Takotsubo syndrome (TTS) is characterized by acute, transient dysfunction of the myocardium (Figures 1A to 1D), most commonly precipitated by emotional or physical triggers. Although our understanding of pathophysiological mechanisms remains incomplete, contributing factors include excessive sympathetic stimulation and catecholamine excess leading to microcirculatory dysfunction, cardiomyocyte injury, and myocardial edema.1 Catecholamine surges affect β-adrenergic receptor (βAR) signaling, which results in impaired contractility and the distinctive wall motion abnormalities associated with TTS, and also has profound effects on electrophysiological properties.1,2 Electrocardiographic abnormalities comprising QT prolongation with deep T-wave inversions are commonly observed (Figure 1E). Although a prolonged QT interval increases arrhythmic risk among patients with TTS, the rates of ventricular arrhythmia (VA) or sudden cardiac death (SCD) are relatively low across the broad range of TTS patients, with estimated incidences of 4% to 9% during acute hospitalization, but nonetheless contribute significantly to morbidity and mortality.3–5
FIGURE 1. Characteristic Echocardiographic and Electrocardiographic Changes in Takotsubo Syndrome.
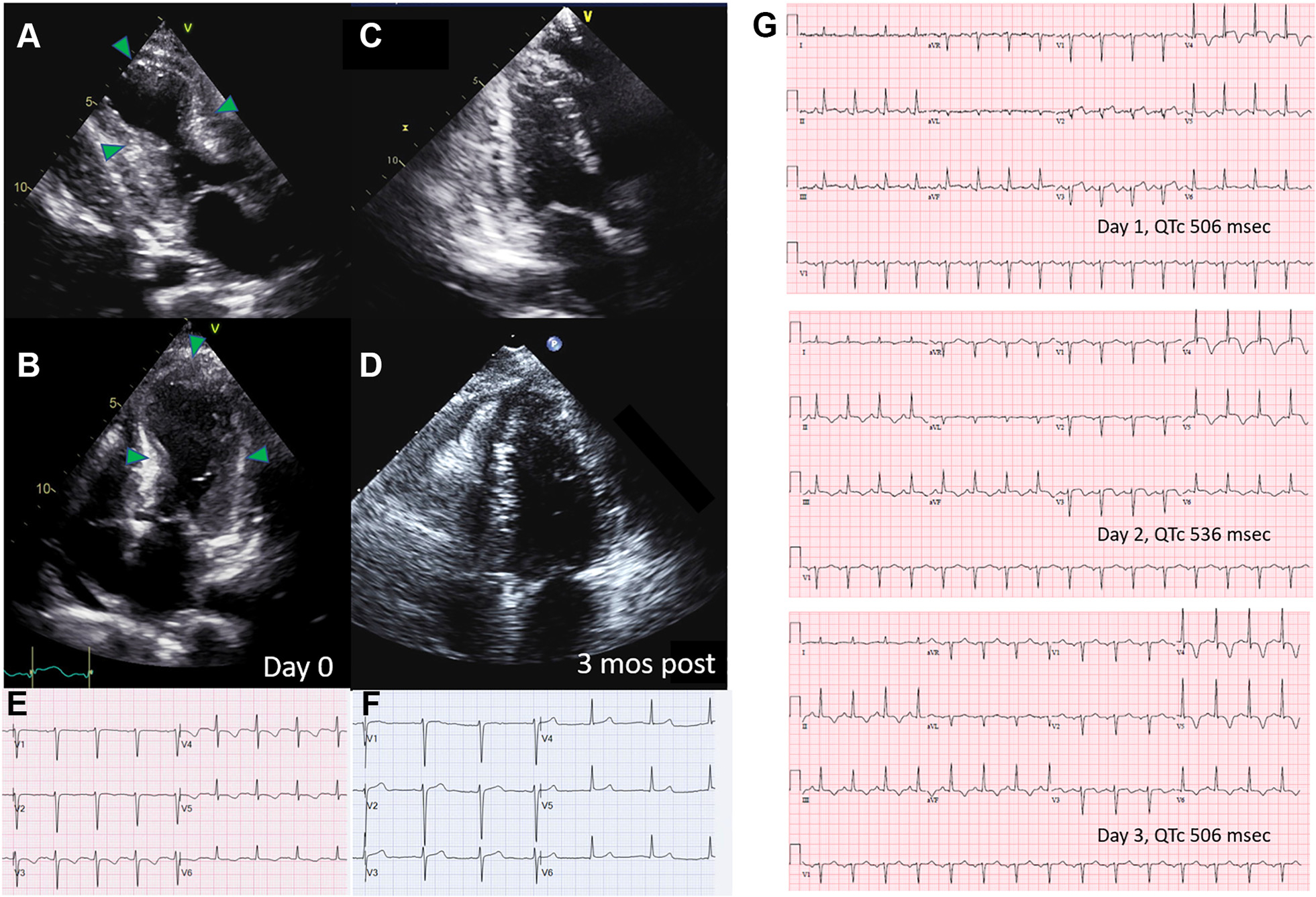
(A and B) Image frames acquired during systole that depict a typical echocardiographic “ballooning” pattern with near akinesis of the mid to apical LV wall segments. Wall motion abnormalities recover (C and D) at variable times following the inciting event. Electrocardiographic changes are seen at the time of the acute event, which tend to be most pronounced in the precordial leads and commonly comprise deep T-wave inversions with QT prolongation (E). Electrocardiographic changes also resolve over time (F). There is generally marked evolution of electrocardiographic features in the acute period (G), here characterized initially by pronounced ST-segment elevations and T-wave inversions followed by normalization of the ST segments but with lengthening of the QT interval and deep T-wave inversions, which improve but may persist for a variable duration of time.3
There are several potential mechanisms whereby TTS increases the risk for VA/SCD.1 Catecholamine overstimulation of βAR results in intracellular calcium overload, which can promote arrhythmogenesis because of delayed after-depolarization triggered activity. Both microvascular dysfunction and myocardial edema may contribute to electrical inhomogeneity by causing repolarization abnormalities, including prolongation of the QT interval. Early after-depolarizations in the setting of prolonged action potentials are another potential source of triggered proarrhythmic ventricular activity. Arrhythmogenesis may also result from catecholamine-induced abnormal automaticity of myocardial cells, which normally do not play a pacemaker role. Although the usual anatomic substrate for re-entrant arrhythmias, namely, fibrosis or scar, is not a prominent feature of TTS, particularly in the acute stages, the presence of myocardial edema may predispose to functional re-entry by creating differential zones of electrical conductivity. However, there is currently no validated risk predictor to identify the TTS patients who are most likely to experience an in-hospital VA event.
In this issue of JACC: Clinical Electrophysiology, Del Buono et al6 conducted a retrospective review of electronic health records from a single U.S. institution to identify patients specifically with the apical form of TTS and to assess whether electrocardiographic QT interval duration could predict the subsequent occurrence of in-hospital VA. Of 154 patients identified over a 7-year period, 105 met criteria for the apical variant or typical form of TTS,3 of whom 82% were women and 32% identified as Black or African American. In only 6% was an emotional trigger documented. A physical stressor was ascribed in 78%, most commonly postoperative respiratory distress in 37%, infection in 34%, central nervous system event in 16%, and trauma in 11%. Inotropic support was administered to 35% of patients, vasopressors to 39%, and mechanical support to 7%. At least 1 VA episode occurred in 11% of patients (n = 10), of whom 6 died of their arrhythmia. Overall in-hospital mortality was 18%. An electrocardiogram was acquired within 24 hours of admission in 57% and between 25 and 96 hours in 32%, with median unadjusted QT interval of 360 milliseconds (95% CI: 320–420 milliseconds). Unadjusted QT intervals did not differ between those with and without VA. However, after correction with Fridericia’s formula, patients with VA had longer QTc than those with no VA (470 milliseconds vs 417 milliseconds; P = 0.03). Area under the receiver operator curve was modest at 0.70 for predicting VA using a QTc threshold of 460 milliseconds.
Several study limitations should be highlighted. The retrospective design limited the availability of key data elements. Only 1 electrocardiogram was obtained for each patient with timing of the acquisition that was quite variable (from admission to 96 hours after) and of unclear proximity to the precipitating event triggering TTS. There is generally marked evolution of electrocardiographic changes in the subacute phase of TTS (Figures 1E to 1G) with QT prolongation occurring within 1 to 3 days of the triggering event and peaking at 2 to 6 days.7 Hence, serial and consistently timed electrocardiograms are vital for comparing and interpreting potential group differences and prognostic implications of the QT interval, in addition to appropriate QT interval correction formulas. Although the severity of QT prolongation may identify a high-risk cohort, whether the risks and long-term consequences of interventions to shorten the QT interval such as sympathectomy warrant further investigation, as suggested by the authors, remains to be seen. In this syndrome, QT prolongation and myocardial abnormalities are transient, other mechanisms for arrhythmogenesis play a role and evolve over time but were not investigated, and VA risk is still relatively low, particularly among those less sick. Finally, more granular details regarding the inciting triggering event or combination of events are also lacking but are important for predicting disease trajectory and interpreting the findings in the context of other studies.
Indeed, what is most striking and informative about the study is the patient cohort composition. Much of the published literature in TTS is limited by collections of very heterogeneous subtypes of TTS, while it is increasingly clear that prognosis depends on clinical and anatomic subtypes.3,8 In primary TTS, patients seek medical care for acute cardiac symptoms, generally in the setting of an emotional trigger. The demographic is most commonly postmenopausal women who may have increased susceptibility because of abnormal microvascular function and increased sympathetic tone.9 In contrast, secondary TTS tends to occur in patients already hospitalized for other medical or psychiatric conditions or surgical procedures, and the catecholamine surge ensues as a complication of the primary disorder or its treatment. Secondary TTS typically has a worse prognosis than primary TTS, not only because of underlying comorbidities in these hospitalized patients, but perhaps also because of the fact that a larger sympathetic stimulus may be required to generate TTS among those with inherently lower susceptibility.9 The classic apical ballooning pattern is also associated with worse short-term outcomes.10
Notably, the cohort compiled by Del Buono6 does not in fact represent a typical U.S. TTS demographic, nor are the results generalizable broadly to the TTS population at large. In most series, the proportion of women is higher, comprising 90% of patients, and patients of Black or African-American descent account for only 10% of cases.9 The current study is unique in that a relatively homogeneous cohort was identified with an overwhelming predominance of secondary TTS patients. These patients had severely reduced left ventricular ejection fraction (median 25%) with a disproportionately high requirement for inotropes, vasopressors, and mechanical support and rates of in-hospital mortality and VA/SCD that well exceeded those reported previously (historically, 1%-4.5% for in-hospital mortality and 4%-9% for VA/SCD).3 The value of the current study lies in its ability to hone in on a more precise estimate of VA and emphasize its greater incidence among high-acuity patients with secondary TTS, low left ventricular ejection fraction, and classic apical ballooning and to highlight a possible association between QT duration and VA in this specific population. It further underscores the need for well-phenotyped, homogeneous cohorts of TTS with systematic, serial, prospective data collection to advance our understanding of disease course, prognosis, and mechanisms. Clinicians are also reminded that not all TTS patients have a benign clinical trajectory. In-hospital morbidity and mortality, particularly from VA, are elevated among this subset with secondary TTS who would benefit from heightened vigilance and monitoring; avoidance of QT prolonging agents and inotropes whenever possible; and consideration of judicious beta-blockade in hemodynamically stable, nonbradycardic patients with QTc <500 milliseconds to reduce tachyarrhythmic risk.3
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr Wu is supported by National Institutes of Health/National Heart, Lung, and Blood Institute R01HL132181. Dr Wittstein has reported that he has no relationships relevant to the contents of this paper to disclose.
Footnotes
Editorials published in JACC: Clinical Electrophysiology reflect the views of the authors and do not necessarily represent the views of JACC: Clinical Electrophysiology or the American College of Cardiology.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Lyon AR, Citro R, Schneider B, et al. Pathophysiology of takotsubo syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:902–921. [DOI] [PubMed] [Google Scholar]
- 2.Moller C, Eitel C, Thiele H, Eitel I, Stiermaier T. Ventricular arrhythmias in patients with takotsubo syndrome. J Arrhythm. 2018;34:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on takotsubo syndrome: a position statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. [DOI] [PubMed] [Google Scholar]
- 4.Pelliccia F, Pasceri V, Patti G, et al. Long-term prognosis and outcome predictors in takotsubo syndrome: a systematic review and meta-regression study. JACC Heart failure. 2019;7:143–154. [DOI] [PubMed] [Google Scholar]
- 5.Manolis AA, Manolis TA, Melita H, Manolis AS. Takotsubo syndrome and sudden cardiac death. Angiology. Published online June 6, 2022. 10.1177/00033197221105757 [DOI] [PubMed] [Google Scholar]
- 6.Del Buono MG, Davonte JI, Moroni F, et al. QT prolongation and in-hospital ventricular arrhythmic complications in patients with apical ballooning takotsubo syndrome. J Am Coll Cardiol EP. 2022;8:1500–1510. [DOI] [PubMed] [Google Scholar]
- 7.Singh T, Khan H, Gamble DT, Scally C, Newby DE, Dawson D. Takotsubo syndrome: pathophysiology, emerging concepts, and clinical implications. Circulation. 2022;145:1002–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghadri JR, Kato K, Cammann VL, et al. Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol. 2018;72:874–882. [DOI] [PubMed] [Google Scholar]
- 9.Wittstein IS. Why sex matters in takotsubo syndrome. J Am Coll Cardiol. 2022;79:2094–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stiermaier T, Moller C, Graf T, et al. Prognostic usefulness of the ballooning pattern in patients with takotsubo cardiomyopathy. Am J Cardiol. 2016;118:1737–1741. [DOI] [PubMed] [Google Scholar]


