ABSTRACT
Five siphoviruses were isolated from soil in southeastern Pennsylvania using Microbacterium foliorum. Bacteriophages NeumannU and Eightball have 25 predicted genes, Chivey and Hiddenleaf have 87 genes, and GaeCeo has 60 genes. Based on gene content similarity to sequenced actinobacteriophages, these five phages are distributed across clusters EA, EE, and EF.
ANNOUNCEMENT
Bacteriophages are an abundant and genetically diverse group of viruses that prey on bacteria (1). Here we report on the isolation and characterization of five bacteriophages isolated using Microbacterium foliorum NRRL B-24224.
All bacteriophages were isolated from soil collected in southeastern Pennsylvania (Table 1), using standard methods (2). Soil samples were suspended in peptone-yeast extract-calcium (PYCa) liquid medium and incubated with shaking at 250 rpm for 2 h at 30°C. The wash was then collected by centrifugation and filtered through a 0.22-μm filter, and the filtrate was plated in PYCa soft agar containing M. foliorum and incubated at 30°C for up to 48 h. Each bacteriophage was purified through three rounds of plating, and plaque morphologies are presented in Table 1. Bacteriophage morphology was determined by negative-staining transmission electron microscopy (TEM), and measurements of the capsids and tails were determined manually for a minimum of three particles. All isolated bacteriophages are siphoviruses (Table 1).
TABLE 1.
Bacteriophage, plaque morphology, and genomic characteristicsa
| Phage name | Soil sample collection site | Isolation yr | Plaque morphology | Plaque sizeb (mm) | Capsid sizec (nm) | Tail lengthc (nm) | Approx shotgun coverage (fold) | Genome length (bp) | Genome end characteristic | G+C content (%) | No. of ORFsd | No. of tRNAs | Cluster |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chivey | Garnet Valley, PA, 39.853972 N, 75.479417 W | 2018 | Clear middle, cloudy haloe | 1–1.5 | 59–77 | 154–181 | 2,383 | 56,082 | Circularly permutated | 63.7 | 84 | 0 | EF |
| Hiddenleaf | Garnet Valley, PA, 39.853972 N, 75.479417 W | 2018 | Small and clear | 2 | 68 | 150–168 | 1,334 | 56,082 | Circularly permutated | 63.7 | 84 | 0 | EF |
| Eightball | Chester, PA, 39.841972 N, 75.389932 W | 2020 | Small and clear | 2 | ND | ND | 5,973 | 17,439 | 3′ single-stranded overhang, 5′-CCCGCCCCA-3′ | 68.7 | 25 | 0 | EE |
| NeumannU | Aston, PA, 39.874167 N, 75.440889 W | 2018 | Small and clear | 3–4 | 41 | 100 | 6,452 | 17,445 | 3′ single-stranded overhang, 5′-CCCGCCCCA-3′ | 68.7 | 25 | 0 | EE |
| GaeCeo | Aston, PA, 39.871102 N, 75.436438 W | 2018 | Small and cloudy | 0.9 | 50–58 | 134–146 | 2,726 | 40,168 | Circularly permutated | 63.4 | 60 | 1 | EAf |
“ND” indicates the TEM was not performed.
Based on the size of a minimum of 3 measured plaques.
Based on the measurements of a minimum of 3 particles from a TEM.
ORFs, open reading frames.
Indicates a clear middle of the plaque with a diffuse or cloudy edge.
Subcluster EA9.
Genomic DNA was isolated from phage lysates using a ZnCl2 precipitation method as previously described (2, 3). The DNA was prepared for sequencing using the NEBNext Ultra II FS kit (New England BioLabs) and sequenced using Illumina MiSeq (v3 reagents), yielding ~200,000 single-end 150-base reads. Untrimmed reads were assembled and then checked for completeness using Newbler v2.9 (4) and Consed v29 (5), respectively (6). Phages were assigned to clusters (Table 1) based on at least 35% gene content similarity to sequences in the actinobacteriophage database, phagesDB (7, 8).
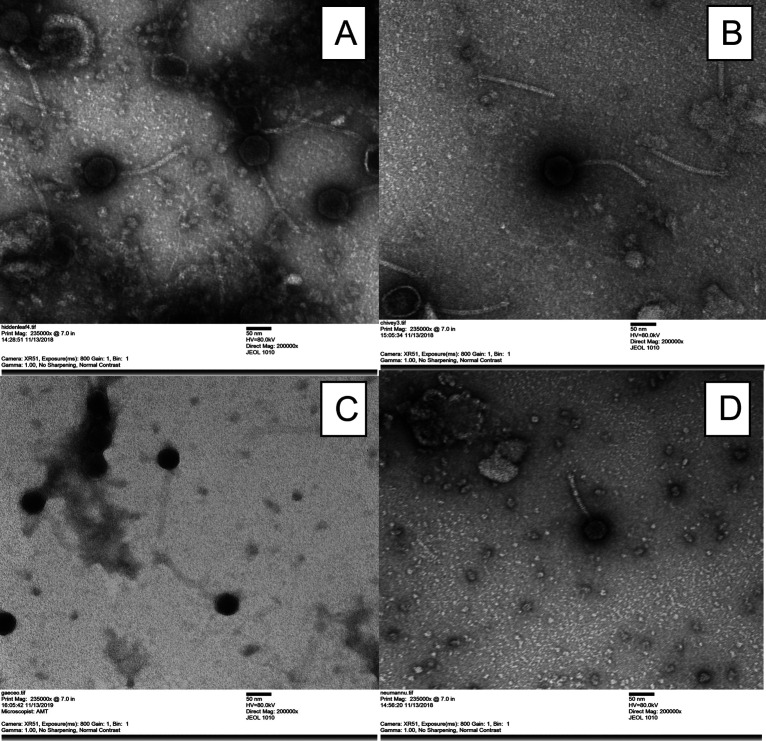
Initial autoannotations of the genome were performed using DNA Master v5.23.6 (http://cobamide2.bio.pitt.edu/computer.htm) embedded with GeneMark v4.28 (9) and Glimmer v3.02b (10) and then refined using Phage Evidence Collection and Annotation Network v20211202 (PECAAN [https://pecaan.kbrinsgd.org/index.html]), Phamerator (11), and Starterator v462 (https://github.com/SEA-PHAGES/starterator). Transmembrane helices were predicted using TMHMM v2.0 (12), DeepTMHMM v1.0.11 (13), TOPCONS v2.0 (https://topcons.cbr.su.se/pred/) (14), and SOSUI v1.11 (15). tRNAs were predicted using ARAGORN v1.2.41 (16) and tRNAscanSE v2.0 (17), and functional assignments were made using BLASTP v2.9 (18) and HHPRED v3.2 (19). All annotations were performed with default parameters. Genome characteristics of each bacteriophage are listed in Table 1, and the bacteriophage morphology is shown in Fig. 1.
FIG 1.
Transmission electron micrographs of bacteriophage morphology. (A) Hiddenleaf, cluster EF; (B) Chivey, cluster EF; (C) GaeCeo, cluster EA9; (D) NeumannU, cluster EE.
NeumannU and Eightball are highly similar, sharing 99.9964% nucleotide identity, and contain 25 predicted genes, of which 22 are transcribed rightwards. The 3 genes that are transcribed leftward (genes 20 to 22) encode DNA-binding proteins. Hiddenleaf and Chivey also share 99.5% nucleotide identity, with all 84 predicted genes transcribed rightwards. GaeCeo has 60 predicted genes, including 1 tRNA tRNAPro, with structure, assembly, and lysis genes occupying the left half of the genome (genes 2 to 27) and transcribed rightwards and DNA metabolism genes (genes 34 to 51) occupying the right half of the genome and transcribed leftwards, with the exception of the rightmost gene (gene 60), which is transcribed rightwards. None of the five phages encode identifiable immunity repressor or integrase functions, and they are therefore likely to be lytic, consistent with the life cycle of other phages in these clusters.
Data availability.
All genomes, NeumannU, Eightball, Chivey, Hiddenleaf, and GaeCeo, are available at GenBank under accession no. MT657332, OK040783, MT684591, MN497954, and MT657343 and Sequence Read Archive (SRA) no. SRX15940725, SRX15940721, SRX15940720, SRX15940723, and SRX15940722, respectively.
ACKNOWLEDGMENTS
We thank Dan Russell and Rebecca Garlena for sequencing and assembly of the bacteriophages in this study and Karen Snetselaar for helping us with the transmission electron microscope work. We also thank Steven Cresawn, Debbie Jacobs-Sera, Graham Hatfull, Viknesh Sivanathan, and other members of the SEA-PHAGES and Howard Hughes Medical Institute for their expertise and support of our research.
Contributor Information
Matthew D. Mastropaolo, Email: mastropm@neumann.edu.
John J. Dennehy, Queens College Department of Biology
REFERENCES
- 1.Pedulla ML, Ford ME, Houtz JM, Karthikeyan T, Wadsworth C, Lewis JA, Jacobs-Sera D, Falbo J, Gross J, Pannunzio NR, Brucker W, Kumar V, Kandasamy J, Keenan L, Bardarov S, Kriakov J, Lawrence JG, Jacobs WR, Hendrix RW, Jr, Hatfull GF. 2003. Origins of highly mosaic mycobacteriophage genomes. Cell 113:171–182. doi: 10.1016/S0092-8674(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 2.Poxleitner M, Pope W, Jacobs-Sera D, Sivanathan V, Hatfull G. 2018. Phage discovery guide. Howard Hughes Medical Institute, Chevy Chase, MD. https://seaphagesphagediscoveryguide.helpdocsonline.com/home. [Google Scholar]
- 3.Santos MA. 1991. An improved method for the small scale preparation of bacteriophage DNA based on phage precipitation by zinc chloride. Nucleic Acids Res 19:5442. doi: 10.1093/nar/19.19.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller JR, Koren S, Sutton G. 2010. Assembly algorithms for next-generation sequencing data. Genomics 95:315–327. doi: 10.1016/j.ygeno.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon D, Green P. 2013. Consed: a graphical editor for next-generation sequencing. Bioinformatics 29:2936–2937. doi: 10.1093/bioinformatics/btt515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell DA. 2018. Sequencing, assembling, and finishing complete bacteriophage genomes. Methods Mol Biol 1681:109–125. doi: 10.1007/978-1-4939-7343-9_9. [DOI] [PubMed] [Google Scholar]
- 7.Russell DA, Hatfull GF. 2017. PhagesDB: the actinobacteriophage database. Bioinformatics 33:784–786. doi: 10.1093/bioinformatics/btw711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pope WH, Mavrich TN, Garlena RA, Guerrero-Bustamante CA, Jacobs-Sera D, Montgomery MT, Russell DA, Warner MH, Hatfull GF, Science Education Alliance-Phage Hunters Advancing Genomics and Evolutionary Science (SEA-PHAGES) . 2017. Bacteriophages of Gordonia spp. display a spectrum of diversity and genetic relationships. mBio 8:e01069-17. doi: 10.1128/mBio.01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besemer J, Borodovsky M. 2005. GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res 33:W451–W454. doi: 10.1093/nar/gki487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delcher AL, Bratke KA, Powers EC, Salzberg SL. 2007. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cresawn SG, Bogel M, Day N, Jacobs-Sera D, Hendrix RW, Hatfull GF. 2011. Phamerator: a bioinformatic tool for comparative bacteriophage genomics. BMC Bioinformatics 12:395. doi: 10.1186/1471-2105-12-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 13.Hallgren J, Tsirigos KD, Pedersen MD, Almagro AJ, Marcatili P, Nielsen H, Krogh A, Winther O. 2022. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv. doi: 10.1101/2022.04.08.487609. [DOI]
- 14.Tsirigos KD, Peters C, Shu N, Käll L, Elofsson A. 2015. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res 43:W401–W407. doi: 10.1093/nar/gkv485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirokawa T, Boon-Chieng S, Mitaku S. 1998. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 16.Laslett D, Canback B. 2004. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res 32:11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res 44:W54–W57. doi: 10.1093/nar/gkw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Söding J, Biegert A, Lupas AN. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 33:W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All genomes, NeumannU, Eightball, Chivey, Hiddenleaf, and GaeCeo, are available at GenBank under accession no. MT657332, OK040783, MT684591, MN497954, and MT657343 and Sequence Read Archive (SRA) no. SRX15940725, SRX15940721, SRX15940720, SRX15940723, and SRX15940722, respectively.