Abstract
Rationale
Genetic studies suggest that SOX17 (SRY-related HMG-box 17) deficiency increases pulmonary arterial hypertension (PAH) risk.
Objectives
On the basis of pathological roles of estrogen and HIF2α (hypoxia-inducible factor 2α) signaling in pulmonary artery endothelial cells (PAECs), we hypothesized that SOX17 is a target of estrogen signaling that promotes mitochondrial function and attenuates PAH development via HIF2α inhibition.
Methods
We used metabolic (Seahorse) and promoter luciferase assays in PAECs together with the chronic hypoxia murine model to test the hypothesis.
Measurements and Main Results
Sox17 expression was reduced in PAH tissues (rodent models and from patients). Chronic hypoxic pulmonary hypertension was exacerbated by mice with conditional Tie2-Sox17 (Sox17EC−/−) deletion and attenuated by transgenic Tie2-Sox17 overexpression (Sox17Tg). On the basis of untargeted proteomics, metabolism was the top pathway altered by SOX17 deficiency in PAECs. Mechanistically, we found that HIF2α concentrations were increased in the lungs of Sox17EC−/− and reduced in those from Sox17Tg mice. Increased SOX17 promoted oxidative phosphorylation and mitochondrial function in PAECs, which were partly attenuated by HIF2α overexpression. Rat lungs in males displayed higher Sox17 expression versus females, suggesting repression by estrogen signaling. Supporting 16α-hydroxyestrone (16αOHE; a pathologic estrogen metabolite)–mediated repression of SOX17 promoter activity, Sox17Tg mice attenuated 16αOHE-mediated exacerbations of chronic hypoxic pulmonary hypertension. Finally, in adjusted analyses in patients with PAH, we report novel associations between a SOX17 risk variant, rs10103692, and reduced plasma citrate concentrations (n = 1,326).
Conclusions
Cumulatively, SOX17 promotes mitochondrial bioenergetics and attenuates PAH, in part, via inhibition of HIF2α. 16αOHE mediates PAH development via downregulation of SOX17, linking sexual dimorphism and SOX17 genetics in PAH.
Keywords: pulmonary arterial hypertension, hypoxia-inducible factor 2α, 16α-hydroxyestrone, SRY-related HMG-box 17, metabolism
At a Glance Commentary
Scientific Knowledge on the Subject
Genetic studies have yielded novel candidate genes associated with the development of pulmonary arterial hypertension (PAH). One of the top genes from these studies is SOX17 (SRY-related HMG-box 17). Absent from the field are studies that experimentally validate whether SOX17 contributes to the development of PAH.
What This Study Adds to the Field
Our study adds novel information about SOX17 regulation and function. Specifically, we experimentally validate the role of SOX17 in the development of murine pulmonary hypertension. For the first time, we demonstrate that estrogen signaling regulates SOX17 expression. We further show that SOX17 mediates metabolic function in endothelial cells. These data connect well-established PAH pathways with SOX17, linking sex, SOX17 genetics, and metabolism.
Pulmonary arterial hypertension (PAH) is a rare disease characterized by endothelial cell (EC) proliferation, mitochondrial dysfunction, and obliterative pulmonary vascular remodeling, leading to increased pulmonary vascular resistance and subsequent right ventricular (RV) failure (1). Despite major advances in its management, mortality rates remain unacceptably high. Genetics contribute to the risk of developing PAH (1). Recently, a genome-wide association study demonstrated the contribution from common genetic variation in PAH, highlighting a region upstream of SOX17 (SRY-related HMG-box 17) linked with disease risk (2). In parallel, rare deleterious mutations of SOX17 were also identified to cause PAH (3, 4). On the basis of prior literature, SOX17 is predominantly an EC-specific transcription factor that has significant roles in pulmonary vascular morphogenesis, cardiovascular development, and inflammatory lung injury, all relevant processes in the development of PAH (5). Taken together, although these published data implicate a causal role, whether and how SOX17 deficiency leads to the development of PAH is unknown.
PAH is a sexually dimorphic disease with female predominance (6). Sex differences are attributed, in major part, to the role of sex hormones and, importantly, estrogen metabolites in PAH pathophysiology (6, 7). From work in patients with PAH, estrogenic activity is associated with the penetrance of PAH (8, 9). Paradoxically, although female subjects are at an increased risk of disease, they exhibit better survival than their male counterparts (10, 11). In contrast to patients with PAH, animal studies of pulmonary hypertension (PH) produce additional complexity to these observations and, in part, conflicting results (12). Cumulatively, discrepancies between patients and animal models of PAH are commonly referred to as the “estrogen paradox” (7). The estrogen paradox is explained partly by the complexity of 17β-estradiol (E2) metabolism and the potential tissue-specific roles of the diverse estrogen metabolites (6). For example, the most abundant circulating estrogen, E2, appears to exert protective effects in the right ventricle in a variety of rodent models of PH (7). In contrast, 16α-hydroxyestrone (16αOHE), an estrogen metabolite with greater estrogenic activity, contributes to the obliterative pulmonary vascular remodeling in animal models (13–16). In addition to exerting proliferative, angiogenic, and inflammatory properties, 16αOHE concentrations are increased in patients with PAH (9, 17). On the basis of consistent disease-promoting effects, 16αOHE is an appealing target for additional studies on understanding disease mechanisms and the development of PAH therapeutics. Furthermore, links among these sex differences, estrogen signaling, and SOX17 remain unknown.
Impaired mitochondrial bioenergetics and shifts to Warburg metabolism are also considered hallmarks of PAH pathology, with established links to hyperproliferative remodeling (18, 19). Induction of sustained, normoxic HIF2α (hypoxia-inducible factor 2α) signaling in smooth muscle cells has also been shown to enhance glycolysis (20), while its reduction in pulmonary artery ECs (PAECs) was shown to promote spontaneous and severe murine PAH (21). Furthermore, its role in PAEC mitochondrial bioenergetics, including oxidative phosphorylation and the tricarboxylic acid (TCA) cycle, remains unclear, while the way in which SOX17-mediated genetic mechanisms intersect with mitochondrial bioenergetics and HIF2α in ECs is also unknown.
We now investigate the functional role of Sox17 deficiency in the development of murine PH. We further hypothesize that SOX17 is a downstream target of estrogen signaling that regulates HIF2α in PAECs, promoting mitochondrial function and attenuating PAH development. We dissect the interactions between 16αOHE-mediated estrogen signaling, regulation of SOX17, and HIF2α signaling to establish the first link between sexual dimorphism, EC metabolism, and SOX17 genetics in PAH. Some of the results of these studies have been previously reported in the form of an abstract (22, 23).
Methods
Patient Cohorts
Only patients with group 1 PAH defined by right heart catheterization measurements, including mean pulmonary arterial pressure ⩾ 25 mm Hg, pulmonary capillary wedge pressure ⩽ 15 mm Hg, and pulmonary vascular resistance ⩾ 3 Wood units, were included from all cohorts. Patients from all three cohorts gave consent and were prospectively recruited for participation in the respective study in accordance with the institutional review board.
PAH Biobank genetic, metabolomic, and multiomic analyses
Target SNP association analyses were performed using a previously published cohort from the National Biological Sample and Data Repository for Pulmonary Arterial Hypertension (PAH Biobank; www.pahbiobank.org), described in detail in prior reports (2, 24). Eligible cases were prospectively recruited from PH centers across North America, including biobanking between October 3, 2012, and March 14, 2016. Clinical data were collected from each center, including demographics, diagnostic right heart catheterization, and 6-minute-walk distance (6MWD). Genotype data were cleaned as previously described (2).
Plasma samples from subjects in PAH Biobank were further profiled for analysis of TCA cycle metabolites by liquid chromatography–mass spectrometry using a Vanquish UPLC coupled to a high-resolution, Q Exactive orbitrap mass spectrometer (Thermo Fisher Scientific), as previously described (25, 26). Quality control analysis was performed, and spectral data were extracted. Data were then batch median normalized with correction for median absolute deviation. Metabolite peaks were then compressed for multiple adducts and in source fragments. Aligned and filtered metabolites involved in the TCA cycle were subsequently used for statistical analyses, as described below.
SOX17 genetics and metabolites involved in the TCA cycle
Associations between log10-transformed plasma metabolite (citrate) concentration and clinical and hemodynamic indices (including mean right atrial pressure, mean pulmonary arterial pressure, pulmonary arterial systolic and diastolic pressures, pulmonary capillary wedge pressure, and 6MWD) were tested in R (https://www.r-project.org) from patients of European genetic ancestry, adjusting for sex, age at enrollment, PAH vintage, and the first two principal components. We also tested for association between plasma metabolite (TCA cycle metabolites) concentrations and two SOX17 variants previously reported to increase disease risk (rs10103692 and rs13266183) using a multiple linear regression model in PLINK (https://zzz.bwh.harvard.edu/plink/contact.shtml#cite). This metabolite quantitative trait locus (mQTL) analysis additively coded for the G allele for rs10103692 and was also adjusted for sex, age at enrollment, PAH vintage, and the first two principal components. After multiple testing corrections, the required P value for significance was <0.025 (0.05/2 independent SNPs).
Human PAECs from the Pulmonary Hypertension Breakthrough Initiative
PAECs were acquired from the Pulmonary Hypertension Breakthrough Initiative, whose methods are previously detailed (27). Briefly, patients with PAH and control subjects were prospectively enrolled starting in 2006. PAECs were isolated and cultured from lungs of patients with PAH (collected at the time of lung transplantation for PAH) and from those of control subjects (from failed donor lungs).
Patient lung staining
Paraffin-embedded lung tissues from deidentified subjects with PAH (collected at the time of autopsy or transplantation for PAH) and control lungs (from subjects who died of other non–lung disease–related causes, collected at the time of autopsy) between 2003 and 2015 were obtained from the University of Arizona Department of Pathology after approval by the institutional review board.
Murine/Rodent Hypoxic PH Models
All experimental protocols were approved by the Animal Care and Use Committees at the University of Arizona and Indiana University.
Mice
Adult (8–12 weeks of age) male and female C57BL/6N mice were used for all experiments. Exposure to normoxia and chronic hypoxia (4 weeks, n = 10–16) was used as a model for murine PH, as previously described (21). Additional details are provided in the online supplement, and primers are listed in Table E1 in the online supplement.
Generation of inducible conditional Tie2-Sox17 knockout mice
Sox17fl/fl mice were purchased from The Jackson Laboratory (#031712). We generated inducible conditional Tie2-specific deletion of Sox17 (Sox17EC−/−) in mice using Cre-Lox technology. We administered Tie2Cre-ERT2+/Sox17fl/fl mice with 1 mg (100 μl) tamoxifen once a day for five days consecutively and then rested for two to four weeks to induce Sox17 deletion. Tie2Cre-ERT2−/Sox17fl/fl mice were used as control animals.
Generation of inducible conditional Sox17 transgenic overexpression mice
To generate inducible conditional Tie2-specific Sox17 transgenic overexpression (Sox17Tg) mice, we used Tet-O-Sox17 mice, generated using an Ins2-rtTA mouse line and a line in which SOX17 expression is regulated by the tetracycline transactivator (Tet-On system). Tet-O-Sox17 mice were crossed with Tie2Cre mice, and the resultant Tet-O-Sox17+Cre-positive mice were administered doxycycline via drinking water at a dose of 2 mg/ml for 2 weeks to induce Tie2-specific overexpression of Sox17 (Sox17Tg). Tet-O-Sox17+Cre-negative mice were used as control mice.
16αOHE administration in mice
16αOHE (Steraloids Inc.) was administered to mice (cage control and Sox17Tg) at a dose of 1 mg/kg/d for 1 week via subcutaneous injections daily (28). 16αOHE was dissolved in 95% ethanol and then diluted with water to 50% ethanol. Ethanol was used as the vehicle.
Generation of estrogen receptor α loss-of-function mutant rat model
Lung homogenate samples of ERα (estrogen receptor α) loss-of-function (ERα−/−) mutant rats (wild-type [WT] and after 4 weeks of exposure to monocrotaline [MCT]) were generously provided by the Lahm laboratory as previously described (29).
Gas Chromatography–Mass Spectrometry Analysis of TCA Metabolites and Bioinformatic Analyses
PAECs were cultured for 6 hours in a medium composed of glucose- and glutamine-free Dulbecco’s modified Eagle medium (Gibco), 10% dialyzed fetal bovine serum, 5.5 mM U-13C6-glucose (CLM-1396-PK, 99%; Cambridge Isotope Laboratories), and 2 mM l-glutamine. TCA metabolites were then analyzed as described in the online supplement. Comprehensive metabolomic data analysis was performed using the online MetaboAnalyst 5.0 platform (https://www.metaboanalyst.ca/). Metabolic flux data normalization was performed by generalized log transformation and Pareto data scaling. For multivariate statistical analysis, partial least squares discriminant analysis (PLS-DA) was applied. A PLS regression was performed using the plsr function provided by the R pls package. Classification and cross-validation were both performed using the corresponding wrapper function offered in the caret package incorporated into MetaboAnalyst 5.0. Correlation analysis was used to visualize the overall correlations between two groups (control and Sox17-overexpressed sets), and the clustering result is shown as a heatmap. Hierarchical clustering was performed with the hclust function in package stat. A summary of enriched metabolite sets was generated and helped generate a visual pathway analysis. Finally, significantly altered flux comparing control and Sox17-overexpressed sets were analyzed and helped construct a TCA cycle diagram.
Seahorse Analysis of Mitochondrial Bioenergetics
Mitochondrial bioenergetics was measured using the XF24 Analyzer (Seahorse Biosciences) and the XF Cell Mito Stress Test Kit (Agilent Technology) per our published procedures (30).
Statistical Analysis
Data are represented as mean ± SEM. Data normalization for gas chromatography–mass spectrometry (GC-MS) analysis was performed by generalized log transformation and Pareto data scaling. For multivariate statistical analysis, principal component analysis and PLS-DA were applied. Student’s unpaired t test was used unless the sample size was less than seven per group, in which case we used a nonparametric test. The Pearson correlation coefficient was analyzed to determine an association between ESR1 (estrogen receptor 1) and SOX17 gene expression in PAECs. Multivariate linear regression analyses were used for genetic associations in R and PLINK. All analyses were performed using Prism 8 software (GraphPad Software). A P value of <0.05 was considered to indicate statistical significance.
Additional details on methods are provided in the online supplement.
Results
SOX17 Expression Is Reduced in PAH
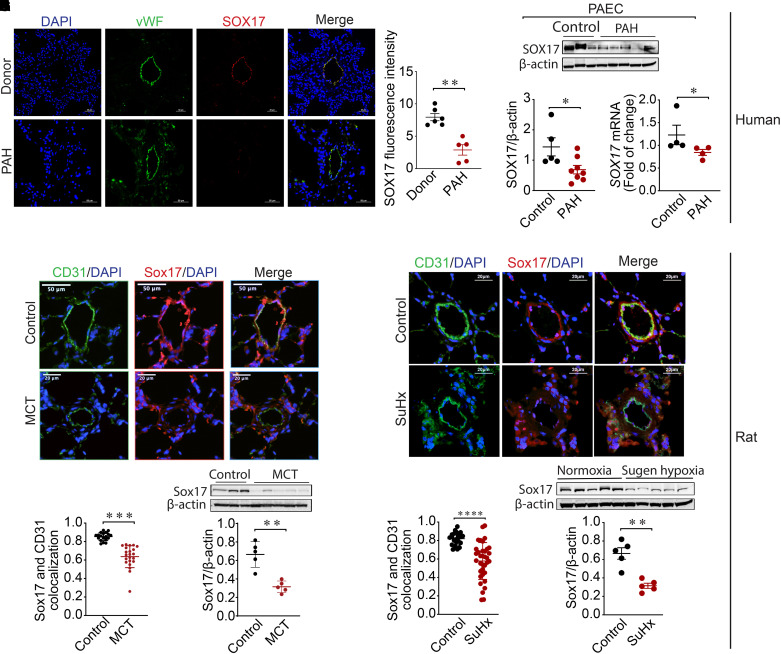
Immunostaining with antibodies against von Willebrand factor, a marker for ECs and SOX17, revealed decreased expression of SOX17 in the endothelium of PAH compared with non-PAH control human lung sections (Figures 1A, 1B, and E1). Both mRNA and protein concentrations of SOX17 were significantly reduced in human PAECs isolated from PAH compared with control subjects (Figures 1C–1E; see Table E2). Mirroring this pattern in patients, immunofluorescence costaining against Sox17 and CD31 (cluster of differentiation 31), a marker to stain ECs, revealed reduced EC-specific Sox17 in rats exposed to MCT (Figures 1F and 1G) and Sugen–hypoxia (Figures 1I and 1J) compared with control rats. Western blots revealed reduced SOX17 expression in whole-lung homogenates from MCT (Figure 1H; see Table E3) and Sugen–hypoxia (Figure 1K; see Table E4) rat models with PH compared with control animals. Cumulatively, this expression profiling reveals reduced endothelial-specific SOX17 in PAH.
Figure 1.
SOX17 (SRY-related HMG-box 17) is downregulated in pulmonary arterial hypertension (PAH). (A and B) Representative (A) and quantitative (B) immunofluorescence staining for DAPI (blue), vWF (green), and SOX17 (red) in human lungs from a non-PAH donor (n = 6) and patients with PAH (n = 5). (C) Western blot demonstrating SOX17 protein concentrations in pulmonary artery endothelial cells (PAECs) isolated from non-PAH control and subjects with PAH. (D) Densitometric analysis of SOX17 normalized to β-actin in PAECs from control subjects (n = 5) and patients with PAH (n = 8). (E) Real-time PCR analysis showing mRNA fold change of SOX17 normalized to actin in PAECs from control (n = 4) subjects and subjects with PAH (n = 4). (F) Representative immunofluorescence images showing DAPI (blue), CD31 (cluster of differentiation 31) (green), and Sox17 (red) staining in lung sections from control and monocrotaline (MCT)–exposed rats. (G) Quantitative immunofluorescence of SOX17 in control and MCT-exposed rat samples. (H) Western blot showing protein concentrations of Sox17 in MCT versus control rat lungs and densitometric analysis of Sox17 normalized to β-actin in MCT (n = 5) and control (n = 5) rats. (I) Representative immunofluorescence images showing DAPI (blue), CD31 (green), and Sox17 (red) staining in lung sections from control and rats exposed to Sugen–hypoxia (SuHx). (J) Quantitative immunofluorescence of SOX17 in control and SuHx-exposed rat samples. (K) Western blot showing the protein concentrations of Sox17 in lungs from SuHx versus normoxic control rats and densitometric analysis of Sox17 normalized to β-actin in SuHx (n = 5) and normoxic control (n = 5) rats. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 versus controls. vWF = von Willebrand factor.
Sox17 Deficiency Promotes Chronic Hypoxia-induced PH
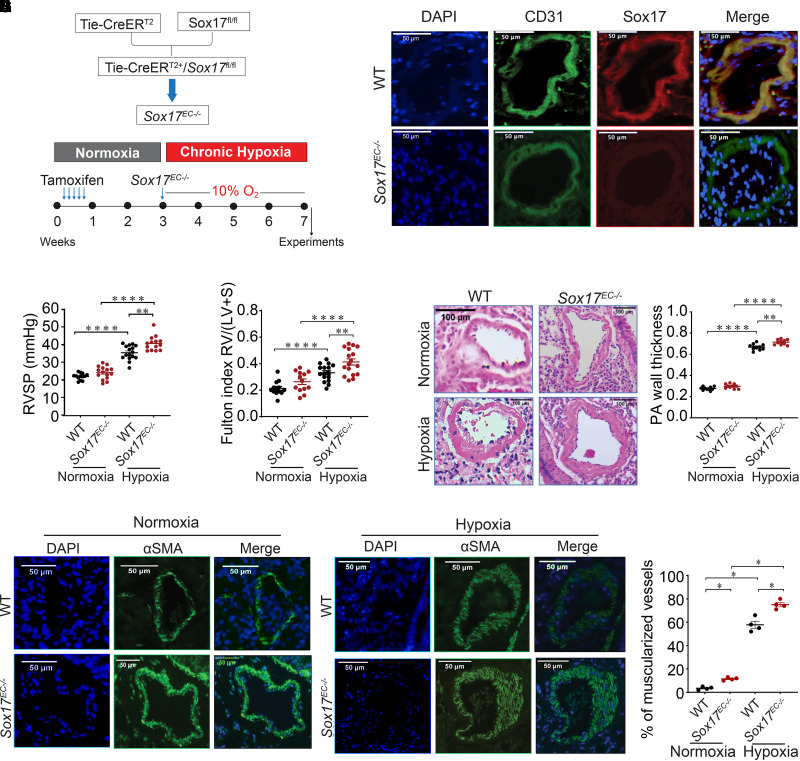
To investigate the functional role of SOX17 in PAH, Sox17EC−/− and cage control mice were exposed to chronic hypoxia (10% oxygen) for 4 weeks to induce PH (Figure 2A). Both mice strains were also exposed to room air as normoxic control. Immunofluorescence costaining with SOX17 and CD31, an EC marker, confirmed deletion of Sox17 in PAECs with absent staining of Sox17EC−/− mice compared with control animals, consistent with their prior reported use in murine models of acute lung injury (31) (Figure 2B). Under normoxic conditions, there was a trend toward increases in basal RV systolic pressure (RVSP) in Sox17EC−/− compared with control mice (P = 0.06). With chronic hypoxia exposure, Sox17EC−/− mice revealed statistically significant increases in RVSP compared with control animals (Figure 2C). The Fulton index, a marker of RV hypertrophy, was increased in Sox17EC−/− mice compared with control animals under both normoxic and hypoxic conditions (Figure 2D).
Figure 2.
Endothelial deficiency of Sox17 (SRY-related HMG-box 17) (Sox17EC−/−) augments chronic hypoxia-induced murine pulmonary hypertension (PH). (A) Schema that reflects modeling of murine hypoxic PH. (B) Representative immunostaining against Sox17 (red) and CD31 (green) in mouse lung section from control and Sox17EC−/− mice (scale bar, 50 μm). (C) RVSP measurements in wild-type (WT) and Sox17EC−/− mice under normoxia and hypoxia (n = 10–16 per group). (D) Fulton index (RV/[LV + S]) measurements in WT control and Sox17EC−/− mice exposed to normoxia or hypoxia (n = 10–16 per group). (E) Representative hematoxylin and eosin images of PAs (categorized into diameter ⩽ 50 μm and diameter > 50 μm) showing wall thickness in WT and Sox17EC−/− mice under normoxia or hypoxia. (F) Summarized data showing PA wall thickness (wall area/total area) in WT and Sox17EC−/− mice. (G) Representative αSMA (α-smooth muscle actin) staining in WT and Sox17EC−/− mice under normoxic conditions. (H) Representative αSMA staining in WT and Sox17EC−/− mice under hypoxic conditions. (I) Percentage of muscularized vessels in control and Sox17EC−/− mice under normoxia and hypoxia (n = 4 per group) and quantitative immunofluorescence of αSMA in mouse lung samples. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, and ****P < 0.0001. CD31 = cluster of differentiation 31; PA = pulmonary artery; RV/(LV + S) = ratio of right ventricular weight to the sum of left ventricular and septal weight; RVSP = right ventricular systolic pressure.
Sox17 Deficiency Augments Pulmonary Vascular Remodeling in Hypoxic Mice
Histological assessments to measure pulmonary arterial wall thickness (PAWT) were performed using hematoxylin and eosin staining. Sox17EC−/− mice exhibited an increase in PAWT under both normoxia and chronic hypoxia compared with control mice (Figures 2E and 2F). αSMA (α-smooth muscle actin) staining was also performed to measure the medial thickness on lung sections from WT control and Sox17EC−/− mice. An increase in the percentage of muscularized vessels was detected in the pulmonary arteries of Sox17EC−/− mice compared with control animals in both normoxic and hypoxic conditions (Figures 2G–2I). These in vivo data suggest that the deficiency of Sox17 in ECs promotes hypoxic PH in mice.
SOX17 Promotes Oxidative Phosphorylation and Mitochondrial Function in PAECs
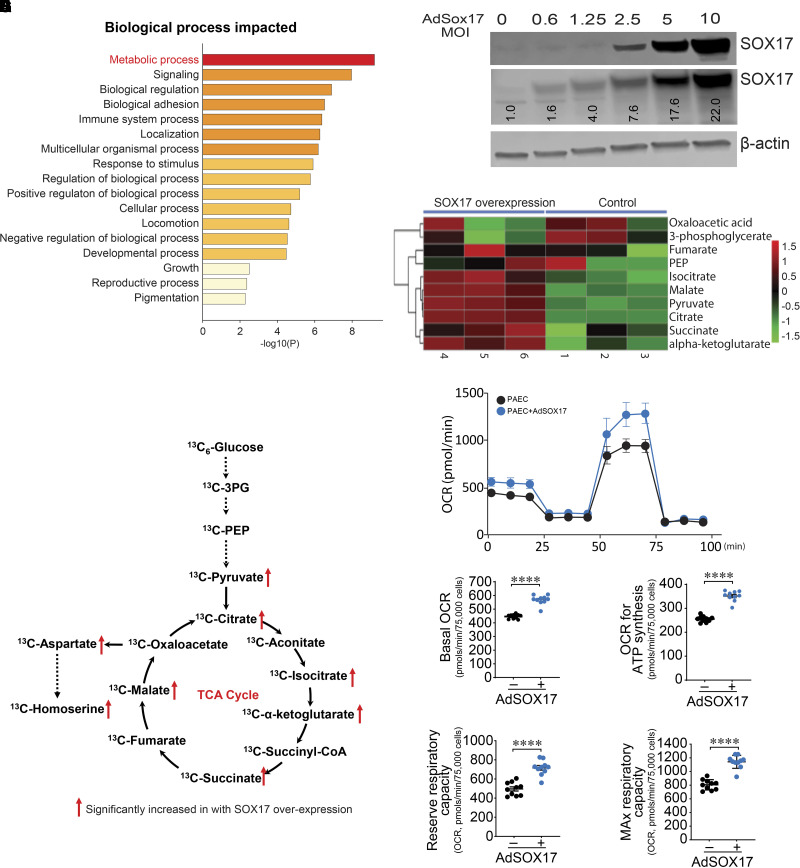
To understand how EC-derived Sox17 deficiency mediates exacerbation of PH, we compared protein expression profiles after silencing SOX17 (siSOX17) and scrambled RNA (scRNA) in PAECs (see Figure E2A) using quantitative mass spectrometry. We detected 7,424 proteins using a scoring system that scores the peptides for confident assignment to known proteins. We next performed differential protein expression analysis between scRNA and siSOX17. Using a nominal P value of <0.05 (Student’s t test), we identified 321 proteins (201 upregulated and 120 downregulated; see Figures E2B and E2C and Tables E5 and E6). We performed enrichment analysis on this latter set of 321 differential proteins using Metascape analysis to identify statistically enriched Gene Ontology pathways. Although no differentially expressed protein met the Bonferroni or Q-value threshold, pathway analysis highlighted metabolism as one of the top potential targets of SOX17 deficiency (Figure 3A).
Figure 3.
SOX17 (SRY-related HMG-box 17) augments oxidative phosphorylation and mitochondrial function in endothelial cells. (A) Gene Ontology analysis highlights metabolic processes as the top targets of SOX17 deficiency. (B) PAECs were transduced with increasing multiplicities of infection (MOIs) of AdSOX17. Western blot analysis was used to confirm the overexpression of SOX17. An MOI of 20 was used. (C) A heatmap illustrating alterations in metabolite concentrations in SOX17-overexpressed endothelial cells (ECs) compared with control ECs. (D) Targeted gas chromatography–mass spectrometry analysis identifies increases in TCA cycle metabolites in SOX17 overexpressing ECs. (E–I) Increasing SOX17 expression enhances mitochondrial bioenergetics (E), as determined by increases in basal oxygen consumption rate (OCR) (F), OCR for ATP generation (G), and reserve (H) and MAX (I) respiratory capacity. Data are represented as mean ± SEM. ****P < 0.0001. 3PG = 3-phosphoglyceric acid; AdSOX17 = adenovirus containing human SOX17; CoA = coenzyme A; MAX = maximal; PAEC = pulmonary artery endothelial cell; PEP = phosphoenolpyruvate; TCA = tricarboxylic acid.
We next overexpressed SOX17 in PAECs using an adenovirus (Figure 3B) and investigated the consequence on TCA cycle cellular metabolites using 13C-metabolic flux analyses. Mass isotope distributions of central carbon metabolites were analyzed using GC-MS. Flux and tracing results were analyzed using the MetaboAnalyst platform. Both principal component analysis and PLS-DA plots showed a clear cluster distinction between control (adenovirus–green fluorescent protein–plasmid alone) and SOX17-overexpressed (adenovirus–green fluorescent protein–SOX17) groups (see Figures 3EA and E3B). Values from GC-MS analysis were used to develop a heatmap depicting the TCA metabolites altered by SOX17 overexpression (Figure 3C). The heatmap generated from hierarchical clustering provides an overview of metabolites significantly decreased (green) and increased (red). Among the 10 TCA metabolites, 6 metabolites were significantly higher in SOX17-overexpressed PAECs (Figure 3D). These metabolites were identified as pyruvate, citrate, isocitrate, α-ketoglutarate, succinate, malate, homoserine, and 3-phosphoglyceric acid (see Figure E4). To confirm the effects of the 13C-metabolic flux analyses, we further characterized cellular bioenergetics status using a Seahorse (Seahorse Biosciences) bioanalyzer coupled with a mitostress test. The Seahorse data indicated that SOX17 overexpression enhanced mitochondrial bioenergetics (Figure 3E), as shown by real-time measurements of mitochondrial oxygen consumption rates on the mitochondrial biogenetic profile. In SOX17-overexpressed PAECs, basal respiration (Figure 3F) and the amount of oxygen consumed for ATP generation (Figure 3G) were significantly increased, together with reserve respiratory capacity (Figure 3H) and maximal respiratory capacity (Figure 3I) compared with control (see Figure E5).
SOX17 Attenuates HIF2α-mediated Inhibition of Oxidative Phosphorylation and Mitochondrial Function in PAECs
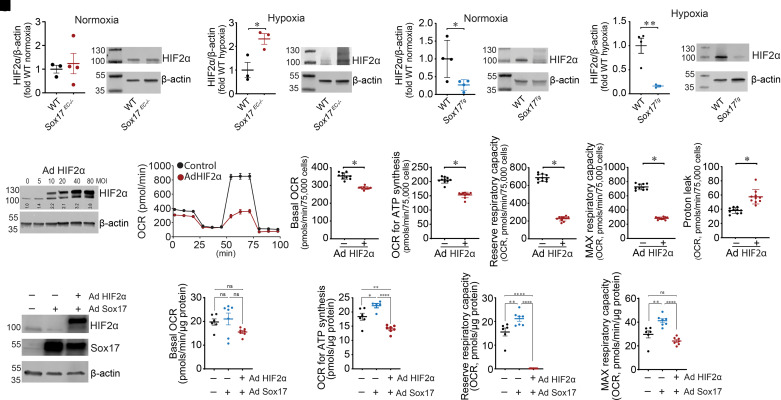
To dissect how SOX17 promotes mitochondrial function and oxidative phosphorylation, we next evaluated whether PAEC-derived HIF2α is a potential downstream mediator, given its established role in EC pathology in murine PH. Although they were unaffected in Sox17EC−/− mice under normoxic conditions (Figure 4A), HIF2α protein concentrations were significantly increased under hypoxic conditions (Figure 4B). Conversely, HIF2α protein concentrations were significantly decreased in Sox17Tg mice in both normoxic (Figure 4C) and hypoxic (Figure 4D) conditions. To further investigate the role of HIF2α on mitochondrial bioenergetics, we overexpressed HIF2α in PAECs using an adenovirus (Figure 4E) and again used a Seahorse bioanalyzer coupled with a mitostress test to investigate changes in cellular bioenergetics. Our Seahorse data indicate that HIF2α overexpression disrupted mitochondrial bioenergetics (Figure 4F). In HIF2α-overexpressed PAECs, basal respiration (Figure 4G), the amount of oxygen consumed for ATP generation (Figure 4H), reserve respiratory capacity (Figure 4I), and maximal respiratory capacity (Figure 4J) were all significantly decreased, whereas the proton leak was elevated (Figure 4K). Furthermore, the overexpression of HIF2α reversed the positive effects of SOX17 overexpression (Figure 4L) on mitochondrial bioenergetics, as shown by decreases in basal respiration (Figure 4M), the amount of oxygen consumed for ATP generation (Figure 4N), reserve respiratory capacity (Figure 4O), and maximal respiratory capacity (Figure 4P) when HIF2α and SOX17 were coexpressed compared with SOX17 alone.
Figure 4.
SOX17 (SRY-related HMG-box 17) attenuates HIF2α (hypoxia-inducible factor 2α)–mediated inhibition of oxidative phosphorylation and mitochondrial function in endothelial cells. (A and B) HIF2α protein concentrations are unchanged in Sox17EC−/− mice under normoxic conditions (A) but significantly increased by hypoxia (B). (C and D) HIF2α protein concentrations are significantly decreased in Sox17Tg mice under both normoxic (C) and hypoxic (D) conditions. (E) Western blot analysis was used to confirm overexpression of HIF2α. An MOI of 40 was used. (F–K) Increasing HIF2α expression attenuates mitochondrial bioenergetics (F), as determined by decreases in basal oxygen consumption rate (OCR) (G), OCR for ATP generation (H), reserve (I) and maximal (J) respiratory capacity, and increases in the proton leak (K). (L–P) Coexpressing HIF2α reverses the positive effect of SOX17 overexpression on mitochondrial bioenergetics; (L) Western blot indicating overexpression of SOX17 and HIF2α. HIF2α coexpression with SOX17 decreases basal respiration (M), amount of oxygen consumed for ATP generation (N), reserve respiratory capacity (O), and maximal respiratory capacity (P) compared with SOX17 overexpression alone. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, and ****P < 0.0001. Ad = adenovirus; MAX = maximal; MOI = multiplicity of infection; ns = not significant; WT = wild-type.
Sex-Specific SOX17 Expression
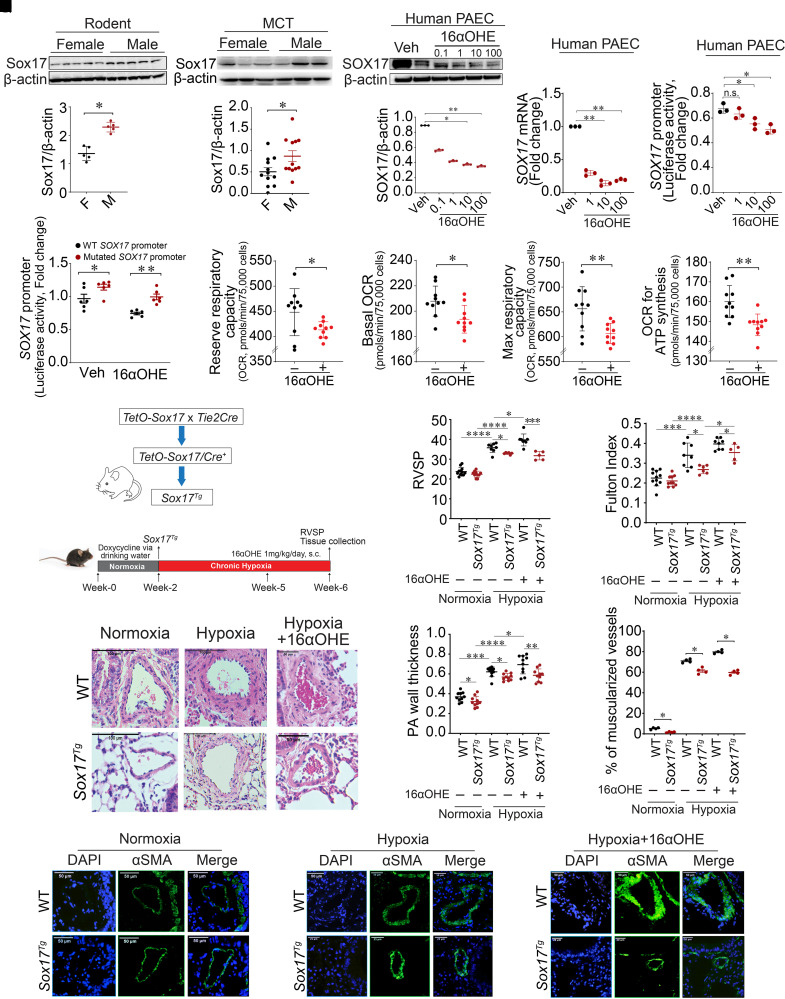
We next evaluated how sex differences influence SOX17 expression and signaling. Sex differences were determined in normoxic and hypoxic WT and Sox17EC−/− mice. Changes in RVSP and the Fulton index between control and Sox17 deficiency trended higher in male compared with female mice (see Figure E6). Notably, Sox17 protein concentrations were significantly reduced in female compared with male lungs at baseline and after MCT in rats as well as after 4 weeks of hypoxic exposure in mice (Figures 5A–5D; see Figure E7). On the basis of lower SOX17 expression in females, we hypothesized estrogen-mediated regulation and, specifically, repression of SOX17 expression.
Figure 5.
Endothelial cell–derived SOX17 (SRY-related HMG-box 17) is a transcriptional target of 16α-hydroxyestrone (16αOHE) and attenuates hypoxic pulmonary hypertension (PH) in mice. (A) Sox17 expression in female (n = 5) and male (n = 5) rat lungs at baseline. (B) Densitometric graph of Sox17 expression normalized to β-actin in female and male rat lungs. (C) Representative western blot showing Sox17 expression in female and male rat lungs exposed to monocrotaline (MCT). (D) Densitometric graph of Sox17 expression normalized to β-actin in female (n = 12) and male (n = 12) rat lungs exposed to MCT. (E) Representative western blot showing protein expression of SOX17 in pulmonary artery endothelial cells (PAECs) treated with 16αOHE (0.1–100 nM). (F) Densitometric analysis of SOX17 normalized to β-actin in vehicle (Veh)– and 16αOHE-treated PAECs. (G) mRNA expression of SOX17 in PAECs treated with 16αOHE (1–100 nM). (H) A dual-luciferase assay was used to measure relative promoter luciferase activities. 16αOHE exposure reduced SOX17 promoter activity compared with Veh in PAECs transfected with pGL3-SOX17 promoter plasmid. (I) Dual-luciferase assay showing luciferase activities of wild-type (WT) and mutated SOX17 promoter in Veh- and 16αOHE-treated PAECs. (J–M) We exposed human PAECs to 16αOHE (10 nM) and measured mitochondrial bioenergetics. Real-time measurements of mitochondrial OCRs on the mitochondrial biogenetic profile, including basal respiration (K) and the amount of oxygen consumed for ATP generation (M), were reduced, together with reserve respiratory capacity (J) and Max respiratory capacity (L). (N) Schema that reflects modeling of murine hypoxic PH. (O and P) RVSP (O) and Fulton index (ratio of right ventricular weight to the sum of left ventricular and septal weight) (P) measurements in WT and Sox17Tg mice under normoxia, hypoxia, or hypoxia with or without 16αOHE. (Q) Representative hematoxylin and eosin images of lung sections showing pulmonary artery wall thickness (PAWT) in WT and Sox17Tg mice. (R) Summarized data showing PAWT measurements (wall area/total area) in WT and Sox17Tg mice. (S) Percentage of muscularized vessels in WT and Sox17Tg mice under normoxia, hypoxia, or hypoxia with or without 16αOHE (n = 4 per group). (T) Representative αSMA staining in WT and Sox17Tg mice under normoxic or hypoxic conditions with or without 16αOHE. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. αSMA = α-smooth muscle actin; F = female; M = male; Max = maximal; OCRs = oxygen consumption rates; PA = pulmonary arterial; RVSP = right ventricular systolic pressure; s.c. = subcutaneous.
Reciprocal SOX17 Responses to Two Estrogen Metabolites
Next, we screened the effects of two well-studied estrogen metabolites, E2 and 16αOHE exposure, on SOX17 expression. We first found that E2 increased SOX17 expression in vivo (see Figures E8A–E8I). Human PAECs were then separately treated with different concentrations of both 16αOHE (0.1–100 nM) versus vehicle (ethanol) for 48 hours. 16αOHE suppressed SOX17 at concentrations as low as 1 nM. Western blotting confirmed significant decreases in SOX17 protein expression after 16αOHE exposure (Figures 5E and 5F). Using real-time PCR, SOX17 mRNA concentrations were reduced after exposure to 16αOHE in a dose-dependent manner (Figure 5G). These data confirm that 16αOHE represses SOX17 expression in PAECs, and therefore, we focused our additional studies on 16αOHE.
Effect of 16αOHE on SOX17 Promoter Activity in PAECs
A SOX17 promoter (3,000 bp) was fused with the luciferase reporter gene (pGL3-Basic) to construct a pGL3-SOX17 promoter plasmid. After validating the transfection of pGL3-SOX17 promoter plasmid in PAECs using a dual-luciferase assay (see Figure E9), the transfected PAECs were treated with increasing concentrations of 16αOHE (1, 10, and 100 nM) versus ethanol as a vehicle for a period of 48 hours. The relative luciferase activity of the SOX17 promoter was decreased in a dose-dependent manner after exposure to 16αOHE compared with vehicle (Figure 5H). To further dissect these luciferase assay observations, we evaluated the human SOX17 promoter using the PROMO database and identified the presence of five consensus estrogen response element (ERE) sequences (5′-GGTCAnnnTGACC-3′). Four of the five were located ∼2,500–3,000 bp from the transcription start site, while one was located within 100 bp (see Figure E10). We mutated four of the five distal sites that were located more than 2,500 bp from the transcription start site and remeasured luciferase activity (see Figure E9). Human PAECs were again transfected with both WT and mutated SOX17 promoters. After 48 hours, the cells were treated with 16αOHE and vehicle, and relative luciferase activity was measured. WT promoter activity was consistently decreased after 16αOHE exposure compared with vehicle (Figure 5I). Mutated SOX17 promoter activity was increased compared with WT after 16αOHE exposure but was lower than vehicle (Figure 5I). These results suggest that 16αOHE-mediated SOX17 transcriptional repression involves ERE sites on the SOX17 promoter.
Effect of 16αOHE on EC Metabolism
To understand the link between metabolism and 16αOHE-exacerbated PH, we exposed human PAECs to 16αOHE (10 nM, 24 hours) and measured mitochondrial bioenergetics. Real-time measurements of mitochondrial oxygen consumption rates (Figures 5J–5M) on the mitochondrial biogenetic profile, including basal respiration and the amount of oxygen consumed for ATP generation, were reduced together with reserve respiratory capacity and maximal respiratory capacity.
Sox17 Attenuates Hypoxic PH
To study the role of Sox17 in 16αOHE-mediated exacerbation of hypoxic PH in vivo, inducible conditional Tie2-specific Sox17 transgenic overexpressing (Sox17Tg) mice were used and tested under a chronic hypoxia PH model. Sox17Tg and control mice were exposed to either normoxia or chronic hypoxia and administered either 16αOHE or vehicle (Figure 5N). Immunofluorescence costaining with SOX17 and CD31, an EC marker, reconfirmed increased expression of Sox17 in ECs of the lungs of Sox17Tg mice compared with control animals, consistent with their prior reported use (32) (see Figure E11).
First, with chronic hypoxia exposure, Sox17Tg mice revealed mild reductions in RVSP compared with control animals (Figure 5O). The Fulton index was also reduced in Sox17Tg mice compared with control animals under both normoxic and hypoxic conditions (Figure 5P). Sox17Tg mice also demonstrated reductions in PAWT under chronic hypoxia compared with control mice (Figures 5Q and 5R).
Second, consistent with prior reports (8, 15), 16αOHE-exposed cage control mice exhibited increases in RVSP and the Fulton index under normoxia (see Figures E12A–E12C). Hematoxylin and eosin staining was performed to assess PAWT in 16αOHE- and vehicle-treated mice. PAWT was also increased in normoxic cage control mice that received 16αOHE compared with vehicle (see Figures E12D and E12E). Expression of αSMA was also increased in mice exposed to 16αOHE compared with vehicle (see Figure E12F). Hypoxia further exacerbated these findings in control mice with 16αOHE exposure (Figures 5O–5T).
Third, compared with cage control animals, Sox17Tg mice exhibited reductions in RVSP and the Fulton index despite exposure to both 16αOHE treatment and chronic hypoxia (Figures 5O–5T). Taken together, these results suggest that EC-derived Sox17 attenuates hypoxic PH in vivo.
16αOHE Transcriptionally Represses SOX17 Promoter via ERα
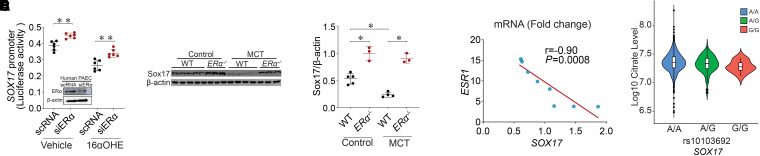
To further dissect 16αOHE-mediated estrogen signaling in the regulation of SOX17, we leveraged prior observations elucidating the canonical genomic pathway of estrogen signaling (33). Specifically, this pathway is defined by the formation of a dimeric complex after binding of estrogen metabolites to ERs (ERα and ERβ), which subsequently binds to EREs located on the promoters of target genes to regulate gene transcription. Both enhancement and repression of target genes have been reported (34–36) with this signaling cascade. On the basis of prior work on the role of ERα in mediating pathologic 16αOHE effects (13), we prioritized studying ERα in the regulation of SOX17. Human PAECs were cotransfected with a pGL3-SOX17 promoter plasmid and siRNA against ERα (siERα) or scRNA. Cells were then treated with 16αOHE versus vehicle (ethanol) followed by measurement of luciferase activity. Despite exposure to 16αOHE, SOX17 promoter activity was rescued in siERα-exposed PAECs compared with scRNA-transfected PAECs (Figure 6A). These in vitro data further support the role of 16αOHE-mediated transcriptional repression of SOX17 expression and implicate ERα.
Figure 6.
16α-Hydroxyestrone (16αOHE) represses SOX17 (SRY-related HMG-box 17) promoter through ERα (estrogen receptor α). (A) pGL3-SOX17 promoter plasmid was cotransfected with scrambled RNA (scRNA) or siRNA against ERα (siERα) in pulmonary artery endothelial cells (PAECs) and later exposed to 16αOHE versus vehicle. Dual-luciferase assay shows the relative luciferase activity of SOX17 promoter; representative western blot ERα protein concentrations in scRNA- and siERα-transfected human PAECs to confirm silencing. (B) Western blot showing Sox17 protein concentrations in wild-type (WT) (n = 5) and ERα−/− (n = 3) control rats and WT (n = 4) and ERα−/− (n = 3) monocrotaline (MCT) rats. (C) Densitometric analysis of Sox17 normalized to β-actin in ERα−/− control and MCT rats compared with WT. (D) Correlation analysis between ESR1 and SOX17 mRNA (fold change) showing a negative correlation in PAECs from patients with pulmonary arterial hypertension (PAH). Pearson correlation coefficient (r) and P value are shown. (E) rs10103692 was significantly associated with reduced citrate concentrations (metabolite quantitative trait locus, n = 1,326, β = −2.04 × 106 for each copy of G allele, SD = 0.75 × 105, P = 0.007, for non–log10-transformed citrate concentrations, n = 1,165 for AA, n = 150 for AG, and n = 10 for GG) in subjects of European ancestry after adjusting for age, sex, time between diagnosis and blood draw (PAH vintage), and population stratification. Data are represented as mean ± SEM. *P < 0.05 and **P < 0.01. ESR1 = estrogen receptor 1.
Sox17 Expression in ERα Loss-of-Function (ERα−/−) Mutant Rat Model
To confirm ERα-mediated repression of Sox17 in vivo, Sox17 expression was measured in whole-lung homogenates from male and female ERα−/− mutant rats treated with MCT. Supporting in vitro data on ERα-mediated Sox17 repression, Sox17 expression was significantly higher in ERα−/− control animals compared with WT control animals among baseline non–MCT-treated rats (Figure 6B; see Table E7). Although SOX17 concentrations were significantly reduced in MCT-exposed rats, we also observed a trend toward increases in SOX17 concentrations in MCT-exposed ERα−/− (MCT+ ERα−/−) compared with WT rats (Figure 6C). These results further support the role of ERα-mediated repression of SOX17.
Consistent with experimental data, we also found an inverse relationship between ESR1 and SOX17 gene expression in human PAECs derived from subjects with PAH. Specifically, ESR1 gene expression was increased, whereas SOX17 concentrations were decreased in PAECs from patients with PAH, indicating a negative correlation (Figure 6D).
SOX17 Genetics (rs10103692) and Plasma Citrate Concentrations
As SOX17 augments TCA cycle metabolite flux and mitochondrial bioenergetics in vitro, we investigated whether genetic variants in the SOX17 locus that promote the risk of PH are associated with reduced concentrations of TCA cycle–related metabolites in patients with PAH. Specifically, we previously reported that rs10103692 was one of the top SOX17 variants associated with PAH risk (P = 5.13 × 10−15; odds ratio for G allele, 1.8) in patients from the PAH Biobank (2). We next assessed whether this variant was also associated with reduced plasma concentrations of TCA cycle metabolites (those identified in samples from the PAH Biobank). This work was based on plasma TCA cycle metabolite data from 1,326 subjects of European genetic ancestry with group 1 PAH in the PAH Biobank who were matched with available genome-wide association study genotyping data (see Table E8). Although there were no associations with isocitrate, malate, and fumarate concentrations, we found that rs10103692 was significantly associated with plasma citrate concentrations (mQTL; Figure 6E; P = 0.007; the disease risk G allele reduces citrate concentration) after adjusting for age, sex, PAH vintage, and population stratification. To assess the validity of our association between rs10103692 and citrate concentration, we further performed a permutation test with 10,000 permutations of the mQTL, adjusting for sex, age at enrollment, PAH vintage, and two principal components. The observed permuted P value was 0.01, which was significant. Finally, although not associated with hemodynamic indices, plasma citrate concentrations (transformed to log10) were positively associated with 6MWD in linear regression analyses after adjusting for age, sex, and PAH vintage (P = 0.009; see Table E9).
Discussion
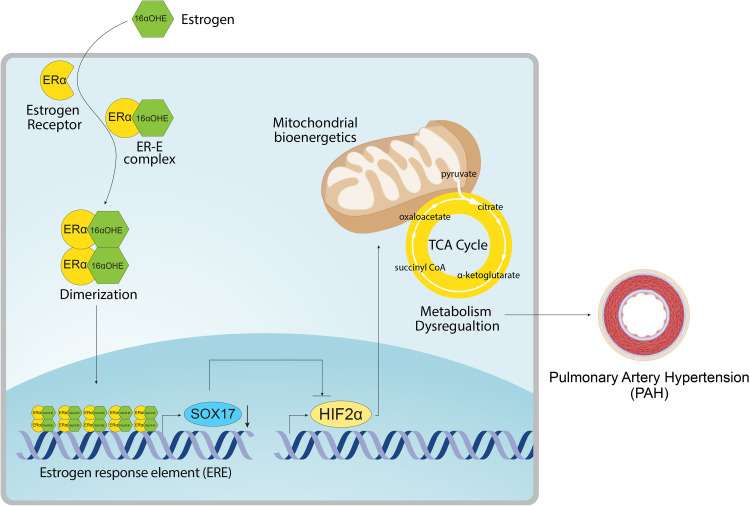
The development of PAH is characterized by mitochondrial dysfunction, genetic contribution, and sexual dimorphism, including a role for estrogen signaling (6). Although genetic data implicate a causal role for SOX17 deficiency in the development of PAH (2, 3), the function of SOX17 in PAH pathogenesis and the links to both mitochondrial dysfunction and estrogen signaling remain unknown. For the first time, the present study reports that 1) 16αOHE represses endothelial SOX17 transcription, 2) endothelial Sox17 attenuates 16αOHE-mediated exacerbation of murine PH, and 3) endothelial SOX17 inhibits HIF2α expression and promotes mitochondrial function. Moreover, 16αOHE-mediated SOX17 repression involves ERα signaling on the SOX17 promoter, supporting contribution from the classical genomic estrogen signaling in PAH (6). Genetic analyses with the SOX17 enhancer variant in patients further bolster the signaling cascade involving SOX17 and metabolism in PAH. These cumulative data highlight a novel, potential mechanistic axis involving 16αOHE-ERα-SOX17-HIF2α in the development of PAH (Figure 7) and suggest interactions among estrogen signaling, SOX17 genetics, EC metabolism, and the sexual dimorphism observed in PAH.
Figure 7.
Summary schema. The schema reveals the overall proposed mechanism demonstrated in this work. Specifically, we report a novel signaling cascade involving 16αOHE-mediated repression of SOX17 (SRY-related HMG-box 17) expression and promoter activity via ERα. To understand the protective effects of SOX17 in vivo, we further connect SOX17-mediated inhibition of HIF2α with enhanced oxidative phosphorylation and mitochondrial function in endothelial cells. 16αOHE = 16α-hydroxyestrone; CoA = coenzyme A; ERα = estrogen receptor α; HIF2α = hypoxia-inducible factor 2α; TCA = tricarboxylic acid.
Consistent with prior literature (37), we also found SOX17 expression is predominantly localized to the lung endothelium and is decreased across PAH tissues from animal models and patients. Specific ablation of Sox17 in ECs and hematopoietic cells using Tie2 promoter augments chronic hypoxia-induced murine PH and related vascular remodeling. In contrast, increased Sox17 in these cells attenuates hypoxic PH development. Given the use of the Tie2 promoter, the role of hematopoietic cells in SOX17 function is unknown. Moreover, although EC expression is more dominant on the basis of our staining data, Sox17 expression in other vascular cells, such as smooth muscle cells, may also play a role in PAH. Mechanisms of the role of EC-derived SOX17 were further dissected by evaluating HIF2α as a downstream mediator of SOX17 and mitochondrial dysfunction. Our data show that SOX17 reduces HIF2α expression. Although we do not have evidence to suggest there is a direct repressive link, an alternative possibility is through an indirect mechanism. Specifically, in silico analyses predict SOX consensus sites on the PHD2 (prolyl-4-hydroxylase 2) promoter, a major regulator of HIF concentrations. Although there is no current evidence, SOX17 may augment PHD2 transcription, which can lead to reduced HIF2α concentrations. This latter suggestion will be important to study in future studies.
Significant published work has accumulated, demonstrating that as in cancer, there is a critical metabolic component to PH development (38, 39). Whether metabolic dysfunction precedes PAH development or is a consequence (or partly both ways) remains unclear. Our data show that not only is HIF2α overexpression sufficient to disrupt mitochondrial bioenergetics, but it also acts in a dominant fashion to reverse the positive effects of SOX17 on mitochondrial function. Although the mechanism by which HIF2α inhibits mitochondrial function is unclear, it potentially involves the loss of PPARγ (peroxisome proliferator activated receptor γ), as our prior work has shown that the increase in HIF2α associated with PH results in a decrease in PPARγ expression (40). As PPARγ is a positive regulator of mitochondrial bioenergetics, its loss would attenuate mitochondrial bioenergetics through its ability to stimulate the carnitine shuttle (30). TGF-β (transforming growth factor-β) signaling is increased in PH (41), and as our data have shown that TGF-β1 decreases PPARγ (30), this pathway may be the upstream mediator of the Sox17–HIF2α axis. HIF2α is also known to target proliferation (42), production of vasoconstrictors, and arginase (43). Further studies will be required to examine these possibilities and what role SOX17 deficiency has on other nonmetabolic roles of HIF2α. Taken together, these data represent the first functional validation of published genetic data on the pathologic role of SOX17 deficiency in PAH development, in part, via repressing HIF2α and enhancing mitochondrial function.
We found reduced Sox17 expression in the lungs of female compared with male rats. We were not able to evaluate SOX17 expression differences in male versus female lungs from patients with PAH, because of small available sample sizes from male subjects. Nonetheless, susceptibility to PH varies by sex and with animal models, and reduced Sox17 expression in female rats is consistent with clinical observations of increased prevalence of PAH in female patients and further suggests that SOX17 deficiency may mediate the increased disease risk in female patients. Although male patients are at reduced risk of developing PAH, they are known to have a higher mortality rate than female patients. Whether and how SOX17 may influence right heart failure and disease outcomes with PAH is unknown. They also highlight sex-specific hormonal differences that may regulate SOX17 expression in the lungs. Estrogen signaling has been considered a canonical mediator of sex differences in PAH. Estrogen metabolism is dysregulated in PAH, and accumulation of proproliferative and inflammatory metabolites such as 16αOHE contribute to the pathogenesis of experimental PAH (44, 45). To further dissect the origins of reduced Sox17 concentrations in PAH female rats, we studied 16αOHE, given its disease-promoting effects across animal and human studies. Both in vitro and in vivo data validate an inverse relationship between 16αOHE signaling and SOX17 concentrations in PAH. 16αOHE significantly repressed SOX17 mRNA and protein concentrations starting at 1 nM, a concentration that was previously shown to stimulate proliferation of human pulmonary arterial smooth muscle cells via ERα and promote vascular remodeling in PAH (13). Promoter luciferase assays further suggest 16αOHE-mediated SOX17 regulation likely occurs via the repression of putative ERE sites in the SOX17 promoter. Moreover, in vivo data confirm that Sox17 partly attenuates 16αOHE-mediated murine hypoxic PH. The protective effects of SOX17 transgene appear to be modest, highlighting other SOX17-independent pathologic signaling by 16αOHE. Moreover, the role of 16αOHE in directly mediating HIF2α signaling and its effects on mitochondrial function remain unknown. Although we recognize that tamoxifen can shed protection via inhibition of ER signaling in Sox17 knockout animals, we administered tamoxifen to control for comparison and, furthermore, administered doxycycline, not tamoxifen, to Sox17Tg mice. These cumulative data confirm regulatory and functional roles for the 16αOHE-SOX17 axis in PAH.
16αOHE binds irreversibly to ERs and exerts mitogenic and inflammatory actions, promoting PAH, whereas E2 appears to be mixed and potentially protective in PAH (45, 46). In contrast to 16αOHE, our data suggest that E2 positively regulates SOX17 expression. These contrasting data suggest that estrogen-mediated regulation of SOX17 expression is complex and likely depends, in part, on a specific estrogen metabolite. And yet our ERα rat data demonstrate that ERα repressed SOX17 and inversely regulated SOX17 promoter activity. Notably, this may reflect the fact that 16αOHE has a greater capacity to activate ERs than E2 (33, 46). ERα-mediated transcription is complex and regulated to different degrees, involving interactions among ligand, receptor, DNA sequence, cofactors, chromatin context, and post-translational modifications (34). Our ERα data further bolster evidence of the genomic pathway of estrogen signaling in PAH. A prior study in PAH demonstrated that the expression of BMPR2 (bone morphogenetic protein receptor type 2) is similarly decreased via ERα-mediated repression of the BMPR2 promoter (35). Although ERα may improve RV remodeling directly (29), our data combined with other published data (36) support a therapeutic role of endothelial ERα inhibition in animal models of PH, in part, via SOX17. Consistent with this idea, a small phase I clinical trial examining the role of fulvestrant (a selective ER degrader) for the treatment of PAH (NCT 02911844) reported reduced, albeit nonsignificant given the sample size, concentrations of 16αOHE (P = 0.22) and a trend toward improved stroke volume (P = 0.07) with the drug (47). These results further suggest that blockade of 16αOHE-ERα may improve PAH. The role of ERα, in particular, in PAH remains unclear, with some studies linking it to the development of PAH (36, 48, 49) and fewer studies demonstrating protective effects in the right ventricle (29, 50). Part of this discrepancy may result from the tissue-specific expression of ERα, mainly RV versus pulmonary vasculature origins. Although we demonstrate a repressive role for ERα in the regulation of SOX17, other ERs were not evaluated in the present study.
Our mQTL analysis further translates the relationship between SOX17 and oxidative phosphorylation in patients. Given that citrate represents a canonical metabolite in the TCA cycle, the association between a disease risk SNP in SOX17 and reduced plasma citrate concentrations further supports a potential underlying mechanism between disease risk for PAH and TCA cycle metabolites moderated by SOX17.
Conclusions
Validating several genetic studies in patients, SOX17 deficiency augments preclinical PAH. On the basis of differential SOX17 expression in the lungs of male and female rats, we uncover a novel mechanistic axis involving 16αOHE-mediated repression of SOX17 expression and promoter activity via ERα using a systems biology–like approach encompassing a spectrum of in vitro and in vivo to patient-level data. To understand the protective effects of SOX17, we further connect SOX17-mediated inhibition of HIF2α with enhanced oxidative phosphorylation and mitochondrial function in ECs. The link between SOX17 to estrogen signaling and mitochondrial function in PAH bridges established clinical observations of sexual dimorphism to newly discovered candidate genes in PAH, implicating a new PAH therapeutic target.
Footnotes
Supported by NIH grants R01 HL136603 and R01 HL160941 (W.C.N. and A.A.D.); American Heart Association grant 14CRP18910051 (A.A.D.); the American Thoracic Society Foundation/Pulmonary Hypertension Association (A.A.D.); NIH grant R01 HL60190 (S.M.B.); NIH grant R01 HL137282 (S.M.B.); NIH grant R01 HL134610 (S.M.B.); NIH grant P01 HL142212 (S.M.B.); NIH grant PR01 HL146369 (S.M.B.); NIH grant R24 HL105333 (M.W.P. and W.C.N.); National Key R&D Program of China grant 2019YFE0119400; Natural Science Foundation of China grants 81970052, 82170057, and 82000055 (H.T.); NIH grant R01ES030197 (H.G.); NIH grants R01 HL156993 and R01 HL158686 (J.H.K.); NHLBI grant 1R01HL144727 (T.L.); VA Merit Review Award 2 I01 BX002042 (T.L.); and Canadian Institutes of Health Research grant SVB-145555 (K.R.C. and D.J.S.).
Author Contributions: S.S. contributed to the concept of this study, data collection, drafting of the manuscript, and data analysis. X.S., Y.J., and H.G. contributed to method development, data collection, and data analysis. T.-H.S.-A. contributed to data collection, drafting of the manuscript, and data analysis. M.Y. and J.H.K. contributed to method development and data analysis. Q.L. contributed to data collection and data analysis. Y. Sun, S.L., T.C., A.F., P.R.L., M.J., and M.A. contributed to data collection. A.L.F. contributed samples and data collection. A.C., K.A.L., M.W.P., K.R.C., D.J.S., T.L., and W.C.N. contributed samples and data collection. H.T. contributed to data collection and analysis. H.C. contributed mice and to data collection. A.D.M. contributed to data collection and drafting of the manuscript. S.M.B. contributed to the concept and design of the study, method development, data collection, drafting of the manuscript, and data analysis. A.A.D. contributed to the concept and design of this study, method development, data collection, drafting of the manuscript, and data analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202203-0450OC on March 13, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Karnes JH, Wiener HW, Schwantes-An TH, Natarajan B, Sweatt AJ, Chaturvedi A, et al. Genetic admixture and survival in diverse populations with pulmonary arterial hypertension. Am J Respir Crit Care Med . 2020;201:1407–1415. doi: 10.1164/rccm.201907-1447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, et al. UK NIHR BioResource Rare Diseases Consortium; UK PAH Cohort Study Consortium; US PAH Biobank Consortium Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med . 2019;7:227–238. doi: 10.1016/S2213-2600(18)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gräf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun . 2018;9:1416. doi: 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu N, Welch CL, Wang J, Allen PM, Gonzaga-Jauregui C, Ma L, et al. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med . 2018;10:56. doi: 10.1186/s13073-018-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Y, Wharton J, Walters R, Vasilaki E, Aman J, Zhao L, et al. The pathophysiological role of novel pulmonary arterial hypertension gene SOX17. Eur Respir J . 2021;58:2004172. doi: 10.1183/13993003.04172-2020. [DOI] [PubMed] [Google Scholar]
- 6. Sun Y, Sangam S, Guo Q, Wang J, Tang H, Black SM, et al. Sex differences, estrogen metabolism and signaling in the development of pulmonary arterial hypertension. Front Cardiovasc Med . 2021;8:719058. doi: 10.3389/fcvm.2021.719058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hester J, Ventetuolo C, Lahm T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr Physiol . 2019;10:125–170. doi: 10.1002/cphy.c190011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fessel JP, Chen X, Frump A, Gladson S, Blackwell T, Kang C, et al. Interaction between bone morphogenetic protein receptor type 2 and estrogenic compounds in pulmonary arterial hypertension. Pulm Circ . 2013;3:564–577. doi: 10.1086/674312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, et al. Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J . 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evans JD, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med . 2016;4:129–137. doi: 10.1016/S2213-2600(15)00544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foppa M, Arora G, Gona P, Ashrafi A, Salton CJ, Yeon SB, et al. Right ventricular volumes and systolic function by cardiac magnetic resonance and the impact of sex, age, and obesity in a longitudinally followed cohort free of pulmonary and cardiovascular disease: the Framingham Heart Study. Circ Cardiovasc Imaging . 2016;9:e003810. doi: 10.1161/CIRCIMAGING.115.003810. [DOI] [PubMed] [Google Scholar]
- 12. Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol . 2014;307:L7–L26. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 13. Hood KY, Montezano AC, Harvey AP, Nilsen M, MacLean MR, Touyz RM. Nicotinamide adenine dinucleotide phosphate oxidase-mediated redox signaling and vascular remodeling by 16α-hydroxyestrone in human pulmonary artery cells: implications in pulmonary arterial hypertension. Hypertension . 2016;68:796–808. doi: 10.1161/HYPERTENSIONAHA.116.07668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, et al. Estrogen metabolite 16α-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation . 2016;133:82–97. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, et al. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation . 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 16. Fishman J, Martucci C. Biological properties of 16 alpha-hydroxyestrone: implications in estrogen physiology and pathophysiology. J Clin Endocrinol Metab . 1980;51:611–615. doi: 10.1210/jcem-51-3-611. [DOI] [PubMed] [Google Scholar]
- 17. Denver N, Homer NZM, Andrew R, Harvey KY, Morrell N, Austin ED, et al. Estrogen metabolites in a small cohort of patients with idiopathic pulmonary arterial hypertension. Pulm Circ . 2020;10:2045894020908783. doi: 10.1177/2045894020908783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab . 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 19. Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest . 2018;128:3704–3715. doi: 10.1172/JCI120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol . 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 21. Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, et al. Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol . 2018;314:L256–L275. doi: 10.1152/ajplung.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sangam S, Schwantes-An T-H, Zagorski J, Shi Y, Morrisroe S, Cook T, et al. 16α-Hydroxyestrone downregulates SOX17 during the development of PAH [abstract] Circulation . 2021;144:A12319. [Google Scholar]
- 23. Sangam S, Gupta A, Yilmaz S, Yuan JXJ, Langlais P, Desai AA. Endothelial targets of SOX17 in pulmonary arterial hypertension: a quantitative proteomic approach [abstract] Am J Respir Crit Care Med . 2019;199:A6748. [Google Scholar]
- 24. Zhu N, Pauciulo MW, Welch CL, Lutz KA, Coleman AW, Gonzaga-Jauregui C, et al. PAH Biobank Enrolling Centers’ Investigators Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med . 2019;11:69. doi: 10.1186/s13073-019-0685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kantz ED, Tiwari S, Watrous JD, Cheng S, Jain M. Deep neural networks for classification of LC-MS spectral peaks. Anal Chem . 2019;91:12407–12413. doi: 10.1021/acs.analchem.9b02983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watrous JD, Niiranen TJ, Lagerborg KA, Henglin M, Xu YJ, Rong J, et al. Directed non-targeted mass spectrometry and chemical networking for discovery of eicosanoids and related oxylipins. Cell Chem Biol . 2019;26:433–442.e4. doi: 10.1016/j.chembiol.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stearman RS, Bui QM, Speyer G, Handen A, Cornelius AR, Graham BB, et al. Systems analysis of the human pulmonary arterial hypertension lung transcriptome. Am J Respir Cell Mol Biol . 2019;60:637–649. doi: 10.1165/rcmb.2018-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lotinun S, Westerlind KC, Kennedy AM, Turner RT. Comparative effects of long-term continuous release of 16 alpha-hydroxyestrone and 17 beta-estradiol on bone, uterus, and serum cholesterol in ovariectomized adult rats. Bone . 2003;33:124–131. doi: 10.1016/s8756-3282(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 29. Frump AL, Albrecht M, Yakubov B, Breuils-Bonnet S, Nadeau V, Tremblay E, et al. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Invest . 2021;131:e129433. doi: 10.1172/JCI129433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun X, Lu Q, Yegambaram M, Kumar S, Qu N, Srivastava A, et al. TGF-β1 attenuates mitochondrial bioenergetics in pulmonary arterial endothelial cells via the disruption of carnitine homeostasis. Redox Biol . 2020;36:101593. doi: 10.1016/j.redox.2020.101593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Zhang L, Marsboom G, Jambusaria A, Xiong S, Toth PT, et al. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nat Commun. 2019;10:2126. doi: 10.1038/s41467-019-10134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jonatan D, Spence JR, Method AM, Kofron M, Sinagoga K, Haataja L, et al. Sox17 regulates insulin secretion in the normal and pathologic mouse β cell. PLoS One . 2014;9:e104675. doi: 10.1371/journal.pone.0104675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lewis JS, Thomas TJ, Klinge CM, Gallo MA, Thomas T. Regulation of cell cycle and cyclins by 16alpha-hydroxyestrone in MCF-7 breast cancer cells. J Mol Endocrinol . 2001;27:293–307. doi: 10.1677/jme.0.0270293. [DOI] [PubMed] [Google Scholar]
- 34. Welboren WJ, Sweep FC, Span PN, Stunnenberg HG. Genomic actions of estrogen receptor alpha: what are the targets and how are they regulated? Endocr Relat Cancer . 2009;16:1073–1089. doi: 10.1677/ERC-09-0086. [DOI] [PubMed] [Google Scholar]
- 35. Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ . 2012;3:6. doi: 10.1186/2042-6410-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med . 2014;190:456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yao Y, Yao J, Boström KI. SOX transcription factors in endothelial differentiation and endothelial-mesenchymal transitions. Front Cardiovasc Med . 2019;6:30. doi: 10.3389/fcvm.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu Q, Chan SY. Mitochondrial and metabolic drivers of pulmonary vascular endothelial dysfunction in pulmonary hypertension. Adv Exp Med Biol . 2017;967:373–383. doi: 10.1007/978-3-319-63245-2_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi Y, Gu C, Zhao T, Jia Y, Bao C, Luo A, et al. Combination therapy with rapamycin and low dose imatinib in pulmonary hypertension. Front Pharmacol . 2021;12:758763. doi: 10.3389/fphar.2021.758763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest . 2014;124:3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun . 2017;8:15494. doi: 10.1038/ncomms15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ . 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cowburn AS, Crosby A, Macias D, Branco C, Colaço RD, Southwood M, et al. HIF2α-arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci USA . 2016;113:8801–8806. doi: 10.1073/pnas.1602978113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morris H, Denver N, Gaw R, Labazi H, Mair K, MacLean MR. Sex differences in pulmonary hypertension. Clin Chest Med . 2021;42:217–228. doi: 10.1016/j.ccm.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 45. Tofovic SP, Jackson EK. Estradiol metabolism: crossroads in pulmonary arterial hypertension. Int J Mol Sci . 2019;21:116. doi: 10.3390/ijms21010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swaneck GE, Fishman J. Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci USA . 1988;85:7831–7835. doi: 10.1073/pnas.85.21.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kawut SM, Pinder D, Al-Naamani N, McCormick A, Palevsky HI, Fritz J, et al. Fulvestrant for the treatment of pulmonary arterial hypertension. Ann Am Thorac Soc . 2019;16:1456–1459. doi: 10.1513/AnnalsATS.201904-328RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, et al. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol . 2010;298:H1235–H1248. doi: 10.1152/ajpheart.00254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wright AF, Ewart MA, Mair K, Nilsen M, Dempsie Y, Loughlin L, et al. Oestrogen receptor alpha in pulmonary hypertension. Cardiovasc Res . 2015;106:206–216. doi: 10.1093/cvr/cvv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng TC, Philip JL, Tabima DM, Kumari S, Yakubov B, Frump AL, et al. Estrogen receptor-α prevents right ventricular diastolic dysfunction and fibrosis in female rats. Am J Physiol Heart Circ Physiol . 2020;319:H1459–H1473. doi: 10.1152/ajpheart.00247.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]