Abstract
Rationale
Dyspnea is often a persistent symptom after acute coronavirus disease (COVID-19), even if cardiac and pulmonary function are normal.
Objectives
This study investigated diaphragm muscle strength in patients after COVID-19 and its relationship to unexplained dyspnea on exertion.
Methods
Fifty patients previously hospitalized with COVID-19 (14 female, age 58 ± 12 yr, half of whom were treated with mechanical ventilation, and half of whom were treated outside the ICU) were evaluated using pulmonary function testing, 6-minute-walk test, echocardiography, twitch transdiaphragmatic pressure after cervical magnetic stimulation of the phrenic nerve roots, and diaphragm ultrasound. Diaphragm function data were compared with values from a healthy control group.
Measurements and Main Results
Moderate or severe dyspnea on exertion was present at 15 months after hospital discharge in approximately two-thirds of patients. No significant pulmonary function or echocardiography abnormalities were detected. Twitch transdiaphragmatic pressure was significantly impaired in patients previously hospitalized with COVID-19 compared with control subjects, independent of initial disease severity (14 ± 8 vs. 21 ± 3 cm H2O in mechanically ventilated patients vs. control subjects [P = 0.02], and 15 ± 8 vs. 21 ± 3 cm H2O in nonventilated patients vs. control subjects [P = 0.04]). There was a significant association between twitch transdiaphragmatic pressure and the severity of dyspnea on exertion (P = 0.03).
Conclusions
Diaphragm muscle weakness was present 15 months after hospitalization for COVID-19 even in patients who did not require mechanical ventilation, and this weakness was associated with dyspnea on exertion. The current study, therefore, identifies diaphragm muscle weakness as a correlate for persistent dyspnea in patients after COVID-19 in whom lung and cardiac function are normal.
Clinical trial registered with www.clinicaltrials.gov (NCT 04854863).
Keywords: coronavirus, diaphragm muscle strength, pulmonary function, dyspnea
At a Glance Commentary
Scientific Knowledge on the Subject
Up to one-third of coronavirus disease (COVID-19) survivors report some degree of dyspnea that cannot be explained by routine clinical diagnostic measures, including pulmonary function tests and cardiac evaluation. Therefore, the pathophysiological basis for the “long COVID” symptoms and how long they persist is not yet fully understood.
What This Study Adds to the Field
This study used state-of-the-art in-depth techniques to determine diaphragm muscle strength in patients 15 months after hospitalization for COVID-19 and its relationship to otherwise unexplained dyspnea on exertion. It is, therefore, the first study to: 1) demonstrate that diaphragm muscle weakness is present 15 months after hospitalization for COVID-19 independent of initial disease severity (i.e., even in patients who did not require mechanical ventilation); and 2) identify diaphragm muscle weakness as a correlate for persistent dyspnea in patients after COVID-19 in whom lung and cardiac function are normal.
It is now more than 2 years since the beginning of the coronavirus disease (COVID-19) pandemic (1). Therefore, a substantial population has recovered from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (2–5). Some of these individuals experience a range of symptoms and abnormalities that may persist for more than several months after recovery from acute illness (2–4, 6). The new terms “long COVID” and “post-COVID syndrome” have been used to describe these findings. However, whether these abnormalities are unique to COVID-19, how long they persist, and their underlying pathophysiology are not well understood, especially in patients in whom invasive mechanical ventilation was necessary (inevitable) to manage acute COVID-19. Some patients with COVID-19 treated with invasive mechanical ventilation remain symptomatic after hospital discharge, with dyspnea on exertion being one of the most frequent symptoms even when cardiac and pulmonary function are within normal limits (6, 7).
This raises the question as to what might be causing exertional dyspnea in these patients. The presence of diaphragm weakness in patients who survived COVID-19 has been suggested (8) but remains unproven by objective measures. This lack of research on diaphragm muscle weakness in COVID-19 survivors is striking for two reasons. First, postmortem studies have documented the extrapulmonary presence of SARS-CoV-2, including immune-mediated skeletal muscle myopathy (9). Second, prolonged hospitalization, especially with mechanical ventilation, may independently predispose to diaphragm atrophy or weakness (10, 11).
Our group had previously reported diaphragm dysfunction with impaired volitional diaphragm function and control as a potential determinant of exertional dyspnea after COVID-19 illness in a hypothesis-generating research letter (12). To further investigate the potential role of diaphragm dysfunction on otherwise unexplained dyspnea, the present study used a multimodal approach using state-of-the-art assessments, including both volitional and nonvolitional invasive measures, to determine diaphragm muscle strength in patients previously hospitalized for the management of COVID-19 and its relationship to exertional dyspnea.
Methods
Study Design and Participants
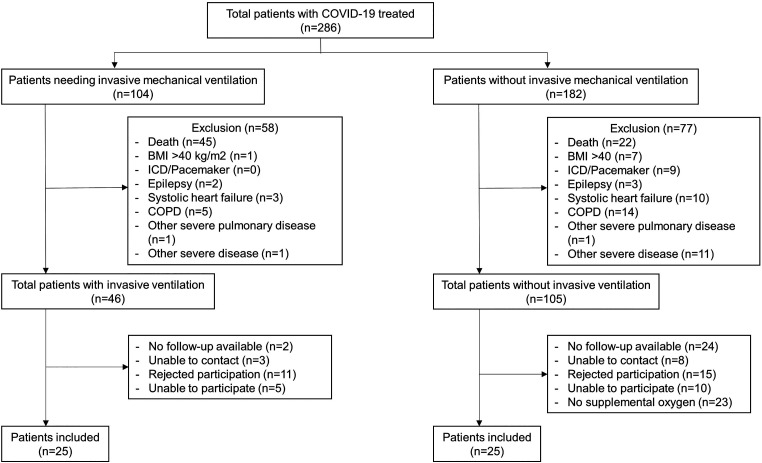
This prospective noninterventional study (NCT04854863) included patients who had been hospitalized at RWTH University Hospital Aachen (Aachen, Germany) between February 2020 and April 2021 for the management of COVID-19 and required supplemental oxygen therapy and/or invasive mechanical ventilation. Patients consented to attend one research visit approximately 15 months after discharge. Those with comorbidities that are known to cause dyspnea on exertion (such as treated systolic heart failure, anemia, heart defects including valve disorders, chronic obstructive pulmonary disease, or neuromuscular disorders) were excluded (Figure 1).
Figure 1.
CONSORT diagram. BMI = body mass index; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; ICD = implantable cardioverter/defibrillator.
For comparison of diaphragm muscle strength, patients previously hospitalized with COVID-19 were matched 3:1 with a control group of healthy subjects (recruited before the COVID-19 pandemic with identical technical equipment and standardization of the investigations) for age, sex, body mass index, and comorbidities.
The study was approved by the local ethics committee, and written informed consent was obtained from all patients. The trial was conducted according to Good Clinical Practice and the Principles of the Declaration of Helsinki 2002.
ICU Stay Routine Follow-Up
The severity of acute respiratory distress syndrome on the day of intubation was determined using the Berlin Definition (13). Laboratory parameters, arterial blood gas analysis, and ventilation variables, including the Po2/FiO2 ratio, were extracted from medical records. At follow-up approximately 15 months later, patients underwent pulmonary function testing, ECG, and transthoracic echocardiography. Serum, plasma, and whole blood samples were obtained and analyzed.
With support from a trained study team, patients answered clinical questionnaires to determine dyspnea (Medical Research Council dyspnea scale [14], Borg dyspnea scale [15]) and fatigue (modified Fatigue Severity Index [16]). Whole-body plethysmography (MasterLab, Viasys) was performed according to current guidelines (17, 18) before and after bronchodilation (diffusion capacity of carbon monoxide was determined after bronchodilation only). Samples for capillary blood gas analysis were taken from the arterialized earlobe of all patients while breathing room air without supplemental oxygen (ABL 800 flex, Radiometer). Borg dyspnea scale scores were determined before and after a 6-minute-walk test without supplemental oxygen (17, 18). Patients were then classified into three subgroups based on the severity of reported dyspnea after the 6-minute-walk test: mild or no dyspnea (Borg dyspnea scale score, 0–1; Medical Research Council dyspnea scale score, I), moderate dyspnea (Borg dyspnea scale score, 2–5; Medical Research Council dyspnea scale score, II/III), or severe dyspnea (Borg dyspnea scale score, ⩾6; Medical Research Council dyspnea scale score, IV/V).
Phrenic Nerve Stimulation Studies
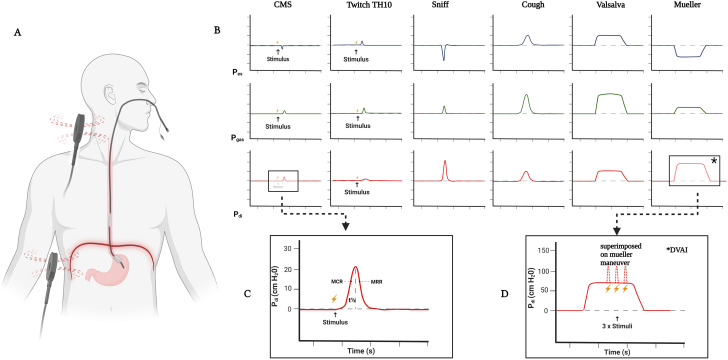
Twitch transdiaphragmatic pressure was recorded and analyzed using balloon catheters (Cooper Surgical) transnasally inserted into the stomach and the distal esophagus as previously described (Figure 2) (19). Magnetic phrenic nerve stimulation was used for invasive measurement of twitch transdiaphragmatic pressure and for phrenic nerve conduction studies as previously described (19). After transnasal placement of the catheter, a few sniff maneuvers were performed to confirm the position of esophageal and gastral transducers. Posterior cervical magnetic stimulation was performed with the subject in the seated position. Stimuli were delivered using a MagPro Compact magnetic stimulator equipped with a 2 Tesla 12 cm C-100 circular coil (MagVenture) (19). For posterior cervical magnetic stimulation, the coil was placed at C7 and then moved up toward C6 until the highest reproducible twitch transdiaphragmatic pressure was obtained (19). At least five stimuli were delivered to achieve the highest possible twitch transdiaphragmatic pressure showing <10% variation from the preceding two stimulations (19). Supramaximality of magnetic stimuli (with 0.1 millisecond duration each and 2.0 Tesla maximum magnetic field output) was achieved by judging the relationship between stimulation intensity and the amplitude of twitch transdiaphragmatic pressure (Figure 2). To minimize twitch potentiation resulting from previous voluntary diaphragm activation, there was a resting period of 5 minutes (with no speaking and no maneuvers, and only quiet breathing) before the stimulation. Between two twitches, a period of at least 30 seconds was ensured. The state of FRC was achieved by requesting the participants to hold their breath after a normal, passive exhalation and demonstrating it. Stimulation at FRC was determined by visual observation of abdominal movements combined with visualization of pressure curves on a large flat screen to reproducibly generate a state of FRC (19).
Figure 2.
Experimental setup. (A) Subject in the respiratory physiology lab with transnasal placement of double-balloon catheter measuring pressure from esophageal and gastral sensors for the calculation of transdiaphragmatic pressure; magnetic coil placement for delivery of cervical magnetic stimulation (CMS) and TH10 is shown. (B) Curves during different voluntary and nonvoluntary maneuvers. Readings from Pes and Pgas sensors and calculated Pdi are shown. (C) Representative twitch pressure recording after a CMS and further in-depth analysis of a twitch curve; pressure amplitude, duration of the pressure deflection, maximum rate of contraction (MRC), maximum rate of relaxation (MRR), and half relaxation time (t1/2) were analyzed. MRC is defined as the positive peak of the pressure derivative as a function of time (i.e., the steepest slope of the inclining twitch diaphragmatic pressure [twPDI] curve) and reflects the maximum velocity of diaphragm contraction. MRR is defined as the negative peak of the pressure derivative over time and measures the initial part of the pressure decay, reflecting maximum velocity of muscle relaxation. Both MRC and MRR were adjusted for twPDI. Finally, t1/2 was defined as the time taken for twPDI amplitude to decrease by 50% from the maximum. (D) CMS twitches superimposed on voluntary contraction and voluntary transdiaphragmatic pressure; performed on Mueller maneuver (negative esophageal pressure and positive gastric pressure). The DVAI reflects the percentage of diaphragmatic muscle mass activated by voluntary effort or the extent of diaphragmatic activation during any given inspiratory effort. DVAI = diaphragm voluntary activation index; Pdi = diaphragmatic pressure; Pes = esophageal pressure; Pgas = gastric pressure; TH10 = tenth vertebra. Created with BioRender.com.
Invasive Inspiratory Muscle Strength Measurements
After performing cervical magnetic stimulation, subjects were also instructed to repeatedly perform a maximum sniff maneuver and a maximum Mueller maneuver as measures of volitional diaphragm muscle strength to achieve maximum deflection of the transdiaphragmatic pressure curve (19). The highest of five consecutive efforts was taken for analysis (19). Reduced twitch transdiaphragmatic pressure reflecting diaphragm muscle strength impairment was defined as a value <16 cm H2O (which has previously been defined as the lower limit of normal [19]).
Twitch Superimposition
The diaphragm voluntary activation index reflects the percentage of diaphragmatic muscle mass activated by voluntary effort or the extent of diaphragmatic activation during any given inspiratory effort. This parameter has been proposed for assessment of central drive to the diaphragm (19). First, maximum twitch transdiaphragmatic pressure at FRC and maximum voluntary transdiaphragmatic pressure were determined, the latter by encouraging the subject to perform a maximum inspiratory effort against an occluded airway at FRC. Repetitive increasing stimuli were then deployed during voluntary inspiration (still with the airway occluded). During isovolumetric activation of the diaphragm, twitch interpolation was specifically timed by visual determination of 100% of the individual maximum voluntary transdiaphragmatic pressure (Figure 2) (19).
Invasive Expiratory Muscle Strength Measurement
The lower thoracic nerve roots were magnetically stimulated at the tenth vertebra with rostrocaudal adjustment of the coil position (by no more than two vertebrae) to achieve the highest reproducible twitch gastric pressure (20). Stimulation intensity was 100% of the maximum magnetic output with no threshold testing because it has been established that, unlike magnetic phrenic nerve stimulation, supramaximal stimulation of the lower thoracic (expiratory) nerves is not possible (21). Stimulation was performed at FRC. The lower thoracic nerve roots were stimulated at the tenth vertebra with clear instruction to go up and down (by no more than two vertebrae) to identify the position where the highest reproducible twitch gastric pressure could be achieved (Figure 2). Subjects were then also instructed to repeatedly perform a maximum cough and a maximum Valsalva maneuver as volitional invasive metrics of expiratory muscle strength. Cough, Valsalva, and twitch gastric pressure were recorded using the same technical setup described above.
Diaphragm Ultrasound
Diaphragm ultrasound was performed on the right hemidiaphragm as previously described (22). Briefly, a portable ultrasound device (LOGIQ S8-XD, GE Healthcare) with a 10-MHz linear transducer was used for evaluation of diaphragm thickness in the zone of apposition. The diaphragm thickening ratio (DTR) was calculated as thickness at TLC divided by thickness at FRC (22).
Statistical Analysis
Statistical analyses were performed using Sigma Plot software (Version 13.0, Systat). The primary endpoint was a reduction in twitch transdiaphragmatic pressure after supramaximal magnetic stimulation of the phrenic nerve roots. Assuming a two-sided significance of 0.05 (α) and 80% power (β), a sample size of 25 subjects per group was calculated to allow detection of a 25% difference in twitch transdiaphragmatic pressure between (each of the two groups of) patients previously hospitalized with COVID-19 and normal values from the healthy control group that were determined previously (19, 20).
Data are expressed as mean and SD if normally distributed or as median and interquartile range. For comparisons between two groups, Fisher’s exact t test or the Mann-Whitney U test was used as appropriate. Comparisons between patient subgroups based on exertional dyspnea severity were performed using one-way ANOVA with Tukey post hoc test for pairwise comparisons when normal distribution could be assumed. Otherwise, the Kruskal-Wallis test with Bonferroni post hoc tests was used. In contrast to single t tests, in which this was not applicable, Bonferroni correction was used for (post hoc) t tests performed after three patient subgroups had been compared using one-way ANOVA. For all analyses, a P value of ⩽0.05 was considered statistically significant.
Results
Study Participants
Of 286 patients with severe COVID-19 who required invasive mechanical ventilation in the ICU or received supplemental oxygen only and were seen for follow-up in the outpatient clinic, 50 fulfilled all inclusion criteria and were reassessed at 15 months after hospital discharge (Figure 1 and Table 1).
Table 1.
Baseline Characteristics, Medical History, and Characteristics during ICU Stay for the Overall Study Population and in Patient Subgroups Based on the Requirement for Invasive Mechanical Ventilation during Hospitalization
| Patients Previously Hospitalized with COVID-19 |
||||
|---|---|---|---|---|
| Total (n = 50) | Ventilated (n = 25) | Nonventilated (n = 25) | Control Subjects (n = 9) | |
| Male sex | 36 (72) | 19 (76) | 17 (68) | 5 (60) |
| Age, yr | 58.06 ± 12.43 | 58.75 ± 8.62 | 57.36 ± 15.50 | 57.11 ± 10.47 |
| Postdischarge time, mo | 14.80 ± 7.33 | 16.23 ± 4.35 | 14.63 ± 4.16 | — |
| Height, cm | 175 ± 10 | 176 ± 8 | 174 ± 11 | 178 ± 8 |
| Weight, kg | 88.76 ± 17.84 | 95.20 ± 18.49 | 82.32 ± 14.89 | 78.33 ± 7.50 |
| BMI, kg/m2 | 28.88 ± 5.03 | 30.63 ± 5.73 | 27.14 ± 3.52 | 24.84 ± 1.71 |
| Total MFIS score | 31.04 ± 19.72 | 36.60 ± 21.61 | 25.48 ± 16.20 | — |
| Comorbidities | ||||
| COPD | 0 (0) | 0 (0) | 0 (0) | — |
| Bronchial asthma | 4 (8) | 2 (8) | 2 (8) | — |
| Hypertension | 28 (56) | 12 (48) | 16 (64) | — |
| Systolic heart failure | 0 (0) | 0 (0) | 0 (0) | — |
| Atrial fibrillation | 2 (4) | 1 (4) | 1 (4) | — |
| Chronic kidney disease | 6 (12) | 3 (12) | 3 (12) | — |
| Diabetes mellitus | 9 (18) | 4 (16) | 5 (20) | — |
| In-hospital period | ||||
| Length of stay, d | 31.72 ± 26.97 | 50.08 ± 27.35 | 13.36 ± 5.84 | — |
| Oxygen supplementation | 50 (100) | 25 (100) | 25 (100) | — |
| Characteristics during ICU stay | ||||
| P/F ratio at (ICU) admission | — | 135.32 ± 45.17 | 247.68 ± 37.26 | — |
| Mean P/F ratio halfway through total ventilation duration | — | 217.44 ± 57.92 | N/A | — |
| Duration of ventilation, d | — | 36.44 ± 22.30 | — | — |
| Duration of ICU stay, d | — | 36.60 ± 30.07 | — | — |
| Patients on ECMO | — | 8 (32) | — | — |
| Duration of ECMO, d* | — | 15 (10.50–24.50) | — | — |
| Prone positioning | — | 21 (84) | — | — |
| Continuous NMB >6 h at any point | — | 13 (52) | — | — |
| Duration of NMB, d | — | 10.08 ± 8.15 | — | — |
| Catecholamine therapy | — | 24 (96) | — | — |
| Duration of catecholamine therapy, d* | — | 16 (4.50–34.00) | — | — |
| CRRT | — | 15 (60) | — | — |
| Antibiotic therapy | — | 24 (96) | 4 (16) | — |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; CRRT = continuous renal replacement therapy; ECMO = extracorporeal membrane oxygenation; MFIS = modified fatigue impact scale; N/A = not applicable; NMB = neuromuscular blockade; P/F = partial pressure of oxygen/fraction of inspired oxygen.
Data are presented as n (%) or mean ± SD unless otherwise noted.
Median (interquartile range) values for nonnormally distributed data.
Twenty-five patients met the Berlin definition (13) for severe acute respiratory distress syndrome and required invasive mechanical ventilation. The majority of these (84%) also required prone positioning, nearly one-third (32%) received extracorporeal membrane oxygenation therapy, and nearly two-thirds (60%) developed acute renal failure requiring continuous renal replacement therapy (Table 1). The mean duration of hospitalization in these patients was 50 ± 27 days (Table 1). The remaining 25 patients were not treated in the ICU but needed supplemental oxygen therapy during hospitalization (Table 1). None of the nonventilated patients received noninvasive ventilation therapy at any point during their hospital admission. This subgroup had a mean hospital stay of 13 ± 6 days (P < 0.0001 vs. ventilated patients) (Table 1).
Twenty-seven patients (54%) participated in pulmonary rehabilitation programs after discharge. The rate of participation in pulmonary rehabilitation was higher in ventilated patients (n = 22; 88%) than in nonventilated patients (n = 5; 20%). Except for six patients (12%), most were able to return to their previous daily activities or work.
Dyspnea and Fatigue
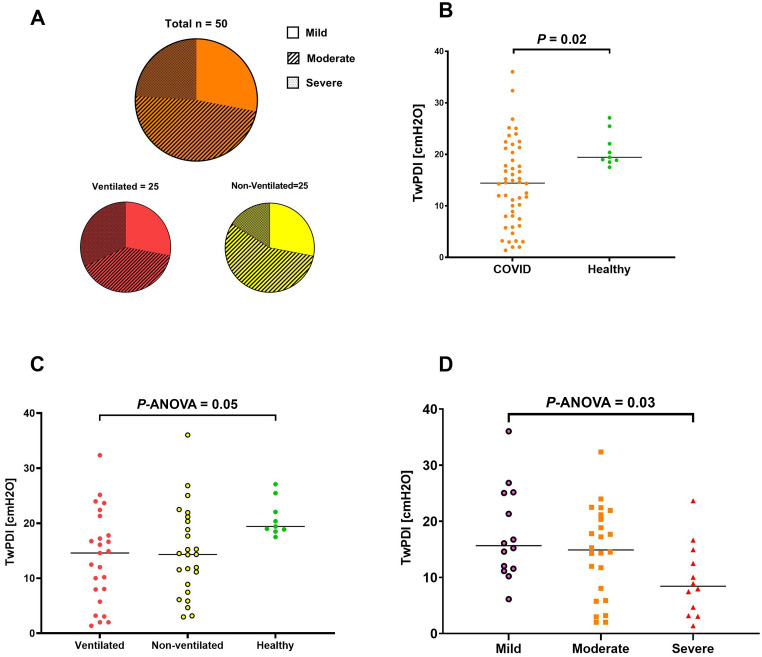
Outpatient clinic follow-up occurred at approximately 15 months (432 ± 124 d) after discharge. Regarding patient-reported outcomes, the severity of exertional dyspnea at follow-up was no/mild in 14 patients (28%), moderate in 24 patients (48%), and severe in 12 patients (24%). Severe dyspnea on exertion was reported by eight patients (32%) in the ventilated group and four patients (16%) in the nonventilated group. Moderate dyspnea was reported by 10 ventilated patients (40%) and 14 nonventilated patients (56%), and 7 patients in each group (28%) reported mild or no dyspnea (Figure 3). Performance on the 6-minute-walk test was similarly impaired in both ventilated and nonventilated patients previously hospitalized with COVID-19, with no significant between-group difference (see Table E1 in the online supplement). Both groups also experienced fatigue, although patients from the ventilated group had a significantly greater degree of fatigue than those who did not require ventilation (P = 0.04) (Table E1). Dyspnea severity was significantly associated with both distances achieved in the 6-minute-walk test and fatigue scores (both P < 0.01) (Table E2). At follow-up, the vaccination rate of patients receiving at least two doses was very high (84% vs. 88%) in both cohorts.
Figure 3.
The impact of diaphragm muscle weakness on exertional dyspnea at 15 months after hospitalization for coronavirus disease (COVID-19). (A) Proportion of patients with different degrees of exertional dyspnea severity in the total cohort and in subgroups who did or did not undergo invasive mechanical ventilation. (B) Differences in twitch diaphragmatic pressure (twPDI) after posterior cervical magnetic stimulation (CMS) in patients previously hospitalized for COVID-19 compared with healthy control subjects. (C) Differences in twPDI after posterior CMS in patient subgroups who did or did not undergo invasive mechanical ventilation and in healthy control subjects. (D) Differences in twPDI after posterior CMS in patient subgroups based on the severity of exertional dyspnea.
Clinical Follow-Up
At follow-up, no significant abnormalities were identified based on pulmonary function tests, capillary blood gas analysis, or transthoracic echocardiography (Tables E1 and E2). White blood cells, platelets, and creatine kinase varied significantly across subgroups based on dyspnea severity, but all values were within normal limits (Table E2). There were no associations between the findings of pulmonary function testing, capillary blood gas analysis, or echocardiography dyspnea severity (Table E2).
Diaphragm Muscle Strength
Twitch diaphragmatic pressure after posterior cervical magnetic stimulation was significantly lower in patients previously hospitalized with COVID-19 than in healthy control subjects (P = 0.02), irrespective of whether patients had been ventilated or not (Figure 3). There was a significant correlation between twitch diaphragmatic pressure and the severity of dyspnea on exertion (P = 0.03) (Figure 3 and Table 2).
Table 2.
Diaphragm Muscle Strength at 15 Months after Hospitalization for Coronavirus Disease in Patient Subgroups Based on the Severity of Dyspnea on Exertion at 12-Month Follow-Up
| Exertional Dyspnea Severity |
||||
|---|---|---|---|---|
| None/Mild (n = 14; 28%) | Moderate (n = 24; 48%) | Severe (n = 12; 24%) | P Value* | |
| Age, yr | 55.36 ± 11.08 | 55.43 ± 11.78 | 66.46 ± 12.30 | 0.60† |
| Male sex, n (%) | 12 (86) | 15 (63) | 9 (75) | — |
| BMI, kg/m2 | 27.73 ± 2.74 | 29.33 ± 6.23 | 29.34 ± 4.50 | 0.61 |
| mMRC dyspnea scale score | 1.00 ± 0.00 | 2.04 ± 0.75 | 3.58 ± 1.08 | <0.001 † ‡ § |
| Nonvolitional invasive RMS | ||||
| CMS twPDI, cm H2O | 17.73 ± 8.16 | 14.51 ± 8.07 | 9.53 ± 6.52 | 0.03 § |
| CMS twPes, cm H2O | −9.94 ± 5.38 | −6.39 ± 4.27 | −6.34 ± 6.19 | 0.09 |
| CMS twPgas, cm H2O | 7.79 ± 5.49 | 7.90 ± 5.84 | 3.20 ± 2.57 | 0.03 ‡ |
| CMS MRR normalized, cm H2O/ms | −8.65 ± 3.28 | −10.85 ± 6.81 | −12.52 ± 8.82 | 0.33 |
| CMS MCR normalized, cm H2O/ms | 21.89 ± 6.98 | 21.54 ± 8.33 | 21.06 ± 11.78 | 0.97 |
| CMS t1/2, ms | 72.14 ± 38.06 | 74.17 ± 44.81 | 57.78 ± 37.97 | 0.59 |
| CMS time to peak, ms | 67.64 ± 37.93 | 68.17 ± 46.46 | 51.61 ± 25.45 | 0.61 |
| twPgas TH10, cm H2O | 27.46 ± 17.16 | 21.53 ± 18.85 | 17.99 ± 15.70 | 0.40 |
| Volitional invasive RMS | ||||
| Sniff PDI, cm H2O | 91.86 ± 29.11 | 79.34 ± 26.86 | 63.25 ± 24.06 | 0.03 § |
| Sniff Pes, cm H2O | −65.69 ± 26.25 | −65.88 ± 24.13 | −49.74 ± 19.29 | 0.13 |
| Mueller PDI, cm H2O | 83.96 ± 43.12 | 71.11 ± 40.74 | 50.31 ± 28.08 | 0.10 |
| Mueller Pes, cm H2O | −46.81 ± 21.32 | −58.34 ± 33.78 | −41.03 ± 20.20 | 0.19 |
| Valsalva Pgas, cm H2O | 222.44 ± 70.89 | 144.66 ± 71.69 | 115.65 ± 78.26 | <0.001 ‡ § |
| Cough Pgas, cm H2O | 183.86 ± 60.73 | 153.69 ± 74.48 | 121.22 ± 69.60 | 0.09 |
| Neural control | ||||
| DVAI, % | 53.07 ± 29.99 | 52.21 ± 34.02 | 48.58 ± 30.59 | 0.93 |
| Diaphragm ultrasound | ||||
| Amplitude TB, cm | 1.70 ± 0.50 | 1.80 ± 0.39 | 1.93 ± 0.76 | 0.55 |
| Velocity TB, cm/s | 1.73 ± 0.96 | 1.59 ± 0.50 | 1.73 ± 0.93 | 0.81 |
| Sniff velocity, cm/s | 7.95 ± 2.04 | 7.24 ± 2.02 | 6.97 ± 3.78 | 0.61 |
| Thickness at FRC, cm | 0.23 ± 0.06 | 0.18 ± 0.04 | 0.20 ± 0.06 | 0.06 |
| Thickness at TLC, cm | 0.42 ± 0.12 | 0.38 ± 0.09 | 0.38 ± 0.12 | 0.44 |
| DTR | 1.88 ± 0.26 | 2.10 ± 0.36 | 1.86 ± 0.33 | 0.06 |
Definition of abbreviations: CMS = cervical magnetic stimulation (of the phrenic nerve roots); COVID-19 = coronavirus disease; DTR = diaphragm thickening ratio; DVAI = diaphragm voluntary activation index; MCR = maximum contraction rate; mMRC = Modified Medical Research Council; MRR = maximum relaxation rate; PDI = transdiaphragmatic pressure; Pes = esophageal pressure; Pgas = gastric pressure; RMS = respiratory muscle strength; TB = tidal breathing; twPDI = twitch transdiaphragmatic pressure; twPes = twitch esophageal pressure; twPgas = twitch gastric pressure; twPgas TH10 = twitch gastric pressure (in response to magnetic stimulation of the expiratory nerve roots).
Data are presented as mean ± SD unless otherwise noted. Bold indicates P < 0.05.
ANOVA.
Significant differences (P < 0.05) within paired t tests between moderate and severe dyspnea groups, if ANOVA is significantly different (P < 0.05).
Significant differences (P < 0.05) within paired t tests between mild and moderate dyspnea groups, if ANOVA is significantly different (P < 0.05).
Significant differences (P < 0.05) within paired t tests between mild and severe dyspnea groups, if ANOVA is significantly different (P < 0.05).
Volitional measurements of diaphragmatic pressure were not reduced in patients compared with control subjects and did not differ significantly between ventilated and nonventilated patients (Table 3). Significant group differences were seen in the diaphragm voluntary activation index between the ventilated/nonventilated patients previously hospitalized with COVID-19 compared with healthy control subjects (P ANOVA = 0.04; Table 3).
Table 3.
Diaphragm Muscle Strength in Control Subjects and at 15 Months after Hospitalization in Ventilated or Nonventilated Patients with COVID-19
| Control Subjects (n = 9) |
COVID-19 (Ventilated) (n = 25) |
COVID-19 (Nonventilated) (n = 25) |
P Value* | |
|---|---|---|---|---|
| Age, yr | 57.11 ± 10.46 | 57.36 ± 15.50 | 58.75 ± 8.62 | 0.90 |
| Male sex, n (%) | 6 (67) | 19 (76) | 17 (68) | |
| BMI, kg/m2 | 24.84 ± 1.71 | 30.63 ± 5.73 | 27.14 ± 3.52 | 0.002 † ‡ |
| Nonvolitional invasive RMS | ||||
| CMS twPDI, cm H2O | 20.92 ± 3.32 | 13.65 ± 8.37 | 14.79 ± 8.09 | 0.05 |
| CMS twPes, cm H2O | −12.19 ± 3.87 | −6.59 ± 5.06 | −7.37 ± 5.24 | 0.02 ‡ |
| CMS twPgas, cm H2O | 8.67 ± 2.95 | 6.84 ± 6.04 | 6.63 ± 4.89 | 0.59 |
| CMS MRR normalized, cm H2O/ms | −9.73 (−12.66 to −6.49) | −9.44 (−11.97 to −7.24) | −10.07 (−14.01 to −5.73) | 0.55 |
| CMS MCR normalized, cm H2O/ms | 20.73 ± 7.73 | 22.42 ± 10.16 | 20.73 ± 7.73 | 0.34 |
| CMS t1/2, ms | 110.00 (90.00 to 195.00) | 75.00 (50.00 to 100.00) | 50.00 (30.00 to 80.00) | 0.001 ‡ § |
| CMS time to peak, ms | 100.00 (75.00 to 140.00) | 60.00 (50.00 to 75.00) | 60.00 (35.50 to 75.75) | 0.002 § |
| twPgas TH10, cm H2O | 30.72 (18.33 to 55.82) | 17.38 (6.55 to 24.12) | 23.51 (11.23 to 32.10) | 0.20 |
| Volitional invasive RMS | ||||
| Sniff PDI, cm H2O | 91.80 ± 17.68 | 75.59 ± 29.38 | 82.38 ± 27.37 | 0.29 |
| Sniff Pes, cm H2O | −65.00 ± 17.27 | −58.69 ± 24.48 | −65.22 ± 24.04 | 0.58 |
| Mueller PDI, cm H2O | 87.18 ± 37.53 | 62.04 ± 36.22 | 77.39 ± 42.82 | 0.19 |
| Mueller Pes, cm H2O | −35.12 ± 32.68 | −43.91 ± 23.89 | −58.00 ± 31.09 | 0.08 |
| Valsalva Pgas, cm H2O | 111.24 (84.32 to 153.79) | 128.45 (88.12 to 171.43) | 195.04 (108.81 to 247.15) | 0.62 |
| Cough Pgas, cm H2O | 116.35 (94.19 to 157.82) | 127.79 (90.82 to 177.69) | 173.49 (133.41 to 219.39) | 0.10 |
| Neural control | ||||
| DVAI, % | 68.69 ± 21.65 | 45.34 ± 27.44 | 61.10 ± 36.97 | 0.04 |
| Diaphragm ultrasound | ||||
| Amplitude TB, cm | 1.52 ± 0.75 | 1.69 ± 0.60 | 1.92 ± 0.47 | 0.16 |
| Velocity TB, cm/s | 1.14 ± 0.52 | 1.36 ± 0.50 | 1.96 ± 0.89 | 0.002 † § |
| Sniff velocity, cm/s | 8.11 ± 3.05 | 6.75 ± 3.11 | 8.01 ± 1.86 | 0.19 |
| Thickness at FRC, cm | 0.19 ± 0.06 | 0.19 ± 0.04 | 0.21 ± 0.07 | 0.66 |
| Thickness at TLC, cm | 0.61 ± 0.06 | 0.38 ± 0.100 | 0.40 ± 0.12 | <0.001 ‡ § |
| DTR | 3.39 ± 1.32 | 1.96 ± 0.42 | 2.01 ± 0.27 | <0.001 ‡ § |
Definition of abbreviations: CMS = cervical magnetic stimulation (of the phrenic nerve roots); COVID-19 = coronavirus disease; DTR = diaphragm thickening ratio; DVAI = diaphragm voluntary activation index; MCR = maximum contraction rate; MRR = maximum relaxation rate; PDI = transdiaphragmatic pressure; Pes = esophageal pressure; Pgas = gastric pressure; RMS = respiratory muscle strength; TB = tidal breathing; twPDI = twitch transdiaphragmatic pressure; twPes = twitch esophageal pressure; twPgas = twitch gastric pressure; twPgas TH10 = twitch gastric pressure (in response to magnetic stimulation of the expiratory nerve roots).
Data are presented as means ± SD or median and first and third quartile unless otherwise noted. Bold indicates P < 0.05.
ANOVA.
Significant differences (P < 0.05) within paired t tests between ventilated and nonventilated groups, if ANOVA is significantly different (P < 0.05).
Significant differences (P < 0.05) within paired t tests between ventilated and control groups, if ANOVA is significantly different (P < 0.05).
Significant differences (P < 0.05) within paired t tests between nonventilated and control groups, if ANOVA is significantly different (P < 0.05).
Diaphragm Ultrasound
Among the ultrasound parameters, only DTR was significantly reduced in both ventilated and nonventilated COVID-19 cohorts compared with healthy control subjects (Table 3). No significant differences were seen in any ultrasound values between subgroups based on dyspnea severity (Table 2). We did not observe any diaphragm atrophy using ultrasound measurements in our cohort (Tables 2 and 3). However, diaphragm dysfunction was found using ultrasound measurements in 80% of our patients, and the DTR was below the lower limit of normal (2.2 as previously reported [22]) in 40 patients.
Expiratory Muscle Strength
There were no significant differences between twitch gastric pressure after magnetic stimulation of the abdominal muscles and twitch gastric pressure induced by a maximum voluntary cough (Tables 2 and 3).
Discussion
This study showed that patients previously hospitalized for COVID-19 have diaphragm weakness 15 months after discharge, irrespective of whether or not acute care included mechanical ventilation. The study findings also indicate that diaphragm weakness is associated with the occurrence of exertional dyspnea. Therefore, diaphragm weakness might explain exertional dyspnea reported by patients with long COVID in the absence of other pulmonary or cardiac function abnormalities.
Our findings support previous studies that did not find any significant pulmonary function test abnormalities in patients who survived moderate to severe COVID-19 (3–5). However, it is not surprising that standard pulmonary function tests do not detect changes in diaphragm muscle strength. Steier and colleagues showed that in-depth techniques of respiratory musculature assessment increase the accuracy of diagnosing diaphragm weakness by up to 40% (23). Using gold standard, invasive techniques, the present study showed that diaphragm muscle weakness was present 15 months after COVID-19. The reduction in twitch transdiaphragmatic pressure in patients with COVID-19 found in the present study is likely to be clinically relevant because it is similar to reductions reported in patients with neuromuscular disease with severe dyspnea (24, 25).
Furthermore, we have shown that diaphragm muscle weakness was related to exertional dyspnea, and, therefore, diaphragm muscle weakness is a potential pathophysiological correlate of dyspnea on exertion in patients previously hospitalized for COVID-19. However, a direct causal relationship cannot be directly proven, and a symptom as complex in its pathophysiology as exertional dyspnea may still show a multifactorial origin. Nevertheless, a direct effect of COVID-19 on the diaphragm seems plausible, especially in light of data from postmortem autopsy studies that showed potential direct viral infiltration or associated immunomodulatory changes of the diaphragm with development of fibrosis in patients who died after infection with SARS-CoV-2 (26). Therefore, the present study extends these findings by showing a clinical impact of COVID-19 on the human diaphragm on a long-term basis. We noted diaphragm dysfunction in patients who were and those who were not mechanically ventilated during their index hospitalization. This is relevant because there is the potential for critical illness–induced diaphragm dysfunction in mechanically ventilated patients (27). Critical illness–induced diaphragm dysfunction has been shown to be very common in the first week of invasive mechanical ventilation (10, 28). However, our follow-up took place over a much longer time frame (15 months after discharge), and it is not clear whether critical illness–induced diaphragm dysfunction would persist for >12 months. Nevertheless, critical illness–induced diaphragm dysfunction might be a potential confounding factor because invasive measurement of transdiaphragmatic pressure (twitch transdiaphragmatic pressure) after magnetic stimulation of the phrenic nerve roots in mechanically ventilated patients showed impaired contractility of the diaphragm compared with healthy individuals (28, 29). In addition, our finding of diaphragm weakness in patients previously hospitalized with COVID-19 who had not been mechanically ventilated suggests a virus-specific pathogenetic mechanism rather than a ventilator-specific mechanism for diaphragm dysfunction. Although no significant differences in DlCO and DlCO/Va were seen between the dyspnea subgroups, a downward trend was seen (especially in DlCO) paralleling dyspnea severity. These differences might represent persisting pulmonary vascular or interstitial damage that may at least partly contribute to dyspnea symptoms, as indicated in recent studies (30, 31). However, in contrast to the present findings on diaphragm muscle weakness, impairment in DlCO did not translate into persistent dyspnea in the present study (30, 31).
The main factor limiting 6-minute-walk distance was dyspnea, but most patients also reported a degree of fatigue, which might have contributed to a further reduction in the 6-minute-walk distance achieved. In addition, the role of peripheral muscle strength should not be underestimated, even if this was only reported by a small proportion of patients (5/50; 10%) during the 6-minute-walk test.
The growing number of individuals infected with SARS-CoV-2 worldwide, and therefore the proportion of previously infected patients who experience persistent symptoms (so-called long COVID), is an emerging public health issue. Often, persistent symptoms cannot be linked to a specific pathophysiological correlate, making targeted management extremely difficult. Therefore, the identification of a possible underlying mechanism for exertional dyspnea at 15 months after recovery from COVID-19 is clinically relevant. First, it may be reassuring for patients to have a possible explanation for their persistent symptom (dyspnea) after COVID-19. Second, respiratory muscle training has been shown to be effective in other groups of patients with diaphragm muscle weakness and therefore represents a potential therapeutic intervention in this setting (32, 33).
Our study has some limitations. First, we selected a specific patient population that did not have any underlying cardiac or pulmonary disease that could act as a potential confounder and explain the perceived dyspnea. On the other hand, the control group did not have respiratory failure and serious illness at the same time period. Second, the ventilated patient cohort included a subset of patients who underwent extracorporeal membrane oxygenation, all of whom were treated with paralytics and six of whom received steroids. This is a potential confounder because it can lead to critical illness neuropathy and myopathy. Additional factors that could contribute to diaphragm weakness include phrenic nerve neuropathy due to immune mechanisms, preexisting risk factors, use of antiviral drugs, or bedding in the ICU. Furthermore, this observational study does not address whether the changes observed in diaphragmatic muscle strength are specifically attributable to COVID-19 rather than more general postinfection myopathy after an acute lung injury. Specifically designed studies with control subjects who have survived non-COVID pneumonia are required to gain further insights into the pathophysiology. However, it also remains clear that regardless of the specificity of COVID-19, the extent of diaphragm muscle weakness and its clear association with otherwise unexplained persistent dyspnea is a significant finding, particularly because the large number of patients who have had COVID worldwide are likely to impose a considerable burden on modern healthcare systems.
Conclusions
This study demonstrated a pathophysiological mechanism, namely diaphragm muscle weakness, underlying otherwise unexplained exertional dyspnea in patients previously hospitalized for COVID-19. Additional research is needed to determine whether specific interventions targeting diaphragm muscle weakness, such as inspiratory muscle training, could be an effective intervention to address exertional dyspnea in patients with long COVID.
Acknowledgments
Acknowledgment
The authors thank all the volunteers and patients with coronavirus disease (COVID-19) whose cooperation made this study possible. They also thank Mrs. Merite Emrulai for analyzing patient-related data. English language editing assistance was provided by Nicola Ryan, an independent medical writer. The authors also thank Dr. Gerold Kierstein (AD Instruments, Oxford, United Kingdom) for his help in performing an analysis of twitch transdiaphragmatic pressure gradients after cervical stimulation of the phrenic nerve roots.
Footnotes
Supported by the RWTH Aachen Faculty of Medicine START Grant supporting the junior research group around Dr. Jens Spiesshoefer.
Author Contributions: B.R., J.S., J.F., B.J., and M.S. collected the data. B.R. wrote the manuscript. B.R., J.S., and M.D. contributed significantly to the study design. The manuscript was revised by all other authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202206-1243OC on January 3, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med . 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med . 2022;10:761–775. doi: 10.1016/S2213-2600(22)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spruit MA, Holland AE, Singh SJ, Tonia T, Wilson KC, Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society and American Thoracic Society-coordinated international task force. Eur Respir J . 2020;56:2002197. doi: 10.1183/13993003.02197-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med . 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jamil S, Mark N, Carlos G, Cruz CSD, Gross JE, Pasnick S. Diagnosis and management of COVID-19 disease. Am J Respir Crit Care Med . 2020;201:P19–P20. doi: 10.1164/rccm.2020C1. [DOI] [PubMed] [Google Scholar]
- 6. Daher A, Balfanz P, Cornelissen C, Müller A, Bergs I, Marx N, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med . 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daher A, Cornelissen C, Hartmann NU, Balfanz P, Müller A, Bergs I, et al. Six months follow-up of patients with invasive mechanical ventilation due to COVID-19 related ARDS. Int J Environ Res Public Health . 2021;18:5851. doi: 10.3390/ijerph18115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hennigs JK, Huwe M, Hennigs A, Oqueka T, Simon M, Harbaum L, et al. Respiratory muscle dysfunction in long-COVID patients. Infection . 2022;50:1391–1397. doi: 10.1007/s15010-022-01840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aschman T, Schneider J, Greuel S, Meinhardt J, Streit S, Goebel HH, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol . 2021;78:948–960. doi: 10.1001/jamaneurol.2021.2004. [DOI] [PubMed] [Google Scholar]
- 10. Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med . 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 11. Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care . 2010;14:R127. doi: 10.1186/cc9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spiesshoefer J, Friedrich J, Regmi B, Geppert J, Jörn B, Kersten A, et al. Diaphragm dysfunction as a potential determinant of dyspnea on exertion in patients 1 year after COVID-19-related ARDS. Respir Res . 2022;23:187. doi: 10.1186/s12931-022-02100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin Definition. JAMA . 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. BMJ . 1959;2:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc . 1982;14:377–381. [PubMed] [Google Scholar]
- 16. Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci . 1994;21:9–14. [PubMed] [Google Scholar]
- 17. Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J . 2019;53:1801214. doi: 10.1183/13993003.01214-2018. [DOI] [PubMed] [Google Scholar]
- 18. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spiesshoefer J, Henke C, Herkenrath S, Brix T, Randerath W, Young P, et al. Transdiapragmatic pressure and contractile properties of the diaphragm following magnetic stimulation. Respir Physiol Neurobiol . 2019;266:47–53. doi: 10.1016/j.resp.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 20. Spiesshoefer J, Henke C, Kabitz HJ, Nofer JR, Mohr M, Evers G, et al. Respiratory muscle and lung function in lung allograft recipients: association with exercise intolerance. Respiration . 2020;99:398–408. doi: 10.1159/000507264. [DOI] [PubMed] [Google Scholar]
- 21. Kyroussis D, Polkey MI, Mills GH, Hughes PD, Moxham J, Green M. Simulation of cough in man by magnetic stimulation of the thoracic nerve roots. Am J Respir Crit Care Med . 1997;156:1696–1699. doi: 10.1164/ajrccm.156.5.9702008. [DOI] [PubMed] [Google Scholar]
- 22. Spiesshoefer J, Herkenrath S, Henke C, Langenbruch L, Schneppe M, Randerath W, et al. Evaluation of respiratory muscle strength and diaphragm ultrasound: normative values, theoretical considerations, and practical recommendations. Respiration . 2020;99:369–381. doi: 10.1159/000506016. [DOI] [PubMed] [Google Scholar]
- 23. Steier J, Kaul S, Seymour J, Jolley C, Rafferty G, Man W, et al. The value of multiple tests of respiratory muscle strength. Thorax . 2007;62:975–980. doi: 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henke C, Spiesshoefer J, Kabitz HJ, Herkenrath S, Randerath W, Brix T, et al. Respiratory muscle weakness in facioscapulohumeral muscular dystrophy. Muscle Nerve . 2019;60:679–686. doi: 10.1002/mus.26717. [DOI] [PubMed] [Google Scholar]
- 25. Spiesshoefer J, Henke C, Kabitz HJ, Brix T, Görlich D, Herkenrath S, et al. The nature of respiratory muscle weakness in patients with late-onset Pompe disease. Neuromuscul Disord . 2019;29:618–627. doi: 10.1016/j.nmd.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 26. Shi Z, de Vries HJ, Vlaar APJ, van der Hoeven J, Boon RA, Heunks LMA, et al. Dutch COVID-19 Diaphragm Investigators Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med . 2021;181:122–124. doi: 10.1001/jamainternmed.2020.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med . 2017;43:1441–1452. doi: 10.1007/s00134-017-4928-4. [DOI] [PubMed] [Google Scholar]
- 28. Sklar MC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Association of low baseline diaphragm muscle mass with prolonged mechanical ventilation and mortality among critically ill adults. JAMA Netw Open . 2020;3:e1921520. doi: 10.1001/jamanetworkopen.2019.21520. [DOI] [PubMed] [Google Scholar]
- 29. Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, et al. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med . 2001;29:1325–1331. doi: 10.1097/00003246-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 30. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet . 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med . 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J . 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 33. Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care . 2011;15:R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]