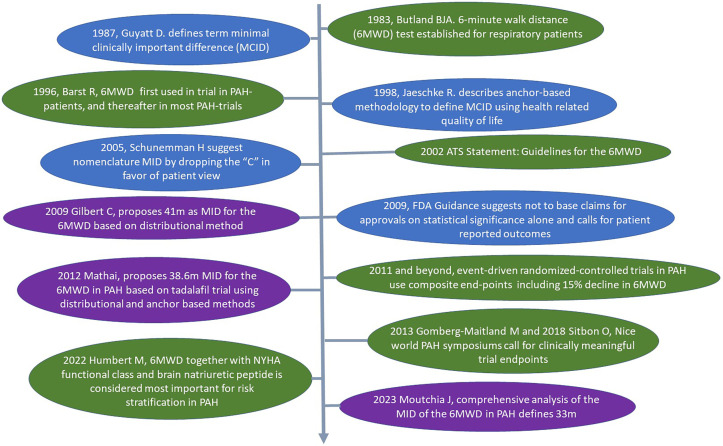
Simple tests that measure the distance walked on an even floor have been used as an objective measure to assess physical performance since the early 1960s (1). Because the 6-minute-walk distance (6MWD) test performed as well as 12-minute-walk tests (2), was easier to administer and was well tolerated by patients, this approach moved into clinical practice for respiratory patients (Figure 1). In 2002, the American Thoracic Society published a statement to guide implementation of the 6WMD in clinical practice (3). The first randomized controlled clinical trial investigating the efficacy of intravenous epoprostenol in patients with pulmonary arterial hypertension (PAH) included the 6MWD as an endpoint, and the 6MWD was thereafter used as the primary outcome measure in many PAH clinical studies. Most often, change in absolute distance walked between baseline and study completion is reported to demonstrate the effect of an intervention. More recently, the percentage deterioration of the 6MWD, mostly as a decrease in 6MWD by 15%, has been included together with hospitalization and death as part of a composite endpoint in event-driven trials (4, 5). The 6MWD is widely used not only as an endpoint in research studies but also to monitor clinical progress of patients with PAH at the point of care. Thus, the 6MWD is included in all major registries, along with symptoms, World Health Organization functional class, and brain natriuretic peptide concentration, as a surrogate for pulmonary hemodynamics. Overall, the 6MWD is a core measure for risk assessment that guides therapy selection and escalation for patients with PAH (6).
Figure 1.
The arrow from top to bottom signifies the timeline over the last 40 years. The ovals linked to the timeline contain important studies on the implementation of the 6-minute-walk distance (6MWD) in respiratory medicine, especially in the field of pulmonary arterial hypertension (green), the concept of MID to address meaningful outcome measures for patients (blue), and published studies on minimal important difference of the 6MWD in patients with pulmonary arterial hypertension (purple). FDA = Food and Drug Administration; MCID = minimal clinically important difference; MID = minimal important difference; NYHA = New York Heart Association; PAH = pulmonary arterial hypertension.
Over the past several years, many more tests, including different imaging, assessments of exercise capacity, or physiological measures, became available in respiratory medicine and were proposed as markers of risk and trial endpoints. With every measure, the question arose to which degree a change over time would be perceived by patients as meaningful, and the term “minimal clinically important difference” (MCID) was introduced by Guyatt and colleagues (7). MCID is preferentially determined if well-validated instruments to assess a patient’s quality of life were integrated into clinical trials to anchor changes of specific tests to changes of patient-reported outcomes (7). MCID defined by these anchor-based methods integrating patients’ perceptions are clearly preferred to distributional methods, which calculate MCID from statistical variance (7–10). The MCID of endpoints should be integrated in sample size calculation and thus should have a direct implementation in trial design. It was later suggested that the “C” in MCID should be abandoned because it is not the “clinical” view that is the important measure but the health-related quality of life as a pure patient-centered outcome measure, but this request was not followed by a majority of publications (11, 12). Thus, the M(C)ID was defined as the smallest difference in score in the outcome of interest that patients were likely to perceive as important, as either beneficial or harmful, and that leads, together with caregivers, to consideration of a change in disease management (11). It was this patient-centeredness that was claimed in the subsequent 2009 U.S. Food and Drug Administration guidance for industry and that directed medical research and many clinical trials toward inclusion of patient-reported outcome measures, which also allow definition of MCID (13).
In the PAH field, working groups for the World Symposium on Pulmonary Hypertension meetings in 2013 and 2018 called for trial endpoints that are meaningful for patients (5, 14). The first study on MCID that focused on 6MWD defined a change by +41 m; however, this was calculated using distributional methods (15). A second study using an anchor-based method found that +38.5 m in 6MWD met the criteria for MCID (16). Both of these approaches were based on single randomized trials, with the first using a distributional method and the second using an anchor-based method based on the 36-item Short Form Health Survey quality of life questionnaire.
In this issue of the Journal, Moutchia and colleagues (pp. 1070–1079) performed a comprehensive evaluation of the MCID in 6MWD based on meta-analysis including datasets of 2,404 patients enrolled in various randomized controlled trials and validated their findings using real-life registry data of 537 adult patients enrolled in the Pulmonary Hypertension Association Registry (17). The present analysis, based on a very large number of trial participants in the training dataset, revealed an MCID in the 6MWD of +33 m, whereas the validation cohort suggested an MCID for 6MWD of +36 m, which was very similar. The large sample size allowed the investigators to perform different subgroup analyses, which interestingly revealed that the MCID did not differ by age, sex, race, pulmonary hypertension etiology, body mass index, use of background therapy, or World Health Organization functional class. However, percentage change from baseline 6MWD, which has been used in several outcome-driven randomized clinical trials in the field of PAH, revealed considerable heteroscedasticity, suggesting that using this measure in composite endpoints most likely will have different implications for MCID than absolute 6MWD. It should also be mentioned that the presently determined MCID of the 6MWD for individual patient response and +24 m as the group mean difference in PAH trials is based on studies that included patients with a 6MWD of 150–450 m, and thus data reported by Moutchia and colleagues may not apply to more severe PAH, in which 6MWD is less than this range, or to fit patients with preserved exercise capacity, in whom cardiopulmonary exercise testing with assessment of the peak oxygen uptake may be better suited as an outcome measure (18).
The presently discussed paper once again underscores the importance of including quality of life as an outcome measure in clinical trials. Measuring quality of life as a patient-reported outcome in clinical practice and as a trial endpoint is important to determine and verify MCID for other tests, including pulmonary hemodynamics, imaging, or cardiopulmonary exercise testing. The comprehensive establishment of the MCID of the 6MWD using a sound method based on a large collective of patients in clinical trials and validation in a real-life registry will aid in the design of future clinical trials that aim to advance novel therapies toward regulatory approval for PAH by relying on meaningful outcome measures for patients who walk between 150 and 450 m, regardless of age, sex, body mass index, race, underlying etiology, and background therapies (17). However, the search for a valid and significant trial endpoint for a remaining broad collective of patients with PAH outside of this 6MWD range is ongoing and also which measures would best be included in event-driven trials. Until this holy grail endpoint is determined, it seems reasonable to include a combination of measures, including patient-reported outcomes, biomarkers, exercise capacity, imaging, and hemodynamics, in future PAH trials, which will hopefully help to define MCID for other measures and significant trial endpoints to improve the quality of life and prognosis of patients with PAH worldwide.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202301-0143ED on February 15, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Balke B.A simple field test for the assessment of physical fitness. 1963. pp. 1–8. [PubMed]
- 2. Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) . 1982;284:1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med . 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 4. Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. Primary Pulmonary Hypertension Study Group A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med . 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 5. Sitbon O, Gomberg-Maitland M, Granton J, Lewis MI, Mathai SC, Rainisio M, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J . 2019;53:1801908. doi: 10.1183/13993003.01908-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS Scientific Document Group 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J . 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 7. Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis . 1987;40:171–178. doi: 10.1016/0021-9681(87)90069-5. [DOI] [PubMed] [Google Scholar]
- 8. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials . 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 9. Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care . 1999;37:469–478. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 10. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med . 2014;189:250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- 11. Schünemann HJ, Guyatt GH. Commentary—goodbye M(C)ID! Hello MID, where do you come from? Health Serv Res . 2005;40:593–597. doi: 10.1111/j.1475-6773.2005.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schünemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ) COPD . 2005;2:81–89. doi: 10.1081/copd-200050651. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH) Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. 2009.
- 14. Gomberg-Maitland M, Bull TM, Saggar R, Barst RJ, Elgazayerly A, Fleming TR, et al. New trial designs and potential therapies for pulmonary artery hypertension. J Am Coll Cardiol . 2013;62:D82–D91. doi: 10.1016/j.jacc.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbert C, Brown MCJ, Cappelleri JC, Carlsson M, McKenna SP. Estimating a minimally important difference in pulmonary arterial hypertension following treatment with sildenafil. Chest . 2009;135:137–142. doi: 10.1378/chest.07-0275. [DOI] [PubMed] [Google Scholar]
- 16. Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med . 2012;186:428–433. doi: 10.1164/rccm.201203-0480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moutchia J, McClelland RL, Al-Naamani N, Appleby DH, Blank K, Grinnan D, et al. Minimal clinically important difference in the six-minute walk distance for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med . 2023;207:1070–1079. doi: 10.1164/rccm.202208-1547OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Appenzeller P, Gautschi F, Müller J, Lichtblau M, Saxer S, Schneider SR, et al. Prediction of maximal oxygen uptake from 6-min walk test in pulmonary hypertension. ERJ Open Res . 2022;8:00664-2021. doi: 10.1183/23120541.00664-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]