Abstract
Introduction:
The incidence and factors related to early cognitive impairment (ECI) after mild traumatic brain injury (mTBI) in pediatric trauma patients (PTPs) are unknown. Prior data in the adult population demonstrated an ECI incidence of 51% after mTBI and strong correlation with initial Glasgow Coma Scale (GCS) and Brain Injury Guidelines (BIG) category. Therefore, we hypothesized that ECI is common after mTBI in PTPs and associated with initial GCS and BIG category.
Methods:
A single-center, retrospective review of PTPs (age 8–17) from 2015–2019 with intracranial hemorrhage and mTBI (GCS 13–15) was performed. Primary outcome was ECI, defined as Ranchos Los Amigos score <8. Comparisons between ECI and non-ECI groups regarding injury severity score (ISS), demographics, and cognitive and clinical outcomes were evaluated using chi-square statistics and Wilcoxon rank sum tests. Odds of ECI were evaluated using multivariable logistic regression.
Results:
From 47 PTPs with mTBI, 19 (38.3%) had ECI. ECI patients had a higher ISS than non-ECI patients (19.7 vs. 12.6, p=0.003). Injuries involving motor vehicles were more often related to ECI than non-auto-involved mechanisms (55% vs. 15%, p=0.005). Lower GCS (OR 6.60, 95% CI 1.34–32.51, p=0.02), higher ISS (1.12, 1.01–1.24, p=0.030), and auto-involved injuries (6.06, 1.15–31.94, p=0.030) were all associated with increased risk of ECI. There was no association between BIG category and risk of ECI (p>0.05).
Conclusion:
Nearly 40% of PTPs with mTBI suffer from ECI. Lower initial GCS, higher ISS, and auto-involved mechanism of injury were associated with increased risk of ECI. BIG category was not associated with ECI in pediatric patients.
Study Type:
Retrospective, prognostic study
Level of Evidence:
Level III
Keywords: Traumatic brain injury (TBI), cognitive impairment, intracranial hemorrhage (ICH), pediatrics
Introduction
Traumatic brain injury (TBI) in children and adolescents remains a significant cause of morbidity and mortality worldwide (1,2). There were nearly three million emergency department visits for children with minor TBI in the United States between 2005 and 2009 (3). Furthermore, chronic complications are common in survivors of childhood brain injury. Up to 25% of children will suffer from persistent post-concussive symptoms, and some children show impaired verbal comprehension up to ten years after mild TBI (4–9). However, the majority of research on pediatric TBI has been focused on prevention of secondary injury or prognosis of post-concussive symptoms, and little is known about the incidence and predictive factors for early cognitive impairment (ECI) following mild TBI (5).
While knowing the significant burden associated with pediatric brain injuries helps to prognosticate the likelihood of recovery, it does little to identify those patients that may benefit from early cognitive intervention or therapies. A prior study by Delaplain et al. demonstrated that over half of adult trauma patients with intracranial hemorrhage (ICH) and mild TBI had ECI (10). In addition, they found that Glasgow Coma Scale (GCS) and Brain Injury Guidelines (BIG) category were associated with an increased risk of ECI and could be used as an indicator for patients who would benefit from prompt cognitive evaluation and intervention.
This study sought to identify factors related to ECI for pediatric trauma patients with mild TBI in the setting of ICH or skull fracture. It was hypothesized that the incidence of ECI in pediatric trauma patients is common and, similarly to adults, can be predicted by GCS and BIG score.
Methods
A. Study Design
Institutional Review Board approval was obtained from the University of California, Irvine to retrospectively review pediatric trauma patients with mild TBI. Mild TBI was defined as a presenting GCS between 13–15 with radiographic evidence of skull fracture or ICH, including subdural hematoma (SDH), epidural hematoma (EDH), subarachnoid hemorrhage (SAH), intraparenchymal hemorrhage (IPH), intraventricular hemorrhage (IVH), or diffuse axonal injury (DAI). The study included patients 8–18 years of age who were admitted between 2015–2019, were found to have mild TBI, and underwent at least one cognitive assessment during their hospitalization. Patients were excluded if a cognitive evaluation was not performed (n=4) or if a Rancho score was not reported in the cognitive evaluation (n=2). Patients under eight years old were excluded for ease of comparison with the intent of ensuring reliable cognitive evaluation among the pediatric cohort.
Study data were collected and managed using REDCap (11). Information collected included demographics such as age and sex, mechanism of injury, presenting symptoms and history, interventions for intracranial hypertension (intracranial pressure monitor placement, craniotomy, use of mannitol, or use of hypertonic saline), and initial GCS. In addition, outcomes and complications were recorded including seizures, hospital length of stay (LOS), intensive care (ICU) LOS, discharge location, 30-day readmission, mortality, follow-up information, and persistent TBI symptoms. Injury-related data including loss of consciousness and the injury severity score (ISS) were also obtained. Initial head computed tomography (CT) findings were also collected, including type and size of ICH and details of skull fracture. Information regarding interval change was collected when repeat head imaging was performed based solely on clinician discretion, as directed by the consulting neurosurgeon. All imaging findings were then reported based on the formal radiologist read. Rotterdam score and BIG criteria were then calculated based on these results (12,13).
Patient information such as presence of baseline cognitive deficits and results of cognitive evaluation scores was also recorded. Although multiple versions of pediatric cognitive evaluations are used in clinical settings, the adult Rancho Los Amigos (RLA) scale was the most universally reported for our patient population and was therefore chosen as the primary endpoint for this study. At our institution, it is standard practice that all pediatric patients undergo cognitive evaluation if they have cross-sectional imaging consistent with intracranial hemorrhage or skull fracture. Evaluations were completed by a licensed speech-language pathologist with significant pediatric trauma experience within 24 hours, unless the patient was intubated, intoxicated, or otherwise unable to participate in evaluation. If the patient received continued intervention or if there was concern regarding the accuracy of the original evaluation (e.g. related to recent narcotics), cognitive testing was repeated later in the hospital stay. If patients received narcotics (codeine, fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, and tramadol) in the 24 hours prior to cognitive evaluation, proximity to testing and morphine milligram equivalents (MME) were determined.
B. Statistical Analysis
The primary outcome was ECI, defined as an adult RLA score < 8. Injury categories were combined to create a binary variable for mechanism: motor-vehicle (i.e., auto or motorcycle) or other (e.g., assault, fall, etc.). Demographic, cognitive, and clinical data between the ECI and non-ECI groups were compared using Chi-square statistics and Wilcoxon rank sum tests for categorical and continuous variables, respectively. Odds of ECI were estimated using logistic regression models which included variables that were significantly associated with ECI at p<0.10. Akaike information criterion (AIC) and Bayesian information criterion (BIC) statistics were used to select the final multivariable model. All analyses were performed in Stata 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Results
A. Demographics & Patient Characteristics
Of 47 pediatric patients with mild TBI, a total of 18 (38.3%) had ECI. When compared to non-ECI patients, ECI patients had lower GCS (14.3 vs. 14.8, p=0.010), and of the subcategories, the largest difference was in the verbal score (4.4 vs. 4.9, p=0.020). In addition, the ECI group had more severe injuries, on average, than the non-ECI group (ISS 19.7 vs. 12.6, p=0.0031) and were more likely to have sustained a motor-vehicle related mechanism of injury (83.3% vs. 41.3%, p=0.005). A summary of demographic information is in Table 1.
Table 1:
Comparison of patient characteristics for patients with and without ECI.
| Characteristic | No ECI (n=29) | ECI (n=18) | p-value* |
|---|---|---|---|
| Mean (SD)/Count (%) | Mean (SD)/Count (%) | ||
| Demographics | |||
| Age | 14.6 (2.7) | 13.9 (3.3) | 0.61 |
| Sex | 0.49 | ||
| Female | 6 (20.7) | 6 (33.3) | |
| Male | 22 (75.9) | 12 (66.7) | |
| ISS | 12.6 (7.4) | 19.7 (10.2) | 0.0031 |
| AIS-head | 3.1 (0.8) | 3.2 (1.0) | 0.69 |
| Mechanism of Injury | 0.12 | ||
| MVC | 8 (27.6) | 7 (38.9) | |
| MCC | 0 (0.0) | 1 (5.6) | |
| Auto vs. pedestrian | 3 (10.3) | 4 (22.2) | |
| Auto vs. bicycle | 1 (3.5) | 3 (16.7) | |
| Assault | 3 (10.3) | 1 (5.6) | |
| Fall | 7 (24.1) | 1 (5.6) | |
| Other | 7 (24.1) | 1 (5.6) | |
| Presentation | |||
| LOC | 23 (79.3) | 16 (88.9) | 0.49 |
| Exam | |||
| GCS score | 0.02 | ||
| 13 | 0 (0.0) | 3 (16.67) | |
| 14 | 5 (17.24) | 6 (33.33) | |
| 15 | 24 (82.76) | 9 (50.0) | |
| GCS sub-categories | |||
| eye opening | 4.0 (0.2) | 4 (0) | 1.0 |
| verbal | 4.9 (0.4) | 4.4 (0.7) | 0.02 |
| motor | 6 (0) | 5.9 (0.2) | 0.74 |
ECI (early cognitive impairment), SD (standard deviation), ISS (injury severity score), AIS (abbreviated injury score), MVC (motor vehicle crash), MCC (motorcycle crash), LOC (loss of consciousness)
P values were calculated for each continuous variable using Wilcoxon rank sum tests (age, ISS, AIS, GCS). P values for categorial variables were reported using Pearson’s χ2 (remaining variables).
B. Imaging
All patients underwent at least one head CT scan and 70.2% (n=33) underwent a repeat head CT during their hospital stay. The most common type of hemorrhage was SAH (59.5%) followed by SDH (48.9%). Only one patient in the ECI group had an IVH, and no patients in the non-ECI group had an IVH. Of those patients who underwent a repeat CT scan, 72.7% had no interval change while 21.2% had an increased size of hemorrhage and 9.1% had a new type of ICH. When compared to patients without ECI, patients with ECI did not show any difference between incidence of skull fracture, displacement of skull fracture, or average Rotterdam score (all p>0.05, data in Table 2).
Table 2:
Summary of CT findings
| Characteristic | No ECI (n=29) | ECI (n=18) | p-value* |
|---|---|---|---|
| Mean (SD)/Count (%) | Mean (SD)/Count (%) | ||
| Skull fracture | 13 (44.8) | 8 (53.3) | 0.59 |
| Displaced | 2 (15.4) | 2 (25.0) | 0.59 |
| ICH | |||
| Subdural | 14 (48.3) | 9 (50.0) | 0.91 |
| Size (mm) | 4 (1.85) | 8.57 (9.64) | 0.21 |
| Epidural | 7 (24.1) | 2 (11.1) | 0.27 |
| Size (mm) | 10.1 (6.9) | 12 (7.1) | 0.61 |
| SAH | 16 (55.2) | 12 (66.7) | 0.44 |
| Trace | 13 (44.8) | 10 (55.6) | 0.73 |
| Localized | 0 (0.0) | 0 (0.0) | |
| Scattered | 3 (10.3) | 2 (11.1) | |
| IPH | 5 (17.2) | 0 (0) | 0.06 |
| Max Size (mm) | 6 | -- | |
| 1 Location | 3 | 0 | |
| 2 Locations | 1 | 0 | |
| 3+ Locations | 0 | 0 | |
| IVH | 0 (0) | 1 (5.6) | 0.20 |
| Scoring | |||
| Rotterdam score (median/IQR) | 2 (1–3) | 2.5 (1–4) | 0.06 |
| BIG category | |||
| 1 | 7 (24.1) | 7 (38.9) | 0.28 |
| 2 | 12 (41.4) | 3 (16.7) | 0.08 |
| 3 | 10 (34.5) | 8 (44.4) | 0.59 |
| Repeat CT head | |||
| Performed (yes) | 19 (65.5) | 14 (77.8) | 0.37 |
| No new findings | 12 (41.4) | 12 (66.7) | 0.09 |
| Increased size of hemorrhage | 6 (20.7) | 1 (5.6) | 0.16 |
| New type of hemorrhage | 2 6.9) | 1 (5.6) | 0.86 |
CT (computed tomography), ECI (early cognitive impairment), SD (standard deviation), ICH (intracranial hemorrhage), SDH (subdural hemorrhage), EDH (epidural hemorrhage), SAH (subarachnoid hemorrhage), IPH (intraparenchymal hemorrhage), IQR (interquartile range), BIG (Brain Injury Guidelines)
P values were calculated for each continuous variable using Wilcoxon rank sum tests (Rotterdam score). P values for categorial variables were reported using Pearson’s χ2 (remaining variables).
C. Cognitive Evaluation
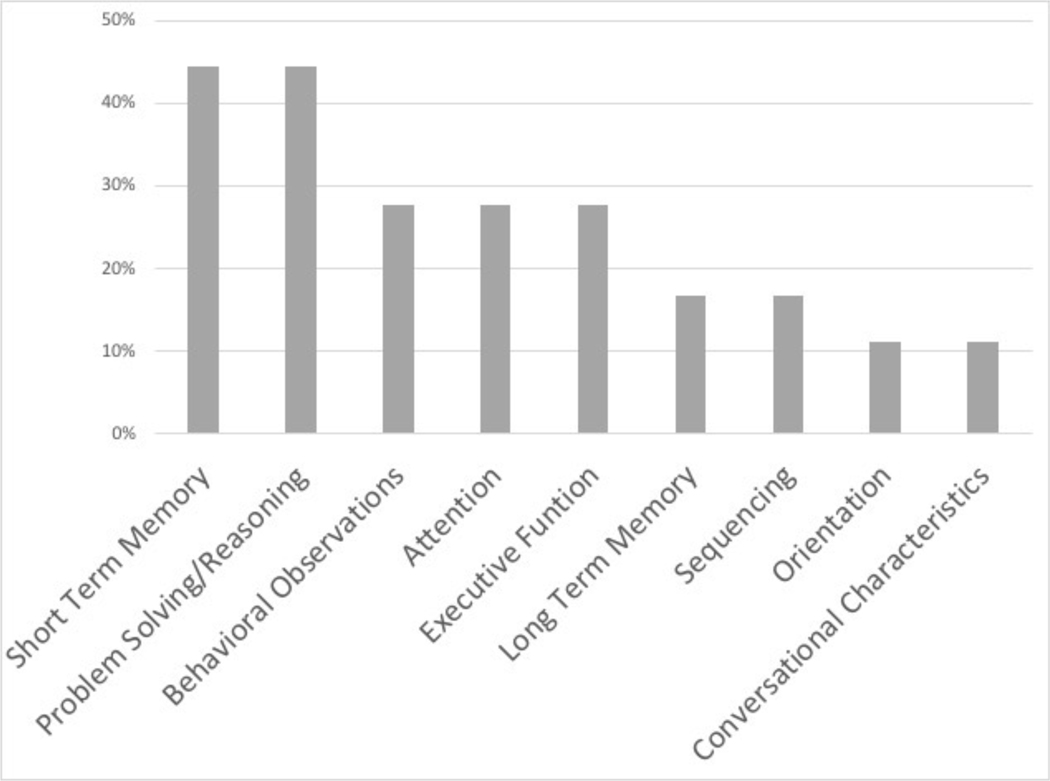
Of 47 patients presenting with mild TBI, only two patients had a pre-existing cognitive deficit, and one of these was found to have concomitant ECI. Of those patients with an abnormal initial cognitive evaluation, 11 (61.1%) underwent at least one additional evaluation prior to discharge. While the average RLA score on repeat evaluation did increase in ECI patients (6.4 initial, 7.3 repeat), the majority did not return to baseline at discharge (63.6%). The most common areas of RLA deficit on initial evaluation were short term memory and problem solving/reasoning (both 44.4%), and 77.8 of ECI patients had a deficit in more than one area (Figure 1).
Figure 1.
Areas of deficit on Rancho Los Amigos cognitive evaluation
When compared to patients without ECI, patients with ECI had no differences in administration of narcotic pain medications with regard to percentage receiving medication (50% vs. 62%), average time interval between administration and cognitive evaluation (8.6 hours vs. 6.7 hours), or average MME (4.1 vs. 4.5) (all p>0.05).
D. Interventions & Outcomes
Of all participants in the study, only 10.6% (n=5) underwent a craniotomy. The two patients in the non-ECI group who required a craniotomy received no additional interventions. While one of the three ECI patients underwent craniotomy alone, two also received mannitol and hypertonic saline (Table 3). No patients from either the ECI or non-ECI group received anti-platelet or anticoagulation agents.
Table 3.
Comparison of interventions between ECI and non-ECI patients
| Intervention | No ECI (n=29) | ECI (n=18) | p-value* |
|---|---|---|---|
| Mean (SD)/Count (%) | Mean (SD)/Count (%) | ||
| Seizure prophylaxis | 8 (28.6) | 4 (22.2) | 0.63 |
| Craniotomy/craniectomy | 2 (6.9) | 3 (16.7) | 0.29 |
| EVD | 0 (0) | 0 (0) | -- |
| Bolt | 0 (0) | 1 (5.6) | 0.20 |
| Mannitol | 0 (0) | 2 (11.1) | 0.07 |
| Hypertonic saline | 0 (0) | 2 (11.1) | 0.07 |
ECI (early cognitive impairment), SD (standard deviation), EVD (external ventricular drain)
P values were calculated using Pearson’s χ2.
There were no mortalities or seizures for pediatric patients in either the ECI or non-ECI group during index hospitalization, and no patients were readmitted to the hospital within 30 days of their discharge. Two patients in the non-ECI group returned to the ED within 30 days for reasons unrelated to their mild TBI, and one patient from the ECI group was discharged with a persistent neurological deficit. Patients with ECI had a greater average hospital (5.6 vs. 2.6 days, p=0.002) and ICU (2.6 vs. 1.7 days, p=0.02) LOS. However, there was no difference in rate of discharge home or incidence of persistent TBI symptoms following discharge (all p>0.05, data in Table 4).
Table 4.
Outcomes for ECI vs. non-ECI patients
| Outcome | No ECI (n=29) | ECI (n=18) | p-value* |
|---|---|---|---|
| Mean (SD)/Count (%) | Mean (SD)/Count (%) | ||
| Hospital LOS | 2.6 (1.5) | 5.6 (3.9) | 0.002 |
| ICU LOS | 1.7 (0.8) | 2.6 (1.9) | 0.02 |
| Discharge location | 0.24 | ||
| Home | 25 (86.2) | 12 (70.6) | |
| Acute care facility | 3 (10.3) | 2 (11.7) | |
| Acute rehab facility | 1 (3.5) | 3 (16.7) | |
| Follow up appt. | 11 (37.9) | 4 (22.2) | 0.26 |
| Memory loss | 1 (3.5) | 1 (5.6) | 0.73 |
| Headache | 4 (13.8) | 3 (16.7) | 0.79 |
| Other TBI symptoms | 3 (10.3) | 2 (11.1) | 0.93 |
ECI (early cognitive impairment), SD (standard deviation), LOS (length of stay), ICU (intensive care unit), Follow up appt. (follow up appointment), TBI (traumatic brain injury)
P values were calculated for each continuous variable using Wilcoxon rank sum tests (Hospital LOS and ICU LOS). P values for categorial variables were reported using Pearson’s χ2 (remaining variables).
E. Risk of early cognitive impairment
In a multivariable logistic regression model (Table 5) a one point lower initial GCS score was associated with a more than seven-fold increase in the estimated odds of ECI after controlling for ISS and motor vehicle-related mechanism of injury (OR 6.60, 95% CI 1.34–32.51, p=0.02). In addition, a one point increase in ISS was associated with an increased risk of ECI using the same model (OR 1.12, 95%CI 1.01–1.24, p=0.03). Any injury involving a motor vehicle was also found to increase the associated risk of ECI by more than six-fold (OR 6.06, 95%CI 1.15–31.94, p=0.03). Other variables, including BIG category and Rotterdam score, were not associated with risk of ECI.
Table 5.
Multivariable logistic regression for risk of ECI
| Variable | Adjusted OR | 95% CI | p-value |
|---|---|---|---|
| ISS (per unit increase) | 1.12 | 1.01–1.24 | 0.03 |
| GCS (per unit decrease) | 6.60 | 1.34–32.51 | 0.02 |
| Motor vehicle MOI | 6.06 | 1.15–31.94 | 0.03 |
ECI (early cognitive impairment), OR (odds ratio), CI (confidence interval), ISS (injury severity score), GCS (Glasgow Coma Scale), MOI (mechanism of injury)
Discussion
TBI is one of the leading causes of death in children and adolescents, and the long-term disability among survivors can be devastating. Identifying youth at risk of cognitive impairment is critical, but the incidence and risk factors for ECI have not been specifically examined in pediatric patients with apparently mild TBI. While, similar to adults, a reduction in GCS was associated with an increased risk of ECI, there was no association with BIG category. Instead, the overall severity of injuries (as measured by ISS) and injury mechanism were associated with risk of ECI.
The correlation of severity of overall injury and post-TBI symptoms has been previously demonstrated in regard to persistent post-concussive symptoms (6,14). However, our study evaluates more than concussive symptoms, as the most common findings of ECI included problem solving and reasoning deficiencies. These subtle findings may not be readily identified by parents or even in brief physician-patient encounters, emphasizing the importance of a detailed cognitive evaluation in the setting of mild TBI for pediatric patients.
Although our study found the incidence of ECI in pediatric mild TBI patients was less than adult patients (approximately 40% vs. 50%, respectively), this still represents a large portion of the pediatric trauma population. In addition, over 60% of these patients had a need for continuing therapy following discharge. Furthermore, this study suggests the incidence of ECI following mild TBI in pediatric patients may be higher than estimates of other post-mild TBI disability seen by Rivara et al. (15). However, this difference in incidence may be attributed to the timeline for evaluation of deficits, with our study identifying impairment during acute hospitalization and Rivara et al. including disability identified at 12 months after injury. As such, this study identifies a potential gap in recognition of disability that may negatively impact the child’s performance at school or in social settings.
Conventional evaluations regarding the degree or severity of TBI include GCS and BIG category, among others. While these findings have clearly demonstrated roles in the evaluation of brain injury for adults, less is known about their ability to predict ECI in children. The findings of this study are consistent with a study by Nesiama et al. which demonstrated that GCS does have a high association with functional outcomes in children following TBI as measured by Glasgow Outcome Score and Disability Rating Scale (16). This suggests that GCS may be utilized not only for neurologic evaluation in the acute setting, but also as an indicator to identify patients who are at risk of developing ECI as a result of their injuries.
To the knowledge of these authors, this is the first study to evaluate if the BIG category could predict ECI in a pediatric population. Contrary to the previous adult study by Delaplain et al., our study did not show a correlation between BIG category and ECI in pediatric mild TBI patients (10). This may be attributable to the low sample size in this study, as BIG category has been validated for use in pediatric patients (17). Alternatively, it is possible that while BIG category is useful for determining the need for neurosurgical consultation and/or admission, it may not have a correlation with cognitive outcomes in pediatric trauma patients who have heightened neuroplasticity and overall superior outcomes compared to adults (18). Future, more adequately powered prospective studies are needed to definitively determine the utility of BIG to predict ECI.
This study also demonstrated ISS to be associated with risk of ECI, suggesting that children with more extensive physical injuries are at greater risk for developing ECI. Although the per unit of ISS finding appears to be modest, it does suggest screening to be important especially in the severely injured child.
This study has several limitations, including those inherent to a single-center, retrospective study. The small sample size is a significant limitation and given the single-center nature of the study it requires further multicenter evaluation before results can be generalized to a larger population. However, this was the full extent of data we had available and we are therefore unable to remedy this substantial limitation without a multicenter design. While adult RLA scores are commonly used at our institution and other authors have published the successful application of these scores to the pediatric population, a scoring system designed specifically for children, such as Glasgow Outcome Scale Extended Pediatrics or the Pediatric Quality of Life Inventory, may have changed the incidence or risk factors for ECI (19,20). Furthermore, this study was limited to the index hospitalization and a 30-day follow-up interval within our health system. Many patients live far from our hospital or are mandated by their insurance to receive outpatient medical care at other organizations, and thus were unable to receive follow-up care at our institution. Details of follow-up care from outside providers and a longer follow-up interval would have provided additional useful details. In addition, follow-up visits did not include reassessment of the RLA scale, limiting the conclusions which can be drawn regarding longevity of the impact of ECI and importance of early intervention.
In conclusion, this study demonstrates that ECI affects almost 40% of pediatric patients with mild TBI and either ICH or skull fracture. Motor vehicle related injury and GCS score, particularly the verbal subcategory, may be useful to predict risk of ECI in pediatric patients who have sustained mild TBI. While we were unable to determine the proportion of patients with ECI that will go on to have long-term cognitive impairment, early identification and treatment of at-risk children is critical to prevent potential developmental and social complications among pediatric trauma patients (21). In the absence of the ability to screen all pediatric trauma patients with mild TBI, which the authors would recommend, practitioners should utilize these findings to guide decisions regarding which pediatric trauma patients are most likely to benefit from formal cognitive evaluation.
Funding:
There were no external sources of funding related to this project.
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest related to this project.
References
- 1.Appavu B, Foldes ST, Adelson PD. Clinical trials for pediatric traumatic brain injury: definition of insanity? J Neurosurg Pediatr. 2019. 01;23(6):661–9. [DOI] [PubMed] [Google Scholar]
- 2.Dewan MC, Mummareddy N, Wellons JC, Bonfield CM. Epidemiology of Global Pediatric Traumatic Brain Injury: Qualitative Review. World Neurosurg. 2016. Jul;91:497–509.e1. [DOI] [PubMed] [Google Scholar]
- 3.Mannix R, O’Brien MJ, Meehan WP. The epidemiology of outpatient visits for minor head injury: 2005 to 2009. Neurosurgery. 2013. Jul;73(1):129–34; discussion 134. [DOI] [PubMed] [Google Scholar]
- 4.Lumba-Brown A, Yeates KO, Sarmiento K, Breiding MJ, Haegerich TM, Gioia GA, Turner M, Benzel EC, Suskauer S, Giza CC, et al. Diagnosis and Management of Mild Traumatic Brain Injury in Children: A Systematic Review. JAMA Pediatr. 2018. 01;172(11):e182847. [DOI] [PubMed] [Google Scholar]
- 5.Lumba-Brown A, Yeates KO, Sarmiento K, Breiding MJ, Haegerich TM, Gioia GA, Turner M, Benzel EC, Suskauer S, Giza CC, et al. Centers for Disease Control and Prevention Guideline on the Diagnosis and Management of Mild Traumatic Brain Injury Among Children. JAMA Pediatr. 2018. 01;172(11):e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell DR, Zemek R, Brilliant AN, Mannix RC, Master CL, Meehan WP. Identifying Persistent Postconcussion Symptom Risk in a Pediatric Sports Medicine Clinic. Am J Sports Med. 2018;46(13):3254–61. [DOI] [PubMed] [Google Scholar]
- 7.Ewing-Cobbs L, Cox CS, Clark AE, Holubkov R, Keenan HT. Persistent Postconcussion Symptoms After Injury. Pediatrics. 2018;142(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor HG, Dietrich A, Nuss K, Wright M, Rusin J, Bangert B, Minich N, Yeates K. Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology. 2010. Mar;24(2):148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics. 2012. Feb;129(2):e254–261. [DOI] [PubMed] [Google Scholar]
- 10.Delaplain PT, Albertson S, Grigorian A, Williams B, Smith M, Inaba K, Lekawa M, Nahmias J. Early cognitive impairment is common after intracranial hemorrhage with mild traumatic brain injury. J Trauma Acute Care Surg. 2020;89(1):215–21. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. Apr;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maas AIR, Hukkelhoven CWPM, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005. Dec;57(6):1173–82; discussion 1173–1182. [DOI] [PubMed] [Google Scholar]
- 13.Joseph B, Friese RS, Sadoun M, Aziz H, Kulvatunyou N, Pandit V, Wynne J, Tang A, O’Keeffe T, Rhee P. The BIG (brain injury guidelines) project: defining the management of traumatic brain injury by acute care surgeons. J Trauma Acute Care Surg. 2014. Apr;76(4):965–9. [DOI] [PubMed] [Google Scholar]
- 14.Meehan WP, Mannix RC, Stracciolini A, Elbin RJ, Collins MW. Symptom severity predicts prolonged recovery after sport-related concussion, but age and amnesia do not. J Pediatr. 2013. Sep;163(3):721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivara FP, Koepsell TD, Wang J, Temkin N, Dorsch A, Vavilala MS, Durbin D, Jaffe K. Incidence of disability among children 12 months after traumatic brain injury. Am J Public Health. 2012. Nov;102(11):2074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nesiama J-AO, Pirallo RG, Lerner EB, Hennes H. Does a prehospital Glasgow Coma Scale score predict pediatric outcomes? Pediatr Emerg Care. 2012. Oct;28(10):1027–32. [DOI] [PubMed] [Google Scholar]
- 17.Azim A, Jehan FS, Rhee P, O’Keeffe T, Tang A, Vercruysse G, Kulvatunyou N, Latifi R, Joseph B. Big for small: Validating brain injury guidelines in pediatric traumatic brain injury. J Trauma Acute Care Surg. 2017;83(6):1200–4. [DOI] [PubMed] [Google Scholar]
- 18.Ismail FY, Fatemi A, Johnston MV. Cerebral plasticity: Windows of opportunity in the developing brain. Eur J Paediatr Neurol. 2017. Jan;21(1):23–48. [DOI] [PubMed] [Google Scholar]
- 19.McCauley SR, Wilde EA, Anderson VA, Bedell G, Beers SR, Campbell TF, Chapman S, Ewing-Cobbs L, Gerring J, Gioia G, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J Neurotrauma. 2012. Mar 1;29(4):678–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel N, West M, Wurster J, Tillman C. Pediatric traumatic brain injuries treated with decompressive craniectomy. Surg Neurol Int. 2013;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minney MJ, Roberts RM, Mathias JL, Raftos J, Kochar A. Service and support needs following pediatric brain injury: perspectives of children with mild traumatic brain injury and their parents. Brain Inj. 2019;33(2):168–82. [DOI] [PubMed] [Google Scholar]