ABSTRACT
Group B Streptococcus (GBS) is a pervasive neonatal pathogen accounting for a combined half a million deaths and stillbirths annually. The most common source of fetal or neonatal GBS exposure is the maternal microbiota. GBS asymptomatically colonizes the gastrointestinal and vaginal mucosa of 1 in 5 individuals globally, although its precise role in these niches is not well understood. To prevent vertical transmission, broad-spectrum antibiotics are administered to GBS-positive mothers during labor in many countries. Although antibiotics have significantly reduced GBS early-onset neonatal disease, there are several unintended consequences, including an altered neonatal microbiota and increased risk for other microbial infections. Additionally, the incidence of late-onset GBS neonatal disease remains unaffected and has sparked an emerging hypothesis that GBS-microbe interactions in developing neonatal gut microbiota may be directly involved in this disease process. This review summarizes our current understanding of GBS interactions with other resident microbes at the mucosal surface from multiple angles, including clinical association studies, agriculture and aquaculture observations, and experimental animal model systems. We also include a comprehensive review of in vitro findings of GBS interactions with other bacterial and fungal microbes, both commensal and pathogenic, along with newly established animal models of GBS vaginal colonization and in utero or neonatal infection. Finally, we provide a perspective on emerging areas of research and current strategies to design microbe-targeting prebiotic or probiotic therapeutic intervention strategies to prevent GBS disease in vulnerable populations.
KEYWORDS: vaginal microbiota, group B Streptococcus, probiotic, microbe-microbe interactions, Streptococcus agalactiae
INTRODUCTION
Group B Streptococcus (GBS), or Streptococcus agalactiae, is a Gram-positive bacterium that is commonly found at the mucosa of the human gastrointestinal and vaginal tracts, along with the gastrointestinal tract of bovine species. Although typically an asymptomatic colonizer, GBS may disseminate to other tissues peripartum, such as the placental-fetal unit in pregnancy, the neonate during labor and delivery, or the mammary gland during lactation. In these environments, GBS can cause severe disease, including chorioamnionitis, preterm birth, stillbirth, neonatal sepsis or meningitis, or clinical mastitis (1–3). GBS is also an agent of skin and soft tissue infections, urinary tract infections, and sepsis; nonpregnant adults with underlying conditions such as diabetes or cardiovascular disease are at increased risk for such infections (4, 5). The global GBS colonization incidence in pregnancy is 18% (with regional variations from 11% to 35%) at any single point in time (6, 7). Colonization rates in nonpregnant adults are similar to those in pregnant individuals (8); however, cross-sectional studies may underestimate transient GBS colonization over time. In a 5-month longitudinal study of nonpregnant women, 79% of subjects were vaginally colonized with GBS in at least one of six visits (9). Similarly, in a pregnancy and postpartum study, the cumulative carriage rate of GBS was 54% (10). While GBS colonization may fluctuate within an individual and GBS strains may be eradicated or replaced with new GBS strains (11, 12), more typically, individuals are consistently colonized by the same strain or “clone” as determined by pulsed-field gel electrophoresis (10).
Current measures to prevent GBS neonatal disease consist of maternal recto-vaginal screening between 35 and 37 weeks of gestation, or risk factor screening, and administration of intrapartum antibiotic prophylaxis (IAP) to GBS+ or at-risk individuals. This intervention has decreased GBS early-onset disease (EOD) from 1 in 500 live births (13) to 0.34 cases per 1,000 live births in the United States (14). The majority (~60%) of EOD cases occur in term infants born to women who were GBS− at the time of maternal screening, highlighting the need for further protocol optimization or implementation of additional testing upon hospital admission (14). Despite the exposure of ~30% of U.S. infants to IAP (14), universal screening and prophylactic treatment have not reduced late-onset disease (LOD) incidence. GBS LOD incidence has remained constant in North America and parts of Europe (15–18) and may even be on the rise in some regions (19, 20). GBS transfer between humans most often occurs through vertical transmission from mother to infant peripartum (21, 22) or through breastfeeding (23), although cocolonization between sexual partners or transmission through cohabitation has been reported (24, 25). GBS transmission in other species is less well characterized but can occur in dairy herds (26, 27) and in wild aquatic (28, 29) or aquaculture (30) settings. Since not all GBS-colonized individuals experience disease, it is imperative to understand how host-specific factors can protect against or aggravate complications arising from GBS carriage.
GBS AND THE HUMAN MICROBIOTA
Within its host, GBS does not exist in isolation. As a frequent member of the human gastrointestinal and vaginal microbiota, GBS has ample opportunity for microbial interactions. Although GBS can be a transient or permanent resident member of the human microbiota, it is not yet clear whether GBS is truly a commensal in this environment. Clinical and experimental studies examining GBS synergistic and antagonistic interactions with other microbes are summarized in Table 1 and Table 2 respectively.
TABLE 1.
Summary of synergistic interactions between GBS and other microbes from clinical and experimental studiesa
| Host or model | Interaction | Population or condition/intervention | Sample type or medium | Cohort or sample size | Study type | Key finding(s) | Reference |
|---|---|---|---|---|---|---|---|
| Humans | Bifidobacterim spp. | Newborns of GBS− and GBS+ (and IAP-treated) mothers | Stool | 52 newborns | Cross-sectional | IAP-exposed newborns have decreased gastrointestinal bifidobacteria, of which some strains were found to have antibacterial properties against GBS. | 51 |
| Candida albicans | Nonpregnant | Urine and urogenital discharge | >2,400 participants | Cross-sectional | Urogenital GBS was coisolated with enterococci, Staphylococcus saprophyticus, and C. albicans. | 193 | |
| Nonpregnant | Not specified | 56 participants | Retrospective | GBS was most common bacterial coisolate in a cohort of recurrent vulvovaginal candidiasis patient samples. | 194 | ||
| Pregnant | Vaginal wash | 13,914 participants | Prospective | Candida colonization was accompanied by GBS in 36.8% of samples collected. | 195 | ||
| Pregnant | Vaginal swab | 150 participants | Cross-sectional | Candida spp. were found in 14.9% of GBS+ women compared to 2.9% of GBS− women. | 41 | ||
| Pregnant | Vaginal swab | 623 participants | Cross-sectional | Of the 7% of healthy vaginal samples in which GBS was detected, C. albicans was coisolated in 54.5% of those samples. | 42 | ||
| Candida spp. | Pregnant | Vaginal swab | 7,742 participants | Cross-sectional | Risk of cervicovaginal colonization with GBS was increased with dual GBS and Candida sp. colonization. | 196 | |
| Pregnant | Vaginal swab | 405 participants | Prospective | Risk of GBS colonization was increased in women cocolonized with Candida. | 43 | ||
| Pregnant | Rectal and vaginal swab | 100 participants | Cross-sectional | GBS prevalence was increased in women cocolonized with Candida. | 197 | ||
| Pregnant | Vaginal swab | 221 participants | Longitudinal | GBS and Candida were coisolated in 26.6% of samples. | 198 | ||
| Candida spp. | Pregnant | Vaginal swab | 542 participants | Cross-sectional | GBS and Candida were coisolated in 36% of GBS+ samples. | 132 | |
| Enterococcus spp. | |||||||
| Staphylococcus aureus | |||||||
| Candida albicans | Pregnant and nonpregnant | Vaginal swab | 430 participants | Cross-sectional | GBS had a higher risk of detection in samples with C. albicans (37.0%) and E. coli (24.6%) than in those without dual colonization. Conversely, E. coli had a higher risk of detection in GBS+ (43.3%) vs GBS− samples. | 44 | |
| Escherichia coli | |||||||
| Escherichia coli | Nonpregnant | Midstream and catheter urine | 202 paired samples | Cross-sectional | GBS and E. coli were cocultured from midstream urine and catheter urine collections. | 167 | |
| Pregnant | Urine | 821 participants | Retrospective | GBS and E. coli were cocultured from uterine cultures taken during nonelective cesarean sections. | 163 | ||
| Nonpregnant | Endocervical swab | 61 participants | Randomized trial | Of participants using a candidate HIV prophylactic, the GBS concn was inversely associated with inhibitory activity against E. coli. | 165 | ||
| Escherichia coli | Nonpregnant | Rectal and vaginal swab | 1,248 participants | Prospective | Rectal GBS colonization is a predictor of GBS vaginal colonization. Other risk factors for GBS vaginal colonization include concurrent vaginal E. coli or yeast colonization. | 162 | |
| Yeast | |||||||
| Escherichia coli | Women diagnosed with vulvovaginitis | Vaginal swab | 4 participants | Case study | GBS was coisolated with E. coli and S. aureus. | 148 | |
| Staphylococcus aureus | |||||||
| Escherichia coli | Women diagnosed with tubo-ovarian abscess | Laparotomy drainage | 11 participants | Case study | GBS was coisolated with multiple aerobic and anaerobic microbes. | 164 | |
| Eubacterium lentum | |||||||
| Porphyromonas spp. | |||||||
| Prevotella spp. | |||||||
| Salmonella sp. | |||||||
| Staphylococcus spp. | |||||||
| Streptococcus viridans | |||||||
| Atopobium vaginae | Pregnant | Vaginal swab | 248 participants | Prospective | GBS+ samples correlated with different species depending on thresholds set and analysis via cultivation or WGS. | 46 | |
| Bifidobacterium spp. | |||||||
| Megasphaera sp. | |||||||
| Prevotella spp. | |||||||
| Clostridiaceae | Infants of GBS+ mothers | Stool | 298 participants | Longitudinal | Once adjusted for demographic parameters and use of IAP, GBS+ status in mothers was associated with variation in gut microbiota composition in samples from the 6-mo visit. | 50 | |
| Enterococcaceae | |||||||
| Ruminococcaceae | |||||||
| Bacteroidetes | Nonpregnant | Vaginal swab | 66 participants | Cross-sectional | After filtering for taxa with a logarithmic discriminant analysis score of ≥3, 10 taxa were positively associated with GBS+ status. Note that Streptococcus as a correlate has been omitted since it was not distinguished from GBS. | 33 | |
| Bacteroidia | |||||||
| Bacteroidales | |||||||
| Clostridia | |||||||
| Clostridiales | |||||||
| Megasphaera | |||||||
| Prevotella | |||||||
| Prevotellaceae | |||||||
| Veillonellaceac | |||||||
| Prevotella spp. | Diabetic patient | Scrotal abscess | 1 participant | Case study | GBS and Prevotella were cocultured. | 187 | |
| Pregnant | Vaginal swab | 72 participants | Prospective longitudinal | Of the women who received IAP for GBS, Prevotella relative abundance increases postpartum. | 185 | ||
| Eubacterium siraeum | Nonpregnant | Vaginal lavage specimen | 432 participants | Cross-sectional | After filtering for taxa with a logarithmic discriminant analysis score of ≥2.5, 8 taxa were positively associated with GBS+ status. | 32 | |
| Finegoldia magna | |||||||
| Prevotella bivia | |||||||
| Prevotella melaninogenica | |||||||
| Peptostreptococcus anaerobiius | |||||||
| Staphylococcus spp. | |||||||
| Veillonella spp. | |||||||
| Staphylococcus aureus | Pregnant | Vaginal swab | 4,025 participants | Cross-sectional | GBS+ status was associated with reduced coculture with Gram-positive cocci, anaerobes, and fungi, but also an increased association with specific microbes. | 40 | |
| Klebsiella pneumoniae | |||||||
| Staphylococcus aureus | Nonpregnant | Sterile site culture | 19,512 samples | Population-based surveillance | S. aureus was the most common coisolate (46.2%) in GBS polymicrobial sepsis cases. | 139 | |
| Nonpregnant | Blood | 94 cases | Retrospective | GBS was most commonly coisolated with S. aureus or K. pneumonia. | 142 | ||
| Patients diagnosed with pneumonia | Blood and respiratory samples | 1,791 cases | Retrospective | Of those identified with polymicrobial cultures, 20% were coisolated with S. aureus and 7% with P. aeruginosa | 143 | ||
| Patients diagnosed with bone or joint infection | Deep surgical sample, joint aspirate, or blood | 26 cases | Retrospective | GBS was found more often in polymicrobial than monomicrobial infections. Staphylococcus spp. (S. aureus and S. ludgunesis) and E. faecalis were found in 56% and 19% of samples, respectively. | 144 | ||
| Men | Skin, urinary or respiratory tract, blood, abscess, joint, bone, or unknown | 23 cases | Retrospective | Of patients with bacteremia, GBS was coisolated with B. fragilis, other beta-hemolytic Streptococcus spp., S. aureus, and Providencia stuartii. | 140 | ||
| Nonpregnant adults and neonates | Blood | 18 cases | Retrospective | S. aureus was the most common coisolate in GBS bacteremia. | 141 | ||
| Infants | Nasopharyngeal swab | 1,200 participants | Cross-sectional | Risk of GBS colonization increased with the detection of S. aureus. | 137 | ||
| Pregnant | Rectal and vaginal swab | 2,963 samples | Prospective | S. aureus prevalence was increased in GBS+ samples. | 127 | ||
| Pregnant women and neonates | Rectal and vaginal swab | 2,702 women and 2,789 infants | Retrospective | Risk of S. aureus colonization increased with the detection of GBS. | 131 | ||
| Pregnant | Rectal and vaginal swab | 2,921 samples | Prospective | GBS colonization was positively associated with S. aureus. | 130 | ||
| Pregnant | Vaginal swab | 6,626 participants | Cross-sectional | Risk of S. aureus colonization increased in patients with GBS. | 128 | ||
| Pregnant | Rectal and vaginal swab | 5,732 participants | Prospective | Risk of S. aureus colonization increases with positive GBS colonization status. | 129 | ||
| Trichomonas vaginalis | Patients with vaginitis | Vaginal swab | 327 participants | Cross-sectional | GBS was positively associated with T. vaginalis infection. | 35 | |
| Cows (Holstein) | Aeromonas spp. | Dairy herds | Milk | 7 cows | Cross-sectional | GBS− subclinical mastitis milk was dominant in Firmicutes and was associated with an increase in 2 taxa. | 3 |
| Chryseobacterium spp. | |||||||
| Mice (KK-Ay) | Klebsiella pneumoniae | Laboratory colony | Submandibular abscess and uterine tissue | 2 mice | Case study | Of the cultures taken from submandibular abscesses, K. pneumoniae was coisolated. Of the 1 uterine culture, S. aureus was coisolated. | 119 |
| Staphylococcus aureus | |||||||
| Rats | Staphylococcus spp. | Pups from MWF-hDTR laboratory colony | Spleen, kidney, and heart | 4 rats | Case study | GBS was cocultured with coagulase-negative Staphylococcus spp. in limb abscesses, and E. coli was coisolated from a liver. | 120 |
| Escherichia col | |||||||
| Escherichia coli | Female adults from Long-Evans laboratory colony | Spleen, vaginal swab, cervical, nares, lung, brain, uterine, fetal, cardiac thrombus | 2 rats | Case study | GBS was coisolated with multiple microbes from different tissue sites or lavage specimens. | 121 | |
| Enterobacter aerogenes | |||||||
| Enterococcus spp. | |||||||
| Staphylococcus sciuri | |||||||
| Streptococcus mitis | |||||||
| Streptococcus oralis | |||||||
| Exptl models | Interaction | Condition/intervention | Sample type or medium | Sample size | Key finding(s) | Reference | |
| In vivo | |||||||
| Mice (CD-1) | Akkermansia municiphila | GBS challenge following pretreatment or cocolonization with A. muciniphila | Vaginal lavage specimen | Not specified | GBS persistence in the vaginal lumen was increased in mice dually colonized with A. muciniphila. | 175 | |
| Nile tilapia | Akkermansia spp. | Oral gavage with GBS strain YM001 | Intestinal tissue | 30 fish | Administration of YM001 caused temporary and nonlethal changes in the intestinal gut microbiota, making it a safe vaccine. | 106 | |
| Bacteroides spp. | |||||||
| Mice | |||||||
| C57BL/6J | Candida albicans | Transurethral coinfection of C. albicans and GBS | Bladder | 14–18 mice/group | C. albicans increased GBS colonization and epithelial adherence through hypha-specific adhesin Als3. | 200 | |
| Vaginal coinoculation of C. albicans and GBS | Vaginal lavage specimen | 6 mice/group | The vaginal mucosal Th17 response to C. albicans is reduced in the presence of GBS. | 199 | |||
| C3H/HeN and C3H/HeJ | Escherichia coli | Urinary tract coexposure to GBS and UPEC | Bladder | >10 mice/group | Immune response against UPEC is dampened by GBS sialic acid host immune signaling. | 168 | |
| C57BL/6J | Gardnerella vaginalis | Vaginal coinoculation of GBS and G. vaginalis | Maternal vaginal, uterine, and placental tissues | 31 mice | Risk for vaginal colonization, ascension into the uterus, and presence in both the maternal and fetal sides of the placenta increase in the presence of G. vaginalis. | 192 | |
| Rats (Wistar) | Prevotella bivia | Uterine inoculation with P. bivia and GBS | Uterine fluid | 10 rats/group | P. bivia uterine infection is increased by GBS. | 186 | |
| In vitro | Candida albicans | C. albicans and GBS coculture in vitro | Spider agar and YPD | Triplicate | GBS suppresses hyphal formation through reduction of EFG1/Hwp1 expression. | 199 | |
| Cocultivation of C. albicans and GBS | Yeast nitrogen base | Triplicate | GBS BspA protein promoted association of GBS with C. albicans hyphae. | 201 | |||
| Cocultivation of C. albicans and GBS and cocolonization of vaginal epithelial cells | Keratinocyte serum-free medium | Triplicate | Interaction between GBS and C. albicans increases with hyphal form via C. albicans adhesin Als3 and GBS Bsp adhesins. | 202 | |||
| Cocultivation of C. albicans and GBS | Blood agar | 110 C. albicans strains | C. albicans and GBS demonstrate cohemolytic (CAMP factor like) activity. | 203 | |||
| Escherichia coli | Cross-feeding assays on solid media and liquid culture | M17 glucose agar | Not specified | E. coli produces ubiquinone, menaquinones, and other metabolites that may be utilized by GBS. | 166 | ||
| Staphylococcus aureus | Culture of S. aureus with supernatants from bacteria associated with dysbiosis and health | BHI | Not specified | GBS supernatant increased S. aureus tst gene expression encoding toxic shock syndrome protein TSST-1. | 145 | ||
| Coculture of GBS with bacterial species commonly found in female genital tract | TSB agar | Not specified | S. aureus was not inhibited by GBS isolates. | 146 | |||
sp., single unspecified or unknown species of a genus; spp., more than one unspecified or unknown species of a genus; IAP, intrapartum antibiotic prophylaxis; UPEC, uropathogenic E. coli; YPD, yeast extract-peptone-dextrose; BHI, brain heart infusion; TSB, tryptic (or Trypticase) soy broth.
TABLE 2.
Summary of antagonistic interactions between GBS and other microbes from clinical and experimental studiesa
| Host or model | Interaction | Population or condition/intervention | Sample type or medium | Cohort or sample size | Study type | Key finding(s) | Reference |
|---|---|---|---|---|---|---|---|
| Humans | BVAB1 | Nonpregnant | Vaginal lavage | 428 samples | Cross-sectional | The abundances of 6 taxa were negatively associated with GBS+ status. Communities clustered according to GBS status on principal-component analysis, but the contribution of GBS status to overall variance was small. GBS presence was associated with community state type IV subgroup analysis, where 40% of subgroup A samples were GBS+ compared to 17% in subgroup IV-B. | 32 |
| BVAB2 | |||||||
| Dialister sp. type 2 | |||||||
| Megasphaera sp. type 1 | |||||||
| Prevotella genogroup 3 | |||||||
| Prevotella genogroup 4 | |||||||
| Candida spp. | Pregnant | Vaginal swab | 4,025 participants | Cross-sectional | From a collection of strains isolated from vaginal samples; 8 taxa were associated with the GBS-negative group. | 40 | |
| Enterococcus faecalis | |||||||
| Lactobacillus spp. | |||||||
| Peptostreptococcus spp. | |||||||
| Prevotella spp. | |||||||
| Staphylococcus spp. (coagulase negative) | |||||||
| Streptococcus spp. (microaerophilic) | |||||||
| Lactobacillus helveticus | Infants of GBS+ mothers | Stool | 86 samples | Cross-sectional | α diversity did not differ based on GBS status. Communities clustered similarly on principal-component analysis regardless of GBS status. The abundances of 4 taxa were negatively associated with GBS+ status. | 49 | |
| Lactobacillus mudanjiangensis | |||||||
| Lactobacillus paracasei | |||||||
| Staphylococcus lugdunensis | |||||||
| Bacilli (class) | Nonpregnant | Vaginal swab | 66 participants | Cross-sectional | α diversity did not differ based on GBS status. Communities clustered according to GBS status on principal-component analysis, but the contribution of GBS status to overall variance was small. LEfSe analysis revealed 5 taxa negatively associated with GBS+ status. | 33 | |
| Firmicutes (phylum) | |||||||
| Lactobacillaceae (family) | |||||||
| Lactobacillales (order) | |||||||
| Lactobacillus spp. | Nonpregnant | Vaginal swab | 191 participants | Longitudinal with intervention | In vaginal samples classified by amt of lactobacilli, GBS prevalence was lower in samples with avg or large amt of high lactobacilli vs samples with small amt of lactobacilli. | 9 | |
| Nonpregnant receiving IVF | Vaginal swab | 285 participants | Prospective | Small amounts of Lactobacilli, classified as <104 CFU, were associated with GBS positive status. | 211 | ||
| Lactobacillus spp. | Pregnant | Vaginal swab | 1,860 samples | Retrospective cross-sectional | Presence of lactobacilli, specifically L. crispatus, was negatively correlated with GBS+ status. | 191 | |
| Lactobacillus crispatus | |||||||
| Lactobacillus reuteri and Lactobacillus rhamnosus | Pregnant | Vaginal and rectal swab | 110 participants | Prospective with intervention | 42.9% of GBS+ participants treated with a combination of L. rhamnosus and L. reuteri early in pregnancy became GBS− by the time of delivery, while 18% became GBS− in placebo group. | 278 | |
| Pregnant | Vaginal secretions | 155 participants | Longitudinal with intervention | The group receiving oral Lactobacillus probiotic treatment had significantly reduced incidence of premature rupture of membranes but did not significant impact GBS clearance. | 279 | ||
| Lactobacillus salivarius | Nonpregnant and pregnant | Vaginal and rectal swab | 54 participants | Longitudinal with intervention | Of GBS+ participants treated with L. salivarius, 78% became rectally GBS− and 68% became vaginally GBS−, while none became rectally or vaginally GBS− in the control group. | 284 | |
| Lactobacillus sp. | Pregnant | Vaginal swab | 150 participants | Cross-sectional | From a collection of strains isolated from vaginal samples, Lactobacillus species were associated with the GBS− status. | 41 | |
| Lactobacillus crispatus | Pregnant | Vaginal swab | 243 samples | Longitudinal | The relative abundances of 2 taxa negatively co-occurred with GBS presence. | 46 | |
| Neisseria spp. | |||||||
| Aerococcus spp. | Pregnant | Vaginal swab | 94 participants | Cross-sectional | Aerococcus relative abundance was lower in the GBS+ group. | 45 | |
| Cows (Holstein) | Acinetobacter spp. | Dairy herds | Milk | 12 cows | Cross-sectional | The relative abundances of 4 genera were lower in the GBS+ group. | 103 |
| Corynebacterium spp. | |||||||
| Microbacterium spp. | |||||||
| Stenotrophomonas spp. | |||||||
| Lactobacillus spp. | Dairy herds | Milk | 40 cows | Cross-sectional | As determined by qPCR, Lactobacillus quantity was negatively correlated with GBS quantity. | 212 | |
| Exptl models | Interaction | Condition/intervention | Sample type or medium | Sample size | Key finding(s) | Reference | |
| In vivo | |||||||
| Mice | |||||||
| BALB/c | Lactobacillus reuteri | Vaginal L. reuteri administration | Vaginal washes | 14 mice | L. reuteri inhibited GBS vaginal colonization. | 213 | |
| C57BL/6J | Staphylococcus spp. | Vaginal inoculation with GBS | Vaginal swab | 72 mice | Upon GBS colonization, Staphylococcus-dominant vaginal microbiota were less stable. | 122 | |
| Staphylococcus succinus | Vaginal inoculation with GBS | Vaginal swab | 32 mice | S. succinus relative abundance decreased over time in GBS infection. | 123 | ||
| CD-1 | Streptococcus salivarius | Vaginal inoculation with GBS and treatment with S. salivarius | Vaginal swab | Not specified | S. salivarius reduced GBS vaginal colonization. | 206 | |
| Nile tilapia | Brevinema spp. | Oral inoculation with attenuated GBS strain | Intestinal tissues | 105 fish | Attenuated GBS temporarily impacted gut microbiome by reducing diversity and changing composition. | 106 | |
| Cetobacterium spp. | |||||||
| Romboutsia spp. | |||||||
| Enterococcus faecium | Treatment with E. faecium and i.p. injection with GBS | Stool | 45 fish | E. faecium treatment reduced mortality in GBS-infected fish. | 297 | ||
| Lactobacillus rhamnosus and Lactococcus lactis subsp. lactis | Treatment with L. rhamnosus and L. lactis and i.p. injection with GBS | Intestinal tissues | 720 fish | L. rhamnosus and L. lactis treatment increased GBS disease resistance. | 298 | ||
| Clostridium butyricum | Treatment with C. butyricum and i.p. injection with GBS | 225 fish | C. butyricum treatment reduced mortality in GBS-infected fish. | 299 | |||
| Bacillus cereus and Bacillus subtilus | Treatment with B. cereus and/or B. subtilis and i.p. injection with GBS | 100 fish | Combined probiotic and B. cereus-only treatments reduced mortality in GBS-infected fish. | 300 | |||
| In vitro | |||||||
| Diphtheroids | Agar overlay inhibition assay | Strains grown in TSB with 5% sheep blood, assay on TSB agar | Duplicate | GBS inhibited the growth of many, but not all, tested bacterial strains found in the vaginal tract. | 146 | ||
| Enterococcus spp. | |||||||
| Gardnerella vaginalis | |||||||
| Group A Streptococcus | |||||||
| Group B Streptococcus (other than test strain) | |||||||
| Group C or G Streptococcus | |||||||
| Lactobacillus spp. | |||||||
| Peptostreptococcus spp. | |||||||
| Streptococcus spp. (alpha-hemolytic) | |||||||
| Streptococcus spp. (nonhemolytic) | |||||||
| Bacillus altitudinis | Agar overlay inhibition assay | Isolates derived from intestinal tissues; assay on LB agar | Triplicate | Some fish gut bacterial isolates inhibited GBS growth. | 301 | ||
| Bacillus amyloliquefaciens | |||||||
| Bacillus pumilus | |||||||
| Streptomyces rutgersensis | |||||||
| Bifidobacterium breve | Agar overlay inhibition assay | Bifidobacterium in TPY; GBS in BHI | Triplicate | Some bifidobacterial strains inhibited GBS growth. | 51 | ||
| Bifidobacterium longum subsp. longum | |||||||
| Streptococcus salivarius | Coculture and deferred antagonism | THB and Columbia blood agar | Triplicate | S. salivarius inhibits the growth of GBS in coculture and through secreted substrates. | 206 | ||
| Transwell coculture; cell-free supernatant treatment | THB | Triplicate | GBS inhibited growth of S. salivarius via coculture and supernatant, but inhibition was reversed by galacto-oligosaccharide treatment. | 207 | |||
| Lactobacillus acidophilus | Bacteriocin treatment of GBS culture | BHI | Not specified | L. acidophilus bacteriocin inhibited GBS growth. | 222 | ||
| Lactobacillus fermentum | Agar well diffusion assay | TSB agar | Not specified | L. fermentum bacteriocin inhibited GBS growth. | 218 | ||
| Lactobacillus crispatus | Cell-free supernatant treatment | TSB | Triplicate | L. reuteri and L. gasseri supernatants inhibited GBS growth; all 3 species supernatants inhibited GBS biofilm formation and association with human endometrial stromal cells. | 214 | ||
| Lactobacillus gasseri | |||||||
| Lactobacillus reuteri | |||||||
| Lactobacillus acidophilus | GBS adhesion assay with Lactobacillus inhibition by exclusion, competition, or displacement | LAPTg | Triplicate | Lactobacillus strains inhibited GBS adhesion to human vaginal epithelial cells. | 215 | ||
| Lactobacillus paracasei | |||||||
| Lactobacillus crispatus | GBS adhesion assay with Lactobacillus inhibition by exclusion, competition, or displacement | Not specified | Triplicate | All L. crispatus and L. gasseri strains inhibited GBS adhesion to human vaginal epithelial cells by exclusion, competition, and displacement. | 216 | ||
| Lactobacillus gasseri | |||||||
| Lactobacillus crispatus | Coculture and cell-free supernatant treatment | Horse blood agar and MRS with l-cysteine | Duplicate | Multiple Lactobacillus strains and supernatants inhibited GBS growth. | 220 | ||
| Lactobacillus gasseri | |||||||
| Lactobacillus vaginalis | |||||||
| Lactobacillus fermentum | Streak-diffusion agar inhibition assay | MRS and TSB | Duplicate | Lactobacillus bacteriocins inhibited GBS growth. | 223 | ||
| Lactobacillus rhamnosus | |||||||
| Lactobacillus fermentum | Agar well diffusion assay | MRS and TSB | Triplicate | Bacteriocins from either Lactobacillus strain and a combination of their bacteriocins inhibited growth of most GBS strains. | 224 | ||
| Lactobacillus rhamnosus | |||||||
| Lactobacillus paracasei subsp. paracasei | Microdilution antimicrobial assay | MRS and TSB | Triplicate | Lactobacillus biosurfactant inhibited GBS growth. | 225 | ||
| Lactobacillus gasseri | Agar well diffusion assay | LAPTg | Not specified | Lactobacillus supernatants inhibited GBS growth. | 227 | ||
| Lactobacillus salivarius | |||||||
| Lactobacillus salivarius (multiple strains) | Agar overlay, agar well diffusion, coaggregation, and coculture assays | MRS | Triplicate for agar diffusion. Not specified for other assays. | GBS growth was inhibited in agar overlay with L. salivarius, but not in agar well diffusion using L. salivarius supernatants. Some L. salivarius strains coaggregated with some GBS strains. Compared to GBS monoculture, most L. salivarius strains interfered with GBS growth in co-culture. | 284 | ||
BVAB1 and BVAB2, bacterial vaginosis-associated bacterium 1 and 2; sp., single unspecified or unknown species of a genus; spp., more than one unspecified or unknown species of a genus; IVF, in vitro fertilization; i.p., intraperitoneal; subsp., subspecies; TSB, tryptic (or Trypticase) soy broth; LB, Luria-Bertani (or lysogeny broth); TPY, tryptone-peptone-yeast; BHI, brain heart infusion; THB, Todd Hewitt broth; LAPTg, cultivation medium composed of 1.5% peptone, 1% tryptone, 1% glucose, 1% yeast extract, and 0.1% Tween 80; MRS, de Man Rogosa Sharpe.
Due to the importance of GBS vaginal colonization as a risk factor for neonatal disease, the majority of GBS-microbe interactions that have been identified so far are in the context of the vaginal tract. The human vaginal microbiome across demographics clusters into five distinct community state types (CSTs) based on sequencing of the 16S rRNA gene V3-V4 region (31). Four CSTs are dominated by a single Lactobacillus species, and one community (CST IV) is instead dominated by a mixture of anaerobic or facultative anaerobic organisms (31). In 16S rRNA sequencing-based nonpregnant vaginal studies, GBS colonization was observed across all five CSTs, but GBS abundance was highest in a non-Lactobacillus dominant CST, IV-A (32) (recently reclassified as CST IV-C1 [31]). Additionally, GBS+ individuals displayed lower relative abundance of Lactobacillus than GBS− women (33) (Table 2). Inverse correlations between GBS and Lactobacillus species abundance have been corroborated in a microbial culture-based study (9). Several taxa correlate with GBS colonization status, although results differ between studies, potentially due to differences in cohort demographics or sequencing methodologies (e.g., taxonomy based on 16S rRNA V1-V2 versus V3-V4 sequences). In one study, GBS+ individuals displayed higher levels of certain Prevotella, Megasphaera, and Streptococcus species than GBS− individuals (33) (Table 1). In another study, linear discriminant analyses identified positive associations with GBS and Staphylococcus spp., Prevotella bivia, and Streptococcus spp. and negative associations with GBS and bacterial vaginosis associated bacterium 1 (BVAB1), BVAB2, and other Prevotella and Megasphaera subgroups (32). In terms of pathogenesis, a culture-based study of nonpregnant women did not detect any differences in bacterial vaginosis (BV) incidence between GBS+ and GBS− individuals (34). Furthermore, detection of vaginal pathogens, including Candida albicans, Neisseria gonorrhoeae, Trichomonas vaginalis, and Chlamydia trachomatis, was not influenced by GBS colonization status (34); however, in another study, GBS colonization was enriched in individuals with T. vaginalis infection (35).
Pregnancy studies also report mixed clinical findings, which may be driven by biological differences across cohorts, including subject demographics and gestational length at the time of sampling, which are both known to impact the vaginal microbiota composition (36–38). Methodological differences ranging from targeted cultivation or quantitative PCR (qPCR) to broader sequencing approaches, including 16S rRNA amplicon profiling or whole-genome sequencing, may also influence study outcomes. As with nonpregnant women, GBS colonization itself does not indicate an altered or aberrant vaginal microbiota or clinical disease, such as BV, but distinct correlations with individual taxa and GBS have been reported. One culture-based study of 3,596 pregnant women found that patients diagnosed with BV had lower GBS colonization rates than non-BV controls (39). In another culture-based study of 4,025 pregnant women, midgestation GBS colonization was associated with lower levels of Lactobacillus, Prevotella, and Candida and higher levels of Staphylococcus (40) (Table 1 and Table 2). A smaller (150 subjects) midgestation culture-based study also observed reduced Lactobacillus in GBS+ subjects, but in contrast, reported GBS coisolation with C. albicans (41). In culture-based studies of late-gestation samples, GBS was coisolated with C. albicans (42) or individuals with candidosis (43). In a longitudinal pregnancy study of 42 women sampled at each trimester, no significant differences in levels of Lactobacillus, Bifidobacterium, or Candida were observed between GBS+ and GBS− individuals as measured by cultivation and qPCR of target species (11). In another qPCR-based study consisting of both pregnant and nonpregnant subjects, GBS was coisolated with C. albicans and Escherichia coli, and GBS+ individuals had a reduced incidence of BV (44). In a 16S rRNA amplicon study of 94 women during late gestation, the GBS+ group displayed decreased Aerococcus and increased Corynebacterium abundance, as well as an increased but nonsignificant abundance of Lactobacillus spp. (45). Finally, in a whole-genome sequencing study conducted in 248 pregnant women, GBS colonization was negatively associated with Lactobacillus crispatus and positively associated with 19 species, including multiple Prevotella spp., Bifidobacterium spp., Atopobium vaginae, and other Streptococcus spp. (46). The vast majority of vaginal studies to date have binned subjects into GBS+ and GBS− groups based on culture results, and thus potential ecologic relationships between GBS abundance and other organisms remain undiscovered. Future studies designed to broadly capture GBS-microbe associations, such as whole-genome sequencing, might establish microbial relationships that hold predictive value for host outcomes or inform therapeutic interventions.
Outside of the context of the vaginal microbiota, there are fewer data reporting GBS associations with the host microbiota. If GBS influences the presence or absence of other organisms, either directly through niche competition or indirectly through host immune or metabolism modulation, it is feasible that GBS presence in the maternal vagina may alter maternal microbial transfer to the infant or the establishment of the infant microbiota. Maternal microbial transfer may be further perturbed by IAP; studies have reported reduced vaginal Lactobacillus spp. in the IAP group compared to women without IAP (47, 48). In general, no large differences in fecal α or β diversity are observed in infants born to GBS+ mothers (49, 50). One study found reduced Staphylococcus lugdunensis and Lactobacillus spp. in infants born to GBS+ mothers; however, IAP effects were not accounted for in this cohort since no significant differences in the rates of IAP between GBS+ and GBS− groups were observed (49). Clinical observations report differences in abundance for taxa including Ruminococcus, Clostridium, Akkermansia, and Bacteriodes in infant fecal samples between infants born to GBS+ mothers and those born to GBS− mothers even when adjusting for other factors such as IAP (50) (Table 1). Bifidobacterium abundance is reduced in the fecal microbiota of infants exposed to IAP through GBS+ mothers (51). Additional disturbances to the infant microbiota following IAP exposure have been reported out to 90 days of life (48, 52–59) and are reviewed elsewhere (60). GBS may be transmitted to the neonate at low levels during delivery, even with IAP administration, although IAP may reduce the severity of or delay LOD (16). Infants may also be exposed postpartum through GBS+ breast milk or colonized caregivers (21, 61, 62). It has been hypothesized that a dysbiotic or delayed maturation of the gastrointestinal microbiota may provide a foothold for GBS to initiate LOD (63). This is supported by observations that GBS appears in infant stool prior to LOD but is undetectable in healthy infants (64) and that empirical antibiotic therapy in low-birthweight infants increases the risk of LOD with GBS and other pathogens (65). Furthermore, animal studies comparing neonatal and adult germfree and conventional mice demonstrate increased susceptibility to GBS translocation in the gut in animals with juvenile or absent microbiota (66).
In the context of host-associated viruses, clinical reports of GBS-viral interactions are rare and limited to co-occurrence observations. HIV infection is a risk factor for GBS invasive disease in nonpregnant adults (67, 68). In pregnancy, infants born to HIV+ mothers have increased risk for GBS LOD (69–71), although no differences in maternal colonization between HIV+ and HIV− women are reported (72–75). This heightened risk may be the result of indirect immune suppression, since HIV+ mothers have lower transplacental transfer of anti-GBS antibodies to their infants than HIV− controls (76–78). Other associations include increased shedding of herpes simplex virus 2 in women with higher GBS vaginal burdens (79), and a case report of respiratory syncytial virus and GBS coinfection (80). Experimental evidence supports glycan-mediated interactions of GBS with viral pathogens, including influenza virus types A and B, parainfluenza virus, and paramyxoviruses (81–83), and prior exposure to influenza A enhances GBS virulence and adherence in vitro and in vivo (84–86). Nevertheless, concurrent or subsequent influenza and group B streptococcal lung infections are not reported in the clinical literature. In terms of bacterial viruses, or bacteriophages, most GBS strains (~70 to 85%) contain 1 to 4 prophages, which carry genes associated with defense, stress response, or virulence (87–91). Although not fully characterized, the retention of genetically similar GBS prophages in certain clonal complexes associated with pathogenesis and colonization suggests a beneficial role in host adaption (87, 90, 91).
GBS AND THE ANIMAL MICROBIOTA
While GBS is most notorious for its role in human disease, GBS colonization and/or disease are reported in many animals, including camels (92), cats and dogs (93), dolphins (94), seals (95), crocodiles (96), and fish (97). Although GBS-microbe associations within animals are not well described, there are several studies describing potential interactions between GBS and other resident or therapeutic microbes. GBS colonizes the gastrointestinal tract in 10 to 30% of dairy cattle (98, 99) and is a common and costly agent of mastitis due to reduction in both milk quality and production (100–102). Even subclinical mastitis causes a decrease in milk production and risk of spreading within the herd, which may lead to greater economic losses than clinical mastitis (3). In 16S rRNA amplicon sequencing studies, GBS-induced subclinical mastitis coincides with increased Streptococcus abundance (3, 103), although delineation between GBS and other Streptococcus species was not determined. Other perturbations to the milk microbiome were inconsistent across studies and include reduced Acinetobacter, Stenotrophomonas, Microbacterium, and Corynebacterium (103) or increased Aeromonas and Chryseobacterium (3) (Table 1 and Table 2). GBS can also cause invasive disease in fish, including Nile tilapia (104) and ya-fish (105). In a live attenuated vaccine study in Nile tilapia, introduction of attenuated GBS strain YM001 temporarily reduced abundance of intestinal Cetobacterium, Romboutsia, and Brevinema and increased that of Bacteroides and Akkermansia; however, these effects resolved within a week (106). In a continuous-flow competitive exclusion culture system of communities derived from farmed tilapia, GBS-inhibiting activity of community supernatants was positively correlated with Cetobacterium and Plesiomonas abundance (107). GBS-microbiome interactions that occur within bovine or fish hosts may be also relevant to zoonotic infections. GBS interspecies transmission to humans may occur as a result of raising livestock (108–111) or through consumption of infected fish (112–114). Indeed, the hypervirulent sequence type 17 lineage, significantly overrepresented in neonatal invasive disease, appears to have recently arisen from a bovine strain (115).
Although not recognized as a common endogenous microbe in laboratory animal species, GBS has been reported in some colonies of mice (116–119) and rats (120, 121). Animal models have also been used to experimentally study GBS-microbe interactions relevant to human colonization and disease. In a mouse model of GBS vaginal colonization, increased GBS uterine ascension was observed in conventional mice with Staphylococcus-dominant vaginal microbiota compared to mice harboring other vaginal taxa (122). Additionally, GBS inoculation induced instability of the vaginal microbiota, including early displacement of endogenous Staphylococcus spp. (122, 123) (Table 2). The murine gastrointestinal tract has also been implicated in GBS pathology. Murine neonatal susceptibility to GBS invasion of the meninges appears to be mediated by an immature gastrointestinal microbiota, since GBS neuroinvasion can be partially mitigated in neonatal mice by transplantation with adult mouse microbes (66). Furthermore, germfree adult mice have enhanced susceptibility to GBS invasion that can be rescued with microbial transplantation (66), although no particular taxa were associated with increased susceptibility or protection in this study. The murine microbiota has also been studied in the context of testing the impact of GBS therapeutic strategies on the endogenous microbiota in preclinical models. In a GBS vaginal colonization murine model, treatment with human milk oligosaccharides reduced GBS vaginal burdens with minimal changes to the endogenous vaginal microbiota (123). In a study assessing the efficacy of a maternal GBS-specific rGAPDH (recombinant glyceraldehyde-3-phosphate dehydrogenase) vaccine on infant GBS colonization and immune and neurologic development, maternal GBS vaccination resulted in distinct pup fecal microbiota through 90 days of life with an increase in Enterobacteriaceae compared to sham controls (124). Of concern, maternal GBS rGAPDH vaccination also was associated with changes in offspring, including altered immune profiles in intestinal and brain tissues combined with aberrant behavior and stress responses (124). Together, these human clinical association studies and preclinical animal models do not reveal large microbial disturbances as a result of GBS colonization as a whole, but rather suggest that GBS may influence cocolonization of individual taxa within the community.
SYNERGISTIC INTERACTIONS WITH GBS AND OTHER MICROBES
GBS and Staphylococcus aureus.
Staphylococcus aureus is a Gram-positive pathobiont that colonizes the skin, nasal passages, and vaginal tract of humans (125, 126). GBS and Staphylococcus spp., including S. aureus, are often coisolated from the vaginal tract of nonpregnant (32) and pregnant (127–132) women, and both organisms are often associated with reduced Lactobacillus and increased incidence of aerobic vaginitis (133, 134) (Table 1). In contrast, several studies have not observed an increased co-occurrence of GBS and S. aureus within the vaginal tract (135, 136). Moreover, GBS and S. aureus are also coisolated from the nasopharynx in infants (137) and in neonatal blood samples (138). In nonpregnant adult GBS invasive disease, S. aureus is the most common co-occurring pathogen in polymicrobial infections, including bacteremia or sepsis (139–142), pneumonia (143), and bone and joint infections (144). Although the underlying mechanisms driving this polymicrobial pathogenesis are unknown, some studies have begun to elucidate GBS-S. aureus interactions. Experimental studies show that GBS increases S. aureus production of toxic shock syndrome toxin-1 by 3-fold in vitro (145) (Fig. 1) and does not inhibit S. aureus growth (146–148). Although the synergistic cohemolytic effects of S. aureus sphingomyelinase C and GBS CAMP factor have been used for decades in clinical microbiology for definitive diagnosis of GBS (149), the contribution of this enhanced hemolysis to host-microbe interactions remains elusive. GBS CAMP factor is dispensable for GBS virulence and colonization in in vitro and in vivo models (147, 150), and no differences were found between CAMP-deficient and wild-type GBS interactions with vaginal epithelial cells in the presence of S. aureus (147). Since individuals suffering from polymicrobial GBS and S. aureus interactions frequently report metabolic and/or immune dysfunction such as diabetes (151), future work should assess GBS-S. aureus interactions in the context of host immunity and host and microbial metabolism.
FIG 1.
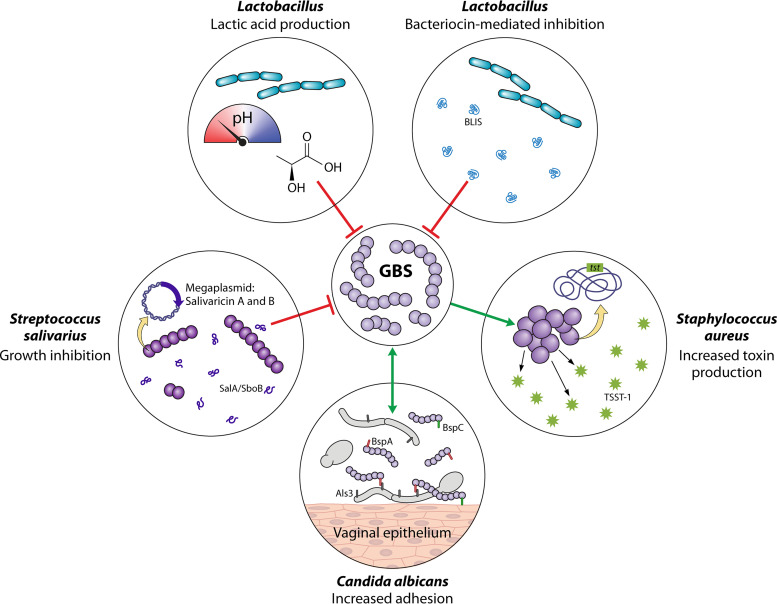
Social network of group B Streptococcus: mechanisms of negative (antagonistic) and positive (synergistic) GBS-microbe interactions. The network is ordered clockwise from the lower left. Streptococcus salivarius K12 produces salivaricin A and B to inhibit GBS growth in vitro and reduce GBS vaginal colonization in vivo (206). Lactic acid production by Lactobacillus spp. reduces pH and inhibits GBS growth in vitro (220, 226, 227). Multiple Lactobacillus spp. produce bacteriocin-like inhibitory substances (BLIS) that elicit bactericidal or inhibitory activity. Examples include L. salivarius CRL 1328 salivaricin (221), a L. acidophilus KS400 bacteriocin (222), an L. fermentum CS57 bacteriocin-like substance (218), and synergistic activity of bacteriocins from L. fermentum L23 and L. rhamnosus L60 (223, 224). GBS antigen I/II family adhesins BspA and BspC facilitate GBS interactions with Candida albicans, in part through hypha-specific ALS3, and promote binding of both organisms to the vaginal epithelium (201, 202). GBS supernatant increases S. aureus expression of tst, the gene encoding toxic shock syndrome toxin-1 (TSST-1), in vitro (145).
GBS and E. coli.
Although E. coli is an almost universal colonizer of the human gut, the vaginal tract is also a reservoir for commensal E. coli strains as well as extraintestinal pathogenic E. coli strains, such as those causing urinary tract infection (152) or neonatal sepsis and meningitis (153, 154). Independently, GBS and E. coli have similar colonization rates in the maternal vaginal tract (155, 156) and are the two leading causes of infant early-onset sepsis and associated mortality, accounting for 70% of all sepsis cases (157). Although GBS EOD incidence has been reduced with maternal screening and IAP, E. coli early-onset sepsis has not been impacted by this intervention, and IAP exposure may be contributing to ampicillin-resistant E. coli infections (157–161). GBS and E. coli are coisolated from the vaginal tract of pregnant (44) and nonpregnant women (44, 162) in some cohorts, but not others (41, 155) (Table 1). GBS and E. coli have also been coisolated from uterine swabs in patients undergoing cesarean sections (163), and rare polymicrobial reproductive tract infections have been reported (164). Although the specific molecular interactions are unknown, vaginal host mediators that inhibit E. coli are reduced in individuals colonized by both E. coli and GBS (165), and GBS does not inhibit E. coli growth in vitro (148). Additionally, cross-feeding of metabolites between GBS and E. coli has been observed in vitro (166). GBS and E. coli may also collude to cause urinary tract infections. Midstream urine samples that test positive for GBS are associated with E. coli-positive urine collected via urinary catheter (167). In animal models of urinary tract infection, GBS presence in the bladder increases E. coli burdens (168), but it is currently unknown whether this is through direct or indirect mechanisms. GBS capsular sialic acids engage sialic acid-binding immunoglobulin-type lectins (Siglecs) on immune cells to repress activation in multiple cell types, including macrophages and neutrophils (168, 169), which may indirectly permit higher E. coli burdens. Future work is needed to characterize the co-occurrence of GBS and pathogenic E. coli in the vaginal tract as a precursor to invasive disease such as urinary tract infection or neonatal sepsis.
GBS and Akkermansia (including A. muciniphila).
A fairly newly described species, Akkermansia muciniphila (170), has been associated with host gut health and positive health outcomes under a variety of conditions, including obesity (171, 172), resistance to immunotherapy (173), and liver disease (174). A recent analysis of the vaginal microbiota in pregnancy observed increased co-occurrence of GBS and A. muciniphila in women that delivered preterm compared to term births (175) (Table 1). In a Nile tilapia study, introduction of an attenuated GBS strain temporarily increased Akkermansia from 1% relative abundance to 28% relative abundance in the intestinal tract 3 days after inoculation, although the species of Akkermansia was not resolved (106). In murine models, vaginal A. muciniphila was identified in GBS-inoculated mice (123, 175), achieving statistically significant coassociation with GBS in one study (175). In silico metabolic modeling predicted multiple cross-feeding compounds between GBS and A. muciniphila, and a murine model demonstrated prolonged GBS vaginal persistence with coexposure to A. muciniphila (175). With recent discoveries of A. muciniphila consumption of host-produced substances, including mucin degradation (170, 176), and the importance of mucins in host defense against GBS in the reproductive tract (177), these interactions suggest mutual synergies at the mucosal surface are important for GBS persistence.
GBS and Prevotella (including P. bivia).
Prevotella spp. are commonly found in the human vaginal and gastrointestinal tracts (178–180). Increased Prevotella species abundance is reported in patients diagnosed with BV (181–183), although the causal role for Prevotella spp. in BV has not been established (184). Vaginal Prevotella species abundance was significantly higher in cohorts of GBS-positive nonpregnant women (32, 33) and postpartum in women that received IAP for GBS (185). Incongruously, pregnant cohorts show mixed results with both positive (46) and negative (40) correlations of P. bivia with GBS (Table 1 and Table 2). These mixed results may in part be due to the inability to delineate specific Prevotella species in many of the sequence-based studies. Functionally, the combination of GBS and P. bivia resulted in more bacterial invasion of the uterus than either species alone in a rat model of pyometra (186). Reports of GBS and Prevetolla interactions outside the female reproductive tract are rare. In one case report, GBS and P. bivia were coisolated from a scrotal abscess in a diabetic patient (187). Direct interactions between GBS and P. bivia and other Prevotella spp. have not been well characterized experimentally and should be addressed in future studies.
GBS and Gardnerella vaginalis.
While Gardnerella vaginalis is considered a common member of the human vaginal microbiota (178), increased abundance of G. vaginalis is observed in individuals diagnosed with BV (181, 183, 188). Recent work has identified distinct genetic signatures between G. vaginalis isolated from healthy women and those from women with BV (188, 189), suggesting a divergence into strains with enhanced or reduced pathogenic capability (190). Clinical studies reporting GBS and G. vaginalis coisolation are rare, and none has identified co-occurrence with GBS and G. vaginalis in pregnant women (41, 191). In a murine pregnancy model, coinoculation of GBS and G. vaginalis increased the likelihood of identifying both organisms in the maternal uterus and fetal placenta (192) (Table 1 and Table 2). However, in vitro results show GBS inhibits G. vaginalis growth (146), suggesting that GBS-Gardnerella interactions may be more complex than a neutralistic relationship and may depend on the pathogenic potential of each individual strain.
GBS and Candida albicans.
Candida albicans is a frequent opportunistic pathogen of multiple human mucosal surfaces, including the mouth, skin, gut, and urogenital tract. GBS and C. albicans are frequently coisolated from the vaginal tract of nonpregnant (44, 193, 194) and pregnant (42, 44, 132, 195–198) women. In a murine model of vaginal colonization, GBS presence in the vaginal tract enhances both fungal burdens and proinflammatory cytokines; however, GBS also suppresses hypha formation through reduced expression of EFG1/Hwp1 and dampens the vaginal mucosal Th17 response (199). In the urinary tract, the presence of C. albicans promotes GBS adherence to the bladder epithelium and GBS bladder persistence in vivo through a mechanism dependent on C. albicans hypha-specific adhesin Als3 (200). Two members of the GBS Bsp adhesion family, BspA and BspC, not only enhance GBS adherence to the vaginal epithelium, but also facilitate C. albicans vaginal adherence, in part through binding Als3 (201, 202) (Fig. 1). Other molecular interactions between GBS and C. albicans include production of a CAMP-like cohemolytic activity in vitro (203). Unlike other Streptococcus spp., GBS does not appear to use Candida mannan as a nutrient source in vitro (204). The mounting clinical evidence supporting GBS and C. albicans co-occurrence and emerging molecular interactions compel continued characterization of the interspecies interactions for these two important human opportunistic pathogens.
ANTAGONISTIC INTERACTIONS WITH GBS AND OTHER MICROBES
GBS and other Streptococcus spp.
The most notable antagonistic interactions that GBS encounters may be with other members of its own species or closely related Streptococcus spp. In the majority of clinical samples, only one GBS strain is present (11), although cocolonization with multiple GBS has been reported (205). In vitro, GBS strains can inhibit growth of other GBS strains or the growth of other beta-hemolytic Streptococcus spp., including group A, C, and G Streptococcus spp. (146), although the mechanism for this inhibition is unknown. Streptococcus salivarius K12 production of salivaricin A and salivaricin B reduces GBS growth in vitro, and S. salivarius administration reduces GBS vaginal persistence in a murine model (206) (Table 2 and Fig. 1). GBS can also inhibit the growth of S. salivarius ATCC 19258, but this growth advantage can be inverted by addition of galacto-oligosaccharide or galactose (207). Aside from influencing growth, GBS can use short hydrophobic peptide (SHP) signaling pheromones to influence biofilm formation in other Streptococcus spp. (208). The impact of this signaling on colonization or pathogenesis is still unknown. Direct interactions may further influence colonization success of either organism. For example, GBS coaggregation with Streptococcus mutans, an oral Streptococcus, has been observed in vitro (209). Understanding how GBS competes with or collaborates with other streptococci is an important facet of developing long-term strategies to modulate GBS colonization at the mucosal surface.
GBS and Bifidobacterium.
As mentioned previously, maternal IAP to prevent GBS transmission to neonates is associated with changes to both the maternal and infant microbiomes. In particular, Bifidobacterium spp. are reduced in infants exposed to IAP (51, 56–59) and this perturbation is proportional to the length of IAP exposure (56). Inverse correlations of Bifidobacterium spp. and GBS have also been observed in the murine gut in mice fed high-amylose maize, suggesting interspecies antagonism between these two organisms may occur in vivo (118). In vitro, GBS growth is inhibited by Bifidobacterium breve and Bifidobacterium longum subsp. longum through secreted products of an unknown type (51) (Table 2). These observations support the development of Bifidobacterium spp. as an appealing probiotic intervention in the neonatal period due to the combined risk of GBS late-onset disease in GBS-colonized infants and the beneficial associations of Bifidobacterium spp. in the infant gut (210).
GBS and Lactobacillus.
GBS and Lactobacillus spp. are often inversely abundant in the human vaginal tract in nonpregnant (9, 211) and pregnant (41, 191) individuals (Table 2). Additionally, GBS and Lactobacillus spp. are inversely associated in the bovine mammary gland, with higher GBS/Lactobacillus ratios observed in more severe forms of subclinical mastitis (212). In an experimental murine model, repeated vaginal administration of L. reuteri reduced GBS vaginal colonization, suggesting a potential causative effect (213). These inverse associations may be in part due to competition for the same geographic niche within the host, depending on the tissue or organ. While Lactobacillus gasseri facilitates GBS association with decidualized human endometrial stromal cells (214), Lactobacillus acidophilus, Lactobacillus paracasei, and L. gasseri reduce GBS adherence to vaginal epithelial cells in vitro (215, 216). Direct antagonism is another potential explanation of GBS and Lactobacillus species inverse correlations. Lactobacillus spp. inhibit GBS growth (214, 217–220) and biofilm formation in vitro (214). Examples of Lactobacillus-produced products with inhibitory activity toward GBS include L. salivarius class IIb bacteriocin salivaricin CRL 1328 (221), an L. acidophilus bacteriocin (222), a bacteriocin-like substance in Lactobacillus fermentum CS57 (218), synergistic activity of bacteriocins from L. fermentum and Lactobacillus rhamnosus (223, 224), biosurfactant production by L. paracasei (225), and Lactobacillus species production of lactic acid since pH neutralization removes GBS inhibitory activity (220, 226, 227) (Fig. 1). Additionally, nonproteinaceous but cell-contact-dependent inhibitory effects have been reported (220). Lactobacillus production of another antimicrobial substance, hydrogen peroxide, does not correlate with GBS inhibition (227). Indeed, GBS appears equipped to neutralize both acid and oxidative stress from Lactobacillus spp., in part through expression of a pH-regulated NRAMP Mn2+/Fe2+ transporter, MntH (228), an NADH peroxidase (229), a superoxide dismutase, SodA (230), and a glutathione synthase (231). One in vitro study found that multiple Lactobacillus spp. could coaggregate with GBS and enhanced GBS binding to mucin (232), while other studies have not noted GBS-Lactobacillus coaggregation (219). Additionally, GBS may counter with its own antimicrobial activity since GBS inhibition of Lactobacillus growth has been reported in vitro (146, 217). The robust clinical and experimental evidence supporting Lactobacillus species and GBS antagonism and the importance of Lactobacillus in supporting a healthy vaginal mucosa compel the continuing search for and refinement of Lactobacillus-based approaches to control GBS vaginal colonization.
PERSPECTIVES ON THERAPEUTIC DEVELOPMENT
GBS-microbe interactions in susceptible hosts.
While frequently isolated from the healthy adult gastrointestinal or vaginal tract in 1 in 5 individuals, the vast majority of GBS invasive disease is borne by specific subsets of the human population, including newborns, pregnant women, the elderly, and immune-comprised individuals, such as those with diabetes. While it is paramount to understand GBS-microbe interactions in those most at risk for GBS invasive disease, there is a paucity of clinical and experimental data from these susceptible populations. Clinical data suggest that GBS blooms in the neonatal gut prior to GBS LOD (64), but the dynamics of the gut microbiota during this process are unknown. Coinciding with the susceptibility window to GBS LOD, the neonatal gut microbiota displays lower α diversity during the first few months of life than at older ages (233). On the other side of the human life span, increased GBS colonization in elderly individuals has been observed in some studies (234), but not others (235–237). Elderly populations show higher incidence of GBS invasive disease and all-cause mortality following GBS infection than younger groups (5, 237–241). Changes to the aging gut microbiota vary across cohorts, which may be attributed in part to subject demographics, comorbidities/medications, and living conditions, but tend to include increased α diversity with age, increased relative abundance of Akkermansia and Escherichia, and decreased relative abundance of Faecalibacterium, Prevotella, and Bacteroides (242). It is currently unknown whether these changes to the elderly microbiota create a more favorable environment for GBS colonization or invasion of host tissues. Other age-associated conditions may be aggravated by GBS colonization. In a study of postmenopausal women, CST IV-A, a CST with enriched GBS colonization, is associated with mild to moderate vulvovaginal atrophy, although no individual taxa were associated with clinical findings in this study (243).
Diabetes mellitus is consistently identified as a risk factor for GBS invasive disease in nonpregnant adults (4, 67, 139, 237–239, 244–250) and pregnant adults (251), and significantly increased rates of GBS rectovaginal colonization in diabetic individuals have been observed in some studies (251–259), but not others (234, 260–269). Clinical studies demonstrate alterations to the gastrointestinal microbiota in diabetic individuals, which include increased relative abundance of Ruminococcus, Fusobacterium, and Blautia and decreased relative abundance of Bifidobacterium and Bacteroides (270). Vaginal microbiome studies, although rarer, report differences in pregnant diabetic individuals, including altered α and β diversity and differential abundance of taxa, but findings were not consistent between cohorts (271, 272). There have been few experimental model studies on GBS in diabetic hosts to date, including GBS invasive disease (119, 273–275), urinary tract infection (276), or diabetic wound infection (277), but it is not yet clear whether diabetic-induced perturbation of the host microbiota facilitates enhanced susceptibility to GBS colonization and infection. To develop tailored therapies for the most susceptible populations, it is important to consider host immune and metabolic factors shaping the microbiota and influencing colonization by opportunistic pathogens, including GBS.
Emerging and future therapeutic avenues.
Due to the abundance of in vitro observations supporting Lactobacillus species inhibition of GBS growth, many clinical studies examining probiotics to control GBS have incorporated Lactobacillus spp. Unfortunately, most studies to date report null or mixed results and many are underpowered pilot studies. Four studies have evaluated an oral probiotic combination of L. rhamnosus GR-1 and L. reuteri RC-14 intervention on GBS colonization during pregnancy. Ho et al. observed a significant decrease of GBS colonization in the intervention group compared to the placebo group (278) (Table 2). Although no differences in GBS colonization prevalence were observed in the second study by Liu et al., potentially beneficial effects such as reduced antibiotic use, reduced incidence of premature rupture of membranes, and decreased GBS abundances by sequencing were reported in the probiotic intervention group compared to the nonintervention group (279). In the third and fourth studies, no differences between the intervention and placebo groups were observed and additional challenges in enrollment and compliance were reported (280, 281). Several other probiotic formulations have been tested, but the routes and lengths of probiotic administration vary across studies. Oral administration of Florajen3 (L. acidophilus, Bifidobacterium lactis, and Bifidobacterium longum) from week 28 of pregnancy to labor onset did not impact GBS clearance in pregnant women in two separate cohorts (282, 283). Oral administration of L. salivarius CECT 9145 from weeks 26 to 38 of pregnancy significantly increased GBS clearance from rectal and vaginal samples from GBS+ women compared to placebo-treated GBS+ women, and reduced GBS burdens over time were observed in women who remained colonized during probiotic treatment (284). A clinical trial with L. rhamnosus HN001 has listed maternal GBS colonization as a secondary outcome; however, results related to GBS colonization from this cohort have not yet been reported (285). A proprietary Enterococcus faecium-based probiotic tested in pregnant women resulted in a 6% decreased GBS colonization rate (286). Local vaginal application of L. plantarum did not reduce GBS vaginal colonization in a placebo-controlled, double-blind study of nonpregnant women (9). Finally, in a study testing the combination of Lactobacillus jensenii, L. crispatus, L. rhamnosus, and L. gasseri as a twice-daily oral probiotic for 14 days beginning at 33 to 37 weeks of pregnancy, no differences in GBS colonization rates were observed between intervention and placebo groups (287). A clinical trial investigating the combination of L. rhamnosus, L. reuteri, and S. salivarius K12 on GBS colonization at delivery as a primary outcome is actively recruiting (288). Bacteriophages or their products are also of interest as a targeted therapy to control GBS colonization and/or disease. Bacteriophage lysins display in vitro lytic activity toward GBS, and administration reduces GBS vaginal colonization and lethality in vivo (89, 289–294). Maternal GBS colonization rates vary across regions (6, 7), as do burdens of invasive GBS disease (295, 296). While the contribution of GBS-microbe dynamics to regional variation is currently unknown, this is certainly a point to consider for microbe-based GBS therapy development and implementation.
Another active area of probiotic research for GBS is in the context of preventing invasive disease in the aquaculture setting. One study found that probiotic feeding of E. faecium reduced GBS mortality in Nile tilapia, but this effect appears to be independent of the microbiota since E. faecium treatment did not alter the fish gut microbiome profile (297) (Table 2). Another probiotic study found treatment with either L. rhamnosus, Lactococcus lactis subsp. lactis, or a combination of the two protected against GBS lethal infection and correlated with an increased intestinal abundance of Proteobacteria, including Escherichia-Shigella spp. and Achromobacter spp. (298). Several other studies have identified probiotics that reduce GBS mortality and modulate the host microbiota in tilapia: Li et al. found that dietary Clostridium butyricum was accompanied by decreased abundance of Cetobacterium spp. and an increase in Bacillis spp. (299), and Xia et al. found that treatment with Bacillus cereus was accompanied by a decrease in abundance of Cetobacterium and an increase in Rhizobium (300). Prebiotic approaches may also have potential impacts on GBS colonization or infection. Feeding woody forages to tilapia encouraged the growth of several organisms, including Bacillus spp., which demonstrate antagonistic activity toward GBS in vitro (301). In coculture studies with GBS and S. salivarius, addition of either galacto-oligosaccharide or galactose inhibited GBS growth (207). Based on the recent surge in probiotic and prebiotic interest in preventing GBS invasive disease in humans and animals, widespread use of microbe-based therapies for GBS may be on the near horizon.
CONCLUSIONS
The diversity of environments that GBS adapts to, from the nares of a camel (92) to the mammary gland of a dairy cow (102), from the maternal vaginal tract (6) to the gut of an infant (64), all of which have distinct resident microbes, implies that GBS has developed numerous microbial interactions that are either synergistic or antagonistic in nature. One major challenge in advancing our understanding of these interspecies interactions is, although there are many clinical studies reporting incidence of GBS and other pathobionts, more often than not, co-occurrences of GBS and these other organisms within subjects are not reported. Future GBS research, in both clinical and experimental settings, should endeavor to address the other microbes present or absent at the host site of interest across the full range of susceptible populations from the newborn to the elderly. Incorporation of new experimental models to study GBS interactions with complex microbial communities in vitro (107, 302) or in humanized microbiota animal models in vivo (303) may provide a clearer picture of GBS-microbe interactions with translational value. Several recent discoveries provide opportunities to describe GBS-microbe-host interactions with further mechanistic insight, including identification of a GBS type 7 secretion system and its heterogeneous potential toxin and immunity effectors (304, 305) and identification of GBS regulatory factors, such as the two-component system SaeRS, that sense the host environment to drive transcriptional adaptions (306). Finally, recognition of the ability of GBS to acquire nutrients within the host environment, such as degradation of physiologically relevant carbohydrates, including glycogen (307, 308) or fructose (309), provides a starting point for understanding GBS metabolism within the host and in competition with other microbes. If prebiotic/probiotic-based therapies are going to be perfected and implemented, we need to identify microbes that can outfight, outbind, and/or outeat GBS within the host niche.
ACKNOWLEDGMENTS
We thank Lamisha Shah for helpful discussions with generating the manuscript outline.
M.E.M. was supported by an NIH T32 award (GM136554) and an NIH F31 award (AI167538). Research was supported through a Burroughs Wellcome Fund Next Gen Pregnancy Initiative (NGP10103) award and an NIH R01 award (DK128053) and R21 award (AI169231) to K.A.P. The funders had no role in the preparation of or the decision to submit the work for publication.
Biographies

Marlyd E. Mejia grew up in Southern California, where she received multiple AS degrees from Moorpark College and her B.S. in Immunology and Microbiology from the University of California Irvine. Her research experience ranges from captive-rearing endangered butterflies, to creating repositories of photometric star data, to assessing cross-feeding interactions between microbes contributing to cystic fibrosis pathologies under the mentorship of Dr. Jana Johnson, Dr. Farisa Morales, and Dr. Katrine Whiteson, respectively. In 2019, she began her doctoral training at Baylor College of Medicine. Now a Ph.D. candidate in the lab of Dr. Katy Patras, her work focuses on developing translationally relevant mouse models for research on the reproductive microbiome. She hopes to understand the role vaginal microbes play during asymptomatic pathogen colonization (specifically that of group B Streptococcus) via direct microbe-microbe interactions or indirect host-microbe interactions mediated by endogenous microbiota priming the immune system.

Clare M. Robertson is a Ph.D. student in the laboratory of Dr. Katy Patras at Baylor College of Medicine. She received her B.S. in Biological Sciences at The University of Alabama. As an undergraduate researcher in the laboratory of Dr. Ryan Earley, she studied the genetics and evolution of life history traits in mangrove rivulus fish. As a research technician in the laboratory of Dr. Robert Britton at Baylor College of Medicine, she focused on growing previously uncultivated microbes from the human gut and studied the impact of fructose sugar on infant gut microbiota. In the Patras lab, she focuses on group B Streptococcus pathogenicity and ecology in the context of the vaginal microbiota.

Kathryn A. Patras grew up in the Midwest, where she received a B.S. in Animal Science from the University of Nebraska—Lincoln while working under Dr. Jennifer Wood. She completed her Ph.D. at San Diego State University with Dr. Kelly Doran and postdoctoral fellowship at University of California San Diego with Dr. Victor Nizet. In 2020, she joined Baylor College of Medicine as an Assistant Professor with appointments in Molecular Virology and Microbiology and the Alkek Center of Metagenomics and Microbiome Research. The goal of her research program is to understand how the immune system and the microbiota interact within the female urogenital tract. Her group uses newly developed models to study why individuals with certain conditions, such as pregnancy or diabetes, are more susceptible to urogenital infection. These studies seek to characterize the functional role of the female urogenital microbiota with ultimate application to both disease pathogenesis and overall women’s health.
Contributor Information
Kathryn A. Patras, Email: katy.patras@bcm.edu.
Karen M. Ottemann, University of California at Santa Cruz Department of Microbiology and Environmental Toxicology
REFERENCES
- 1.Patras KA, Nizet V. 2018. Group B streptococcal maternal colonization and neonatal disease: molecular mechanisms and preventative approaches. Front Pediatr 6:27. 10.3389/fped.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotiw M, Zhang GW, Daggard G, Reiss-Levy E, Tapsall JW, Numa A. 2003. Late-onset and recurrent neonatal group B streptococcal disease associated with breast-milk transmission. Pediatr Dev Pathol 6:251–256. 10.1007/s10024-001-0276-y. [DOI] [PubMed] [Google Scholar]
- 3.Kaczorowski L, Powierska-Czarny J, Wolko L, Piotrowska-Cyplik A, Cyplik P, Czarny J. 2022. The influence of bacteria causing subclinical mastitis on the structure of the cow's milk microbiome. Molecules 27:1829. 10.3390/molecules27061829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jump RLP, Wilson BM, Baechle D, Briggs JM, Banks RE, Song S, Zappernick T, Perez F. 2019. Risk factors and mortality rates associated with invasive group B Streptococcus infections among patients in the US Veterans Health Administration. JAMA Netw Open 2:e1918324. 10.1001/jamanetworkopen.2019.18324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro-Torne A, Curcio D, Moisi JC, Jodar L. 2021. Burden of invasive group B Streptococcus disease in non-pregnant adults: a systematic review and meta-analysis. PLoS One 16:e0258030. 10.1371/journal.pone.0258030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, Tam WH, Madhi SA. 2016. Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis 16:1076–1084. 10.1016/S1473-3099(16)30055-X. [DOI] [PubMed] [Google Scholar]
- 7.Russell NJ, Seale AC, O'Driscoll M, O'Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, Lawn JE, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Le Doare K, Madhi SA, Rubens CE, Schrag S, Sobanjo-Ter Meulen A, Vekemans J, Saha SK, Ip M, GBS Maternal Colonization Investigator Group . 2017. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 65:S100–S111. 10.1093/cid/cix658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kassel MN, Janssen S, Kofman S, Brouwer MC, van de Beek D, Bijlsma MW. 2021. Prevalence of group B streptococcal colonization in the healthy non-pregnant population: a systematic review and meta-analysis. Clin Microbiol Infect 27:968–980. 10.1016/j.cmi.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Ronnqvist PD, Forsgren-Brusk UB, Grahn-Hakansson EE. 2006. Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet Gynecol Scand 85:726–735. 10.1080/00016340600578357. [DOI] [PubMed] [Google Scholar]
- 10.Hansen SM, Uldbjerg N, Kilian M, Sorensen UB. 2004. Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants. J Clin Microbiol 42:83–89. 10.1128/JCM.42.1.83-89.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brzychczy-Wloch M, Pabian W, Majewska E, Zuk MG, Kielbik J, Gosiewski T, Bulanda MG. 2014. Dynamics of colonization with group B streptococci in relation to normal flora in women during subsequent trimesters of pregnancy. New Microbiol 37:307–319. [PubMed] [Google Scholar]
- 12.Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA. 2014. Serotype-specific acquisition and loss of group B streptococcus recto-vaginal colonization in late pregnancy. PLoS One 9:e98778. 10.1371/journal.pone.0098778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuchat A. 1998. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev 11:497–513. 10.1128/CMR.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, Mohle-Boetani J, Gershman K, Schaffner W, Petit S, Zansky SM, Morin CA, Spina NL, Wymore K, Harrison LH, Shutt KA, Bareta J, Bulens SN, Zell ER, Schuchat A, Schrag SJ. 2009. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med 360:2626–2636. 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 15.Nanduri SA, Petit S, Smelser C, Apostol M, Alden NB, Harrison LH, Lynfield R, Vagnone PS, Burzlaff K, Spina NL, Dufort EM, Schaffner W, Thomas AR, Farley MM, Jain JH, Pondo T, McGee L, Beall BW, Schrag SJ. 2019. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr 173:224–233. 10.1001/jamapediatrics.2018.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berardi A, Rossi C, Lugli L, Creti R, Bacchi Reggiani ML, Lanari M, Memo L, Pedna MF, Venturelli C, Perrone E, Ciccia M, Tridapalli E, Piepoli M, Contiero R, Ferrari F, GBS Prevention Working Group, Emilia-Romagna . 2013. Group B streptococcus late-onset disease: 2003–2010. Pediatrics 131:e361–e368. 10.1542/peds.2012-1231. [DOI] [PubMed] [Google Scholar]
- 17.Slotved HC, Hoffmann S. 2020. The epidemiology of invasive group B Streptococcus in Denmark from 2005 to 2018. Front Public Health 8:40. 10.3389/fpubh.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma A, Thompson LA, Corsiatto T, Hurteau D, Tyrrell GJ. 2021. Epidemiological characterization of group B Streptococcus infections in Alberta, Canada: an update from 2014 to 2020. Microbiol Spectr 9:e01283-21. 10.1128/Spectrum.01283-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergadi E, Manoura A, Chatzakis E, Karavitakis E, Maraki S, Galanakis E. 2018. Changes in the incidence and epidemiology of neonatal group B streptococcal disease over the last two decades in Crete, Greece. Infect Dis Rep 10:7744. 10.4081/idr.2018.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata M, Matsubara K, Matsunami K, Miyairi I, Kasai M, Kai M, Katayama Y, Maruyama T, Le Doare K. 2022. Epidemiology of group B streptococcal disease in infants younger than 1 year in Japan: a nationwide surveillance study 2016–2020. Eur J Clin Microbiol Infect Dis 41:559–571. 10.1007/s10096-021-04396-y. [DOI] [PubMed] [Google Scholar]
- 21.Berardi A, Rossi C, Creti R, China M, Gherardi G, Venturelli C, Rumpianesi F, Ferrari F. 2013. Group B streptococcal colonization in 160 mother-baby pairs: a prospective cohort study. J Pediatr 163:1099–1104.e1. 10.1016/j.jpeds.2013.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Berardi A, Rossi C, Guidotti I, Vellani G, Lugli L, Bacchi Reggiani ML, Ferrari F, Facchinetti F, Ferrari F. 2014. Factors associated with intrapartum transmission of group B Streptococcus. Pediatr Infect Dis J 33:1211–1215. 10.1097/INF.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 23.Li A, Fang M, Hao D, Wu Q, Qian Y, Xu H, Zhu B. 2022. Late-onset sepsis in a premature infant mediated by breast milk: mother-to-infant transmission of group B Streptococcus detected by whole-genome sequencing. Infect Drug Resist 15:5345–5352. 10.2147/IDR.S381466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning SD, Neighbors K, Tallman PA, Gillespie B, Marrs CF, Borchardt SM, Baker CJ, Pearlman MD, Foxman B. 2004. Prevalence of group B streptococcus colonization and potential for transmission by casual contact in healthy young men and women. Clin Infect Dis 39:380–388. 10.1086/422321. [DOI] [PubMed] [Google Scholar]
- 25.Manning SD, Tallman P, Baker CJ, Gillespie B, Marrs CF, Foxman B. 2002. Determinants of co-colonization with group B streptococcus among heterosexual college couples. Epidemiology 13:533–539. 10.1097/00001648-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Skarbye AP, Krogh MA, Denwood M, Bjerring M, Ostergaard S. 2021. Effect of enhanced hygiene on transmission of Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus dysgalactiae in dairy herds with automatic milking systems. J Dairy Sci 104:7195–7209. 10.3168/jds.2020-19635. [DOI] [PubMed] [Google Scholar]
- 27.Barsi F, Carra E, Ricchi M, Gnali G, Pisoni G, Russo S, Filippi A, Arrigoni N, Zadoks RN, Garbarino C. 2022. Circulation of Streptococcus agalactiae ST103 in a free stall Italian dairy farm. Appl Environ Microbiol 88:e00383-22. 10.1128/aem.00383-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowater RO, Forbes-Faulkner J, Anderson IG, Condon K, Robinson B, Kong F, Gilbert GL, Reynolds A, Hyland S, McPherson G, Brien JO, Blyde D. 2012. Natural outbreak of Streptococcus agalactiae (GBS) infection in wild giant Queensland grouper, Epinephelus lanceolatus (Bloch), and other wild fish in northern Queensland, Australia. J Fish Dis 35:173–186. 10.1111/j.1365-2761.2011.01332.x. [DOI] [PubMed] [Google Scholar]
- 29.Jafar QA, Sameer AZ, Salwa AM, Samee AA, Ahmed AM, Al-Sharifi F. 2008. Molecular investigation of Streptococcus agalactiae isolates from environmental samples and fish specimens during a massive fish kill in Kuwait Bay. Pak J Biol Sci 11:2500–2504. 10.3923/pjbs.2008.2500.2504. [DOI] [PubMed] [Google Scholar]
- 30.Amal MN, Zamri-Saad M, Siti-Zahrah A, Zulkafli AR. 2013. Transmission of Streptococcus agalactiae from a hatchery into a newly established red hybrid tilapia, Oreochromis niloticus (L.) × Oreochromis mossambicus (Peters), farm. J Fish Dis 36:735–739. 10.1111/jfd.12056. [DOI] [PubMed] [Google Scholar]
- 31.France MT, Ma B, Gajer P, Brown S, Humphrys MS, Holm JB, Waetjen LE, Brotman RM, Ravel J. 2020. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8:166. 10.1186/s40168-020-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen GH, Randis TM, Desai PV, Sapra KJ, Ma B, Gajer P, Humphrys MS, Ravel J, Gelber SE, Ratner AJ. 2017. Group B Streptococcus and the vaginal microbiota. J Infect Dis 216:744–751. 10.1093/infdis/jix395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu X, Zhao C, Yang J, Wei X, Zhang J, Liang C, Gai Z, Zhang C, Zhu D, Wang Y, Zhang L. 2019. Group B Streptococcus colonization induces Prevotella and Megasphaera abundance-featured vaginal microbiome compositional change in non-pregnant women. PeerJ 7:e7474. 10.7717/peerj.7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honig E, Mouton JW, van der Meijden WI. 2002. The epidemiology of vaginal colonisation with group B streptococci in a sexually transmitted disease clinic. Eur J Obstet Gynecol Reprod Biol 105:177–180. 10.1016/S0301-2115(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 35.Chiu SF, Huang PJ, Cheng WH, Huang CY, Chu LJ, Lee CC, Lin HC, Chen LC, Lin WN, Tsao CH, Tang P, Yeh YM, Huang KY. 2021. Vaginal microbiota of the sexually transmitted infections caused by Chlamydia trachomatis and Trichomonas vaginalis in women with vaginitis in Taiwan. Microorganisms 9:1864. 10.3390/microorganisms9091864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Zhai Q, Wang J, Ma X, Xing B, Fan H, Gao Z, Zhao F, Liu W. 2022. Variation of the vaginal microbiome during and after pregnancy in Chinese women. Genomics Proteomics Bioinformatics 20:322–333. 10.1016/j.gpb.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korza G, Ozols J. 1988. Complete covalent structure of 60-kDa esterase isolated from 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced rabbit liver microsomes. J Biol Chem 263:3486–3495. 10.1016/S0021-9258(18)69097-0. [DOI] [PubMed] [Google Scholar]
- 38.MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, Lehne B, Arulkumaran S, Brown R, Teoh TG, Holmes E, Nicoholson JK, Marchesi JR, Bennett PR. 2015. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 5:8988. 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorsen P, Jensen IP, Jeune B, Ebbesen N, Arpi M, Bremmelgaard A, Moller BR. 1998. Few microorganisms associated with bacterial vaginosis may constitute the pathologic core: a population-based microbiologic study among 3596 pregnant women. Am J Obstet Gynecol 178:580–587. 10.1016/S0002-9378(98)70442-9. [DOI] [PubMed] [Google Scholar]
- 40.Kubota T, Nojima M, Itoh S. 2002. Vaginal bacterial flora of pregnant women colonized with group B streptococcus. J Infect Chemother 8:326–330. 10.1007/s10156-002-0190-X. [DOI] [PubMed] [Google Scholar]
- 41.Altoparlak U, Kadanali A, Kadanali S. 2004. Genital flora in pregnancy and its association with group B streptococcal colonization. Int J Gynaecol Obstet 87:245–246. 10.1016/j.ijgo.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Bayo M, Berlanga M, Agut M. 2002. Vaginal microbiota in healthy pregnant women and prenatal screening of group B streptococci (GBS). Int Microbiol 5:87–90. 10.1007/s10123-002-0064-1. [DOI] [PubMed] [Google Scholar]
- 43.Rocchetti TT, Marconi C, Rall VL, Borges VT, Corrente JE, da Silva MG. 2011. Group B streptococci colonization in pregnant women: risk factors and evaluation of the vaginal flora. Arch Gynecol Obstet 283:717–721. 10.1007/s00404-010-1439-8. [DOI] [PubMed] [Google Scholar]
- 44.Cools P, Jespers V, Hardy L, Crucitti T, Delany-Moretlwe S, Mwaura M, Ndayisaba GF, van de Wijgert JH, Vaneechoutte M. 2016. A multi-country cross-sectional study of vaginal carriage of group B streptococci (GBS) and Escherichia coli in resource-poor settings: prevalences and risk factors. PLoS One 11:e0148052. 10.1371/journal.pone.0148052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rick AM, Aguilar A, Cortes R, Gordillo R, Melgar M, Samayoa-Reyes G, Frank DN, Asturias EJ. 2017. Group B streptococci colonization in pregnant Guatemalan women: prevalence, risk factors, and vaginal microbiome. Open Forum Infect Dis 4:ofx020. 10.1093/ofid/ofx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pace RM, Chu DM, Prince AL, Ma J, Seferovic MD, Aagaard KM. 2021. Complex species and strain ecology of the vaginal microbiome from pregnancy to postpartum and association with preterm birth. Med (N Y) 2:1027–1049. 10.1016/j.medj.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roesch LF, Silveira RC, Corso AL, Dobbler PT, Mai V, Rojas BS, Laureano AM, Procianoy RS. 2017. Diversity and composition of vaginal microbiota of pregnant women at risk for transmitting group B Streptococcus treated with intrapartum penicillin. PLoS One 12:e0169916. 10.1371/journal.pone.0169916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou P, Zhou Y, Liu B, Jin Z, Zhuang X, Dai W, Yang Z, Feng X, Zhou Q, Liu Y, Xu X, Zhang L. 2020. Perinatal antibiotic exposure affects the transmission between maternal and neonatal microbiota and is associated with early-onset sepsis. mSphere 5:e00984-19. 10.1128/mSphere.00984-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li YF, Gong XL, Chen SX, Wang K, Jiang YH. 2021. Deviations in the gut microbiota of neonates affected by maternal group B Streptococcus colonization. BMC Microbiol 21:140. 10.1186/s12866-021-02204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassidy-Bushrow AE, Sitarik A, Levin AM, Lynch SV, Havstad S, Ownby DR, Johnson CC, Wegienka G. 2016. Maternal group B Streptococcus and the infant gut microbiota. J Dev Orig Health Dis 7:45–53. 10.1017/S2040174415001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aloisio I, Mazzola G, Corvaglia LT, Tonti G, Faldella G, Biavati B, Di Gioia D. 2014. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol 98:6051–6060. 10.1007/s00253-014-5712-9. [DOI] [PubMed] [Google Scholar]
- 52.Arboleya S, Sanchez B, Milani C, Duranti S, Solis G, Fernandez N, de los Reyes-Gavilan CG, Ventura M, Margolles A, Gueimonde M. 2015. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr 166:538–544. 10.1016/j.jpeds.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 53.Jaureguy F, Carton M, Panel P, Foucaud P, Butel MJ, Doucet-Populaire F. 2004. Effects of intrapartum penicillin prophylaxis on intestinal bacterial colonization in infants. J Clin Microbiol 42:5184–5188. 10.1128/JCM.42.11.5184-5188.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nogacka A, Salazar N, Suarez M, Milani C, Arboleya S, Solis G, Fernandez N, Alaez L, Hernandez-Barranco AM, de Los Reyes-Gavilan CG, Ventura M, Gueimonde M. 2017. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome 5:93. 10.1186/s40168-017-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazzola G, Murphy K, Ross RP, Di Gioia D, Biavati B, Corvaglia LT, Faldella G, Stanton C. 2016. Early gut microbiota perturbations following intrapartum antibiotic prophylaxis to prevent group B streptococcal disease. PLoS One 11:e0157527. 10.1371/journal.pone.0157527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stearns JC, Simioni J, Gunn E, McDonald H, Holloway AC, Thabane L, Mousseau A, Schertzer JD, Ratcliffe EM, Rossi L, Surette MG, Morrison KM, Hutton EK. 2017. Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Sci Rep 7:16527. 10.1038/s41598-017-16606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corvaglia L, Tonti G, Martini S, Aceti A, Mazzola G, Aloisio I, Di Gioia D, Faldella G. 2016. Influence of intrapartum antibiotic prophylaxis for group B Streptococcus on gut microbiota in the first month of life. J Pediatr Gastroenterol Nutr 62:304–308. 10.1097/MPG.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 58.Imoto N, Kano C, Aoyagi Y, Morita H, Amanuma F, Maruyama H, Nojiri S, Hashiguchi N, Watanabe S. 2021. Administration of beta-lactam antibiotics and delivery method correlate with intestinal abundances of Bifidobacteria and Bacteroides in early infancy, in Japan. Sci Rep 11:6231. 10.1038/s41598-021-85670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aloisio I, Quagliariello A, De Fanti S, Luiselli D, De Filippo C, Albanese D, Corvaglia LT, Faldella G, Di Gioia D. 2016. Evaluation of the effects of intrapartum antibiotic prophylaxis on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariable 16S rDNA regions. Appl Microbiol Biotechnol 100:5537–5546. 10.1007/s00253-016-7410-2. [DOI] [PubMed] [Google Scholar]
- 60.Garcia VR. 2021. Impact of intrapartum antibiotic prophylaxis for group B Streptococcus on the term infant gut microbiome: a state of the science review. J Midwife Womens Health 66:351–359. 10.1111/jmwh.13245. [DOI] [PubMed] [Google Scholar]
- 61.Hsu JF, Chen CL, Lee CC, Lien R, Chu SM, Fu RH, Chiang MC, Yang CY, Lai MY, Wu IH, Yen YS, Chiu CH. 2019. Characterization of group B Streptococcus colonization in full-term and late-preterm neonates in Taiwan. Pediatr Neonatol 60:311–317. 10.1016/j.pedneo.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 62.Filleron A, Lombard F, Jacquot A, Jumas-Bilak E, Rodiere M, Cambonie G, Marchandin H. 2014. Group B streptococci in milk and late neonatal infections: an analysis of cases in the literature. Arch Dis Child Fetal Neonatal Ed 99:F41–F47. 10.1136/archdischild-2013-304362. [DOI] [PubMed] [Google Scholar]
- 63.Kolter J, Henneke P. 2017. Codevelopment of microbiota and innate immunity and the risk for group B streptococcal disease. Front Immunol 8:1497. 10.3389/fimmu.2017.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carl MA, Ndao IM, Springman AC, Manning SD, Johnson JR, Johnston BD, Burnham CA, Weinstock ES, Weinstock GM, Wylie TN, Mitreva M, Abubucker S, Zhou Y, Stevens HJ, Hall-Moore C, Julian S, Shaikh N, Warner BB, Tarr PI. 2014. Sepsis from the gut: the enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin Infect Dis 58:1211–1218. 10.1093/cid/ciu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. 2011. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr 159:720–725. 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Travier L, Alonso M, Andronico A, Hafner L, Disson O, Lledo PM, Cauchemez S, Lecuit M. 2021. Neonatal susceptibility to meningitis results from the immaturity of epithelial barriers and gut microbiota. Cell Rep 35:109319. 10.1016/j.celrep.2021.109319. [DOI] [PubMed] [Google Scholar]
- 67.Farley MM, Harvey RC, Stull T, Smith JD, Schuchat A, Wenger JD, Stephens DS. 1993. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N Engl J Med 328:1807–1811. 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- 68.Civljak R, Lisic M, Begovac J, Maretic T, Planinc D. 2001. Meningitis and endocarditis caused by group B streptococcus in a human immunodeficiency virus (HIV) infected patient. Croat Med J 42:572–575. [PubMed] [Google Scholar]
- 69.Epalza C, Goetghebuer T, Hainaut M, Prayez F, Barlow P, Dediste A, Marchant A, Levy J. 2010. High incidence of invasive group B streptococcal infections in HIV-exposed uninfected infants. Pediatrics 126:e631–e638. 10.1542/peds.2010-0183. [DOI] [PubMed] [Google Scholar]
- 70.Dangor Z, Lala SG, Cutland CL, Koen A, Jose L, Nakwa F, Ramdin T, Fredericks J, Wadula J, Madhi SA. 2015. Burden of invasive group B Streptococcus disease and early neurological sequelae in South African infants. PLoS One 10:e0123014. 10.1371/journal.pone.0123014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cutland CL, Schrag SJ, Thigpen MC, Velaphi SC, Wadula J, Adrian PV, Kuwanda L, Groome MJ, Buchmann E, Madhi SA. 2015. Increased risk for group B Streptococcus sepsis in young infants exposed to HIV, Soweto, South Africa, 2004–2008. Emerg Infect Dis 21:638–645. 10.3201/eid2104.141562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Beitune P, Duarte G, Maffei CM, Quintana SM, De Sa Rosa ESAC, Nogueira AA. 2006. Group B Streptococcus carriers among HIV-1 infected pregnant women: prevalence and risk factors. Eur J Obstet Gynecol Reprod Biol 128:54–58. 10.1016/j.ejogrb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Morgan JA, Hankins ME, Callais NA, Albritton CW, Vanchiere JA, Betcher RE, Lewis DF. 2021. Group B Streptococcus rectovaginal colonization and resistance patterns in HIV-positive compared to HIV-negative pregnant patients. Am J Perinatol 10.1055/s-0041-1739356. [DOI] [PubMed] [Google Scholar]
- 74.Gray KJ, Kafulafula G, Matemba M, Kamdolozi M, Membe G, French N. 2011. Group B Streptococcus and HIV infection in pregnant women, Malawi, 2008–2010. Emerg Infect Dis 17:1932–1935. 10.3201/eid1710.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shah M, Aziz N, Leva N, Cohan D. 2011. Group B Streptococcus colonization by HIV status in pregnant women: prevalence and risk factors. J Womens Health (Larchmt) 20:1737–1741. 10.1089/jwh.2011.2888. [DOI] [PubMed] [Google Scholar]
- 76.Le Doare K, Allen L, Kampmann B, Heath PT, Taylor S, Hesseling AC, Gorringe A, Jones CE. 2015. Anti-group B Streptococcus antibody in infants born to mothers with human immunodeficiency virus (HIV) infection. Vaccine 33:621–627. 10.1016/j.vaccine.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dangor Z, Kwatra G, Izu A, Adrian P, van Niekerk N, Cutland CL, Adam Y, Velaphi S, Lala SG, Madhi SA. 2015. HIV-1 is associated with lower group B Streptococcus capsular and surface-protein IgG antibody levels and reduced transplacental antibody transfer in pregnant women. J Infect Dis 212:453–462. 10.1093/infdis/jiv064. [DOI] [PubMed] [Google Scholar]
- 78.Dzanibe S, Adrian PV, Mlacha SZK, Dangor Z, Kwatra G, Madhi SA. 2017. Reduced transplacental transfer of group B Streptococcus surface protein antibodies in HIV-infected mother-newborn dyads. J Infect Dis 215:415–419. 10.1093/infdis/jiw566. [DOI] [PubMed] [Google Scholar]
- 79.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. 2005. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis 40:1422–1428. 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 80.Barton MS, Spencer H, Johnson DP, Crook TW, Frost PA, Castillo-Galvan R, Creech CB. 2020. Group B Streptococcus meningitis in an infant with respiratory syncytial virus detection. J Pediatr 225:259–262. 10.1016/j.jpeds.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Hosaka Y, Ikeura A, Harada Y, Kuroda K, Hamayasu H, Suzuki T, Yamada K, Kawase Y, Suzuki Y. 2000. Binding of influenza type A viruses to group B Streptococcus and haemagglutination by virus-bound bacteria. J Electron Microsc (Tokyo) 49:765–773. 10.1093/oxfordjournals.jmicro.a023870. [DOI] [PubMed] [Google Scholar]
- 82.Hosaka Y, Kuroda K, Ikeura A, Iwamoto T, Suzuki Y. 1998. Binding of influenza and paramyxoviruses to group B Streptococcus with the terminal sialyl-galactose linkage. J Electron Microsc (Tokyo) 47:169–174. 10.1093/oxfordjournals.jmicro.a023574. [DOI] [PubMed] [Google Scholar]
- 83.Tong J, Fu Y, Wu NH, Rohde M, Meng F, Valentin-Weigand P, Herrler G. 2018. Sialic acid-dependent interaction of group B streptococci with influenza virus-infected cells reveals a novel adherence and invasion mechanism. Cell Microbiol 10.1111/cmi.12818. [DOI] [PubMed] [Google Scholar]
- 84.Jones WT, Menna JH. 1982. Influenza type A virus-mediated adherence of type 1a group B streptococci to mouse tracheal tissue in vivo. Infect Immun 38:791–794. 10.1128/iai.38.2.791-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones WT, Menna JH, Wennerstrom DE. 1983. Lethal synergism induced in mice by influenza type A virus and type Ia group B streptococci. Infect Immun 41:618–623. 10.1128/iai.41.2.618-623.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanford BA, Shelokov A, Ramsay MA. 1978. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J Infect Dis 137:176–181. 10.1093/infdis/137.2.176. [DOI] [PubMed] [Google Scholar]
- 87.van der Mee-Marquet N, Diene SM, Barbera L, Courtier-Martinez L, Lafont L, Ouachee A, Valentin AS, Santos SD, Quentin R, Francois P. 2018. Analysis of the prophages carried by human infecting isolates provides new insight into the evolution of group B Streptococcus species. Clin Microbiol Infect 24:514–521. 10.1016/j.cmi.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 88.Lichvarikova A, Soltys K, Szemes T, Slobodnikova L, Bukovska G, Turna J, Drahovska H. 2020. Characterization of clinical and carrier Streptococcus agalactiae and prophage contribution to the strain variability. Viruses 12:1323. 10.3390/v12111323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Furfaro LL, Payne MS, Chang BJ. 2020. Host range, morphological and genomic characterisation of bacteriophages with activity against clinical Streptococcus agalactiae isolates. PLoS One 15:e0235002. 10.1371/journal.pone.0235002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Renard A, Barbera L, Courtier-Martinez L, Dos Santos S, Valentin AS, Mereghetti L, Quentin R, van der Mee-Marquet NL. 2019. phiD12-like livestock-associated prophages are associated with novel subpopulations of Streptococcus agalactiae infecting neonates. Front Cell Infect Microbiol 9:166. 10.3389/fcimb.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salloum M, van der Mee-Marquet N, Valentin-Domelier AS, Quentin R. 2011. Diversity of prophage DNA regions of Streptococcus agalactiae clonal lineages from adults and neonates with invasive infectious disease. PLoS One 6:e20256. 10.1371/journal.pone.0020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seligsohn D, Crestani C, Gitahi N, Lejon Flodin E, Chenais E, Zadoks RN. 2021. Investigation of extramammary sources of group B Streptococcus reveals its unusual ecology and epidemiology in camels. PLoS One 16:e0252973. 10.1371/journal.pone.0252973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yildirim AO, Lammler C, Weiss R, Kopp P. 2002. Pheno- and genotypic properties of streptococci of serological group B of canine and feline origin. FEMS Microbiol Lett 212:187–192. 10.1111/j.1574-6968.2002.tb11265.x. [DOI] [PubMed] [Google Scholar]
- 94.Evans JJ, Pasnik DJ, Klesius PH, Al-Ablani S. 2006. First report of Streptococcus agalactiae and Lactococcus garvieae from a wild bottlenose dolphin (Tursiops truncatus). J Wildl Dis 42:561–569. 10.7589/0090-3558-42.3.561. [DOI] [PubMed] [Google Scholar]
- 95.Delannoy CM, Crumlish M, Fontaine MC, Pollock J, Foster G, Dagleish MP, Turnbull JF, Zadoks RN. 2013. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol 13:41. 10.1186/1471-2180-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bishop EJ, Shilton C, Benedict S, Kong F, Gilbert GL, Gal D, Godoy D, Spratt BG, Currie BJ. 2007. Necrotizing fasciitis in captive juvenile Crocodylus porosus caused by Streptococcus agalactiae: an outbreak and review of the animal and human literature. Epidemiol Infect 135:1248–1255. 10.1017/S0950268807008515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen SL. 2019. Genomic insights into the distribution and evolution of group B Streptococcus. Front Microbiol 10:1447. 10.3389/fmicb.2019.01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cobo-Angel C, Jaramillo-Jaramillo AS, Lasso-Rojas LM, Aguilar-Marin SB, Sanchez J, Rodriguez-Lecompte JC, Ceballos-Marquez A, Zadoks RN. 2018. Streptococcus agalactiae is not always an obligate intramammary pathogen: molecular epidemiology of GBS from milk, feces and environment in Colombian dairy herds. PLoS One 13:e0208990. 10.1371/journal.pone.0208990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jorgensen HJ, Nordstoga AB, Sviland S, Zadoks RN, Solverod L, Kvitle B, Mork T. 2016. Streptococcus agalactiae in the environment of bovine dairy herds—rewriting the textbooks? Vet Microbiol 184:64–72. 10.1016/j.vetmic.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 100.Keefe GP. 1997. Streptococcus agalactiae mastitis: a review. Can Vet J 38:429–437. [PMC free article] [PubMed] [Google Scholar]
- 101.Zadoks RN, Middleton JR, McDougall S, Katholm J, Schukken YH. 2011. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J Mammary Gland Biol Neoplasia 16:357–372. 10.1007/s10911-011-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomes F, Henriques M. 2016. Control of bovine mastitis: old and recent therapeutic approaches. Curr Microbiol 72:377–382. 10.1007/s00284-015-0958-8. [DOI] [PubMed] [Google Scholar]
- 103.Tong J, Zhang H, Zhang Y, Xiong B, Jiang L. 2019. Microbiome and metabolome analyses of milk from dairy cows with subclinical Streptococcus agalactiae mastitis—potential biomarkers. Front Microbiol 10:2547. 10.3389/fmicb.2019.02547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mian GF, Godoy DT, Leal CA, Yuhara TY, Costa GM, Figueiredo HC. 2009. Aspects of the natural history and virulence of S. agalactiae infection in Nile tilapia. Vet Microbiol 136:180–183. 10.1016/j.vetmic.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 105.Geng Y, Wang KY, Huang XL, Chen DF, Li CW, Ren SY, Liao YT, Zhou ZY, Liu QF, Du ZJ, Lai WM. 2012. Streptococcus agalactiae, an emerging pathogen for cultured ya-fish, Schizothorax prenanti, in China. Transbound Emerg Dis 59:369–375. 10.1111/j.1865-1682.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 106.Li M, Li L, Huang T, Liu Y, Lei A, Ma C, Chen F, Chen M. 2018. Effects of attenuated S. agalactiae strain YM001 on intestinal microbiota of tilapia are recoverable. Front Microbiol 9:3251. 10.3389/fmicb.2018.03251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Melo-Bolivar JF, Ruiz Pardo RY, Hume ME, Nisbet DJ, Rodriguez-Villamizar F, Alzate JF, Junca H, Villamil Diaz LM. 2019. Establishment and characterization of a competitive exclusion bacterial culture derived from Nile tilapia (Oreochromis niloticus) gut microbiomes showing antibacterial activity against pathogenic Streptococcus agalactiae. PLoS One 14:e0215375. 10.1371/journal.pone.0215375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Manning SD, Springman AC, Million AD, Milton NR, McNamara SE, Somsel PA, Bartlett P, Davies HD. 2010. Association of group B Streptococcus colonization and bovine exposure: a prospective cross-sectional cohort study. PLoS One 5:e8795. 10.1371/journal.pone.0008795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cobo-Angel CG, Jaramillo-Jaramillo AS, Palacio-Aguilera M, Jurado-Vargas L, Calvo-Villegas EA, Ospina-Loaiza DA, Rodriguez-Lecompte JC, Sanchez J, Zadoks R, Ceballos-Marquez A. 2019. Potential group B Streptococcus interspecies transmission between cattle and people in Colombian dairy farms. Sci Rep 9:14025. 10.1038/s41598-019-50225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carra E, Russo S, Micheli A, Garbarino C, Ricchi M, Bergamini F, Bassi P, Prosperi A, Piva S, Cricca M, Schiavo R, Merialdi G, Serraino A, Arrigoni N. 2021. Evidence of common isolates of Streptococcus agalactiae in bovines and humans in Emilia Romagna region (Northern Italy). Front Microbiol 12:673126. 10.3389/fmicb.2021.673126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sorensen UBS, Klaas IC, Boes J, Farre M. 2019. The distribution of clones of Streptococcus agalactiae (group B streptococci) among herdspersons and dairy cows demonstrates lack of host specificity for some lineages. Vet Microbiol 235:71–79. 10.1016/j.vetmic.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 112.Chau ML, Chen SL, Yap M, Hartantyo SHP, Chiew PKT, Fernandez CJ, Wong WK, Fong RK, Tan WL, Tan BZY, Ng Y, Aung KT, Mehershahi KS, Goh C, Kang JSL, Barkham T, Leong AOK, Gutierrez RA, Ng LC. 2017. Group B Streptococcus infections caused by improper sourcing and handling of fish for raw consumption, Singapore, 2015–2016. Emerg Infect Dis 23:2002–2010. 10.3201/eid2312.170596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalimuddin S, Chen SL, Lim CTK, Koh TH, Tan TY, Kam M, Wong CW, Mehershahi KS, Chau ML, Ng LC, Tang WY, Badaruddin H, Teo J, Apisarnthanarak A, Suwantarat N, Ip M, Holden MTG, Hsu LY, Barkham T, Singapore Group B Streptococcus Consortium . 2017. 2015 epidemic of severe Streptococcus agalactiae sequence type 283 infections in Singapore associated with the consumption of raw freshwater fish: a detailed analysis of clinical, epidemiological, and bacterial sequencing data. Clin Infect Dis 64:S145–S152. 10.1093/cid/cix021. [DOI] [PubMed] [Google Scholar]
- 114.Foxman B, Gillespie BW, Manning SD, Marrs CF. 2007. Risk factors for group B streptococcal colonization: potential for different transmission systems by capsular type. Ann Epidemiol 17:854–862. 10.1016/j.annepidem.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bisharat N, Crook DW, Leigh J, Harding RM, Ward PN, Coffey TJ, Maiden MC, Peto T, Jones N. 2004. Hyperinvasive neonatal group B streptococcus has arisen from a bovine ancestor. J Clin Microbiol 42:2161–2167. 10.1128/JCM.42.5.2161-2167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Geistfeld JG, Weisbroth SH, Jansen EA, Kumpfmiller D. 1998. Epizootic of group B Streptococcus agalactiae serotype V in DBA/2 mice. Lab Anim Sci 48:29–33. [PubMed] [Google Scholar]
- 117.Schenkman DI, Rahija RJ, Klingenberger KL, Elliott JA, Richter CB. 1994. Outbreak of group B streptococcal meningoencephalitis in athymic mice. Lab Anim Sci 44:639–641. [PubMed] [Google Scholar]
- 118.Chiou WC, Lai WH, Cai YL, Du ML, Lai HM, Chen JC, Huang HC, Liu HK, Huang C. 2022. Gut microbiota-directed intervention with high-amylose maize ameliorates metabolic dysfunction in diet-induced obese mice. Food Funct 13:9481–9495. 10.1039/d2fo01211a. [DOI] [PubMed] [Google Scholar]
- 119.Koga T, Aoki W, Fujii M, Satou K, Ikeda Y. 2017. Spontaneous infection caused by Streptococcus agalactiae in KK-A(y) mice. Comp Med 67:416–419. [PMC free article] [PubMed] [Google Scholar]
- 120.Shuster KA, Hish GA, Selles LA, Chowdhury MA, Wiggins RC, Dysko RC, Bergin IL. 2013. Naturally occurring disseminated group B Streptococcus infections in postnatal rats. Comp Med 63:55–61. [PMC free article] [PubMed] [Google Scholar]
- 121.Bodi Winn C, Bakthavatchalu V, Esmail MY, Feng Y, Dzink-Fox J, Richey L, Perkins SE, Nordberg EK, Fox JG. 2018. Isolation and molecular characterization of group B Streptococcus from laboratory Long-Evans rats (Rattus norvegicus) with and without invasive group B streptococcal disease. J Med Microbiol 67:97–109. 10.1099/jmm.0.000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vrbanac A, Riestra AM, Coady A, Knight R, Nizet V, Patras KA. 2018. The murine vaginal microbiota and its perturbation by the human pathogen group B Streptococcus. BMC Microbiol 18:197. 10.1186/s12866-018-1341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mejia ME, Ottinger S, Vrbanac A, Babu P, Zulk JJ, Moorshead D, Bode L, Nizet V, Patras KA. 2022. Human milk oligosaccharides reduce murine group B Streptococcus vaginal colonization with minimal impact on the vaginal microbiota. mSphere 7:e00885-21. 10.1128/msphere.00885-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonifacio Andrade E, Lorga I, Roque S, Geraldo R, Mesquita P, Castro R, Simoes-Costa L, Costa M, Faustino A, Ribeiro A, Correia-Neves M, Trieu-Cuot P, Ferreira P. 2022. Maternal vaccination against group B Streptococcus glyceraldehyde-3-phosphate dehydrogenase leads to gut dysbiosis in the offspring. Brain Behav Immun 103:186–201. 10.1016/j.bbi.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 125.Laux C, Peschel A, Krismer B. 2019. Staphylococcus aureus colonization of the human nose and interaction with other microbiome members. Microbiol Spectr 10.1128/microbiolspec.GPP3-0029-2018. [DOI] [PubMed] [Google Scholar]
- 126.Charlier C, Cretenet M, Even S, Le Loir Y. 2009. Interactions between Staphylococcus aureus and lactic acid bacteria: an old story with new perspectives. Int J Food Microbiol 131:30–39. 10.1016/j.ijfoodmicro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 127.Chen KT, Huard RC, Della-Latta P, Saiman L. 2006. Prevalence of methicillin-sensitive and methicillin-resistant Staphylococcus aureus in pregnant women. Obstet Gynecol 108:482–487. 10.1097/01.AOG.0000227964.22439.e3. [DOI] [PubMed] [Google Scholar]
- 128.Ghanim N, Alchyib O, Morrish D, Tompkins D, Julliard K, Visconti E, Hoskins IA. 2011. Maternal-neonatal outcome with Staphylococcus aureus rectovaginal colonization. J Reprod Med 56:421–424. [PubMed] [Google Scholar]
- 129.Andrews WW, Schelonka R, Waites K, Stamm A, Cliver SP, Moser S. 2008. Genital tract methicillin-resistant Staphylococcus aureus: risk of vertical transmission in pregnant women. Obstet Gynecol 111:113–118. 10.1097/01.AOG.0000298344.04916.11. [DOI] [PubMed] [Google Scholar]
- 130.Top KA, Huard RC, Fox Z, Wu F, Whittier S, Della-Latta P, Saiman L, Ratner AJ. 2010. Trends in methicillin-resistant Staphylococcus aureus anovaginal colonization in pregnant women in 2005 versus 2009. J Clin Microbiol 48:3675–3680. 10.1128/JCM.01129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Top KA, Buet A, Whittier S, Ratner AJ, Saiman L. 2012. Predictors of Staphylococcus aureus rectovaginal colonization in pregnant women and risk for maternal and neonatal infections. J Pediatric Infect Dis Soc 1:7–15. 10.1093/jpids/pis001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dechen TC, Sumit K, Ranabir P. 2010. Correlates of vaginal colonization with group B streptococci among pregnant women. J Glob Infect Dis 2:236–241. 10.4103/0974-777X.68536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qi W, Li H, Wang C, Li H, Zhang B, Dong M, Fan A, Han C, Xue F. 2021. Recent advances in presentation, diagnosis and treatment for mixed vaginitis. Front Cell Infect Microbiol 11:759795. 10.3389/fcimb.2021.759795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Donders GGG, Bellen G, Grinceviciene S, Ruban K, Vieira-Baptista P. 2017. Aerobic vaginitis: no longer a stranger. Res Microbiol 168:845–858. 10.1016/j.resmic.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 135.Tomlinson MW, Schmidt NM, Rourke JW, Jr, McDonald J. 2011. Rectovaginal Staphylococcus aureus colonization: is it a neonatal threat? Am J Perinatol 28:673–676. 10.1055/s-0031-1276732. [DOI] [PubMed] [Google Scholar]
- 136.Chen KT, Campbell H, Borrell LN, Huard RC, Saiman L, Della-Latta P. 2007. Predictors and outcomes for pregnant women with vaginal-rectal carriage of community-associated methicillin-resistant Staphylococcus aureus. Am J Perinatol 24:235–240. 10.1055/s-2007-976551. [DOI] [PubMed] [Google Scholar]
- 137.Foster-Nyarko E, Kwambana B, Aderonke O, Ceesay F, Jarju S, Bojang A, McLellan J, Jafali J, Kampmann B, Ota MO, Adetifa I, Antonio M. 2016. Associations between nasopharyngeal carriage of group B Streptococcus and other respiratory pathogens during early infancy. BMC Microbiol 16:97. 10.1186/s12866-016-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Asghar S, Khan JA, Mahmood MS, Arshad MI. 2020. A cross-sectional study of group B Streptococcus-associated sepsis, coinfections, and antibiotic susceptibility profile in neonates in Pakistan. Adv Neonatal Care 20:E59–E69. 10.1097/ANC.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 139.Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T, Schrag SJ. 2009. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin Infect Dis 49:85–92. 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- 140.Vergbese A, Mireault K, Arbeit RD. 1986. Group B streptococcal bacteremia in men. Rev Infect Dis 8:912–917. 10.1093/clinids/8.6.912. [DOI] [PubMed] [Google Scholar]
- 141.Eskandarian N, Neela V, Ismail Z, Puzi SM, Hamat RA, Desa MN, Nordin SA. 2013. Group B streptococcal bacteremia in a major teaching hospital in Malaysia: a case series of eighteen patients. Int J Infect Dis 17:e777–e780. 10.1016/j.ijid.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 142.Huang PY, Lee MH, Yang CC, Leu HS. 2006. Group B streptococcal bacteremia in non-pregnant adults. J Microbiol Immunol Infect 39:237–241. [PubMed] [Google Scholar]
- 143.Banks RE, Wilson BM, Bej T, Briggs JM, Song S, Goto M, Jump RLP, Perez F. 2022. Similar mortality among United States veterans with invasive and noninvasive pneumonia due to group B Streptococcus. Open Forum Infect Dis 9:ofac051. 10.1093/ofid/ofac051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Loubet P, Koumar Y, Lechiche C, Cellier N, Schuldiner S, Kouyoumdjian P, Lavigne JP, Sotto A. 2021. Clinical features and outcome of Streptococcus agalactiae bone and joint infections over a 6-year period in a French university hospital. PLoS One 16:e0248231. 10.1371/journal.pone.0248231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.MacPhee RA, Miller WL, Gloor GB, McCormick JK, Hammond JA, Burton JP, Reid G. 2013. Influence of the vaginal microbiota on toxic shock syndrome toxin 1 production by Staphylococcus aureus. Appl Environ Microbiol 79:1835–1842. 10.1128/AEM.02908-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chaisilwattana P, Monif GR. 1995. In vitro ability of the group B streptococci to inhibit Gram-positive and Gram-variable constituents of the bacterial flora of the female genital tract. Infect Dis Obstet Gynecol 3:91–97. 10.1155/S1064744995000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ballard MB, Mercado-Evans V, Marunde MG, Nwanosike H, Zulk J, Patras KA. 2021. Group B Streptococcus CAMP factor does not contribute to interactions with the vaginal epithelium and is dispensable for vaginal colonization in mice. Microbiol Spectr 9:e01058-21. 10.1128/Spectrum.01058-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Monif GR. 1999. Semiquantitative bacterial observations with group B streptococcal vulvovaginitis. Infect Dis Obstet Gynecol 7:227–229. 10.1155/S1064744999000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tapsall JW, Phillips EA. 1987. Presumptive identification of group B streptococci by rapid detection of CAMP factor and pigment production. Diagn Microbiol Infect Dis 7:225–228. 10.1016/0732-8893(87)90010-1. [DOI] [PubMed] [Google Scholar]
- 150.Hensler ME, Quach D, Hsieh CJ, Doran KS, Nizet V. 2008. CAMP factor is not essential for systemic virulence of group B Streptococcus. Microb Pathog 44:84–88. 10.1016/j.micpath.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li X, Du Z, Tang Z, Wen Q, Cheng Q, Cui Y. 2022. Distribution and drug sensitivity of pathogenic bacteria in diabetic foot ulcer patients with necrotizing fasciitis at a diabetic foot center in China. BMC Infect Dis 22:396. 10.1186/s12879-022-07382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brannon JR, Dunigan TL, Beebout CJ, Ross T, Wiebe MA, Reynolds WS, Hadjifrangiskou M. 2020. Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat Commun 11:2803. 10.1038/s41467-020-16627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martinez de Tejada B, Stan CM, Boulvain M, Renzi G, Francois P, Irion O, Schrenzel J. 2010. Development of a rapid PCR assay for screening of maternal colonization by group B Streptococcus and neonatal invasive Escherichia coli during labor. Gynecol Obstet Invest 70:250–255. 10.1159/000314014. [DOI] [PubMed] [Google Scholar]
- 154.Birgy A, Mariani-Kurkdjian P, Bidet P, Doit C, Genel N, Courroux C, Arlet G, Bingen E. 2013. Characterization of extended-spectrum-beta-lactamase-producing Escherichia coli strains involved in maternal-fetal colonization: prevalence of E. coli ST131. J Clin Microbiol 51:1727–1732. 10.1128/JCM.03255-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barcaite E, Bartusevicius A, Tameliene R, Maleckiene L, Vitkauskiene A, Nadisauskiene R. 2012. Group B streptococcus and Escherichia coli colonization in pregnant women and neonates in Lithuania. Int J Gynaecol Obstet 117:69–73. 10.1016/j.ijgo.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 156.Spaetgens R, DeBella K, Ma D, Robertson S, Mucenski M, Davies HD. 2002. Perinatal antibiotic usage and changes in colonization and resistance rates of group B streptococcus and other pathogens. Obstet Gynecol 100:525–533. 10.1016/s0029-7844(02)02068-9. [DOI] [PubMed] [Google Scholar]
- 157.Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. 2014. Early-onset neonatal sepsis. Clin Microbiol Rev 27:21–47. 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ecker KL, Donohue PK, Kim KS, Shepard JA, Aucott SW. 2013. The impact of group B streptococcus prophylaxis on late-onset neonatal infections. J Perinatol 33:206–211. 10.1038/jp.2012.76. [DOI] [PubMed] [Google Scholar]
- 159.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. 2008. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics 121:689–696. 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 160.Puopolo KM, Eichenwald EC. 2010. No change in the incidence of ampicillin-resistant, neonatal, early-onset sepsis over 18 years. Pediatrics 125:e1031–e1038. 10.1542/peds.2009-1573. [DOI] [PubMed] [Google Scholar]
- 161.Schrag SJ, Hadler JL, Arnold KE, Martell-Cleary P, Reingold A, Schuchat A. 2006. Risk factors for invasive, early-onset Escherichia coli infections in the era of widespread intrapartum antibiotic use. Pediatrics 118:570–576. 10.1542/peds.2005-3083. [DOI] [PubMed] [Google Scholar]
- 162.Meyn LA, Krohn MA, Hillier SL. 2009. Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am J Obstet Gynecol 201:76.e1–76.e7. 10.1016/j.ajog.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sgayer I, Gur T, Glikman D, Rechnitzer H, Bornstein J, Wolf MF. 2020. Routine uterine culture swab during cesarean section and its clinical correlations: a retrospective comparative study. Eur J Obstet Gynecol Reprod Biol 249:42–46. 10.1016/j.ejogrb.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 164.Cohen CR, Gravelle L, Symekher S, Waiyaki P, Stamm WE, Kiehlbauch JA. 2003. Etiology of persistent tubo-ovarian abscess in Nairobi, Kenya. Infect Dis Obstet Gynecol 11:45–51. 10.1155/S1064744903000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pellett Madan R, Dezzutti CS, Rabe L, Hillier SL, Marrazzo J, McGowan I, Richardson BA, Herold BC, Microbicide Trials Network Biomedical Sciences Working Group, MTN 004 Protocol Team . 2015. Soluble immune mediators and vaginal bacteria impact innate genital mucosal antimicrobial activity in young women. Am J Reprod Immunol 74:323–332. 10.1111/aji.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Franza T, Delavenne E, Derre-Bobillot A, Juillard V, Boulay M, Demey E, Vinh J, Lamberet G, Gaudu P. 2016. A partial metabolic pathway enables group B streptococcus to overcome quinone deficiency in a host bacterial community. Mol Microbiol 102:81–91. 10.1111/mmi.13447. [DOI] [PubMed] [Google Scholar]
- 167.Hooton TM, Roberts PL, Cox ME, Stapleton AE. 2013. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med 369:1883–1891. 10.1056/NEJMoa1302186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kline KA, Schwartz DJ, Gilbert NM, Hultgren SJ, Lewis AL. 2012. Immune modulation by group B Streptococcus influences host susceptibility to urinary tract infection by uropathogenic Escherichia coli. Infect Immun 80:4186–4194. 10.1128/IAI.00684-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carlin AF, Lewis AL, Varki A, Nizet V. 2007. Group B streptococcal capsular sialic acids interact with Siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol 189:1231–1237. 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Derrien M, Vaughan EE, Plugge CM, de Vos WM. 2004. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54:1469–1476. 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 171.Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, Anjos T, Marti-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. 2010. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr 104:83–92. 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 172.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Dore J, Cani PD, Clement K. 2016. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65:426–436. 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 173.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragon L, Jacquelot N, Qu B, Ferrere G, Clemenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. 2018. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359:91–97. 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 174.Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, Enrich B, Ciocan D, Macheiner S, Mayr L, Drach M, Moser P, Moschen AR, Perlemuter G, Szabo G, Cassard AM, Tilg H. 2018. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67:891–901. 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 175.Burcham LR, Burcham ZM, Akbari MS, Metcalf JL, Doran KS. 2022. Interrelated effects of zinc deficiency and the microbiome on group B streptococcal vaginal colonization. mSphere 7:e00264-22. 10.1128/msphere.00264-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hagi T, Belzer C. 2021. The interaction of Akkermansia muciniphila with host-derived substances, bacteria and diets. Appl Microbiol Biotechnol 105:4833–4841. 10.1007/s00253-021-11362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Burcham LR, Bath JR, Werlang CA, Lyon LM, Liu N, Evans C, Ribbeck K, Doran KS. 2022. Role of MUC5B during group B streptococcal vaginal colonization. mBio 13:e00039-22. 10.1128/mbio.00039-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108(Suppl 1):4680–4687. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van de Wijgert JH, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, Jespers V. 2014. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One 9:e105998. 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Dore J, MetaHIT Consortium, Antolin M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, et al. 2011. Enterotypes of the human gut microbiome. Nature 473:174–180. 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson KE, Xia Y, Xiang C. 2010. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics 11:488. 10.1186/1471-2164-11-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zozaya-Hinchliffe M, Lillis R, Martin DH, Ferris MJ. 2010. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol 48:1812–1819. 10.1128/JCM.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis 16(Suppl 4):S273–S281. 10.1093/clinids/16.Supplement_4.S273. [DOI] [PubMed] [Google Scholar]
- 184.Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ. 2012. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 160:267–282. 10.1016/j.trsl.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Severgnini M, Morselli S, Camboni T, Ceccarani C, Laghi L, Zagonari S, Patuelli G, Pedna MF, Sambri V, Foschi C, Consolandi C, Marangoni A. 2022. A deep look at the vaginal environment during pregnancy and puerperium. Front Cell Infect Microbiol 12:838405. 10.3389/fcimb.2022.838405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mikamo H, Kawazoe K, Izumi K, Watanabe K, Ueno K, Tamaya T. 1998. Studies on the pathogenicity of anaerobes, especially Prevotella bivia, in a rat pyometra model. Infect Dis Obstet Gynecol 6:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Watanabe H, Norimatsu Y, Ohno Y. 2021. Scrotal abscess in a Japanese patient caused by Prevotella bivia and Streptococcus agalactiae, successfully treated with cefazolin and amoxicillin: a case report. Int Med Case Rep J 14:475–481. 10.2147/IMCRJ.S321547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Janulaitiene M, Paliulyte V, Grinceviciene S, Zakareviciene J, Vladisauskiene A, Marcinkute A, Pleckaityte M. 2017. Prevalence and distribution of Gardnerella vaginalis subgroups in women with and without bacterial vaginosis. BMC Infect Dis 17:394. 10.1186/s12879-017-2501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Harwich MD, Alves JM, Buck GA, Strauss JF, Patterson JL, Oki AT, Girerd PH, Jefferson KK. 2010. Drawing the line between commensal and pathogenic Gardnerella vaginalis through genome analysis and virulence studies. BMC Genomics 11:375. 10.1186/1471-2164-11-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cornejo OE, Hickey RJ, Suzuki H, Forney LJ. 2018. Focusing the diversity of Gardnerella vaginalis through the lens of ecotypes. Evol Appl 11:312–324. 10.1111/eva.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Starc M, Lucovnik M, Vrlic PE, Jeverica S. 2022. Protective effect of Lactobacillus crispatus against vaginal colonization with group B streptococci in the third trimester of pregnancy. Pathogens 11:980. 10.3390/pathogens11090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gilbert NM, Foster LR, Cao B, Yin Y, Mysorekar IU, Lewis AL. 2021. Gardnerella vaginalis promotes group B Streptococcus vaginal colonization, enabling ascending uteroplacental infection in pregnant mice. Am J Obstet Gynecol 224:530.e1–530.e17. 10.1016/j.ajog.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Malek-Jafarian M, Hosseini FS, Ahmadi AR. 2015. Pattern of infection and antibiotic activity among Streptococcus agalactiae isolates from adults in Mashhad, Iran. Rep Biochem Mol Biol 3:89–93. [PMC free article] [PubMed] [Google Scholar]
- 194.Buchta V, Spacek J. 2004. Microbiological findings in patients with recurrent vulvovaginal candidiasis in the Hradec Kralove Faculty Hospital 1995–2002. Ceska Gynekol 69:7–14. (In Czech.) [PubMed] [Google Scholar]
- 195.Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA, Vaginal Infections and Prematurity Study Group . 1998. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am J Obstet Gynecol 178:374–380. 10.1016/S0002-9378(98)80028-8. [DOI] [PubMed] [Google Scholar]
- 196.Regan JA, Klebanoff MA, Nugent RP, Vaginal Infections and Prematurity Study Group . 1991. The epidemiology of group B streptococcal colonization in pregnancy. Obstet Gynecol 77:604–610. [PubMed] [Google Scholar]
- 197.Dilrukshi GN, Kottahachchi J, Dissanayake D, Pathiraja RP, Karunasingha J, Sampath MKA, Vidanage UA, Fernando SSN. 2021. Group B Streptococcus colonisation and their antimicrobial susceptibility among pregnant women attending antenatal clinics in tertiary care hospitals in the Western Province of Sri Lanka. J Obstet Gynaecol 41:1–6. 10.1080/01443615.2020.1716313. [DOI] [PubMed] [Google Scholar]
- 198.Ghaddar N, El Roz A, Ghssein G, Ibrahim JN. 2019. Emergence of vulvovaginal candidiasis among Lebanese pregnant women: prevalence, risk factors, and species distribution. Infect Dis Obstet Gynecol 2019:5016810. 10.1155/2019/5016810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yu XY, Fu F, Kong WN, Xuan QK, Wen DH, Chen XQ, He YM, He LH, Guo J, Zhou AP, Xi YH, Ni LJ, Yao YF, Wu WJ. 2018. Streptococcus agalactiae inhibits Candida albicans hyphal development and diminishes host vaginal mucosal TH17 response. Front Microbiol 9:198. 10.3389/fmicb.2018.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shing SR, Ramos AR, Patras KA, Riestra AM, McCabe S, Nizet V, Coady A. 2020. The fungal pathogen Candida albicans promotes bladder colonization of group B Streptococcus. Front Cell Infect Microbiol 9:437. 10.3389/fcimb.2019.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rego S, Heal TJ, Pidwill GR, Till M, Robson A, Lamont RJ, Sessions RB, Jenkinson HF, Race PR, Nobbs AH. 2016. Structural and functional analysis of cell wall-anchored polypeptide adhesin BspA in Streptococcus agalactiae. J Biol Chem 291:15985–16000. 10.1074/jbc.M116.726562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pidwill GR, Rego S, Jenkinson HF, Lamont RJ, Nobbs AH. 2018. Coassociation between group B Streptococcus and Candida albicans promotes interactions with vaginal epithelium. Infect Immun 86:e00669-17. 10.1128/IAI.00669-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pakshir K, Bordbar M, Zomorodian K, Nouraei H, Khodadadi H. 2017. Evaluation of CAMP-like effect, biofilm formation, and discrimination of Candida africana from vaginal Candida albicans species. J Pathog 2017:7126258. 10.1155/2017/7126258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fultz R, Ticer T, Glover J, Stripe L, Engevik MA. 2022. Select streptococci can degrade Candida mannan to facilitate growth. Appl Environ Microbiol 88:e02237-21. 10.1128/aem.02237-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Khatami A, Randis TM, Tavares L, Gegick M, Suzman E, Ratner AJ. 2019. Vaginal co-colonization with multiple group B Streptococcus serotypes. Vaccine 37:409–411. 10.1016/j.vaccine.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Patras KA, Wescombe PA, Rosler B, Hale JD, Tagg JR, Doran KS. 2015. Streptococcus salivarius K12 limits group B Streptococcus vaginal colonization. Infect Immun 83:3438–3444. 10.1128/IAI.00409-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moore RE, Thomas HC, Manning SD, Gaddy JA, Townsend SD. 2022. Galacto-oligosaccharide supplementation modulates pathogen-commensal competition between Streptococcus agalactiae and Streptococcus salivarius. Chembiochem 23:e202100559. 10.1002/cbic.202100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cook LC, LaSarre B, Federle MJ. 2013. Interspecies communication among commensal and pathogenic streptococci. mBio 4:e00382-13. 10.1128/mBio.00382-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu T, Liu J, Liu J, Yang R, Lu X, He X, Shi W, Guo L. 2020. Interspecies interactions between Streptococcus mutans and Streptococcus agalactiae in vitro. Front Cell Infect Microbiol 10:344. 10.3389/fcimb.2020.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lewis ZT, Mills DA. 2017. Differential establishment of Bifidobacteria in the breastfed infant gut. Nestle Nutr Inst Workshop Ser 88:149–159. 10.1159/000455399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ricci S, De Giorgi S, Lazzeri E, Luddi A, Rossi S, Piomboni P, De Leo V, Pozzi G. 2018. Impact of asymptomatic genital tract infections on in vitro fertilization (IVF) outcome. PLoS One 13:e0207684. 10.1371/journal.pone.0207684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Qiao J, Kwok L, Zhang J, Gao P, Zheng Y, Guo Z, Hou Q, Huo D, Huang W, Zhang H. 2015. Reduction of Lactobacillus in the milks of cows with subclinical mastitis. Benef Microbes 6:485–490. 10.3920/BM2014.0077. [DOI] [PubMed] [Google Scholar]
- 213.De Gregorio PR, Juarez Tomas MS, Leccese Terraf MC, Nader-Macias ME. 2015. Preventive effect of Lactobacillus reuteri CRL1324 on group B Streptococcus vaginal colonization in an experimental mouse model. J Appl Microbiol 118:1034–1047. 10.1111/jam.12739. [DOI] [PubMed] [Google Scholar]
- 214.Shiroda M, Aronoff DM, Gaddy JA, Manning SD. 2020. The impact of Lactobacillus on group B streptococcal interactions with cells of the extraplacental membranes. Microb Pathog 148:104463. 10.1016/j.micpath.2020.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zarate G, Nader-Macias ME. 2006. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett Appl Microbiol 43:174–180. 10.1111/j.1472-765X.2006.01934.x. [DOI] [PubMed] [Google Scholar]
- 216.He Y, Niu X, Wang B, Na R, Xiao B, Yang H. 2020. Evaluation of the inhibitory effects of Lactobacillus gasseri and Lactobacillus crispatus on the adhesion of seven common lower genital tract infection-causing pathogens to vaginal epithelial cells. Front Med (Lausanne) 7:284. 10.3389/fmed.2020.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Strus M, Malinowska M, Heczko PB. 2002. In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis. J Reprod Med 47:41–46. [PubMed] [Google Scholar]
- 218.Sabia C, Anacarso I, Bergonzini A, Gargiulo R, Sarti M, Condo C, Messi P, de Niederhausern S, Iseppi R, Bondi M. 2014. Detection and partial characterization of a bacteriocin-like substance produced by Lactobacillus fermentum CS57 isolated from human vaginal secretions. Anaerobe 26:41–45. 10.1016/j.anaerobe.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 219.Boris S, Suarez JE, Vazquez F, Barbes C. 1998. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun 66:1985–1989. 10.1128/IAI.66.5.1985-1989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marziali G, Foschi C, Parolin C, Vitali B, Marangoni A. 2019. In-vitro effect of vaginal lactobacilli against group B Streptococcus. Microb Pathog 136:103692. 10.1016/j.micpath.2019.103692. [DOI] [PubMed] [Google Scholar]
- 221.Juarez Tomas MS, Saralegui Duhart CI, De Gregorio PR, Vera Pingitore E, Nader-Macias ME. 2011. Urogenital pathogen inhibition and compatibility between vaginal Lactobacillus strains to be considered as probiotic candidates. Eur J Obstet Gynecol Reprod Biol 159:399–406. 10.1016/j.ejogrb.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 222.Gaspar C, Donders GG, Palmeira-de-Oliveira R, Queiroz JA, Tomaz C, Martinez-de-Oliveira J, Palmeira-de-Oliveira A. 2018. Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Expr 8:153. 10.1186/s13568-018-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ruiz FO, Gerbaldo G, Asurmendi P, Pascual LM, Giordano W, Barberis IL. 2009. Antimicrobial activity, inhibition of urogenital pathogens, and synergistic interactions between lactobacillus strains. Curr Microbiol 59:497–501. 10.1007/s00284-009-9465-0. [DOI] [PubMed] [Google Scholar]
- 224.Ruiz FO, Gerbaldo G, Garcia MJ, Giordano W, Pascual L, Barberis IL. 2012. Synergistic effect between two bacteriocin-like inhibitory substances produced by lactobacilli strains with inhibitory activity for Streptococcus agalactiae. Curr Microbiol 64:349–356. 10.1007/s00284-011-0077-0. [DOI] [PubMed] [Google Scholar]
- 225.Gudina EJ, Rocha V, Teixeira JA, Rodrigues LR. 2010. Antimicrobial and antiadhesive properties of a biosurfactant isolated from Lactobacillus paracasei ssp. paracasei A20. Lett Appl Microbiol 50:419–424. 10.1111/j.1472-765X.2010.02818.x. [DOI] [PubMed] [Google Scholar]
- 226.Acikgoz ZC, Gamberzade S, Gocer S, Ceylan P. 2005. Inhibitor effect of vaginal lactobacilli on group B streptococci. Mikrobiyol Bul 39:17–23. (In Turkish.) [PubMed] [Google Scholar]
- 227.De Gregorio PR, Tomas MSJ, Terraf MCL, Nader-Macias MEF. 2014. In vitro and in vivo effects of beneficial vaginal lactobacilli on pathogens responsible for urogenital tract infections. J Med Microbiol 63:685–696. 10.1099/jmm.0.069401-0. [DOI] [PubMed] [Google Scholar]
- 228.Shabayek S, Bauer R, Mauerer S, Mizaikoff B, Spellerberg B. 2016. A streptococcal NRAMP homologue is crucial for the survival of Streptococcus agalactiae under low pH conditions. Mol Microbiol 100:589–606. 10.1111/mmi.13335. [DOI] [PubMed] [Google Scholar]
- 229.Korir ML, Flaherty RA, Rogers LM, Gaddy JA, Aronoff DM, Manning SD. 2018. Investigation of the role that NADH peroxidase plays in oxidative stress survival in group B Streptococcus. Front Microbiol 9:2786. 10.3389/fmicb.2018.02786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Poyart C, Pellegrini E, Gaillot O, Boumaila C, Baptista M, Trieu-Cuot P. 2001. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect Immun 69:5098–5106. 10.1128/IAI.69.8.5098-5106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Walker EA, Port GC, Caparon MG, Janowiak BE. 2019. Glutathione synthesis contributes to virulence of Streptococcus agalactiae in a murine model of sepsis. J Bacteriol 201:e00367-19. 10.1128/JB.00367-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.do Carmo MS, Noronha FM, Arruda MO, Costa EP, Bomfim MR, Monteiro AS, Ferro TA, Fernandes ES, Giron JA, Monteiro-Neto V. 2016. Lactobacillus fermentum ATCC 23271 displays in vitro inhibitory activities against Candida spp. Front Microbiol 7:1722. 10.3389/fmicb.2016.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Roswall J, Olsson LM, Kovatcheva-Datchary P, Nilsson S, Tremaroli V, Simon MC, Kiilerich P, Akrami R, Kramer M, Uhlen M, Gummesson A, Kristiansen K, Dahlgren J, Backhed F. 2021. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 29:765–776.e3. 10.1016/j.chom.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 234.Martins ER, Nascimento do OD, Marques Costa AL, Melo-Cristino J, Ramirez M. 2022. Characteristics of Streptococcus agalactiae colonizing nonpregnant adults support the opportunistic nature of invasive infections. Microbiol Spectr 10:e0108222. 10.1128/spectrum.01082-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Baldan R, Droz S, Casanova C, Knabben L, Huang DJ, Brulisauer C, Kind AB, Krause E, Mauerer S, Spellerberg B, Sendi P. 2021. Group B streptococcal colonization in elderly women. BMC Infect Dis 21:408. 10.1186/s12879-021-06102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Edwards MS, Rench MA, Palazzi DL, Baker CJ. 2005. Group B streptococcal colonization and serotype-specific immunity in healthy elderly persons. Clin Infect Dis 40:352–357. 10.1086/426820. [DOI] [PubMed] [Google Scholar]
- 237.Edwards MS, Baker CJ. 2005. Group B streptococcal infections in elderly adults. Clin Infect Dis 41:839–847. 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- 238.Collin SM, Shetty N, Lamagni T. 2020. Invasive group B Streptococcus infections in adults, England, 2015–2016. Emerg Infect Dis 26:1174–1181. 10.3201/eid2606.191141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Graux E, Hites M, Martiny D, Maillart E, Delforge M, Melin P, Dauby N. 2021. Invasive group B Streptococcus among non-pregnant adults in Brussels-Capital Region, 2005–2019. Eur J Clin Microbiol Infect Dis 40:515–523. 10.1007/s10096-020-04041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ, Active Bacterial Core surveillance/Emerging Infections Program Network . 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065. 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 241.Dahl MS, Tessin I, Trollfors B. 2003. Invasive group B streptococcal infections in Sweden: incidence, predisposing factors and prognosis. Int J Infect Dis 7:113–119. 10.1016/S1201-9712(03)90006-3. [DOI] [PubMed] [Google Scholar]
- 242.Badal VD, Vaccariello ED, Murray ER, Yu KE, Knight R, Jeste DV, Nguyen TT. 2020. The gut microbiome, aging, and longevity: a systematic review. Nutrients 12:3759. 10.3390/nu12123759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, Viscidi RP, Burke AE, Ravel J, Gravitt PE. 2014. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 21:450–458. 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pitts SI, Maruthur NM, Langley GE, Pondo T, Shutt KA, Hollick R, Schrag SJ, Thomas A, Nichols M, Farley M, Watt JP, Miller L, Schaffner W, Holtzman C, Harrison LH. 2018. Obesity, diabetes, and the risk of invasive group B streptococcal disease in nonpregnant adults in the United States. Open Forum Infect Dis 5:ofy030. 10.1093/ofid/ofy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Arias B, Kovacec V, Vigliarolo L, Suarez M, Tersigni C, Lopardo H, Mollerach M, Bonofiglio L. 2022. Epidemiology of invasive infections caused by Streptococcus agalactiae in Argentina. Microb Drug Resist 28:322–329. 10.1089/mdr.2021.0071. [DOI] [PubMed] [Google Scholar]
- 246.Camuset G, Picot S, Jaubert J, Borgherini G, Ferdynus C, Foucher A, Maiza JC, Fels O, Poyart C, Poubeau P, Gerardin P. 2015. Invasive group B streptococcal disease in non-pregnant adults, Reunion Island, 2011. Int J Infect Dis 35:46–50. 10.1016/j.ijid.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 247.Phoompoung P, Pirogard N, Leelaporn A, Angkasekwinai N. 2021. Incidence of invasive group B Streptococcus (iGBS) infections and the factors associated with iGBS mortality in adults during 2013–2017: a retrospective study at Thailand's largest national tertiary referral center. Ann Med 53:715–721. 10.1080/07853890.2021.1930138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Francois Watkins LK, McGee L, Schrag SJ, Beall B, Jain JH, Pondo T, Farley MM, Harrison LH, Zansky SM, Baumbach J, Lynfield R, Snippes Vagnone P, Miller LA, Schaffner W, Thomas AR, Watt JP, Petit S, Langley GE. 2019. Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008–2016. JAMA Intern Med 179:479–488. 10.1001/jamainternmed.2018.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jenkins PJ, Clement ND, Gaston P, Breusch S, Simpson H, Dave J. 2010. Invasive group B streptococcal disease in an orthopaedic unit. J Hosp Infect 76:231–233. 10.1016/j.jhin.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 250.Ballard MS, Schonheyder HC, Knudsen JD, Lyytikainen O, Dryden M, Kennedy KJ, Valiquette L, Pinholt M, Jacobsson G, Laupland KB, International Bacteremia Surveillance Collaborative . 2016. The changing epidemiology of group B streptococcus bloodstream infection: a multi-national population-based assessment. Infect Dis (Lond) 48:386–391. 10.3109/23744235.2015.1131330. [DOI] [PubMed] [Google Scholar]
- 251.Edwards JM, Watson N, Focht C, Wynn C, Todd CA, Walter EB, Heine RP, Swamy GK. 2019. Group B Streptococcus (GBS) colonization and disease among pregnant women: a historical cohort study. Infect Dis Obstet Gynecol 2019:5430493. 10.1155/2019/5430493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ramos E, Gaudier FL, Hearing LR, Del Valle GO, Jenkins S, Briones D. 1997. Group B streptococcus colonization in pregnant diabetic women. Obstet Gynecol 89:257–260. 10.1016/s0029-7844(96)00489-9. [DOI] [PubMed] [Google Scholar]
- 253.Venkatesh KK, Vladutiu CJ, Strauss RA, Thorp JM, Stringer JSA, Stamilio DM, Hughes BL, Dotters-Katz S. 2020. Association between maternal obesity and group B Streptococcus colonization in a national U.S. cohort. J Womens Health (Larchmt) 29:1507–1512. 10.1089/jwh.2019.8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nowakowska M, Jarosz-Chobot P. 2004. Streptococcus group B (GBS)—characteristic, occurrence in children and adolescents with type 1 diabetes mellitus. Pol J Microbiol 53:17–22. [PubMed] [Google Scholar]
- 255.Ji Y, Zhao C, Ma XX, Peppelenbosch MP, Ma Z, Pan Q. 2019. Outcome of a screening program for the prevention of neonatal early-onset group B Streptococcus infection: a population-based cohort study in Inner Mongolia, China. J Med Microbiol 68:803–811. 10.1099/jmm.0.000976. [DOI] [PubMed] [Google Scholar]
- 256.Obata-Yasuoka M, Hamada H, Yoshikawa H. 2012. Impaired glucose tolerance during pregnancy: possible risk factor for vaginal/anorectal colonization by group B Streptococcus. J Obstet Gynaecol Res 38:1233–1233. 10.1111/j.1447-0756.2012.01885.x. [DOI] [PubMed] [Google Scholar]
- 257.Matorras R, Garcia-Perea A, Usandizaga JA, Omenaca F. 1988. Recto-vaginal colonization and urinary tract infection by group B Streptococcus in the pregnant diabetic patient. Acta Obstet Gynecol Scand 67:617–620. 10.3109/00016348809004274. [DOI] [PubMed] [Google Scholar]
- 258.Bey M, Pastorek JG, II, Miller JM, Jr. 1992. Group B streptococcal colonization in the diabetic gravida patient. Am J Perinatol 9:425–427. 10.1055/s-2007-999280. [DOI] [PubMed] [Google Scholar]
- 259.Akpaka PE, Henry K, Thompson R, Unakal C. 2022. Colonization of Streptococcus agalactiae among pregnant patients in Trinidad and Tobago. IJID Reg 3:96–100. 10.1016/j.ijregi.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Piper JM, Georgiou S, Xenakis EM, Langer O. 1999. Group B streptococcus infection rate unchanged by gestational diabetes. Obstet Gynecol 93:292–296. [DOI] [PubMed] [Google Scholar]
- 261.Raimer K, O'Sullivan MJ. 1997. Influence of diabetes on group B Streptococcus colonization in the pregnant patient. J Matern Fetal Med 6:120–123. [DOI] [PubMed] [Google Scholar]
- 262.Siqueira F, Ferreira EM, de Matos Calderon I, Dias A. 2019. Prevalence of colonisation by group B streptococcus in pregnant patients in Taguatinga, Federal District, Brazil: a cross-sectional study. Arch Gynecol Obstet 299:703–711. 10.1007/s00404-019-05040-z. [DOI] [PubMed] [Google Scholar]
- 263.Place K, Rahkonen L, Nupponen I, Kruit H. 2021. Vaginal streptococcus B colonization is not associated with increased infectious morbidity in labor induction. Acta Obstet Gynecol Scand 100:1501–1510. 10.1111/aogs.14154. [DOI] [PubMed] [Google Scholar]
- 264.Gopal Rao G, Hiles S, Bassett P, Lamagni T. 2019. Differential rates of group B streptococcus (GBS) colonisation in pregnant women in a racially diverse area of London, UK: a cross-sectional study. BJOG 126:1347–1353. 10.1111/1471-0528.15648. [DOI] [PubMed] [Google Scholar]
- 265.Lukic A, Napoli A, Santino I, Bianchi P, Nobili F, Ciampittiello G, Nardone MR, Santomauro M, Di Properzio M, Caserta D. 2017. Cervicovaginal bacteria and fungi in pregnant diabetic and non-diabetic women: a multicenter observational cohort study. Eur Rev Med Pharmacol Sci 21:2303–2315. [PubMed] [Google Scholar]
- 266.Najmi N, Jehan I, Sikandar R, Zuberi NF. 2013. Maternal genital tract colonisation by group-B streptococcus: a hospital based study. J Pak Med Assoc 63:1103–1107. [PubMed] [Google Scholar]
- 267.Hadavand S, Ghafoorimehr F, Rajabi L, Davati A, Zafarghandi N. 2015. Frequency of group B streptococcal colonization in pregnant women aged 35–37 weeks in clinical centers of Shahed University, Tehran, Iran. Iran J Pathol 10:120–126. [PMC free article] [PubMed] [Google Scholar]
- 268.Allen U, Nimrod C, Macdonald N, Toye B, Stephens D, Marchessault V. 1999. Relationship between antenatal group B streptococcal vaginal colonization and premature labour. Paediatr Child Health 4:465–469. 10.1093/pch/4.7.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Casey JI, Maturlo S, Albin J, Edberg SC. 1982. Comparison of carriage rates of group B streptococcus in diabetic and nondiabetic persons. Am J Epidemiol 116:704–708. 10.1093/oxfordjournals.aje.a113453. [DOI] [PubMed] [Google Scholar]
- 270.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. 2020. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51:102590. 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang J, Zheng J, Shi W, Du N, Xu X, Zhang Y, Ji P, Zhang F, Jia Z, Wang Y, Zheng Z, Zhang H, Zhao F. 2018. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 67:1614–1625. 10.1136/gutjnl-2018-315988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cortez RV, Taddei CR, Sparvoli LG, Angelo AGS, Padilha M, Mattar R, Daher S. 2019. Microbiome and its relation to gestational diabetes. Endocrine 64:254–264. 10.1007/s12020-018-1813-z. [DOI] [PubMed] [Google Scholar]
- 273.Edwards MS, Fuselier PA. 1983. Enhanced susceptibility of mice with streptozotocin-induced diabetes to type II group B streptococcal infection. Infect Immun 39:580–585. 10.1128/iai.39.2.580-585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Puliti M, Bistoni F, Orefici G, Tissi L. 2006. Exacerbation of group B streptococcal sepsis and arthritis in diabetic mice. Microbes Infect 8:2376–2383. 10.1016/j.micinf.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 275.Alves J, Madureira P, Baltazar MT, Barros L, Oliveira L, Dinis-Oliveira RJ, Andrade EB, Ribeiro A, Vieira LM, Trieu-Cuot P, Duarte JA, Carvalho F, Ferreira P. 2015. A safe and stable neonatal vaccine targeting GAPDH confers protection against group B Streptococcus infections in adult susceptible mice. PLoS One 10:e0144196. 10.1371/journal.pone.0144196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Patras KA, Coady A, Babu P, Shing SR, Ha AD, Rooholfada E, Brandt SL, Geriak M, Gallo RL, Nizet V. 2020. Host cathelicidin exacerbates group B Streptococcus urinary tract infection. mSphere 5:e00932-19. 10.1128/mSphere.00932-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Keogh RA, Haeberle AL, Langouet-Astrie CJ, Kavanaugh JS, Schmidt EP, Moore GD, Horswill AR, Doran KS. 2022. Group B Streptococcus adaptation promotes survival in a hyperinflammatory diabetic wound environment. Sci Adv 8:eadd3221. 10.1126/sciadv.add3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ho M, Chang YY, Chang WC, Lin HC, Wang MH, Lin WC, Chiu TH. 2016. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce group B Streptococcus colonization in pregnant women: a randomized controlled trial. Taiwan J Obstet Gynecol 55:515–518. 10.1016/j.tjog.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 279.Liu Y, Huang Y, Cai W, Li D, Zheng W, Xiao Y, Liu Y, Zhao H, Pan S. 2020. Effect of oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on vaginal Group B Streptococcus colonization and vaginal microbiome in late pregnancy. Nan Fang Yi Ke Da Xue Xue Bao 40:1753–1759. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Olsen P, Williamson M, Traynor V, Georgiou C. 2018. The impact of oral probiotics on vaginal group B Streptococcal colonisation rates in pregnant women: a pilot randomised control study. Women Birth 31:31–37. 10.1016/j.wombi.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 281.Sharpe M, Shah V, Freire-Lizama T, Cates EC, McGrath K, David I, Cowan S, Letkeman J, Stewart-Wilson E. 2021. Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on group B Streptococcus colonization during pregnancy: a midwifery-led double-blind randomized controlled pilot trial. J Matern Fetal Neonatal Med 34:1814–1821. 10.1080/14767058.2019.1650907. [DOI] [PubMed] [Google Scholar]
- 282.Hanson L, Vande Vusse L, Duster M, Warrack S, Safdar N. 2014. Feasibility of oral prenatal probiotics against maternal group B Streptococcus vaginal and rectal colonization. J Obstet Gynecol Neonatal Nurs 43:294–304. 10.1111/1552-6909.12308. [DOI] [PubMed] [Google Scholar]
- 283.Hanson L, VandeVusse L, Forgie M, Malloy E, Singh M, Scherer M, Kleber D, Dixon J, Hryckowian AJ, Safdar N. 2023. A randomized controlled trial of an oral probiotic to reduce antepartum group B Streptococcus colonization and gastrointestinal symptoms. Am J Obstet Gynecol MFM 5:100748. 10.1016/j.ajogmf.2022.100748. [DOI] [PubMed] [Google Scholar]
- 284.Martin V, Cardenas N, Ocana S, Marin M, Arroyo R, Beltran D, Badiola C, Fernandez L, Rodriguez JM. 2019. Rectal and vaginal eradication of Streptococcus agalactiae (GBS) in pregnant women by using Lactobacillus salivarius CECT 9145, a target-specific probiotic strain. Nutrients 11:810. 10.3390/nu11040810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Barthow C, Wickens K, Stanley T, Mitchell EA, Maude R, Abels P, Purdie G, Murphy R, Stone P, Kang J, Hood F, Rowden J, Barnes P, Fitzharris P, Craig J, Slykerman RF, Crane J. 2016. The Probiotics in Pregnancy Study (PiP Study): rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth 16:133. 10.1186/s12884-016-0923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Di Pierro F, Parolari A, Brundu B, Nigro R. 2016. Positive clinical outcomes derived from using a proprietary mixture of selected strains during pregnancy. Acta Biomed 87:259–265. [PMC free article] [PubMed] [Google Scholar]
- 287.Farr A, Sustr V, Kiss H, Rosicky I, Graf A, Makristathis A, Foessleitner P, Petricevic L. 2020. Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Sci Rep 10:19745. 10.1038/s41598-020-76896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.ClinicalTrials.gov. 2022. Oral probiotic supplementation in pregnancy to reduce group B Streptococcus colonization (OPSiP). https://clinicaltrials.gov/ct2/show/NCT03407157. Accessed 1 November 2022.
- 289.Bocanova L, Psenko M, Barak I, Halgasova N, Drahovska H, Bukovska G. 2022. A novel phage-encoded endolysin EN534-C active against clinical strain Streptococcus agalactiae GBS. J Biotechnol 359:48–58. 10.1016/j.jbiotec.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 290.Cheng Q, Nelson D, Zhu S, Fischetti VA. 2005. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother 49:111–117. 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shan Y, Yang N, Teng D, Wang X, Mao R, Hao Y, Ma X, Fan H, Wang J. 2020. Recombinant of the staphylococcal bacteriophage lysin CHAP(k) and its elimination against Streptococcus agalactiae biofilms. Microorganisms 8:216. 10.3390/microorganisms8020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pritchard DG, Dong S, Baker JR, Engler JA. 2004. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology (Reading) 150:2079–2087. 10.1099/mic.0.27063-0. [DOI] [PubMed] [Google Scholar]
- 293.Huang L, Luo D, Gondil VS, Gong Y, Jia M, Yan D, He J, Hu S, Yang H, Wei H. 2020. Construction and characterization of a chimeric lysin ClyV with improved bactericidal activity against Streptococcus agalactiae in vitro and in vivo. Appl Microbiol Biotechnol 104:1609–1619. 10.1007/s00253-019-10325-z. [DOI] [PubMed] [Google Scholar]
- 294.Oechslin F, Daraspe J, Giddey M, Moreillon P, Resch G. 2013. In vitro characterization of PlySK1249, a novel phage lysin, and assessment of its antibacterial activity in a mouse model of Streptococcus agalactiae bacteremia. Antimicrob Agents Chemother 57:6276–6283. 10.1128/AAC.01701-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, Madrid L, Blencowe H, Cousens S, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Ip M, Le Doare K, Madhi SA, Rubens CE, Saha SK, Schrag SJ, Sobanjo-Ter Meulen A, Vekemans J, Lawn JE. 2017. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 65:S200–S219. 10.1093/cid/cix664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gonçalves BP, Procter SR, Paul P, Chandna J, Lewin A, Seedat F, Koukounari A, Dangor Z, Leahy S, Santhanam S, John HB, Bramugy J, Bardají A, Abubakar A, Nasambu C, Libster R, Sánchez Yanotti C, Horváth-Puhó E, Sørensen HT, van de Beek D, Bijlsma MW, Gardner WM, Kassebaum N, Trotter C, Bassat Q, Madhi SA, Lambach P, Jit M, Lawn JE, Søgaard KK, van Kassel MN, Snoek L, de Gier B, van der Ende A, Hahné SJM, Harden LM, Ghoor A, Mbatha S, Lowick S, Laughton B, Jaye T, Lala SG, Sithole P, Msayi J, Kumalo N, Msibi TN, Arumugam A, Murugesan N, Rajendraprasad N, Priya M, et al. 2022. Group B streptococcus infection during pregnancy and infancy: estimates of regional and global burden. Lancet Glob Health 10:e807–e819. 10.1016/S2214-109X(22)00093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Suphoronski SA, de Souza FP, Chideroli RT, Mantovani Favero L, Ferrari NA, Ziemniczak HM, Goncalves DD, Lopera Barrero NM, Pereira UP. 2021. Effect of Enterococcus faecium as a water and/or feed additive on the gut microbiota, hematologic and immunological parameters, and resistance against francisellosis and streptococcosis in Nile tilapia (Oreochromis niloticus). Front Microbiol 12:743957. 10.3389/fmicb.2021.743957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Xia Y, Lu M, Chen G, Cao J, Gao F, Wang M, Liu Z, Zhang D, Zhu H, Yi M. 2018. Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 76:368–379. 10.1016/j.fsi.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 299.Li H, Zhou Y, Ling H, Luo L, Qi D, Feng L. 2019. The effect of dietary supplementation with Clostridium butyricum on the growth performance, immunity, intestinal microbiota and disease resistance of tilapia (Oreochromis niloticus). PLoS One 14:e0223428. 10.1371/journal.pone.0223428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Xia Y, Wang M, Gao F, Lu M, Chen G. 2020. Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim Nutr 6:69–79. 10.1016/j.aninu.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wu F, Chen B, Liu S, Xia X, Gao L, Zhang X, Pan Q. 2020. Effects of woody forages on biodiversity and bioactivity of aerobic culturable gut bacteria of tilapia (Oreochromis niloticus). PLoS One 15:e0235560. 10.1371/journal.pone.0235560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Melo-Bolivar JF, Ruiz Pardo RY, Junca H, Sidjabat HE, Cano-Lozano JA, Villamil Diaz LM. 2022. Competitive exclusion bacterial culture derived from the gut microbiome of Nile tilapia (Oreochromis niloticus) as a resource to efficiently recover probiotic strains: taxonomic, genomic, and functional proof of concept. Microorganisms 10:1376. 10.3390/microorganisms10071376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mejia ME, Mercado-Evans V, Zulk J, Ottinger S, Ruiz K, Ballard MB, Britton RA, Patras KA. 2023. Vaginal microbial dynamics and pathogen colonization in a humanized microbiota mouse model. BioRxiv. https://www.biorxiv.org/content/10.1101/2023.02.09.527909v1. [DOI] [PMC free article] [PubMed]
- 304.Spencer BL, Tak U, Mendonca JC, Nagao PE, Niederweis M, Doran KS. 2021. A type VII secretion system in group B Streptococcus mediates cytotoxicity and virulence. PLoS Pathog 17:e1010121. 10.1371/journal.ppat.1010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Spencer BL, Job AM, Roberston CM, Hameed ZA, Serchejian C, Wiafe-Kwakye CS, Mendonça JC, Apolonio MA, Nagao PE, Neely MN, Korotkova N, Korotkov KV, Patras KA, Doran KS. 2023. Heterogeneity of the group B streptococcal type VII secretion system and influence on colonization of the female genital tract. bioRxiv. https://www.biorxiv.org/content/10.1101/2023.01.25.525443v1. [DOI] [PMC free article] [PubMed]
- 306.Cook LCC, Hu H, Maienschein-Cline M, Federle MJ. 2018. A vaginal tract signal detected by the group B Streptococcus SaeRS system elicits transcriptomic changes and enhances murine colonization. Infect Immun 86:e00762-17. 10.1128/IAI.00762-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Santi I, Pezzicoli A, Bosello M, Berti F, Mariani M, Telford JL, Grandi G, Soriani M. 2008. Functional characterization of a newly identified group B Streptococcus pullulanase eliciting antibodies able to prevent alpha-glucans degradation. PLoS One 3:e3787. 10.1371/journal.pone.0003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Davis GH, Pham AV, Triscott MX. 1982. Polysaccharase activity in Streptococcus agalactiae (group B streptococci). J Gen Microbiol 128:1381–1384. [DOI] [PubMed] [Google Scholar]
- 309.Faralla C, Metruccio MM, De Chiara M, Mu R, Patras KA, Muzzi A, Grandi G, Margarit I, Doran KS, Janulczyk R. 2014. Analysis of two-component systems in group B Streptococcus shows that RgfAC and the novel FspSR modulate virulence and bacterial fitness. mBio 5:e00870-14. 10.1128/mBio.00870-14. [DOI] [PMC free article] [PubMed] [Google Scholar]