Abstract
The use of alkynes as vinylmetal pronucleophiles in intermolecular enantioselective metal-catalyzed carbonyl and imine reductive couplings to form allylic alcohols and amines is surveyed. Related hydrogen auto-transfer processes, wherein alcohols or amines serve dually as reductants and carbonyl or imine proelectrophiles, also are cataloged, as are applications in target-oriented synthesis. These processes represent an emerging alternative to the use of stoichiometric vinylmetal reagents or Nozaki-Hiyama-Kishi (NHK) reactions in carbonyl and imine alkenylation.
Keywords: Alkyne, Reductive Coupling, Enantioselective, Allylic Alcohol, Allylic Amine, Hydrogen Transfer
Graphical Abstract

1. Introduction to Alkyne-Mediated C═X (X = O, NR) Vinylation
Catalytic enantioselective additions of vinyl anions to carbonyl compounds and imines to form allylic alcohols and allylic amines represent an important subset of carbonyl addition reactions.1,2 Among methods of this type, Noyori-type vinylzinc additions3,4 and Nozaki-Hiyama-Kishi reactions5 of vinyl halide pronucleophiles are utilized most frequently. The vinylic reactants utilized in these processes invariably derive from alkynes. Hence, over the past two decades substantial effort has been devoted to the development of direct enantioselective metal-catalyzed reductive couplings of alkynes to carbonyl compounds and imines and, more recently, related hydrogen auto-transfer protocols (Figure 1).6,7,8
Figure 1.
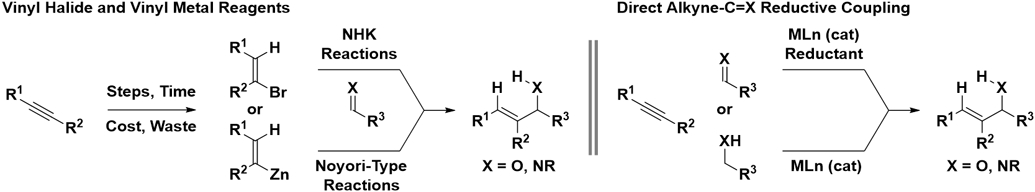
Strategies for catalytic enantioselective vinylation of carbonyl compounds and imines.
Here, intermolecular enantioselective metal-catalyzed reductive couplings of alkynes with carbonyl compounds and imines to form allylic alcohols and allylic amines, respectively, are surveyed. Corresponding enantioselective reductive cyclizations of acetylenic carbonyl compounds and imines are not covered.9,10 Discussion is limited to processes that result in C─H and C─C bond formation via formal addition of H2 across the alkyne pronucleophile and C═X (X = O, NR) π-bond. Hence, multicomponent intermolecular alkylative6a,7d,11 alkyne-C═X (X = O, NR) reductive couplings are not covered (nor are related alkylative/arylative12 or borylative/silylative13 cyclization processes). Finally, metal-catalyzed reductive couplings of alkynes with carbon dioxide are beyond the scope of this monograph.14 Content is organized on the basis of alkyne structure (1,3-enynes and 1,3-diynes vs alkyl-and aryl-substituted alkynes) and then on the basis of metal catalyst.
2. 1,3-Enyne and 1,3-Diyne-C═X (X = O, NR) Reductive Coupling
2.1. Rhodium
Hydrogenation of 1,3-enynes or 1,3-diynes in the presence of activated aldehydes or ketones using cationic rhodium catalysts modified by chiral chelating bis(phosphine) ligands promotes reductive coupling to form enantiomerically enriched secondary and tertiary allylic alcohols as single regioisomers with complete control of alkene geometry (Scheme 1).15 Remarkably, although cationic rhodium complexes are widely utilized as catalysts for alkene hydrogenation,16 competing hydrogenation of π-unsaturated products is not observed. This stems from the fact that the reactants are more π-acidic than the products and, consequently, are better ligands for the low-valent rhodium catalyst. Therefore, upon full consumption of the carbonyl partner, excess enyne reactant binds the catalyst to suppress product hydrogenation. Hence, beyond the reductive coupling of enynes with pyruvates (Scheme 1A),15a glyoxalates (Scheme 1C),15d or certain heterocyclic aldehydes or ketones (Scheme 1B),15c 1,3-diyne-glyoxalate reductive coupling15b can be achieved without further reaction (over-reduction or coupling) of the 1,3-enyne-containing products (Scheme 1D).
Scheme 1.
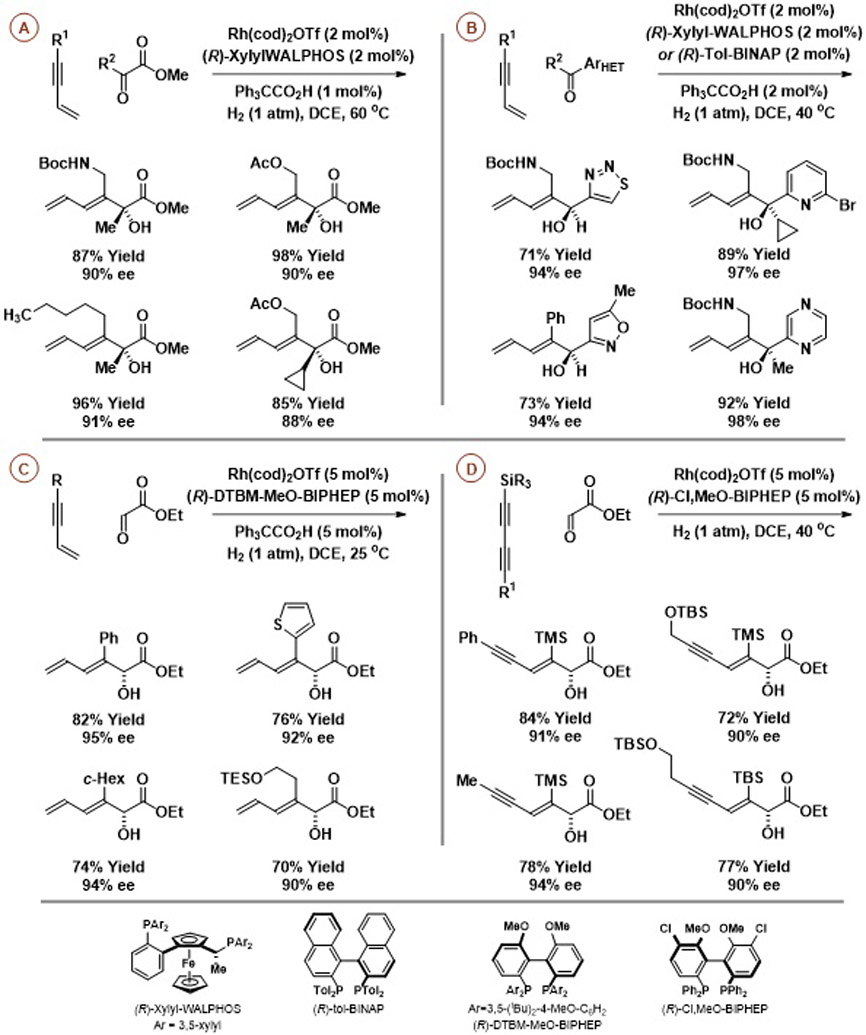
Enantioselective rhodium-catalyzed reductive coupling of 1,3-enynes or 1,3-diynes with activated aldehydes or ketones mediated by hydrogen.
The indicated mechanism for rhodium-catalyzed reductive coupling of conjugated alkynes with activated carbonyl compounds mediated by hydrogen is supported by isotopic labelling15a and computational studies (Scheme 2).17 The square planar cationic rhodium complex I has two vacant coordination sites, which enables the binding of both enyne and carbonyl compound and, in turn, their oxidative coupling to form an oxarhodacyclopentene. Direct σ-bond metathesis of the oxarhodacyclopentene and hydrogen, which occurs by way of the 4-centered transition structure II, can be slow. Brønsted acid co-catalysts, typically carboxylic acids, are often required to enhance rate and conversion via protonolytic cleavage of the oxarhodacyclopentene by way of the 6-centered transition structure III. σ-Bond metathesis of the resulting rhodium carboxylate with hydrogen can now occur more rapidly through the 6-centered transition structure IV.18 Reductive elimination releases the product of reductive coupling and regenerates low-valent rhodium to close the catalytic cycle.
Scheme 2.
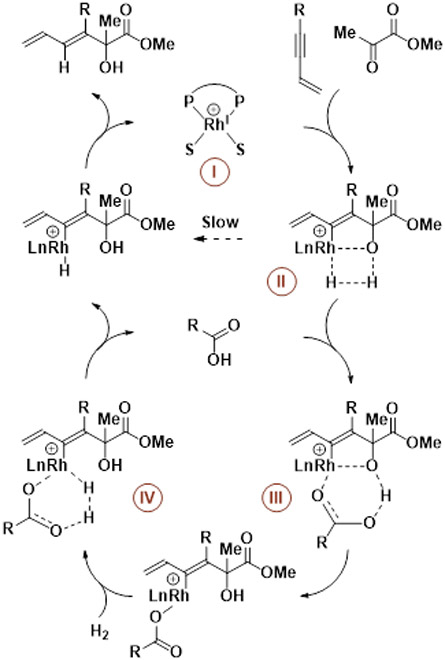
Mechanism for rhodium-catalyzed reductive coupling of conjugated alkynes with activated carbonyl compounds mediated by hydrogen.
Related rhodium-catalyzed reductive couplings of conjugated enynes or diynes with activated imines mediated by hydrogen occur under essentially identical conditions, but in the absence of Brønsted acid co-catalysts (Scheme 3).19 Whereas ethyl (N-tert-butanesulfinyl)iminoacetate provides optimal levels of asymmetric induction for enyne pronucleophiles, ethyl (N-2,4,6-triisopropylbenzenesulfinyl)iminoacetate is required for diyne pronucleophiles. Both enynes and diynes undergo C─C coupling with complete levels of regioselectivity. Although these reactions are not enantioselective, this chiral auxiliary-based method provides access to chiral nonracemic allylic amines in the form of nonproteogenic amino acid esters.
Scheme 3.
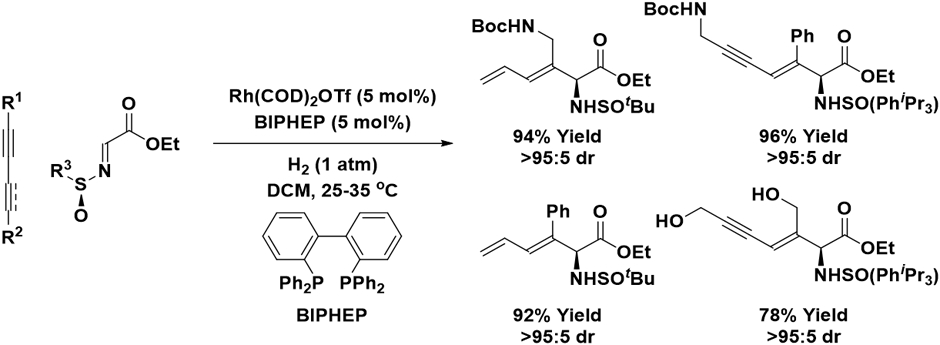
Diastereoselective rhodium-catalyzed reductive coupling of 1,3-enynes or 1,3-diynes with ethyl (N-tert-butanesulfinyl)iminoacetate mediated by hydrogen.
The exceptional functional group tolerance and stereoselectivity of these rhodium-catalyzed hydrogen-mediated alkyne-carbonyl reductive couplings is demonstrated by their application to the total synthesis of bryostatin 7 and preparation of related seco-B-ring analogs (Scheme 4).20 The bryostatins are a family of structurally complex marine macrolides that bind the C1 domain of protein kinase C (PKC) isozymes, antagonizing most biological responses of the phorbol esters, classic PKC activators that are generally tumor promoting. To construct the bryostatin C-ring and simplified congeners, hydrogen-mediated reductive coupling of the indicated glyoxals and 1,3-enynes was conducted using a cationic rhodium catalyst modified by (R)-tol-BINAP. This process forges the C20-C21 bond with complete control of regioselectivity and alkene geometry, as well as good levels of catalyst-directed diastereoselectivity vis-à-vis control of the C20 carbinol stereochemistry.
Scheme 4.
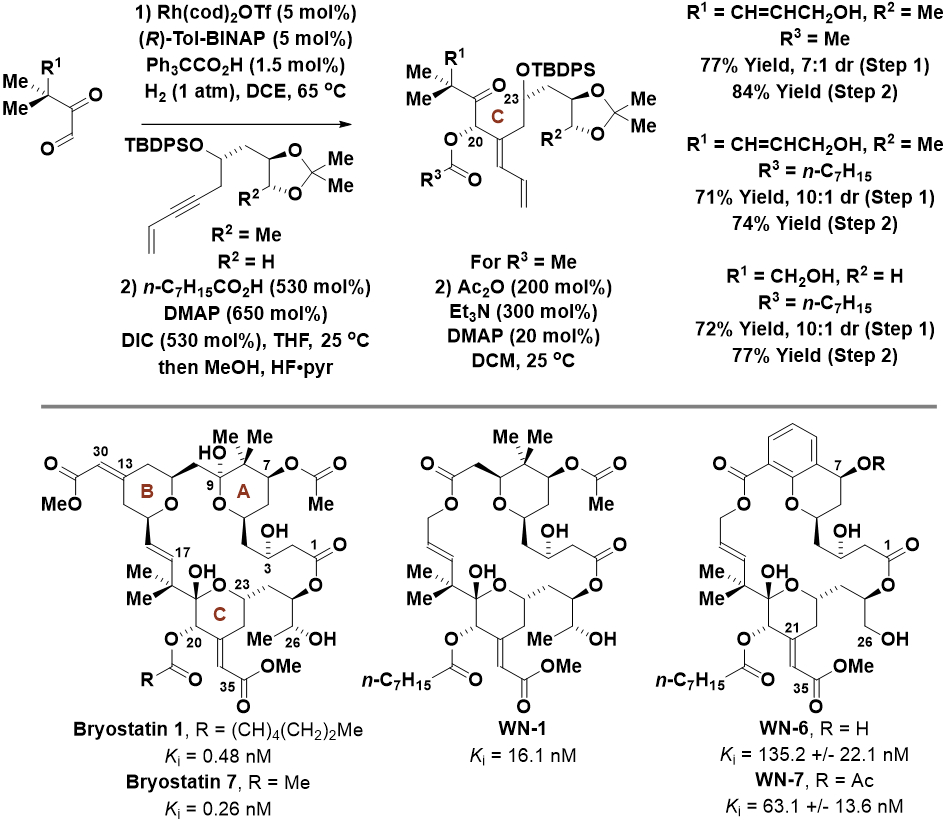
Synthesis of bryostatin 7 and selected seco-B-ring analogs via asymmetric rhodium-catalyzed reductive coupling of 1,3-enynes with glyoxals mediated by hydrogen.
2.2. Nickel
Like their rhodium-catalyzed counterparts,14,19,20 enantioselective nickel-catalyzed carbonyl reductive couplings of conjugated enynes occur with complete control of regioselectivity and alkene geometry to furnish dienyl allylic alcohols (Scheme 5).21 These processes are applicable to aldehydes (Scheme 5B) and, quite remarkably, unactivated ketones (Scheme 5A). However, triethylborane, a pyrophoric liquid, is required as terminal reductant and modest yields and enantioselectivities are observed upon use of the indicated monodentate P-chiral ferrocenyl phosphine ligand. Nevertheless, these data support the prospect of developing highly enantioselective reductive couplings of enynes with unactivated ketones, which would represent a significant achievement.
Scheme 5.
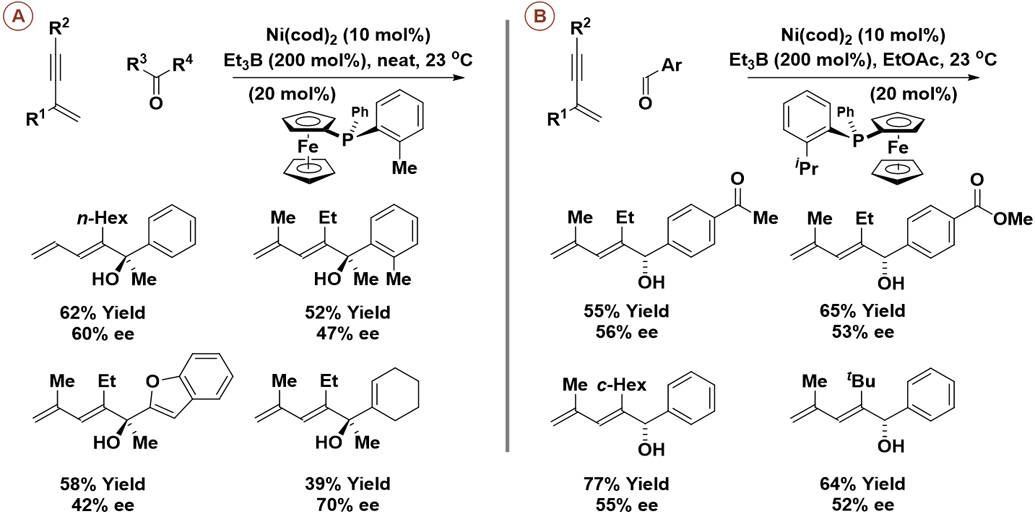
Enantioselective nickel-catalyzed reductive coupling of 1,3-enynes with aldehydes or ketones mediated by triethylborane.
3. Nonconjugated and Aryl Alkyne-C═X (X = O, NR) Reductive Coupling
3.1. Rhodium
Hydrogenation of acetylene, an abundant feedstock (>10 x 106 tons/yr),22 in the presence of carbonyl compounds (Scheme 6A) or imines (Scheme 6B) provides products of reductive C─C coupling in the form of (Z)-dienyl allylic alcohols and amines, respectively.23a,b Here, a cationic rhodium complex with the BAr4F counterion, tetrakis[3,5-bis(CF3)phenyl]borate, was employed as catalyst. Using enantiomeric rhodium catalysts ligated by (R)- and (S)-MeO-BIPHEP, L-glyceraldehyde acetonide is converted to the indicated diastereomeric adducts with good levels of catalyst-directed asymmetric induction and, therefrom, all eight L-hexoses (Scheme 6A).23c As corroborated by deuterium labelling and ESI mass spectrometric analyses, these processes occur via oxidative coupling of acetylene to furnish a rhodacyclopentadiene followed by insertion of the C═X (X = O, NSO2Ar) π-bond (Scheme 6C).23d Carboxylic acid co-catalyzed hydrogenolysis of the resulting oxa/aza-rhodacycloheptadiene releases the product and returns rhodium to its low valent form. This method was applied to the total syntheses of (+)-trienomycins A and F (not shown).23e
Scheme 6.
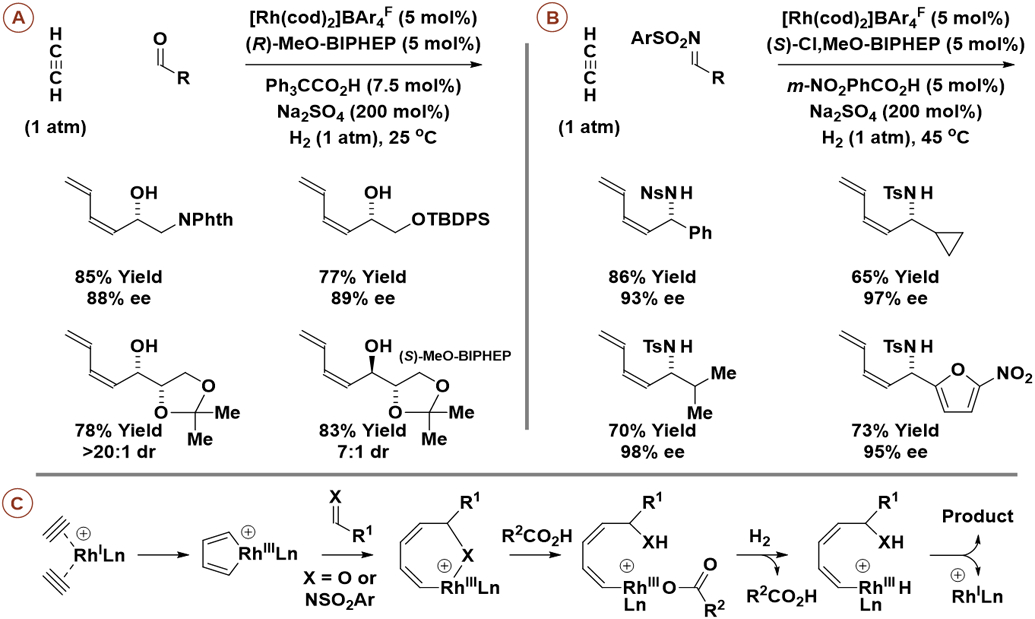
Enantioselective and diastereoselective rhodium-catalyzed reductive coupling of acetylene with carbonyl compounds or imines mediated by hydrogen.
3.2. Iridium
Cationic iridium complexes modified by chiral chelating bis(phosphine) ligands catalyze enantioselective reductive couplings of alkyl-substituted alkynes with imines mediated by hydrogen (Scheme 7).24 These reactions proceed via stereoselective alkyne-C═NSO2Ar oxidative coupling to form the indicated aza-iridacyclopentene that suffers carboxylic acid co-catalyzed hydrogenolysis to release the allylic amine. In reactions of non-symmetric alkynes, there exists a pronounced preference for placement of the larger alkyne substituent distal to the sterically demanding metal center to promote high levels of contrasteric regioselectivity in the C─C bond forming event.
Scheme 7.
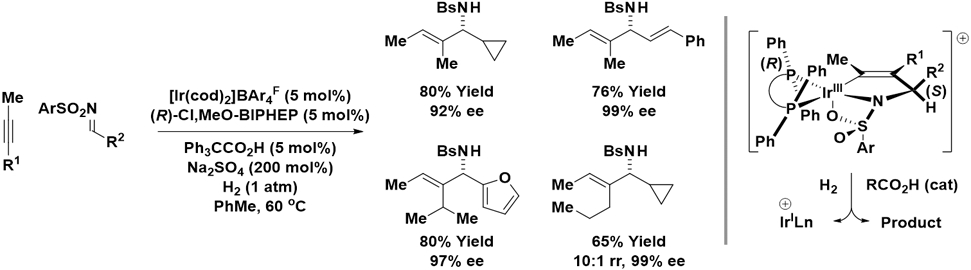
Enantioselective iridium-catalyzed reductive coupling of nonconjugated alkynes with N-arylsulfonyl aldimines mediated by hydrogen.
3.3. Ruthenium
Pursuant to the development of non-asymmetric processes,25a enantioselective ruthenium-catalyzed carbonyl vinylations via hydrogen auto-transfer recently were disclosed (Scheme 8).25b Using an iodide-bound ruthenium-JOSIPHOS catalyst and 2-butyne as the vinyl donor, structurally diverse primary alcohols are converted to the corresponding chiral allylic alcohols with excellent levels of enantioselectivity and complete control of alkene geometry. Deuterium labelling experiments corroborate a catalytic cycle in which alcohol dehydrogenation forms an aldehyde and a ruthenium hydride, which upon alkyne hydrometallation delivers a nucleophilic vinylruthenium species that participates in carbonyl addition. Beyond interactions with the chiral ligand, the absolute stereochemical course of vinylation is influenced by formyl CH···I[Ru] and CH···O≡C[Ru] hydrogen bonding26,27 and iodide-dependent stereogenicity of the ruthenium center.28
Scheme 8.
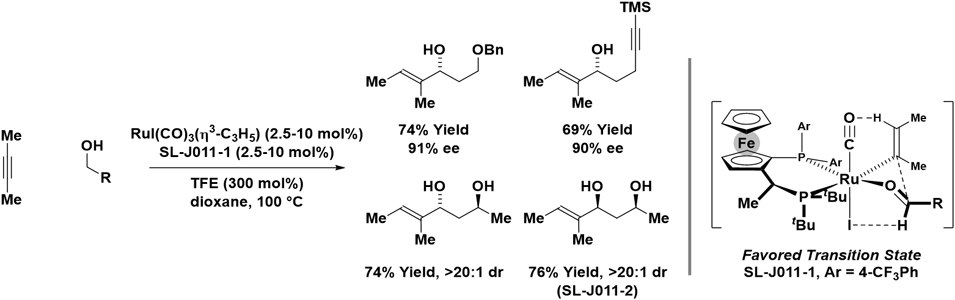
Enantioselective and diastereoselective ruthenium-catalyzed reductive coupling of 2-butyne with aldehydes via hydrogen auto-transfer from alcohol reactants.
3.4. Cobalt
Cobalt catalysts modified by (S,S)-BDPP promote the Hantzsch ester-mediated reductive coupling of alkynes with aldehydes upon irradiation with visible light in the presence of an organic photoredox catalyst (Scheme 9).29 Although irradiation and use of a mass-intensive reductant (the Hantzsch ester) are required, nonsymmetric alkynes undergo C─C coupling to furnish the allylic alcohols with excellent control of regio- and enantioselectivity. A key feature of the reaction mechanism involves photo-mediated electron transfer from Hantzsch ester to 4-CzlPN. The resulting anion radical of 4-CzlPN affects reduction of cobalt(II) to cobalt(I), which promotes alkyne-carbonyl oxidative coupling. The authors posit that formal hydrogenolysis of the cobalt(III) oxametallacyclopentene occurs through successive protonation-electron transfer-protonation events to releases product and regenerate cobalt(II).
Scheme 9.
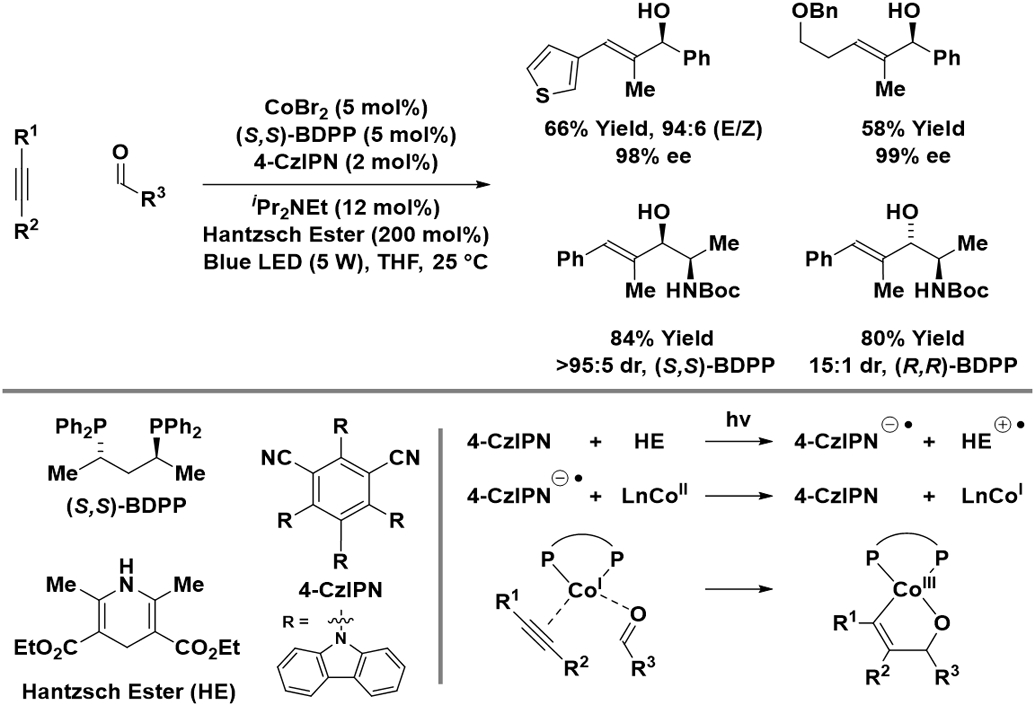
Enantioselective and diastereoselective cobalt-catalyzed reductive coupling of alkynes with aldehydes mediated by Hantzsch ester.
3.5. Nickel
In 2003, Jamison reported enantioselective nickel-catalyzed alkyne-aldehyde reductive couplings mediated by triethylborane (Scheme 10A).30a Using a zero-valent nickel catalyst modified by (+)-(neomenthyl)diphenylphosphino, (+)-NMDPP, aryl-substituted alkynes and α-branched aldehydes are transformed to highly enantiomerically enriched trisubstituted allylic alcohols with complete control of regioselectivity and alkene geometry. In parallel studies by Montgomery, chiral NHC-modified nickel-catalysts were developed for enantioselective alkyne-aldehyde reductive couplings mediated by silane.30b,d Initial work led to the design of the ligand NHC-I, for which the N-aryl moieties are geared conformationally with respect to the 1,2-diphenylethylenediamine-containing tetrahydroimidazole core to induce an element of axial chirality (Scheme 10B).30b However, in subsequent studies, the less complex ligand NHC-II was found to be superior. Upon use of NHC-II in combination with tert-butyldimethylsilane as reductant, the reductive coupling products are formed as their TBS ethers with good control of regio- and enantioselectivity (Scheme 10C).30d Finally, as demonstrated in work by the Scheidt laboratory, nickel catalysts modified by the planar chiral ferrocene-containing NHC ligand, NHC-III, are also effective in enantioselective silane-mediated alkyne-aldehyde reductive coupling (Scheme 10D).30c Several total syntheses of complex secondary metabolites were accomplished using stereoselective nickel-catalyzed alkyne-carbonyl reductive couplings (Scheme 11).31 These applications in target-oriented synthesis involve reductive cyclizations and, with one exception, exploit substrate-directed asymmetric induction using achiral nickel catalysts, thus falling outside the purview of this monograph. Nevertheless, selected target structures are indicated for the benefit of the interested reader.
Scheme 10.
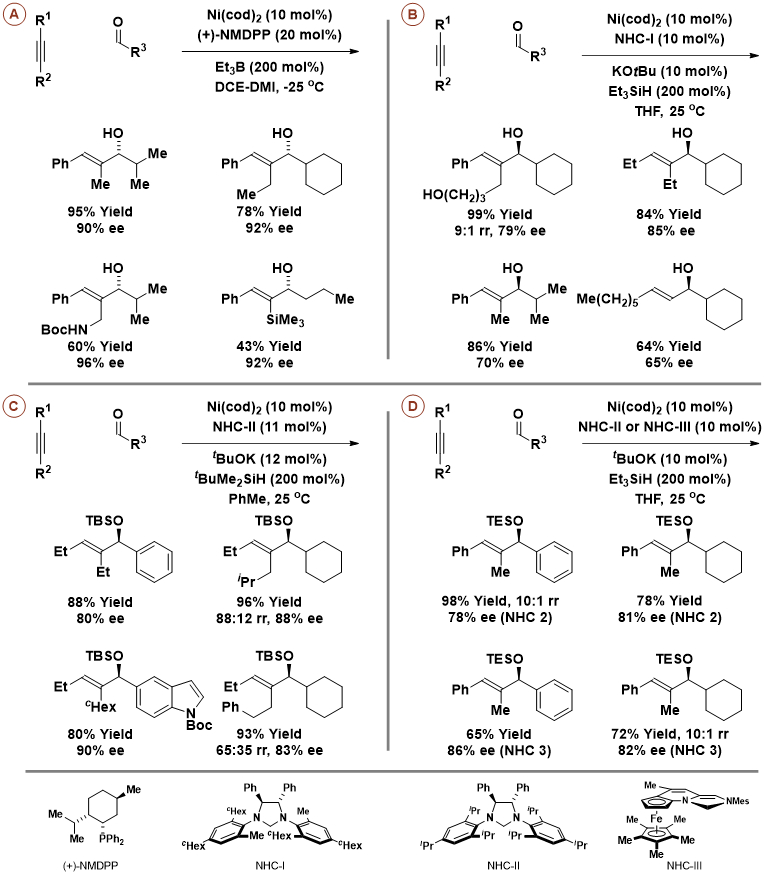
Enantioselective nickel-catalyzed reductive coupling of alkynes with aldehydes mediated by boranes or silanes.
Scheme 11.
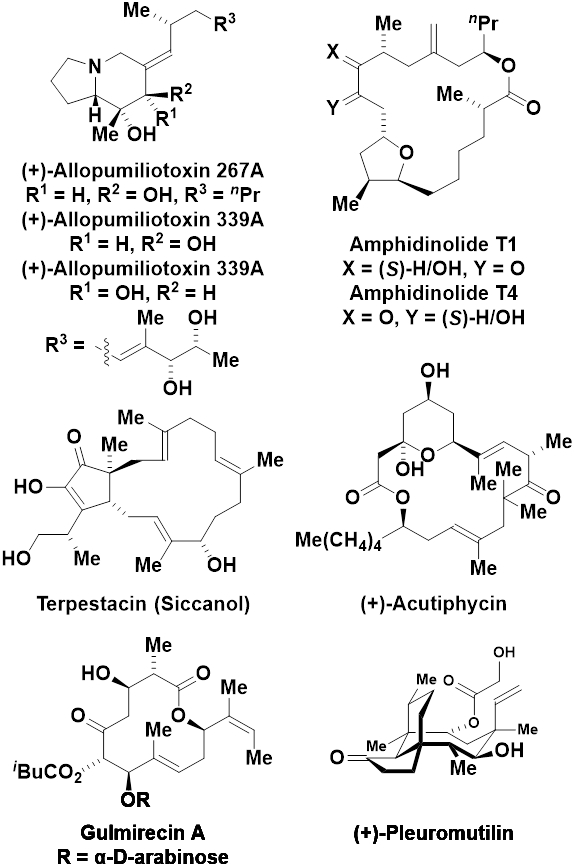
Natural products prepared via nickel-catalyzed alkyne-carbonyl reductive coupling.
Redox-neutral alkyne-aldehyde couplings were achieved by the Shi laboratory using alcohol reactants under the conditions of nickel-catalyzed hydrogen auto-transfer (Scheme 12A).32a Optimal results were obtained using symmetric alkynes in combination with a nickel catalyst modified by the chiral N-heterocyclic carbene ligand, (R,R,R,R)-SIPE, developed by the authors’ group. Triphenyl phosphite was required as secondary ligand to inhibit isomerization of the alkene-containing products. Related redox-neutral alkyne-imine couplings from amine reactants are especially challenging due to the endothermic nature of amine dehydrogenation. Despite this challenge, the Shi laboratory identified conditions for such nickel-catalyzed hydrogen auto-transfer processes.32b,c Specifically, using a nickel-catalyst modified by the indicated P-chiral monodentate phosphine ligand, amines bearing a mesitylene-2-sulfonyl (Mts) protecting group engage in redox-neutral C─C coupling with aryl-substituted alkynes to furnish the corresponding allylic sulfonamides with good levels of regio- and enantioselectivity (Scheme 12B). These reactions proceed by way of an aza-nickelacyclopentene, which upon transfer hydrogenolysis from the amine reactant releases product and nickel(0) along with the requisite imine to close the catalytic cycle (not shown).
Scheme 12.
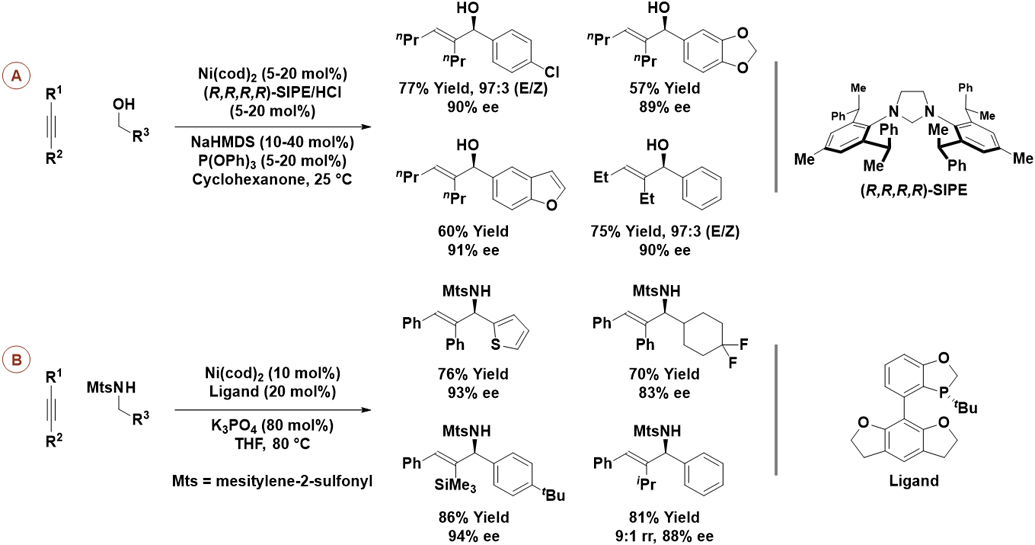
Enantioselective nickel-catalyzed reductive coupling of alkynes with aldehydes or aldimines via hydrogen auto-transfer from alcohol or amine reactants, respectively.
4. Conclusion and Outlook
From the inception of organic chemistry as a discrete field of research to modern day drug discovery, carbonyl and imine addition mediated by premetalated reagents continues to serve as one of the foremost methods for C─C bond formation.33 However, the organometallic reagents typically used in such processes pose issues of safety, selectivity, cost and waste. Metal-catalyzed carbonyl reductive coupling has emerged as an alternative to stoichiometric organometallic reagents in an ever-increasing array of carbonyl addition reactions. As demonstrated by the transformations described herein, metal-catalyzed alkyne-carbonyl/imine reductive couplings and related hydrogen auto-transfer reactions of alcohol and amine proelectrophiles may serve as alternatives to classical carbonyl and imine alkenylations; the Noyori-type vinylzinc additions3,4 and Nozaki-Hiyama-Kishi reactions5 of vinyl halide pronucleophiles. Although considerable progress has been made, numerous unmet challenges remain. For example, catalysts capable of promoting highly enantioselective alkenylations from terminal alkyne pronucleophiles remain highly underrepresented and, in the case of hydrogen auto-transfer reactions, are entirely unknown. It is the authors’ hope the present state-of-the art summary will expedite progress toward this and other challenges in this growing area of research.
ACKNOWLEDGMENTS
The Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM069445) are acknowledged for financial support.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).For an introduction to the broad topic of carbonyl and imine addition, see: Comprehensive Organic Synthesis, 2nd ed.; Knochel P; Molander GA, Eds.; Elsevier: Oxford, 2014; Vols. 1 and 2. [Google Scholar]
- (2).(a) For selected reviews on the catalytic enantioselective addition of vinyl anions to carbonyl compounds and imines, see: Wipf P; Kendall C Novel Applications of Alkenyl Zirconocenes. Chem. Eur. J 2002, 8, 1778–1784. [DOI] [PubMed] [Google Scholar]; (b) Kauffman MC; Walsh PJ In Science of Synthesis: Stereoselective Synthesis; Molander GA, Ed.; Georg Thieme Verlag KG: Stuttgart, 2011; Vol. 2, pp 449–495. [Google Scholar]; (c) Lumbroso A; Cooke ML; Breit B Catalytic Asymmetric Synthesis of Allylic Alcohols and Derivatives and their Applications in Organic Synthesis. Angew. Chem. Int. Ed 2013, 52, 1890–1932. [DOI] [PubMed] [Google Scholar]
- (3).For a recent review on catalytic enantioselective vinylzinc additions to aldehydes, see: Bauer T Enantioselective Dialkylzinc-Mediated Alkynylation, Arylation and Alkenylation of Carbonyl Groups. Coord. Chem. Rev 2015, 299, 83–150. [Google Scholar]
- (4).(a) For selected examples of catalytic enantioselective vinylzinc additions to aldehydes, see: Oppolzer W; Radinov RN Catalytic Asymmetric Synthesis of Secondary (E)-Allyl Alcohols from Acetylenes and Aldehydes via (1-Alkenyl)zinc Intermediates. Helv. Chim. Acta 1992, 75, 170–173. [Google Scholar]; (b) Oppolzer W; Radinov RN Synthesis of (R)-(−)-Muscone by an Asymmetrically Catalyzed Macrocyclization of an ω-Alkynal. J. Am. Chem. Soc 1993, 115, 1593–1594. [Google Scholar]; (c) Soai K; Takahashi K Asymmetric Alkenylation of Chiral and Prochiral Aldehydes Catalysed by Chiral or Achiral Amino Alcohols: Catalytic Diastereoselective Synthesis of Protected erythro-Sphingosine and Enantioselective Synthesis of Chiral Diallyl Alcohols. J. Chem. Soc., Perkin Trans 1 1994, 1257–1258. [Google Scholar]; (d) Oppolzer W; Radinov RN; De Brabander J Total Synthesis of the Macrolide (+)-Aspicilin by an Asymmetrically Catalyzed Macrocyclization of an ω-Alkynal Ester. Tetrahedron Lett 1995, 36, 2607–2610. [Google Scholar]; (e) Wipf P; Ribe S Zirconocene-Zinc Transmetalation and in Situ Catalytic Asymmetric Addition to Aldehydes. J. Org. Chem 1998, 63, 6454–6455. [Google Scholar]; (f) Oppolzer W; Radinov RN; El-Sayed E Catalytic Asymmetric Synthesis of Macrocyclic (E)-Allylic Alcohols from ω-Alkynals via Intramolecular 1-Alkenylzinc/Aldehyde Additions. J. Org. Chem 2001, 66, 4766–4770. [DOI] [PubMed] [Google Scholar]; (g) Dahmen S; Bräse S [2,2]Paracyclophane-Based N,O-Ligands in Alkenylzinc Additions to Aldehydes. Org. Lett 2001, 3, 4119–4122. [DOI] [PubMed] [Google Scholar]; (h) Chen YK; Lurain AE; Walsh PJ A General, Highly Enantioselective Method for the Synthesis of D and L α-Amino Acids and Allylic Amines. J. Am. Chem. Soc 2002, 124, 12225–12231. [DOI] [PubMed] [Google Scholar]; (i) Ji J-X; Qiu L-Q; Yip CW; Chan ASC A Convenient, One-Step Synthesis of Optically Active Tertiary Aminonaphthol and Its Applications in the Highly Enantioselective Alkenylations of Aldehydes. J. Org. Chem 2003, 68, 1589–1590. [DOI] [PubMed] [Google Scholar]; (j) Lurain AE; Walsh PJ A Catalytic Asymmetric Method for the Synthesis of γ-Unsaturated β-Amino Acid Derivatives. J. Am. Chem. Soc 2003, 125, 10677–10683. [DOI] [PubMed] [Google Scholar]; (k) Jeon S-J; Chen YK; Walsh PJ Catalytic Asymmetric Synthesis of Hydroxy Enol Ethers: Approach to a Two-Carbon Homologation of Aldehydes. Org. Lett 2005, 7, 1729–1732. [DOI] [PubMed] [Google Scholar]; (l) Lauterwasser F; Gall J; Höfener S; Bräse S Second-Generation N,O-[2.2]Paracyclophane Ketimine Ligands for the Alkenylzinc Addition to Aliphatic and Aromatic Aldehydes: Scope and Limitations. Adv. Synth. Catal 2006, 348, 2068–2074. [Google Scholar]; (m) Salvi L; Jeon S-J; Fisher EL; Carroll PJ; Walsh PJ Catalytic Asymmetric Generation of (Z)-Disubstituted Allylic Alcohols. J. Am. Chem. Soc 2007, 129, 16119–16125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) DeBerardinis AM; Turlington M; Pu L Activation of Vinyl Iodides for the Highly Enantioselective Addition to Aldehydes. Angew. Chem. Int. Ed 2011, 50, 2368–2370. [DOI] [PubMed] [Google Scholar]
- (5).(a) For selected reviews on enantioselective Nozaki-Hiyama-Kishi vinylations, see: Hargaden GC; Guiry PJ The Development of the Asymmetric Nozaki–Hiyama–Kishi Reaction. Adv. Synth. Catal 2007, 349, 2407–2424. [Google Scholar]; (b) Tian Q; Zhang G Recent Advances in the Asymmetric Nozaki–Hiyama–Kishi Reaction. Synthesis 2016, 48, 4038–4049. [Google Scholar]; (c) Gil A; Albericio F; AÁlvarez M Role of the Nozaki–Hiyama–Takai–Kishi Reaction in the Synthesis of Natural Products. Chem. Rev 2017, 117, 8420–8446. [DOI] [PubMed] [Google Scholar]
- (6).(a) For general reviews on metal-catalyzed carbonyl reductive coupling, see: Montgomery J Nickel-Catalyzed Reductive Cyclizations and Couplings. Angew. Chem. Int. Ed 2004, 43, 3890–3908. [DOI] [PubMed] [Google Scholar]; (b) Kimura M; Tamaru Y Nickel-Catalyzed Reductive Coupling of Dienes and Carbonyl Compounds. Top. Curr. Chem 2007, 279, 173–207. [Google Scholar]; (c) Moragas T; Correa A; Martin R Metal-Catalyzed Reductive Coupling Reactions of Organic Halides with Carbonyl-Type Compounds. Chem. Eur. J 2014, 20, 8242–8258. [DOI] [PubMed] [Google Scholar]; (d) Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Metal-Catalyzed Reductive Coupling of Olefin-Derived Nucleophiles: Reinventing Carbonyl Addition. Science. 2016, 354, aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Holmes M; Schwartz LA; Krische MJ Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev 2018, 118, 6026–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) For selected reviews on metal-catalyzed alkyne-carbonyl reductive coupling, see: Skucas E; Ngai M-Y; Komanduri V; Krische MJ Enantiomerically Enriched Allylic Alcohols and Allylic Amines via C─C Bond-Forming Hydrogenation: Asymmetric Carbonyl and Imine Vinylation. Acc. Chem. Res 2007, 40, 1394–1401. [DOI] [PubMed] [Google Scholar]; (b) Montgomery J; Sormunen GJ Nickel-Catalyzed Reductive Couplings of Aldehydes and Alkynes. Top. Curr. Chem 2007, 279, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Iida H; Krische MJ Catalytic Reductive Coupling of Alkenes and Alkynes to Carbonyl Compounds and Imines Mediated by Hydrogen. Top. Curr. Chem 2007, 279, 77–104. [Google Scholar]; (d) Moslin RM; Miller-Moslin K; Jamison TF Regioselectivity and Enantioselectivity in Nickel-Catalysed Reductive Coupling Reactions of Alkynes. Chem. Commun 2007, 4441–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Liu P; Houk KN Theoretical Studies of Regioselectivity of Ni- and Rh-Catalyzed C─C Bond Forming Reactions with Unsymmetrical Alkynes. Inorg. Chim. Acta 2011, 369, 2–14. [Google Scholar]; (f) Jackson EP; Malik HA; Sormunen GJ; Baxter RD; Liu P; Wang H; Shareef A-R; Montgomery J Mechanistic Basis for Regioselection and Regiodivergence in Nickel-Catalyzed Reductive Couplings. Acc. Chem. Res 2015, 48, 1736–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) For selected reviews on carbonyl addition and imine addition via hydrogen auto-transfer, see: Ketcham JM; Shin I; Montgomery TP; Krische MJ Catalytic Enantioselective C─H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon. Angew. Chem. Int. Ed 2014, 53, 9142–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Perez F; Oda S; Geary LM; Krische MJ Ruthenium Catalyzed Transfer Hydrogenation for C─C Bond Formation: Hydrohydroxyalkylation and Hydroaminoalkylation via Reactant Redox Pairs. Top. Curr. Chem 2016, 374, 365–387. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Santana CG; Krische MJ From Hydrogenation to Transfer Hydrogenation to Hydrogen Auto-Transfer in Enantioselective Metal-Catalyzed Carbonyl Reductive Coupling: Past, Present and Future. ACS Catal. 2021, 11, 5572–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) For selected reviews on metal-catalyzed reductive cyclization, see: Krische MJ, Jang H-Y Metal Catalyzed Reductive Cyclization (C═C, C≡C, C═O Bonds) In Comprehensive Organometallic Chemistry III.; Mingos M, Crabtree R, Eds. Elsevier: Oxford, 2007, Vol. 10, pp 493–536. [Google Scholar]; (b) Tanaka K; Tajima Y Transition-Metal-Catalyzed Cyclization of Alkynals via Oxametallacycle Intermediates. Eur. J. Org. Chem 2012, 3715–3725. [Google Scholar]
- (10).(a) For selected examples of enantioselective metal-catalyzed reductive cyclization of acetylenic carbonyl compounds, see: Rhee JU; Krische MJ Highly Enantioselective Reductive Cyclization of Acetylenic Aldehydes via Rhodium Catalyzed Asymmetric Hydrogenation. J. Am. Chem. Soc 2006, 128, 10674–10675. [DOI] [PubMed] [Google Scholar]; (b) Tanaka R; Noguchi K; Tanaka K Rhodium-Catalyzed Asymmetric Reductive Cyclization of Heteroatom-Linked 5-Alkynals with Heteroatom-Substituted Acetaldehydes. J. Am. Chem. Soc 2010, 132, 1238–1239. [DOI] [PubMed] [Google Scholar]; (c) Fu W; Nie M; Wang A; Cao Z; Tang W Highly Enantioselective Nickel-Catalyzed Intramolecular Reductive Cyclization of Alkynones. Angew. Chem. Int. Ed 2015, 54, 2520–2524. [DOI] [PubMed] [Google Scholar]
- (11).(a) For selected reviews on metal-catalyzed intermolecular alkylative alkyne-mediated carbonyl and imine alkenylations, see: Ikeda S Reaction of Alkynes. In Modern Organonickel Chemistry; Tamaru Y, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005; pp 102–136. [Google Scholar]; (b) Eppe G; Didier D; Marek I Stereocontrolled Formation of Several Carbon─Carbon Bonds in Acyclic Systems. Chem. Rev 2015, 115, 9175–9206. [DOI] [PubMed] [Google Scholar]; (c) Standley EA; Tasker SZ; Jensen KL; Jamison TF Nickel Catalysis: Synergy between Method Development and Total Synthesis. Acc. Chem. Res 2015, 48, 1503–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu W; Kong W Ni-Catalyzed Stereoselective Difunctionalization of Alkynes. Org. Chem. Front 2020, 7, 3941–3955. [Google Scholar]; (e) Ghosh S; Chakrabortty R; Ganesh V Dual Functionalization of Alkynes Utilizing the Redox Characteristics of Transition Metal Catalysts. ChemCatChem. 2021, 13, 4262–4298. [Google Scholar]
- (12).(a) For selected examples of enantioselective metal-catalyzed alkylative cyclization of acetylenic carbonyl compounds, see: Shintani R; Okamoto K; Otomaru Y; Ueyama K; Hayashi T Catalytic Asymmetric Arylative Cyclization of Alkynals: Phosphine-Free Rhodium/Diene Complexes as Efficient Catalysts. J. Am. Chem. Soc 2005, 127, 54–55. [DOI] [PubMed] [Google Scholar]; (b) Song J; Shen Q; Xu F; Lu X Cationic Pd(II)-Catalyzed Enantioselective Cyclization of Aroylmethyl 2-Alkynoates Initiated by Carbopalladation of Alkynes with Arylboronic Acids. Org. Lett 2007, 9, 2947–2950. [DOI] [PubMed] [Google Scholar]; (c) Li Y; Xu M-H Rhodium-Catalyzed Asymmetric Tandem Cyclization for Efficient and Rapid Access to Underexplored Heterocyclic Tertiary Allylic Alcohols Containing a Tetrasubstituted Olefin. Org. Lett 2014, 16, 2712–2715. [DOI] [PubMed] [Google Scholar]; (d) Clarke C; Incerti-Pradillos CA; Lam HW Enantioselective Nickel-Catalyzed anti-Carbometallative Cyclizations of Alkynyl Electrophiles Enabled by Reversible Alkenylnickel E/Z Isomerization. J. Am. Chem. Soc 2016, 138, 8068–8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) For selected examples of enantioselective metal-catalyzed silylative and borylative cyclization of acetylenic carbonyl compounds, see: Wang C; Ge S Versatile Cobalt-Catalyzed Enantioselective Entry to Boryl-Functionalized All-Carbon Quaternary Stereogenic Centers. J. Am. Chem. Soc 2018, 140, 10687–10690. [DOI] [PubMed] [Google Scholar]; (b) He C-Y; Xie L-B; Ding R; Tian P; Lin G-Q Copper-Catalyzed Asymmetric Silylative Cyclization of Cyclohexadienone-Containing 1,6-Enynes. Tetrahedron. 2019, 75, 1682–1688. [Google Scholar]; (c) Zanghi JM; Liu S; Meek SJ Enantio- and Diastereoselective Synthesis of Functionalized Carbocycles by Cu-Catalyzed Borylative Cyclization of Alkynes with Ketones. Org. Lett 2019, 21, 5172–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) For selected reviews on metal-catalyzed reductive carboxylation of alkynes, see: Braunstein P; Matt D; Nobel D Reactions of Carbon Dioxide with Carbon─Carbon Bond Formation Catalyzed by Transition-Metal Complexes. Chem. Rev 1988, 88, 747–764. [Google Scholar]; (b) Tsuji Y; Fujihara T Carbon Dioxide as a Carbon Source in Organic Transformation: Carbon─Carbon Bond Forming Reactions by Transition-Metal Catalysts. Chem. Commun 2012, 48, 9956–9964. [DOI] [PubMed] [Google Scholar]; (c) Zhang L; Hou Z N-Heterocyclic Carbene (NHC)-Copper Catalysed Transformations of Carbon Dioxide. Chem. Sci 2013, 4, 3395–3403. [Google Scholar]; (d) Tortajada A; Juliá-Hernández F; Böerjesson M; Moragas T; Martin R Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem. Int. Ed 2018, 57, 15948–15982. [DOI] [PubMed] [Google Scholar]
- (15).(a) Kong J-R; Ngai M-Y; Krische MJ Highly Enantioselective Direct Reductive Coupling of Conjugated Alkynes and α-Ketoesters via Rhodium-Catalyzed Asymmetric Hydrogenation. J. Am. Chem. Soc 2006, 128, 718–719. [DOI] [PubMed] [Google Scholar]; (b) Cho C-W; Krische MJ α-Hydroxy Esters via Enantioselective Hydrogen-Mediated C─C Coupling: Regiocontrolled Reactions of Silyl-Substituted 1,3-Diynes. Org. Lett 2006, 8, 3873–3876. [DOI] [PubMed] [Google Scholar]; (c) Komanduri V; Krische MJ Enantioselective Reductive Coupling of 1,3-Enynes to Heterocyclic Aromatic Aldehydes and Ketones via Rhodium Catalyzed Asymmetric Hydrogenation: Mechanistic Insight into the Role of Brønsted Acid Additives. J. Am. Chem. Soc 2006, 128, 16448–16449. [DOI] [PubMed] [Google Scholar]; (d) Hong Y-T; Cho C-W; Skucas E; Krische MJ Enantioselective Reductive Coupling of 1,3-Enynes to Glyoxalates Mediated by Hydrogen: Asymmetric Synthesis of β,γ-Unsaturated α-Hydroxy Esters. Org. Lett 2007, 9, 3745–3748. [DOI] [PubMed] [Google Scholar]
- (16).(a) For selected reviews on enantioselective hydrogenation in the synthesis of pharmaceutical ingredients, see: Ager DJ; de Vries AHM; de Vries JG Asymmetric Homogeneous Hydrogenations at Scale. Chem. Soc. Rev 2012, 41, 3340–3380. [DOI] [PubMed] [Google Scholar]; (b) Etayo P; Vidal-Ferran A Rhodium-Catalysed Asymmetric Hydrogenation as a Valuable Synthetic Tool for the Preparation of Chiral Drugs. Chem. Soc. Rev 2013, 42, 728–754. [DOI] [PubMed] [Google Scholar]
- (17).Liu P; Krische MJ; Houk KN Mechanism and Origins of Regio- and Enantioselectivities in Rh(I)-Catalyzed Hydrogenative Couplings of 1,3-Diynes and Activated Carbonyl Partners: Intervention of a Cumulene Intermediate. Chem. Eur. J 2011, 17, 4021–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Musashi Y; Sakaki S Theoretical Study of Rhodium(III)- Catalyzed Hydrogenation of Carbon Dioxide into Formic Acid. Significant Differences in Reactivity among Rhodium(III), Rhodium(I), and Ruthenium(II) Complexes. J. Am. Chem. Soc 2002, 124, 7588–7603. [DOI] [PubMed] [Google Scholar]
- (19).Kong J-R; Cho C-W; Krische MJ Hydrogen-Mediated Reductive Coupling of Conjugated Alkynes with Ethyl (N-Sulfinyl)iminoacetates: Synthesis of Unnatural α-Amino Acids via Rhodium-Catalyzed C─C Bond Forming Hydrogenation. J. Am. Chem. Soc 2005, 127, 11269–11276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Cho C-W; Krische MJ Enantioselective Reductive Coupling of Alkynes and α-Ketoaldehydes via Rhodium-Catalyzed Hydrogenation: An Approach to Bryostatin Substructures. Org. Lett 2006, 8, 891–894 [DOI] [PubMed] [Google Scholar]; (b) Lu Y; Woo SK; Krische MJ Total Synthesis of Bryostatin 7 via C─C Bond-Forming Hydrogenation. J. Am. Chem. Soc 2011, 133, 13876–13879. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Andrews Ian P.; Ketcham JM; Blumberg PM; Kedei N; Lewin NE; Krische MJ Synthesis of seco-B-Ring Bryostatin Analogue WN-1 via C─C Bond Forming Hydrogenation: Critical Contribution of the B-Ring in Determining Bryostatin- and PMA-Like Properties. J. Am. Chem. Soc 2014, 136, 13209–13216. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ketcham JM; Volchkov I; Chen T-Y; Blumberg PM; Kedei N; Lewin NE; Krische MJ Evaluation of Chromane-Based Bryostatin Analogues Prepared via Hydrogen-Mediated C─C Bond Formation: Potency Does Not Confer Bryostatin-Like Biology. J. Am. Chem. Soc 2016, 138, 13415–13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Miller KM; Jamison TF Highly Regioselective, Catalytic Asymmetric Reductive Coupling of 1,3-Enynes and Ketones. Org. Lett 2005, 7, 3077–3080. [DOI] [PubMed] [Google Scholar]; (b) Miller KM; Colby EA; Woodin KS; Jamison TF Asymmetric Catalytic Reductive Coupling of 1,3-Enynes and Aromatic Aldehydes. Adv. Synth. Catal 2005, 347, 1533–1536. [Google Scholar]
- (22).Suisui Z; J Li.; G Li.; Y Nie.; L Qiang.; B Bai.; Ma. X Life Cycle Assessment of Acetylene Production from Calcium Carbide and Methane in China. J. Clean. Prod 2021, 322, 129055. [Google Scholar]
- (23).(a) Kong J-R; Krische MJ Catalytic Carbonyl (Z)-Dienylation via Multicomponent Reductive Coupling of Acetylene to Aldehydes and α-Ketoesters Mediated by Hydrogen: Carbonyl Insertion into Cationic Rhodacyclopentadienes. J. Am. Chem. Soc 2006, 128, 16040–16041. [DOI] [PubMed] [Google Scholar]; (b) Skucas E; Kong JR; Krische MJ Enantioselective Reductive Coupling of Acetylene to N-Arylsulfonyl Imines via Rhodium Catalyzed C─C Bond Forming Hydrogenation: (Z)-Dienyl Allylic Amines. J. Am. Chem. Soc 2007, 129, 7242–7243. [DOI] [PubMed] [Google Scholar]; (c) Han SB; Kong J-R; Krische MJ Catalyst-Directed Diastereoselectivity in Hydrogenative Couplings of Acetylene to α-Chiral Aldehydes: Formal Synthesis of All Eight L-Hexoses. Org. Lett 2008, 10, 4133–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Williams VM; Kong JR; Ko BJ; Mantri Y; Brodbelt JS; Baik M-H; Krische MJ ESI-MS, DFT and Synthetic Studies on the H2-Mediated Coupling of Acetylene: Insertion of C═X Bonds into Rhodacyclopentadienes and Brønsted Acid Cocatalyzed Hydrogenolysis of Organorhodium Intermediates. J. Am. Chem. Soc 2009, 131, 16054–16062. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Del Valle DJ; Krische MJ Total Synthesis of (+)-Trienomycins A and F via C─C Bond-Forming Hydrogenation and Transfer Hydrogenation. J. Am. Chem. Soc 2013, 135, 10986–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).(a) Barchuk A; Ngai M-Y; Krische MJ Allylic Amines via Iridium Catalyzed C─C Bond Forming Hydrogenation: Imine Vinylation in the Absence of Stoichiometric Byproducts or Metallic Reagents. J. Am. Chem. Soc 2007, 129, 8432–8433. [DOI] [PubMed] [Google Scholar]; (b) Ngai M-Y; Barchuk A; Krische MJ Enantioselective Iridium Catalyzed Imine Vinylation: Optically Enriched Allylic Amines via Alkyne-Imine Reductive Coupling Mediated by Hydrogen. J. Am. Chem. Soc 2007, 129, 12644–12645. [DOI] [PubMed] [Google Scholar]
- (25).(a) Patman RL; Chaulagain MR; Williams VM; Krische MJ Direct Vinylation of Alcohols or Aldehydes Employing Alkynes as Vinyl Donors: A Ruthenium Catalyzed C─C Bond Forming Transfer Hydrogenation. J. Am. Chem. Soc 2009, 131, 2066–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ortiz E; Chang Y-H; Shezaf JZ; Shen W; Krische MJ Stereo- and Site-Selective Conversion of Primary Alcohols to Allylic Alcohols via Ruthenium-Catalyzed Hydrogen Auto-Transfer Mediated by 2-Butyne. J. Am. Chem. Soc 2022, 144, 8861–8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).DFT calculations corroborate intervention of formyl hydrogen bonds to the iodide counterion in the transition state for carbonyl addition in related enantioselective ruthenium-JOSIPHOS-catalyzed carbonyl (α-aryl)allylations: Ortiz E; Shezaf JZ; Chang Y-H; alves TP Huang K-W; Krische MJ Understanding Halide Counterion Effects in Enantioselective Ruthenium-Catalyzed Carbonyl (α-Aryl)allylation: Alkynes as Latent Allenes and Trifluoroethanol-Enhanced Turnover in the Conversion of Ethanol to Higher Alcohols via Hydrogen Auto-Transfer. J. Am. Chem. Soc 2021, 143, 16709–16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).(a) A survey of single crystal X-ray diffraction data reveals the persistence of ruthenium CH···O≡C[Ru] hydrogen bonds in vinylruthenium carbonyl complexes. For selected examples, see: Werner H; Esteruelas MA; Otto H Insertion Reactions of the 16-Electron Complexes MHCl(CO)(P-i-Pr3)2 (M = Ru, Os) with Alkynes. The X-ray Crystal Structure of Os((E)-CH═PCHPh)Cl(CO)(P-i-Pr3)2. Organometallics. 1986, 5, 2295–2299. [Google Scholar]; (b) Torres MR; Vegas A; Santos A; Ros J Insertion Reactions of Acetylenes with Hydridocarbonyl-Chlorotris(triphenylphosphine)ruthenium(II). X-Ray Structure of Carbonylchloro(cis-1,2-diphenylethenyl)bis(triphenylphosphine) ruthenium(II) J. Organomet. Chem 1986, 309, 169–177. [Google Scholar]; (c) Romero A; Santos A; Vegas A Reactions of [Ru(CO)ClH(Me2Hpz)(PR3)2] (Me2Hpz = 3,5-dimethylpyrazole; R = Ph, p-tolyl) with Acetylenes. The Crystal Structure of [Ru(CO)Cl(HC═CHCMe3)(Me2HPz)(PPh3)2] and [Ru(CO)(MeO2CC═CHCO2Me)(HCO3)(PPh3)2]. Organometallics. 1988, 7, 1988–1993. [Google Scholar]; (d) Torres MR; Perales A; Loumrhari H; Ros J Carbonylation of Alkenyl-Complexes of Ruthenium. The Formation of Complexes Containing, Alkene or Acyl Ligands, and the Reaction of the Acyl Complexes with Methanol. J. Organomet. Chem 1990, 384, 61–64. [Google Scholar]; (e) Maddock SM; Rickard CEF; Roper WR; Wright LJ Insertion of Ethyne into the Ru-Si Bonds of Coordinatively Unsaturated Ruthenium Silyl Complexes. X-ray Crystal Structures of Ru(CH═CHSiMe2OEt)Cl(CO)2(PPh3)2 and [Ru(CH═CHSiMe2OH)(CN-p-tolyl)(CO)(PPh3)2]ClO4. Organometallics. 1996, 15, 1793–1803. [Google Scholar]; (f) Wilton-Ely JDET; Wang M; Honarkhah SJ; Tocher DA Ruthenium Hydride and Vinyl Complexes Supported by Nitrogen–Oxygen Mixed-Donor Ligands. Inorganica Chim. Acta 2005, 358, 3218–3226. [Google Scholar]; (g) Wilton-Ely JDET; Wang M; Benoit DM; Tocher DA Spectroscopic, Structural and Theoretical Investigation of Alkenyl Ruthenium Complexes Supported by Sulfur–Nitrogen Mixed-Donor Ligands. Eur. J. Inorg. Chem 2006, 15, 3068–3078. [Google Scholar]; (h) Maurer J; Linseis M; Sarkar B; Schwederski B; Niemeyer M; Kaim W; Záliš S; Anson C; Zabel M; Winter RF Ruthenium Complexes with Vinyl, Styryl, and Vinylpyrenyl Ligands: A Case of Non-Innocence in Organometallic Chemistry. J. Am. Chem. Soc 2008, 130, 259–268. [DOI] [PubMed] [Google Scholar]; (i) Wu XH; Jin S; Liang JH; Li ZY; Yu GA; Liu SH Synthesis, Characterization, and Substituent Effects of Binuclear Ruthenium Vinyl Complexes [RuCl(CO)(PMe3)3]2(μ-CH═CH─Ar─CH═CH). Organometallics. 2009, 28, 2450–2459. [Google Scholar]; (j) Ou Y-P; Jiang C; Wu D; Xia J; Yin J; Jin S; Yu G-A; Liu SH Synthesis, Characterization, and Properties of Anthracene-Bridged Bimetallic Ruthenium Vinyl Complexes [RuCl(CO)(PMe3)3]2(μ-CH═CH-anthracene-CH═CH). Organometallics 2011, 30, 5763–5770. [Google Scholar]; (k) Toscani A; Marín-Hernández C; Moragues ME; Sancenón F; Dingwall P; Brown NJ; Martínez-Máñez R; White AJP; Wilton-Ely JDET Ruthenium(II) and Osmium(II) Vinyl Complexes as Highly Sensitive and Selective Chromogenic and Fluorogenic Probes for the Sensing of Carbon Monoxide in Air Chem. Eur. J 2015, 21, 14529–14538. [DOI] [PubMed] [Google Scholar]
- (28).(a) For reviews on halide effects in metal catalysis, see: Maitlis PM; Haynes A; James BR; Catellani M; Chiusoli GP Iodide Effects in Transition Metal Catalyzed Reactions. Dalton Trans 2004, 3409–3419. [DOI] [PubMed] [Google Scholar]; (b) Fagnou K; Lautens M Halide Effects in Transition Metal Catalysis. Angew. Chem. Int. Ed 2002, 41, 26–47. [DOI] [PubMed] [Google Scholar]
- (29).Li Y-L; Zhang S-Q; Chen J; Xia J-B Highly Regio- and Enantioselective Reductive Coupling of Alkynes and Aldehydes via Photoredox Cobalt Dual Catalysis. J. Am. Chem. Soc 2021, 143, 7306–7313. [DOI] [PubMed] [Google Scholar]
- (30).(a) Miller KM; Huang WS; Jamison TF Catalytic Asymmetric Reductive Coupling of Alkynes and Aldehydes: Enantioselective Synthesis of Allylic Alcohols and α-Hydroxy Ketones. J. Am. Chem. Soc 2003, 125, 3442–3443. [DOI] [PubMed] [Google Scholar]; (b) Chaulagain MR; Sormunen GJ; Montgomery J New N-Heterocyclic Carbene Ligand and Its Application in Asymmetric Nickel-Catalyzed Aldehyde/Alkyne Reductive Couplings. J. Am. Chem. Soc 2007, 129, 9568–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Check CT; Jang KP; Schwamb CB; Wong AS; Wang MH; Scheidt KA Ferrocene-Based Planar Chiral Imidazopyridinium Salts for Catalysis. Angew. Chem., Int. Ed 2015, 54, 4264–4268. [DOI] [PubMed] [Google Scholar]; (d) Wang H; Lu G; Sormunen GJ; Malik HA; Liu P; Montgomery J NHC Ligands Tailored for Simultaneous Regio- and Enantiocontrol in Nickel-Catalyzed Reductive Couplings. J. Am. Chem. Soc 2017, 139, 9317–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).(a) Tang X-Q; Montgomery J Nickel Catalysis in the Stereoselective Preparation of Quinolizidine, Pyrrolizidine, and Indolizidine Alkaloids: Total Synthesis of (+)-Allopumiliotoxin 267. J. Am. Chem. Soc 1999, 121, 6098–6099. [Google Scholar]; (b) Tang X-Q; Montgomery J Nickel-Catalyzed Preparation of Bicyclic Heterocycles: Total Synthesis of (+)-Allopumiliotoxin 267A, (+)-Allopumiliotoxin 339A, and (+)-Allopumiliotoxin 339B. J. Am. Chem. Soc 2000, 122, 6950–6954. [Google Scholar]; (c) Colby EA; O'Brien KC; Jamison TF Synthesis of Amphidinolide T1 via Catalytic, Stereoselective Macrocyclization. J. Am. Chem. Soc 2004, 126, 998–999. [DOI] [PubMed] [Google Scholar]; (d) Chan J; Jamison TF Enantioselective Synthesis of (−)-Terpestacin and Structural Revision of Siccanol Using Catalytic Stereoselective Fragment Couplings and Macrocyclizations. J. Am. Chem. Soc 2004, 126, 10682–10691. [DOI] [PubMed] [Google Scholar]; (e) Colby EA; O'Brie KC; Jamison TF Total Syntheses of Amphidinolides T1 and T4 via Catalytic, Stereoselective, Reductive Macrocyclizations. J. Am. Chem. Soc 2005, 127, 4297–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Moslin RM; Jamison TF Total Synthesis of (+)-Acutiphycin. J. Org. Chem 2007, 72, 9736–9745. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zeng M; Murphy SK; Herzon SB Development of a Modular Synthetic Route to (+)-Pleuromutilin, (+)-12-epi-Mutilins, and Related Structures. J. Am. Chem. Soc 2017, 139, 16377–16388. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Kitahata S; Katsuyama A; Ichikawa S A Synthesis Strategy for the Production of a Macrolactone of Gulmirecin A via a Ni(0)-Mediated Reductive Cyclization Reaction. Org. Lett 2020, 22, 2697–2701. [DOI] [PubMed] [Google Scholar]
- (32).(a) Cai Y; Zhang J-W; Li F; Liu J-M; Shi S-L Nickel/N-Heterocyclic Carbene Complex-Catalyzed Enantioselective Redox-Neutral Coupling of Benzyl Alcohols and Alkynes to Allylic Alcohols. ACS Catal. 2019, 9, 1–6. [Google Scholar]; (b) Li L; Liu Y-C; Shi H Nickel-Catalyzed Enantioselective α-Alkenylation of N-Sulfonyl Amines: Modular Access to Chiral α-Branched Amines. J. Am. Chem. Soc 2021, 143, 4154–4161. [DOI] [PubMed] [Google Scholar]; (c) Yan X-B; Li L; Wu W-Q; Xu L; Li K; Liu Y-C; Shi H Ni-Catalyzed Hydroalkylation of Olefins with N-Sulfonyl Amines. Nat. Commun 2021, 12, 5881; [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Analysis of >9 million patents from the pharmaceutical industry demonstrate that carbonyl addition (alongside the Suzuki coupling) is among the most widely utilized methods for C─C bond formation in process R&D: Schneider N; Lowe DM; Sayle RA; Tarselli MA; Landrum GA Big Data from Pharmaceutical Patents: A Computational Analysis of Medicinal Chemists’ Bread and Butter. J. Med. Chem 2016, 59, 4385–4402. [DOI] [PubMed] [Google Scholar]


