ABSTRACT
Chlamydia trachomatis is an obligate intracellular bacterial pathogen that causes ocular and urogenital infections in humans. The ability of C. trachomatis to grow intracellularly in a pathogen-containing vacuole (known as an inclusion) depends on chlamydial effector proteins transported into the host cell by a type III secretion system. Among these effectors, several inclusion membrane proteins (Incs) insert in the vacuolar membrane. Here, we show that human cell lines infected by a C. trachomatis strain deficient for Inc CT288/CTL0540 (renamed IncM) displayed less multinucleation than when infected by IncM-producing strains (wild type or complemented). This indicated that IncM is involved in the ability of Chlamydia to inhibit host cell cytokinesis. The capacity of IncM to induce multinucleation in infected cells was shown to be conserved among its chlamydial homologues and appeared to require its two larger regions predicted to be exposed to the host cell cytosol. C. trachomatis-infected cells also displayed IncM-dependent defects in centrosome positioning, Golgi distribution around the inclusion, and morphology and stability of the inclusion. The altered morphology of inclusions containing IncM-deficient C. trachomatis was further affected by depolymerization of host cell microtubules. This was not observed after depolymerization of microfilaments, and inclusions containing wild-type C. trachomatis did not alter their morphology upon depolymerization of microtubules. Overall, these findings suggest that IncM may exert its effector function by acting directly or indirectly on host cell microtubules.
KEYWORDS: bacterial pathogenesis, Chlamydia, effectors, Incs, centrosomes
INTRODUCTION
The phylum Chlamydiae comprises obligate intracellular Gram-negative bacteria that parasitize eukaryotic hosts, ranging from single cell amoebae to diverse animals (1). Among Chlamydiae, the Chlamydia genus includes several species that are pathogenic for humans (e.g., C. trachomatis and C. pneumoniae) and other mammals, such as mice (C. muridarum), cats (C. felis), and guinea pigs (C. caviae) (2). The most-studied Chlamydia species is C. trachomatis, the leading cause of preventable blindness (trachoma; caused by serovars A to C) (3) and the most prevalent cause of sexually transmitted bacterial infections (mainly caused by serovars D to K) (4). C. trachomatis serovars L1 to L3 are also sexually transmitted but are less common and cause invasive lymphogranuloma venereum (LGV) (2, 4).
Chlamydiae are characterized by a developmental cycle involving the interconversion between infectious, but nonreplicative, elementary bodies (EBs) and noninfectious, but replicative, reticulate bodies (RBs) (5, 6). EBs drive their entry into host cells, and the internalized chlamydiae reside then within a pathogen-containing vacuole, which is propelled along microtubules toward the centrosome by the minus-end-directed cytoplasmic dynein 1 (dynein) motor protein. Within the vacuole, EBs differentiate into RBs, which then multiply, leading to vacuolar expansion and formation of a large compartment, known as an inclusion, that eventually occupies most of the host cell cytoplasm. The inclusion is surrounded and protected by a meshwork of actin filaments, intermediate filaments, and microtubules (7, 8). Furthermore, as chlamydial infection of host cells progresses, the Golgi complex fragments and distributes into intact ministacks around the inclusion (9). At a certain stage in the cycle, RBs differentiate asynchronously back into EBs. The newly formed infectious EBs ultimately egress either by extrusion of the entire inclusion or after host cell lysis (5).
Completion of the developmental cycle by Chlamydiae requires a type III secretion (T3S) system (10). This protein transport mechanism is present in many Gram-negative bacterial pathogens and enables the delivery into eukaryotic cells of bacterially produced effector proteins that can interfere with a multitude of host cell processes (11). While much remains to be understood about the functions of chlamydial effectors, they mediate progress through the different stages of the developmental cycle (5, 12).
Chlamydial T3S effectors include inclusion membrane proteins (Incs), characterized by at least one bilobal hydrophobic motif mediating their insertion in the inclusion membrane (5, 12–14). Incs are present in all Chlamydiae (13, 15, 16), but only some Incs are conserved among Chlamydia species (13, 17). However, apart from the characteristic hydrophobic motif, Incs from the same Chlamydia species are normally unrelated to each other. C. trachomatis encodes >35 Incs (12, 13, 18, 19), which have been shown, for example, to interfere with host cell vesicular (20–22) and nonvesicular transport (23, 24), to control inclusion stability and modulate host cell death (25, 26), to subvert the host cell cytoskeleton and alter Golgi structure (8, 27), and mediate chlamydial host cell egress (28–30). Furthermore, several Incs concentrate at distinct regions within the inclusion membrane (12, 31). These inclusion microdomains have been described to localize near the centrosome (31) and are believed to mediate interactions with the centrosome, microtubules, and actin cytoskeleton (31, 32).
A consequence of the infection of tissue culture cells with Chlamydia is the induction of multinucleation because of the inhibition of host cell cytokinesis (33–35). We previously described that C. trachomatis Inc CT288 can interact with the centrosomal protein coiled-coil domain containing 146 (CCDC146), which is recruited to the periphery of the inclusion in a CT288-independent manner (36). Here, we found that CT288 mediates the induction of host cell multinucleation in C. trachomatis-infected cells and, therefore, named it Inc mediating multinucleation (IncM). Further experiments showed that IncM interferes with centrosome positioning and Golgi distribution around the inclusion, and it is important for inclusion morphology and stability.
RESULTS
Early in the C. trachomatis developmental cycle, IncM localizes in patches at the inclusion membrane that do not localize near the centrosome.
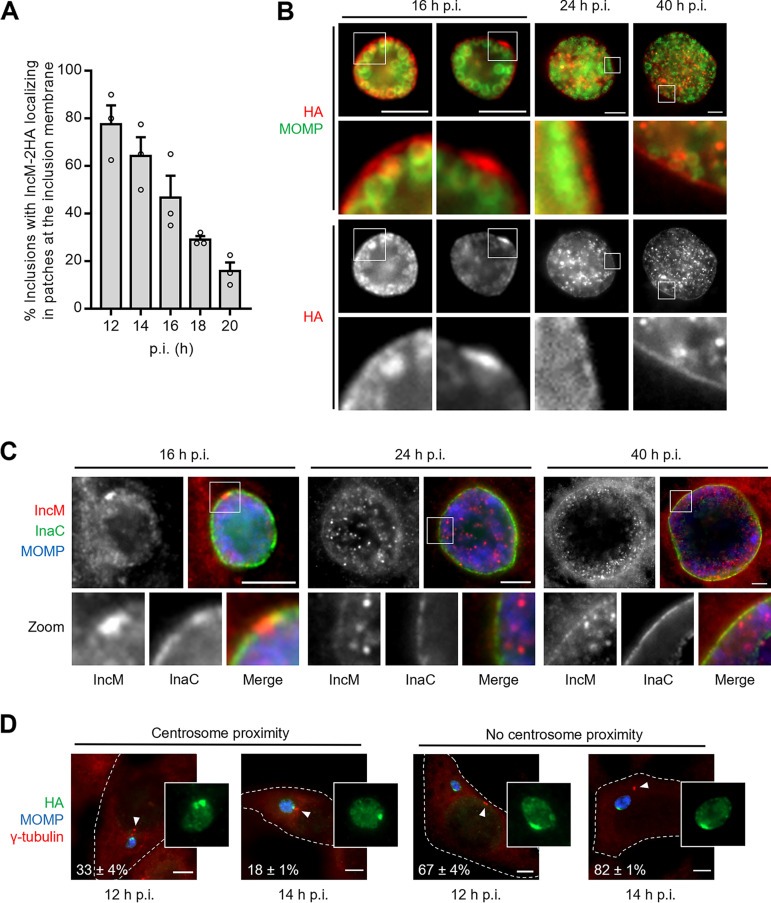
We started by analyzing the localization of IncM during the C. trachomatis developmental cycle. For this, HeLa cells were infected for 12, 14, 16, 18, 20, 24, and 40 h with a C. trachomatis strain with incM disrupted by a group II intron (36) and carrying a plasmid encoding IncM C-terminally tagged with a double hemagglutinin tag [incM::aadA(pIncM-2HA)]. The hybrid incM-2HA gene in the plasmid is expressed from the incM promoter. The infected cells were then fixed and analyzed by immunofluorescence microscopy. At 12 h postinfection (p.i.), IncM-2HA appeared in patches at the inclusion membrane in about 80% of the infected cells (Fig. 1A; see also Fig. 1D). This agreed with a previous study that showed that IncM localizes at inclusion microdomains (18). However, the frequency of infected cells showing IncM-2HA appearing in these patches decreased with the course of infection (Fig. 1A and B). At 16 h p.i., IncM-2HA appeared in patches in about half of the infected cells, while the remaining infected cells revealed a more uniform localization of IncM-2HA around the inclusion (Fig. 1A and B). At 24 h p.i., IncM-2HA was detected uniformly around the inclusion, and its localization in patches was not detected (Fig. 1B). Furthermore, particularly from about 24 h p.i., IncM-2HA was also detected within the inclusion (Fig. 1B). At 40 h p.i., a dim immunofluorescence signal of IncM-2HA could still be detected around the inclusion, but the stronger signal was within the inclusion (Fig. 1B). This lack of accumulation of IncM-2HA at the inclusion membrane is not a consequence of reduced protein levels as host cell infection progresses (see Fig. S1A in the supplemental material). Furthermore, although the levels of plasmid-encoded IncM-2HA were ~8-fold higher than those of chromosomally encoded endogenous IncM (Fig. S1B and C), the localization of IncM-2HA at different times of infection of HeLa cells was identical to endogenous IncM in cells infected by the C. trachomatis LGV serovar L2 strain 434/Bu (L2/434) (Fig. 1C). To analyze if the IncM-2HA patches localized in the proximity of the centrosome, HeLa cells were infected with the incM::aadA(pIncM-2HA) strain for 12 and 14 h. Analysis by immunofluorescence microscopy did not reveal a particular association between the IncM-2HA patches and the centrosomes, as only a minority of these patches was detected near γ-tubulin-immunolabeled centrosomes (Fig. 1D).
FIG 1.
IncM concentrates transiently at patches in the inclusion membrane. HeLa cells were infected at a multiplicity of infection of 0.3 for the indicated times with C. trachomatis strain incM::aadA harboring a plasmid encoding IncM with a C-terminal 2HA epitope tag (pIncM-2HA) (A, B, and D) or with the L2/434 strain (C). The infected cells were fixed with methanol, immunolabeled with anti-C. trachomatis MOMP (green) and anti-HA (red) antibodies (A and B), anti-MOMP (blue), anti-IncM (red), and anti-InaC (green) antibodies (C), or anti-MOMP (blue), anti-HA (green), and γ-tubulin (centrosomes; red) (D) antibodies and analyzed by fluorescence microcopy. (A) Enumeration of the number of inclusions showing IncM-2HA localizing in patches at the inclusion membrane at different times postinfection (p.i.). (B) Representative images of the localization of IncM-2HA during C. trachomatis infection of HeLa cells at different times p.i. (C) Images of the localization of endogenous IncM during C. trachomatis infection of HeLa cells at different times p.i. (D) Illustrative images and enumeration of the number of IncM-2HA patches at the inclusion membrane at 12 and 14 h p.i. that localized within ~2 μm from the centrosome (centrosome proximity) or not (no centrosome proximity); arrowheads point to the centrosomes. For panels A and D, values represent means ± standard errors of the mean, n = 3, ≥40 cells (A) or ≥20 cells (D) per experiment. Scale bars, 5 μm.
In general, these experiments confirmed the localization of IncM at the inclusion membrane, although this localization was not as striking as for other Incs, such as CT813/InaC (Fig. 1C). We also confirmed the localization of IncM in patches at the inclusion membrane that resembled inclusion microdomains (18). However, this was only observed until ~20 h p.i. and, differently from what has been described for inclusion microdomains (31), the IncM patches were normally not detected near the host cell centrosome.
IncM contributes to multinucleation of C. trachomatis-infected host cells.
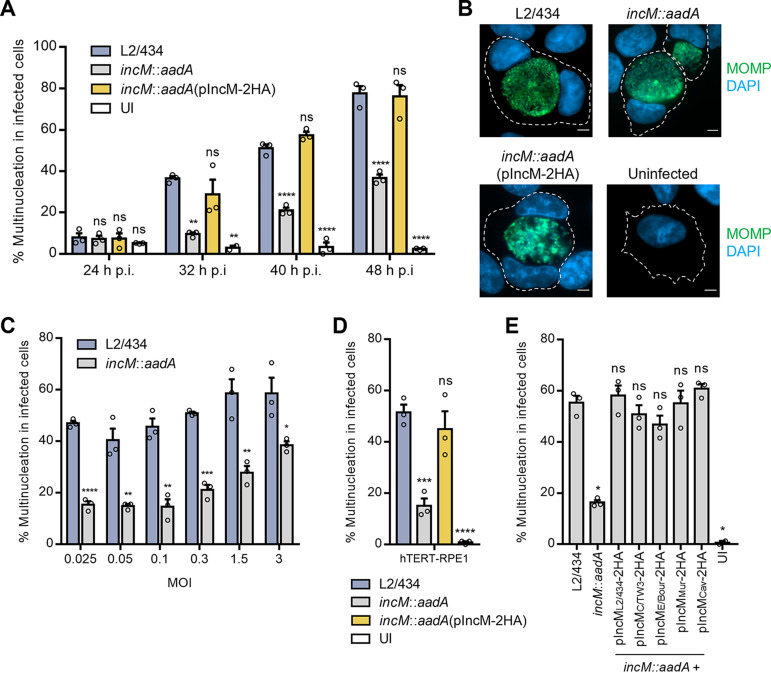
Although the IncM patches at the inclusion membrane were normally not detected near the host cell centrosome, the previously described capacity of IncM to associate with a human centrosomal protein (CCDC146) (36) led us to investigate if IncM could have a role in the ability of C. trachomatis to interfere with the host cell cycle, and specifically to inhibit cytokinesis, which results in multinucleated infected cells (33, 34, 37). For this, HeLa cells were left uninfected or infected with L2/434, incM::aadA, or incM::aadA(pIncM-2HA) strains for 24, 32, 40, and 48 h. The cells were then fixed and analyzed by immunofluorescence microscopy. As expected, the number of multinucleated cells increased with the progression of infection with the wild-type L2/434 strain (Fig. 2A). However, cells infected by the IncM-deficient incM::aadA strain were significantly less multinucleated, while cells infected by the complemented incM::aadA(pIncM-2HA) strain showed an identical frequency of multinucleated cells as L2/434-infected cells (Fig. 2A and B). This IncM-dependent defect in the capacity of C. trachomatis to induce host cell multinucleation was observed at different multiplicities of infection (Fig. 2C). Moreover, as HeLa are cancerous cells transformed by human papillomavirus (HPV), the capacity of IncM to induce multinucleation was further confirmed after infection of the diploid, nontransformed, and immortalized retinal pigment epithelial cell line hTERT-RPE1 (Fig. 2D).
FIG 2.
IncM is involved in the ability of C. trachomatis to induce multinucleation in infected cells. HeLa (A, B, C, and E) or hTERT-RPE1 (D) cells were left uninfected (UI) or were infected at a MOI of 0.1 (except for panel C) with C. trachomatis strain L2/434, incM::aadA, or incM::aadA harboring a plasmid encoding 2HA-tagged IncM from L2/434 (pIncM-2HA) (A, B, and E), from 2HA-tagged IncM orthologues from C. trachomatis strains C/TW3 or E/Bour, or from C. muridarum Nigg or C. caviae GPIC (E). The cells were fixed with methanol, immunolabeled with anti-C. trachomatis MOMP (green) antibodies, and nuclei were stained with DAPI (blue) and analyzed by fluorescence microcopy. (A) At the indicated times postinfection (p.i.), HeLa cells with more than one nucleus were enumerated. (B) Images representative of HeLa cells uninfected or infected for 40 h with the indicated C. trachomatis strains. Scale bars, 5 μm. (C and E) Enumeration of HeLa cells with more than one nucleus infected for 40 h with the indicated C. trachomatis strains and at the MOIs shown. (D) At 40 h p.i., uninfected (UI) and infected hTERT-RPE1 cells with more than one nucleus were enumerated. Values represent means ± standard errors of the means, n = 3, ≥70 cells per experiment. P values were obtained using a one-way analysis of variance (ANOVA) and Dunnett’s post hoc test analysis relative to the cells infected by the L2/434 strain (A, D, and E) or by a two-tailed t test (C). ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
In summary, IncM contributes to the ability of C. trachomatis to induce multinucleation in infected cells, a known consequence of chlamydial inhibition of host cell cytokinesis (33).
The ability of IncM to induce multinucleation in C. trachomatis-infected cells is conserved among its homologues.
IncM from C. trachomatis LGV strains (such as L2/434) contains 18 unique residues (of a total of 564 residues) that are different from its homologues in ocular or urogenital strains (38). Furthermore, previous studies identified IncM homologues in C. muridarum, C. felis, C. caviae, and C. pneumoniae (39). As different C. trachomatis serovars (34) and Chlamydia species (35) have been shown to induce multinucleation in infected cells, we next tested if IncM homologues from serovar C (96% amino acid identity with IncM from L2/434), urogenital serovar E (96% identity), C. muridarum (64% identity), and C. caviae (23% identity) were able to complement the defect in induction of host cell multinucleation exhibited by the C. trachomatis incM::aadA strain. For this, we transformed the incM::aadA strain with plasmids encoding the homologues of IncM C-terminally tagged with 2HA, whose encoding hybrid genes were all under the control of the incM promoter. Infection of HeLa cells for 30 h followed by immunoblotting confirmed that the generated C. trachomatis strains produced IncM proteins that migrated on SDS-PAGE at the expected molecular mass (Fig. S1D). Furthermore, immunofluorescence microscopy analyses of cells infected for 16, 24, or 40 h revealed that the IncM homologues tested localized at the inclusion membrane as IncM from the L2/434 strain, including the appearance in patches at the inclusion membrane at 16 h p.i. (Fig. S2). Infection of HeLa cells for 40 h followed by immunofluorescence microscopy analyses revealed that all IncM homologues tested could rescue the defect in inducing multinucleation displayed by the incM::aadA strain (Fig. 2E). Thus, the ability of L2/434 IncM to induce multinucleation in infected host cells is conserved among its homologues in other C. trachomatis serovars and Chlamydia species.
The ability of IncM to induce multinucleation in infected cells appears to require both of its host cytosol-exposed larger regions.
IncM from the L2/434 strain possesses two bilobal transmembrane domains, from amino acid residues 34 to 88 and from residues 243 to 288 (Fig. S3A), which should mediate its insertion in the inclusion membrane. It can be predicted that its N-terminal region (residues 1 to 33), likely containing the T3S signal, and its central (residues 89 to 242) and C-terminal (residues 289 to 564) regions are exposed to the host cell cytosol (Fig. S3B).
Aiming to understand which regions of IncM are required to induce host cell multinucleation in infected cells, we transformed the incM::aadA strain with plasmids encoding C-terminally 2HA-tagged truncated IncM proteins lacking its C-terminal region (IncM1-242-2HA) or its central region (IncMΔ89-288-2HA and IncMΔ34-242-2HA) and one of its bilobed transmembrane domains (Fig. S3A). The hybrid incM-2HA-truncated genes in the plasmid were all expressed from the incM promoter. Infection of HeLa cells for 30 h followed by immunoblotting analysis confirmed that the generated C. trachomatis strains produced IncM proteins that migrated on SDS-PAGE at the expected molecular mass (Fig. S1E). Furthermore, infection of HeLa cells for 16, 24, and 40 h followed by immunofluorescence analysis revealed that IncM1-242-2HA and IncMΔ89-288-2HA, but not IncMΔ34-242-2HA, can localize at the inclusion membrane (Fig. S4A). This suggested that the bilobal transmembrane domain of IncM between residues 34 and 88 is required for T3S delivery and/or its insertion at the inclusion membrane. However, IncM1-242-2HA did not concentrate at the inclusion membrane as well as full-length IncM did (Fig. S4A). We also did not detect IncM1-242-2HA and IncMΔ89-288-2HA proteins in patches at the inclusion membrane at 16 h p.i. (Fig. S4A), as seen for full-length IncM (Fig. 1B). Infection of HeLa cells for 40 h followed by immunofluorescence microscopy analyses revealed that none of the truncated IncM proteins analyzed (IncM1-242-2HA, IncMΔ89-288-2HA, or IncMΔ34-242-2HA) was able to rescue the defect in inducing multinucleation displayed by the incM::aadA strain (Fig. S4B). Therefore, both the central region (residues 89 to 242) and C-terminal region (residues 289 to 654) of IncM predicted to be exposed at the host cell cytosol (Fig. S3) appeared to be required for its ability to induce multinucleation in infected host cells. However, we cannot exclude that lack of complementation after production and delivery of IncM1-242-2HA and IncMΔ89-288-2HA results from a deficient accumulation or folding of these truncated proteins at the inclusion membrane.
IncM does not influence localization of the inclusion during host cell interphase or telophase.
Next, we sought to understand how the activity of IncM could inhibit host cell cytokinesis and result in increased multinucleation in infected host cells. Previous studies indicated that inhibition of host cell cytokinesis by C. trachomatis is caused by chlamydial effector-dependent localization of the inclusion at the cell equator during telophase (37). Other studies indicated that steric effects of the inclusion do not account for chlamydial inhibition of cytokinesis (33), which instead is a result of centrosomal defects and early mitotic exit due to the action of chlamydial protease/proteasome-like activity factor (CPAF) and of other effector(s) (33, 35).
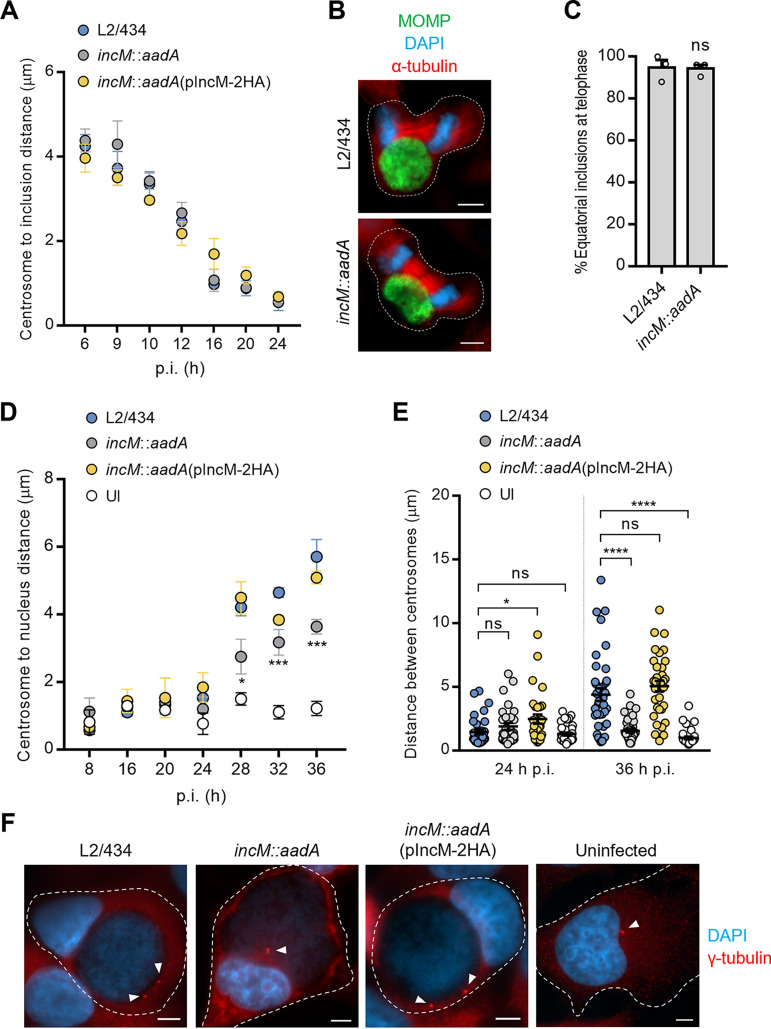
We started by analyzing whether there was an IncM-dependent difference in the overall localization of the C. trachomatis inclusion during infection. HeLa cells were infected with the L2/434, incM::aadA, or incM::aadA(pIncM-2HA) strains for 6, 9, 10, 12, 16, 20, and 24 h followed by immunofluorescence microscopy analysis of the localization of the inclusion relative to the host cell centrosome. This revealed no difference in the position of inclusions of incM::aadA C. trachomatis by comparison to the L2/434 and incM::aadA(pIncM-2HA) inclusions (Fig. 3A). Furthermore, infection of HeLa cells with the L2/434 or incM::aadA strains for 24 h followed by immunofluorescence microscopy analysis of cells at telophase indicated that in both cases the inclusion mostly localized at the cell equator (Fig. 3B and C). Therefore, mislocalization of the inclusion is likely not the cause for the defect of the incM::aadA strain in inhibiting host cell cytokinesis that leads to multinucleated infected cells.
FIG 3.
IncM contributes to host cell centrosome positioning in infected cells. HeLa cells were infected at a multiplicity of infection of 0.1 with C. trachomatis strains L2/434, incM::aadA, or incM::aadA harboring a plasmid encoding IncM with a C-terminal 2HA epitope tag (pIncM-2HA), as indicated. Cells were fixed with methanol at the indicated times postinfection (p.i.) (A, D, and E) or only at 24 h p.i. (B and C), immunolabeled with anti-C. trachomatis MOMP and γ-tubulin (centrosomes) antibodies, and stained for nuclei with DAPI (blue). (A) The distance of the centrosomes to the closest point of the inclusion was measured by fluorescence microscopy using Fiji (75). (B) Representative infected cells show the localization of the inclusion during telophase. Scale bars, 5 μm. (C) Quantification of the position of the inclusion in telophase cells relative to the division plane (equatorial versus polar). (D) The distance of the centrosomes to the closest point in the nucleus was measured using Fiji (75). (E) The distance between centrosomes was measured using Fiji (75). (F) Representative uninfected (UI) and infected cells at 36 h p.i showing centrosome positioning; arrowheads point to the centrosomes. Scale bars, 5 μm. Values represent means ± standard errors of the means, n = 3, ≥30 cells per experiment. P values were obtained by a two-tailed t test (C) or by one-way ANOVA and Dunnett’s post hoc test analysis relative to cells infected by the L2/434 strain (D and E). ns, not significant; *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
The mitotic index is not altered in cells infected by wild-type C. trachomatis.
To analyze if IncM induces host cell multinucleation by promoting an early anaphase onset that leads to premature mitotic exit, HeLa cells were left uninfected or were infected for 24 or 40 h by IncM-producing [L2/434 or incM::aadA(pIncM-2HA)] or IncM-deficient (incM::aadA) C. trachomatis. Then, the number of mitotic cells was enumerated by looking for cells with mitotic spindles and condensed DNA. By comparison to uninfected cells, our results did not show significant differences in the mitotic index of cells infected by IncM-producing C. trachomatis (Fig. S5). Therefore, we did not observe the previously reported early mitotic exit of cells infected by wild-type C. trachomatis (33). However, at 40 h p.i., cells infected by IncM-deficient incM::aadA appeared to be delayed in a phase similar to prometaphase and therefore presented a significantly higher mitotic index relative to uninfected cells (Fig. S5A). These cells also exhibited a perturbed spindle organization (Fig. S5B). However, this IncM-dependent mitotic abnormality was not observed in hTERT-RPE1 cells (Fig. S5C). As these cells also showed an IncM-dependent multinucleation defect when infected by C. trachomatis (Fig. 2D), the mitotic delay observed in HeLa cells after infection with the incM::aadA strain was cell type dependent and unlikely to be a reason for the reduced inhibition of cytokinesis seen in cells infected with this IncM-deficient strain.
IncM contributes to altered host cell centrosome positioning in cells infected by C. trachomatis.
As previously mentioned, centrosomal defects (overduplication and mispositioning) promoted by C. trachomatis infection have been indicated as contributing factors for the inhibition of cytokinesis that leads to host cell multinucleation (35). Therefore, we compared the number of centrosomes between cells infected by IncM-producing [L2/434 or incM::aadA(pIncM-2HA)] or IncM-deficient (incM::aadA) C. trachomatis. After analysis by immunofluorescence microscopy of the infected cells, and similar to previous reports (35, 40, 41), from 40 h p.i., cells infected by the IncM-producing strains showed more centrosomes per cell than did cells infected by the IncM-deficient strain or uninfected cells (Fig. S6A). However, centrosome supernumerary was often seen only in multinucleated cells (Fig. S6B and C). To clarify this, we quantified the centrosome:nuclei ratio, which revealed no difference between cells infected by IncM-producing or IncM-deficient strains and uninfected cells (Fig. S6B). Similar observations were made in infected hTERT-RPE1 cells (Fig. S6D and E). These findings were different from those of previous studies (35), but in agreement with a recent report (42), indicating that supernumerary centrosomes are probably a consequence, and not a cause, of inhibition of host cell cytokinesis by C. trachomatis.
Next, to test if IncM interferes with centrosome localization, HeLa cells were infected by IncM-producing [L2/434 or incM::aadA(pIncM-2HA)] or IncM-deficient (incM::aadA) strains for 8, 16, 20, 24, 28, or 36 h. After immunofluorescence microscopy analysis, the distance between the centrosome and the nearest point of the nucleus was measured. This showed that in infected cells, from 24 h p.i., the centrosomes were seen gradually farther away from the nucleus (Fig. 3D and F). At 28, 32, and 36 h p.i., the centrosome was found significantly closer to the nucleus in cells infected by the IncM-deficient incM::aadA strain than with cells infected by the IncM-producing strains (wild type and complemented) (Fig. 3D and F). However, at 28 and 36 h p.i., the centrosome-nucleus distance in cells infected by all three strains continued to be significantly higher than in uninfected cells (Fig. 3D), indicating that other chlamydial effectors should be involved in modulating centrosome positioning in C. trachomatis-infected cells. Similar observations were made in infected hTERT-RPE1 cells (Fig. S7A). Furthermore, measuring the distance between centrosomes in uninfected and infected HeLa cells revealed that at 36 h p.i. centrosomes were less clustered in cells infected by IncM-producing strains than in cells infected by the IncM-deficient incM::aadA strain or in uninfected cells (Fig. 3E). We also observed that, at 24 h p.i., the distance between centrosomes was significantly larger in cells infected by the incM::aadA(pIncM-2HA)-complemented strain than in cells infected by the L2/434 strain (Fig. 3E).
All together, these data indicated that IncM interferes with centrosome positioning during infection, which should contribute to its ability to inhibit cytokinesis.
IncM contributes to host cell Golgi distribution during infection by C. trachomatis.
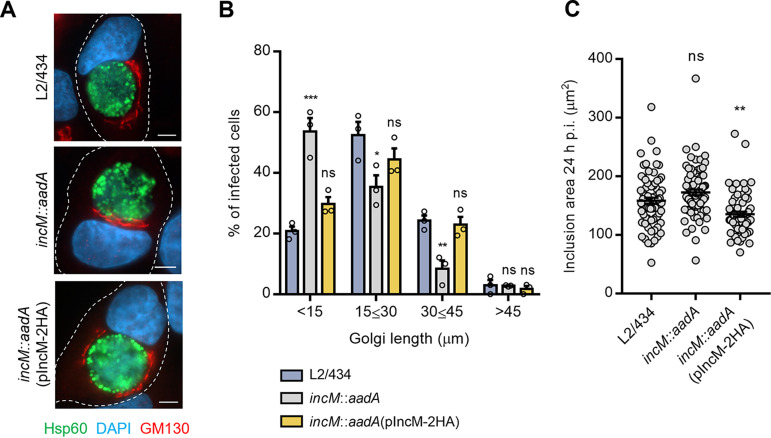
In mammalian cells in interphase, the centrosome is normally surrounded by the Golgi complex (43). Because in Chlamydia-infected cells the Golgi fragments and distributes around the inclusion (9) and because IncM modulates centrosome positioning, we investigated the distribution of the Golgi in cells infected by IncM-producing or -deficient C. trachomatis. For this, HeLa cells were infected for 24 h with L2/434, incM::aadA, or incM::aadA(pIncM-2HA) strains followed by immunofluorescence microscopy and quantitative analysis of Golgi distribution around the inclusion. This revealed that the Golgi complex was less dispersed around the inclusion in cells infected by IncM-deficient C. trachomatis (incM::aadA) than in cells infected by IncM-producing strains [L2/434 or incM::aadA(pIncM-2HA)] (Fig. 4A and B). Measurement of the area of the analyzed inclusions revealed that the observed difference in Golgi dispersion was not due to differences in inclusion size (Fig. 4C). Thus, IncM contributes to Golgi distribution around the inclusion in cells infected by C. trachomatis.
FIG 4.
IncM modulates Golgi distribution around the inclusion. HeLa cells were infected at a multiplicity of infection of 0.3 with C. trachomatis strain L2/434, incM::aadA, or incM::aadA harboring a plasmid encoding IncM with a C-terminal 2HA epitope tag (pIncM-2HA). Cells were fixed with methanol at 24 h postinfection and immunolabeled with anti-GM130 (cis-Golgi network; red) and anti-C. trachomatis Hsp60 (green) antibodies, stained for nuclei with DAPI (blue), and analyzed by fluorescence microscopy. (A) Representative infected cells showing Golgi distribution around the inclusion. Scale bars, 5 μm. (B) The Golgi distribution length around the inclusion was measured using Fiji (75). (C) The area of the inclusion was measured by tracing the inclusion using Fiji (75). Values represent means ± standard errors of the means, n = 3, ≥30 cells per experiment. P values were obtained using one-way ANOVA and Dunnett’s post hoc test analysis relative to cells infected by the L2/434 strain. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IncM controls inclusion morphology and stability.
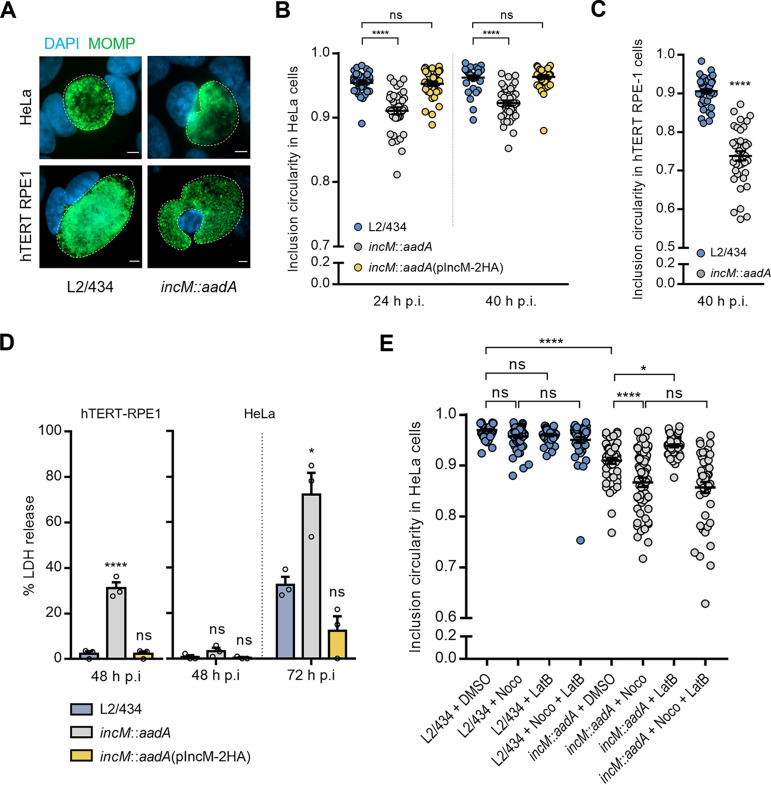
In the course of the experiments described above, we noticed that in cells infected by the incM::aadA strain, the inclusions appeared less circular than in cells infected by the L2/434 strain (Fig. 5A). To analyze this directly, HeLa cells were infected with the L2/434, incM::aadA, or incM::aadA(pIncM-2HA) strains for 24 and 40 h. Then, immunofluorescence microscopy followed by quantitative analysis of circularity confirmed the different geometry of the IncM-deficient incM::aadA inclusions relative to that of the IncM-producing L2/434 and incM::aadA(pIncM-2HA) inclusions (Fig. 5A and B). Similar observations were made in infected hTERT-RPE1 cells (Fig. 5A and C), where this IncM-dependent defect was particularly evident (Fig. 5A and C). Also, infected hTERT-RPE1 cells with disrupted inclusions and cytosolic chlamydiae were easily seen when infected by the IncM-deficient incM::aadA strain, but not when infected by the wild-type L2/434 strain (Fig. S7B). As cytosolic release of chlamydiae resulting from inclusion rupture is known to lead to host cell lysis (25, 44), we quantified the release of host cell lactate dehydrogenase (LDH) into the culture supernatant of hTERT-RPE1 cells infected for 48 h with the L2/434, incM::aadA, or incM::aadA(pIncM-2HA) strains. This revealed an ~10-fold increase in the release of LDH in cells infected by the IncM-deficient incM::aadA strain compared to that of the IncM-producing L2/434 or incM::aadA(pIncM-2HA) strains (Fig. 5D). Similar observations were made in infected HeLa cells, although the increase in LDH release was not as pronounced and was only detected after infection with IncM-deficient C. trachomatis for 72 h (Fig. 5D). Together, these results indicated that IncM participates in the control of inclusion morphology and stability.
FIG 5.
IncM modulates inclusion morphology and integrity. HeLa (A, B, D, and E) or hTERT-RPE1 (C) cells were infected at a MOI of 0.1 (A, B, and E) or 0.5 (D) with C. trachomatis strain L2/434, incM::aadA, or incM::aadA harboring a plasmid encoding IncM with a C-terminal 2HA epitope tag (pIncM-2HA). Cells were fixed with methanol, immunolabeled with anti-C. trachomatis MOMP (green) antibodies, stained for the nuclei with DAPI (blue), and analyzed by fluorescence microscopy. (A) Representative images of the inclusion morphology in HeLa and hTERT-RPE1 cells infected by the indicated strains for 40 h. Scale bars, 5 μm. (B and C) Cells were fixed at 24 and 40 h p.i. (B) or only at 40 h p.i. (C), and the circularity of each inclusion was assessed using Fiji (75). (D) HeLa and hTERT-RPE1 cells were infected at an MOI of 0.5. At the indicated times p.i., the release of host LDH into the supernatant of infected HeLa cells was measured using a CytoScan LDH cytotoxicity assay kit (G-Biosciences). As described in Materials and Methods, the values (percentages) of LDH released were related to the amount of LDH activity in uninfected cells after their lysis with Triton X-100. (E) Cells were fixed at 30 h p.i. after being treated with nocodazole (Noco) and/or latrunculin B (LatB), as described in Materials and Methods, or with the solvent DMSO as control, and the circularity of each inclusion was assessed using Fiji (75). Values represent means ± standard errors of the means, n = 3, ≥30 inclusions per experiment (B, C, and E). P values were obtained using one-way ANOVA and Dunnett’s (B, C, and D) or Tukey’s (E) post hoc test analyses; statistical comparisons are only shown for cases mentioned in the main text. ns, not significant; *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
We thought that the different morphology and reduced stability of the IncM-deficient inclusions could be related to a defect in the cytoskeletal networks around the inclusion (7, 8). However, by immunofluorescence microscopy of infected cells, we did not detect visible differences between phalloidin-stained actin filaments, tubulin-immunolabeled microtubules, or vimentin-immunolabeled intermediate filaments surrounding the inclusion of IncM-producing or IncM-deficient C. trachomatis (Fig. S8A). To further analyze this, we tested how treating infected HeLa cells with microfilament and microtubule depolymerizing agents affected inclusion circularity. Depolymerization of microfilaments with latrunculin B did not affect the circularity of inclusions of IncM-producing (L2/434) C. trachomatis and slightly increased the circularity of IncM-deficient (incM::aadA) inclusions (Fig. 5E and Fig. S8B). Strikingly, depolymerization of the microtubules with nocodazole worsened the circularity defect of incM::aadA inclusions, while having no impact on IncM-producing (L2/434) inclusions (Fig. 5E). Simultaneous depolymerization of both microfilaments and microtubules led to inclusions whose morphology was indistinguishable from when only microtubules were depolymerized (Fig. 5E).
Overall, this indicated that IncM-deficient inclusions are more sensitive to microtubule depolymerization than inclusions of wild-type C. trachomatis, suggesting that IncM may modulate microtubules.
DISCUSSION
In this study, we show that IncM participates in the known abilities of C. trachomatis to induce host cell multinucleation (34) (a consequence of inhibition of host cell cytokinesis [33]), to interfere with centrosome positioning (40), and to distribute the Golgi around the inclusion (9, 45). Furthermore, we found that inclusions containing IncM-deficient C. trachomatis have altered morphology. As we also observed inclusion rupture and premature host cell lysis in cells infected by IncM-deficient C. trachomatis, the simplest explanation is that the defect in inclusion morphology reflects a defect in inclusion stability leading to rupture of its membrane. This altered morphology of inclusions containing IncM-deficient C. trachomatis appears to be specifically sensitive to microtubule depolymerization. As host cell cytokinesis and centrosome and Golgi positioning all depend on microtubules (43, 46, 47), we propose that IncM acts directly or indirectly on host cell microtubules. For the indirect role, IncM could act on the centrosome, the major microtubule-organizing center, which would then lead to altered microtubule cytoskeleton. As centrosome positioning itself depends on the activity of microtubule motors (47), another possibility is that IncM acts on dynein or kinesins, or on one of the many proteins that regulate their activity or that associate with microtubules. Still, there is specificity in the microtubule-related processes targeted by IncM, as we showed that this effector did not influence the microtubule-dependent positioning of the C. trachomatis inclusion throughout the developmental cycle. A potential mechanism by which IncM may modulate microtubule-associated functions could be through its binding to the human centrosomal protein CCDC146 (36). However, the low expression levels of CCDC146 (36) and the lack of functional studies on this protein have thus far prevented us from examining this further. In summary, while the mechanism of action of IncM remains to be elucidated, overall, our work contributes to a better understanding of how C. trachomatis Incs interfere with host cell processes.
In a recent study, cells infected by C. trachomatis deficient for Dre1 (Inc CT192/CTL0444) (48) displayed similar phenotypes (host cell multinucleation, altered centrosome positioning, and Golgi distribution) as cells infected by IncM-deficient C. trachomatis. Dre1 binds the dynactin complex (49), a cofactor required for the activity of dynein (50), and recruits it to the periphery of the inclusion membrane (48). The situation where a bacterial effector acts on microtubule-related processes and cells infected by mutant strains display several related phenotypes is evocative of the Salmonella enterica serovar Typhimurium effectors SseF and SseG, which control positioning of the Salmonella-containing vacuoles (SCVs) that normally cluster near the centrosome and Golgi (51–53). The activity of these effectors has been proposed to involve control of microtubule molecular motors (52) and/or tethering of the SCVs to the Golgi (54, 55). Akin to C. trachomatis and to IncM and Dre1, S. Typhimurium inhibits host cell cytokinesis in an SseF- and SseG-dependent manner (56).
Other C. trachomatis Incs, CT223/CTL0476 (known as IPAM, for inclusion protein acting on microtubules [8]), CT224/CTL0477, and CT225/CTL0477A, have been suggested to inhibit host cell cytokinesis, based on the consequences of their ectopic production in mammalian cells (57). A CT224-deficient C. trachomatis strain did not reveal a growth phenotype; further analyses were not described (25). C. trachomatis strains deficient for IPAM or CT225 were not reported, but IPAM was shown to bind a centrosomal protein (CEP170) (8). CEP170 controls assembly of microtubules around the inclusion, indicating that IPAM has a prominent role in this process (8). Other C. trachomatis Incs have been shown or suggested to interfere with microtubule-related processes. The best studied is InaC (CT813/CTL0184), which, besides its role in assembly of microfilaments around the inclusion (27, 58, 59), also controls Golgi distribution around the inclusion (27), as IncM does. Through host cell small GTPases ARF1 and ARF4, InaC induces stabilizing posttranslational modification of microtubules that serve as scaffold for Golgi distribution around the inclusion (27). Furthermore, CT850/CTL0223 binds the dynein light chain DYNLT1 and may mediate inclusion positioning (32); the homologue of IncB (CT232/CTL0484) in C. psittaci binds Snapin, which may connect inclusions to microtubules through dynein (60). It can be predicted that further analyses of chlamydiae deficient for IPAM, CT224, CT225, IncB, or CT850 should also reveal phenotypes in infected cells similar to those related to IncM. Besides Incs, the chlamydial CPAF protease has been shown to inhibit host cell cytokinesis in C. trachomatis-infected cells (33, 35). Finally, another chlamydial non-Inc effector (CteG) (61, 62) has been reported to induce centrosome amplification by binding to centrin-2, a structural component of centrosomes (63). However, CteG seems dispensable for the ability of C. trachomatis to induce host cell multinucleation (63). How the activities of these Incs (IPAM, CT224, CT225, IncB, InaC, and CT850), CPAF, and possibly CteG may relate to the functions of IncM is presently unknown.
We also found that inclusions containing IncM-deficient chlamydiae were morphologically different and less stable than inclusions of IncM-producing chlamydiae, a phenotype which is detectable at late stages of the developmental cycle (from 48 h p.i.). Other Incs have also been shown to play a role in inclusion stability. Inclusions of CT229/CpoS-, IncC-, InaC-, or CT383-deficient C. trachomatis are also less stable; however, in these cases, increased inclusion lysis followed by host cell death is detectable from 18 to 24 h p.i. (25, 59), earlier than for IncM. While the distinct morphology of IncM-deficient inclusions does indicate a role of IncM in mediating inclusion stability, we cannot formally exclude that the two observations are unlinked, e.g., that IncM participates instead in a putative mechanism that prevents untimely chlamydial lytic exit (62). Thus, several Incs may cooperate to promote inclusion stability and ensure host cell survival throughout the chlamydial developmental cycle.
Most of the Incs that interfere, or may interfere, with microtubule-related processes have been shown to accumulate at inclusion microdomains, which are reported to localize near the host cell centrosome (18, 31). Besides IncM (18), this is at least the case of IPAM, CT224, IncB, IncC, and CT850 (18, 31). Other Incs shown to localize at inclusion microdomains are CT101 (known as MrcA [29]), CT222, and CT228 (18, 31). IncM was also reported to localize at the inclusion microdomains. Although we confirmed that IncM concentrates at discrete patches in the inclusion membrane, this localization was transient, and the patches were not markedly associated with the host cell centrosome. The localization of IncM at the inclusion membrane varies along the chlamydial developmental cycle, and at later stages the protein is found mostly within the inclusion. This is consistent with a previous study indicating its abundance in EBs (64). A change in the localization of bacterial effector proteins during infection has been associated with a diversification of their functions (65). Thus, IncM could have additional roles during the chlamydial developmental cycle besides those described in this work. For other Incs that have been shown to appear in inclusion microdomains (IPAM, CT224, IncB, IncC, MrcA, CT222, and CT228), the analysis of their localization has been reported at a single time point, at 18 h (18) or at 24 h p.i. (31), and it is unclear whether such localization is also transient. Therefore, our detailed analysis of IncM localization suggested that inclusion microdomains may have a different composition, or even disappear, as the chlamydial infectious cycle progresses. Regardless of this, we speculate that IncM and other Incs reported to localize at inclusion microdomains may cooperate, and even interact, to exert their function on host cell microtubules or more generally on cytoskeleton-associated processes. Therefore, IncM is necessary, but likely not sufficient, to promote host cell multinucleation, Golgi and centrosome positioning, and for the morphology and stability of the inclusion.
Host cell multinucleation in Chlamydia-infected cells has been linked to C. trachomatis being a cofactor for HPV in the development of cervical cancer (66, 67). It is probable that C. trachomatis did not evolve to interfere with specific host cell division machinery to block cytokinesis, as it mostly infects noncycling terminally differentiated cells in vivo (5, 33). Instead, as suggested by our work and other related studies (48), inhibition of cytokinesis by C. trachomatis is likely a consequence of the action of several effectors on processes that enable completion of the chlamydial developmental cycle and which are also required for host cell division (e.g., cytoskeleton dynamics or vesicular trafficking), leading to the phenotype of multinucleation in cultured cells infected by C. trachomatis.
In a previous study, analysis of IncM-deficient C. trachomatis revealed a slight defect in chlamydial growth in a tissue culture infection model and in vivo in a mouse model (25). In contrast, in our previous characterization of the incM::aadA strain, we did not detect a chlamydial growth phenotype (36). Given the likely role of IncM in controlling inclusion stability, which was more evident in hTERT-RPE1 cells than in HeLa cells, it seems reasonable that the exact experimental conditions (e.g., cell line, host cell and chlamydial clone, and precise methodology to collect and analyze infectious particles) can influence the detection of slightly more or less infectious IncM-deficient chlamydiae within host cells. Furthermore, as discussed above, the function of IncM could be partially redundant with other Incs, which may explain why a clearer intracellular growth defect was not detected for IncM-deficient C. trachomatis.
In summary, we have shown that infection of mammalian cells with IncM-deficient C. trachomatis leads to several phenotypes (host cell multinucleation, defects in centrosome and Golgi positioning, and inclusion morphology and stability) linked to microtubule-associated processes, thereby contributing to the overall understanding of the functions of chlamydial Incs. Future studies are required to understand the molecular mechanism of action of IncM and how it relates with several other Incs and chlamydial proteins with similar phenotypes and likely acting on similar host cell processes.
MATERIALS AND METHODS
DNA manipulation, plasmids, and primers.
The plasmids used in this work and their main characteristics are described in Table S1 in the supplemental material. The DNA primers used in their construction are listed in Table S2. Plasmids were constructed and purified using standard molecular biology procedures. Genomic DNA of C. caviae (strain GPIC) and C. muridarum (strain Nigg) were gifts from Agathe Subtil and Ian Clarke, respectively. Genomic DNA of C. trachomatis strains C/TW3 and E/Bour was a gift from João Paulo Gomes. The backbone plasmids used in this work included p2TK2-SW2 (68), a cloning vector suitable for transformation of C. trachomatis, and its derivative, pVector[Pgp4+]/pSVP247 (62, 69), which enables the expression of genes encoding proteins with a C-terminal 2HA tag in C. trachomatis. The accuracy of the nucleotide sequence of all the relevant parts of the constructed plasmids was confirmed by DNA sequencing.
Mammalian cell lines.
HeLa 229 and Vero cells (both from European Collection Authenticated of Cell Cultures [ECACC]) were maintained in Dulbecco’s modified Eagle medium (DMEM; Corning) (with 4.5 g/liter glucose, l-glutamine, and sodium pyruvate) supplemented with heat-inactivated 10% (vol/vol) fetal bovine serum (FBS; Thermo Fisher Scientific) at 37°C in a humidified atmosphere of 5% (vol/vol) CO2. hTERT-RPE1 (from ECACC) were maintained in DMEM–nutrient mixture F-12 (DMEM–F-12; Thermo Fisher Scientific) (with GlutaMAX) supplemented with heat-inactivated 10% (vol/vol) FBS at 37°C in a humidified atmosphere of 5% (vol/vol) CO2. Cells were checked for Mycoplasma by conventional PCR, as described elsewhere (70).
Bacterial strains and growth conditions.
Escherichia coli NEB 10β (New England Biolabs) was used for construction and purification of plasmids, E. coli BL21(DE3) (Novagen) was used for recombinant protein production, and methylation-deficient E. coli ER2925 (New England Biolabs) was used to amplify and purify plasmids for transformation of C. trachomatis. E. coli strains were routinely grown in liquid or solid lysogeny broth (LB) medium with the appropriate antibiotics. Plasmids were introduced into E. coli by electroporation.
The C. trachomatis strains used and generated in this work are listed in Table S3. C. trachomatis strains were propagated in HeLa 229 cells using standard techniques (71). C. trachomatis transformants were generated essentially as described by Agaisse and Derré (68), followed by clone isolation by plaque purification using Vero cells, as previously described (72). Chlamydia stocks were tested for Mycoplasma by conventional PCR (70) followed by Sanger sequencing.
Infection of HeLa 229 cells with C. trachomatis.
Infection of mammalian cells by C. trachomatis was done as previously described (69). Briefly, HeLa cells were seeded the day before the infection in the appropriate format tissue culture (multiwell plates or flasks). For immunofluorescence experiments, cells were seeded onto 13-mm glass coverslips. The day after seeding, cells were washed with Hank's balanced salt solution (Thermo Fisher Scientific), and the C. trachomatis inoculum was added at the indicated multiplicities of infection (MOIs) for 30 to 60 min. At that point, the inoculum was removed and replaced by DMEM supplemented with 10% (vol/vol) FBS and appropriate antibiotics. This was considered the time zero of infection.
Recombinant protein production and purification.
E. coli BL21(DE3) carrying a pET28b+ derived plasmid encoding full-length IncM with 6 histidines fused at its N terminus (6×His-IncM) was used for recombinant protein expression by autoinduction (73). For this, a preinoculum was prepared by inoculating fresh colonies of E. coli into LB supplemented with the appropriate antibiotics and incubating for 4 h at 37°C with agitation of 180 rpm. Then, the preinoculum was diluted 1/200 in autoinduction medium [1 mM MgSO4, 1× 5052] (0.5% [vol/vol] glycerol, 0.05% [wt/vol] glucose, and 0.2% [wt/vol] α-lactose), 1× NPS salts [0.33% (wt/vol) (NH4)2SO4, 0.68% (wt/vol) KH2PO4, and 0.71% (wt/vol) NaHPO4], and the culture was incubated at 26°C for 24 h, with agitation at 180 rpm. The bacterial cells were harvested by centrifugation at 2,500 × g for 10 min at 4°C and washed twice with ice-cold phosphate-buffered saline (PBS). The bacterial cell pellet from each liter of culture was resuspended in 20 mL of ice-cold lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole [pH 8.0], 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail [Amresco, VWR]), and cells were lysed by sonication (80% amplitude, 0.5 cycle; 10 cycles of 1 min, with a 1-min interval). The lysates were centrifuged at 3,000 × g for 30 min at 4°C, after which the supernatant was transferred into a new tube and centrifuged at 20,000 × g for 1 h at 4°C. To solubilize the protein, the pellet was resuspended in ice-cold lysis buffer with 1% (wt/vol) n-dodecyl β-d-maltoside (DDM; Glyco Biochemicals) and incubated 2 h at 4°C under stirring. From this point, 1% (wt/vol) DDM was added to all solutions. The solubilized protein was centrifuged at 20,000 × g for 1 h at 4°C, after which the supernatants were loaded onto a nickel resin column (Thermo Fisher Scientific) preequilibrated with lysis buffer. The protein was allowed to interact with the resin for 1 h at 4°C with end-over-end mixing before allowing the supernatant to flow through. The column was washed 2 times with 1 mL of washing buffer (50 mM NaH2PO4, 300 mM NaCl, 50 mM imidazole [pH 8.0]), after which the protein was eluted using elution buffer (50 mM NaH2PO4, 300 mM NaCl, 400 mM imidazole [pH 8.0]).
Antibodies and dyes.
To generate rabbit polyclonal anti-IncM serum, purified 6×His-IncM was used to immunize a New Zealand White rabbit (Davids Biotechnologie, Germany). The anti-IncM serum was affinity purified on 0.5 mg of 6×His-IncM immobilized on a nitrocellulose membrane. The affinity-purified antibodies were eluted from the membrane with 100 mM glycine-HCl (pH 2.5) and quantified using a NanoDrop 1000 system.
For immunoblotting, the following primary antibodies were used: rat anti-HA (clone 3F10; Roche; diluted 1:1,000), mouse anti-chlamydial Hsp60 (clone A57-B9; Thermo Fisher Scientific; 1:1,000), and mouse anti-α-tubulin (clone B-5-1-2; Sigma-Aldrich; 1:1,000). Anti-mouse and anti-rat secondary antibodies were all horseradish peroxidase-conjugated (GE Healthcare and Jackson ImmunoResearch; diluted 1:10,000).
For immunofluorescence microscopy, the following primary antibodies were used: goat anti-Chlamydia major outer membrane protein (MOMP; Meridian; 1:200), mouse anti-Chlamydia Hsp60 (A57-B90; Invitrogen; 1:200), rat anti-HA (3F10; Roche; 1:200), rabbit anti-GM130 (EP892Y; Abcam; 1:200), mouse anti-γ-tubulin antibody (GTU-88; Sigma-Aldrich; 1:200), mouse anti-α-tubulin antibody (B-5-1-2; Sigma-Aldrich; 1:200), mouse anti-vimentin (v9; Sigma; 1:200), rabbit anti-IncM (1:10), mouse anti-CT813/InaC (a gift from Guangming Zhong; 1:200) (19, 74), mouse anti-myc (9E10; Calbiochem; 1:200), and goat anti-C. trachomatis fluorescein isothiocyanate-conjugated polyclonal antibody (Millipore; 1:150). The secondary antibodies were all diluted 1:200: rhodamine RedTM-X-conjugated anti-rat, AF568-conjugated anti-mouse (Thermo Fisher Scientific), AF488-conjugated anti-mouse (Jackson ImmunoResearch), AF488-conjugated anti-goat (Thermo Fisher Scientific), DyLight 405 conjugated anti-goat (Jackson ImmunoResearch), and AF568-conjugated anti-rabbit (Thermo Fisher Scientific). 4′,6-Diamidino-2-phenylindole (DAPI; 1:30,000) was used to label DNA, and actin staining was carried out by incubating HeLa cells with phalloidin-Alexa 555 (Thermo Fisher Scientific; 1:100).
Drug treatments.
For disruption of microtubules, infected cells were placed at 4°C for 20 min, followed by addition of 10 μM nocodazole (Sigma-Aldrich; stock solution at 5 mg/mL in dimethyl sulfoxide [DMSO]) and incubation for 2 h at 37°C before fixation. For disruption of the actin cytoskeleton, 0.5 μM latrunculin B (Calbiochem; stock solution at 1 mg/mL in DMSO) was added to cells for 30 min before fixation.
Immunoblotting.
To prepare total mammalian cell extracts for protein production assessment, HeLa 229 cells were collected by trypsinization and centrifuged for 5 min, 2,200 × g, at 4°C. The pellet was washed twice with ice-cold PBS and immediately resuspended and boiled in SDS-PAGE Laemmli buffer (SDS loading buffer). The samples were resolved by 12% SDS-PAGE, followed by immunoblotting. Proteins in the gel were transferred onto nitrocellulose membranes (Bio-Rad) and blocked in 4% (wt/vol) dried skimmed milk diluted in PBS containing 0.1% (vol/vol) Tween 20. The membranes were probed with primary and horseradish peroxidase-conjugated secondary antibodies and detected using SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific) by exposure to Amersham Hyperfilm ECL (GE Healthcare).
Immunofluorescence microscopy.
For immunofluorescence microscopy, cells were either fixed with 4% (wt/vol) paraformaldehyde (PFA) for 15 min at room temperature or with methanol for 5 to 10 min at −20°C. Immunostaining was performed by incubating the cells in the coverslips individually for 1 h with primary and secondary antibodies previously diluted in PBS and 10% (vol/vol) horse serum (when cells were fixed with PFA, 0.1% [vol/vol] Triton X-100 was added). Next, cells were washed in PBS and H2O, sequentially, and mounted onto glass slides using Aqua-poly/mount mounting medium (Polysciences). Samples were analyzed using a wide-field fluorescence microscope (Zeiss Axio Imager D2), and images were processed using Fiji software.
After immunostaining, the following steps were performed: (i) patches of IncM-2HA at the inclusion membrane were considered in proximity of γ-tubulin-immunolabeled centrosomes if the centrosome was within a ~2-μm radius from the patches; (ii) to quantify cell multinucleation and centrosome amplification, ≥70 infected cells/assay were counted with the fluorescence microscope for the number of nuclei and centrosomes; (iii) to measure centrosome recruitment and inclusion positioning, a line between each centrosome and the closest point in the nucleus or the inclusion, respectively, was drawn using Fiji (≥30 cells/assay) (75); (iv) to quantify the morphology of the inclusion, using Fiji (75), a line was traced around each inclusion and the circularity value was measured (≥30 cells/assay); (v) to quantify the mitotic index, the DNA condensation along with the bipolar spindles were used to evaluate the cell cycle phase of the cells and identify mitotic cells (≥250 cells/assay).
Cell cytotoxicity assays.
The supernatants of infected HeLa cells were assayed for released LDH with the CytoScan LDH cytotoxicity assay kit (G-Biosciences), as described previously (62). The multiplicity of infection and time p.i. used in these assays were based on optimization experiments described recently (62). In brief, the amount of LDH activity released from uninfected cells and detected in uninfected cells after lysis with 1% (vol/vol) Triton X-100 was determined and corresponded to the minimal and maximal values, respectively. The percentage of LDH released in each infected sample was calculated as 100 × [(LDH activity released from infected cells) − (LDH activity released from uninfected cells)/(LDH activity after lysis of uninfected cells with Triton X-100) − (LDH activity released from uninfected cells)]. Absorbance at 490 nm was measured in a SpectraMax 190 microplate reader (Molecular Devices), and data were acquired using SoftMax Pro 7.1 software (Molecular Devices).
Statistical analyses.
Statistical analyses were done using GraphPad Prism, version 7.00 for Windows (GraphPad Software, San Diego CA, USA). Data are presented as means ± standard errors of the means of 3 or more independent replicates. Statistical tests are specified in the legend of each figure. Differences between data sets were considered significant if P was <0.05.
ACKNOWLEDGMENTS
We thank Irina Franco for critical reading of the manuscript and Agathe Subtil, Guangming Zhong, Ian Clarke, and João Paulo Gomes for gifts of antibodies and chlamydial genomic DNA. This work was supported by Fundação para a Ciência e a Tecnologia (FCT) in the scope of grant PTDC/IMI-MIC/1300/2014 and projects UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences, UCIBIO, and LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy, i4HB. M.P.L. and I.S.P. were supported by PhD fellowships SFRH/BD/144284/2019 and SFRH/BD/129756/2017, respectively, funded by FCT. J.N.B. was supported by PhD fellowship PD/BD/128214/2016 within the scope of the PhD program Molecular Biosciences (PD/00133/2012), also funded by FCT.
Footnotes
Supplemental material is available online only.
Contributor Information
Luís Jaime Mota, Email: ljmota@fct.unl.pt.
Craig R. Roy, Yale University School of Medicine
REFERENCES
- 1.Collingro A, Kostlbacher S, Horn M. 2020. Chlamydiae in the environment. Trends Microbiol 28:877–888. 10.1016/j.tim.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Nunes A, Gomes JP. 2014. Evolution, phylogeny, and molecular epidemiology of Chlamydia. Infect Genet Evol 23:49–64. 10.1016/j.meegid.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Taylor HR, Burton MJ, Haddad D, West S, Wright H. 2014. Trachoma. Lancet 384:2142–2152. 10.1016/S0140-6736(13)62182-0. [DOI] [PubMed] [Google Scholar]
- 4.O'Connell CM, Ferone ME. 2016. Chlamydia trachomatis genital infections. Microb Cell 3:390–403. 10.15698/mic2016.09.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elwell C, Mirrashidi K, Engel J. 2016. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol 14:385–400. 10.1038/nrmicro.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelrahman YM, Belland RJ. 2005. The chlamydial developmental cycle. FEMS Microbiol Rev 29:949–959. 10.1016/j.femsre.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Kumar Y, Valdivia RH. 2008. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe 4:159–169. 10.1016/j.chom.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumoux M, Menny A, Delacour D, Hayward RD. 2015. A Chlamydia effector recruits CEP170 to reprogram host microtubule organization. J Cell Sci 128:3420–3434. 10.1242/jcs.169318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuer D, Lipinski AR, Machuy N, Karlas A, Wehrens A, Siedler F, Brinkmann V, Meyer TF. 2009. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature 457:731–735. 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- 10.Mueller KE, Plano GV, Fields KA. 2014. New frontiers in type III secretion biology: the Chlamydia perspective. Infect Immun 82:2–9. 10.1128/IAI.00917-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner S, Grin I, Malmsheimer S, Singh N, Torres-Vargas CE, Westerhausen S. 2018. Bacterial type III secretion systems: a complex device for the delivery of bacterial effector proteins into eukaryotic host cells. FEMS Microbiol Lett 365. 10.1093/femsle/fny201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugalhão JN, Mota LJ. 2019. The multiple functions of the numerous Chlamydia trachomatis secreted proteins: the tip of the iceberg. Microb Cell 6:414–449. 10.15698/mic2019.09.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dehoux P, Flores R, Dauga C, Zhong G, Subtil A. 2011. Multi-genome identification and characterization of Chlamydiae-specific type III secretion substrates: the Inc proteins. BMC Genomics 12:109. 10.1186/1471-2164-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockey DD, Scidmore MA, Bannantine JP, Brown WJ. 2002. Proteins in the chlamydial inclusion membrane. Microbes Infect 4:333–340. 10.1016/s1286-4579(02)01546-0. [DOI] [PubMed] [Google Scholar]
- 15.Heinz E, Rockey DD, Montanaro J, Aistleitner K, Wagner M, Horn M. 2010. Inclusion membrane proteins of Protochlamydia amoebophila UWE25 reveal a conserved mechanism for host cell interaction among the Chlamydiae. J Bacteriol 192:5093–5102. 10.1128/JB.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kebbi-Beghdadi C, Pilloux L, Croxatto A, Tosetti N, Pillonel T, Greub G. 2019. A predation assay using amoebae to screen for virulence factors unearthed the first W. chondrophila inclusion membrane protein. Sci Rep 9:19485. 10.1038/s41598-019-55511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutter EI, Martens C, Hackstadt T. 2012. Evolution and conservation of predicted inclusion membrane proteins in chlamydiae. Comp Funct Genomics 2012:362104. 10.1155/2012/362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber MM, Bauler LD, Lam J, Hackstadt T. 2015. Expression and localization of predicted inclusion membrane proteins in Chlamydia trachomatis. Infect Immun 83:4710–4718. 10.1128/IAI.01075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Chen C, Chen D, Wu Y, Zhong Y, Zhong G. 2008. Characterization of fifty putative inclusion membrane proteins encoded in the Chlamydia trachomatis genome. Infect Immun 76:2746–2757. 10.1128/IAI.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cingolani G, McCauley M, Lobley A, Bryer AJ, Wesolowski J, Greco DL, Lokareddy RK, Ronzone E, Perilla JR, Paumet F. 2019. Structural basis for the homotypic fusion of chlamydial inclusions by the SNARE-like protein IncA. Nat Commun 10:2747. 10.1038/s41467-019-10806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elwell CA, Czudnochowski N, von Dollen J, Johnson JR, Nakagawa R, Mirrashidi K, Krogan NJ, Engel JN, Rosenberg OS. 2017. Chlamydia interfere with an interaction between the mannose-6-phosphate receptor and sorting nexins to counteract host restriction. Elife 6. 10.7554/eLife.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faris R, Merling M, Andersen SE, Dooley CA, Hackstadt T, Weber MM. 2019. Chlamydia trachomatis CT229 subverts Rab GTPase-dependent CCV trafficking pathways to promote chlamydial infection. Cell Rep 26:3380–3390.e5. 10.1016/j.celrep.2019.02.079. [DOI] [PubMed] [Google Scholar]
- 23.Stanhope R, Flora E, Bayne C, Derre I. 2017. IncV, a FFAT motif-containing Chlamydia protein, tethers the endoplasmic reticulum to the pathogen-containing vacuole. Proc Natl Acad Sci USA 114:12039–12044. 10.1073/pnas.1709060114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derre I, Swiss R, Agaisse H. 2011. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog 7:e1002092. 10.1371/journal.ppat.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber MM, Lam JL, Dooley CA, Noriea NF, Hansen BT, Hoyt FH, Carmody AB, Sturdevant GL, Hackstadt T. 2017. Absence of specific Chlamydia trachomatis inclusion membrane proteins triggers premature inclusion membrane lysis and host cell death. Cell Rep 19:1406–1417. 10.1016/j.celrep.2017.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sixt BS, Bastidas RJ, Finethy R, Baxter RM, Carpenter VK, Kroemer G, Coers J, Valdivia RH. 2017. The Chlamydia trachomatis inclusion membrane protein CpoS counteracts STING-mediated cellular surveillance and suicide programs. Cell Host Microbe 21:113–121. 10.1016/j.chom.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wesolowski J, Weber MM, Nawrotek A, Dooley CA, Calderon M, St Croix CM, Hackstadt T, Cherfils J, Paumet F. 2017. Chlamydia hijacks ARF GTPases to coordinate microtubule posttranslational modifications and Golgi complex positioning. mBio 8. 10.1128/mBio.02280-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutter EI, Barger AC, Nair V, Hackstadt T. 2013. Chlamydia trachomatis inclusion membrane protein CT228 recruits elements of the myosin phosphatase pathway to regulate release mechanisms. Cell Rep 3:1921–1931. 10.1016/j.celrep.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen PH, Lutter EI, Hackstadt T. 2018. Chlamydia trachomatis inclusion membrane protein MrcA interacts with the inositol 1,4,5-trisphosphate receptor type 3 (ITPR3) to regulate extrusion formation. PLoS Pathog 14:e1006911. 10.1371/journal.ppat.1006911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop RC, Derre I. 2022. The Chlamydia trachomatis inclusion membrane protein CTL0390 mediates host cell exit via lysis through STING activation. Infect Immun 10.1128/iai.00190-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mital J, Miller NJ, Fischer ER, Hackstadt T. 2010. Specific chlamydial inclusion membrane proteins associate with active Src family kinases in microdomains that interact with the host microtubule network. Cell Microbiol 12:1235–1249. 10.1111/j.1462-5822.2010.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mital J, Lutter EI, Barger AC, Dooley CA, Hackstadt T. 2015. Chlamydia trachomatis inclusion membrane protein CT850 interacts with the dynein light chain DYNLT1 (Tctex1). Biochem Biophys Res Commun 462:165–170. 10.1016/j.bbrc.2015.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown HM, Knowlton AE, Grieshaber SS. 2012. Chlamydial infection induces host cytokinesis failure at abscission. Cell Microbiol 14:1554–1567. 10.1111/j.1462-5822.2012.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greene W, Zhong G. 2003. Inhibition of host cell cytokinesis by Chlamydia trachomatis infection. J Infect 47:45–51. 10.1016/s0163-4453(03)00039-2. [DOI] [PubMed] [Google Scholar]
- 35.Brown HM, Knowlton AE, Snavely E, Nguyen BD, Richards TS, Grieshaber SS. 2014. Multinucleation during C. trachomatis infections is caused by the contribution of two effector pathways. PLoS One 9:e100763. 10.1371/journal.pone.0100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almeida F, Luis MP, Pereira IS, Pais SV, Mota LJ. 2018. The human centrosomal protein CCDC146 binds Chlamydia trachomatis inclusion membrane protein CT288 and is recruited to the periphery of the Chlamydia-containing vacuole. Front Cell Infect Microbiol 8:254. 10.3389/fcimb.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun HS, Wilde A, Harrison RE. 2011. Chlamydia trachomatis inclusions induce asymmetric cleavage furrow formation and ingression failure in host cells. Mol Cell Biol 31:5011–5022. 10.1128/MCB.05734-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almeida F, Borges V, Ferreira R, Borrego MJ, Gomes JP, Mota LJ. 2012. Polymorphisms in Inc proteins and differential expression of inc genes among Chlamydia trachomatis strains correlate with invasiveness and tropism of lymphogranuloma venereum isolates. J Bacteriol 194:6574–6585. 10.1128/JB.01428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutter EI, Bonner C, Holland MJ, Suchland RJ, Stamm WE, Jewett TJ, McClarty G, Hackstadt T. 2010. Phylogenetic analysis of Chlamydia trachomatis Tarp and correlation with clinical phenotype. Infect Immun 78:3678–3688. 10.1128/IAI.00515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knowlton AE, Brown HM, Richards TS, Andreolas LA, Patel RK, Grieshaber SS. 2011. Chlamydia trachomatis infection causes mitotic spindle pole defects independently from its effects on centrosome amplification. Traffic 12:854–866. 10.1111/j.1600-0854.2011.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson KA, Tan M, Sutterlin C. 2009. Centrosome abnormalities during a Chlamydia trachomatis infection are caused by dysregulation of the normal duplication pathway. Cell Microbiol 11:1064–1073. 10.1111/j.1462-5822.2009.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang K, Munoz KJ, Tan M, Sutterlin C. 2021. Chlamydia and HPV induce centrosome amplification in the host cell through additive mechanisms. Cell Microbiol 23:e13397. 10.1111/cmi.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascanzoni F, Iannitti R, Colanzi A. 2022. Functional coordination among the Golgi complex, the centrosome and the microtubule cytoskeleton during the cell cycle. Cells 11:354. 10.3390/cells11030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr MC, Gomez GA, Ferguson C, Tanzer MC, Murphy JM, Yap AS, Parton RG, Huston WM, Teasdale RD. 2017. Laser-mediated rupture of chlamydial inclusions triggers pathogen egress and host cell necrosis. Nat Commun 8:14729. 10.1038/ncomms14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackstadt T, Rockey DD, Heinzen RA, Scidmore MA. 1996. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J 15:964–977. 10.1002/j.1460-2075.1996.tb00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Addi C, Bai J, Echard A. 2018. Actin, microtubule, septin and ESCRT filament remodeling during late steps of cytokinesis. Curr Opin Cell Biol 50:27–34. 10.1016/j.ceb.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Burakov A, Nadezhdina E, Slepchenko B, Rodionov V. 2003. Centrosome positioning in interphase cells. J Cell Biol 162:963–969. 10.1083/jcb.200305082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherry J, Dolat L, McMahon E, Swaney DL, Bastidas RJ, Johnson JR, Valdivia RH, Krogan NJ, Elwell CA, Engel JN. 2022. Chlamydia trachomatis effector Dre1 interacts with dynactin to reposition host organelles during infection. bioRxiv. https://www.biorxiv.org/content/10.1101/2022.04.15.488217v1.
- 49.Mirrashidi KM, Elwell CA, Verschueren E, Johnson JR, Frando A, Von Dollen J, Rosenberg O, Gulbahce N, Jang G, Johnson T, Jager S, Gopalakrishnan AM, Sherry J, Dunn JD, Olive A, Penn B, Shales M, Cox JS, Starnbach MN, Derre I, Valdivia R, Krogan NJ, Engel J. 2015. Global mapping of the Inc-human interactome reveals that retromer restricts Chlamydia infection. Cell Host Microbe 18:109–121. 10.1016/j.chom.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reck-Peterson SL, Redwine WB, Vale RD, Carter AP. 2018. The cytoplasmic dynein transport machinery and its many cargoes. Nat Rev Mol Cell Biol 19:382–398. 10.1038/s41580-018-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salcedo SP, Holden DW. 2003. SseG, a virulence protein that targets Salmonella to the Golgi network. EMBO J 22:5003–5014. 10.1093/emboj/cdg517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrahams GL, Muller P, Hensel M. 2006. Functional dissection of SseF, a type III effector protein involved in positioning the Salmonella-containing vacuole. Traffic 7:950–965. 10.1111/j.1600-0854.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 53.Deiwick J, Salcedo SP, Boucrot E, Gilliland SM, Henry T, Petermann N, Waterman SR, Gorvel JP, Holden DW, Meresse S. 2006. The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect Immun 74:6965–6972. 10.1128/IAI.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu XJ, Liu M, Holden DW. 2016. Salmonella effectors SseF and SseG interact with mammalian protein ACBD3 (GCP60) to anchor Salmonella-containing vacuoles at the Golgi network. mBio 7. 10.1128/mBio.00474-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramsden AE, Holden DW, Mota LJ. 2007. Membrane dynamics and spatial distribution of Salmonella-containing vacuoles. Trends Microbiol 15:516–524. 10.1016/j.tim.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Santos AJM, Durkin CH, Helaine S, Boucrot E, Holden DW. 2016. Clustered intracellular Salmonella enterica serovar Typhimurium blocks host cell cytokinesis. Infect Immun 84:2149–2158. 10.1128/IAI.00062-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alzhanov DT, Weeks SK, Burnett JR, Rockey DD. 2009. Cytokinesis is blocked in mammalian cells transfected with Chlamydia trachomatis gene CT223. BMC Microbiol 9:2. 10.1186/1471-2180-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kokes M, Dunn JD, Granek JA, Nguyen BD, Barker JR, Valdivia RH, Bastidas RJ. 2015. Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of Chlamydia. Cell Host Microbe 17:716–725. 10.1016/j.chom.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haines A, Wesolowski J, Ryan NM, Monteiro-Bras T, Paumet F. 2021. Cross talk between ARF1 and RhoA coordinates the formation of cytoskeletal scaffolds during Chlamydia infection. mBio 12:e0239721. 10.1128/mBio.02397-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bocker S, Heurich A, Franke C, Monajembashi S, Sachse K, Saluz HP, Hanel F. 2014. Chlamydia psittaci inclusion membrane protein IncB associates with host protein Snapin. Int J Med Microbiol 304:542–553. 10.1016/j.ijmm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Pais SV, Key CE, Borges V, Pereira IS, Gomes JP, Fisher DJ, Mota LJ. 2019. CteG is a Chlamydia trachomatis effector protein that associates with the Golgi complex of infected host cells. Sci Rep 9:6133. 10.1038/s41598-019-42647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pereira IS, Pais SV, Borges V, Borrego MJ, Gomes JP, Mota LJ. 2022. The type III secretion effector CteG mediates host cell lytic exit of Chlamydia trachomatis. Front Cell Infect Microbiol 12:902210. 10.3389/fcimb.2022.902210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steiert B, Icardi CM, Faris R, Klingelhutz AJ, Yau PM, Weber MM. 2022. The Chlamydia trachomatis type III secreted effector protein CteG induces centrosome amplification through interactions with centrin-2. bioRxiv. https://www.biorxiv.org/content/10.1101/2022.06.23.496711. [DOI] [PMC free article] [PubMed]
- 64.Saka HA, Thompson JW, Chen YS, Kumar Y, Dubois LG, Moseley MA, Valdivia RH. 2011. Quantitative proteomics reveals metabolic and pathogenic properties of Chlamydia trachomatis developmental forms. Mol Microbiol 82:1185–1203. 10.1111/j.1365-2958.2011.07877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel JC, Hueffer K, Lam TT, Galan JE. 2009. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell 137:283–294. 10.1016/j.cell.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith JS, Munoz N, Herrero R, Eluf-Neto J, Ngelangel C, Franceschi S, Bosch FX, Walboomers JM, Peeling RW. 2002. Evidence for Chlamydia trachomatis as a human papillomavirus cofactor in the etiology of invasive cervical cancer in Brazil and the Philippines. J Infect Dis 185:324–331. 10.1086/338569. [DOI] [PubMed] [Google Scholar]
- 67.Zhu H, Shen Z, Luo H, Zhang W, Zhu X. 2016. Chlamydia trachomatis infection-associated risk of cervical cancer: a meta-analysis. Medicine (Baltimore) 95:e3077. 10.1097/MD.0000000000003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agaisse H, Derre I. 2013. A C. trachomatis cloning vector and the generation of C. trachomatis strains expressing fluorescent proteins under the control of a C. trachomatis promoter. PLoS One 8:e57090. 10.1371/journal.pone.0057090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.da Cunha M, Pais SV, Bugalhao JN, Mota LJ. 2017. The Chlamydia trachomatis type III secretion substrates CT142, CT143, and CT144 are secreted into the lumen of the inclusion. PLoS One 12:e0178856. 10.1371/journal.pone.0178856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uphoff CC, Drexler HG. 2011. Detecting mycoplasma contamination in cell cultures by polymerase chain reaction. Methods Mol Biol 731:93–103. 10.1007/978-1-61779-080-5_8. [DOI] [PubMed] [Google Scholar]
- 71.Scidmore MA. 2006. Cultivation and laboratory maintenance of Chlamydia trachomatis. CP Microbiology 00:Unit 11A.1. 10.1002/9780471729259.mc11a01s00. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen BD, Valdivia RH. 2013. Forward genetic approaches in Chlamydia trachomatis. J Vis Exp 23:e50636. 10.3791/50636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Studier FW. 2005. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234. 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 74.Hamaoui D, Cosse MM, Mohan J, Lystad AH, Wollert T, Subtil A. 2020. The Chlamydia effector CT622/TaiP targets a nonautophagy related function of ATG16L1. Proc Natl Acad Sci USA 117:26784–26794. 10.1073/pnas.2005389117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3 and Fig. S1 to S8. Download iai.00405-22-s0001.pdf, PDF file, 1.2 MB (1.2MB, pdf)