Abstract
Mechanistic target of rapamycin complex 1 (mTORC1) regulates cell growth and metabolism in response to multiple nutrients, including the essential amino acid leucine1. Recent work in cultured mammalian cells established the Sestrins as leucine-binding proteins that inhibit mTORC1 signaling during leucine deprivation2,3, but their role in the organismal response to dietary leucine remains elusive. Here, we find that Sestrin null flies (Sesn−/−) fail to inhibit mTORC1 or activate autophagy after acute leucine starvation and have impaired development and a shortened lifespan on a low-leucine diet. Knock-in flies expressing a leucine-binding-deficient Sestrin mutant (SesnL431E) have reduced, leucine-insensitive mTORC1 activity. Notably, we find that flies can discriminate between food with or without leucine, and preferentially feed and lay progeny on leucine-containing food. This preference depends on Sestrin and its capacity to bind leucine. Leucine regulates mTORC1 activity in glial cells, and a knockdown of Sesn in these cells reduces the ability of flies to detect leucine-free food. Thus, nutrient sensing by mTORC1 is necessary for flies not only to adapt to, but also to detect, a diet deficient in an essential nutrient.
Introduction
The protein kinase mTORC1 regulates growth and metabolism in response to diverse signals, including growth factors and nutrients like amino acids1. Amino acids activate mTORC1 by promoting its translocation to the lysosomal surface, where its essential activator Rheb resides4–6. The heterodimeric Rag GTPases, which are under the control of several multi-component protein complexes, including GATOR1 and GATOR27, regulate the lysosomal localization of mTORC14,5. GATOR1 is a GTPase activating protein for RagA and RagB and is necessary for amino acid deprivation to inhibit mTORC1 signaling8,9. By contrast, GATOR2 is required for amino acids to activate mTORC1 and directly interacts with several of the amino acid sensors so far discovered, indicating that it acts as a nutrient-sensing hub despite its still unknown biochemical function7.
Amongst the proteogenic amino acids, leucine is the best-established activator of mTORC110–13. Work in cultured mammalian cells has shown that leucine controls mTORC1 by regulating the interaction of GATOR2 with the Sestrin family of proteins3,14,15, which are repressors of mTORC1 signaling16,17. Human Sestrin1 and Sestrin2 bind leucine at affinities consistent with the leucine concentration needed to activate mTORC1 and are required for leucine deprivation to inhibit mTORC1 signaling3. Moreover, a Sestrin2 mutant that does not bind leucine fails to dissociate from GATOR2 in the presence of leucine, and in cells expressing this mutant, mTORC1 activity remains low even when the cells are cultured in leucine-replete conditions2,3. Despite the evidence that Sestrin is a leucine sensor for the mTORC1 pathway in cultured mammalian cells, the roles of Sestrin-mediated leucine sensing in the physiology of an intact organism remain largely unexplored.
Although much of the work on leucine sensing has been in mammalian systems, Sestrin and the core nutrient-sensing machinery, including the Rag GTPases, GATOR1, and GATOR2, are conserved in most invertebrates, including the fly Drosophila melanogaster18. Unlike in mammals, flies express only one gene for Sestrin (Sesn)16, greatly facilitating the in vivo study of leucine sensing by mTORC1. Here, we show that Sestrin and its leucine-binding pocket are required for leucine to regulate mTORC1 activity in fly tissues in vivo and for flies to detect and adapt to leucine-deficient diets.
Results
Fly mTORC1 senses leucine in vivo via Sestrin
In an equilibrium binding assay, Drosophila Sestrin bound leucine with a dissociation constant (Kd) of about 100 μM (Fig. 1a), an affinity several fold lower than those of human Sestrin1 and Sestrin2 (Kd values of about 15–20 μM)3. This reduced affinity is probably the result of a difference between the leucine-binding pockets of human and fly Sestrin. Structural studies show that in human Sestrin2 a tryptophan (W444) forms the floor of the pocket, but in the fly protein, the analogous residue is a leucine (L431), a smaller residue that when introduced into human Sestrin2 (W444L) is sufficient to reduce its leucine-binding capacity by several fold2. The low leucine affinity of fly Sestrin is consistent with the observation that fly hemolymph has substantially higher amino acid concentrations than human plasma18,19, a difference probably reflected intracellularly. Like the analogous mutant of human Sestrin2 (W444E), fly Sestrin L431E does not bind leucine (Fig. 1b).
Fig. 1: Drosophila Sestrin binds GATOR2 and regulates mTORC1 in vivo in response to dietary leucine.

a, Data from an equilibrium binding assay showing that purified FLAG-Sestrin bound leucine, Kd~100 μM. Values are mean ± SD of three technical replicates from a representative experiment.
b, The L431E mutation blocks leucine binding by Drosophila Sestrin. HA-tagged wild-type Sestrin and Sestrin(L431E) were prepared from HEK293T cells expressing the appropriate cDNAs. The binding assays were performed as in (a). Values are mean ± SD of three technical replicates from a representative experiment. The P values were determined using an unpaired t test with Welch correction, and the Holm-Šídák multiple comparison method.
c, Leucine starvation enhances the Sestrin-GATOR2 interaction. FLAG-immunoprecipitates (IPs) were prepared from S2R+ cells stably expressing FLAG-tagged und (negative control) or WDR59 (a GATOR2 component) and starved or not of leucine. IPs and lysates were analyzed by immunoblotting for indicated proteins. Addition to the IPs of 1 mM of leucine, but not other amino acids, disrupted the Sestrin-GATOR2 interaction.
d, Dietary leucine regulates in vivo the interaction of Sestrin with GATOR2 depending on the leucine-binding site of Sestrin. Immunoprecipitates were prepared from lysates of fat bodies from Wild-type (OreR) or SesnL431E larvae expressing the MYC-tagged control protein GFP or the MYC-tagged GATOR2 component WDR24 in the fat body (lpp-gal4). Animals were fed the indicated diets for 4.5 hours before sample collection. Amino-acid-replete: chemically defined diet containing all amino acids; leucine-free or valine-free: chemically defined diet lacking leucine or valine, respectively.
e, Sestrin binding to leucine regulates mTORC1 signaling in vivo. Shown are immunoblots of Sestrin, S6K and phospho-S6K in fat bodies prepared as in (d) from larvae with indicated genotypes. Nprl2 and Mio encode core components of the GATOR1 and GATOR2 complexes, respectively. Dietary composition and feeding period were as in (d).
Data are representative of three (a, b) or two (c-e) independent experiments with similar results.
To examine whether leucine regulates the interaction of fly Sestrin with GATOR2, we stably expressed in Drosophila S2R+ cells a Flag-tagged control protein (und, the Drosophila ortholog of mammalian metap2, methionyl aminopeptidase) or WDR59, one of the five core components of the GATOR2 complex. Sestrin co-immunoprecipitated with GATOR2, but not und, and removal of leucine from the cell medium strongly enhanced the interaction. The addition of leucine, but not isoleucine, valine, or methionine, to the immunoprecipitates was sufficient to release Sestrin from GATOR2 (Fig. 1c). Thus, like the human protein, fly Sestrin binds to GATOR2 in a fashion that is specifically disrupted by leucine.
To extend our work in vivo, we generated flies that ectopically express MYC-tagged WDR24, another core component of GATOR2 (lpp>myc-WDR24 flies), in the fat body, and are either wild type at the Sesn locus or have a knock-in mutation causing the L431E substitution that renders Sestrin unable to bind leucine (SesnL431E). For a period of 4.5 h, we fed third instar larvae a chemically defined diet (see Methods and Extended Data Table 1–4 for details) containing all proteogenic amino acids (amino acid replete) or the same diet lacking just leucine (leucine free) or valine (valine free). Regardless of genotype, larvae eating the leucine- or valine-fee diets had reduced levels of leucine or valine, respectively (Extended Data Fig. 1a, b). In lysates prepared from isolated fat bodies, endogenous Sestrin co-immunoprecipitated with GATOR2, but not a control protein (GFP-MYC), and deprivation of leucine, but not valine, strongly boosted the interaction. In contrast, Sestrin L431E bound equally well to GATOR2 under all dietary conditions, consistent with the mutant being leucine insensitive (Fig. 1d). In cultured cells and in fat bodies, we observed that Sestrin has multiple isoforms (Fig. 1c, d), probably the result of differential splicing16.
In wild-type larvae, feeding of the diet free in leucine, but not valine, inhibited mTORC1 in the fat body, as assessed by the phosphorylation of S6K, a canonical mTORC1 substrate. The loss of Sestrin (Sesn−/−) did not impact mTORC1 activity in larvae eating the amino-acid-replete diet, but completely prevented the inhibition of mTORC1 normally caused by leucine deprivation (Fig. 1e). Sestrin was also required for the leucine-free diet to activate autophagy, a process suppressed by mTORC1, as monitored by the formation of mCherry-Atg8a-positive puncta (Extended Data Fig. 1c). In SesnL431E larvae, mTORC1 activity was low relative to that in wild-type larvae and also unaffected by leucine deprivation, indicating that the leucine-binding mutant of Sestrin acts as a non-repressible inhibitor of mTORC1 (Fig. 1e). Notably, mTORC1 signaling was inhibited in Sesn−/− larvae deprived of all food to a similar extent as wild-type larvae (Extended Data Fig. 1d), which is consistent with work in cultured mammalian cells showing that Sestrin has a specific role in transmitting leucine availability to mTORC13,14. Last, in larvae lacking a component of GATOR1 (Nprl2−/−) or GATOR2 (Mio−/−), the absence of dietary leucine did not impact mTORC1 activity and it remained as hyperactive or suppressed, respectively, as when the larvae were fed the amino-acid-replete diet (Fig. 1e). Consistent with mTORC1 promoting Sesn transcription as part of a feedback loop16,20, Nprl2−/− and Mio−/− flies had increased and decreased Sestrin levels, respectively (Fig. 1e). Collectively, these results show that dietary leucine modulates mTORC1 in vivo and that this regulation requires Sestrin and its leucine-binding pocket as well as the GATOR1 and GATOR2 complexes.
Sestrin mediates adaption to low-leucine diets
We reasoned that Sestrin-mediated suppression of mTORC1 helps animals adapt to and thus survive a diet low in leucine. We first tried to test this idea by feeding larvae food lacking leucine, but all larvae, independently of genotype, died within 2–3 days of starting the diet, consistent with leucine being an essential amino acid required for larval growth. When given food containing one-tenth of the normal leucine content, ~40% of wild-type larvae survived over a period of 16 days (Fig. 2a, b). In contrast, only ~10% of Sesn−/− larvae did so (Fig. 2b). Moreover, the surviving larvae grew to a much smaller size than their wild-type counterparts (Fig. 2c), a defect rescued by the expression of wild-type Sestrin from the ubiquitous Tubulin-Gal4, Tubulin-Gal80ts promoter (Fig. 2c). When fed the standard laboratory diet, Sesn−/− and wild-type larvae developed indistinguishably (Extended Data Fig. 2a).
Fig. 2: Drosophila require Sestrin to adapt to a low-leucine diet.

a, b, Loss of Sestrin reduces survival during development upon leucine starvation. The bar charts show survival (%) of larvae raised for 10 days on a chemically defined diet containing 10% of the leucine in the control diet. The P values were determined using two-proportion z-test (two-sided). The bars show the percentage of surviving larvae in each genotype and the error bars represent the 95% Wald confidence interval.
c, Sestrin is required for larval growth on a low-leucine diet. Shown are age-synchronized animals of the indicated genotypes raised 9 days on either an amino-acid-replete diet or a reduced (10%)-leucine diet. Scale bar, 1 mm.
d-i, Loss of Sestrin reduces survival of adult flies upon leucine starvation. Sesn−/− animals show reduced lifespan when fed a diet lacking leucine (0% leucine). Survival curves of age-synchronized adult male and female animals of the indicated genotypes fed the indicated diets. (c) nWT(w1118)=157; nSesn−/−=217; (d) nWT(w1118)=221; nSesn−/−=225; (e) nWT(w1118)=206; nSesn−/−=203; (f) nWT(w1118)=205; nSesn−/−=226; (g) nWT(w1118)=222; nSesn−/−=230; (h) nWT(w1118)=221; nSesn−/−=228. See statistics in Supplementary Data 1 and Methods.
(a-i), Data are representative of three independent experiments with similar results.
Consistent with previous work showing that adult flies can live for weeks on a diet lacking any amino acid source21, our observations showed that wild-type flies also survived for many weeks on a leucine-free diet (Fig. 2e, h, Extended Data Fig. 2c, f, and Supplementary Data 1). As with larvae, adult flies also required Sestrin to adapt to leucine scarcity, as Sesn−/− male and female animals had greatly shortened lifespans on the leucine-free, but not amino-acid-replete, diet (Fig. 2d, e, g, h, and Supplementary Data 1). On the other hand, Sesn−/− flies had slightly shorter lifespans than wild-type counterparts when eating the valine-free food (Fig. 2f, i, and Supplementary Data 1), a diet on which the activity of processes controlled by mTORC1, such as protein synthesis and autophagy, would be expected to impact survival. When the SesnL431E flies were fed the same chemically defined diets, they survived similarly to the wild-type flies (Extended Data Fig. 2b–g, and Supplementary Data 1). Consistent with the chronic suppression of mTORC1 signaling, SesnL431E larvae reared on the standard laboratory diet developed more slowly than wild-type ones (Extended Data Fig. 2h).
We monitored mTORC1 activity in whole-fly lysates of female and male adult flies that had been fasted overnight and then refed for 90 min with the chemically defined diets used above. The loss of Sestrin prevented the inhibition of mTORC1 caused by the leucine-free diet in male and female flies (Extended Data Fig. 3a, b).
We further focused on oogenesis, a physiological trait that is known to be regulated by diet22. Moreover, diet is known to regulate ovarian function through the GATOR1-GATOR2 complexes21,23–25, and Mio, the gene for one of the components of GATOR2, was so named because mutations in it result in a missing oocyte phenotype26. We found that mTORC1 activity was strongly increased in the ovaries of Sesn−/− flies eating the standard laboratory diet, and as in larval fat bodies (Fig. 1e), it was suppressed in the ovaries of SesnL431E flies (Extended Data Fig. 3c).
When fed the amino-acid-replete or valine-free diet, Sesn−/− and wild-type flies had ovaries of similar sizes, but the loss of Sestrin greatly reduced ovarian size in flies under conditions of acute leucine deprivation (Extended Data Fig. 3d, e), again pointing to a specific role for Sestrin in adapting to leucine scarcity. The ovaries of the SesnL431E flies were equally small on all of the diets (Extended Data Fig. 3d, e), consistent with a role for mTORC1 in the control of gonad development. SesnL431E flies also had reduced fecundity as they laid fewer eggs than wild-type flies (Extended Data Fig. 3f). Eggs from wild-type, SesnL431E, and Sesn−/− flies had comparable hatching rates, suggesting that Sestrin does not impact fertility (Extended Data Fig. 3g). Collectively, these data reveal that in larvae and adult flies Sestrin promotes survival on a low-leucine diet and has a particularly important role in controlling ovarian size and function.
Sestrin regulates feeding behavior
Having established that Sestrin is important for flies to adapt to and survive on diets low in leucine, we examined whether flies also require Sestrin to detect and thus avoid food that is poor in leucine. To do so, we developed an assay to test whether adult flies prefer eating leucine-rich over leucine-poor food. The experimental set-up consisted of 15 female and 5 male flies in a bottle containing 2 apple pieces, the first painted with a solution of one or more amino acids and the second with an appropriate control (Fig. 3a). Each also contained a trace amount of a unique DNA oligonucleotide, which served as a barcode for measuring the food consumption, an approach previously described27 and that we validated (Extended Data Fig. 4a–c and Methods). We chose apple as the base food because it is carbohydrate rich and protein poor28, allowing us to set up food choices that have different amino acid compositions but the same content of sugars. Apples are reported to contain very little leucine and valine29–31.
Fig. 3: Flies prefer to eat leucine-containing food in a fashion that depends on the capacity of Sestrin to bind leucine.

a, A schematic of the two-choice food preference assay (see Methods for details). AA, amino acids.
b, Wild-type female animals develop a preference for leucine over the course of several hours. Data show the fold-difference in relative food intake for the leucine-coated compared to water-coated apples. n≥11 per time point.
c, Rapamycin prevents flies from developing a preference for the leucine-coated apple. n≥5 per condition.
d-f, SesnL431E and Sesn−/− animals fail to develop a preference for the leucine-containing apple. (d, e), n≥4 per condition; (f) n≥6 per condition.
g, Immunoblotting for Sestrin following knockdown of Sesn in adult flies. Akt serves as a loading control.
h, Ubiquitous knockdown of Sesn reduces the preference of adult female flies for leucine. Data show the fold-difference in food intake for the leucine-coated apple relative to the water-coated apple. n≥5 per condition.
i, The approach used to achieve temporal control of Sesn knockdown in (j, k).
j, Sesn immunoblot showing Gal80ts-mediated depletion of Sestrin in adult, but not developing, animals. Extracts were prepared from flies raised at indicated temperatures. S6K serves as a loading control. Note that heat shock induces Sestrin protein levels in control flies.
k, Knockdown of Sestrin during adulthood is sufficient to decrease the preference of female flies for leucine-containing apples. n≥13 per condition.
(a, i) created with BioRender.com. (b, c, d-f, h, k), Values are mean ± SD of biological replicates from a representative experiment. Each experiment was repeated three (d-k) or two (b, c) times with similar results. Statistical analyses: one-way ANOVA followed by Dunnett’s multiple comparisons test (b), two-way ANOVA followed by Šídák’s multiple comparisons test (c-e), one-way ANOVA followed by Šídák’s multiple comparisons test (f), and two-tailed unpaired t-test (h, k).
We found that wild-type female flies prefer to eat apples coated with leucine rather than water. This preference emerges after the flies have been eating the food for about 6 h and increases to 5 to 6-fold by 24 h, the time point we used in subsequent experiments (Fig. 3b). The preference for leucine is concentration dependent (Extended Data Fig. 4d) and not every amino acid elicits a preference, as flies do not distinguish between apples coated with valine or water (Extended Data Fig. 4e). Given a choice between equal amounts of leucine and valine, flies still prefer leucine, suggesting that the leucine preference is not simply the result of a nitrogen imbalance (Extended Data Fig. 4e). Moreover, the leucine preference requires differential mTORC1 activity, as when flies were fed the mTORC1 inhibitor rapamycin, they no longer showed a preference (Fig. 3c). Rapamycin treatment also lowered the total amount of food consumed by the flies (Extended Data Fig. 4f), consistent with previous reports32,33.
Remarkably, neither Sesn−/− nor SesnL431E females—both of which have leucine-insensitive mTORC1 signaling—had a preference for leucine as they ate similar amounts of leucine-rich and -poor foods (Fig. 3d, e and Extended Data Fig. 4g). However, the two Sesn mutants probably differ in the total amount of food each ate. The amount of food (leucine-rich or leucine-poor) that Sesn−/− females ate was similar to the amount of leucine-rich food consumed by wild-type (w1118) flies (Extended Data Fig. 4h). The opposite was true for SesnL431E female flies. These flies ate an amount of food (leucine-rich or leucine-poor) similar to the amount of leucine-poor food consumed by the wild-type (OreR) flies (Extended Data Fig. 4i). That SesnL431E files, which have low mTORC1 signaling, eat less food than wild-type controls is consistent with rapamycin causing a reduction in food consumption (Extended Data Fig. 4f). Whole-body re-expression in the Sesn−/− female flies of Sestrin driven by Tub>Gal4 partially restored the leucine preference of the animals (Extended Data Fig. 4j).
We also examined whether flies can distinguish between foods with a more subtle difference in amino acid composition: an apple coated with the 20 proteogenic amino acids versus just 19 of them (that is, lacking only leucine). Indeed, this was the case and this preference was also absent in the Sesn−/− and SesnL431E flies (Fig. 3f). Valine again served as a control: when removed from the 20-amino-acid cocktail, neither wild-type nor Sesn mutant flies showed preference for the valine-containing food (Extended Data Fig. 4k).
To obtain temporal control of Sestrin suppression, we generated a conditional knockdown system using a short hairpin RNA (shRNA) targeting Sesn. Ubiquitous expression of the shRNA reduced Sestrin protein levels (Fig. 3g), and as expected, the preference of the flies for the leucine-containing food (Fig. 3h). Using a temperature-sensitive shRNA driver, we suppressed Sestrin specifically during adulthood (Fig. 3i, j). This too reduced their leucine preference (Fig. 3k), indicating that the acute loss of Sestrin in adult flies is sufficient to impact the leucine preference. Notably, the temperature shift to 29°C increased Sestrin levels (Fig. 3j), consistent with previous work showing that multiple stresses induce its transcription17,34. Thus, female flies can readily detect food lacking leucine even if it contains sugars and other amino acids. This ability requires Sestrin and its capacity to bind leucine.
To further analyze the physiological relevance of leucine sensing via the Sestrin-mTORC1 axis, we tested the impact of both leucine and Sestrin on the choice between low- and high-protein diets: apple coated with a low or high amount of yeast extract, which is a complex type of food and the major protein source for laboratory-raised flies. Wild-type flies had a strong preference for the apple with a higher protein content. The addition of leucine to the protein-poor food reduced the preference of wild-type female flies for the protein-rich food, but only minimally impacted the preference of the SesnL431E mutants (Extended Data Fig. 5a). Sesn−/− mutants had a similar trend (Extended Data Fig. 5b), but it was not statistically significant. Together, these data suggest that flies use leucine sensing through the Sestrin-mTORC1 axis as a proxy for the food protein content.
Sestrin regulates egg-laying behavior
We found that female flies prefer to lay eggs on the leucine-coated apples. To explore this further, we put 15 female and 5 male flies in the assay bottle and 24 h later counted the number of eggs on each piece of apple (Extended Data Fig. 6a). In an initial test, we found that flies laid many more eggs on an apple piece painted with a yeast suspension instead of water, consistent with yeast being a food rich in nutrients and the olfactory cues that attract flies35–38 (Extended Data Fig. 6b).
Wild-type flies that had been deprived of protein overnight deposited 5 to 6-fold more eggs on an apple piece coated with the 20 proteogenic amino acids instead of water (Extended Data Fig. 6c, d, f). Flies had a similar, albeit smaller (threefold), preference for leucine-coated apple, and this preference was more profound when the flies had been starved for protein. Importantly, flies did not distinguish between apple pieces painted with the same substance (Extended Data Fig. 6d, f).
We found that SesnL431E mutant flies lacked a strong preference for laying eggs on the apple coated with leucine and had a reduced preference for the apple with the 20 amino acids (Extended Data Fig. 6e), although the total number of eggs SesnL431E mutant flies laid was about 25% reduced compared to that for the wild-type flies (Extended Data Fig. 3f). This altered egg-laying behavior was also observed in the Sesn−/− flies, which laid similar number of eggs as the wild-type animals (Extended Data Fig. 6g). Furthermore, the wild-type flies mildly preferred to deposit eggs on an apple piece painted with the 20 proteogenic amino acids instead of 19 (that is, lacking leucine), a much more complex choice, and this ability was reduced in the SesnL431E flies (Extended Data Fig. 6h). When facing the same complex choice, Sesn−/− flies did not show a statistically significant different behavior compared to the wild-type flies (Extended Data Fig. 6h), which might reflect the subtleness and noise of this complex choice set-up. Consistent with the leucine preference we observed in the food choice assay, we found that female flies also laid fewer eggs on food lacking leucine, and this capacity requires the intact leucine-binding pocket of Sestrin. This finding might reflect an active choice for egg deposition or the amount of time that flies spend on each apple owing to their preference for eating leucine-containing food.
Glial Sestrin regulates leucine preference
To determine in which tissue(s) Sestrin is required for flies to distinguish between food with or without leucine, we suppressed Sestrin with the Sesn shRNA under the control of a variety of cell-type-specific Gal4 drivers. Notably, Sesn knockdown specifically in glial cells (repo-Gal4) was sufficient to reduce the preference of flies for the leucine-containing food to a similar extent as when it was expressed ubiquitously (da-Gal4; Fig. 4a). In contrast, Sesn knockdown in many other tissues, including the fat body and muscle, did not impact the leucine preference. It is important to note that the intrinsic capacity of each Gal4 driver line to distinguish between food with or without leucine varied considerably (Extended Data Fig. 7a), probably owing to their different genetic backgrounds. Thus, although we are confident that the preference of flies for leucine-containing food requires Sestrin in glial cells, we are cautious in ruling out contributions from other tissues, particularly those examined with driver lines with intrinsically lower leucine preferences, such as the pan (elav-Gal4) and dopaminergic and cholinergic (ddc-Gal4) neuronal lines (Extended Data Fig. 7a).
Fig. 4: Sestrin-regulated mTORC1 signaling in glial cells controls the preference of flies for leucine-containing food.
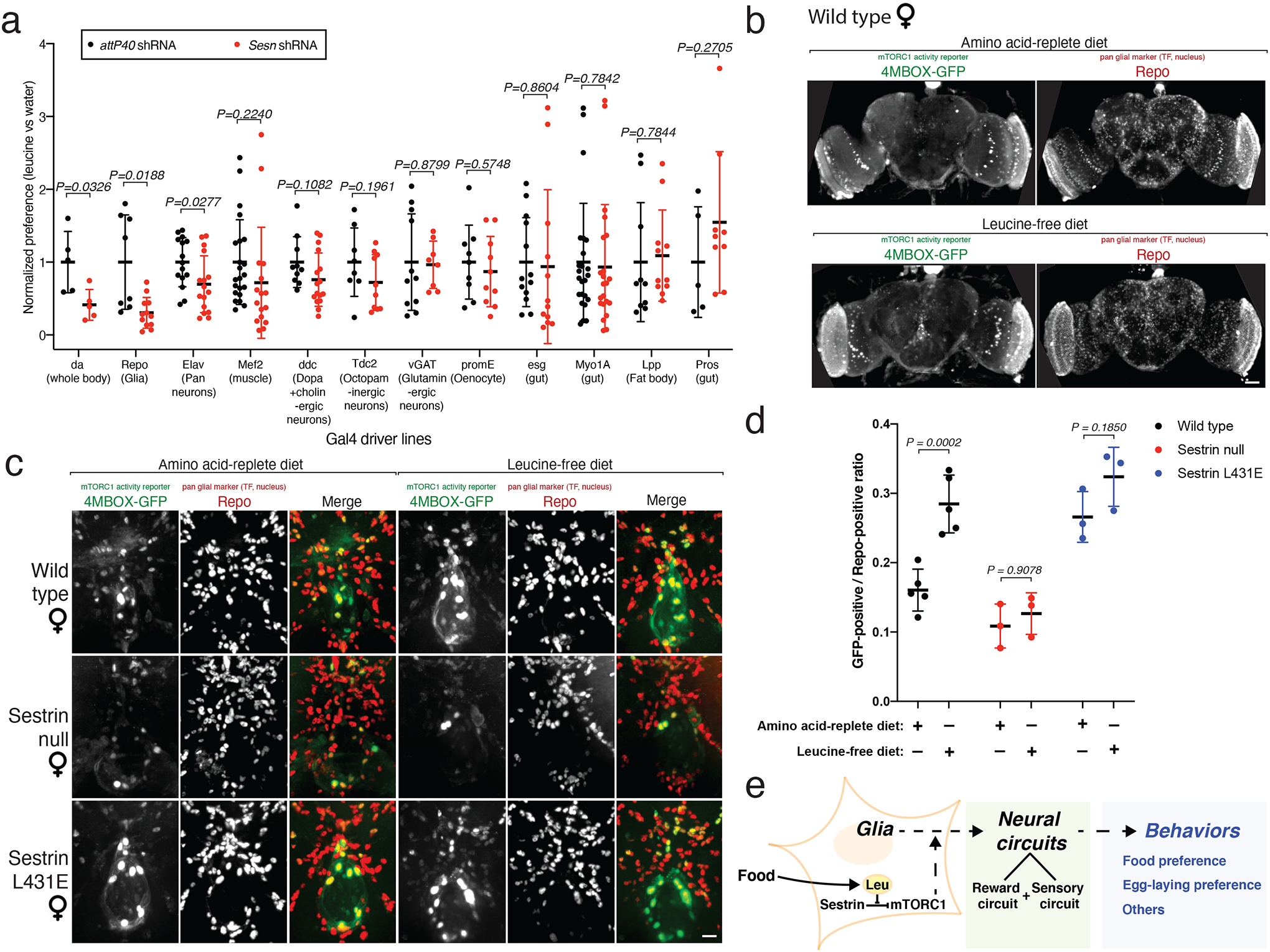
a, A genetic screen identifies a role for Sesn in glial cells in mediating the leucine preference. Sesn RNA-mediated interference was performed in various tissues with the indicated Gal4 lines. Knockdown of Sesn in glial cells (Repo-Gal4) and ubiquitously (da-Gal4), but not in other tissues, reduces the preference for the leucine-coated versus water-coated apple. For each Gal4 line, data are normalized to the leucine preference of control flies. See non-normalized data in Extended Data Figure 7a. n≥5 per condition.
b, c, Confocal projection of brains of adult female flies of the indicated genotypes expressing 4MBOX-GFP, a reporter for the MITF transcription factor that is negatively regulated by mTORC1. Animals were fed the indicated diets for 1 day and brains were stained for GFP and Repo. Images in (b) and (c) were taken with 10X and 40x objectives, respectively. Scale bars in (b) and (c) represent 50 μm and 10 μm, respectively.
d, In wild-type flies, but not SesnL431E or Sesn−/−flies, leucine starvation increases the number of GFP-positive peri-oesophageal glial cells. Each point represents the ratio of the number of GFP- to Repo-positive cells in the oesophageal area of one fly brain. n≥3 per condition.
e, Proposed role of the Sestrin-mTORC1 pathway in regulating the preference of flies for leucine-containing food.
(a, d), Values are mean ± SD of technical replicates from a representative experiment. Data are representative of three independent experiments with similar results. Statistical analysis was performed using two-tailed unpaired t test (a), and two-way ANOVA followed by Šídák’s multiple comparisons test (d).
Consistent with an important role for glial Sestrin in regulating the leucine preference, expression of wild-type Sestrin just in glial cells in Sestrin-null flies partially rescued the defect in detecting leucine-poor food (Extended Data Fig. 7b). In wild-type flies, expression in the glial cells of either wild-type Sestrin or Sestrin(L431E) decreased the leucine preference, consistent with the inhibition of mTORC1 caused by Sestrin overexpression (Extended Data Fig. 7b). Indeed, overexpression under the control of repo-Gal4 of TSC1 and TSC2—well established inhibitors of mTORC1 signaling—was also sufficient to decrease the leucine preference (Extended Data Fig. 7c).
Analyses of a single-cell RNA-sequencing dataset indicated that Sestrin is expressed in most glial subtypes39 (Extended Data Fig. 7d). Expression of the Sesn shRNA under the control of Gal4 driver lines that target subtypes of glial cells revealed that none caused as strong a suppression of the leucine preference as with the pan-glial driver repo-Gal4 (Extended Data Fig. 7e), although Wrapper-Gal4-driven Sesn knockdown led to a partial reduction of the leucine preference. Thus, multiple glial subtypes probably participate in mediating the leucine preference.
Given the importance of glial Sestrin in mediating the leucine preference, we examined mTORC1 signaling in glial cells in the brains of adult female flies. To do so, we used a line expressing a GFP-based reporter for the MITF transcription factor40, which is the Drosophila ortholog of mammalian TFEB41. mTORC1 suppresses MITF so that upon mTORC1 inhibition, MITF activity increases41 and drives GFP expression. In wild-type flies, starvation for total protein activated, as indicated by elevated GFP expression, MITF in Repo-positive glial cells, particularly in those surrounding the oesophagus (Extended Data Fig. 7f). Remarkably, starvation for just leucine also increased the number of peri-oesophageal GFP-positive glial cells (Fig. 4b–d and Extended Data Fig. 8a, b). In contrast, in Sesn−/− flies, leucine starvation did not increase the number of peri-oesophageal GFP-positive glial cells, which were few in number irrespective of the diet (Fig. 4c, d and Extended Data Fig. 8a, b). In SesnL431E flies, there were many peri-oesophageal GFP-positive glial cells, and, like in Sesn−/− flies, leucine starvation did not increase their numbers (Fig. 4c, d and Extended Data Fig. 8a, b). Notably, quantification of GFP-positive cells in the mushroom body and optic lobe areas showed that, unlike in peri-oesophageal glial cells, the mTORC1 activity in these cells did not significantly respond to acute dietary treatments (Extended Data Fig. 8b–e). Thus, dietary leucine regulates mTORC1 signaling in a subset of glial cells in a fashion that depends on Sestrin and its capacity to bind leucine, and this regulation correlates with the ability of flies to distinguish between food that is rich or poor in leucine.
Discussion
We show that Drosophila melanogaster requires Sestrin to regulate mTORC1 signaling in response to dietary leucine, survive a leucine-poor diet, and control leucine-sensitive physiological measures such as food choice and ovarian size. Flies with a point mutation that eliminates the leucine-binding capacity of Sestrin(L431E) have suppressed, leucine-insensitive mTORC1 signaling. Moreover, whereas wild-type flies can live on leucine-free diets for weeks, flies lacking Sestrin die much faster. In all, our results establish Sestrin as a physiologically relevant leucine sensor in vivo. Recently, Lu et al. reported complementary findings of an amino acid-sensing role of Sestrin upstream of mTORC1 in the control of Drosophila development, fecundity and longevity42.
We find that Sestrin and its leucine-binding pocket are required for the preference of adult female flies for consuming, as well as laying eggs on, leucine-rich instead of leucine-poor food even when it contains sugars and other amino acids. To our knowledge, the ability of flies to choose food that is rich in leucine over food that lacks leucine but still retains a complex set of other nutrients has not been previously documented, although such behavior has been reported in mice43. When given a starker choice than we provided—a pure sugar, such as sucrose or glucose, versus an individual amino acid—flies prefer to eat a variety of essential amino acids in sex- and developmental stage-dependent fashions44–46.
There has been a long-standing interest in understanding the mechanisms that enable animals, including flies and rodents43,47, to prefer diets rich in protein. A variety of mechanisms in flies have been implicated, including amino acid transporters44, taste receptors45,48,49, GCN250, serotonin51 and dopamine signaling50,52, sex peptide receptor53, microbiome54, as well as mTOR and S6K51,53. How these mechanisms coordinate together to impact organismal protein detection in the diet remains unclear.
Our work raises several questions for future study. One such question concerns whether there is crosstalk between the food preference behavior controlled by glial cells and acute changes in ovarian size caused by nutritional stress. Another question is whether female flies actively choose to lay more eggs on the leucine-containing food because it has the nutrients needed for larval growth, or whether the apparent preference simply reflects the amount of time they spend on it owing to their dietary preference. As it takes flies many hours to distinguish between leucine-containing and leucine-free food (Fig. 3b), it seems unlikely that the alterations in Sestrin eliminate the preference for leucine by substantially interfering with the capacity of flies to taste leucine. Rather, we favor the idea that leucine, through Sestrin-mTORC1, turns on a neuronal reward circuit that drives food consumption (see potential model in Fig. 4e). Previous work has identified a set of dopaminergic neurons that controls protein hunger51 and it will be interesting to examine whether Sestrin-mediated leucine-sensitive mTORC1 signaling can impact these cells. In this regard, it is intriguing that the preference for leucine requires the expression of Sestrin in glia as there is increasing evidence that glial cells can be key intermediates between an environmental signal and its modulation of a neuronal circuit55–57. Last, it will be interesting to investigate why mTORC1 activity in a set of peri-oesophageal glial cells is particularly sensitive to Sestrin-dependent regulation by dietary leucine.
Materials and Methods
Materials
Reagents were obtained from the following sources: HRP-labeled anti-rabbit secondary antibody and the antibodies against Drosophila Phospho-70 S6 Kinase (Thr398) (#9209), Akt (#9272), phosphor-ERK (#9101), ERK (#4695), Akt (#9272), MYC (#2278), and the FLAG (#2368) epitope from Cell Signaling Technology (CST); Anti-Green Fluorescent protein (GFP) antibody from Aves Labs (GFP-1020); 8D12 Anti-Repo antibody from Developmental Studies Hybridoma Bank (DSHB); Alexa 488-, 568-, and 647-conjugated secondary antibodies and Complete Protease Cocktail from Roche; Schneider’s medium and Inactivated Fetal Bovine Serum (IFS) from Invitrogen; amino-acid-free Schneider’s medium from US Biologicals; [3H]-leucine from American Radiolabeled Chemicals, Inc.; leucine from Sigma (L8912); rapamycin from LC Laboratories (#R-5000); and Reliance One-Step Multiplex RT-qPCR Supermix from BIO-RAD. Fresh apples (Gala) were from Star Market. The dS6K antibody was a gift from Mary Stewart (North Dakota State University) and the Drosophila Sestrin antibody one from Jun Hee Lee (University of Michigan).
Methods
Tissue culture
Drosophila S2R+ cells were cultured in Schneider’s medium with 10% inactivated fetal bovine serum at 25°C and 5% CO2. The S2R+ cell line was obtained from the Drosophila RNAi Screening Center/Transgenic RNAi Project Functional Genomics Resources and Drosophila Research & Screening Center-Biomedical Technology Research Resource at Harvard Medical School. It has been molecularly validated by DNA and RNA sequencing (see Table 2 of a recent authentication58).
Suspension FreeStyle 293F cells were obtained from Thermal Fisher Scientific and cultured in FreeStyle 293 expression medium (ThermoFisher (12338018)), supplemented with 100 IU/ml penicillin and 100μg/ml Streptomycin, at a shaking speed of 125 rpm at 37°C and 8% CO2, 80% humidity. No mycoplasma contamination was detected using PCR.
Lysis of cells, tissues, and flies and Immunoprecipitations
Cells were rinsed with cold PBS and lysed in lysis buffer (1% Triton, 10 mM β-glycerol phosphate, 10 mM pyrophosphate, 40 mM HEPES pH 7.4, 2.5 mM MgCl2 and 1 tablet of EDTA-free protease inhibitor [Roche] (per 25 ml buffer)). Cell lysates were cleared by centrifugation in a microcentrifuge (15,000 rpm for 10 minutes at 4°C). Cell lysate samples were prepared by the addition of 5X sample buffer (0.242 M Tris, 10% SDS, 25% glycerol, 0.5 M DTT, and bromophenol blue), resolved by 8%−12% SDS-PAGE, and analyzed by immunoblotting.
Dissected tissues and whole flies were crushed physically utilizing a bead beater in 1% Triton lysis buffer (same as above). The resulting lysates were cleared by centrifugation in a microcentrifuge (15,000 rpm for 10 minutes at 4°C) and analyzed as above. For anti-FLAG immunoprecipitations, the anti-FLAG M2 affinity gel (Sigma #A2220) was washed with lysis buffer three times and then resuspended to a ratio of 50:50 affinity gel to lysis buffer. 25 μL of a well-mixed slurry was added to cleared lysates and incubated at 4°C in a shaker for 90–120 minutes. For anti-Myc immunoprecipitations, magnetic anti-Myc beads (Pierce) were washed three times with lysis buffer. 30 μL of resuspended beads in lysis buffer was added to cleared lysates and incubated at 4°C in a shaker for 90–120 minutes. Immunoprecipitates were washed three times; once with lysis buffer and twice with lysis buffer with 500 mM NaCl. Immunoprecipitated proteins were denatured by addition of 50 μL of SDS-containing sample buffer (0.121 M Tris, 5% SDS, 12.5% glycerol, 0.25 M DTT, and bromophenol blue) and heated in boiling water for 5 minutes. Denatured samples were resolved by 8%−12% SDS-PAGE, and analyzed by immunoblotting.
Leucine-binding assay and Kd calculation
For radiolabeled leucine-binding assays using FLAG-tagged Drosophila Sestrin, suspension HEK293F cells were seeded at 2.5 million cells/ml, and transfected with the pRK5-FLAG-Sestrin cDNA using polyethylenimine. 72 hours after transfection, cells were rinsed one time in cold PBS and lysed in 1% Triton lysis buffer (1% Triton, 40 mM Hepes pH 7.4, 2.5 mM MgCl2 and 1 tablet of EDTA-free protease inhibitor [Roche] per 25 ml buffer). Following an anti-FLAG immunoprecipitation, the beads were washed 4 times with lysis buffer containing 500 mM NaCl and then incubated for one hour on ice in cytosolic buffer (0.1% Triton, 40 mM HEPES pH 7.4, 10 mM NaCl, 150 mM KCl, 2.5 mM MgCl2) with the indicated amount of [3H]-leucine and unlabeled leucine. After one hour, the beads were aspirated dry and rapidly washed four times with binding wash buffer (0.1% Triton, 40 mM HEPES pH 7.4, 300 mM NaCl, 2.5 mM MgCl2). The beads were aspirated dry again and resuspended in 80 μl of cytosolic buffer. Each sample was mixed well and then 15 μl aliquots were separately quantified using a TriCarb scintillation counter (Perkin Elmer). This process was repeated in pairs for each sample, to ensure similar incubation and wash times for all samples analyzed across different experiments.
The affinity for leucine of Drosophila FLAG-Sestrin was determined by first normalizing the bound [3H]-labeled leucine concentrations across three separate binding assays performed with varying amounts of cold leucine. These values were plotted and fit to a hyperbolic equation (Cheng-Prusoff equation) to estimate the IC50 value. The Kd value was derived from the IC50 value using the equation:
In vitro GATOR2-Sestrin dissociation assay
Drosophila S2 cells stably expressing Flag-tagged Drosophila WDR59 were leucine-starved for 1 hour or kept in full media were lysed and subjected to anti-FLAG immunoprecipitations as described above. The GATOR2-Sestrin complexes immobilized on the FLAG beads were washed twice in lysis buffer with 250 mM NaCl, and then incubated for 25 minutes in 0.3 ml of cytosolic buffer (0.1% Triton, 40 mM HEPES pH7.4, 10 mM NaCl, 150 mM KCl, 2.5 mM MgCl2) with the indicated concentrations of leucine or other amino acids in the cold. The beads were then washed three times in the cytosolic buffer. The FLAG-tagged WDR59 and the amount of Sestrin that remained bound to the beads was assayed by SDS-PAGE and immunoblotting.
Liquid chromatography-mass spectrometry (LC-MS)-based metabolomics and quantification of metabolite abundances
LC/MS-based metabolomics were performed and analyzed as previously described59,60 using 500 nM isotope-labeled internal standards. Briefly, an 80% methanol extraction buffer with 500 nM isotope-labeled internal standards was used for whole fly metabolite extraction. Samples were briefly vortexed, dried by vacuum centrifugation, and stored at −80°C until analyzed. On the day of analysis, samples were resuspended in 100 μL of LC/MS grade water and insoluble material was cleared by centrifugation at 15,000 rpm. The supernatant was then analyzed as previously described by LC/MS59,60.
Fly stocks and maintenance
All flies were reared at 25°C and 60% humidity with a 12 hours on/off light cycle on standard laboratory food (12.7 g/L deactivated yeast, 7.3 g/L soy flour, 53.5 g/L cornmeal, 0.4 % agar, 4.2 g/L malt, 5.6 % corn syrup, 0.3 % propionic acid, 1% tegosept/ethanol). The following stocks were used: nprl1 21, Mio1 26, Sesn8A11 16, Lpp-gal4 (gift from S. Eaton and P. Léopold); PromE-Gal461, yw,hs-Flp; mCherry–Atg8a; Act>CD2>GAL4, UAS–nlsGFP/TM6B (gift from Eric Baehrecke), hsFlp; act>CD2>Gal4, UAS nlsGFP62, and w; UAS-sfGFPMODC-3xMyc63. Elav-Gal4 (#458), Repo-Gal4 (#7415), Mef2-Gal4 (27390), ddc-gal4 (#7010), Tdc2-Gal4 (#9313), vGAT-Gal4 (#58980), attP40 (#36304), attP2 (#36303) and Sesn RNAi (#64027) were obtained from the Bloomington Drosophila Stock Center. Da-Gal4, esg-Gal4, Myo1A-Gal4, Pros-Gal4 were constructed (Perrimon lab stocks).
UAS-Sesn, UAS-Sesn-L431E, UAS-Myc-WDR24 were constructed using the Gateway system. cDNAs were cloned in entry plasmids used for the LR clonase reaction (Invitrogen, 11791–020), with the destination vector pWALIUM10-roe64 or equivalent (Frederik Wirtz-Peitz, unpublished data). The plasmids were then microinjected into embryos for ϕ31-mediated recombination at attP2 or attP40 landing sites, as per standard procedures to create transgenic flies. attP40, attP2, W1118 and OreR were used as controls.
In Fig. 2c, the larvae of genotype w; Sesn−/−; tubulin-Gal4, tubulin-Gal80ts/UAS-Sesn were raised at a mildly permissive temperature (25°C) to express relatively physiological levels of the Sestrin cDNA in Sestrin-null larvae.
SesnL431E knock-in flies were generated with CRISPR/Cas9 technology to achieve di-nucleotide replacement at the endogenous locus. A single strand oligo donor (ssODN) was used, containing the codon change (CTG>GAG) flanked by 20 bp homology arms (Sequence: ACCAAGGACTACGATAGTGTGGAGGTCGAGCTGCAGGACAGTGA). A single sgRNA with a cutting site abutting the nucleotide replacement locus (sequence F/R: GTCGCAAGGACTACGATAGTGTGC/ AAACGCACACTATCGTAGTCCTTG) was cloned in the pCFD3 expression vector as in a previous report65. pCFD3-sgRNA and ssODN were injected into nos-Cas9 embryos and emerging adults crossed to Sco/Cyo. Progenies were screened by sequencing heterozygous animals (5–10 animals/ founder cross) (PCR/Sequencing primers: Forward primer: CGACGACTACGACTATGGCGAA; Reverse primer: GCATGTGTGGGTATGTGTGTGGT). Individual stocks were established, and backcrossed 9 times onto a control OreR background (using the same PCR and sequencing primers as above for genotyping).
Synthetic fly food formulation and preparation
Drosophila diet formulations were derived from previous recipes66,67 with the following modifications: (1) the type of Agar (Micropropagation Agar-Type II; Caisson Laboratories #A037), (2) the final percentage of Agar (1%), (3) the amount of sucrose (25 g/L of food), and (4) the amino acids that were added to stock solutions before or after autoclaving68 whose order is described below. The amino acid composition of the diet including the concentrations of leucine, isoleucine, and valine were based on the exome-matched (i.e. the concentrations used for a given amino acid correspond with the prevalence of exons for that amino acid in the Drosophila genome) and Drosophila diet formulation developed in a previous study67 that was found to be optimal for growth and fecundity without compromising lifespan. The rationale for which amino acids were part of the autoclaving process was based on solubility considerations68.
The complete procedure, formula, and stock solutions for food production are as follows:
Procedure:
Prepare “Part 1” mixture (see Extended Data Tables 1, 3 and 4);
Stir using stir bar;
Autoclave “Part 1” mixture for 15 minutes;
Prepare “Part 2” mixture (see Extended Data Tables 2–4) and set aside;
Remove “Part 1” mixture from autoclave, then combine with “Part 2” Mixture and stir, make sure to mix well;
Quickly pipette food into Drosophila vials (5–10 mL food/vial);
Allow food to solidify/cool for roughly an hour, then cover vials (either with cotton plugs or with plastic wrap) and store food at 4°C.
Food is good for about 3 weeks at 4°C (will shrink and pull away from sides of vials due to evaporation).
Note:
After autoclaving, “Part 1” mixture containing agar can start solidifying (both before and after the two mixtures are combined, but combining the two mixtures will cause food to cool down and solidify fast). Quickly combine and pour food while autoclaved mixture is still hot to avoid this. Adding water to the autoclave tray and
keeping the “Part 1” mixture in this hot water until ready to combine and pour help prevent premature solidification.
Catalog Numbers for reagents not listed in Extended Data Tables 1–4:
Sucrose: Sigma, S7903
Agar: Caisson, A037
Propionic acid: Sigma, P5561
Stocks can be stored at 4°C for several months unless otherwise specified.
Generation of clones expressing the Sesn shRNA
Clones were generated by crossing yw,hs-flp; mCherry–Atg8a; Act>CD2>GAL4, UAS–nlsGFP/TM6B with the indicated UAS lines. Progeny of the relevant genotype was reared at 25°C and spontaneous clones were generated in the fat body due to the leakiness of the heat-shock flipase (hs-flp).
Food preference assay
Determination of relative food consumption from two different food sources using unique DNA oligomers was performed as previously reported27.
DNA Oligomer 1:
5’ACCTACACGCTGCGCAACCGAGTCATGCCAATATAAGCAGATTAGCATTACTTTGAGCAACGTATCGGCGATCAGTTCGCCAGCAGTTGTAATGAGCCCC-3’
Forward qPCR Primer 1 – 5’ – GCAACCGAGTCATGCCAATA – 3’
Reverse qPCR Primer 1 – 5’ – TTACAACTGCTGGCGAACTG – 3’
DNA Oligomer 2:
5’GGGCAGCAGGATAACTCGAATGTCTTAGTGCTAGAGGCTTGGGGCGTGTAAGTGTATCG AAGAAGTTCGTGTTAAACGCTTTGGAATGACTGTAATGTAG-3’
Forward qPCR Primer 2 – 5’ – CAGCAGGATAACTCGAATGTCTTA – 3’
Reverse qPCR Primer 2 – 5’ – CAGTCATTCCAAAGCGTTTAACA – 3’
Genomic Cyp1 qPCR primers:
Cyp1 Forward qPCR primer – 5’ – ACCAACCACAACGGCACTG – 3’
Cyp1 Reverse qPCR primer – 3’ – TGCTTCAGCTCGAAGTTCTCATC – 5’
The DNA oligomers and their corresponding qPCR primers were purchased from Integrated DNA Technologies (IDT) with 4 nmole per tube and diluted in nuclease-free water to final stocks with a DNA concentration of 3.5 μg/μl.
Spray and clean the surface of fresh Gala apples using 70% ethanol. Fresh Gala apple pieces (~1 g) that contain both a piece of peel and pulp were cut on a clean field using a knife, and both the knife and field were precleaned by 70% ethanol. Two apple pieces with similar shape and weight were placed in the opposite corners of a 6 oz (177 ml; 57L × 57W × 103H (in mm)) clean Drosophila bottle. 100 μL solutions that contained one DNA oligomer (final concentration is 3.5 ng/μL) and substances (i.e., sterile water, amino acid solutions, etc.) were placed evenly on top of the apple pieces and allowed to soak in for 1.5–2 hours. Age-synchronized adult flies (15 females and 5 males) were flipped into these assay bottles and allowed to feed ad libitum on the apples for the indicated times in the time course experiments (Fig 3b and Extended Data Fig 4g) and for 24 hours in the other food preference experiments.
CO2-anesthetized flies were collected using a tweezer. From each bottle, two tubes of female flies were collected with five flies per tube. Five flies were homogenized for each qPCR sample. Homogenization was performed using a beads beater in the cold after adding 250 μL of squishing buffer (10 mM Tris-HCl pH 8.2, 1 mM EDTA, 1 mM NaCl) and 0.5 μL of 20 mg/ml proteinase K (Thermofisher Scientific # AM2546). The whole fly lysates were digested at 37 °C for 30–40 minutes after homogenization followed by proteinase K inactivation at 95 °C for 5 minutes. The samples were centrifuged for 10 minutes at 15,000 rpm at room temperature and 2 μL of the supernatant was loaded in each qPCR reaction in a 96-well qPCR plate. We used the SYBR green qPCR master mix from BIO-RAD and a CFX96 Touch Real-Time PCR Detection System with a Tm=60°C and 40 cycles per run.
Genomic Cyp1 qPCR Ct values were used to control for extraction efficiency. For every batch of samples, an average of Cyp1 qPCR Ct values was taken and all samples beyond −/+ 0.5 away from the average were discarded. Standard curves for each DNA oligomer 1 and 2 were generated, and the amount of DNA oligomer from each tube of flies was calculated by fitting their Ct values to the standard curves. The preference index was generated by dividing the calculated DNA oligomer 1 amount by the calculated DNA oligomer 2 amount.
To remove external oligomer that may stick to the outside of the flies, we used a four-step protocol described previously27: (1) a ten-minute wash with 10% Contrex AP Powdered labware detergent (Cat #5204, Decon Laboratories, Inc.); (2) a five-minute wash in ddH2O; (3) a two-minute wash in 30% bleach; and (4) a five-minute wash in ddH2O. All washes are performed in a 1500 uL microfuge tube with continuous rocking at room temperature.
For Figure 3c and Extended Data Figure 4f, we fed the flies with food containing either 25 μM Rapamycin or 25 μM ethanol for 2 days prior either protein starvation overnight or not (including 25 μM Rapamycin or 25 μM ethanol). Then for the final choice assay, 25 μM of Rapamycin or 25 μM ethanol was added to both apple pieces in the container.
Immunofluorescence assays
Fat bodies from 96 hours AEL (after egg laying) larvae were dissected in phosphate-buffered saline (PBS) at room temperature, fixed 25–30 min in 4% formaldehyde, washed twice for 10 min in PBS 0.3% Triton (PBST), blocked 30 min (PBST, 5% BSA, 2% FBS, 0.02% NaN3), incubated with primary antibodies in the blocking buffer overnight, and washed 4 times for 15 min. Secondary antibodies diluted 1:500 in PBST were added for 1 hour and tissues washed 4 times before mounting in Vectashield (Vector Laboratories) containing DAPI. Brains from 5–10 days old adult female flies were dissected and processed as in a previous study69.
Images for Figure 2c and Extended Data Figure 3d were acquired on Zeiss Axio Zoom V16. Images for Figure 4b, 4c, Extended Data Figure 1c, Extended Data Figure 7f, and Extended Data Figures 8b, 8c were acquired on a Zeiss AxioVert200M microscope with a 63X or 40X oil immersion objective or 10X objective and a Yokogawa CSU-22 spinning disk confocal head with a Borealis modification (Spectral Applied Research/Andor) and a Hamamatsu ORCA-ER CCD camera. The MetaMorph software package (Molecular Devices) was used to control the hardware and image acquisition. The excitation lasers used to capture the images were 405 nm, 488 nm, and 561 nm. Images for Extended Data Figures 6b, 6c were acquired on iPhone XR camera via a binocular microscope.
Egg laying preference assay
The set-up for the egg laying preference assay was identical as the food preference assay. Instead of collecting female flies for qPCR analyses, the two apple pieces were removed from the bottle and examined under a binocular microscope. The number of eggs on each apple piece was determine.
Ovary size quantification
Ovaries were dissected in PBS and bright field images were acquired using a Zeiss axio zoom v16 scope. The size of the ovaries was quantified using the average area of individual ovaries on ImageJ.
Developmental timing
Three-day-old crosses were used for 3–4 hour periods of egg collection on standard lab food. Newly hatched L1 larvae were collected 24 hours later for synchronized growth using the indicated diets at a density of 30 animals/vial. The time to develop was monitored by counting the number of animals that underwent pupariation, every two hours in fed conditions, or once/twice a day in starved conditions. The time at which half the animals had undergone pupariation is reported. For larvae developmental timing experiments, 10% leucine chemically-defined diet was used because complete leucine starvation quickly caused lethality before any size comparison across genotypes can be efficiently and meaningfully performed.
Lifespan experiments
To generate age-synchronized adult flies, larvae were raised on lab food at low density, transferred to fresh food upon emerging as adults and mated for 48h. Animals were anaesthetized with low levels of CO2 and sorted at a density of 25 flies/vial. Each condition examined used 8–10 vials of flies. Flies were transferred to fresh vials three times per week at which point deaths were also scored. For adult flies, leucine-free diet or valine-free diet was used.
Statistical analyses
For non-survival experiments, two-tailed unpaired t tests, multiple t tests, one-way or two-way ANOVA analyses followed my post hoc tests were used for comparison between two groups in GraphPad Prism (GraphPad Software v.9). All comparisons were two-sided unless specified. All analyzed P values are indicated for each made comparison within all figure panels. P values of less than 0.05 were considered to indicate statistical significance.
For survival comparisons in Figure 2a and 2b, two proportion z tests were performed. Pupariation percentage (Extended Data Figure 2a, h) data were compared using permutation tests, where the test statistic was the difference in mean pupariation times of the two genotypes. The distribution of the test statistic under the null hypothesis was estimated by simulating 100 million rearrangements of the data. Permutation tests were performed in R (script available in Supplementary Data 2). Results for all statistical analyses were summarized in source data files corresponding to each figure.
Analysis of survival data
All data were complete and uncensored. Kaplan-Meier estimates of the survival function were plotted and used to compute median survival times. Log-rank tests were used to compare survival distributions, and univariate Cox proportional hazard analysis (with ties handled by Efron approximation) was used to compute hazard ratios between Sestrin mutant versus wild-type flies within individual dietary conditions. To examine the interaction between genotype and diet (specifically using the alternative hypothesis that the lifespan defect of Sestrin mutant versus wild-type flies is exacerbated on a leucine-free compared to a valine-free diet), one-tailed Wald tests were conducted on the interaction coefficients generated by two-factor with interaction Cox proportional hazard models (with ties handled by Efron approximation). All statistical analyses on survival data were performed in R (script available in Supplementary Data 3).
Extended Data
Extended Data Fig. 1: Validation of chemically-defined diets and loss of Sestrin phenotypes in larval fat bodies.
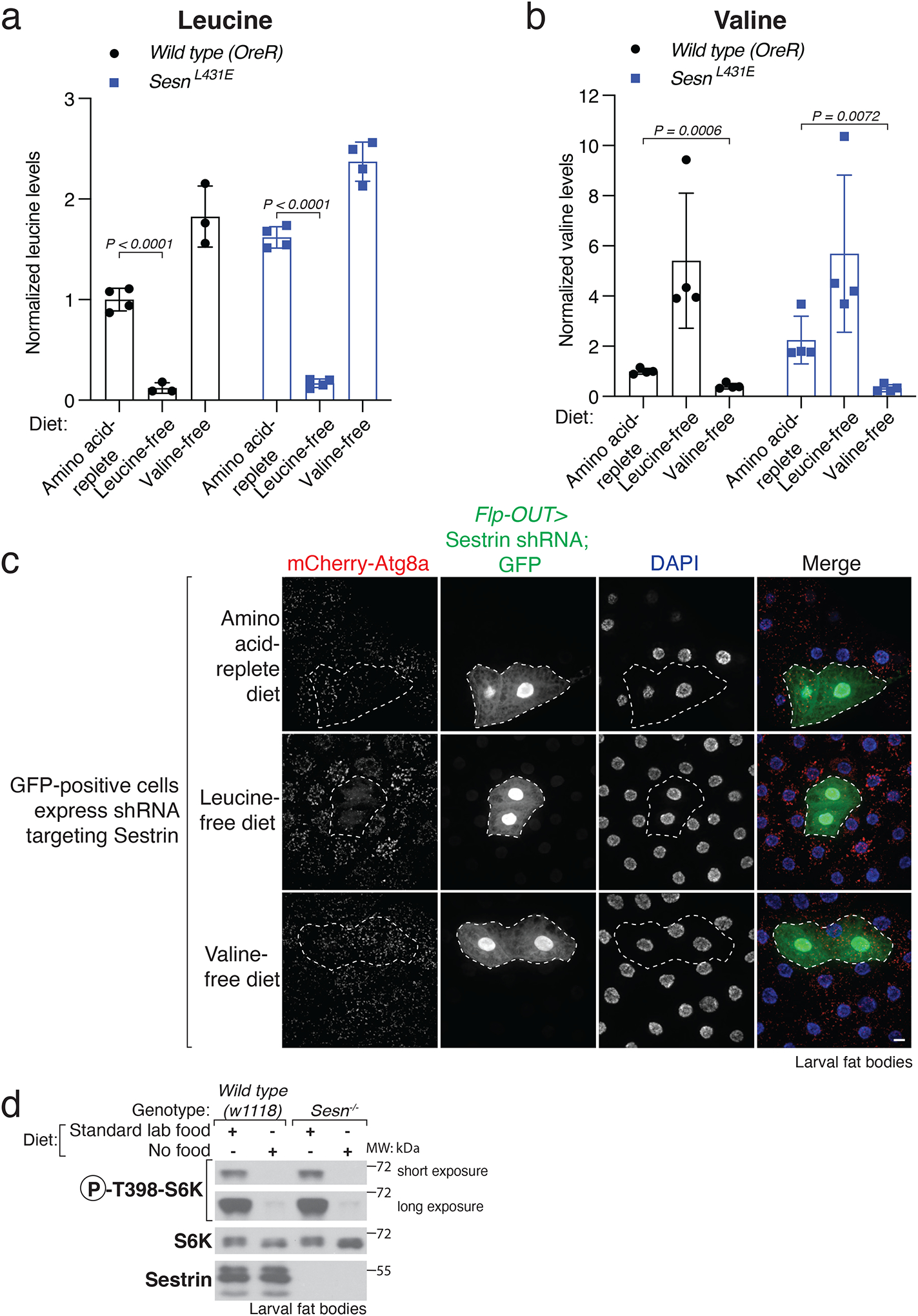
a, b, Drosophila larvae eating chemically-defined diets lacking individual amino acids have reduced levels of the missing amino acid. Relative levels of leucine (a) and valine (b) measured by LC-MS/MS in whole larval extracts of Wild-type (OreR) or SesnL431E larvae fed the indicated diet for 4.5 hours. Values are mean ± SD of technical replicates from a representative experiment. n=4 independent biological samples. Two samples from wild type (OreR) leucine-free and valine-free, respectively, failed to yield decent peaks for leucine levels, thus discarded. Multiple unpaired t tests, Holm-Šídák multiple comparison method.
c, Sesn knockdown prevents autophagy induction upon leucine deprivation. Fat body cells in mid-third instar larvae expressing mCherry-Atg8a were fed the indicated diets for 4.5 hours. The Sesn RNAi was expressed in clones of cells (GFP, outlined) with a FLP-out system70. Scale bar, 10 μm.
d, Loss of Sestrin does not affect the inhibition of mTORC1 caused by the deprivation of all food. Immunoblot analyses of phospho-S6K and S6K in adult female flies in the fed state or starved of all food for 1 day.
Extended Data Fig. 2: The SesnL431E mutation does not affect adult fly lifespan on the chemically defined diets but does mildly delay larvae development, while loss of Sestrin does not affect larvae development.
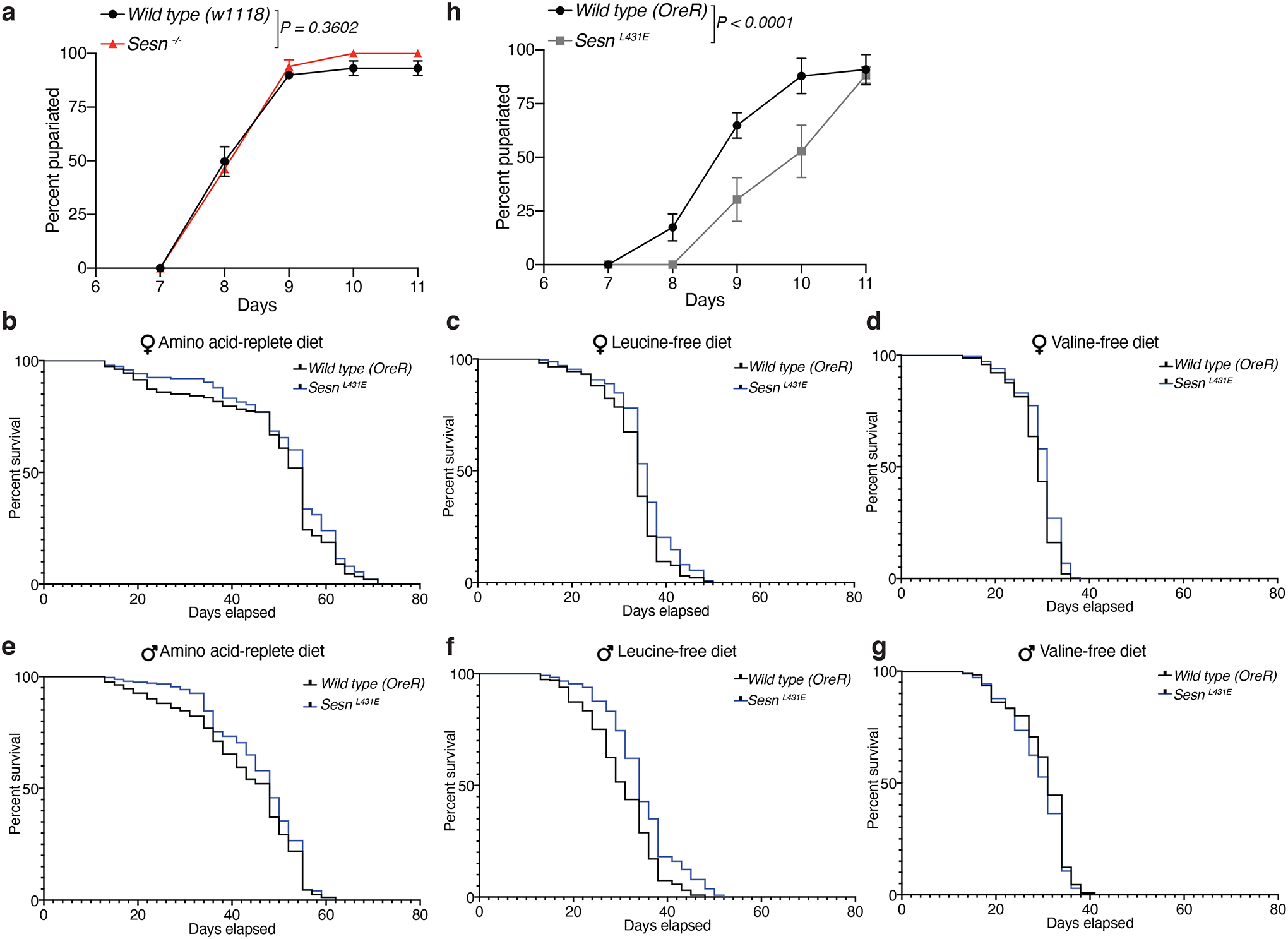
a, Loss of Sestrin does not the affect development of larva feeding on a complete diet. Time to pupariation for w1118 and Sesn −/− larvae fed the standard yeast-based diet.
b-g, Survival curved for animals of the indicated sex and genotypes fed the indicated chemically-defined diets. (a) nWT(OreR)=235; n(SesnL431E)=238; (b) nWT(OreR)=233; n(SesnL431E)=237; (c) nWT(OreR)=242; n(SesnL431E)=248; (d) nWT(OreR)=242; n(SesnL431E)=240; (e) nWT(OreR)=229; n(SesnL431E)=243; (f) nWT(OreR)=245; n(SesnL431E)=245. See statistics in Supplementary Data 1 and methods.
h. SesnL431E larvae raised on a standard yeast-based diet are developmentally delayed.
Data are representative of three independent experiments with similar results. Statistical analysis was performed using a permutation test on the difference of the mean pupariation times of the two genotypes (a, h).
Extended Data Fig. 3: Sestrin-mediated mTORC1 signaling in ovaries.
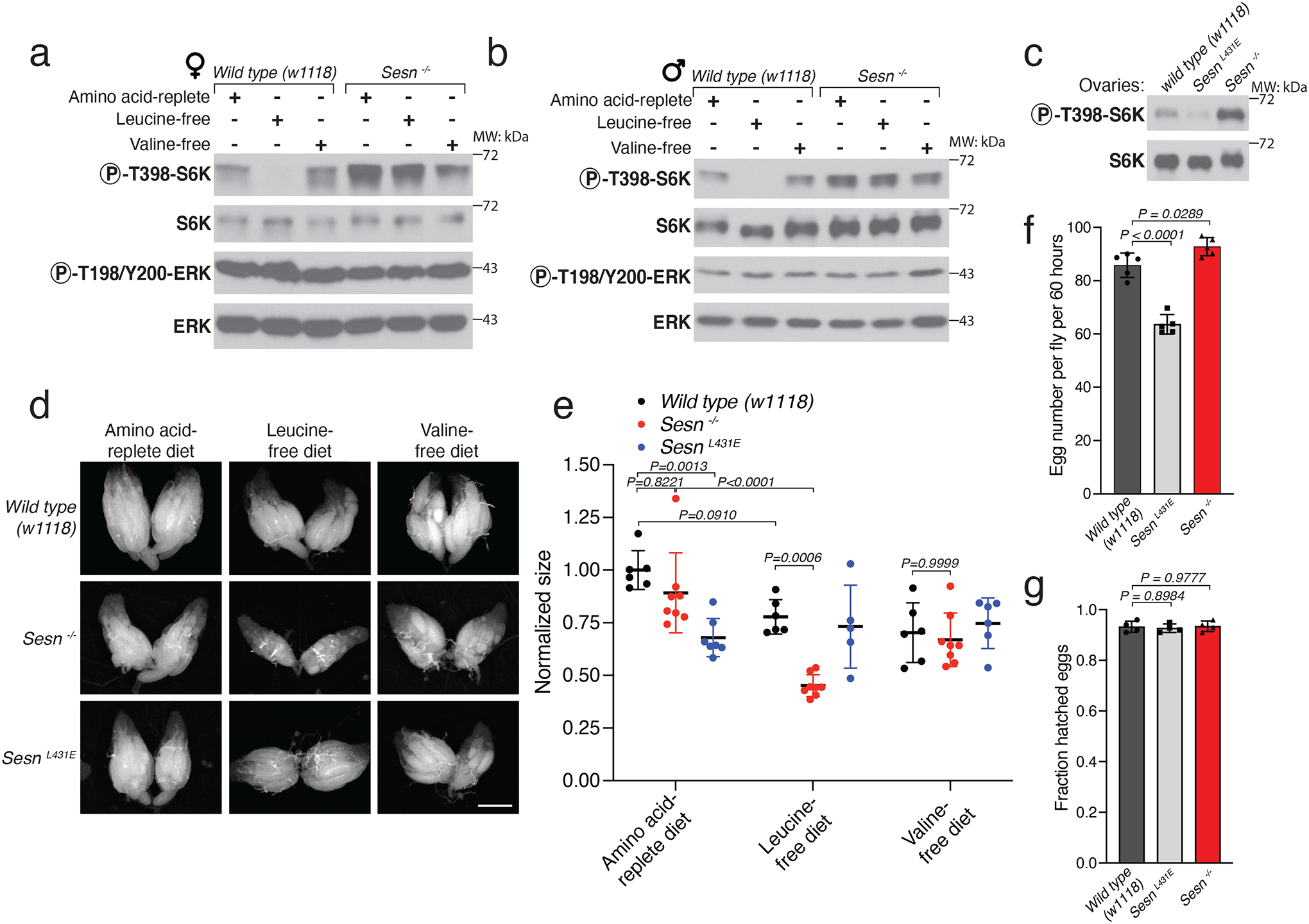
a, b, Sestrin mediates leucine-sensing by mTORC1 in adult animals. Immunoblot analyses of whole adult animals of the indicated sex and genotype following overnight starvation and 1.5 hours of refeeding with the indicated diets.
c, In flies feeding a standard diet and lacking Sestrin or expressing the leucine-binding deficient Sestrin mutant (L431E), mTORC1 activity is increased or decreased, respectively. Lysates were prepared from isolated ovaries from animals of the indicated genotypes and fed a standard yeast-based diet.
d, e, Loss of Sestrin accelerates the reduction in ovary size caused by leucine starvation. (d) Ovarian size in females of the indicated genotypes fed the indicated diets for 24 hours. Results are quantified in (e). Scale bar, 500 μm.
f, g, SesnL431E flies have reduced fecundity but not fertility. (f) Number of eggs laid over a period of 60 hours by females of the indicated genotypes maintained on the standard yeast-based diet. (g) Hatching rate of eggs laid in the same conditions as in (f).
(e, f, g) Values are mean ± SD of technical replicates from a representative experiment. (e) n=6 (Wild type (w1118)), 8 (Sesn −/−), 7 (SesnL431E amino acid-replete diet), 5 (SesnL431E leucine-free diet), and 6 (SesnL431E valine-free diet). (f) n=5. (g) n=4. Data are representative of three independent experiments with similar results. Statistical analysis was performed using two-way ANOVA followed by Tukey’s multiple comparisons test (e), and one-way ANOVA (f, g) followed by Dunnett’s multiple comparisons test.
Extended Data Fig. 4: Sestrin mediates the preference for leucine-containing food and influences total food intake.
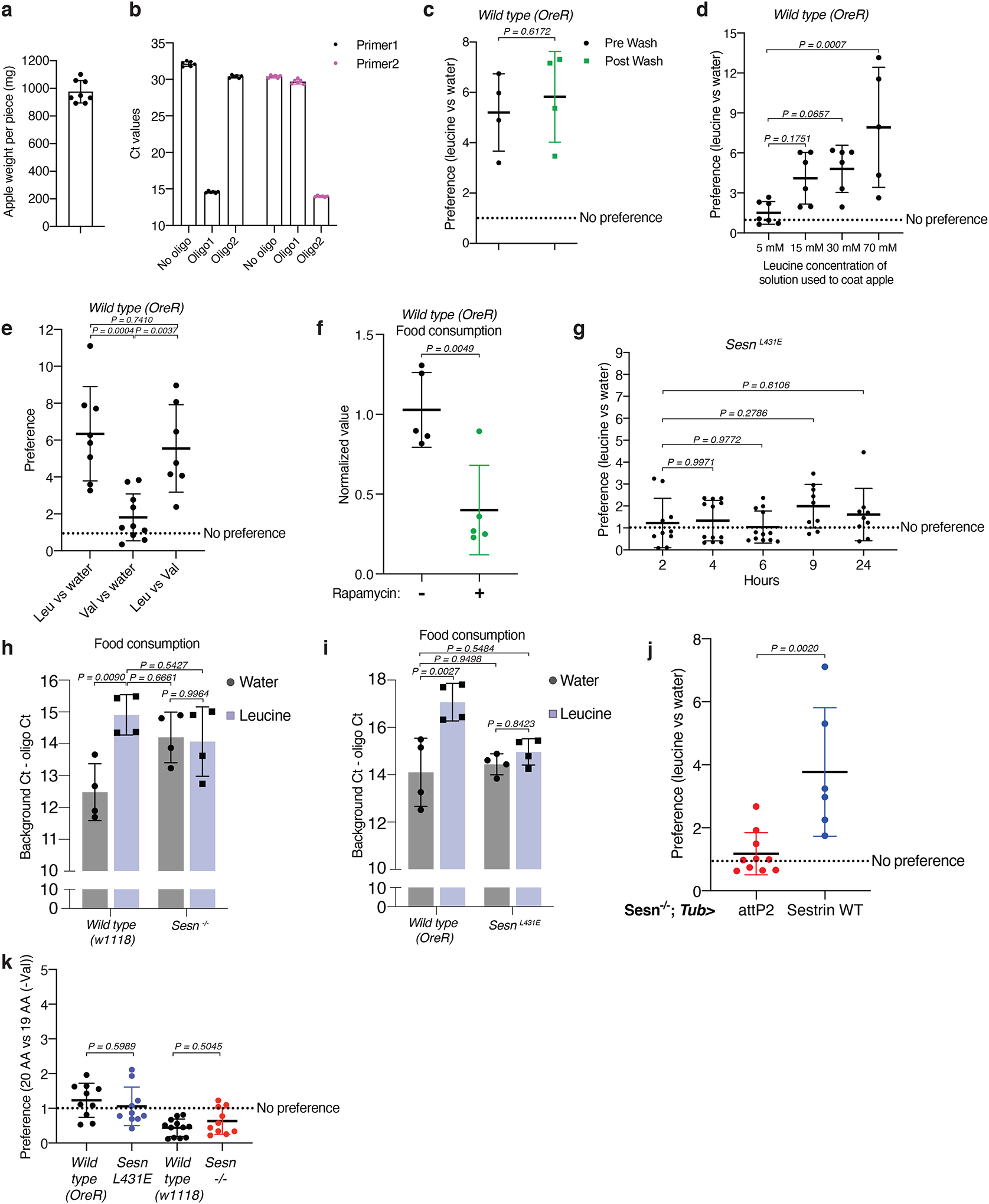
a-c, Characterization of the methods used in the food two-choice assay. (a) Measurement of the weight of the apple pieces used in the assay. n=8. (b) Background qPCR signal determination for each oligonucleotide barcode used in assay. n=6 for each condition. (c) The qPCR signals used to determine the leucine preference of the wild-type flies come primarily from internal DNA oligonucleotides instead of external ones that might contaminate the outside of the body of female flies. qPCR for oligonucleotide barcodes in a leucine versus water choice assay before and after washing animals as previously described27. n=4 for both pre and post wash conditions.
d, Preference of the flies for apple pieces painted with the indicated leucine concentrations. Animals were given a choice between leucine- or water-coated apples. Indicated leucine concentrations (5 mM, 15 mM, 30 mM, and 70 mM) were the solution concentrations used to coat apples. The final concentration on the food should be ~10 times more diluted. n (5 mM) = 7, n (15 mM and 30 mM) = 6, n (70 mM) = 5.
e, Adult female flies do not have a preference for valine- versus water-painted apple pieces. Wild-type (OreR) animals were given indicated food choices and the preference fold-difference was shown. n (leucine vs water) = 8, n (valine vs water) = 10, n (leucine vs valine) = 7.
f, Rapamycin treatment reduces fly food consumption. Vehicle or Rapamycin pre-treated animals were given a choice between leucine- or water-coated apples. For the Rapamycin group during the choice assay, animals were fed on apples painted with Rapamycin in addition to either leucine or water. Data show the normalized values of food consumption. n=5 for both conditions.
g, SesnL431E animals do not have a preference for valine- over water-painted apple. Animals were given a choice between valine- or water-coated apples and food preference was measured at the indicated time points. Data show the fold-difference in relative food intake for the valine-coated apple compared to the water-coated apple. n=10 (2 hrs), 12 (4 hrs), 12 (6 hrs), 9 (9 hrs), and 9 (24 hrs).
h,i, SesnL431E animals have decreased food intake regardless of the leucine content of the food (h), and Sesn −/− animals have increased food intake regardless of the leucine content of the food (i). n=4 for all conditions.
j, Whole-body re-expression of wild-type Sestrin driven by Tub>Gal4 is sufficient to partially restore the preference for leucine-containing food of Sesn −/− adult female flies. Animals with indicated genotypes were given the choice between leucine- or water- coated apples. Data show the preference of fold-difference. n (attP2) = 10, n (Sestrin WT) = 6.
k, Adult female flies do not develop a preference for valine-containing apple regardless of their genotype. Animals with indicated genotypes were given the choice between leucine- or water- coated apples. Data show the preference of fold-difference. n (Wild type OreR, SesnL431E, Sesn−/−) = 10, n (Wild type w1118) = 12.
Values are mean ± SD of technical replicates from a representative experiment. Data are representative of three independent experiments with similar results. Statistical analysis was performed using two-tailed unpaired t test (c, f, j), one-way ANOVA followed by Dunnett’s multiple comparisons test (d, g), one-way ANOVA followed by Tukey’s multiple comparisons test (e), two-way ANOVA followed by Tukey’s multiple comparisons test (h, i), and one-way ANOVA followed by Šídák’s multiple comparisons test (k).
Extended Data Fig. 5: Leucine-sensing via the Sestrin-mTORC1 axis contributes to the detection of the protein content of food.

a, Wild-type (OreR) flies prefer food containing a high amount of yeast extract and this preference is reduced by the addition of leucine to food containing a low amount of yeast extract. SesnL431E flies have a reduced preference for the food containing a high amount of the yeast extract and the addition of leucine has minimal impact on the preference. How the food preference index was calculated is described in the methods. n (Wild type OreR, no leucine)=5, n (Wild type OreR, with leucine)=7, n (SesnL431E, no leucine)=6, n (SesnL431E, with leucine)= 9.
b, As in (a) a choice experiment for wild type w1118 and Sesn −/− flies. n (Wild type w1118, no leucine)=9, n (Wild type w1118, no leucine)=8, n (Sesn−/−, no leucine)=9, n (Sesn−/−, with leucine)= 12.
Values are mean ± SD of technical replicates from a representative experiment. Data are representative of three independent experiments with similar results. Statistical analysis was performed using two-tailed unpaired t test, Holm-Šídák method.
Extended Data Fig. 6: Flies prefer to lay eggs on leucine-containing food in a fashion that requires the leucine-binding capacity of Sestrin.
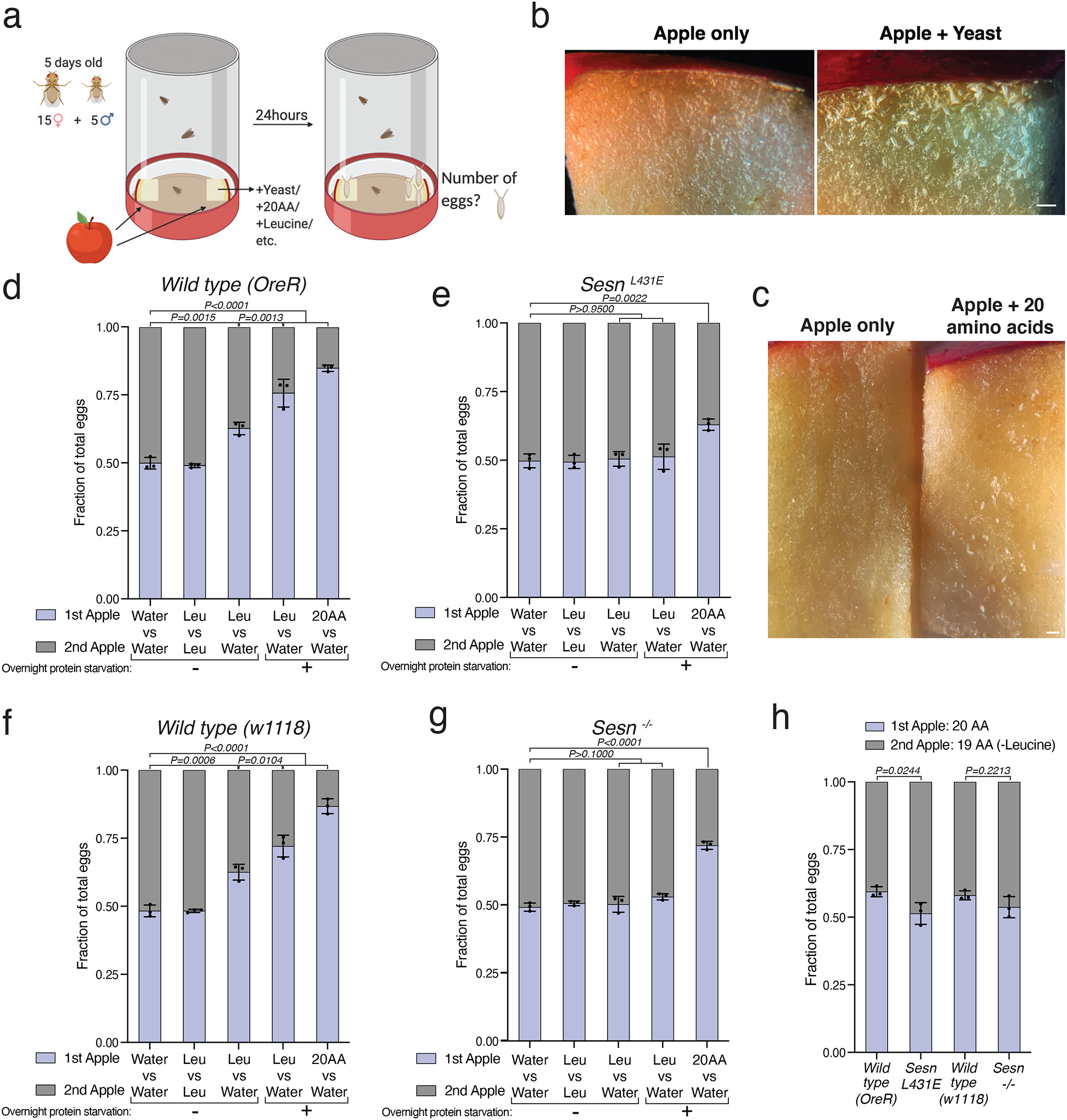
a, Schematic of the setup used in the egg-laying preference assay. Two identical apple pieces were painted with solutions containing different substances and placed on opposite sides of a container. Animals were allowed to feed ad libitum over the course of the assay and the number of eggs deposited on each apple was counted after 24 hours.
b, c, Wild-type flies prefer to lay eggs on yeast- or amino acid-painted apples over water-painted apples. Scale bars, 1 mm.
d-h, SesnL431E and Sesn −/− animals do not prefer to lay eggs on the leucine-containing apple.
(a) created with BioRender.com. Values are mean ± SD of three technical replicates from a representative experiment. Data are representative of two independent experiments with similar results. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test (d-g), and Šídák’s multiple comparisons test (h).
Extended Data Fig. 7: Sestrin-regulated mTORC1 signaling in glial cells controls the preference of flies for leucine-containing food.

a, Same data as in Figure 4a except that the values were not normalized to the values from the flies expressing the control shRNA from each of the indicated drivers. n=5 (da, pros attP40 shRNA; da Sesn shRNA), 8 (repo, tdc2 attP40 shRNA; vGAT Sesn shRNA), 12 (repo, esg Sesn shRNA), 15 (Elav attP40 shRNA), 16 (Elav, Mef2, ddc Sesn shRNA), 22 (Mef2 attP40 shRNA; Myo1A Sesn shRNA), 10 (ddc, Lpp attP40 shRNA; tdc2, promE Sesn shRNA), 11 (vGAT attP40 shRNA; Lpp Sesn shRNA), 9 (promE attP40 shRNA; pros Sesn shRNA), 13 (esg attP40 shRNA), 24 (Myo1A attP40 shRNA). Each point represents the ratio of the amount of two oligonucleotide barcodes per 5 flies.
b, Expression of wild-type Sestrin under repo-Gal4 driver in Sesn −/− flies is sufficient to partially rescue the leucine preference phenotype. n (repo-attP40 in wild type w1118) = 4, n (other conditions) = 8.
c, Overexpression of TSC1+TSC2 in glial cells using repo-Gal4; Tub-Gal80ts reduces the preference of flies for leucine-containing food. n (attP40) = 16, n (TSC1+2) = 19.
d, The Sesn mRNA (red) is expressed in all classified subtypes of glial cells as indicated by co-expression of a pan glial marker, Repo (green). The single cell RNA sequencing dataset is from a previous study39.
e, The knock-down of Sestrin using a pan glial cell driver (repo-Gal4) reduces the leucine preference of flies much more significantly than a knockdown using drivers for glial subtypes. The knockdown of Sestrin in cortex glial cells using the wrapper-Gal4 driver line significantly decreased the leucine preference of flies. n=8 (repo, 9.GMR50A12, 15.R85G01-Gal4 attP40 shRNA; 9.GMR50A12, 15.R85G01-Gal4 Sesn shRNA), 12 (1.GMR60F04, 2.GMR53B07, 3.GMR55B03, 4.GMR56F03, 5.GMR86E01, 6.GMR53H12, 10.Alrm-Gal4 attP40 shRNA; repo, 2.GMR53B07, 3.GMR55B03, 4.GMR56F03, 5.GMR86E01, 10.Alrm-Gal4, 14.R75H03-Gal4 Sesn shRNA), 10 (7.GMR35E04 attP40 shRNA, 1.GMR60F04 Sesn shRNA), 11 (8.GMR77A03, 11.Wrapper-Gal4, 14.R75H03-Gal4 attP40 shRNA; 6.GMR53H12, 11.Wrapper-Gal4 Sesn shRNA), 28 (12.Eaat1-Gal4 39915, 13.Mdr65-Gal4 attP40 shRNA), 9 (7.GMR35E04, 8.GMR77A03 Sesn shRNA), 24 (12.Eaat1-Gal4 39915 Sesn shRNA), 18 (13.Mdr65-Gal4 Sesn shRNA).
f, Confocal projection of wild-type female brains expressing 4MBOX-GFP fed the standard yeast-based food or starved of protein for 24 hours. Scale bar,10 μm.
Values are mean ± SD of technical replicates from a representative experiment. Data are representative of two independent experiments with similar results. Statistical analysis was performed using two-tailed unpaired t test (a, c, e), and two-way ANOVA followed by Dunnett’s multiple comparisons test (b).
Extended Data Fig. 8: Dietary leucine regulates mTORC1 signaling in glial cells in the peri-esophageal area in a fashion that depends on Sestrin and its capacity to bind leucine.
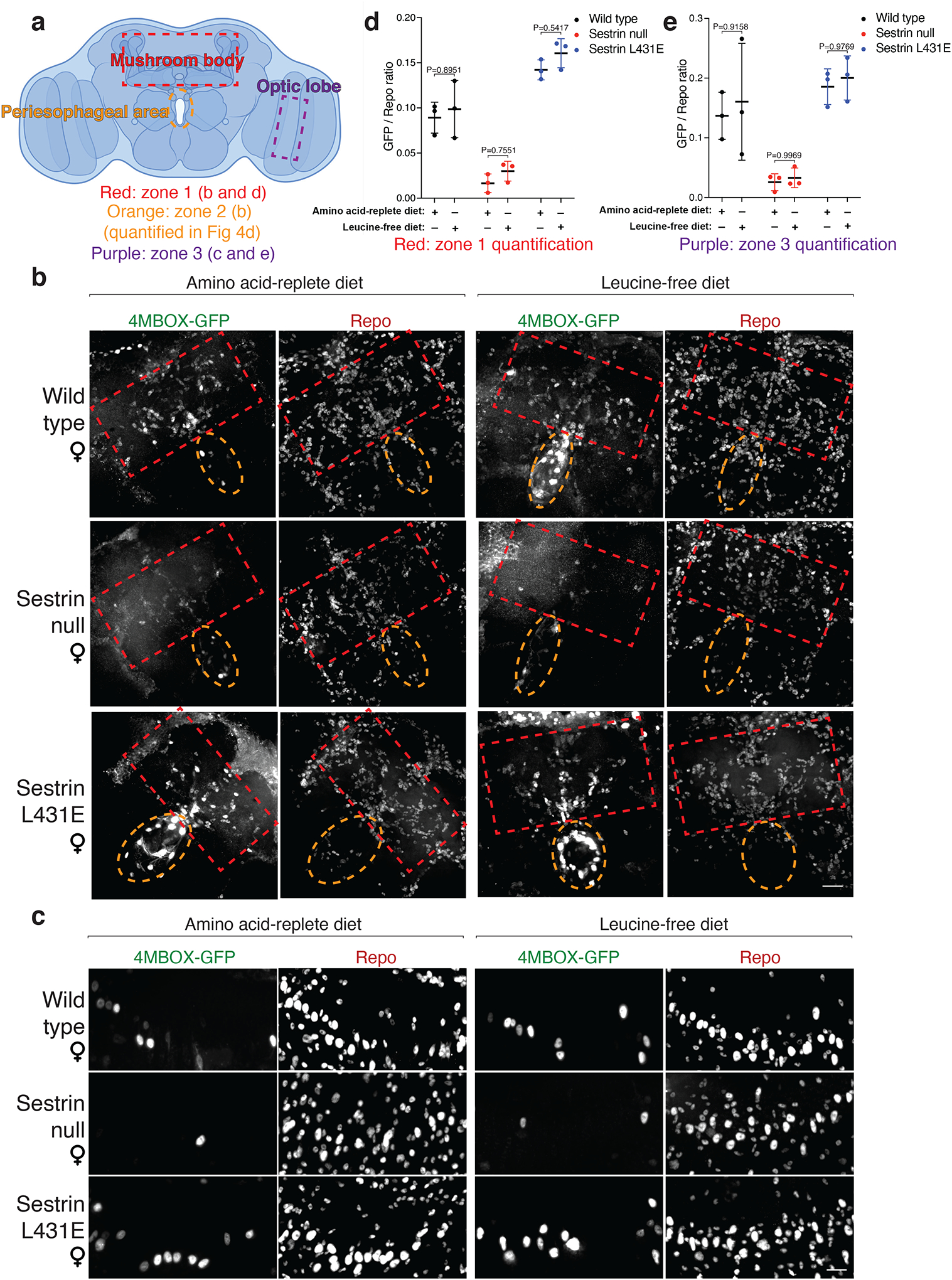
a, Schematic of the areas imaged and quantified for the ratio of GFP-positive cells to Repo-positive cells. The red rectangle represents zone 1, the orange rectangle represents zone 2, and the purple rectangle represents zone 3.
b, Representative confocal images of zone 1 and zone 2 brain areas from wild-type, Sesn −/−, and SesnL431E female flies fed with an amino acid-replete or leucine-free diet. Scale bar, 25 μm. Note: images are reprocessed during revision from the same batch of samples as Figure 4c for the purpose of showing all zones 1, 2, and 3 clearly. The exact fly brains in the representative images and stacks might vary from Figure 4c, despite they are all from the same batch of samples.
c, Representative confocal images of zone 3 brain areas of wild-type, Sesn −/−, and SesnL431E female flies fed an amino acid-replete or leucine-free diet. Scale bar, 10 μm. Note: images are from the same brains shown in (b).
(a) created with BioRender.com. d, e, Quantification of the GFP-positive to Repo-positive ratio in zone 1 (d) and zone 3 (e). n=3 individual brains with indicated dietary treatment and genotype for each condition. Values are mean ± SD of biological replicates from a representative experiment. Data are representative of three independent experiments with similar results. Statistical analysis was performed using two-way ANOVA followed by Šídák’s multiple comparisons test.
Extended Data Table 1:
Chemically defined food “Part 1” mixture
| Part 1 (AUTOCLAVE 15 minutes) | ||
|---|---|---|
| Category | Ingredient | Amount of stock per liter |
| Gelling Agent | Agar-Type II | 10 g |
| Sugar | Sucrose | 25 g |
| Metal Ions | CaCl2*6h2o | 1 mL |
| CuSO4*5h2o | 1 mL | |
| FeSO4*7h2o | 1 mL | |
| MgSO4 (anhydrous) | 1 mL | |
| MnCl2*4h2o | 1 mL | |
| ZnSO4*7h2o | 1 mL | |
| Cholesterol | Cholesterol | 15 mL |
| Amino Acids | Tyrosine | 0.93g |
| Histidine | 50 mL | |
| Isoleucine | 50 mL | |
| Methionine | 50 mL | |
| Phenylalanine | 50 mL | |
| Threonine | 50 mL | |
| Valine | 50 mL | |
| Water | Water (milliQ) | 158 mL |
Extended Data Table 2:
Chemically defined food “Part 2” mixture
| Part 2 | ||
|---|---|---|
| Category | Ingredient | Amount of stock per liter |
| Base | Buffer | 100 ml |
| Amino Acids | Arginine | 10 mL |
| Cysteine | 10 mL | |
| Glutamate | 10 mL | |
| Glycine | 10 mL | |
| Lysine | 10 mL | |
| Proline | 10 mL | |
| Serine | 10 mL | |
| Alanine | 50 mL | |
| Asparagine | 50 mL | |
| Aspartate | 50 mL | |
| Glutamine | 50 mL | |
| Leucine | 50 mL | |
| Tryptophan | 50 mL | |
| Vitamin Solution | see Part 1 | 21 mL |
| Folic Acid | Folic Acid | 1 mL |
| Other Nutrients Solution | see Part 1 | 8 mL |
| Preservatives | Propionic acid | 6 mL |
| methyl 4-hydroxybenzoate | 15 mL | |
Extended Data Table 3:
Amino acid stock solutions
| Amino Acids | Catalog Number | g/50mL | Suspend in: |
|---|---|---|---|
| L-Alanine | Sigma, A7469 | 1.1 | H2O |
| L-Asparagine | Amresco, 94341 | 1.03 | H2O |
| L-Aspartic Acid | Alfa Aesar, A13520 | 1.17 | 0.5N NaOH |
| L-Glutamine | Amresco, 0374 | 1.12 | H2O |
| L-Histidine | Amresco, 1B1164 | 0.65 | H2O |
| L-Isoleucine | Amresco, E803 | 1.12 | H2O |
| L-Leucine | Sigma, L8912 | 2.03 | 0.2N HCl |
| L-Methionine | Amresco, E801 | 0.6 | H2O |
| L-Phenylalanine | Sigma, P5482 | 1.01 | H2O |
| L-Threonine | Sigma, T8441 | 1.11 | H2O |
| L-Tryptophan | Amresco, E800 | 0.32 | H2O |
| L-Valine | Amresco, 1B1102 | 1.2 | H2O |
| L-Arginine HCl | Amresco, 0877 | 8.16 | H2O |
| L-Cysteine | Sigma, 30089 | 1.71 | 1N HCl |
| L-Glutamic acid | Alfa Aesar, A12919 | 7.59 | H2O |
| L-Glycine | Alfa Aesar, A13816 | 3.84 | H2O |
| L-Lysine HCl | Amresco, 0437 | 6.83 | H2O |
| L-Proline | Sigma, P5607 | 4.89 | H2O |
| L-Serine | Sigma, S4311 | 6.89 | H2O |
| L-Tyrosine | Sigma, T8566 | **add Tyr powder | N/A |
Extended Data Table 4:
Other stock solutions
| Vitamin solution | Catalog Number | g/50mL | Suspend in: |
|---|---|---|---|
| Biotin | Sigma, B4501 | 0.001 | H2O |
| Ca pantothenate | Sigma, 21210 | 0.039 | H2O |
| Nicotinic acid | Sigma, N4126 | 0.03 | H2O |
| Pyridoxine HCl | Sigma, P9755 | 0.006 | H2O |
| Riboflavin | Sigma, R4500 | 0.003 | H2O |
| Thiamine (aneurin) | Sigma, T4625 | 0.005 | H2O |
| Folic acid solution | Catalog Number | g/50mL | Suspend in: |
| Folic acid | Sigma F8758 | 0.025 | 0.004N NaOH |
| Other Nutrients solution | Catalog Number | g/50mL | Suspend in: |
| Choline chloride | MP Biomedicals, 194639 | 0.3125 | H2O |
| Inosine | Sigma, I4125 | 0.4065 | H2O |
| Myo-Inositol | Sigma, I7508 | 0.0315 | H2O |
| Uridine | Sigma, U3003 | 0.375 | H2O |
| Methyl 4-hydroxybenzoate solution | Catalog Number | g/50mL | Suspend in: |
| Methyl 4-hydroxybenzoate | Sigma, H3647 | 5 | 95% EtOH |
| Buffer | Catalog Number | 50mL stock | |
| Glacial Acetic Acid | Millipore, AX0074 | 1.5 mL | |
| KH2PO4 | JT Baker, 3246 | 1.5 g | |
| NaHCO3 | Sigma, S8875 | 0.5 g | |
| Water | Up to 50mL | ||
| Metal Ions | Catalog Number | g/50mL | Suspend in: |
| CaCl2*6h2o | Sigma, 21108 | 12.5 | H2O |
| CuSO4*5h2o | Sigma, C7631 | 0.125 | H2O |
| FeSO4*7h2o | Sigma, F7002 | 1.25 | H2O (store −20C) |
| MgSO4 (anhydrous) | Sigma, M7506 | 12.5 | H2O |
| MnCl2*4h2o | Sigma, M3634 | 0.05 | H2O |
| ZnSO4*7h2o | Sigma, Z0251 | 1.25 | H2O |
| Cholesterol solution | Catalog Number | g/50mL | Suspend in: |
| Cholesterol | Sigma, C8253 | 1 | EtOH |
Supplementary Material
Supplementary Data 1: Statistical tests for survival analyses from Figure 2 and Extended Data Figure 5
Supplementary Data 2: Script for permutation test analysis
Supplementary Data 3: Script for statistical analysis of the survival data
Acknowledgements
We thank all members of the Sabatini and Perrimon Labs for helpful insights and suggestions, as well as J. H. Lee, J. D. Levine, M. A. Lilly, F. Pignoni, A. A. Teleman, the Bloomington Drosophila Stock Center (BDSC), and the Developmental Studies Hybridoma Bank (DSHB) for providing fly stocks and reagents. We thank P. Rosen for critical reading of the manuscript and R. Chivukula and T. Wang for experimental suggestions. We thank C. Lewis, B. Chan, and T. Kunchok for performing the LC/MS analyses and L. Parel for help on mutant genotyping. This work was supported by grants from the NIH to D.M.S. (R01 CA103866 and R37 AI47389), N.P. (5P01 CA120964-04 and R01 AR057352), J.W.L. (R01 CA193256), M.L.V. (F30 CA228229 and T32 GM007753), A.E.A. (F31 CA232658), and N.K. (T32 HG002295), the Department of Defense (W81XWH-07-0448) to D.M.S., the American Cancer Society postdoctoral fellowship to M.A.R., the American Cancer Society Research Scholar Award to J.W.L., the Cystinosis Research Foundation to P.J. and N.P., and the Ludwig Center Graduate Fellowship to X.G. rom the Koch Institute for Integrative Cancer Research at MIT. N.P. is investigator of the Howard Hughes Medical Institute.
Footnotes
Competing interests
D.M.S. is a shareholder of Navitor Pharmaceuticals, which is targeting for therapeutic benefit the amino-acid-sensing pathway upstream of mTORC1. J.W.L. advises Raphael Pharmaceuticals, Nanocare Technologies, Petri Biologics, and Restoration Foodworks. M.A.R. is currently employed by Amgen, which has interests in neurodegenerative diseases. These relationships have no overlap with this study. The other authors declare no competing interests.
Supplementary Information is available for this paper.
Data availability
The data that support the findings of this study are available from the corresponding authors and the Whitehead Institute (sabadmin@wi.mit.edu) upon reasonable request.
Reference
- 1.Liu GY & Sabatini DM mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21, 183–203, doi: 10.1038/s41580-019-0199-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxton RA et al. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351, 53–58, doi: 10.1126/science.aad2087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfson RL et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48, doi: 10.1126/science.aab2674 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sancak Y et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501, doi: 10.1126/science.1157535 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim E, Goraksha-Hicks P, Li L, Neufeld TP & Guan KL Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10, 935–945, doi: 10.1038/ncb1753 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buerger C, DeVries B & Stambolic V Localization of Rheb to the endomembrane is critical for its signaling function. Biochem Biophys Res Commun 344, 869–880, doi: 10.1016/j.bbrc.2006.03.220 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Bar-Peled L et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100–1106, doi: 10.1126/science.1232044 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen K, Valenstein ML, Gu X & Sabatini DM Arg-78 of Nprl2 catalyzes GATOR1-stimulated GTP hydrolysis by the Rag GTPases. J Biol Chem 294, 2970–2975, doi: 10.1074/jbc.AC119.007382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen K et al. Architecture of the human GATOR1 and GATOR1-Rag GTPases complexes. Nature 556, 64–69, doi: 10.1038/nature26158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox HL, Pham PT, Kimball SR, Jefferson LS & Lynch CJ Amino acid effects on translational repressor 4E-BP1 are mediated primarily by L-leucine in isolated adipocytes. Am J Physiol 275, C1232–1238, doi: 10.1152/ajpcell.1998.275.5.C1232 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Lynch CJ, Fox HL, Vary TC, Jefferson LS & Kimball SR Regulation of amino acid-sensitive TOR signaling by leucine analogues in adipocytes. J Cell Biochem 77, 234–251, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 12.Dodd KM & Tee AR Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 302, E1329–1342, doi: 10.1152/ajpendo.00525.2011 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Suryawan A et al. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295, E868–875, doi: 10.1152/ajpendo.90314.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JS et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci Rep 5, 9502, doi: 10.1038/srep09502 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chantranupong L et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 9, 1–8, doi: 10.1016/j.celrep.2014.09.014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327, 1223–1228, doi: 10.1126/science.1182228 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye J et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev 29, 2331–2336, doi: 10.1101/gad.269324.115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfson RL & Sabatini DM The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metab 26, 301–309, doi: 10.1016/j.cmet.2017.07.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piyankarage SC, Augustin H, Grosjean Y, Featherstone DE & Shippy SA Hemolymph amino acid analysis of individual Drosophila larvae. Anal Chem 80, 1201–1207, doi: 10.1021/ac701785z (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park Y, Reyna-Neyra A, Philippe L & Thoreen CC mTORC1 Balances Cellular Amino Acid Supply with Demand for Protein Synthesis through Post-transcriptional Control of ATF4. Cell Rep 19, 1083–1090, doi: 10.1016/j.celrep.2017.04.042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Y, Reveal B, Cai W & Lilly MA The GATOR1 Complex Regulates Metabolic Homeostasis and the Response to Nutrient Stress in Drosophila melanogaster. G3 (Bethesda) 6, 3859–3867, doi: 10.1534/g3.116.035337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirth CK, Nogueira Alves A & Piper MD Turning food into eggs: insights from nutritional biology and developmental physiology of Drosophila. Curr Opin Insect Sci 31, 49–57, doi: 10.1016/j.cois.2018.08.006 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Wei Y & Lilly MA The TORC1 inhibitors Nprl2 and Nprl3 mediate an adaptive response to amino-acid starvation in Drosophila. Cell Death Differ. 21, 1460–1468, doi: 10.1038/cdd.2014.63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Y et al. TORC1 regulators Iml1/GATOR1 and GATOR2 control meiotic entry and oocyte development in Drosophila. Proc Natl Acad Sci U S A 111, E5670–5677, doi: 10.1073/pnas.1419156112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senger S et al. The nucleoporin Seh1 forms a complex with Mio and serves an essential tissue-specific function in Drosophila oogenesis. Development 138, 2133–2142, doi: 10.1242/dev.057372 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iida T & Lilly MA missing oocyte encodes a highly conserved nuclear protein required for the maintenance of the meiotic cycle and oocyte identity in Drosophila. Development 131, 1029–1039, doi: 10.1242/dev.01001 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Park A, Tran T & Atkinson NS Monitoring food preference in Drosophila by oligonucleotide tagging. Proc Natl Acad Sci U S A 115, 9020–9025, doi: 10.1073/pnas.1716880115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar P et al. Nutritional characterization of apple as a function of genotype. J Food Sci Technol 55, 2729–2738, doi: 10.1007/s13197-018-3195-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng F, Li M, Ma F & Cheng L Effects of location within the tree canopy on carbohydrates, organic acids, amino acids and phenolic compounds in the fruit peel and flesh from three apple (Malus × domestica) cultivars. Hortic Res 1, 14019, doi: 10.1038/hortres.2014.19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma S et al. Free amino acid composition of apple juices with potential for cider making as determined by UPLC-PDA. Journal of the Institute of Brewing 124, 467–476, doi: 10.1002/jib.519 (2018). [DOI] [Google Scholar]
- 31.Agriculture, U. S. D. o. https://fdc.nal.usda.gov/fdc-app.html#/food-details/171688/nutrients, 2019).
- 32.Hebert M et al. Single rapamycin administration induces prolonged downward shift in defended body weight in rats. PLoS One 9, e93691, doi: 10.1371/journal.pone.0093691 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anisimov VN et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 10, 4230–4236, doi: 10.4161/cc.10.24.18486 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Pasha M, Eid AH, Eid AA, Gorin Y & Munusamy S Sestrin2 as a Novel Biomarker and Therapeutic Target for Various Diseases. Oxid Med Cell Longev 2017, 3296294, doi: 10.1155/2017/3296294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becher PG et al. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct Ecol 26, 822–828, doi: 10.1111/j.1365-2435.2012.02006.x (2012). [DOI] [Google Scholar]
- 36.Becher PG et al. Chemical signaling and insect attraction is a conserved trait in yeasts. Ecol Evol 8, 2962–2974, doi: 10.1002/ece3.3905 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumberger JP A nutritional study of insects, with special reference to microorganisms and their substrata. J Exp Zool 28, 1–81, doi:DOI 10.1002/jez.1400280102 (1919). [DOI] [Google Scholar]
- 38.Steck K et al. Internal amino acid state modulates yeast taste neurons to support protein homeostasis in Drosophila. Elife 7, doi:ARTN e31625 10.7554/eLife.31625 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davie K et al. A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Cell 174, 982–998 e920, doi: 10.1016/j.cell.2018.05.057 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T et al. Mitf is a master regulator of the v-ATPase, forming a control module for cellular homeostasis with v-ATPase and TORC1. J Cell Sci 128, 2938–2950, doi: 10.1242/jcs.173807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouche V et al. Drosophila Mitf regulates the V-ATPase and the lysosomal-autophagic pathway. Autophagy 12, 484–498, doi: 10.1080/15548627.2015.1134081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu J et al. Sestrin is a key regulator of stem cell function and lifespan in response to dietary amino acids. Nat aging 1, 60–72, 10.1038/s43587-020-00001-7 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Leib DE & Knight ZA Re-examination of Dietary Amino Acid Sensing Reveals a GCN2-Independent Mechanism. Cell Rep 13, 1081–1089, doi: 10.1016/j.celrep.2015.09.055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z et al. A post-ingestive amino acid sensor promotes food consumption in Drosophila. Cell Res 28, 1013–1025, doi: 10.1038/s41422-018-0084-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Croset V, Schleyer M, Arguello JR, Gerber B & Benton R A molecular and neuronal basis for amino acid sensing in the Drosophila larva. Sci Rep 6, 34871, doi: 10.1038/srep34871 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kudow N et al. Preference for and learning of amino acids in larval Drosophila. Biol Open 6, 365–369, doi: 10.1242/bio.020412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maurin AC et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab 1, 273–277, doi: 10.1016/j.cmet.2005.03.004 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Ganguly A et al. A Molecular and Cellular Context-Dependent Role for Ir76b in Detection of Amino Acid Taste. Cell Rep 18, 737–750, doi: 10.1016/j.celrep.2016.12.071 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park J & Carlson JR Physiological responses of the Drosophila labellum to amino acids. J Neurogenet 32, 27–36, doi: 10.1080/01677063.2017.1406934 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjordal M, Arquier N, Kniazeff J, Pin JP & Leopold P Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell 156, 510–521, doi: 10.1016/j.cell.2013.12.024 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Vargas MA, Luo N, Yamaguchi A & Kapahi P A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr Biol 20, 1006–1011, doi: 10.1016/j.cub.2010.04.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q et al. Branch-specific plasticity of a bifunctional dopamine circuit encodes protein hunger. Science 356, 534–539, doi: 10.1126/science.aal3245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribeiro C & Dickson BJ Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol 20, 1000–1005, doi: 10.1016/j.cub.2010.03.061 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Henriques SF et al. Metabolic cross-feeding in imbalanced diets allows gut microbes to improve reproduction and alter host behaviour. Nat Commun 11, 4236, doi: 10.1038/s41467-020-18049-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma Z, Stork T, Bergles DE & Freeman MR Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539, 428–432, doi: 10.1038/nature20145 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kottmeier R et al. Wrapping glia regulates neuronal signaling speed and precision in the peripheral nervous system of Drosophila. Nat Commun 11, 4491, doi: 10.1038/s41467-020-18291-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otto N et al. The sulfite oxidase Shopper controls neuronal activity by regulating glutamate homeostasis in Drosophila ensheathing glia. Nat Commun 9, 3514, doi: 10.1038/s41467-018-05645-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mariyappa D et al. A novel transposable element-based authentication protocol for Drosophila cell lines. G3 (Bethesda) 12, doi: 10.1093/g3journal/jkab403 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Birsoy K et al. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 162, 540–551, doi: 10.1016/j.cell.2015.07.016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen WW, Freinkman E, Wang T, Birsoy K & Sabatini DM Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism. Cell 166, 1324–1337 e1311, doi: 10.1016/j.cell.2016.07.040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Billeter JC, Atallah J, Krupp JJ, Millar JG & Levine JD Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991, doi: 10.1038/nature08495 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Karpowicz P, Zhang Y, Hogenesch JB, Emery P & Perrimon N The circadian clock gates the intestinal stem cell regenerative state. Cell Rep 3, 996–1004, doi: 10.1016/j.celrep.2013.03.016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He L, Binari R, Huang J, Falo-Sanjuan J & Perrimon N In vivo study of gene expression with an enhanced dual-color fluorescent transcriptional timer. Elife 8, doi: 10.7554/eLife.46181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ni JQ et al. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods 5, 49–51, doi: 10.1038/nmeth1146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Housden BE, Lin S & Perrimon N Cas9-based genome editing in Drosophila. Methods Enzymol 546, 415–439, doi: 10.1016/B978-0-12-801185-0.00019-2 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Piper MD et al. A holidic medium for Drosophila melanogaster. Nat Methods 11, 100–105, doi: 10.1038/nmeth.2731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piper MDW et al. Matching Dietary Amino Acid Balance to the In Silico-Translated Exome Optimizes Growth and Reproduction without Cost to Lifespan. Cell Metab 25, 1206, doi: 10.1016/j.cmet.2017.04.020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis RW, Botstein D, Roth JR & Cold Spring Harbor Laboratory. Advanced bacterial genetics. (Cold Spring Harbor Laboratory, 1980). [Google Scholar]
- 69.Wu JS & Luo L A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc 1, 2110–2115, doi: 10.1038/nprot.2006.336 (2006). [DOI] [PubMed] [Google Scholar]
- 70.McGuire SE, Le PT, Osborn AJ, Matsumoto K & Davis RL Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765–1768, doi: 10.1126/science.1089035 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data 1: Statistical tests for survival analyses from Figure 2 and Extended Data Figure 5
Supplementary Data 2: Script for permutation test analysis
Supplementary Data 3: Script for statistical analysis of the survival data
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors and the Whitehead Institute (sabadmin@wi.mit.edu) upon reasonable request.


