centre of the bladder.
Glandular lesions
An intriguing group of clinically and morphologically diverse neoplasms, encompassing benign processes to malignant growths.
Inverted papillomas
Rare yet benign neoplasms that is are moderate significance due to their similarity to inverted urothelial carcinoma, which has a much more aggressive prognosis.
Complex of proteins associated with Set1
(COMPAss). Methyltransferase protein complex that controls gene transcription by specifically methylating histone H3 on the lysine at position 4 of the protein.
Immune checkpoint inhibitors
(ICIs). A class of therapies (antibodies) that reactivate dormant or exhausted cytotoxic T cells.
Intravesical instillations
Administration of therapeutics directly to the bladder through a soft catheter inserted through the urethra.
Bacillus Calmette–Guérin
(BCG). A live attenuated strain of Mycobacterium bovis. Used as a vaccine against tuberculosis (first given to patients in 1921). since 1977, it has been used as an immunotherapeutic agent in patients with bladder cancer.
Radical cystectomy
Complete removal of the bladder in cases of advanced muscle-invasive bladder cancer with the aim of removing bladder cancer entirely from the patient.
Androgen deprivation therapy
A clinical approach to inhibit signalling of the hormone androgen, either by chemical or surgical castration, with the goal of preventing androgen-dependent cancer cells from growing.
5α-Reductase
Enzyme that catalyses conversion of testosterone to dihydrotestosterone.
Hydroxyflutamide
An active metabolite of flutamide used as an androgen antagonist.
Urine cytology
A process whereby cells from a patient’s urine are collected and analysed under a microscope to look for cellular abnormalities such as those found in bladder cancer.
Metastatic relapse
Relapse of cancer after treatment with the cancer spreading to distant organs.
Transurethral resection of the bladder
A surgical technique used to harvest tissue samples for pathology analysis and remove non-muscle-invasive bladder cancer tumours.
Photodynamic therapy
A therapy involving localized activation of photosensitive molecules that are selectively taken up by cancer cells, reducing toxicity to normal cells.
Acknowledgements
This work was supported in part by NIH grants CA075115 and CA143971 to D.T.
RELATED LINkS
CBioportal: https://www.cbioportal.org/
NCI Patient-Derived Models Repository (NCI at Frederick, Frederick National Laboratory for Cancer Research, Frederick, MD, USA): https://pdmr.cancer.gov/
Footnotes
Competing interests
D.T. is on the scientific advisory board for Urogen. J.E.D. receives compensation from Invitae. The other authors declare no competing interests.
Peer review information
Nature Reviews Cancer thanks L. Derre, L. Dyrskjøt and C. L. Mendelsohn for their contribution to the peer review of this work.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41568-020-00313-1.
References
- 1.Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Pardo JC et al. Moving towards personalized medicine in muscle-invasive bladder cancer: where are we now and where are we going? Int. J. Mol. Sci 21, 10.3390/ijms21176271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witjes JA et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur. Urol 10.1016/j.eururo.2020.03.055 (2020). [DOI] [PubMed]
- 4.Lindgren D et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res 70, 3463–3472 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Sjodahl G et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res 18, 3377–3386 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Volkmer JP et al. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc. Natl Acad. Sci. USA 109, 2078–2083 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robertson AG et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171, 540–556 e525 (2017). This study reports molecular characterization of 412 patients with MIBC, using multiple TCGA platforms, to identify mutation and expression data on mRNA, long non-coding RNA and microRNA, leading to the identification of five molecular subtypes.
- 8.Damrauer JS et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl Acad. Sci. USA 111, 3110–3115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebouissou S et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci. Transl Med 6, 244ra291 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Choi W et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seiler R et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur. Urol 72, 544–554 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Sjodahl G, Eriksson P, Liedberg F & Hoglund M Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J. Pathol 242, 113–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marzouka NA et al. A validation and extended description of the Lund taxonomy for urothelial carcinoma using the TCGA cohort. Sci. Rep 8, 3737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjodahl G et al. Discordant molecular subtype classification in the basal-squamous subtype of bladder tumors and matched lymph-node metastases. Mod. Pathol 31, 1869–1881 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Tan TZ, Rouanne M, Tan KT, Huang RY & Thiery JP Molecular subtypes of urothelial bladder cancer: results from a meta-cohort analysis of 2411 tumors. Eur. Urol 75, 423–432 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Morera DS et al. Clinical parameters outperform molecular subtypes for predicting outcome in bladder cancer: results from multiple cohorts, including TCGA. J. Urol 203, 62–72 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence MS et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandrov LB et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachakonda PS et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl Acad. Sci. USA 110, 17426–17431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leao R et al. Combined genetic and epigenetic alterations of the TERT promoter affect clinical and biological behavior of bladder cancer. Int. J. Cancer 144, 1676–1684 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtis B et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann. Diagn. Pathol 21, 7–11 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Allory Y et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol 65, 360–366 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Berdik C Unlocking bladder cancer. Nature 551, S34–S35 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Knowles MA & Hurst CD Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 15, 25–41 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Babjuk M et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur. Urol 71, 447–461 (2017). [DOI] [PubMed] [Google Scholar]
- 26.James AC & Gore JL The costs of non-muscle invasive bladder cancer. Urol. Clin. North. Am 40, 261–269 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Abdollah F et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol 37, 219–225 (2013). [DOI] [PubMed] [Google Scholar]
- 28.van Rhijn BW et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J. Clin. Oncol 21, 1912–1921 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Knowles E et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res 66, 7401–7404 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Hernandez S et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J. Clin. Oncol 24, 3664–3671 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Hartmann A et al. Frequent genetic alterations in simple urothelial hyperplasias of the bladder in patients with papillary urothelial carcinoma. Am. J. Pathol 154, 721–727 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedegaard J et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 30, 27–42 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Stroggilos R et al. Proteome-based classification of nonmuscle invasive bladder cancer. Int. J. Cancer 146, 281–294 (2020). [DOI] [PubMed] [Google Scholar]
- 34. Kamoun A et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol 77, 420–433 (2020). This work creates molecular classes of MIBC for clinical application to patient prognosis and treatment regimens.
- 35.de Jong JJ et al. Long non-coding RNAs identify a subset of luminal muscle-invasive bladder cancer patients with favorable prognosis. Genome Med 11, 60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venter JC et al. The sequence of the human genome. Science 291, 1304–1351 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Chan K et al. An APOBEC3A hypermutation signature is distinguishable from the signature of background mutagenesis by APOBEC3B in human cancers. Nat. Genet 47, 1067–1072 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts SA et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat. Genet 45, 970–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinagre J et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun 4, 2185 (2013). [DOI] [PubMed] [Google Scholar]
- 40. Dudley JC et al. Detection and surveillance of bladder cancer using urine tumor DNA. Cancer Discov 9, 500–509 (2019). This study presents of a high-throughput screen to identify tumour DNA from a patient urine sample, with an excellent application to post-treatment surveillance.
- 41.Wu S et al. Whole-genome sequencing identifies ADGRG6 enhancer mutations and FRS2 duplications as angiogenesis-related drivers in bladder cancer. Nat. Commun 10, 720 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nickerson ML et al. Concurrent alterations in TERT, KDM6A, and the BRCA pathway in bladder cancer. Clin. Cancer Res 20, 4935–4948 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K et al. TERT promoter mutations and TERT mRNA but not FGFR3 mutations are urinary biomarkers in Han Chinese patients with urothelial bladder cancer. Oncologist 20, 263–269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng L et al. Telomerase reverse transcriptase (TERT) promoter mutation analysis of benign, malignant and reactive urothelial lesions reveals a subpopulation of inverted papilloma with immortalizing genetic change. Histopathology 69, 107–113 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Pivovarcikova K et al. Comparative study of TERT gene mutation analysis on voided liquid-based urine cytology and paraffin-embedded tumorous tissue. Ann. Diagn. Pathol 24, 7–10 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Pietzak EJ et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur. Urol 72, 952–959 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isharwal S et al. Prognostic value of TERT alterations, mutational and copy number alterations burden in urothelial carcinoma. Eur. Urol. Focus 5, 201–204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kilian A et al. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum. Mol. Genet 6, 2011–2019 (1997). [DOI] [PubMed] [Google Scholar]
- 49.Borah S et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 347, 1006–1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab 99, E1130–E1136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theodorescu D & Cech TR Telomerase in bladder cancer: back to a better future? Eur. Urol 65, 370–371 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Huang DS et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer 51, 969–976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roggisch J, Ecke T & Koch S Molecular identification of telomerase reverse transcriptase (TERT) promotor mutations in primary and recurrent tumors of invasive and noninvasive urothelial bladder cancer. Urol. Oncol 38, 77 e17–e77 e25 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Palsgrove DN et al. Targeted sequencing of plasmacytoid urothelial carcinoma reveals frequent TERT promoter mutations. Hum. Pathol 85, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor AS et al. PAX8 expression and TERT promoter mutations in the nested variant of urothelial carcinoma: a clinicopathologic study with immunohistochemical and molecular correlates. Mod. Pathol 10.1038/s41379-020-0453-z (2020). [DOI] [PubMed]
- 56.Wang CC, Huang CY, Jhuang YL, Chen CC & Jeng YM Biological significance of TERT promoter mutation in papillary urothelial neoplasm of low malignant potential. Histopathology 72, 795–803 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Vail E et al. Telomerase reverse transcriptase promoter mutations in glandular lesions of the urinary bladder. Ann. Diagn. Pathol 19, 301–305 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Cowan M et al. High prevalence of TERT promoter mutations in primary squamous cell carcinoma of the urinary bladder. Mod. Pathol 29, 511–515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng X et al. High frequency of TERT promoter mutation in small cell carcinoma of bladder, but not in small cell carcinoma of other origins. J. Hematol. Oncol 7, 47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roy S et al. Next-generation sequencing-based molecular characterization of primary urinary bladder adenocarcinoma. Mod. Pathol 30, 1133–1143 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Kandimalla R, van Tilborg AA & Zwarthoff EC DNA methylation-based biomarkers in bladder cancer. Nat. Rev. Urol 10, 327–335 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Agger K et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449, 731–734 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Kaneko S & Li X X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci. Adv 4, eaar5598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobatake K et al. Kdm6a deficiency activates inflammatory pathways, promotes M2 macrophage polarization, and causes bladder cancer in cooperation with p53 dysfunction. Clin. Cancer Res 26, 2065–2079 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Ler LD et al. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci. Transl Med 10.1126/scitranslmed.aai8312 (2017). [DOI] [PubMed]
- 66.Shilatifard A The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem 81, 65–95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das C, Lucia MS, Hansen KC & Tyler JK CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 459, 113–117 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duex JE et al. Functional impact of chromatin remodeling gene mutations and predictive signature for therapeutic response in bladder cancer. Mol. Cancer Res 16, 69–77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen J et al. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov 5, 752–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okamura R et al. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J. Immunother. Cancer 10.1136/jitc-2019-000438 (2020). [DOI] [PMC free article] [PubMed]
- 71.Su S et al. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856 e816 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Ozdemir BC et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rhim AD et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Costa A et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33, 463–479 e410 (2018). CAFs are known to provide an immunosuppressive environment, and this study identifies four subsets of CAFs, while identifying the mechanism of immunosuppression in one particular subset.
- 75.Alexa A, Baderca F, Lighezan R & Izvernariu D Myofibroblasts reaction in urothelial carcinomas. Rom. J. Morphol. Embryol 50, 639–643 (2009). [PubMed] [Google Scholar]
- 76. Mezheyeuski A et al. Fibroblasts in urothelial bladder cancer define stroma phenotypes that are associated with clinical outcome. Sci. Rep 10, 281 (2020). Analysing a cohort of 344 patients with bladder cancer, this study uses five fibroblast markers to highlight the need for multiple marker analysis when one is generating a prognosis using fibroblasts.
- 77.Calvete J et al. The coexpression of fibroblast activation protein (FAP) and basal-type markers (CK 5/6 and CD44) predicts prognosis in high-grade invasive urothelial carcinoma of the bladder. Hum. Pathol 91, 61–68 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Schulte J et al. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem. Cell Biol 138, 847–860 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Long X et al. Cancer-associated fibroblasts promote cisplatin resistance in bladder cancer cells by increasing IGF-1/ERbeta/Bcl-2 signalling. Cell Death Dis 10, 375 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tran L & Theodorescu D Determinants of resistance to checkpoint inhibitors. Int. J. Mol. Sci 21, 10.3390/ijms21051594 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez-Lostao L, Anel A & Pardo J How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res 21, 5047–5056 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Pfannstiel C et al. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol. Res 7, 923–938 (2019). [DOI] [PubMed] [Google Scholar]
- 83. Wang L et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat. Commun 9, 3503 (2018). This study finds that non-haematopoietic stromal cells are a major source of EMT-related gene expression and that higher EMT-related and stromal gene expression is associated with poorer response to nivolumab and shorter progression-free and overall survival.
- 84. Mariathasan S et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018). This study illustrates the impact that transforming growth factor-β signalling from fibroblasts and collagen-rich stromal cells has on promoting cytotoxic T cell exclusion in patients with metastatic bladder cancer.
- 85.Salmon H et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest 122, 899–910 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sweis RF et al. Molecular drivers of the non-T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol. Res 4, 563–568 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henze AT & Mazzone M The impact of hypoxia on tumor-associated macrophages. J. Clin. Invest 126, 3672–3679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiu S et al. Tumor-associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci 110, 2110–2118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat. Commun 9, 3826 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeong H et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res 79, 795–806 (2019). [DOI] [PubMed] [Google Scholar]
- 91.Chen Y et al. Tumor-associated macrophages: an accomplice in solid tumor progression. J. Biomed. Sci 26, 78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cassetta L et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 35, 588–602 e510 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mantovani A, Sica A & Locati M Macrophage polarization comes of age. Immunity 23, 344–346 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Takayama H et al. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J. Urol 181, 1894–1900 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Qi Y et al. Tumor-associated macrophages expressing galectin-9 identify immunoevasive subtype muscle-invasive bladder cancer with poor prognosis but favorable adjuvant chemotherapeutic response. Cancer Immunol. Immunother 68, 2067–2080 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hu B et al. Blockade of DC-SIGN+ tumor-associated macrophages reactivates antitumor immunity and improves immunotherapy in muscle-invasive bladder cancer. Cancer Res 80, 1707–1719 (2020). This study identifies of TAMs expressing a dendritic cell-specific C-type lectin which is shown to promote an anti-inflammatory environment. Neutralization of the lectin with an antibody augments pembrolizumab antitumour activity.
- 97.Bernardo ME & Fibbe WE Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, 392–402 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Fu C & Jiang A Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front. Immunol 9, 3059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amodio G et al. Role of myeloid regulatory cells (MRCs) in maintaining tissue homeostasis and promoting tolerance in autoimmunity, inflammatory disease and transplantation. Cancer Immunol. Immunother 68, 661–672 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang M, Diao J & Cattral MS Molecular mechanisms involved in dendritic cell dysfunction in cancer. Cell. Mol. Life Sci 74, 761–776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schneider AK, Chevalier MF & Derre L The multifaceted immune regulation of bladder cancer. Nat. Rev. Urol 16, 613–630 (2019). [DOI] [PubMed] [Google Scholar]
- 102. Chevalier MF et al. Conventional and PD-L1-expressing regulatory T cells are enriched during BCG therapy and may limit its efficacy. Eur. Urol 74, 540–544 (2018). It was discovered that BCG therapy recruits T cells but a significant portion of those are Treg cells, with significant expression of PDL1. This supports trials which look at BCG in combination with ICIs.
- 103.Mu XY et al. RS 504393 inhibits M-MDSCs recruiting in immune microenvironment of bladder cancer after gemcitabine treatment. Mol. Immunol 109, 140–148 (2019). [DOI] [PubMed] [Google Scholar]
- 104.Said N, Sanchez-Carbayo M, Smith SC & Theodorescu D RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J. Clin. Invest 122, 1503–1518 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takeyama Y et al. Myeloid-derived suppressor cells are essential partners for immune checkpoint inhibitors in the treatment of cisplatin-resistant bladder cancer. Cancer Lett 479, 89–99 (2020). [DOI] [PubMed] [Google Scholar]
- 106.Ayari C et al. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette-Guerin immunotherapy. Eur. Urol 55, 1386–1395 (2009). [DOI] [PubMed] [Google Scholar]
- 107.Chevalier MF et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Invest 127, 2916–2929 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baras AS et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology 5, e1134412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee HW et al. Single-cell RNA sequencing reveals the tumor microenvironment and facilitates strategic choices to circumvent treatment failure in a chemorefractory bladder cancer patient. Genome Med 12, 47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sfakianos JP et al. Epithelial plasticity can generate multi-lineage phenotypes in human and murine bladder cancers. Nat. Commun 11, 2540 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oh DY et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 181, 1612–1625 e1613 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Chang Q & Li Y Racial differences in urinary bladder cancer in the united states. Sci. Rep 8, 12521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schinkel JK et al. Overall and recurrence-free survival among black and white bladder cancer patients in an equal-access health system. Cancer Epidemiol 42, 154–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaye DR, Canner JK, Kates M, Schoenberg MP & Bivalacqua TJ Do African American patients treated with radical cystectomy for bladder cancer have worse overall survival? Accounting for pathologic staging and patient demographics beyond race makes a difference. Bladder Cancer 2, 225–234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Casey MF et al. The impact of regionalization of cystectomy on racial disparities in bladder cancer care. J. Urol 194, 36–41 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Danforth KN et al. Disparities in stage at diagnosis in an equal-access integrated delivery system: a retrospective cohort study of 7244 patients with bladder cancer. Clin. Genitourin. Cancer 18, e91–e102 (2020). [DOI] [PubMed] [Google Scholar]
- 117.Dobruch J et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur. Urol 69, 300–310 (2016). [DOI] [PubMed] [Google Scholar]
- 118.Radkiewicz C et al. Sex differences in urothelial bladder cancer survival. Clin. Genitourin. Cancer 18, 26–34 e26 (2020). [DOI] [PubMed] [Google Scholar]
- 119.McGrath M, Michaud DS & De Vivo I Hormonal and reproductive factors and the risk of bladder cancer in women. Am. J. Epidemiol 163, 236–244 (2006). [DOI] [PubMed] [Google Scholar]
- 120.Daugherty SE et al. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP diet and health study. Int. J. Cancer 133, 462–472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pearce MM et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio 5, e01283–e01314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shrestha E et al. Profiling the urinary microbiome in men with positive versus negative biopsies for prostate cancer. J. Urol 199, 161–171 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyamoto H et al. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int 109, 1716–1726 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Miyamoto H et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl Cancer Inst 99, 558–568 (2007). [DOI] [PubMed] [Google Scholar]
- 125.Hsu I et al. Estrogen receptor alpha prevents bladder cancer via INPP4B inhibited Akt pathway in vitro and in vivo. Oncotarget 5, 7917–7935 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hsu I et al. Suppression of ERbeta signaling via ERbeta knockout or antagonist protects against bladder cancer development. Carcinogenesis 35, 651–661 (2014). [DOI] [PubMed] [Google Scholar]
- 127.Shiota M et al. Secondary bladder cancer after anticancer therapy for prostate cancer: reduced comorbidity after androgen-deprivation therapy. Oncotarget 6, 14710–14719 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Izumi K et al. Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget 5, 12665–12674 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shiota M et al. Suppressed recurrent bladder cancer after androgen suppression with androgen deprivation therapy or 5alpha-reductase inhibitor. J. Urol 197, 308–313 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Li P, Chen J & Miyamoto H Androgen receptor signaling in bladder cancer. Cancers 9, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ide H et al. Androgen receptor signaling reduces radiosensitivity in bladder cancer. Mol. Cancer Ther 17, 1566–1574 (2018). [DOI] [PubMed] [Google Scholar]
- 132.Batista R et al. Biomarkers for bladder cancer diagnosis and surveillance: a comprehensive review. Diagnostics 10, 39 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Rhijn BW, van der Poel HG & van der Kwast TH Urine markers for bladder cancer surveillance: a systematic review. Eur. Urol 47, 736–748 (2005). [DOI] [PubMed] [Google Scholar]
- 134.Konety BR Molecular markers in bladder cancer: a critical appraisal. Urol. Oncol 24, 326–337 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Sidransky D et al. Identification of p53 gene mutations in bladder cancers and urine samples. Science 252, 706–709 (1991). [DOI] [PubMed] [Google Scholar]
- 136.Santoni G, Morelli MB, Amantini C & Battelli N Urinary markers in bladder cancer: an update. Front. Oncol 8, 362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miyamoto DT, Mouw KW, Feng FY, Shipley WU & Efstathiou JA Molecular biomarkers in bladder preservation therapy for muscle-invasive bladder cancer. Lancet Oncol 19, e683–e695 (2018). [DOI] [PubMed] [Google Scholar]
- 138.Botezatu I et al. Genetic analysis of DNA excreted in urine: a new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin. Chem 46, 1078–1084 (2000). [PubMed] [Google Scholar]
- 139.Togneri FS et al. Genomic complexity of urothelial bladder cancer revealed in urinary cfDNA. Eur. J. Hum. Genet 24, 1167–1174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kinde I et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res 73, 7162–7167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ward DG et al. Multiplex PCR and next generation sequencing for the non-invasive detection of bladder cancer. PLoS ONE 11, e0149756 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Critelli R et al. Detection of multiple mutations in urinary exfoliated cells from male bladder cancer patients at diagnosis and during follow-up. Oncotarget 7, 67435–67448 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scott SN et al. Next-generation sequencing of urine specimens: A novel platform for genomic analysis in patients with non-muscle-invasive urothelial carcinoma treated with bacille Calmette-Guerin. Cancer Cytopathol 125, 416–426 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Descotes F et al. Non-invasive prediction of recurrence in bladder cancer by detecting somatic TERT promoter mutations in urine. Br. J. Cancer 117, 583–587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Springer SU et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. eLife 7, e32143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stasik S et al. Evaluation of TERT promoter mutations in urinary cell-free DNA and sediment DNA for detection of bladder cancer. Clin. Biochem 64, 60–63 (2019). [DOI] [PubMed] [Google Scholar]
- 147.Hurst CD, Platt FM & Knowles MA Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur. Urol 65, 367–369 (2014). [DOI] [PubMed] [Google Scholar]
- 148. Hosen MI et al. Urinary TERT promoter mutations are detectable up to 10 years prior to clinical diagnosis of bladder cancer: evidence from the Golestan Cohort study. EBioMedicine 53, 102643 (2020). This study performs urinary analysis of TERT promoter mutations in 38 asymptomatic persons who later developed bladder, finding that about 50% of patients exhibited detectable TERT promoter mutations in their urine up to 10 years in advance.
- 149.Stroun M et al. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology 46, 318–322 (1989). [DOI] [PubMed] [Google Scholar]
- 150.Leon SA, Shapiro B, Sklaroff DM & Yaros MJ Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 37, 646–650 (1977). [PubMed] [Google Scholar]
- 151.Razavi P et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med 25, 1928–1937 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stroun M, Anker P, Lyautey J, Lederrey C & Maurice PA Isolation and characterization of DNA from the plasma of cancer patients. Eur. J. Cancer Clin. Oncol 23, 707–712 (1987). [DOI] [PubMed] [Google Scholar]
- 153.Wan JCM et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Christensen E et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J. Clin. Oncol 37, 1547–1557 (2019). [DOI] [PubMed] [Google Scholar]
- 155. Birkenkamp-Demtroder K et al. Genomic alterations in liquid biopsies from patients with bladder cancer. Eur. Urol 70, 75–82 (2016). This work reports molecular characterization of 12 patients with recurring bladder cancer using urine and identifies correlations between next-generation sequencing and Droplet Digital PCR.
- 156.Birkenkamp-Demtroder K et al. Monitoring treatment response and metastatic relapse in advanced bladder cancer by liquid biopsy analysis. Eur. Urol 73, 535–540 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Christensen E et al. Optimized targeted sequencing of cell-free plasma DNA from bladder cancer patients. Sci. Rep 8, 1917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen X et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun 11, 3475 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Babjuk M et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 update. Eur. Urol 76, 639–657 (2019). [DOI] [PubMed] [Google Scholar]
- 160.Messing EM et al. Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence: SWOG S0337 randomized clinical trial. JAMA 319, 1880–1888 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gote V, Sikder S, Sicotte J & Pal D Ocular drug delivery: present innovations and future challenges. J. Pharmacol. Exp. Ther 370, 602–624 (2019). [DOI] [PubMed] [Google Scholar]
- 162.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02720367 (2016). [DOI] [PubMed]
- 163.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03404791 (2018). [DOI] [PubMed]
- 164.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02307487 (2014). [DOI] [PubMed]
- 165.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03558503 (2018). [DOI] [PubMed]
- 166.FDA. FDA approves first therapy for treatment of low-grade upper tract urothelial cancer https://www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-treatment-low-grade-upp er-tract-urothelial-cancer (2020).
- 167.McKiernan JM et al. Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guerin treatment failure. J. Urol 192, 1633–1638 (2014). [DOI] [PubMed] [Google Scholar]
- 168.Seager CM et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev. Res 2, 1008–1014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tamura K et al. Therapeutic effect of intravesical administration of paclitaxel solubilized with poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate) in an orthotopic bladder cancer model. BMC Cancer 15, 317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03053635 (2017). [DOI] [PubMed]
- 171.Monro S et al. Transition metal complexes and photodynamic therapy from a tumor-centered approach: challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev 119, 797–828 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03945162 (2019). [DOI] [PubMed]
- 173.Sylvester RJ, van der MA & Lamm DL Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J. Urol 168, 1964–1970 (2002). [DOI] [PubMed] [Google Scholar]
- 174.Lamm DL et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest oncology group study. J. Urol 163, 1124–1129 (2000). [PubMed] [Google Scholar]
- 175.Peyton CC et al. Updates on the use of intravesical therapies for non-muscle invasive bladder cancer: how, when and what. World J. Urol 37, 2017–2029 (2019). [DOI] [PubMed] [Google Scholar]
- 176.Saluja M & Gilling P Intravesical bacillus Calmette-Guerin instillation in non-muscle-invasive bladder cancer: a review. Int. J. Urol 25, 18–24 (2018). [DOI] [PubMed] [Google Scholar]
- 177.Gupta M, Kates M & Bivalacqua TJ Immunotherapy in nonmuscle invasive bladder cancer: current and emerging treatments. Curr. Opin. Oncol 31, 183–187 (2019). [DOI] [PubMed] [Google Scholar]
- 178.Shore ND et al. Intravesical rAd-IFNalpha/Syn3 for patients with high-grade, bacillus Calmette-Guerin-refractory or relapsed non-muscle-invasive bladder cancer: a phase II randomized study. J. Clin. Oncol 35, 3410–3416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Steinberg RL, Nepple KG, Velaer KN, Thomas LJ & O’Donnell MA Quadruple immunotherapy of bacillus Calmette-Guerin, interferon, interleukin-2, and granulocyte-macrophage colony-stimulating factor as salvage therapy for non-muscle-invasive bladder cancer. Urol. Oncol 35, 670 e677–670 e614 (2017). [DOI] [PubMed] [Google Scholar]
- 180.Bellmunt J et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med 376, 1015–1026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.FDA. FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer (2020).
- 182.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02625961 (2015). [DOI] [PubMed]
- 183.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03171493 (2017). [DOI] [PubMed]
- 184.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT04089163 (2019). [DOI] [PubMed]
- 185.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02773849 (2016). [DOI] [PubMed]
- 186.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03421236 (2018). [DOI] [PubMed]
- 187.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03552796 (2018). [DOI] [PubMed]
- 188.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03022825 (2017). [DOI] [PubMed]
- 189.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03719300 (2018). [DOI] [PubMed]
- 190.Nadal R & Bellmunt J Management of metastatic bladder cancer. Cancer Treat. Rev 76, 10–21 (2019). [DOI] [PubMed] [Google Scholar]
- 191.Hermans TJN et al. Neoadjuvant treatment for muscle-invasive bladder cancer: the past, the present, and the future. Urol. Oncol 36, 413–422 (2018). [DOI] [PubMed] [Google Scholar]
- 192.Tse J, Ghandour R, Singla N & Lotan Y Molecular predictors of complete response following neoadjuvant chemotherapy in urothelial carcinoma of the bladder and upper tracts. Int. J. Mol. Sci 20, 793 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dancik GM & Theodorescu D Pharmacogenomics in bladder cancer. Urol. Oncol 32, 16–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Howell SB, Safaei R, Larson CA & Sailor MJ Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol. Pharmacol 77, 887–894 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kilari D et al. Copper transporter-CTR1 expression and pathological outcomes in platinum-treated muscle-invasive bladder cancer patients. Anticancer Res 36, 495–501 (2016). [PubMed] [Google Scholar]
- 196.Bellmunt J et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann. Oncol 18, 522–528 (2007). [DOI] [PubMed] [Google Scholar]
- 197.Font A et al. BRCA1 mRNA expression and outcome to neoadjuvant cisplatin-based chemotherapy in bladder cancer. Ann. Oncol 22, 139–144 (2011). [DOI] [PubMed] [Google Scholar]
- 198.Teo MY et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin. Cancer Res 23, 3610–3618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim J et al. Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat. Genet 48, 600–606 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02788201 (2016). [DOI] [PubMed]
- 201.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02177695 (2014). [DOI] [PubMed]
- 202.Goldberg H ASCO 2019: SWOG S1314: a randomized phase II study of co-expression extrapolation with neoadjuvant chemotherapy for localized, muscle-invasive bladder cancer https://www.urotoday.com/conference-highlights/asco-2019-annual-meeting/asco-2019-bladder-cancer/113039-asco-2019-swog-s1314-a-randomized-phase-ii-study-of-co-expression-extrapolation-with-neoadjuvant-chemotherapy-for-localized-muscle-invasive-bladder-cancer.html (2019). [DOI] [PMC free article] [PubMed]
- 203.Gallagher DJ et al. Germline single nucleotide polymorphisms associated with response of urothelial carcinoma to platinum-based therapy: the role of the host. Ann. Oncol 24, 2414–2421 (2013). [DOI] [PubMed] [Google Scholar]
- 204.Lim YW et al. Germline genetic polymorphisms influence tumor gene expression and immune cell infiltration. Proc. Natl Acad. Sci. USA 115, E11701–E11710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.di Martino E, Tomlinson DC, Williams SV & Knowles MA A place for precision medicine in bladder cancer: targeting the FGFRs. Future Oncol 12, 2243–2263 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.FDA. FDA approves first targeted therapy for metastatic bladder cancer https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-metastatic-bladder-cancer (2019).
- 207.Javle M et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J. Clin. Oncol 36, 276–282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02657486 (2016). [DOI] [PubMed]
- 209.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT04197986 (2019). [DOI] [PubMed]
- 210.Slater H FDA Grants Breakthrough Therapy Designation to Enfortumab Vedotin Cancernetwork.com. https://www.cancernetwork.com/view/fda-grants-breakthrough-therapy-designation-enfortumab-vedotin (2020)
- 211.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03219333 (2017). [DOI] [PubMed]
- 212.Samstein RM et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet 51, 202–206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cristescu R et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Goodman AM et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther 16, 2598–2608 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hodi FS et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Borghaei H et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med 373, 1627–1639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Herbst RS et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387, 1540–1550 (2016). [DOI] [PubMed] [Google Scholar]
- 218.Fehrenbacher L et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016). [DOI] [PubMed] [Google Scholar]
- 219.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02807636 (2016). [DOI] [PubMed]
- 220.Suzman DL et al. FDA approval summary: atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist 24, 563–569 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Grivas P et al. Immune checkpoint inhibitors as switch or continuation maintenance therapy in solid tumors: rationale and current state. Target. Oncol 14, 505–525 (2019). [DOI] [PubMed] [Google Scholar]
- 222.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03390595 (2018). [DOI] [PubMed]
- 223.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02603432 (2015). [DOI] [PubMed]
- 224.FDA. FDA approves avelumab for urothelial carcinoma maintenance treatment https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-avelumab-urothelial-carcinoma-maintenance-treatment (2020)
- 225. Tu MM et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci. Adv 5, eaav2437 (2019). This study uses functional genomics to find rational and effective therapeutic combinations of small molecules with checkpoint inhibitors in bladder and other cancer types.
- 226.Moad M et al. A novel model of urinary tract differentiation, tissue regeneration, and disease: reprogramming human prostate and bladder cells into induced pluripotent stem cells. Eur. Urol 64, 753–761 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pauli C et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov 7, 462–477 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee SH et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173, 515–528 e517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Puzio-Kuter AM et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev 23, 675–680 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Theodorescu D, Cornil I, Fernandez BJ & Kerbel RS Overexpression of normal and mutated forms of HRAS induces orthotopic bladder invasion in a human transitional cell carcinoma. Proc. Natl Acad. Sci. USA 87, 9047–9051 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Overdevest JB et al. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen regulated. Proc. Natl Acad. Sci. USA 109, E3588–E3596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sottnik JL et al. Elucidating the role of Agl in bladder carcinogenesis by generation and characterization of genetically engineered mice. Carcinogenesis 40, 194–201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zuiverloon TCM, de Jong FC, Costello JC & Theodorescu D Systematic review: characteristics and preclinical uses of bladder cancer cell lines. Bladder Cancer 4, 169–183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Avogbe PH et al. Urinary TERT promoter mutations as non-invasive biomarkers for the comprehensive detection of urothelial cancer. EBioMedicine 44, 431–438 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Russo IJ et al. Toward personalised liquid biopsies for urothelial carcinoma: characterisation of ddPCR and urinary cfDNA for the detection of the TERT 228G>A/T mutation. Bladder Cancer 4, 41–48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hayashi Y et al. Diagnostic potential of TERT promoter and FGFR3 mutations in urinary cell-free DNA in upper tract urothelial carcinoma. Cancer Sci 110, 1771–1779 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Eich ML et al. Incidence and distribution of UroSEEK gene panel in a multi-institutional cohort of bladder urothelial carcinoma. Mod. Pathol 32, 1544–1550 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ward DG et al. Targeted deep sequencing of urothelial bladder cancers and associated urinary DNA: a 23-gene panel with utility for non-invasive diagnosis and risk stratification. BJU Int 124, 532–544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cheng THT et al. Noninvasive detection of bladder cancer by shallow-depth genome-wide bisulfite sequencing of urinary cell-free DNA for methylation and copy number profiling. Clin. Chem 65, 927–936 (2019). [DOI] [PubMed] [Google Scholar]
- 240.Kandori S, Kojima T & Nishiyama H The updated points of TNM classification of urological cancers in the 8th edition of AJCC and UICC. Jpn. J. Clin. Oncol 49, 421–425 (2019). [DOI] [PubMed] [Google Scholar]
- 241.Hoadley KA et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173, 291–304 e296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Choudhury NJ et al. Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J. Clin. Oncol 34, 2165–2171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02872714 (2016). [DOI] [PubMed]
- 244.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02710734 (2016). [DOI] [PubMed]
- 245.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT00030615 (2003). [DOI] [PubMed]
- 246.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03179943 (2017). [DOI] [PubMed]
- 247.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02546661 (2016). [DOI] [PubMed]
- 248.Lloyd-Price J, Abu-Ali G & Huttenhower C The healthy human microbiome. Genome Med 8, 51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Garrett WS Cancer and the microbiota. Science 348, 80–86 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wolfe AJ et al. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol 50, 1376–1383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Burger M et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol 63, 234–241 (2013). [DOI] [PubMed] [Google Scholar]
- 252.Zaghloul MS Bladder cancer and schistosomiasis. J. Egypt. Natl Canc. Inst 24, 151–159 (2012). [DOI] [PubMed] [Google Scholar]
- 253.Adebayo AS et al. The microbiome in urogenital schistosomiasis and induced bladder pathologies. PLoS Negl. Trop. Dis 11, e0005826 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Xu W et al. Mini-review: perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am. J. Clin. Exp. Urol 2, 57–61 (2014). [PMC free article] [PubMed] [Google Scholar]
- 255. Bucevic Popovic V et al. The urinary microbiome associated with bladder cancer. Sci. Rep 8, 12157 (2018). This study compares patients with bladder cancer and 11 healthy individuals and identifies unique urinary microbiota signatures as a potential means of prognosis and surveillance.
- 256.Wu P et al. Profiling the urinary microbiota in male patients with bladder cancer in China. Front. Cell Infect. Microbiol 8, 167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu F et al. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med 8, 6904–6914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Routy B et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018). This study highlights the negative impact antibiotics may have on immune checkpoint blockade and illuminates the potential that faecal microbiota transplantation may have in promoting patient response to immunotherapy.
- 259.Naito S et al. Prevention of recurrence with epirubicin and lactobacillus casei after transurethral resection of bladder cancer. J. Urol 179, 485–490 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


