Abstract
The fruit and seed produced by a small number of crop plants provide the majority of food eaten across the world. Given the growing global population, there is a pressing need to increase yields of these crops without using more land or more chemical inputs. Many of these crops display prominent ‘fruit–flowering feedbacks’, in which fruit produced early in sexual reproductive development can inhibit the production of further fruit by a range of mechanisms. Understanding and overcoming these feedbacks thus presents a plausible route to increasing crop yields ‘for free’. In this review, we define three key types of fruit–flowering feedback, and examine how frequent they are and their effects on reproduction in a wide range of both wild and cultivated species. We then assess how these phenomenologically distinct phenomena might arise from conserved phytohormonal signalling events, particularly the export of auxin from growing organs. Finally, we offer some thoughts on the evolutionary basis for these self-limiting sexual reproductive patterns, and whether they are also present in the cereal crops that fundamentally underpin global diets.
Keywords: Correlative inhibition, dominance, flowering, fruit, plant reproductive development
A review examining how fruit produced early during reproduction can inhibit the formation of new fruit later in development, and why this may be advantageous for plants.
Introduction
There are some ideas that every gardener holds to be true. Ornamental flowers should be ‘dead-headed’ to keep them flowering. Pea pods should be promptly picked to promote production of further fruit. The ‘June drop’ will see some proportion of fruit falling from orchard trees, long before ripening. But conversely, if a tree is allowed to retain too much fruit, the tree might take a break from fruit production in the next year. The typical gardener is probably happy to ignore exactly how and why these effects occur. And yet, in formulating these guidelines, growers nevertheless seem to appreciate something fundamental about the coordination of plant growth and development—that different organs, at different times, can exert a profound inhibitory effect on the production or maintenance of other organs. Indeed, these inter-organ feedbacks are a critical component of the way in which plants organize their architecture so as to grow optimally in relation to resource availability in the environment, providing a developmental framework that is robust and yet highly flexible.
The aim of this review is to explore these feedback-type mechanisms specifically in the context of reproductive development—to explore feedback that occurs between fruit and flowers, and how these allow plants to optimize their reproductive success in an uncertain environment. We will start by trying to define the types of feedback that occur, before considering the mechanisms that underlie these relationships, exploring their adaptive benefits, and assessing the prospects for using this information to improve crop yields.
Fruit-driven feedback
The production of fruit can have several potent effects on the reproductive effort of a mother plant. Since parthenocarpic (unfertilized) fruit can in many cases cause the same or similar effects as fertilized fruit, it is clear that fruit can exert significant feedback in their own right. However, fertilized fruit often have a stronger effect that parthenocarpic fruit (reviewed in Bangerth, 1989), and certainly in non-parthenocarpic varieties the presence of seed is essential for feedback to occur. In this review, we will use ‘fruit’ as a shorthand for ‘fertilized fruit’ unless otherwise stated, and therefore the feedback effects we describe are typically the effects caused by the combined fruit and seed. Fruit-driven effects can be broadly divided into three key types: feedback on flowering in the same reproductive phase (same-phase feedback), feedback on the subsequent reproductive phase (next-phase feedback), and feedback on the growth or retention of other fruit (fruit–fruit feedback) (Fig. 1A)
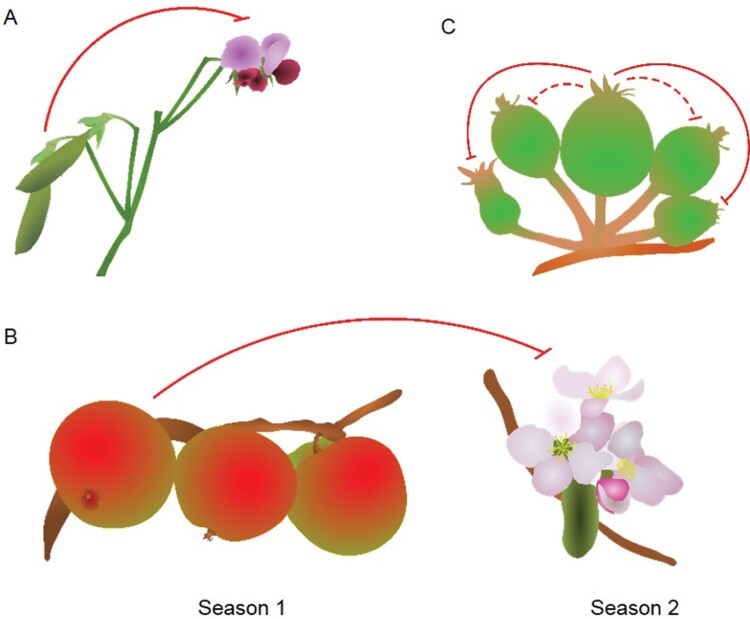
Fig. 1.
The three key types of fruit-driven feedback across species. (A) In pea, developing fruit are capable of inhibiting the formation of later-produced flowers, in same-phase feedback. (B) Apple displays next-phase feedback, where the fruit produced one season can inhibit the production of flowers in the subsequent season, resulting in ‘alternate bearing’. (C) In apple fruit–fruit feedback, the ‘dominant’, ‘king’ fruit inhibits the development of other local fruit, sometimes leading to their abscission from the tree.
Same-phase feedback
The routines of dead-heading and pod-picking illustrate the growers’ intuition that allowing plants to set fruit will somehow limit the ongoing reproductive effort of the plant. However, while the effects of fruit on fruit-set and subsequent reproductive phases are well established in many species in the scientific literature, stimulation of ongoing flowering by fruit removal is, to the best of our knowledge, much less well-described. Indeed, the notion that flower- or fruit-picking promotes ongoing flowering seems largely anecdotal, based on experience of growing peas and beans in particular. Nevertheless, work in the Brassicaceae, and in particular in the model species Arabidopsis, suggests that these effects do occur, although they may be relatively subtle.
Before examining the evidence, it is perhaps useful to define the ways in which plants can continue their reproductive effort. To produce more flowers, plants can initiate more inflorescences (of both lower and higher orders); they can initiate more floral primordia on existing inflorescences; or they can fully develop a larger number of the floral primordia (in essence, they can ‘open more flowers’) (Fig. 1) (González-Suárez et al., 2020). Amongst different species, the structural differences in the arrangement and type of inflorescence may restrict these possibilities. For instance, in species that produce small inflorescences with a relatively fixed number of flowers, the principal option to sustain flowering will be to initiate new inflorescences. Therefore in peas, for example, where two flowers are typically initiated and opened on each inflorescence, only production of new inflorescences can sustain flowering.
The simplest demonstration that fruit-set affects ongoing flowering comes from analysis of male-sterile mutants in Arabidopsis, which do not self-pollinate, and as such do not set seed unless manually cross-pollinated. These mutants, for instance male sterile1 (ms1), flower for a considerably longer period than wild-type, and in the process, initiate more inflorescences than wild-type (Hensel et al., 1994). Those inflorescences in turn produce more total floral primordia than wild-type, and open a higher proportion (~100%) of those primordia (Hensel et al., 1994). The plants, in response to the lack of fertile fruit, seem to ‘throw everything’ into their reproductive effort. The complete removal of fruit from wild-type plants promotes a similar flowering response, as does the lack of viable seed in gametophytic lethal mutants (which are fertile, but only have ~25% seed set) (Hensel et al., 1994).
Further investigation has confirmed and delineated the effects of fruit on Arabidopsis flowering. Fertile fruit located on a given inflorescence exert an inhibitory effect on the outgrowth of subtending, high-order inflorescences on the same branch (Fig. 2A) (Walker et al., 2021). This is part of a more generalized ‘infloretic dominance’, in which the major inflorescences inhibit the activation of their own higher-order, minor inflorescences, and to which the major inflorescence meristem (IM) also contributes (Walker et al., 2021). The effect of each fruit is very small, such that at the beginning of inflorescence lifetime, the effect of fruit is negligible. However, by the time IM arrests, sufficient fruit have usually been formed that their collective effect is sufficient to maintain the inhibition of minor inflorescence activation (Walker et al., 2021). Fruit appear to exert a minimal influence on the activity of the IM on the same inflorescence, such that removal of fruit produced while the IM is active has no discernible effect on the number of floral primordia initiated by the IM (Wang et al., 2020; Walker et al., 2022) (Fig. 2B). Where Arabidopsis fruit do exert a more noticeable effect is on the flower maturation/flower opening process. Arabidopsis inflorescences typically arrest with a cluster of ~15 unopened buds (Hensel et al., 1994), but removal of later-formed fruit is sufficient to promote the opening of almost all of the remaining flowers on the same inflorescence (Ware et al., 2020; Walker et al., 2022). This process does not involve renewed IM activity, and seems to reflect the very localized inhibitory effect that fruit have on the development of nearby floral primordia (Walker et al., 2022) (Fig. 2C).
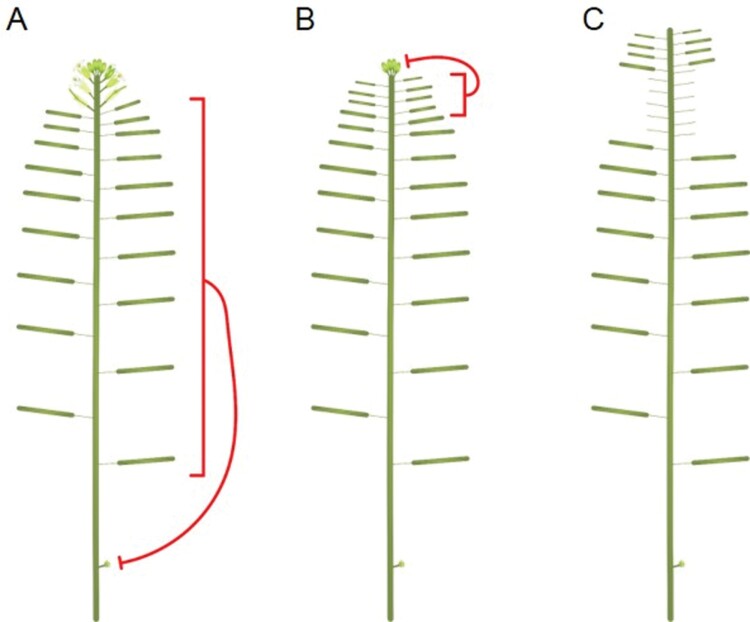
Fig. 2.
Arabidopsis fruit inhibition regulates future flowering. (A) The presence of seed-containing fruit on an inflorescence inhibits the development of buds, which would form a higher-order inflorescence if allowed to develop. (B) Seed-containing fruit proximal to the bud cluster act to inhibit flower development, bringing about inflorescence arrest in Arabidopsis. (C) Removal of fruit proximal to the inflorescence meristem around the time of inflorescence arrest results in the extended opening of floral primordia present within the bud cluster, which would not otherwise develop.
Overall, the data from Arabidopsis support the idea that fruit can inhibit the progression of the ongoing flowering, but the effects of Arabidopsis fruit are rather mild. This might specifically reflect the small size of Arabidopsis fruit, and the short duration of its floral period, or might generally reflect the relevant unimportance of same-phase feedback. Clearly, more experiments are needed across a range of species with different reproductive shoot architectures to establish how generalizable these data are. Pea seems an obvious place to start, since it has long been implicated in these phenomena, and is a highly tractable experimental organism.
Next-phase feedback
Next-phase feedback most obviously occurs in perennial plants in which there is an annual sequence of flowering, with some temporal separation between production of fruit in one year and flowering of the following year, as opposed to repeated waves of flowering during a single year. Next-phase feedback has been particularly studied in commercial fruit trees, in which it can have a major effect on crop yield. In general, based on their annual cycles, two major types of fruit trees can be defined: deciduous and evergreen. While both undergo a synchronous ‘blossoming’ (flower opening) in spring, the induction of flowering differs significantly between them. Flowering induction of deciduous trees usually occurs 80–90 d following anthesis of the previous generation of flowers, most likely due to endogenous developmental cues (Goeckeritz and Hollender, 2021). Inflorescences are initiated, and flower buds form and differentiate before entering paradormancy and spending winter as arrested floral buds. Conversely, floral induction of evergreen subtropical fruit trees occurs during the winter, where the accumulation of sufficient number of cold hours plays a major role in the conversion of vegetative meristems into IMs (Wilkie et al., 2008). Flowering induction in tropical fruit trees also results from endogenous cues, but is not always synchronized to a certain season. In some fruit trees there is clear phase separation, and fruit are harvested prior to the subsequent flowering induction period; for instance, olive and early citrus cultivars. However, in many trees, there is no clear phase separation, and fruit are still present on the tree during or following the subsequent flowering induction; for instance, most citrus cultivars, avocado, and mango. There are even extreme cases where the previous season’s fruit is present during flowering, fruit set and early stages of fruit development, particularly Valencia orange. In all these scenarios, the fruit can exert an inhibitory effect on the subsequent flowering period. Most obviously, these inhibitory effects occur at the level of floral induction, with fruit dampening down the number of inflorescences produced the following season, even if the fruit are no longer present, implying the existence of a remarkable ‘fruit memory’ (Goldschmidt and Sadka, 2021). However, fruit presence can also impact on subsequent stages of flowering, flower development, and even bud break (Verreynne and Lovatt, 2009).
The effect of next-phase fruit feedback is clearly seen in cultivated fruit trees that have an ‘alternate bearing’ (AB) habit (reviewed in Goldschmidt and Sadka, 2021). This refers to the common case in fruit trees when fruit yield of one year inhibits flowering the following year. Moderate yield fluctuations are normal in every cultivar of any fruit species, and occur in response to environmental cues. However, what defines AB is the extreme fluctuation in yield, with heavy fruit load one year (on-crop) followed by very low yield (off-crop) the following year (Fig. 3). Nevertheless, unfavourable climatic conditions, especially during flowering and fruit set of the on-crop year, may cause flowers or fruitlets to drop, and cause a consecutive on-crop year. Additionally, fruit thinning or complete removal (de-fruiting) before the subsequent floral induction period also induces a consecutive on-crop year (back flowering), demonstrating that AB is primarily driven by feedback from fruit. Even in perennial plants without the AB habit, it is likely that fruit-set exerts some feedback on the subsequent reproductive phase, but the phenomenon is most easily visible (and therefore studied) in AB cultivars. De-fruiting or thinning during the on-crop year needs to be performed a significant period before the onset of floral induction, otherwise the plant will ‘remember’ the presence of the fruit even in their absence. How early the de-fruiting needs to be performed is species-dependent. For instance, under the east Mediterranean climate, de-fruiting before October is effective in inducing back flowering in avocado (Ziv et al., 2014). In most citrus cultivars, fruit removal is effective if performed before November (Muñoz-Fambuena et al., 2011). Therefore, it could be concluded that fruit memory in citrus and avocado becomes fixed until about 1 to 2 months before the onset of floral induction. In olive, fruit memory becomes fixed earlier, and fruit removal is effective only if performed before mid- or, at most, the end of August (Dag et al., 2010; Haberman et al., 2017). The mechanisms by which fruit memory affects flowering are still enigmatic. In fact, the mechanistic question can be divided into two: first, how the meristem or the bud senses fruit presence, and second, how this sensing mechanism is translated into a decision-making process: flowering promotion or inhibition. These two topics are discussed below (section ‘Auxin canalization—the origin of dominance?’).
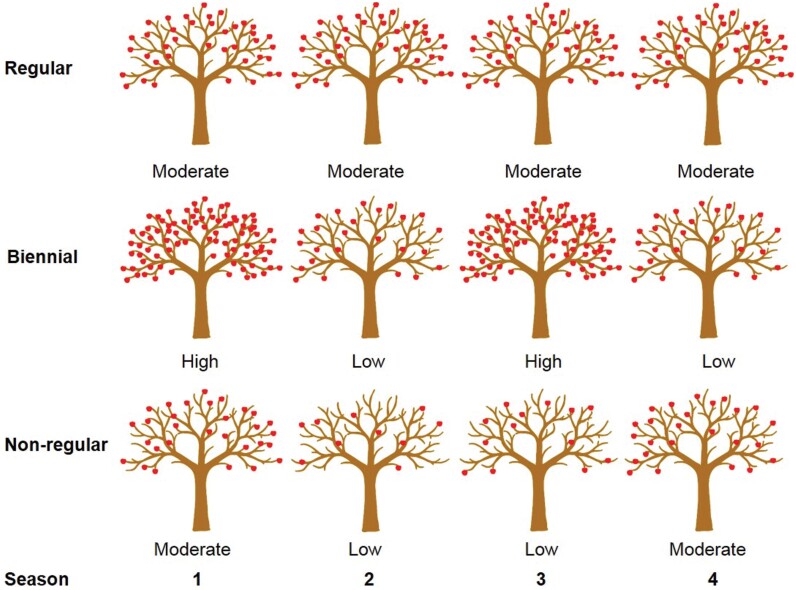
Fig. 3.
Bearing patterns of fruit trees over multiple seasons. Biennial bearing results in alternate years of high and low yield, unlike regular bearing patterns, where yields are more stable and consistent. Non-regular bearing trees tend to have non-regular, less predictable yield from season to season.
The feedback between fruit and flowering plays an important role in allowing perennial plants to balance their growth between vegetative and reproductive development. In most perennials, inflorescences are initiated as specialized lateral branches on vegetative shoots, and therefore annual vegetative growth is a prerequisite for ongoing productive capacity. In many fruit trees, flowers occur on 1-year-old shoots, emphasizing the need for ongoing vegetative capacity (Bangerth, 2009). However, over-flowering may impair vegetative growth, and vice versa. Regular bearing plants seem to use two mechanisms to control a proper balance between vegetation and productivity. The first, fruitlet thinning (see below) provides an important mechanism to control the final number of fruit in each season, which might normally exceed available resources anyway (for instance, ‘Wilking’ mandarin lacks this ability, and if no chemical thinning is applied, the tree may die while carrying many fruit) (Goldschmidt and Sadka, 2021). The second mechanism could be defined as proportional allocation of resources between vegetative growth and reproduction, which occurs under the influence of the remaining fruit. In this context, it can by hypothesized that AB represents a highly simplified strategy to avoid the problem of resource allocation, in which no proportional allocation is required, as the case in regular bearer tree. Resources are allocated one year for vegetative growth (off-crop year) and for productivity the following year (on-crop year). Testing this hypothesis provides a challenge, as it requires full understanding of the meaning of ‘economic’ cost of resource allocation between vegetative and reproductive growths.
From the growers’ perspective, non-regular bearing presents an economic problem, but in nature this might be the norm. Productivity of many forest trees follows a ‘masting’ phenomenon, where extreme but synchronized fluctuations in yield are detected (Kelly and Sork, 2002). Productivity varies between species, with 2–3-year cycles in chestnut, hazelnut, and elm and up to 10–15 years in beeches. The productivity of wild relatives of fruit trees has been reported only in a few cases (reviewed in Goldschmidt, 2013b, 2018). Yield of wild apple (Malus sieversii) varies greatly from tree to tree, and seems to be affected by many environmental parameters, but when trees of this species were planted in an experimental farm, their multi-annual productivity was quite stable, and they did not show a biennial bearing pattern (Goldschmidt, 2013a). Conversely, Polish wild pear (Pyrus pyraster) and wild olive showed clear biennial and non-regular bearing patterns (Goldschmidt, 2013a). The pecan nut (Carya illinoiensis) and macadamia (Macadamia tetraphylla and M. integrifolia) provide examples from recently domesticated fruit trees. In the wild, pecan displays masting and consistent with this, cultivated trees show strong alternate bearing behaviour. Conversely, in the wild, macadamia produces very low and non-regular yield, but when cultivated, the trees show relatively high and stable yield (Goldschmidt, 2013a). These cases suggest that alternate or non-regular bearing is common in the wild, and likely results from the interaction between endogenous and exogenous cues. However, in the wild, productivity varies from tree to tree due to interactions between local conditions and endogenous cues. Only under cultivation, when selected uniform genotypes are grown under homogeneous practices, is the true nature of the production strategy revealed—regular, biennial, or non-regular (Goldschmidt and Sadka, 2021).
Stable and sufficient multi-annual yield is an obvious prerequisite for cultivation of any fruit tree. Therefore, domestication of fruit trees selected against non-regular bearing of any type (Goldschmidt, 2013a, 2018). For instance, the domestication of macadamia, an extreme case of a non-productive wild tree, was based on multiple selections of lines with increased yield. However, selecting against biennial bearing has proved more difficult, with the trait present in many modern cultivars. Molecular markers for regular bearing could therefore be useful in breeding programmes. Indeed, genetic and bioinformatics analyses of segregating populations between a strongly biennial bearing apple cultivar and regular bearer cultivar have identified quantitative trait loci (QTLs) that could explain phenotypic variability associated with biennial bearing, leading to the development of tools to access bearing behaviour, at least during the first year of tree maturity (Guitton et al., 2012; Durand et al., 2013, 2017). However, so far, to the best of our knowledge, these tools are not practically used. Moreover, to the best of our knowledge, these are the only reported attempt to generate marker-assisted breeding for stable yield traits, and no markers have been reported in other species. Indeed, in many breeding and selection programmes, non-regular bearer trees are not discarded if they meet other desired criteria. It should also be considered that for most species and cultivars, efficient horticultural practices to improve productivity have been such that in many cases, the ‘domestication force’ against non-regular bearing is not based on genetic selection, but on horticultural practices.
Fruit feedback on fruit growth and retention
The last major type of fruit-driven feedback is the effect of older fruit on the growth and retention of younger fruit (fruit–fruit feedback, FFF). FFF could theoretically be exerted at three scales: within inflorescences, between inflorescences on the same branch, and between inflorescences on different branches. In annuals, FFF can sometimes be observed at all scales, but in fruit trees FFF is mostly noticed and studied at the intra-inflorescence level and between inflorescences on the same branch. FFF has an important economic impact as it affects the overall number of fruit and it alters the ratio between small and large fruit, thus affecting yield (Cockshull and Ho, 1995).
Cucurbits provide some of the most striking examples of FFF, with a single fruit often inhibiting the development of all subsequently initiated fruit, in both seeded and parthenocarpic varieties (Baniel et al., 2008; Shnaider et al., 2018). Inhibited fruit show a range of fates depending on their position in the hierarchy; older fruit show reduced or stalled growth, but younger fruit actively senesce (Shnaider et al., 2018). Growth of inhibited fruit can, however, be restored by removal of the ‘dominant’ first fruit.
In tomato inflorescences of indeterminate varieties, proximal fruit that are set earlier display a higher rate of assimilate import and have a larger number of cells than distal ones (Bangerth and Ho, 1984; Bohner and Bangerth, 1988). However, when the pollination sequence is manipulated, distal fruit appear earlier and become larger than proximal ones, and if all fruit are pollinated simultaneously, the fruit are the same size (Fig. 4A) (Bangerth and Ho, 1984). Within the inflorescence, fruit set failure is considerably higher in distal positions as compared with proximal ones (Bertin, 1995). Within the same truss, fruit number is also reduced due to abortion in later inflorescences as compared with earlier ones, unless thinning is performed. FFF between trusses is documented in tomato, but to a lesser extent than intra-inflorescence and between inflorescences; some reduction in fruit dry matter and growth rate is detected in later trusses than early ones (Bertin, 1995). While the above early works provide ‘physiological’ evidence for FFF, the accumulation of genetic and transgenic data provide further support. Numerous genes and QTLs are involved in tomato yield and individual fruit weight (reviewed in Ariizumi et al., 2013). For instance, Fruit Weight 2.2 (FW2.2) is a transcription factor that inhibits cell division (Beauchet et al., 2021). Due to sequence variation in its promoter region, it is expressed at higher levels in wild tomatoes than in cultivated lines, causing a small fruit phenotype. Generation of isogenic lines with the two promoter variants allowed assessment of their effect in a reference background (Nesbitt and Tanksley, 2001; Guo and Simmons, 2011). Fruit set is higher in the small fruit genotype, while flower abortion is lower if the number of flowers per inflorescence is greater than six. Interestingly, fruit thinning did not increase fruit weight and size showing the primary effect if FW2.2 is upon individual fruit size, and suggesting that effects on fruit number therefore result from FFF. Altering sink–source ratio was also performed by anti-sense manipulation of sucrose synthase, which along with cell wall invertase, plays an important role in generating sink strength of the fruit by controlling sugar import. The manipulation resulted in reduced fruit set, especially in second and third waves of flowering, and overall reduced number of fruit per plant and per truss (D’Aoust et al., 1999). Regardless of the lower competition, fruit growth rate was also reduced, demonstrating that primary fruit feedback inhibition on later fruit does not always follow a simple inverse relationship between fruit number and size, especially when sink strength of the fruit and sink–source relationships are altered.
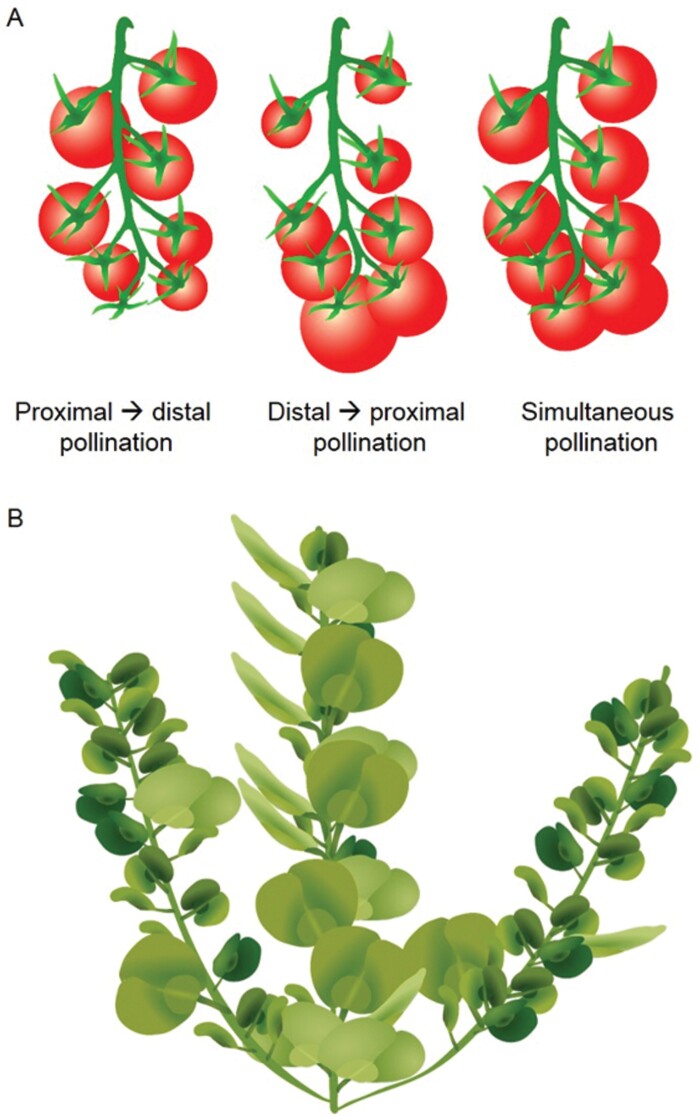
Fig. 4.
Fruit development regulates subsequent fruiting patterns. (A) Fruit size in tomato trusses is often determined by timing of pollination, with the earliest-pollinated flowers producing the largest fruit, regardless of position on the truss. Conversely, if all flowers are pollinated simultaneously, they all develop into fruit of similar mass. (B) Aethionema arabicum displays two distinct fruit morphs, with the primary inflorescence containing the majority of the large-morph fruit. The higher-order inflorescences typically contain predominantly the small-morph fruit. Removal of fruit or inflorescences can alter the distribution of these morphs across the plant, highlighting the dominance relationships between developing fruit (Lenser et al., 2018).
The Brassicaceae represent an interesting case for studying FFF, since some genera clearly display the phenomena, while others do not. For instance, Aethionema species produce two distinct fruit morphs as part of a bet-hedging strategy; larger fruit that immediately dehisce, for seed germination under currently favourable conditions, and smaller fruit that are indehiscent, with seeds for deposition in the long-term seed-bank (Lenser et al., 2016, 2018). The larger fruit morph occurs mostly on main shoot (Fig. 4B), and the proportion of larger fruit could be further induced by side branch removal. Moreover, removal of the larger fruit from the main shoot induced the proportion of larger fruit on the side branches, indicating that indehiscent fruit arise through inhibition by dehiscent ones (Lenser et al., 2018). Thus, Aethionema represents an interesting example where ‘inhibited fruit’ are repurposed as a secondary reproductive strategy. In Brassica species, FFF clearly exists, with many flowers opened later in the reproductive phase failing to set viable fruit under the influence of earlier fruit (Tayo and Morgan, 1975; Bangerth, 1989; Walker et al., 2021). However, in Arabidopsis there is no discernible effect of early fruit on the growth or development of later-initiating fruit (Walker et al., 2021).
In fruit trees, FFF within inflorescences is ubiquitous, and is associated with fruitlet self-thinning, which provides a natural mechanism aimed at regulating yield (Pawar and Rana, 2019). Fruit position within the inflorescence greatly determines its survival, as clearly demonstrated in apple, where about 70% of the fruitlets abscise. Fruitlets at the apical (‘king’) position have ~70% likelihood of surviving and developing into mature fruit, while those in lateral positions within the cluster have 70% likelihood to abscise (Maguylo et al., 2014; Jakopic et al., 2015). Moreover, mature fruit with immediate proximity to the king were smaller relative to both the king and those at lower positions, showing FFF is stronger with proximity to the king. Pedicels of fruit with lowest probability of abscising displayed extra vascular bundles, suggesting they play a role in dominance acquisition (Celton et al., 2014). Inter-inflorescence feedback control is also demonstrated in fruit trees. The overall inverse relationships between the number of fruit and their growth rate, which is also a ubiquitous phenomenon, might be associated with fruit–fruit feedback control, regardless of relative position. In fig, for instance, proximal inflorescences develop into fruit and ripen earlier than distal ones; removal of the latter accelerates the development of the proximal inflorescences (Flaishman, 2022). Feedback control may also be exerted in species that flower and set fruit a few times a year. For instance, lemon trees usually bear large, harvestable fruit, along with small fruit originated from later flowering waves. Although the relationships between these two types of fruit have not been formally investigated, it is well acknowledged by growers that as soon as the large fruit are harvested, growth rate of the small ones is accelerated.
Source–sink versus dominance relationships
A wealth of evidence indicates that fruit can exert strong feedback on flowering and fruit development, but how does this occur? In the next sections, we will focus on potential explanations for these effects. Fruit-driven feedback belongs to a class of phenomena often referred to as ‘correlative inhibition’, in which the growth of one organ (or class of organs) inhibits the growth of other organs (Bangerth, 1989). Probably the best-known class of correlative inhibition is ‘apical dominance’ in which actively growing shoot branches repress the activation of other shoot branches (Thimann and Skoog, 1933; reviewed in Domagalska and Leyser, 2011). There is a long running debate in the literature regarding the extent to which correlative inhibition is either driven by source–sink relationships, or by active ‘dominance’ mechanisms (Bangerth, 1989). The simplest and most intuitive model is the source–sink model, in which correlative inhibition arises because organs act as ‘sink’ organs for assimilates, of which there is a limited supply from ‘source’ organs. On the other hand, dominance mechanisms imply that organs actively inhibit the growth of other organs by signalling processes (Bangerth, 1989). In many ways, this debate reflects a broader debate on the nature of plants: are they passive in their environment, mutely reacting to changes in resource availability in a mechanical way? Or are they active in their environment, using information to proactively plan their growth to both current and future resource availability?
Like many such arguments, the source–sink/dominance debate very likely represents a false dichotomy. There is no reason to doubt that the availability of assimilates within the plant will influence and limit the growth of organs. But equally, to suggest that the only way plants regulate their growth is by waiting to run out of assimilates is to do a disservice to these incredibly complex organisms. There is now a large amount of evidence that plants use long-distance hormonal signalling, among a range of other mechanisms, to coordinate their growth, and there is no reason to persist which such a simplistic model. Indeed, modern formulations of source–sink models for plant growth fully integrate active hormonal signalling (Yu et al., 2015; Chang et al., 2017), and a reasonable argument can be made that one of the major functions of hormonal signalling is to organize the appropriate distribution of the limited supply of assimilates (Perez-Alfocea et al., 2010; Shabala et al., 2016). We take the attitude that, in the context of fruit-driven feedback, both passive source–sink relationships and active dominance mechanisms are likely to play a key role, to different extents in different organs. We will not examine source–sink models further, but will focus on the more active regulatory mechanisms of fruit-feedback.
Auxin canalization—the origin of dominance?
In his seminal review and hypothesis paper on the subject of correlative inhibition, Bangerth (1989) proposed a simple mechanism by which an earlier-initiating organ can actively inhibit the growth of a later-initiating organ, regardless of their relative positions on the plant. He suggested that this ‘primigenic dominance’ arose by ‘auxin transport autoinhibition’ in which a strong auxin flux generated by the dominant organ inhibits auxin flow from a subordinate organ, increasing the hormone level in that organ, and inhibiting its growth (Bangerth, 1989). Thus, in Bangerth’s view, which he supported with a range of experimental observations, the relative ability of an organ to export auxin was key to determining both its own growth and its ability to feed back on the growth of other organs.
Research into the regulation of shoot branching, including the classic apical dominance phenomenon, has subsequently provided strong support for the general concepts proposed by Bangerth. Firstly, the ability of an individual shoot branch to grow is determined, at least in part, by its ability to export auxin (Morris, 1977; Crawford et al., 2010; Balla et al., 2011, 2016), and secondly, the ability of branches to inhibit other branches, and thus the total shoot branching level, is strongly dependent on the auxin transport environment in the shoot system (Bennett et al., 2006, 2016; Crawford et al., 2010; Shinohara et al., 2013; Van Rongen et al., 2019). Developing the auxin transport autoinhibition model further, these auxin-driven effects are usually now proposed to arise from auxin transport canalization, building on the original auxin canalization models for vascular patterning (Sachs, 1969, 1981; Bennett et al., 2014). It is proposed that, in order to export auxin and therefore to grow, branches need to form or maintain a canalized auxin transport link to the main stem, and that their ability to do this will depend on the auxin source strength of the branch and the auxin sink strength of the main stem (Prusinkiewicz et al., 2009). By extension, the ability of branches to inhibit other branches occurs because their auxin export weakens the auxin sink strength of the shared stem, limiting the ability of other branches to form canalized links (Prusinkiewicz et al., 2009). Mathematical modelling demonstrates the plausibility of this (Prusinkiewicz et al., 2009), and it is consistent with a wide range of experimental data, although it must be noted that it is far from universally accepted (reviewed in Waters et al., 2017; Walker and Bennett, 2018).
Nevertheless, the canalization model for apical dominance provides a clear working model for fruit-driven feedback, which can be referred to as ‘carpic dominance’ in this context (Walker and Bennett, 2018). Indeed, carpic dominance was the context in which Bangerth originally framed his auxin transport autoinhibition model for primigenic dominance. There was sufficient evidence to suggest auxin as a causative agent when Bangerth proposed his ideas, but recent work has provided strong additional evidence that auxin transport plays a key role in carpic dominance. For instance, in the context of fruit load or alternate bearing, transcriptomic analysis of on- and off-crop buds in citrus identified induction of auxin polar transport genes in off-crop buds and in the buds of de-fruited on-crop trees soon after de-fruiting (Shalom et al., 2012; Haim et al., 2021). In association with this, indole-3-acetic acid (IAA) levels were higher in on-crop buds than off-crop buds, and this was reduced following de-fruiting (Shalom et al., 2012; Haim et al., 2021). The use of radiolabelled IAA in both citrus and olive has confirmed that auxin flux in the stem is higher when fruit is present and is reduced following de-fruiting (Haim et al., 2021). Moreover, the auxin flux direction originated from the fruit, regardless of its position relative to the bud, and IAA removal from the bud was accelerated by fruit removal, providing further support for a canalization-based mechanism. In Arabidopsis, fruit have been shown to export significant quantities of auxin into the stem (Ware et al., 2020), which progressively weakens auxin transport and increases auxin concentration in the upper inflorescence stem (Goetz et al., 2021). These effects are necessary and sufficient to drive the fruit-induced arrest of the inflorescence (Ware et al., 2020; Goetz et al., 2021). Consistent with this, the inhibitory effect of early fruit on the development of later fruit in Aethionema species can be mimicked by auxin application, implying that auxin might drive this fruit–fruit feedback (Lenser et al., 2018).
However, as with apical dominance, the extent to which auxin export from fruit is the primary explanation for feedback on flowering and fruit development is still open. Considering the limited research tools in fruit trees, especially lack of genetic tools, the establishment of such direct relationships is not trivial. Application of auxin may alter flowering intensity and other flowering traits, such as the duration of bud break and the number of flowers in the inflorescence (Sadka et al., 2022; Martinez-Fuentes et al., 2022). Overall, the effects of auxin treatment are quite mild, probably due to the efficient ability of the plant to remove excess auxin (Eklöf et al., 2000). While establishing more direct relationships between flowering control and auxin is challenging, the canalization model at least provides a testable framework on which to advance studies in this area (Fig. 5).
Fig. 5.
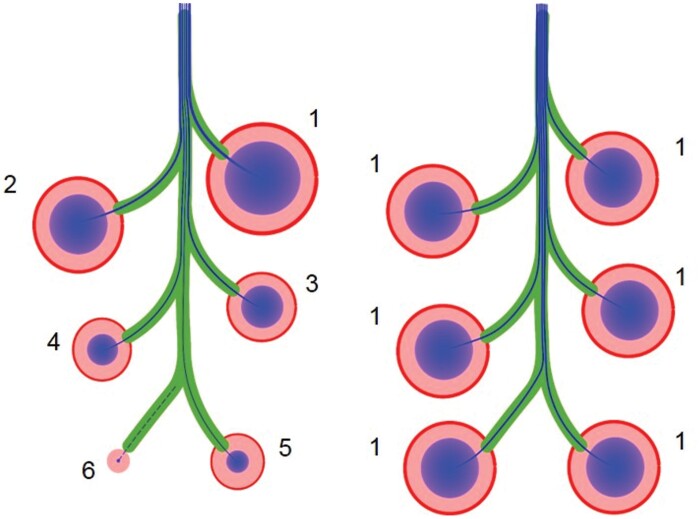
Model for auxin canalization in fruit development. Fruit are numbered in order of their development: sequentially (1–6) or simultaneously (1). Sequential development allows the earlier-developing fruit to export high levels of auxin into the stem, decreasing its sink strength. Subsequent fruit are therefore smaller, as they are unable to export high levels of auxin into the stem due to reaching ‘saturation’. Some fruit may be entirely inhibited from developing if they are unable to canalize to the polar auxin transport stream. Conversely, fruit developing simultaneously are all able to establish links to the polar auxin transport stream, resulting in more even auxin export from the fruit, and uniform fruit size.
While auxin canalization provides the most reasonable scenario for dominance, the question can be asked whether the seed, fruit or both is the source of dominance. Auxin production within the fertilized ovule, and specifically in the endosperm, plays a major role in seed and fruit set (Figueiredo et al., 2015; Guo et al., 2022). Auxin accumulation is detected in fruit tissues during various developmental stages (Nishio et al., 2010; Pattison and Catalá, 2012; McAtee et al., 2013; Feng et al., 2019). In early works, it was modelled that the source of dominance is within the seed (reviewed in Monselise and Goldschmidt, 1982). Apple displays negative correlation between the number of seeds per spur and percentage of flowering spurs (Dennis and Neilsen, 1999; Milyaev et al., 2022). Seed ablation in olive, which did not affect fruit development, also promoted back-flowering, supporting the above notion (Stutte and Martin, 1986; Lavee, 2007). Negative correlation between seed number per fruit and back-flowering intensity was also reported in citrus (Monselise and Goldschmidt, 1982; Davenport, 1990). However, in citrus, opposite trends have also been reported. Satsuma mandarin is seedless, yet it displays strong inhibition of back-flowering, and this is also the case in Ori mandarin, which has a very low number of seeds per fruit (Okuda, 2000; Schneider et al., 2009). Furthermore, it should also be considered that dominance is exerted in parthenocarpic fruits. Therefore, it is likely that both the seed and the fruit provide the source of dominance and their relative contribution is species or even cultivar dependent.
Other hormones
Besides auxin, other hormones play key roles in regulating shoot branching, particularly cytokinin and strigolactones. Cytokinin promotes branching, negating apical dominance, acting both to promote growth of the branch (Dun et al., 2012) and to increase auxin sink strength in the stem (Waldie and Leyser, 2018). Conversely, strigolactones typically repress branching, enhancing apical dominance by inhibiting growth of the branch (Dun et al., 2012) while decreasing sink strength in the stem (Crawford et al., 2010; Shinohara et al., 2013). It therefore seems very likely that other hormones will play a role in modulating fruit-driven feedback. However, in the case of strigolactones, there is little evidence either way. In Arabidopsis and Brassica napus, strigolactone-deficient mutants produce the same number of fruit and seed yield as wild-type, despite their strongly increased number of inflorescences (Walker and Bennett, 2018; Stanic et al. 2021), and undergo inflorescence arrest at the same time as wild-type (Ware et al., 2020), suggesting that strigolactones might not be required for normal fruit-driven feedback, at least in the Brassicaceae. In other species, the effects of strigolactones on reproductive development have not been well studied, and the question thus remains open.
Conversely, there is some evidence for cytokinin acting to oppose fruit-driven feedback in the Brassicaceae. Cytokinin treatment of Aethionema arabicum increases the proportion of large fruit produced on each inflorescence (Lenser et al., 2018), while cytokinin treatment of Arabidopsis delays inflorescence arrest (Merelo et al., 2022; Walker et al., 2022). In part this is because of the effect of cytokinin in prolonging the activity of the inflorescence meristem, but analysis of the rock2 and rock3 mutants with enhanced cytokinin signalling shows that cytokinin also promotes the opening of flowers (after inflorescence meristem arrest) that would normally be prevented by fruit-driven feedback (Ware et al., 2020; Walker et al., 2022). Cytokinin could also reverse first-fruit-inhibition phenomena in cucumber (Baniel et al., 2008). Thus, in terms of mitigating the effects of fruit-driven feedback, enhanced cytokinin seems like an interesting route to explore to improve fruit crop yields.
Gibberellins and abscisic acid (ABA) might also play roles in controlling fruit-driven feedback, particularly in the complex effects of fruit load on flowering. Gibberellins, applied during the flowering induction period, are strong inhibitors of fruit tree flowering (Bangerth, 2009). The effect of external application of gibberellin on the expression of flowering-control genes is well established in several trees (Goldberg-Moeller et al., 2013; Zhang et al., 2019). It might well be that increased auxin levels in the floral buds of on-crop plants induce gibberellin synthesis (as is the case during fruit-set), and therefore flowering inhibition. In cucumber, application of GA could reverse the inhibitory effect of the first fruit on the development of later pollinated ovaries (Baniel et al., 2008). ABA is also involved in flowering control, at least in citrus under stress conditions, and changes in ABA levels have been associated with fruit load in a few fruit tree species, including olive, citrus, and pistachio (Goldschmidt and Sadka 2021). However, the role of ABA in this process is currently unclear, and more investigation is required.
Epigenetic mechanisms and fruit memory
An intriguing question is how fruit trees are able to ‘remember’ previous fruit loads, even when the fruit have been removed. Recent work in citrus suggests an explanation for this remarkable phenomenon. CcMADS19 is a citrus orthologue of the floral repressor FLOWERING LOCUS C (FLC) from Arabidopsis, whose expression was shown to be reciprocal to that of the floral activator FLOWERING LOCUS T (FT) (Agustí et al., 2020). In leaves of on-crop trees, transcript levels of CcMADS19 are high, while those of CcFT are low, and vice versa in leaves of off-crop/de-fruited trees (Agustí et al., 2020). Moreover, when transformed into citrus leaves, CcMADS19 inhibited FT expression. Crucially, the methylation pattern of the CcMADS19 large intron was found to be different in on-crop and off-crop trees, with de-fruited trees showing a similar pattern to off-crop trees (Agustí et al., 2020). Use of a DNA methyltransferase inhibitor was sufficient to induce the expression of CcMADS19 in on-crop trees, while reducing the expression of FT. Furthermore, on-crop trees were also found to have increased histone methylation (H3K4me3) in the CcMADS19 promoter consistent with increased activity, and—consistent with this—to have increased expression of TRITHORAX proteins that act as FLC activators in Arabidopsis (Agustí et al., 2020).
But how do the trees cycle between these epigenetic states, and what is the role of fruit in inducing this? Mesejo et al. (2022) suggest that differences in CcMADS19 histone methylation between ‘old’ leaves and dormant axillary buds are crucial to this effect. In essence, ‘new’ tissues have H3K27me3 marks at the CcMADS19 locus, making the gene inactive, whereas in older tissues the activating H3K4me3 mark predominates at this locus (Mesejo et al., 2022). Removal of fruit, or just girdling of fruit peduncles in on-crop orange trees. results in reduced auxin export from fruit, and leads to sprouting of the dormant axillary buds adjacent to the fruit (Mesejo et al., 2022). These axillary buds produce new leaves, and ultimately undergo flowering and give rise to new inflorescences. Because fruit auxin export inhibits the growth of nearby axillary buds, fruit therefore affect the overall balance of CcMADS19 histone methylation across the tree, with fruit presence leading to a higher proportion of tissue with increased CcMADS19 expression, and fruit absence leading to an increase in tissue with CcMADS19 repression (Mesejo et al., 2022). The growth of new tissues with CcMADS19 repressed then provides a source of CcFT expression to induce flowering the following season, while the absence of new tissues and CcFT expression in on-crop trees leads to vegetative growth occurring the following season instead. These results provide a plausible scenario connecting fruit presence and auxin export to an epigenetic ‘memory’ of the fruit load in citrus, but it remains to be established whether these results are generalizable to other, more distantly related fruit trees.
Measuring reproductive success
While the effects and mechanisms of fruit-driven feedback have been discussed above, an important question that we have skirted so far is why? Why do fruit exert this counter-intuitive inhibitory effect on the further production of fruit? The mostly likely answer is that this feedback allows plants a simple homeostatic mechanism by which to measure their own reproductive success, and to adjust their reproductive development to the circumstances. This reflects the inherent uncertainties that accompany flowering as a reproductive strategy, the main uncertainty being pollination, especially for insect-pollinated species. However, the potential losses of flowers and fruit to wind, rain, and herbivory also count as major uncertainties during reproduction. Amongst these uncertainties, plants must open enough flowers to guarantee a minimum reproductive success, without producing so many fruit that there are insufficient resources for them to all fully develop. The feedbacks described here provide a mechanism to steer a course between these goals. Consistent with overall reproductive strategies, two broad feedback strategies can be identified: over-flowering and under-flowering.
Over-flowering is most appropriate in plants where reproductive effort is largely predetermined, especially where there is a significant time gap between initiation of reproductive structures and actual fruit-set. Spring-blooming fruit trees provide the prime example of this strategy; inflorescences are initiated in autumn, and flower opening is a synchronous event in spring, with little ability to adjust reproductive effort at that point. To ensure sufficient fruit-set despite the uncertainty, there is little option but to ‘over-flower’, to open far more flowers than can ever give rise to successful fruit. Under such circumstances, there is no possibility to use fruit-set to adjust same-phase flowering (which began and ended months before), but there is a strong need for carpic dominance to allow selective shedding of excess fruit. Even with over-flowering and selective fruit-shed mechanisms in play, a wide range of reproductive success might result, and thus next-phase feedback allows plants to adjust their subsequent reproductive effort relative to the eventual success of the preceding reproductive cycle.
By contrast, under-flowering is most appropriate in plants with a continuous reproductive effort, in which events early in reproduction can influence later events. An under-flowerer’s initial effort produces just enough flowers to guarantee minimum seed set if there is ‘perfect’ pollination, thus avoiding unnecessary investment in excess flowers and fruit. However, if pollination is not perfect, a lack of feedback from early fruit will promote the stepwise production of additional inflorescences or flowers to fulfil the minimum requirement (Walker et al., 2022). Since the plant can initiate approximately the correct number of flowers in the first instance, the need for fruit–fruit feedback is greatly reduced. Thus, Arabidopsis represents a clear example of an under-flowering strategy.
There are of course a whole range of possibilities between these two strategies, with B. napus representing a ‘mild over-flowering’ strategy. Flowering in B. napus is continual and adjustable, but seems to contain a large initial effort that produces somewhat too many flowers, if all are successfully pollinated (Tayo & Morgan, 1975). Carpic dominance is therefore present in B. napus to prevent too much fruit-set, but same-phase feedback is also present to allow the initiation of additional inflorescences if fruit-set is too low.
Perspectives
Feedback between fruit and flowering provides important insights into the mechanisms by which plants flexibly and continually adjust their shoot growth to match environmental conditions. As discussed above, very similar dominance mechanisms play a key role in the regulation of vegetative shoot architecture. Indeed, an appealing possibility is that all shoot and reproductive architecture is ultimately regulated by a single, unified dominance mechanism, or at least a suite of fundamentally similar dominance mechanisms. Recent work in pear illustrates how auxin export from leaves is necessary to maintain the paradormancy of autumn-initiated inflorescences (Wei et al, 2022), indicating feedback from vegetative to reproductive organs, and demonstrating for the first time (to our knowledge) that leaves exert dominance over other tissues. Conversely, work in barley might indicate that the initiation of reproductive structures is able to suppress vegetative branching (tillering) in this crop (Zwirek et al, 2019).
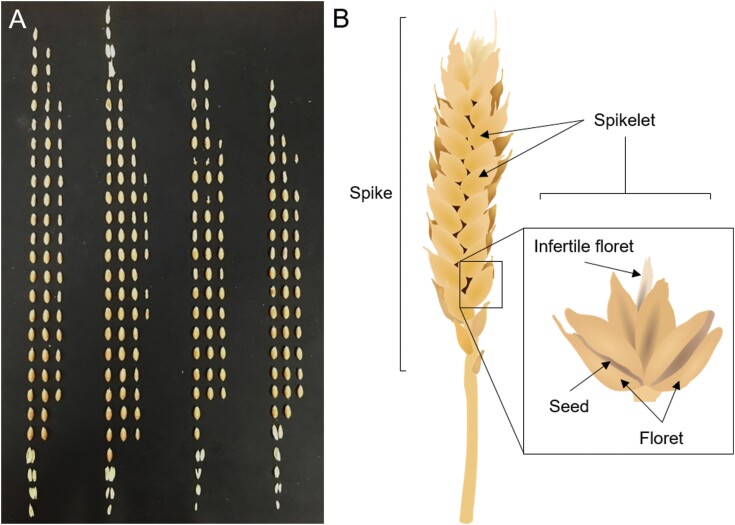
More generally, cereals crops represent an interesting opportunity in terms of fruit-driven feedback. There is no doubting the importance of fruit-driven feedback in commercial fruit trees and horticultural crops, but what about arable crops? Do they also display such feedback, and to what extent could applying the frameworks outlined here to cereals revolutionize our understanding of their yield formation? Bangerth (1989) certainly thought that dominance relationships occur within the ‘spikes’ (grouped inflorescences) of cereals, but despite much interest in understanding the characteristic development and shape of cereal spikes (Wang et al., 2021), there has been little subsequent work on dominance effects in cereals. We can speculate the cereals are over-flowerers, on the basis that the initiation and complete development of spikes occurs a significant time before the spikes emerge into the air, and undergo anthesis and pollination. There is therefore little scope for cereals to produce more flowers except by ‘back-tillering’ during flowering and initiating new, later spikes. Consistent with this idea, we can see that, within a wheat spike, there are strong gradients of grain size (the grains are a caryopsis, a fruit containing a single seed in which the fruit and seed tissues are fused together). This occurs both within spikelets (the small inflorescences that make up the spike), which could indicate older grain inhibiting the growth of younger grain, and between spikelets, which could be indicative of older spikelets inhibiting the growth of later ones (Fig. 6). This would be broadly consistent with an over-flowering strategy. Clearly, more work is needed to explore fruit-driven feedback in the context of cereals; if found to be relevant, there is certainly huge scope to improve grain size across a typical cereal spike.
Fig. 6.
Possible dominance effects in wheat inflorescence development. The seeds from four ‘spikes’ from the same plants have been dissected out (A). Each row represents the seed from an individual spikelet (inflorescence) along the length of the spike (B). The medial spikelets develop first, and have a larger number of fertile flowers (i.e. seeds per spikelet), and larger seed than the apical and basal spikelets. Within each spikelet, the earlier flowers (on the left of each row) from the outer florets of the spikelet (see diagram, right) have larger seed than later-opening flowers.
Mitigating fruit-flower feedback inhibition has long been a subject of intensive research in fruit trees. Numerous approaches have been developed to try and improve fruit tree yields, mostly based on plant growth regulators or alternation of sink–source relations (reviewed in Goldschmidt and Sadka, 2021). For instance, fruitlet thinning during the on-crop year using synthetic auxin compounds is well established in many fruit trees. Manipulating hormonal content during flowering induction period and during flowering time and fruit set is also well documented. Girdling, which induces sugar content in the canopy, might well mitigate previous fruit effects on flowering, and pruning is also a common practice. In line with the notion that auxin canalization is the origin of dominance, use of inhibitors of polar auxin transport, such as tri-iodobenzoic acid, has also been shown to mitigate fruit–flower feedback inhibition (Fichtner et al., 2021). However, deeper understanding of the underlying molecular mechanisms will hopefully in the future allow more stable, genetic manipulation of fruit-tree germplasm, in order to overcome the self-limiting effects controlling fruit–flower feedback without labour-intensive practices.
Contributor Information
Avi Sadka, Department of Fruit Tree Sciences, Institute of Plant Sciences, ARO, The Volcani Institute, Rishon Le’Zion 7528809, Israel.
Catriona H Walker, School of Biology, Faculty of Biological Sciences, University of Leeds, Leeds LS2 9JT, UK.
Dor Haim, Department of Fruit Tree Sciences, Institute of Plant Sciences, ARO, The Volcani Institute, Rishon Le’Zion 7528809, Israel; The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot, 76100, Israel.
Tom Bennett, School of Biology, Faculty of Biological Sciences, University of Leeds, Leeds LS2 9JT, UK.
Rainer Melzer, University College Dublin, Ireland.
Author contributions
All authors contributed to the research and writing of the review article. CHW created the figures.
Conflict of interest
The authors declare they have no conflicting interests.
Funding
AS and DH thank the Fund of the Chief Scientist, Ministry of Agriculture and Rural Development, Israel, for supporting our research on alternate bearing in fruit trees. TB and CHW are supported by BBSRC grant BB/X001423/1.
Data availability
There are no primary data associated with this manuscript.
References
- Agustí M, Mesejo C, Muñoz-Fambuena N, Vera-Sirera F, de Lucas M, Martínez-Fuentes A, Reig C, Iglesias DJ, Primo-Millo E, Blázquez MA.. 2020. Fruit-dependent epigenetic regulation of flowering in Citrus. New Phytologist 225, 376–384. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Shinozaki Y, Fau-Ezura H, Ezura H.. 2013. Genes that influence yield in tomato. Breeding Science 63, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S.. 2011. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. The Plant Journal 65, 571–577. [DOI] [PubMed] [Google Scholar]
- Balla J, Medveďová Z, Kalousek P, Matiješčuková N, Friml J, Reinöhl V, Procházka S.. 2016. Auxin flow-mediated competition between axillary buds to restore apical dominance. Scientific Reports 6, 35955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangerth F. 1989. Dominance among fruits/sinks and the search for a correlative signal. Physiologia Plantarum, 76, 60–614. [Google Scholar]
- Bangerth F, Ho LC.. 1984. Fruit position and fruit set sequence in a truss as factors determining final size of tomato fruits. Annals of Botany 53, 315–320. [Google Scholar]
- Bangerth KF. 2009. Floral induction in mature, perennial angiosperm fruit trees: Similarities and discrepancies with annual/biennial plants and the involvement of plant hormones. Scientia Horticulturae 122, 153–163. [Google Scholar]
- Baniel A, Saraf-levy T, Perl-treves R.. 2008. How does the first fruit inhibit younger fruit set in cucurbits? In: Pitrat M, ed. Cucurbitaceae 2008. Proceedings of the IX EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae, 597–602. [Google Scholar]
- Beauchet A, Gevaudant F, Gonzalez N, Chevalier C.. 2021. In search of the still unknown function of FW2.2/CELL NUMBER REGULATOR, a major regulator of fruit size in tomato. Journal of Experimental Botany 72, 5300–5311. [DOI] [PubMed] [Google Scholar]
- Bennett T, Hines G, Leyser O.. 2014. Canalization: what the flux? Trends in Genetics 30, 41–48. [DOI] [PubMed] [Google Scholar]
- Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljung K, Leyser O.. 2016. Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biology 14, e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O.. 2006. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology 16, 553–563. [DOI] [PubMed] [Google Scholar]
- Bertin N. 1995. Competition for assimilates and fruit position affect fruit set in indeterminate greenhouse tomato. Annals of Botany 75, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohner J, Bangerth F.. 1988. Effects of fruit set sequence and defoliation on cell number, cell size and hormone levels of tomato fruits (Lycopersicon esculentum Mill.) within a truss. Plant Growth Regulation 7, 141–155. [Google Scholar]
- Celton J-M, Dheilly E, Guillou M-C, Simonneau F, Juchaux M, Costes E, Laurens F, Renou J-P.. 2014. Additional amphivasal bundles in pedicel pith exacerbate central fruit dominance and induce self-thinning of lateral fruitlets in apple. Plant Physiology 164, 1930–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TG, Zhu XG, Raines C.. 2017. Source-sink interaction: a century old concept under the light of modern molecular systems biology. Journal of Experimental Botany 68, 4417–4431. [DOI] [PubMed] [Google Scholar]
- Cockshull KE, Ho LC.. 1995. Regulation of tomato fruit size by plant density and truss thinning. Journal of Horticultural Science 70, 395–407. [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O.. 2010. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137, 2905–2913. [DOI] [PubMed] [Google Scholar]
- D’Aoust M-A, Yelle S, Nguyen-Quoc B.. 1999. Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. The Plant Cell 11, 2407–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dag A, Bustan A, Avni A, Tzipori I, Lavee S, Riov J.. 2010. Timing of fruit removal affects concurrent vegetative growth and subsequent return bloom and yield in olive (Olea europaea L.). Scientia Horticulturae 123, 469–472. [Google Scholar]
- Davenport T. 1990. Citrus flowering. Horticultural Reviews 12, 349–408. [Google Scholar]
- Dennis FG, Neilsen JC.. 1999. Physiological factors affecting biennial bearing in tree fruit: the role of seeds in apple. HortTechnology 9, 317–322. [Google Scholar]
- Domagalska MA, Leyser O.. 2011. Signal integration in the control of shoot branching. Nature Reviews Molecular Cell Biology 12, 211–221. [DOI] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA.. 2012. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiology 158, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand J-B, Allard A, Guitton B, van de Weg E, Bink MCAM, Costes E.. 2017. Predicting flowering behavior and exploring its genetic determinism in an apple multi-family population based on statistical indices and simplified phenotyping. Frontiers in Plant Science 8, 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand J-B, Guitton B, Peyhardi J, Holtz Y, Guédon Y, Trottier C, Costes E.. 2013. New insights for estimating the genetic value of segregating apple progenies for irregular bearing during the first years of tree production. Journal of Experimental Botany 64, 5099–5113. [DOI] [PubMed] [Google Scholar]
- Eklöf S, Åstot C, Sitbon F, Moritz T, Olsson O, Sandberg G.. 2000. Transgenic tobacco plants co-expressing Agrobacterium iaa and ipt genes have wild-type hormone levels but display both auxin- and cytokinin-overproducing phenotypes. The Plant Journal 23, 279–284. [DOI] [PubMed] [Google Scholar]
- Feng J, Dai C, Luo H, Han Y, Liu Z, Kang C.. 2019. Reporter gene expression reveals precise auxin synthesis sites during fruit and root development in wild strawberry. Journal of Experimental Botany 70, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner EJ, Chao YY, Ferguson L, Verreynne JS, Tang L, Lovatt CJ.. 2021. Repeating cycles of ON and OFF yields in alternate bearing olive, pistachio and citrus trees – different mechanisms, common solutions. Acta Horticulturae 1315, 1–10. [Google Scholar]
- Figueiredo DD, Batista RA, Roszak PJ, Köhler C.. 2015. Auxin production couples endosperm development to fertilization. Nature Plants 1, 15184. [DOI] [PubMed] [Google Scholar]
- Flaishman MA. 2022. Fig morphology and development. In: Flaishman MA, Askoy U, eds. Advances in fig research and sustainable production. Wallingford, UK: CABI Books, 47–58 [Google Scholar]
- Goeckeritz C, Hollender CA.. 2021. There is more to flowering than those DAM genes: the biology behind bloom in rosaceous fruit trees. Current Opinion in Plant Biology 59, 101995. [DOI] [PubMed] [Google Scholar]
- Goetz M, Rabinovich M, Smith HM.. 2021. The role of auxin and sugar signaling in dominance inhibition of inflorescence growth by fruit load. Plant Physiology 187, 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg-Moeller R, Shalom L, Shlizerman L, Samuels S, Zur N, Ophir R, Blumwald E, Sadka A.. 2013. Effects of gibberellin treatment during flowering induction period on global gene expression and the transcription of flowering-control genes in Citrus buds. Plant Science 198, 46–57. [DOI] [PubMed] [Google Scholar]
- Goldschmidt EE. 2013a. The evolution of fruit tree productivity: a review. Economic Botany 67, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt EE. 2013b. Fruit tree domestication: the evolution of productivity. Hortscience 48, S153–S154. [Google Scholar]
- Goldschmidt EE. 2018. An evolutionary platform for alternate bearing in fruit trees. Acta Horticulturae 1229, 1–8. [Google Scholar]
- Goldschmidt EE, Sadka A.. 2021. Yield alternation: horticulture, physiology, molecular biology and evolution. Horticultural Reviews 48, 363–418 [Google Scholar]
- González-Suárez P, Walker CH, Bennett T.. 2020. Bloom and bust: understanding the nature and regulation of the end of flowering. Current Opinion in Plant Biology 57, 24–30. [DOI] [PubMed] [Google Scholar]
- Guitton B, Kelner J-J, Velasco R, Gardiner SE, Chagné D, Costes E.. 2012. Genetic control of biennial bearing in apple. Journal of Experimental Botany 63, 131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Luo X, Li M, Joldersma D, Plunkert M, Liu Z.. 2022. Mechanism of fertilization-induced auxin synthesis in the endosperm for seed and fruit development. Nature Communications 13, 3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Simmons CR.. 2011. Cell number counts – The fw2.2 and CNR genes and implications for controlling plant fruit and organ size. Plant Science 181, 1–7. [DOI] [PubMed] [Google Scholar]
- Haberman A, Bakhshian O, Cerezo-Medina S, Paltiel J, Adler C, Ben-Ari G, Mercado JA, Pliego-Alfaro F, Lavee S, Samach A.. 2017. A possible role for flowering locus T-encoding genes in interpreting environmental and internal cues affecting olive (Olea europaea L.) flower induction. Plant Cell and Environment 40, 1263–1280. [DOI] [PubMed] [Google Scholar]
- Haim D, Shalom L, Simhon Y, Shlizerman L, Kamara I, Morozov M, Albacete A, Rivero RM, Sadka A.. 2021. Alternate bearing in fruit trees: fruit presence induces polar auxin transport in citrus and olive stem and represses IAA release from the bud. Journal of Experimental Botany 72, 2450–2462. [DOI] [PubMed] [Google Scholar]
- Hensel LL, Michelle AN, Richmond TA, Bleecker AB.. 1994. The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiology 106, 863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakopic J, Zupan A, Eler K, Schmitzer V, Stampar F, Veberic R.. 2015. It’s great to be the King: Apple fruit development affected by the position in the cluster. Scientia Horticulturae 194, 18–25. [Google Scholar]
- Kelly D, Sork VL.. 2002. Mast seeding in perennial plants: why, how, where? Annual Review of Ecology and Systematics, 427–447. [Google Scholar]
- Lavee S. 2007. Biennial bearing in olive (Olea europaea). Annales Series Historia Naturalis 17, 101–112. [Google Scholar]
- Lenser T, Graeber K, Cevik OS, et al. 2016. Developmental control and plasticity of fruit and seed dimorphism in Aethionema arabicum. Plant Physiology 172, 1691–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenser T, Tarkowská D, Novák O, Wilhelmsson PKI, Bennett T, Rensing SA, Strnad M, Theißen G.. 2018. When the BRANCHED network bears fruit: how carpic dominance causes fruit dimorphism in Aethionema. Plant Journal 94, 352–371. [DOI] [PubMed] [Google Scholar]
- Maguylo K, Cook NC, Theron KI.. 2014. Primigenic and positional dominance among reproductive buds in shoots of two apple (Malus × domestica (Borkh.)) cultivars in a warmer and cooler site. Trees 28, 625–634. [Google Scholar]
- Martinez-Fuentes A, Marzal A, Reig C, Mesejo C, Agusti M.. 2022. Auxin reduces flowering in Citrus species by modifying flowering gene expressions in leaves and buds. International Citrus Congress, Mersin, Turkiye (abstract S02P10). [Google Scholar]
- McAtee P, Karim S, Schaffer R, David K.. 2013. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Frontiers in Plant Science 4, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merelo P, González-Cuadra I, Ferrándiz C.. 2022. A cellular analysis of meristem activity at the end of flowering points to cytokinin as a major regulator of proliferative arrest in Arabidopsis. Current Biology 32, 749–762.e3. [DOI] [PubMed] [Google Scholar]
- Mesejo C, Marzal A, Martínez-Fuentes A, Reig C, de Lucas M, Iglesias DJ, Primo-Millo E, Blázquez MA, Agustí M.. 2022. Reversion of fruit-dependent inhibition of flowering in Citrus requires sprouting of buds with epigenetically silenced CcMADS19. New Phytologist 233, 526–533. [DOI] [PubMed] [Google Scholar]
- Milyaev A, Tandron-Moya YA, von Wirén N, Neuwald D, Flachowsky H, Wünsche JN.. 2022. What else don’t we know about biennial bearing? Phytohormone profile of seeds and seed number per fruit differ between a biennial and a non-biennial apple cultivar. Acta Horticulturae 1342, 7–14. [Google Scholar]
- Monselise S, Goldschmidt E.. 1982. Alternate bearing in fruit trees. Horticultural Reviews 4, 128–173. [Google Scholar]
- Morris DA. 1977. Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.). Planta 136, 91–96. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fambuena N, Mesejo C, Carmen González-Mas M, Primo-Millo E, Agustí M, Iglesias DJ.. 2011. Fruit regulates seasonal expression of flowering genes in alternate-bearing ‘Moncada’ mandarin. Annals of Botany 108, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt TC, Tanksley SD.. 2001. fw2.2 directly affects the size of developing tomato fruit, with secondary effects on fruit number and photosynthate distribution. Plant Physiology 127, 575–583. [PMC free article] [PubMed] [Google Scholar]
- Nishio S, Moriguchi R, Ikeda H, Takahashi H, Takahashi H, Fujii N, Guilfoyle TJ, Kanahama K, Kanayama Y.. 2010. Expression analysis of the auxin efflux carrier family in tomato fruit development. Planta 232, 755–764. [DOI] [PubMed] [Google Scholar]
- Okuda H. 2000. A comparison of IAA and ABA levels in leaves and roots of two citrus cultivars with different degrees of alternate bearing. Journal of Horticultural Science & Biotechnology 75, 355–359. [Google Scholar]
- Pattison RJ, Catalá C.. 2012. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. The Plant Journal 70, 585–598. [DOI] [PubMed] [Google Scholar]
- Pawar R, Rana VS.. 2019. Manipulation of source-sink relationship in pertinence to better fruit quality and yield in fruit crops: a review. Agricultural Reviews 40, 200–207. [Google Scholar]
- Perez-Alfocea F, Albacete A, Ghanem ME, Dodd IC.. 2010. Hormonal regulation of source-sink relations to maintain crop productivity under salinity: a case study of root-to-shoot signalling in tomato. Functional Plant Biology 37, 592–603. [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O.. 2009. Control of bud activation by an auxin transport switch. Proceedings of the National Academy of Sciences, USA 106, 17431–17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. 1969. Polarity and the induction of organized vascular tissues. Annals of Botany 33, 263–275. [Google Scholar]
- Sachs T. 1981. The control of the patterned differentiation of vascular tissues. In: Woolhouse HW, ed. Advances in Botanical Research, Vol. 9. Academic Press, 151–262. [Google Scholar]
- Sadka A, Haim D, Shlizerman L, Kamara I.. 2022. Involvement of auxin in the control of annual flowering by fruit load. International Citrus Congress, Mersin, Turkiye (abstract S03021). [Google Scholar]
- Schneider D, Goldway M, Rotman N, Adato I, Stern RA.. 2009. Cross-pollination improves ‘Orri’ mandarin fruit yield. Scientia Horticulturae 122, 380–384. [Google Scholar]
- Shabala S, White RG, Djordjevic MA, Ruan YL, Mathesius U.. 2016. Root-to-shoot signalling: integration of diverse molecules, pathways and functions. Functional Plant Biology 43, 87–104. [DOI] [PubMed] [Google Scholar]
- Shalom L, Samuels S, Zur N, Shlizerman L, Zemach H, Weissberg M, Ophir R, Blumwald E, Sadka A.. 2012. Alternate bearing in citrus: changes in the expression of flowering control genes and in global gene expression in ON- versus OFF-crop trees. PLoS One 7, e46930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O.. 2013. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biology 11, e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnaider Y, Mitra D, Miller G, Baniel A, Doniger T, Kuhalskaya A, Scossa F, Fernie AR, Brotman Y, Perl-Treves R.. 2018. Cucumber ovaries inhibited by dominant fruit express a dynamic developmental program, distinct from either senescence-determined or fruit-setting ovaries. The Plant Journal 96, 651–669. [DOI] [PubMed] [Google Scholar]
- Stanic MA-O, Hickerson NMN, Arunraj R, Samuel MA-O.. 2021. Gene-editing of the strigolactone receptor BnD14 confers promising shoot architectural changes in Brassica napus (canola). Plant Biotechnology Journal 19, 639–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutte GW, Martin GC.. 1986. Effect of killing the seed on return bloom of olive. Scientia Horticulturae 29, 107–113. [Google Scholar]
- Tayo TO, Morgan DG.. 1975. Quantitative analysis of the growth, development and distribution of flowers and pods in oil seed rape (Brassica napus L.). The Journal of Agricultural Science 85, 103–110. [Google Scholar]
- Thimann KV, Skoog F.. 1933. Studies on the growth hormone of plants: III. The inhibiting action of the growth substance on bud development. Proceedings of the National Academy of Sciences, USA 19, 714–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rongen M, Bennett T, Ticchiarelli F, Leyser O.. 2019. Connective auxin transport contributes to strigolactone-mediated shoot branching control independent of the transcription factor BRC1. PLoS Genetics 15, e1008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreynne JS, Lovatt CJ.. 2009. The effect of crop load on budbreak influences return bloom in alternate bearing ‘Pixie’ mandarin. Journal of the American Society for Horticultural Science 134, 299–307. [Google Scholar]
- Waldie T, Leyser O.. 2018. Cytokinin targets auxin transport to promote shoot branching. Plant Physiology 177, 803–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CH, Bennett T.. 2018. Forbidden fruit: Dominance relationships and the control of shoot architecture. Annual Plant Reviews 1, 217–254. [Google Scholar]
- Walker CH, Ware A, Šimura J, Ljung K, Wilson ZA, Bennett T.. 2022. Cytokinin signalling regulates two-stage inflorescence arrest in Arabidopsis. Plant Physiology 191, 479–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CH, Wheeldon CD, Bennett T.. 2021. Integrated dominance mechanisms regulate reproductive architecture in Arabidopsis thaliana and Brassica napus. Plant Physiology 186, 1985–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Yang XJ, Li G.. 2021. Molecular insights into inflorescence meristem specification for yield potential in cereal crops. International Journal of Molecular Sciences 22, 3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kumaishi K, Suzuki T, Ichihashi Y, Yamaguchi N, Shirakawa M, Ito T.. 2020. Morphological and physiological framework underlying plant longevity in Arabidopsis thaliana. Frontiers in Plant Science 11, 600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware A, Walker CH, Šimura J, González-Suárez P, Ljung K, Bishopp A, Wilson ZA, Bennett T.. 2020. Auxin export from proximal fruits drives arrest in temporally competent inflorescences. Nature Plants 6, 699–707. [DOI] [PubMed] [Google Scholar]
- Waters MT, Gutjahr C, Bennett T, Nelson DC.. 2017. Strigolactone signaling and evolution. Annual Review of Plant Biology 68, 291–322. [DOI] [PubMed] [Google Scholar]
- Wei J, Yang Q, Ni J, Gao Y, Tang Y, Bai S, Teng Y.. 2022. Early defoliation induces auxin redistribution, promoting paradormancy release in pear buds. Plant Physiology 190, 2739–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie JD, Sedgley M, Olesen T.. 2008. Regulation of floral initiation in horticultural trees. Journal of Experimental Botany 59, 3215–3228. [DOI] [PubMed] [Google Scholar]
- Yu SM, Lo SF, Ho THD.. 2015. Source-sink communication: regulated by hormone, nutrient, and stress cross-signaling. Trends in Plant Science 20, 844–857. [DOI] [PubMed] [Google Scholar]
- Zhang S, Gottschalk C, van Nocker S.. 2019. Genetic mechanisms in the repression of flowering by gibberellins in apple (Malus × domestica Borkh.). BMC Genomics 20, 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv D, Zviran T, Zezak O, Samach A, Irihimovitch V.. 2014. Expression profiling of flowering locus T-Like gene in alternate bearing ‘Hass’ avocado trees suggests a role for PaFT in avocado flower induction. PLoS One 9, e110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwirek M, Waugh R, McKim SM.. 2019. Interaction between row-type genes in barley controls meristem determinacy and reveals novel routes to improved grain. New Phytologist 221, 1950–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no primary data associated with this manuscript.