Abstract
Valve repair in rheumatic patients poses special problems due to valve deformity and mixed lesions. We present our experience from January 1988 through June 1999, in this retrospective study of 818 patients (377 males). The mean age was 22.8 ± 11.3 years (range, 2 to 70 years). The cause of mitral regurgitation was rheumatic in 718 (88%) patients, congenital in 51, myxomatous in 34, infective in 7, and ischemic in 8. Most patients (64%) were in New York Heart Association functional class III or IV. Congestive heart failure was present in 116 patients (14%).
Reparative procedures included posterior collar annuloplasty (n=710), commissurotomy (n=482), cusp-level chordal shortening (n=237), cusp thinning (n=222), cleft suture (n=166), and cusp excision/plication (n=42).
Operative mortality was 4% (32 patients). Preoperative left ventricular dysfunction, presence of congestive heart failure, and advanced functional class were associated with greater mortality. Follow-up ranged from 1 to 144 months (mean, 44.9 ± 33.2 months) and was 96% complete. Most survivors (70%) had no or trivial mitral regurgitation. Forty patients required reoperation for valve dysfunction. There were 23 (2.8%) late deaths. Actuarial, reoperation-free, and event-free survival at 11 years were 92.6% ± 1.0%, 65.0% ± 10%, and 38% ± 6.0%, respectively. Among the survivors, 85% were in New York Heart Association functional class I.
We conclude that mitral valve repair in rheumatic patients, using current techniques, can effectively correct hemodynamic and functional abnormalities with satisfactory results.
Key words: Heart valve diseases/surgery, mitral valve insufficiency/surgery, mitral valve prolapse/surgery, mitral valve stenosis/surgery, reconstructive surgical procedures, retrospective studies, rheumatic heart disease/surgery, treatment outcome
With standardized and reproducible techniques, mitral valve repair has become the procedure of choice for mitral regurgitation. 1–5 Lower operative mortality rates, better preservation of left ventricular function, freedom from the hazards associated with anticoagulation, and continued growth of the valve in young patients are distinct advantages of mitral valve repair over mitral valve replacement. 6–9 Up to 95% of degenerative mitral valves can be repaired with current techniques, but only 75% of the patients with rheumatic mitral valve disease are amenable to reparative procedures. 1,10 Most of the reports available in the literature have discussed the issue of degenerative mitral valve disease, 3–10 and experience with rheumatic mitral valve disease has been scant. 11–13 In the present study, we present our experience with mitral valve repair, in a predominantly rheumatic population. Some of our surgical techniques were described by us a decade ago. 14–16
Patients and Methods
Eight hundred eighteen consecutive patients undergoing mitral valve reconstruction from January 1988 through June 1999 formed the basis of this retrospective study. The mean age was 22.8 ± 11.3 years (range, 2 to 70 years), and 245 patients were less than 15 years of age. There were 377 males. The cause of mitral regurgitation (MR) was rheumatic in 718 (88%) patients. Other causes included congenital (n=51, associated with atrial septal defect in 47 and with isolated mitral valve cleft in 4), myxomatous (n=34), infective (n=7), and ischemic (n=8). Pure MR was present in 433 patients (53%), and a combined stenotic and regurgitant lesion was present in 385 (47%) patients. Atrial fibrillation was present in 414 (51%) patients. Dyspnea on exertion was the predominant symptom, and 527 patients (64%) were in New York Heart Association (NYHA) functional class III or IV. Controlled or frank congestive heart failure was present in 116 patients (14%).
Preoperative Assessment
Preoperative transthoracic echocardiography was performed in all patients. Patients with suspected aortic valve disease (n=132) underwent cardiac catheterization and cineangiography. Coronary angiography was performed in patients above 40 years of age. Mitral regurgitation was graded by angiography 17 or Doppler echocardiography, or by both. 18 In addition, patients who underwent operation after January 1994 had intraoperative transesophageal echocardi-ography (TEE) through use of the Hewlett-Packard Sonos 1500 or 2500 ultrasound system (Hewlett-Packard Co.; Andover, Mass). In a systematic manner, we assessed the mitral annulus, leaflet thickness and mobility, commissural and chordal fusion, the presence and location of calcific nodules, areas of prolapse and billowing, the direction of the regurgitant jet, and the thickness and length of chordae tendineae.
Surgical Techniques
Either a midsternotomy (n=660) or a right antero-lateral thoracotomy (n=158) approach was used. Moderately hypothermic or normothermic cardiopulmonary bypass was established by ascending aortic and bicaval cannulation. Antegrade, cold blood cardioplegia and topical hypothermia were used for myocardial preservation. The mitral valve was approached through a left atrial incision behind the interatrial groove. After careful evaluation of the mitral valve apparatus, we performed a variety of reparative procedures. These included posterior collar annuloplasty (n=710), commissurotomy (n=482), chordal shortening (n=237), cusp thinning (n=222), chordal fenestration (n=46), cleft suture (n=166), decalcification (n=17), cusp excision/plication (n=42), reattachment of chordae (n=7), chordal transfer (n=9), and neochordae (n=3). Most of these techniques are in accordance with described techniques; 19 however, some were developed by us 14–16 and are described here.
Modified Cooley's Annuloplasty 14 (Fig. 1). The anterior mitral leaflet is measured with a circular valve sizer. A “C”-shaped, 3- to 4-mm-wide collar is fashioned from 0.6-mm-thick polytetrafluoroethylene (PTFE) felt (IMPRA, Inc.; Tempe, Ariz), with the same internal diameter as that of the anterior mitral leaflet. This collar is sutured along the posterior mitral annulus using interrupted sutures. This produces optimal coaptation of the mitral valve leaflets and reduction of annular diameter.
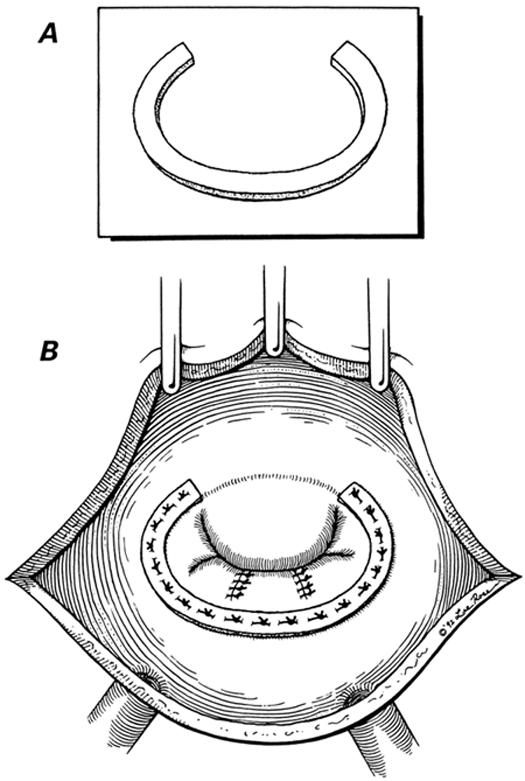
Fig. 1 A) The semicircular polytetrafluoroethylene collar. B) The completed posterior annuloplasty.
(Illustrations by Lee Rose. Reproduced with permission. From Texas Heart Institute Journal 1992;19:107–11.)
Cusp-Level Chordal Shortening. 15 The technique of cusp-level chordal shortening is shown in Figure 2. This effectively shortens the elongated chorda and thus corrects the cuspal prolapse.
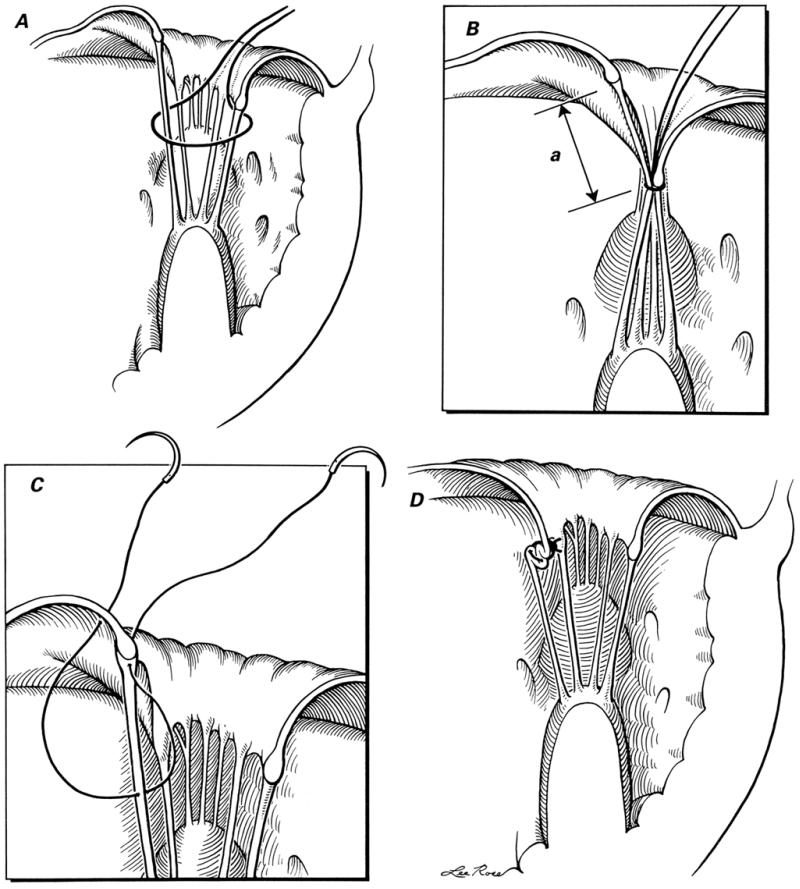
Fig. 2 Technique of chordal shortening: A) a silk suture is passed around both the chorda to be shortened and the opposite (posterior cusp) chorda; B) after the left ventricle has been filled with saline solution, the silk suture is tightened so that the anterior mitral leaflet's degree of prolapse can be assessed and the length of chorda to be shortened (a) can be determined; C) after exposure of the chorda with a traction suture (not shown), a 5-0 polypropylene double-armed suture is taken, and the needle is passed through the chorda and then through the edge of the anterior mitral leaflet close to its point of attachment; and D) the polypropylene suture is tied, shortening the chorda at cusp level.
(Illustrations by Lee Rose. Reproduced with permission. From Texas Heart Institute Journal 1992;19:47–50.)
Leaflet Thinning. 16 After release of fused commissural and subvalvular components, the anterior mitral leaflet is grasped firmly with forceps. The fibrous layer covering the anterior mitral leaflet on the atrial surface is gently peeled from the annulus toward the free edge. When it is completely separated, it is excised at the free edge. A similar procedure is then used to remove the fibrous peel from the posterior mitral leaflet. In patients who were also to undergo aortic valve surgery, this peel was removed from the ventricular aspect of the anterior mitral leaflet.
At the completion of repair, mitral valve competence was assessed by injecting cold saline with a bulb syringe into the left ventricle directly through the mitral valve. Since January 1994, we have also used transesophageal echocardiography to assess mitral valve function intraoperatively.
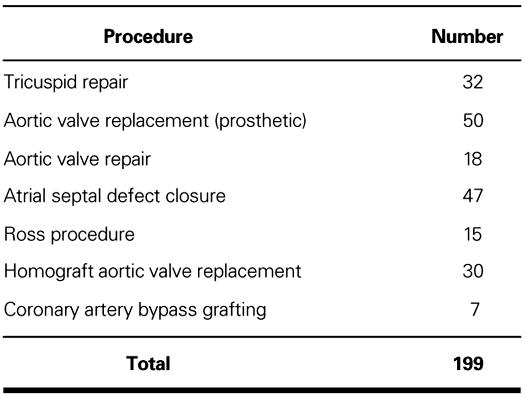
In addition, 199 patients underwent various associated procedures (Table I).
TABLE I. Associated Procedures
Follow-Up. Transthoracic echocardiography was performed before discharge from the hospital and subsequently at regular intervals. Before April 1998, all the patients who had undergone posterior collar annuloplasty received oral anticoagulants (acenocoumarol) for 6 weeks and antiplatelet agents (dipyridamole) for 6 months. Since April 1998, it has been our practice not to prescribe anticoagulants.
Statistical Analysis. Continuous or interval-related variables were expressed as mean ± standard deviation. Categorical variables were expressed as percentages. Univariate analysis was done with the χ2 and Fisher exact tests. Actuarial estimates have been calculated and compared using the Kaplan-Meier technique 20 with Mantel-Cox (log-rank) tests. 21
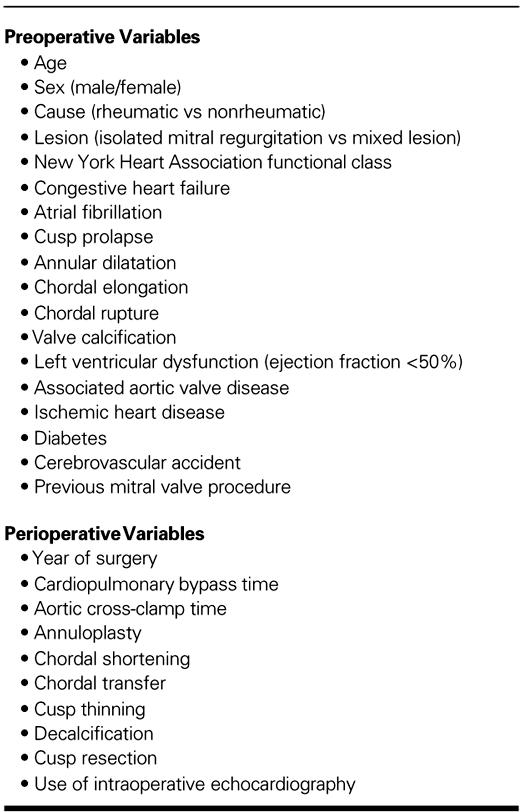
Moderate or severe MR was considered significant. Numerous variables (Table II) were analyzed as predictors of early death, late death, and development of significant MR postoperatively. A step-wise multiple logistic regression analysis was used to reveal independently the predictors of early mortality. Prognostic factors for development of significant MR and for late death have been identified using Cox's proportional hazard model. All statistical analysis was performed with the SPSS for Windows 6.0 software package (SPSS, Inc.; Chicago, Ill).
TABLE II. Variables Included in Multivariable Analyses of Risk Factors for Early Death, Late Death, and Development of Significant Mitral Regurgitation
Results
All patients survived the operation. For isolated mitral valve repair, the mean aortic cross-clamp time was 36.5 ± 11.6 minutes (range, 22 to 71 minutes), and the mean cardiopulmonary bypass time was 45.7 ± 12.4 minutes (range, 34 to 87 minutes). Thirty-six patients (4%) required inotropic support. Patients were ventilated for a period of 14 to 74 hours (median, 22 hours), and the mean stay in the hospital was 6.1 ± 1.8 days.
Early Postoperative Mortality
There were 32 (4%) early deaths (<30 days after surgery). Persistent low-output syndrome was responsible for 15 deaths. Other causes of early death included acute renal failure (n=3), septicemia (n=4), malignant ventricular arrhythmias (n=4), and cerebrovascular accidents (n=6). Most of these patients (n=26) had preoperative left ventricular dysfunction (ejection fraction <50%), and 16 of the patients who died had been in congestive heart failure preoperatively. Logistic regression analysis also identified preoperative left ventricular dysfunction, preoperative congestive heart failure, and advanced NYHA functional class as the significant predictors for early death (Table III).
TABLE III. Risk Factors for Early Death
Late Outcomes
Patients were followed up for 1 to 144 months (mean, 44.9 ± 33.2 months), and follow-up was 96% complete.
Thromboembolic Complications. Twenty-five patients had a thromboembolic complication (0.8 events per 100 patient-years of follow-up) in the late postoperative period. Four of these patients died, 7 recovered with residual weakness, and 6 recovered completely. In 8 patients, the deficit was transient and was classified as a transient ischemic attack. No single factor was found to be associated with incidence of thromboembolism.
Hemolysis. Significant hemolysis, requiring hospitalization and blood transfusion, was seen in 35 patients (1 event per 100 patient-years of follow-up). Among these, 26 patients had significant MR. Sixteen patients with significant MR underwent reoperation, 4 died without further operative intervention, and 6 are awaiting reoperation. In 9 patients with mild or moderate MR, hemolysis gradually subsided.
Infective Endocarditis. Three patients developed subacute infective endocarditis. These patients were treated conservatively and all 3 survived.
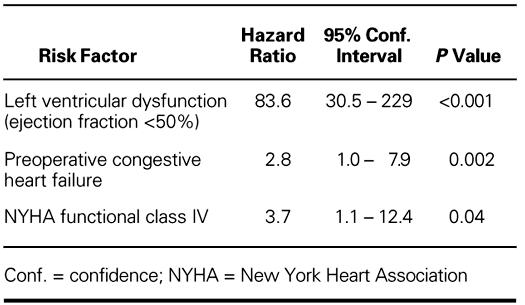
Reoperation. Severe valve dysfunction necessitated reoperation in 40 patients (1.3 events per 100 patient-years of follow-up) after a mean period of 43.9 ± 32.7 months (range, 1 to 132 months). Freedom from reoperation at 11 years was 65.2% ± 10% (Fig. 3).
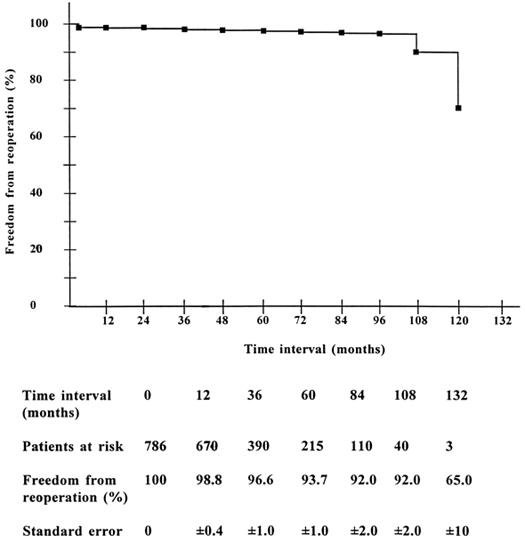
Fig. 3 Freedom from reoperation in operative survivors (Kaplan Meier).
In 4 patients, reoperation was performed within 1 postoperative month, due to failure of sutures holding the annuloplasty felt. In another 18 patients, reoper-ation was performed after an interval of 2 to 24 months. Most of these patients (n=14) had moderate MR at the time of discharge from the hospital, and developed significant MR due either to suture dehiscence (n=8) or to suboptimal repair (n=6). Eighteen patients underwent reoperation after an interval of 25 to 132 months. At reoperation, these patients demonstrated progressive disease in the form of fusion of cusps and chordae and new thickening/contraction of the cusps.
At reoperation, 31 patients had mitral valve replacement with a prosthetic valve, and 2 had mitral valve replacement with a mitral homograft. In 7 patients, the mitral valve was re-repaired. Five patients (12.5%) died after reoperation.
Valve Function. Among the operative survivors, 547 patients (70%) had no or trivial-to-mild mitral regurgitation at the time of their last follow-up visit (mean, 44.9 ± 3.2 months; range, 1 to 144 months). The remaining 30% of patients developed significant MR: moderate in 125 (16%) and severe in 114 (14%). Thirty-seven patients with severe MR underwent reoperation, and 15 others died as a result of intractable congestive heart failure. Three patients developed significant mitral stenosis (valve area <1 cm2); all underwent reoperation.
Eleven-year freedom from moderate or severe MR was 52.3% ± 3.0% (Fig. 4). In about two thirds of the patients (68.5%) with moderate or severe mitral regurgitation, MR developed within the first 24 months after surgery. The instantaneous risk of development of significant MR (hazard function) consisted of a peaking early hazard phase in the 1st postoperative year, followed by a slowly rising late hazard phase after 5 years (Fig. 5).
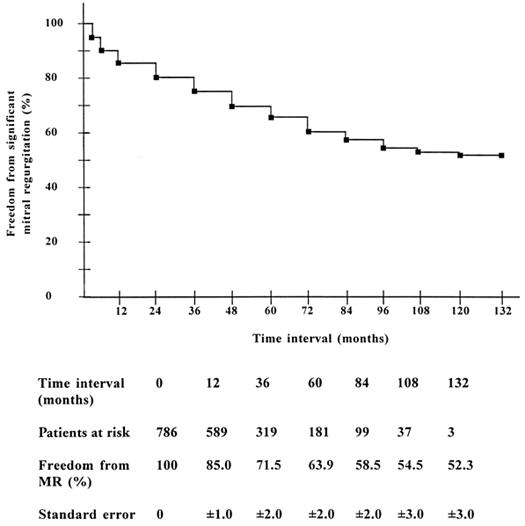
Fig. 4 Freedom from significant mitral regurgitation in operative survivors (Kaplan-Meier).
MR = mitral regurgitation
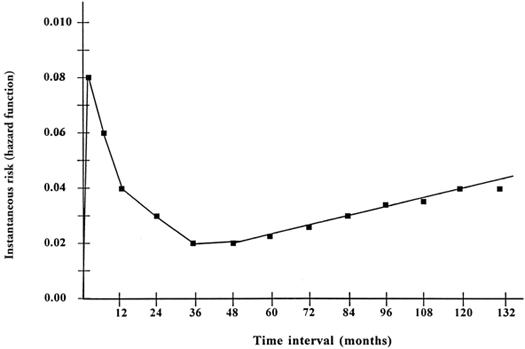
Fig. 5 Instantaneous risk (hazard function) for development of significant mitral regurgitation across time after mitral valve repair.
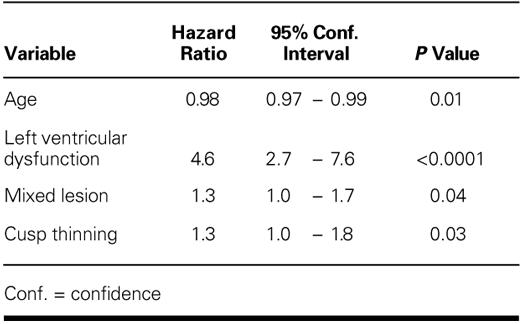
Various risk factors (Table II) were analyzed for the development of significant MR (Fig. 6). The probability of freedom from moderate or severe MR at 11 years was significantly greater in patients with a nonrheumatic cause (nonrheumatic vs rheumatic cause: 72% ± 9.0% vs 51% ± 3%; p = 0.01), with an isolated regurgitant lesion (isolated MR vs mixed lesion: 59% ± 5% vs 48% ± 4%; p = 0.04), and with normal left ventricular function (normal left ventricular function vs left ventricular dysfunction: 55% ± 3% vs 11% ± 10%; p <0.0001). Freedom from moderate or severe MR was significantly reduced if cuspal thinning was required (yes vs no: p = 0.004). In multivariate analysis, rheumatic cause was not identified as an independent risk factor for development of moderate or severe MR; and only younger age, left ventricular dysfunction, mixed lesion, and need for cuspal thinning were identified as independent risk factors (Table IV).
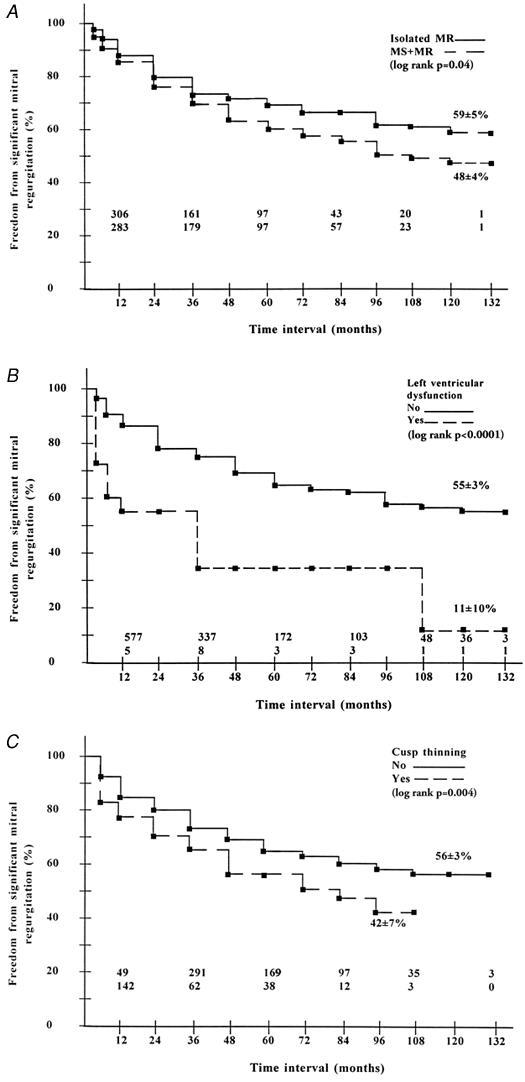
Fig. 6 Influence of pathologic condition and operative technique on freedom from development of significant mitral regurgitation. All presentations are non-risk-adjusted. A) Isolated MR versus mixed lesion. B) Preoperative normal left ventricular function versus left ventricular dysfunction. C) Cuspal thinning versus no cuspal thinning.
MR = mitral regurgitation; MS = mitral stenosis
TABLE IV. Predictors of Development of Significant Mitral Regurgitation (Cox Proportional Hazard Model)
Functional Class. All but 6 patients with no or mild MR were in NYHA class I postoperatively. Left ventricular dysfunction (n=2), progressive aortic valve disease (n=1), failed aortic valve repair (n=2), and severe tricuspid regurgitation (n=1) were responsible for symptoms in these patients.
Among the patients with moderate MR (n=125), the majority (n=118) were symptom free. In those remaining, 4 and 3 patients were in NYHA class III and IV respectively, due to left ventricular dysfunction.
Seventy-five patients with severe MR were in NYHA class III or IV: 37 of these underwent reoperation, 11 died in congestive heart failure, and 27 await reoperation. The remaining 39 patients with severe MR were in class I or II. Three patients with significant mitral stenosis were in NYHA class IV (all underwent reoperation).
Late Death and Survival. There were 23 late deaths (28%). Five patients died at reoperation, and 11 patients died of congestive heart failure secondary to severe MR or left ventricular dysfunction. Cerebrovascular accident was responsible for death in 5 patients. Other causes of death included hepatitis (n=1), septicemia (n=1), and acute gastroenteritis (n=1).
Actuarial survival in the whole group of patients (n=818) was 92.6% ± 1.0% at 11 years (Fig. 7). Survival in patients with left ventricular dysfunction (27.1% ± 10.0% at 11 years) was significantly lower (p <0.001) than that in patients with normal left ventricular function (94.8% ± 4.0% at 11 years). Multivariate analysis also identified preoperative left ventricular dysfunction (hazard ratio, 134.8; 95% confidence interval, 48.6–373.8; p <0.0001), and NYHA functional class IV (hazard ratio, 3.8; 95% confidence interval, 1.4–10.5; p=0.01) as the independent predictors of late death.
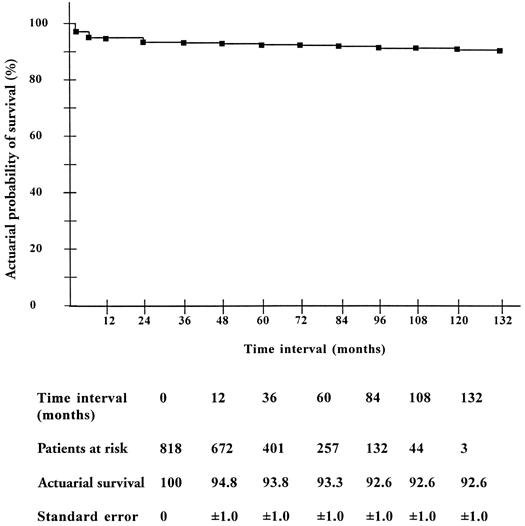
Fig. 7 Actuarial survival (Kaplan-Meier) in patients after mitral valve repair.
In operative survivors, event-free survival (including late death, reoperation, thromboembolism, infective endocarditis, and hemolysis) was 38% ± 6% at 11 years (Fig. 8).
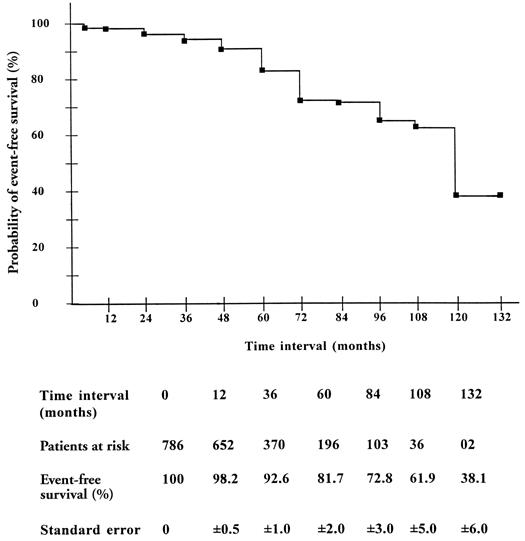
Fig. 8 Event-free survival (Kaplan-Meier) in operative survivors after mitral valve repair.
Discussion
Rheumatic heart disease is the commonest form of cardiovascular ailment that affects younger people in developing countries, especially in India. 22 Either repair or replacement of the regurgitant rheumatic mitral valve has been reported by various investigators. 2,11–16,23,24 Replacement of the diseased mitral valve with a prosthesis is associated with the risks attendant upon anticoagulation, and with suboptimal preservation of ventricular function and reduced survival. 6–9,24 Besides these, poor compliance with an anticoagulation regime, growth, and pregnancy remain important issues in young patient populations, especially in developing countries. Though mitral valve repair has become the procedure of choice for degenerative mitral regurgitation, 1–10 its use for correction of rheumatic mitral regurgitation has remained scant, 11–13,25,26 because repair is technically more difficult and is associated with a high failure rate in this group. 1,2,11–13
Our experience with mitral valve repair in rheumatic patients has been encouraging. 25,26 In consideration of the relative youth of our patients and the problems of anticoagulation, we have tried to repair almost all noncalcified regurgitant valves. Of the survivors, 70% had trivial or mild MR at their last follow-up visit. An additional 16% of survivors had moderate MR, but most of these (118 of 125) were asymptomatic.
In our series, 30% of survivors developed moderate or severe MR. Younger age, mixed lesion, ventricular dysfunction, and cuspal thickening were the important predictors for development of MR. In univariate analysis, rheumatic cause was identified as a risk factor for development of moderate-to-severe MR, but in multivariate analysis it was eliminated, for most patients with a rheumatic cause had either mixed lesions or thickened cusps. Others have also found younger age and mixed lesions as the chief indicators for valve failure. 1,2,11–13
In the majority of patients with moderate or severe MR, the valve failed within the first 2 years after surgery. The instantaneous hazard of developing moderate-to-severe MR was highest in the 1st postoperative year. There was another (but slowly rising) hazard phase after 5 postoperative years (Fig. 5). The early peak in the instantaneous hazard may be attributed to suboptimal repair or to the inherent complexity of the disease process, which often results in gross deformity of the valve. Recurrence and progression of the rheumatic process may have contributed to both the early and late failures. 11–13,25
We have used a number of techniques to repair the valve. The modified Cooley's annuloplasty has proved an easy, reliable, satisfactory, and cost-effective technique over a decade. Cusp-level chordal shortening is also an effective and simple technique for correction of cuspal prolapse in rheumatic patients, because the elongated chorda is usually quite thickened and capable of holding the suture. Cuspal thinning further improves the pliability and mobility of the valve leaflet.
To conclude, valve repair is possible in a large majority of patients with rheumatic mitral regurgitation, and current techniques can effectively correct the hemodynamic and functional abnormalities with satisfactory results.
Acknowledgments
The authors are grateful to consultants and senior residents in the Department of Cardiology for the echocardiographic studies and to Mr. Rajvir for statistical analysis.
Footnotes
Address for reprints: Dr. A. Sampath Kumar, Professor, Dept. of Cardiothoracic & Vascular Surgery, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029, India
References
- 1.Deloche A, Jebara VA, Relland JY, Chauvaud S, Fabiani JN, Perier P, et al. Valve repair with Carpentier techniques. The second decade. J Thorac Cardiovasc Surg 1990;99:990–1002. [PubMed]
- 2.Carpentier A, Chauvaud S, Fabiani JN, Deloche A, Relland J, Lessana A, et al. Reconstructive surgery of mitral valve incompetence: ten-year appraisal. J Thorac Cardiovasc Surg 1980;79:338–48. [PubMed]
- 3.David TE, Armstrong S, Sun Z, Daniel L. Late results of mitral valve repair for mitral valve regurgitation due to degenerative disease. Ann Thorac Surg 1993;56:7–14. [DOI] [PubMed]
- 4.Cohn LH, Couper GS, Aranki SF, Rizzo RJ, Kinchla NM, Collins JJ Jr. Long-term results of mitral valve reconstruction for regurgitation of the myxomatous mitral valve. J Thorac Cardiovasc Surg 1994;107:143–51. [PubMed]
- 5.Galloway AC, Colvin SB, Baumann FG, Esposito R, Vohra R, Harty S, et al. Long-term results of mitral valve reconstruction with Carpentier techniques in 148 patients with mitral insufficiency. Circulation 1988;78(3 Pt 2): I97–105. [PubMed]
- 6.Loop FD. Long-term results of mitral valve repair. Semin Thorac Cardiovasc Surg 1989;1(2):203–10. [PubMed]
- 7.Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation 1995;91:1022–8. [DOI] [PubMed]
- 8.Akins CW, Hilgenberg AD, Buckley MJ, Vlahakes GJ, Torchiana DF, Daggett WM, et al. Mitral valve reconstruction versus replacement for degenerative or ischemic mitral regurgitation. Ann Thorac Surg 1994;58:668–76. [DOI] [PubMed]
- 9.Galloway AC, Colvin SB, Baumann FG, Grossi EA, Ribakove GH, Harty S, et al. A comparison of mitral valve reconstruction with mitral valve replacement: intermediate-term results. Ann Thorac Surg 1989;47:655–62. [DOI] [PubMed]
- 10.Gillinov AM, Cosgrove DM, Lytle BW, Taylor PC, Steward RW, McCarthy PM, et al. Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg 1997;113: 467–75. [DOI] [PubMed]
- 11.Duran CM, Gometza B, Saad E. Valve repair in rheumatic mitral disease: an unsolved problem. J Card Surg 1994;9 (23Suppl):282–5. [DOI] [PubMed]
- 12.Skoularigis J, Sinovich V, Joubert G, Sareli P. Evaluation of the long-term results of mitral valve repair in 254 young patients with rheumatic mitral regurgitation. Circulation 1994;90(5 Pt 2): II-167–74. [PubMed]
- 13.Antunes MJ, Magalhaes MP, Colsen PR, Kinsley RH. Valvuloplasty for rheumatic mitral valve disease. A surgical challenge. J Thorac Cardiovasc Surg 1987;94:44–56. [PubMed]
- 14.Kumar AS, Kumar RV, Shrivastava S, Venugopal P, Sood AK, Gopinath N. Mitral valve reconstruction. Early results of a modified Cooley technique. Tex Heart Inst J 1992; 19:107–11. [PMC free article] [PubMed]
- 15.Kumar AS, Bhan A, Kumar RV, Shrivastava S, Sood AK, Gopinath N. Cusp-level chordal shortening for rheumatic mitral regurgitation. Early results. Tex Heart Inst J 1992; 19:47–50. [PMC free article] [PubMed]
- 16.Kumar AS, Rao PN. Restoration of pliability of the mitral leaflets during reconstruction. J Heart Valve Dis 1995; 4:251–3. [PubMed]
- 17.Grossman W. Profiles in valvular heart disease. In: Baim DS, Grossman W, editors. Cardiac catheterization, angiography, and intervention. 5th ed. Baltimore: Williams & Wilkins; 1996. p. 742–4.
- 18.Helmcke F, Nanda NC, Hsiung MD, Soto B, Adey CK, Goyal RG, et al. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation 1987;75:175–83. [DOI] [PubMed]
- 19.Carpentier A. Cardiac valve surgery—the “French correction”. J Thorac Cardiovasc Surg 1983;86:323–37. [PubMed]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81.
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50:163–70. [PubMed]
- 22.Padmavati S. Epidemiology of cardiovascular disease in India. I. Rheumatic heart disease. Circulation 1962;25: 703–10. [DOI] [PubMed]
- 23.Antunes MJ, Santos LP. Performance of glutaraldehyde-preserved porcine bioprosthesis as a mitral valve substitute in a young population group. Ann Thorac Surg 1984;37: 387–92. [DOI] [PubMed]
- 24.Antunes MJ, Wessels W, Sadowski RG, Schutz JG, Vanderdonck KM, Oliveira JM, et al. Medtronic Hall valve replacement in a third-world population group. A review of the performance of 1000 prostheses. J Thorac Cardiovasc Surg 1988;95:980–93. [PubMed]
- 25.Kumar AS, Rao PN, Saxena A. Results of mitral valve reconstruction in children with rheumatic heart disease. Ann Thorac Surg 1995;60:1044–7. [DOI] [PubMed]
- 26.Kumar AS, Rao PN, Saxena A. Mitral valve reconstruction: eight years' experience in 531 patients. J Heart Valve Dis 1997;6:591–3. [PubMed]


