Abstract
Purpose
We aimed to determine whether interferon gamma-1b prevents hospital-acquired pneumonia in mechanically ventilated patients.
Methods
In a multicenter, placebo-controlled, randomized trial conducted in 11 European hospitals, we randomly assigned critically ill adults, with one or more acute organ failures, under mechanical ventilation to receive interferon gamma-1b (100 µg every 48 h from day 1 to 9) or placebo (following the same regimen). The primary outcome was a composite of hospital-acquired pneumonia or all-cause mortality on day 28. The planned sample size was 200 with interim safety analyses after enrolling 50 and 100 patients.
Results
The study was discontinued after the second safety analysis for potential harm with interferon gamma-1b, and the follow-up was completed in June 2022. Among 109 randomized patients (median age, 57 (41–66) years; 37 (33.9%) women; all included in France), 108 (99%) completed the trial. Twenty-eight days after inclusion, 26 of 55 participants (47.3%) in the interferon-gamma group and 16 of 53 (30.2%) in the placebo group had hospital-acquired pneumonia or died (adjusted hazard ratio (HR) 1.76, 95% confidence interval (CI) 0.94–3.29; P = 0.08). Serious adverse events were reported in 24 of 55 participants (43.6%) in the interferon-gamma group and 17 of 54 (31.5%) in the placebo group (P = 0.19). In an exploratory analysis, we found that hospital-acquired pneumonia developed in a subgroup of patients with decreased CCL17 response to interferon-gamma treatment.
Conclusions
Among mechanically ventilated patients with acute organ failure, treatment with interferon gamma-1b compared with placebo did not significantly reduce the incidence of hospital-acquired pneumonia or death on day 28. Furthermore, the trial was discontinued early due to safety concerns about interferon gamma-1b treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-023-07065-0.
Keywords: Intensive care, Hospital-acquired pneumonia, Interferon-gamma, Immunotherapy, Immunosuppression
Take-home message
| In this randomized clinical trial that included 109 adult critically ill patients requiring invasive mechanical ventilation that was discontinued early for safety concerns, early treatment with interferon gamma-1b vs placebo resulted in an adjusted hazard ratio for hospital-acquired pneumonia or death of 1.76 (95% confidence interval 0.94–3.29; P = 0.08) on day 28. Further studies are necessary to identify patients in which interferon-gamma treatment could be well tolerated. |
Introduction
Hospital-acquired pneumonia (HAP) is a health concern worldwide and a public health priority in Europe. In 2017, the European Centre for Disease Prevention and Control estimated more than 500,000 cases/year in Europe, accounting for a significant proportion of disability, since infected patients lose an average of 7.7 quality-adjusted life years [1]. HAP has significant medical consequences, notably prolonged hospitalization equivalent to 7 extra days on average in the intensive care unit (ICU) and attributable mortality of 10% [2, 3]. With an average cost for each episode of HAP of 40,000 euros, annual expenses for HAP treatment are estimated to reach 8 billion $ in the United States of America [3]. In this context, American, European, and French intensive care societies have published guidelines for preventing and treating HAP [4–6]. However, even after implementing these guidelines, the rates of HAP still routinely reach 30% of critically ill patients hospitalized for more than three days in ICUs [7], which suggests that new approaches are urgently needed.
The involvement of critical illness-related immunosuppression as a risk factor for hospital-acquired infections is well described in ICU patients [8–10]. An emerging trend is that immune restoration could help prevent infection and improve outcomes for this population [11, 12]. While patients with an inherited deficiency in interferon gamma (IFN-γ) are susceptible to respiratory infections [13, 14], it has been shown in experimental models and human samples that the IFN- γ production by immune cells was decreased before and during HAP [15–17]. Following the demonstration that in vitro IFN-γ treatment restores the metabolic activity and the functions of monocytes from critically ill patients [18, 19], several observational cases of rescue therapies with interferon gamma-1b have shown promising effects in patients with protracted infections or difficult-to-treat pathogens [20–22].
The human recombinant interferon gamma-1b for the prevention of hospital-acquired pneumonia in critically ill patients (PREV-HAP) trial was conducted to test the hypothesis that interferon gamma-1b could restore immunity and prevent HAP in critically ill patients under invasive mechanical ventilation.
Methods
Design
We conducted an investigator-initiated multicenter, parallel-group, double-blind, randomized clinical trial to investigate the effects of interferon gamma-1b in critically ill patients at risk of HAP. The study protocol is available in the electronic supplementary material (ESM) 1.
Ethics
The Ethics Committee of Ouest II Angers (France) approved the study protocol in March 2021. This trial complied with the Declaration of Helsinki and was registered in March 2021 (number ClinicalTrial.gov NCT04793568). The patient's legal surrogate provided written informed consent for participation. In agreement with the local laws, patients could be enrolled before the provision of legal surrogate consent if the next of kin could not be informed within the maximum delay for inclusion. When able to provide it, patient’s follow-up consent was requested up to 90 days after inclusion.
Trial sites and study population
The study was conducted at eleven ICUs in France, Spain, and Greece. Patients aged between 18 and 85 years, admitted to the participating sites within the last 48 h, receiving invasive mechanical ventilation, were eligible if presenting with one or more acute organ failures at the time of inclusion among: neurological (Glasgow coma scale < 13 before sedation), hemodynamic (norepinephrine, epinephrine, or any other vasopressor at a dose of ≥ 0.1 μg per kilogram of body weight per minute for at least 6 h), respiratory (PaO2/FiO2 < 200) and renal failure (creatinine blood level > two-fold higher than the basal value and or oliguria < 0.5 mL/kg/h for at least 12 h). Non-inclusion criteria were pregnancy/breastfeeding (legal obligation); hypersensitivity to the interferon gamma-1b; a medical history of pre-existing immunosuppression; severe hepatic insufficiency (Child–Pugh score B or C); liver cytolysis (liver transaminases > 5N); chronic renal failure (Modification of Diet in Renal Disease (MDRD) glomerular filtration rate < 10 ml/min/1.73 m2), persisting coma after resuscitated cardiac arrest, hospitalization for cervical spinal cord injury, a previous episode of hospital-acquired pneumonia during the current hospitalization and sustained hyperlactatemia > 5 mmol/L.
Randomization
Randomization was performed through a secure web-based randomization system. Patients were randomized to interferon gamma-1b (interferon-gamma group) or placebo (placebo group) in fixed blocks of 6, in a 1:1 ratio, with stratification based on the cause of hospitalization (sepsis vs other) and the country (France, Greece, Spain).
Intervention
The study drugs, recombinant interferon gamma-1b (100 μg/0.5 ml vials, IMUKIN®, from Clinigen®) and placebo (normal saline vials), were indiscernible. Participants received five subcutaneous injections of 100 μg of recombinant interferon gamma-1b (interferon-gamma group) or matching placebo (placebo group) from day 1 to day 9 (i.e., one injection every 48 h). The dose and frequency of injections were adapted from the IMUKIN European drug approval (50 µg/m2, three injections/week). The timing was defined to start before the theoretical risk of HAP (48 h after hospitalization) and last for the expected duration of mechanical ventilation (9 days in patients at risk of HAP hospitalized in ICU) [7].
Standard care
To reduce heterogeneity in practice, site medical teams agreed to apply the French and European guidelines for preventing and treating HAP [4, 5]. For consistency, a standardized multimodal prevention approach that included the promotion and semi-recumbent position, the preference for orotracheal over nasotracheal intubation; the reduction of dose and duration of sedatives and analgesics infusions; the early initiation of enteral feeding; and the repeated monitoring of endotracheal tube cuff pressure was recommended.
Primary outcome
The primary outcome was a composite of HAP and all-cause mortality 28 days after inclusion [23, 24]. This composite outcome was chosen to manage the statistical competition between HAP and mortality (i.e., to avoid considering early mortality without HAP as a favorable outcome) [25].
According to European guidelines [5], HAP was defined as pneumonia that occurred 48 h after hospitalization and required at least two clinical signs (body temperature > 38 °C; leukocytosis > 12,000 cells per mL, leucopenia < 4000 cells per mL, or purulent pulmonary secretions) with the appearance of a new infiltrate or worsening of an existing infiltrate on chest radiography, plus semi-quantitative or quantitative positive respiratory fluid cultures. Respiratory samples were obtained before starting any new antimicrobial therapy for pneumonia. HAP were sub-categorized as ventilator-associated pneumonia (VAP) in ICU patients who have been mechanically ventilated for at least 48 h at the time of diagnosis; as ventilated-HAP, if the diagnosis was made more than 48 h after extubating, but invasive ventilation was required for the treatment, or as HAP if the diagnosis was made more than 48 h after extubating and no invasive ventilation was required for the treatment. Early- and late-onset HAP were defined as HAP occurring less than five days and five or more days after hospital admission, respectively.
Ventilator-associated tracheobronchitis was defined with the criteria above, without radiographical signs of new pneumonia.
An adjudication committee composed of intensivists, blinded to the trial-group assignment and not involved in patient recruitment, reviewed all respiratory infection diagnoses. The adjudicators had access to the clinical data, serial results of biological and microbiological analyses, and chest X-rays to confirm or exclude new or persistent infiltrates (the adjudication charter provided by the sponsor is available in ESM 2). The adjudication committee members concluded HAP, tracheobronchitis, or no respiratory infections. The primary endpoint analysis was based on the final diagnosis made by two adjudication committee members who had access to clinical evaluation, bacteriological documentation, chest-X ray, and concomitant antimicrobial therapy. In case of disagreement between the two adjudication committee members, a radiology expert reviewed the chest X-rays before arbitrage by a third adjudication committee member.
Secondary outcomes
The following secondary outcomes were recorded: the rates of the primary outcome components (all-cause mortality at day 28 and HAP at day 28), all-cause mortality at day 90; rates of ventilator-associated tracheobronchitis by day 28 (defined as the association of clinical and bacteriological signs of respiratory infection without appearance of a new infiltrate or worsening of an existing infiltrate on chest radiography [26]) and acute respiratory distress syndrome by day 28 (PaO2/FiO2 < 200 mmHg [27]); durations of mechanical ventilation, ICU hospitalization, and hospital stay; and numbers of antibiotic-free days on day 28 (dead patients being ascribed 0 antibiotic-free days), mechanical ventilation-free days on day 90 (defined as the number of days between day one and day 90 for which living patients breathe spontaneously, dead patients being ascribed 0 mechanical ventilation free days), intensive care unit-free days on day 90 (dead patients being ascribed 0 intensive care unit-free days), hospital-free days on day 90 (dead patients being ascribed 0 hospital-free days). We also collected EuroQol-5D and Short Form-36 on day 28 and day 90 for ancillary medico-economic studies, and Hospital Anxiety and Depression Scale and Satisfaction with Life Scale on day 28 and day 90 were measured to study the suitability of interferon-gamma 1b from the patients' perspective (ancillary study not presented here).
The following post hoc secondary outcomes were defined to investigate the putative mechanism for harm related to HAP severity: (rates of early vs late HAP, lowest PaO2/FiO2 ratio within the seven first days of HAP, rates of pleural empyema or bacteremia associated with HAP); and to other sites of infections: (rates of surgical site infection, urinary tract infection, bacteremia and of invasive candidiasis).
Safety outcomes
The following outcomes were systematically collected on day 15 to investigate interferon gamma-1b tolerance: rates of serious adverse effects and suspected unexpected severe adverse reaction (SUSAR), leukocytosis, neutropenia, lymphopenia, thrombopenia, liver cytolysis (liver transaminases > 5 N), biological pancreatitis (lipase blood level > 3 N), allergic reaction, injection site reaction, and muscle-skeleton symptoms (myalgia, arthralgia, back pain).
Inflammatory protein profiles
Proteins were measured on frozen serum using Olink® Explore 384 Inflammatory panels (Olink Proteomics AB, Uppsala, Sweden) according to the manufacturer's instructions. The raw output data were quality controlled, normalized, and converted into Normalized Protein eXpression (NPX) values, Olink’s proprietary unit of relative abundance. Quality control was performed for each sample plate on the samples (using the spiked internal controls) and the external controls. PLS-DA analyses were carried out using the mixOmics R package [28]. Differential abundant protein between conditions was calculated using a linear model (protein ~ condition) using the R package MatrixEQTL [29], in principal following [30]. Boxplots were generated in R, and heatmaps were generated using the ComplexHeatmap package. Pearson correlation coefficient R was used to generate hierarchical tree distances for this. Correlation networks were generated using the iGraph package based on pair-wise expression similarity estimated with the Pearson correlation coefficient between protein expression levels across patients.
Study monitoring and oversight
The study was monitored on behalf of the sponsor (CHU Nantes). Study initiation visits were performed before site activation. During regular monitoring visits, independent, experienced research staff carried outsourced data verification, monitored data integrity in all participating centers, and verified all informed consent forms. Expected and unexpected serious adverse events were reported blinded to the sponsor for central validation of their severity level and relation to the intervention and whether they were expected. An independent data and safety monitoring board (DSMB), appointed by the sponsor, oversaw ethics, reviewed patient safety, and made recommendations to the sponsor about the research's continuation, modification, or termination. Two safety report evaluations were planned after the inclusion of 50 and 100 patients, respectively. In this phase II trial, DSMB’s mission was to evaluate the safety, but not the futility, of the trial continuation.
Sample size calculation
Based on a nationwide intervention to reduce the risk of HAP [7], we estimated that the rates of the primary outcome in the placebo group should reach 35%. The minimal clinically significant difference in the prevention of HAP in critically ill patients was set between 25 and 40% in recent trials [31, 32]. We calculated that including 200 patients (100 patients/group) should allow the detection of a hazard ratio of 0.625 (37.5%-reduction) with the intervention, with 90% of statistical power and a double-sided type I error α at 5%.
Statistical analysis
The primary analysis was conducted in all randomized patients according to their assigned group (as-randomized population). No multiple imputation of primary outcome was performed because only one datum was missing. As secondary analyses, we also analyzed the primary outcome in a per-protocol population that included only patients treated in full compliance with the protocol drug regimen, with an assessable primary outcome, fulfilling all the inclusion criteria, without consent withdrawal. A modified intention-to-treat analysis, including patients without major non-inclusion criteria, without consent withdrawal, and who received at least one treatment dose, was planned but not performed a posteriori because it included the same population as the as-randomized population.
The primary composite outcome measure was analyzed with a Cox regression model. Such an analysis combining the primary (occurrence of HAP) and competing event (all-cause mortality) into a composite event has been recommended [25]. The primary analysis was adjusted on the stratification criteria and the center as a random effect. Crude estimation was also planned. Data from patients who withdrew consent before 28 days were censored on the withdrawal date.
We estimated the intervention effect on the primary outcome in prespecified subgroups: cause of hospitalization (sepsis vs others), age (< or 65 years), severity upon ICU hospitalization (Simplified Acute Physiology Score (SAPS) II 15–30, 30–45, and > 45), the time between the ICU admission and the first treatment injection (< 24 h; 24–36 h, and 36–48 h), steroid therapy at the time of inclusion (yes or no), and coronavirus disease 2019 (COVID-19) status of the patients included in the study (PCR positive or negative). Interactions between the treatment arm and each subgroup covariate were tested in Cox regression models using the same adjustment as for the primary analysis.
All analyses of secondary outcomes were adjusted on stratification factors and centers as a random effect. All-cause mortality at day 90 was analyzed with a Cox regression model. Categorical data were analyzed with a logistic regression model. The duration of antimicrobial therapy, mechanical ventilation, ICU hospitalization, and hospitalization were analyzed with cause-specific hazard regression models to consider the informative censoring and the competing risk of death. The cumulative incidence functions of each competing event (competition of mortality with durations of antimicrobial therapy, mechanical ventilation, or hospitalization) were estimated. Antibiotic-free days on day 28, mechanical ventilation-free days on day 90, intensive care unit-free days on day 90, and hospital-free days on day 90 were analyzed using a Wilcoxon rank-sum test. The Hodges–Lehmann method was used to estimate the median of differences with 95% confidence intervals (CI) for free-days outcomes. Categorical data, including tolerance outcomes, were analyzed with logistic regression models. The changes from day 1 to 15 after randomization in physiological measurements were compared between the two study groups using mixed models.
Continuous variables were presented as median and quartiles (as mean and standard deviations otherwise), and categorical data were presented as exact numbers and percentages. Missing data were described by the treatment group. Analyses were performed with SAS software (version 9.4, NC, USA). No interim efficacy analysis was performed. Type I error (α) was set at 5%. Because of the potential for type 1 error due to multiple comparisons, analysis findings of secondary endpoints should be interpreted as exploratory.
Results
Patients
At the second safety review, after enrolling 109 patients (54.5% of the planned total recruitment), the DSMB raised concerns regarding the rates of severe adverse events and recommended stopping the trial recruitment because of potential harm in the intervention group (ESM 3, Data safety report).
From April 2021 through October 2021, 109 patients underwent randomization and were followed up for three months (55 patients in the interferon-gamma group and 54 in the placebo group). No patient was included in Spain and Greece, whose centers were activated after the French hospitals and only one month before the study discontinuation. Eight of 109 participants (7.3%) had major protocol violations (6 received less than the five planned treatment injections, 1 received a delayed first injection, 1 withdrew consent) but were kept in the primary analysis (ESM, eFig. 1). Primary outcome data were available for 108 (99%). Characteristics at baseline are reported in Table 1. Trauma and surgical conditions were the most frequent cause of ICU admission, recorded in 51 (44.4%) and 37 (33.9%) patients, respectively. Neurological failure (coma) was the most frequent organ failure at the time of randomization, reported in 91 (83.5%) patients, and 30/108 (27.8%) patients were treated with steroids at the time of randomization (all hydrocortisone). HAP prevention compliance rates are reported in the ESM eTable 1.
Table 1.
Baseline participant characteristics
| Interferon-gamma group (N = 55) | Placebo group (N = 54) | |
|---|---|---|
| Age, years, median (25–75th percentile) | 57 (41–64) | 58 (44–67) |
| Sex, n/N (%) | ||
| Female | 21/55 (38.2) | 16/54 (29.6) |
| Male | 34/55 (61.8) | 38/54 (70.4) |
| Weight, kg, median (25–75th percentile) | 77 (64–83) | 79 (70–94) |
| Body Mass Index, kg/m2, median (25–75th percentile) | 25 (22–29) | 27 (23–31) |
| Race, n/N (%) | ||
| White | 55/55 (100) | 53/54 (98.1) |
| Asian | 0 (0.0) | 1/54 (1.9) |
| African | 0 (0.0) | 0/54 (0) |
| Functional/Chronic Health status, n/N (%) | ||
| 1–2 (normal activity or moderate limitation) | 52/55 (94.5) | 51/54 (94.4) |
| 3–5 (Severe activity limitation to dependent) | 3/55 (5.5) | 3/54 (5.6) |
| Comorbidities, n/N (%) | ||
| Cardiac insufficiency (NYHA > 2, Marked limitation of physical activity) | 1/55 (1.8) | 3/54 (5.6) |
| Chronic kidney failure (MDRD GFR < 50 mL/min) | 0/55 (0) | 0/54 (0) |
| Neurological history | 5/55 (9.1) | 9/54 (16.7) |
| Chronic obstructive pulmonary disease | 1/55 (1.8) | 3/54 (5.6) |
| Active smoking | 15/55 (27.3) | 19/54 (35.2) |
| Diabetes mellitus | 5/55 (9.1) | 6/54 (11.1) |
| Cancer within 5 last years | 1/55 (1.8) | 4/54 (7.4) |
| Main diagnosis leading to hospitalization, n/N (%) | ||
| Trauma | 27/55 (49.1) | 24/54 (44.4) |
| Medical non-septic | 16/55 (29.1) | 22/54 (40.7) |
| Surgical | 9/55 (16.4) | 6/54 (11.1) |
| Sepsis | 3/55 (5.5) | 2/54 (3.7) |
| Main diagnoses on ICU admission, n/N (%) | ||
| Trauma | 27/55 (49.1) | 24/54 (44.4) |
| Surgical | 17/55 (30.9) | 20/54 (37) |
| Medical non septic | 9/55 (16.4) | 6/54 (11.1) |
| Sepsis | 2/55 (3.6) | 4/54 (7.4) |
| Time from hospitalization to randomization, days median (25–75th percentile) | 1 (1–2) | 1 (1–2) |
| Time from ICU admission to randomization, hours median (25–75th percentile) | 31 (18–43) | 24 (17–33) |
| SAPS-II, median (25–75th percentile) | 45 (37–54) | 45 (37–52) |
| SOFA, median (25–75th percentile) | 7 (5–10) | 7 (5–9) |
| Organ failure at inclusion, yes, n/N (%) | ||
| Neurological/comatosea | 48/55 (87.3) | 43/54 (79.6) |
| Hemodynamicb | 20/55 (36.4) | 15/54 (27.8) |
| Respiratoryc | 7/55 (12.7) | 8/54 (14.8) |
| Kidneyd | 3/55 (5.5) | 4/54 (7.4) |
| Laboratory values at baseline, median (25–75th percentile) | ||
| Total Leukocytes, G/L | 11.4 (9.5–15.6) | 12.9 (10–15.9) |
| Lymphocytes, G/L | 0.9 (0.7–1.2) | 0.9 (0.7–1.3) |
| Neutrophils, G/L | 8.9 (7.4–13) | 10.0 (7.6–13) |
| Monocytes, G/L | 0.9 (0.6–1.2) | 0.9 (0.7–1.2) |
| Creatinine, µmol/L | 63 (54–83) | 65 (53–99) |
| Steroid therapy at inclusion (< 48 h), n/N (%) | 18/55 (32.7) | 12/53 (22.6) |
NYHA New York Heart Association, SAPS-II simplified acute physiological score II, SOFA Sequential Organ Failure Assessment
aComatose, defined as Glasgow coma scale < 13 before sedation
bShock, defined as norepinephrine, epinephrine, or any other vasopressor at a dose of ≥ 0.1 μg per kilogram of body weight per minute or ≥ 0.5 mg per hour for at least 6 h
cDefined as PaO2/FiO2 < 200
dCreatinine blood level > twofold higher than the basal value and/or oliguria < 0.5 mL/kg/h for at less 12 h
Primary outcome
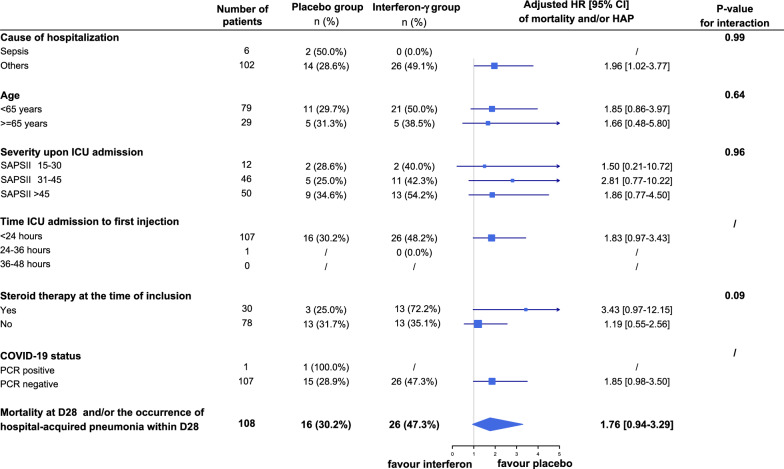
Overall, 26 of 55 participants (47.3%) in the interferon-gamma group and 16 of 53 (30.2%) in the placebo group had died or developed HAP 28 days after inclusion (crude hazard ratio (HR) 1.83, 95% CI 0.98–3.41, P = 0.06; adjusted HR 1.76, 95% CI 0.94–3.29; P = 0.08, Table 2). The treatment effect estimation did not vary significantly across centers (P = 0.27 for interaction, ESM eFig. 2). No interaction between the treatment effect and the six predefined subgroups was observed (Fig. 1). The HR for the primary outcome in non-septic patients was 1.96 (95% CI 1.02–3.77) and 3.43 (95% CI 0.97–12.15) in patients receiving steroid therapy at the time of inclusion.
Table 2.
Outcomes
| Interferon-gamma group | Placebo group | Adj. OR (95% CI)a Adj. HR (95% CI)a or Adj. Difference (95% CI) |
|
|---|---|---|---|
| Primary outcome: HAP or all-cause mortality on day 28, n/N (%) | 26/55 (47.3) | 16/53 (30.2) | 1.76 (0.94–3.29) |
| Components of the primary outcome, n/N (%) | |||
| All-cause mortality at day 28, yes | 7/55 (12.7) | 9/53 (17) | 0.68 (0.25–1.85) |
| HAP, yes | 19/55 (34.5) | 8/53 (15.1) | 2.06 (0.92–4.57) |
| Type of HAP, n/N (%) | |||
| HAP | 0 (0) | 0 (0) | |
| Ventilated-HAP | 0 (0) | 1/8 (12.5) | – |
| Ventilator-Associated Pneumonia | 19/19 (100) | 7/8 (87.5) | |
| Time of first HAP episode, days, median (25-75th percentile) | 3 (3–5) | 3 (2.5–6) | – |
| Early HAP (< day 5), n/N (%) | 12/55 (21.8) | 5/53 (9.4) | 2.53 (0.80–8.03) |
| Late HAP (> = 5 days), n/N (%) | 7/55 (12.7) | 3/53 (5.7) | 2.57 (0.61–10.91) |
| Severity of hospital-acquired pneumonia | |||
| PaO2/FiO2 ratio, median (25–75th percentile) | 158 (110–171) | 124 (107–186) | – |
| Complications of HAP, yes, n/N (%) | |||
| Pleurisy | 0/19 (0) | 1/8 (12.5) | – |
| Bacteriemia | 3/19 (15.8) | 0/8 (0) | – |
| Ventilator-associated tracheobronchitis, yes, n/N (%) | 6/55 (10.9) | 18/54 (34) | 0.24 (0.08–0.68) |
| Acute respiratory distress syndrome at day 28, n/N (%) | 6/49 (12.2) | 2/47 (4.3) | 2.72 (0.33–22.41) |
| If yes, time in days, median (25–75th percentile) | 3 (3–5) | 3 (3–3) | – |
| If yes, lowest PaO2/FiO2, median (25–75th percentile) | 87 (77–110) | 105 (73–136) | – |
| Non-respiratory infections yes, n/N (%) | |||
| Urinary tract infection | 11/55 (20) | 12/53 (22.6) | 0.92 (0.32–2.7) |
| Septicemia, catheter-related infections | 6/55 (10.9) | 9/53 (17) | 0.57 (0.18–1.76) |
| Surgical site infection | 1/55 (1.8) | 4/53 (7.5) | 0.25 (0.03–2.37) |
| Invasive candidiasis | 1/55 (1.8) | 0/53 (0) | – |
| Infection, yes, n/N (%) | 37/55 (67.3) | 37/54 (69.8) | 0.89 (0.38–2.1) |
| Time on antimicrobial therapy, days, median survival (25–75th percentile) | 9 (7–12) | 8 (5–9) | 0.83 (0.56–1.24) |
| Antibiotic-free days at day 28, median (25–75th percentile) | 18 (5–15) | 18.(8–22) | 0 (– 4 to 3)b |
| Time on invasive mechanical ventilation, days, median survival (25–75th percentile) | 13 (7–22) | 14 (7–22) | 1.08 (0.7–1.66) |
| Ventilation-free days at day 90, median (25–75th percentile) | 76 (54–83) | 72 (12–83) | 1 (– 1 to 7)b |
| Time on ICU hospitalisation, days, median survival (25–75th percentile) | 18 (15–26) | 22 (14–28) | 0.95 (0.61–1.47) |
| ICU-free days at day 90, median (25–75th percentile) | 72 (28–77) | 66 (7–78) | 0 (– 3 to 8)b |
| Time on hospitalisation, days, median survival (25–75th percentile) | 50 (31–62) | 47 (36–63) | 1.19 (0.76–1.88) |
| Hospital-free days at day 90, median (25–75th percentile) | 40 (0–63) | 38 (0–63) | 0 (– 5 to 9)** |
| All-cause mortality at day 90, yes, n/N (%) | 13/55 (23.6) | 13/52 (25) | 0.88 (0.4 –1.93) |
HAP hospital-acquired pneumonia
aAdjusted on stratification factor (sepsis or not) as a fixed effect and on centers as a random effect
bMedian of difference were estimated with the Hodges-Lehmann approach
Fig. 1.
Hazard ratio of the primary outcome in prespecified subgroups. Odds ratio of the primary outcome in 6 prespecified subgroups. Square size representing the hazard ratio reflects the relative numbers in each subgroup, and horizontal bars represent 95% confidence intervals. Adjusted HR > 1 means a higher risk of mortality and/or HAP with the intervention. No statistical power analysis was performed and no adjustment were made for multiplicity in the analysis of subgroups. 95% CIs around estimates for subgroup analyses should not be used to infer definitive treatment effects. P values are for heterogeneity of the effect of the trial regimen on the primary outcome in each subgroup. HAP hospital-acquired pneumonia, SAPS-II Simplified Acute Physiological Score II
In the per-protocol analysis, including randomized participants without major study protocol dropout, little change was found in estimating the intervention effect on the primary outcome (adjusted HR 1.66, 95% CI 0.86–3.18).
When considering the components of the primary outcome separately, the mortality rate on day 28 was 12.7% (7 of 49 participants) in the intervention group and 17% (9 of 47) in the placebo group (adjusted HR 0.68, 95% CI 0.25 to 1.85, Table 2). On day 28, 19 (34.5%) patients in the interferon-gamma group had developed HAP, vs 8 (15.1%) in the placebo group (adjusted HR 2.06, 95% CI 0.92–4.57, Table 2). The description of causative pathogens did not find significant differences between the two groups (ESM eTable 2).
Secondary outcomes
Secondary outcomes are described in Table 2. The percentages of patients with acute respiratory distress syndrome on day 28 were 12% (6 of 55) in the interferon-gamma group and 4.3% (2 of 54) in the placebo group (adjusted odds ratio 2.72; 95% CI 0.33–22.41). The numbers of intensive care unit-free days on day 90 were 72 (interquartile range (IQR) 28–77) in the intervention group and 66 (IQR 7–78) in the placebo group (adjusted absolute difference 0 day; 95% CI − 3 to 8). On day 90, 13 (23.6%) patients were dead in the interferon-gamma group, and 13 (25%) patients in the placebo group (adj. HR 0.88; 95% CI 0.40–1.93).
Exploratory post hoc secondary outcomes
HAP severity evaluation and rates of non-respiratory hospital-acquired infections are described in Table 2. The adjusted odds ratio for early and late HAP were 2.53 (95% CI 0.80–8.03) and 2.57 (95% CI 0.61–10.91) with interferon gamma-1b, respectively. The lowest PaO2/FiO2 ratio during HAP was 158 (110–171) in the interferon-gamma group and 124 (107–186) in the placebo group. The rates of urinary tract infections were 20% (11/55 patients) in the interferon group and 22.6% (12/53 patients) with placebo (adj. OR 0.92, 95% CI 0.32–2.70).
Physiological measurements over the first 15 days
The changes from day 1 to day 15 after randomization in physiological measurements were compared between the two study groups. The intervention was not significantly associated with the time course of the mean values of the Sequential Organ Failure Assessment (SOFA) score, PaO2/FiO2 ratio, creatinine blood level, or counts of lymphocytes, neutrophils, and monocytes (ESM eFig. 3 and eTable 4 for the estimations of the time effect and interaction between time and intervention). The intervention was significantly associated with lower gamma-glutamyl transferase concentration but not with body temperature, total leukocyte count, lymphocytes, blood levels of lipase, liver transaminases, alkaline phosphatase, or total bilirubin (ESM eFig. 3).
Adverse events
Twenty-four (43.6%) patients in the interferon-gamma group developed serious adverse events, as compared to 17 (31.5%) patients in the placebo group (p = 0.19, Table 3 and ESM eTable 3 for a complete list of severe adverse events). The mean number of serious adverse events per patient was 0.9 (1.3) in the interferon-gamma group and 0.5 (0.8) in the placebo group (p = 0.14). Biological pancreatitis was recorded in 12 (21.8%) patients receiving interferon-gamma and 7 (13%) patients treated by placebo (p = 0.22).
Table 3.
Adverse events
| Interferon-gamma group (N = 55) | Placebo group (N = 54) | P-values | |
|---|---|---|---|
| Serious adverse events, n (%) | |||
| Number of patients with 1 or more event | 24 (43.6) | 17 (31.5) | 0.19 |
| Numbers of event/patient, mean (SD) | 0.9 (1.3) | 0.5 (0.8) | 0.14 |
| Adverse events, n (%) | |||
| Number of patients with 1 or more event | 52 (94.5) | 51 (94.4) | 1.00 |
| Numbers of event/patient, mean (SD) | 4.1 (3.1) | 2.8 (1.8) | 0.06 |
| General disorder, n (%) | |||
| Pyrexia | 3 (5.5) | 3 (5.6) | 1.00 |
| Systemic inflammatory response | 1 (1.8) | 0 (0) | 1.00 |
| Skin and subcutaneous disorders, n (%) | |||
| Drug eruption | 1 (1.8) | 0 (0) | 1.00 |
| Systemic rash | 0 (0) | 1 (1.9) | 0.50 |
| Digestive adverse events, n (%) | |||
| Liver cytolysis (enzymes > 5 Normal values) | 9 (16.4) | 7 (13) | 0.62 |
| Cholestasis | 4 (7.3) | 3 (5.6) | 1.00 |
| Pancreatitis (lipase > 3 Normal values) | 12 (21.8) | 7 (13) | 0.22 |
| Cardiac adverse events, n (%) | |||
| Atrial fibrillation | 3 (5.5) | 2 (3.7) | 1.00 |
| Bradycardia | 1 (1.8) | 0 (0) | 1.00 |
| Nervous system disorders, n (%) | |||
| Altered level of consciousness | 2 (3.6) | 1 (1.9) | 1.00 |
| Psychiatric disorders | |||
| Agitation, confusion | 3 (5.5) | 2 (3.7) | 1.00 |
| Vascular disorders, n (%) | |||
| Vein thrombosis | 1 (1.8) | 0 (0) | 1.00 |
| Hypotension | 2 (3.6) | 0 (0) | 0.50 |
Inflammatory responses over the first 7 days
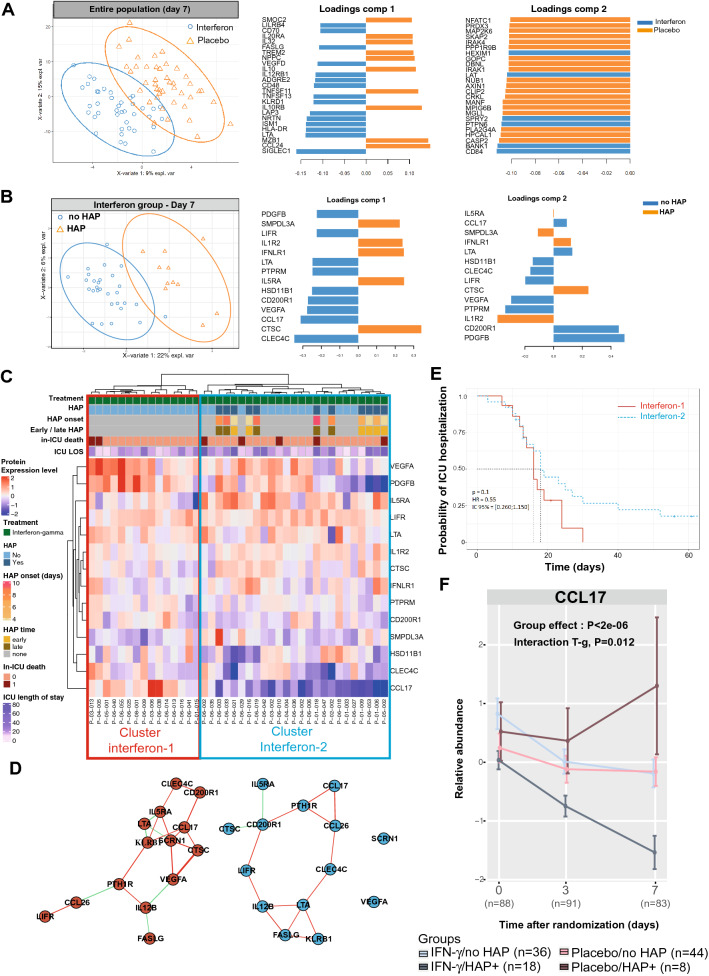
To further understand the clinical response to interferon gamma-1b, we examined the inflammatory blood profile in 10 healthy controls and the 108 patients of the trial. Out of the 384 tested proteins, we found that the inflammatory protein landscape differed between healthy controls and patients at all the tested time points (ESM eFig. 3A). The circulating inflammatory profiles on day 7 differed between the placebo and the interferon groups (Fig. 2A). The proteins the most associated with interferon gamma-1b treatment on day 3 and day 7 were involved in antigen-presenting cell-lymphocyte interactions, including SIGLEC1 (a macrophage-restricted adhesion molecule that mediates binding to lymphocyte) and HLA-DR (alpha chain of antigen-presenting major histocompatibility complex class II essential for antigen presentation by myeloid cells). In contrast, the placebo was associated with IL-10 (a potent anti-inflammatory cytokine) and chemoattractants for T cells (CXCL12 and CCL24) (Fig. 2A and ESM eFig. 3B).
Fig. 2.
Time course of inflammatory profiles. A, ) PLSDA representations of the 384- inflammatory protein distributions on day 7 in (A) all the patients and (B) in the interferon-gamma group. C Unsupervised heatmap of the filtered proteins on day 7 in patients treated with interferon gamma. D Correlation network of the protein from clusters interferon-1 and interferon-2. E Kaplan–Meier estimates of the ICU hospitalization rate among patients of clusters interferon-1 and interferon-2. F Relative expressions of CCL17 in patients with or without HAP, treated with placebo or interferon gamma-1b. Data are presented as mean with standard error of mean. P-values calculated by linear mixed-effects models are reported in eTable 5. Interaction (T-g): Interaction between the effects of time and of groups (treatment)
We then questioned if critically ill patients responded homogeneously to interferon gamma-1b. To respond to this question, we compared the protein profiles in patients of the interferon-gamma group with or without HAP. The inflammatory profiles of patients with or without HAP differed on day 3 and day 7 (Fig. 2B and ESM eFig. 4A, B). Two clusters with different network architectures were defined in the unsupervised heatmap of protein levels in patients treated with interferon gamma-1b (Fig. 2C, D). Supporting the clinical importance of these sub-phenotypes of response to interferon gamma-1b, the rates of HAP were 0% (0/15 patients) in the cluster interferon-1 and 44% (11/25 patients) in the cluster interferon-2 (p = 0.008). The median ICU length of stay was 16 (13–20) days in the interferon-1 and 18 (13–30) days in the interferon-2 cluster (HR 0.55, 95% CI 0.26–1.15, p = 0.10) (Fig. 2E).
Finally, we questioned if the association between this cluster and HAP was also observed in the placebo group. To respond to this question, we investigated the time course of CCL17, a chemokine involved in the CCR4-dependent recruitment of memory T cells, which was the main contributor associated with interferon-2 cluster. Supporting a specific effect of the intervention during the follow-up, CCL17 levels decreased in the interferon group but not in the placebo group (p < 0.002, ESM eFig. 5C). More precisely, the levels of CCL17 decreased in HAP patients treated with interferon-gamma and increased in HAP patients of the placebo group (p < 2e−06 for group effect, Fig. 2F).
Discussion
This multicenter, randomized, placebo-controlled clinical trial involving adults with acute organ failure who received invasive mechanical ventilation in the ICU was discontinued after the second safety analysis by DSMB because of safety concerns with interferon gamma-1b. The predefined subgroup analyses suggested that the tolerance could be lower in non-septic patients and patients receiving steroids at the time of inclusion. This was an unexpected finding, and the secondary outcome analyses—which included the evaluation of blood cell counts, organ failure parameters measured up to day 15, HAP severity evaluation and rates of non-respiratory infections, and inflammatory profiling—determined a putative mechanism for harm. We have also observed an association between a decrease in CCL17 blood levels and respiratory complications during interferon-gamma treatment.
These results differ from those reported in the case series of interferon gamma-1b treatment for the prevention of infection after severe trauma [33] or for the treatment of septic shock [19], refractory infections [20], and difficult-to-treat infections [21]. In these reports, biomarkers associated with immune restoration, such as increased HLA-DR membrane expression by monocytes, usually appeared after three days of treatment and were associated with favorable clinical outcomes. While the timing of initiation, the dose, and the duration regimen were not standardized in most of these case series, this trial tested a high dose of interferon gamma-1b, administered on average within 25 h after admission to the ICU. This timing was chosen because the trial aimed to test interferon gamma-1b for preventing hospital-acquired pneumonia that frequently develops during the first week of hospitalization. However, the interferon gamma-1b treatment effects could significantly differ between early and late injections, given that the immune status of critically ill patients rapidly changes from an early systemic inflammatory response to a late systemic compensatory anti-inflammatory response [34, 35]. The effects of interferon-gamma 1b as rescue therapy for difficult-to-treat late infections remain to be evaluated.
The scientific rationale of this trial was to restore immune competency in patients with critical illness-related immunosuppression [34, 35]. Numerous biomarkers have been developed to define this state biologically, but none is recommended because they are neither readily accessible in critical care nor validated in independent cohorts. We thus treated critically ill patients with clinical risk factors of hospital-acquired infections without performing an immune assessment before inclusion because this strategy increases the treatment feasibility and the extrapolation to other ICUs and countries. The high rates of hospital-acquired infections in the placebo group demonstrate the efficiency of this clinical evaluation in selecting patients at risk of complications.
Immune phenotyping, which is critical for implementing tailored immune therapy, should reach two complementary aims: first, the selection of patients at risk of complications, and second, the prediction of the response to treatment. In this setting, proinflammatory phenotypes (e.g., high blood levels of ferritin or C-reactive proteins) are usually associated with acute organ failure, while immunosuppressive states (usually defined as low HLA-DR expression on circulating monocytes) are associated with secondary infections [36, 37]. Besides this classical dichotomy, we hypothesized that other phenotypes based on blood levels of pro-inflammatory cytokines or host-microbiome interactions could be more efficient in predicting interferon-gamma response [11]. We thus decided to perform a phase II clinical trial with regular safety evaluations to ensure the patient’s safety, associated with a thorough investigation of the inflammatory response to discriminate favorable and unfavorable responses to treatment. While interferon-gamma treatment has been proposed as rescue therapy for difficult-to-treat infections or lung superinfection [21, 38], our results suggest caution before implementation and that a decrease in CCL17 blood levels could help to identify unfavorable responses to interferon-gamma early.
The tolerance and efficacy of a personalized approach to interferon-gamma therapy remain to be investigated in critically ill patients. Our results demonstrated that interferon-gamma treatment could affect differentially proinflammatory and anti-inflammatory subphenotypes of immune response to critical illness. On day 7, the inflammatory response to interferon gamma-1b was driven by proteins involved in antigen-presenting cell-lymphocyte interactions. We found that low levels of CCL17, a chemokine involved in the recruitment of Th2 anti-inflammatory memory T cells, were associated with HAP in the interferon-gamma group. These results suggested that reducing Th2 immune response could be associated with respiration complications in critically ill patients and that monitoring these two inflammatory mediators could help to predict the response to immune therapies in critically ill patients.
The interaction test between the interferon gamma-1b effect and steroid therapy did not reach statistical significance. Still, this pre-planned subgroup analysis suggested a higher risk of the primary outcome with interferon gamma-1b in patients treated with steroids at the time of inclusion. The observations that hydrocortisone increased the blood levels of interferon-γ in septic patients [39] and the production of interferon-γ by natural killer cells in severe trauma patients [40] strengthen the hypothesis that hydrocortisone can increase the immune response to interferon gamma-1b. High levels of CCL17 were also associated with the response to steroid therapy in COVID-19 patients [41], suggesting that interferon-gamma and steroids modulate their immunological effects in critically ill patients.
The trial was discontinued at the second safety analysis due to concern about a higher rate of respiratory complications in patients receiving the treatment. While the liver and pancreatic reactions are recognized adverse effects of interferon gamma-1b, prolonged administrations have not been associated with respiratory toxicity in patients with idiopathic pulmonary fibrosis [42] or tuberculosis [43]. The poor lung tolerance of interferon-gamma in critically ill patients suggests a specific immune response to treatment in this population. The lung recruitment of proinflammatory monocytes that are boosted by interferon-gamma and regulate immune response to pneumonia could have decreased the lung tolerance of interferon gamma-1b treatment in critically ill patients [44, 45]. Moreover, in brain-injured patients, who represent 83.5% of our study population, a decreased monocyte response to interferon was associated with a lower risk of herpes simplex virus pneumonia and favorable neurological outcome [16]. Together with the currently reported increased risk of the primary outcome in the subgroup of non-septic patients, all these observations suggest that decreasing interferon-gamma function could be an appropriate physiologic response to non-septic critical illness.
The intervention altered neither the mortality rate nor the number of ventilator-free days on day 90. These two factors are usually associated with pneumonia severity [46]. This discrepancy suggests that interferon gamma-1b treatment did not induce protracted side effects that would persist after treatment discontinuation. However, this could also be explained by the reduction of the statistical power after the early study termination.
The strengths of our analyses include the use of an indiscernible placebo and blinded adjudication to limit ascertainment bias, a median enrollment time of approximately 25 h after ICU admission, high protocol adherence to a standardized dose regimen, 90-day follow-up, and efficient trial supervision that has ensured timely trial discontinuation to ensure patient safety.
Our study has several limitations. First, interpreting the intervention effect on the primary composite outcome could be challenging, notably because of the severity range of its components. The difference in the primary composite outcome was mainly driven by the rate of HAP in the interferon-gamma-1b group, an event associated with unfavorable outcomes in critically ill patients, but whose attributable mortality is discussed. Second, the study could lack the power to describe all the intervention effects, but the study discontinuation was decided to guarantee the safety of the participating patients. Third, the heterogeneity of the enrolled population, with different causes of organ failures, could explain the lack of clinical efficacy. Notably, 80% of the patients suffered from neurological failure that complicates brain injury, which could be a medical condition with specific lung responses to pathogens and immune therapies [47]. Fourth, because of the potential for type 1 error due to multiple comparisons, secondary endpoints and post hoc analysis findings should be interpreted as exploratory. Fifth, patients were screened in three European countries, but patients were only included in France due to the early study discontinuation. The extrapolation of the results to other countries is thus limited.
In adults with acute organ failure under invasive mechanical ventilation, interferon gamma-1b treatment was not superior to placebo with regard to all-cause mortality or hospital-acquired pneumonia on day 28. Furthermore, the trial was discontinued early for safety concerns since interferon gamma-1b could even be associated with an increased risk of respiratory adverse events. We have observed that the unfavorable profile of interferon gamma-1b for the prevention of HAP is a consequence of its inflammatory modulatory effect, notably by the modulation of CCL17 level, and proposed that selection of patients based on immune monitoring should be considered in future clinical trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the families and patients who participated in the study and the clinical and research staff at all trial sites. We thank Maelle Ningre, Amelie Guisseau, Emilie Le Blanc, Martine Tching-Sin, and Tanguy Roman for their technical and administrative support. Atlanrea study group and the Société Française d’Anesthésie Réanimation (SFAR) Research Network: Nicolas Grillot MD: Service d’Anesthésie Réanimation, CHU Nantes Nantes, France, Karim Asehnoune MD: Service d’Anesthésie Réanimation, CHU Nantes Nantes, France, Alexandre Bourdiol MD: Service d’Anesthésie Réanimation, CHU Nantes Nantes, France, Dominique Demeure dit latte MD: Service d’Anesthésie Réanimation, CHU Nantes Nantes, France, Apostolos Armaganidis MD: Attikon University Hospital, Athens, Greece Athens, France, Nicolas Nesseler MD: Service d’Anesthésie Réanimation, CHU Rennes Rennes, France, Philippe Seguin MD: Service d’Anesthésie Réanimation, CHU Rennes Rennes, France
Author contributions
AR obtained funds from the European Union’s Horizon 2020 research and innovation program to undertake the study. AR, DK, and AT were responsible for conceiving and designing the study, interpreting data, and writing the report. FF performed the statistical analysis and was responsible for data management and statistical analysis. All authors participated in study management, data collection, and data interpretation. All authors have agreed to submit the data for publication.
Funding
Supported by a grant from the European Union’s Horizon 2020 research and innovation program under grant agreement No 847782. The funding organization had no role in designing and conducting the study; collecting, managing, analyzing, and interpreting the data; preparing, reviewing, or approving the manuscript; and deciding to submit the manuscript for publication.
Data availability
Patient-level data and full dataset and statistical code will be available upon reasonable request to the corresponding author. Consent was not obtained, but the presented data are anonymized, and the risk of identification is low. The potential benefits of sharing these data outweigh the potential harm.
Declarations
Conflicts of interest
AR reports receiving grants and consulting fees from Merck and bioMerieux. Other authors declare that they have no conflicts of interest involving the work under consideration for publication. No compensation was received for this study. AT has conflicts of interest with BioMerieux, Pfizer, and MSD (advisory boards or lectures).
Access to data and data analysis
AR had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. FF conducted and was responsible for data analysis.
Data sharing
See electronic supplementary material 6.
Data and Safety Monitoring Board
Filipe Froes (Hospital Pulido Valente, Portugal), Marylaure Gavard (Grenoble University Hospital, France), Bruno Giraudeau (University of Tours, France), Marc Leone (AP/HM, France) and Pedro Póvoa (São Francisco Xavier Hospital, Portugal).
Footnotes
The members of the Atlanrea study group are listed in the Acknowledgements
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Antoine Roquilly, Email: antoine.roquilly@chu-nantes.fr.
the Atlanrea study group and the Société Française d’Anesthésie Réanimation (SFAR) Research Network:
Nicolas Grillot, Karim Asehnoune, Alexandre Bourdiol, Dominique Demeure dit latte, Apostolos Armaganidis, Nicolas Nesseler, and Philippe Seguin
References
- 1.Sipilä PN, Heikkilä N, Lindbohm JV, et al. Hospital-treated infectious diseases and the risk of dementia: a large, multicohort, observational study with a replication cohort. Lancet Infect Dis. 2021;21:1557–1567. doi: 10.1016/s1473-3099(21)00144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bekaert M, Timsit J-F, Vansteelandt S, et al. Attributable mortality of ventilator-associated pneumonia. Am J Resp Crit Care Med. 2012;184:1133–1139. doi: 10.1164/rccm.201105-0867oc. [DOI] [PubMed] [Google Scholar]
- 3.Eber MR, Laxminarayan R, Perencevich EN, Malani A. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Arch Intern Med. 2010;170:347–353. doi: 10.1001/archinternmed.2009.509. [DOI] [PubMed] [Google Scholar]
- 4.Leone M, Bouadma L, Bouhemad B, et al. Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med. 2018;37:83–98. doi: 10.1016/j.accpm.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 6.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roquilly A, Chanques G, Lasocki S, et al. Implementation of French Recommendations for the Prevention and the Treatment of Hospital-acquired Pneumonia: a cluster-randomized trial. Clin Infect Dis. 2020;73:e1601–e1610. doi: 10.1093/cid/ciaa1441. [DOI] [PubMed] [Google Scholar]
- 8.Luyt C-E, Bouadma L, Morris AC, et al. Pulmonary infections complicating ARDS. Intensive Care Med. 2020;46:2168–2183. doi: 10.1007/s00134-020-06292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:nri3552. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:nri.2017.36. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 11.Roquilly A, Torres A, Villadangos JA, et al. Pathophysiological role of respiratory dysbiosis in hospital-acquired pneumonia. Lancet Respir Med. 2019;7:710–720. doi: 10.1016/s2213-2600(19)30140-7. [DOI] [PubMed] [Google Scholar]
- 12.Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126:23–31. doi: 10.1172/jci82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogunovic D, Byun M, Durfee LA, et al. Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorman SE, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113–2121. doi: 10.1016/s0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 15.Roquilly A, McWilliam HEG, Jacqueline C, et al. Local modulation of antigen-presenting cell development after resolution of pneumonia induces long-term susceptibility to secondary infections. Immunity. 2017;47:135–147.e5. doi: 10.1016/j.immuni.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Chaumette T, Cinotti R, Mollé A, et al. Monocyte signature associated with herpes simplex virus reactivation and neurological recovery after brain injury. Am J Resp Crit Care Med. 2022;206:295–310. doi: 10.1164/rccm.202110-2324oc. [DOI] [PubMed] [Google Scholar]
- 17.Roquilly A, David G, Cinotti R, et al. Role of IL-12 in overcoming the low responsiveness of NK cells to missing self after traumatic brain injury. Clin Immunol. 2017;177:87–94. doi: 10.1016/j.clim.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S-C, Scicluna BP, Arts RJW, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol. 2016;17:406–413. doi: 10.1038/ni.3398. [DOI] [PubMed] [Google Scholar]
- 19.Döcke W-D, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 20.Payen D, Faivre V, Miatello J, et al. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. Bmc Infect Dis. 2019;19:931. doi: 10.1186/s12879-019-4526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derungs T, Leo F, Loddenkemper C, Schneider T. Treatment of disseminated nocardiosis: a host–pathogen approach with adjuvant interferon gamma. Lancet Infect Dis. 2021;21:e334–e340. doi: 10.1016/s1473-3099(20)30920-8. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen LS, Hamou ZA, Gastli N, et al. Potential role for interferon gamma in the treatment of recurrent ventilator-acquired pneumonia in patients with COVID-19: a hypothesis. Intensive Care Med. 2021;47:619–621. doi: 10.1007/s00134-021-06377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnstone J, Meade M, Lauzier F, et al. Effect of Probiotics on Incident Ventilator-Associated Pneumonia in Critically Ill Patients. JAMA. 2021;326:1024–1033. doi: 10.1001/jama.2021.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dale CM, Rose L, Carbone S, et al. Effect of oral chlorhexidine de-adoption and implementation of an oral care bundle on mortality for mechanically ventilated patients in the intensive care unit (CHORAL): a multicenter stepped wedge cluster-randomized controlled trial. Intensive Care Med. 2021;47:1295–1302. doi: 10.1007/s00134-021-06475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troendle JF, Leifer ES, Kunz L. Dealing with competing risks in clinical trials: How to choose the primary efficacy analysis? Stat Med. 2018;37:2787–2796. doi: 10.1002/sim.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Loeches I, Povoa P, Rodríguez A, et al. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Medicine. 2015;3:859–868. doi: 10.1016/s2213-2600(15)00326-4. [DOI] [PubMed] [Google Scholar]
- 27.Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 28.Rohart F, Gautier B, Singh A, Cao K-AL. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLos Comput Biol. 2017;13:e1005752. doi: 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–1358. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suhre K, Sarwath H, Engelke R, et al. Identification of robust protein associations with COVID-19 disease based on five clinical studies. Front Immunol. 2022;12:781100. doi: 10.3389/fimmu.2021.781100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.François B, Cariou A, Clere-Jehl R, et al. Prevention of early ventilator-associated pneumonia after cardiac arrest. New Engl J Med. 2019;381:1831–1842. doi: 10.1056/nejmoa1812379. [DOI] [PubMed] [Google Scholar]
- 32.Asehnoune K, Seguin P, Allary J, et al. Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): a double-blind, multicentre phase 3, randomized placebo-controlled trial. Lancet Respir Med. 2014;2:706–716. doi: 10.1016/s2213-2600(14)70144-4. [DOI] [PubMed] [Google Scholar]
- 33.Polk HC, Cheadle WG, Livingston DH, et al. A randomized prospective clinical trial to determine the efficacy of interferon-γ in severely injured patients. Am J Surg. 1992;163:191–196. doi: 10.1016/0002-9610(92)90099-d. [DOI] [PubMed] [Google Scholar]
- 34.Bouras M, Asehnoune K, Roquilly A. Contribution of dendritic cell responses to sepsis-induced immunosuppression and to susceptibility to secondary pneumonia. Front Immunol. 2018;9:2590. doi: 10.3389/fimmu.2018.02590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubio I, Osuchowski MF, Shankar-Hari M, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. 2019;19:e422–e436. doi: 10.1016/s1473-3099(19)30567-5. [DOI] [PubMed] [Google Scholar]
- 36.Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2017 doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 37.Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, et al. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. Bmc Med. 2017;15:172. doi: 10.1186/s12916-017-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feys S, Gonçalves SM, Khan M, et al. Lung epithelial and myeloid innate immunity in influenza-associated or COVID-19-associated pulmonary aspergillosis: an observational study. Lancet Respir Med. 2022 doi: 10.1016/s2213-2600(22)00259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock. Am J Resp Crit Care Med. 2003;167:512–520. doi: 10.1164/rccm.200205-446oc. [DOI] [PubMed] [Google Scholar]
- 40.Roquilly A, Broquet A, Jacqueline C, et al. Hydrocortisone prevents immunosuppression by Interleukin-10+ natural killer cells after trauma-hemorrhage. Crit Care Med. 2014;42:e752–e761. doi: 10.1097/ccm.0000000000000658. [DOI] [PubMed] [Google Scholar]
- 41.Baker JR, Mahdi M, Nicolau DV, et al. Early Th2 inflammation in the upper respiratory mucosa as a predictor of severe COVID-19 and modulation by early treatment with inhaled corticosteroids: a mechanistic analysis. Lancet Respir Med. 2022;10:545–556. doi: 10.1016/s2213-2600(22)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King TE, Albera C, Bradford WZ, et al. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomized, placebo-controlled trial. Lancet. 2009;374:222–228. doi: 10.1016/s0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]
- 43.Gao X-F, Yang Z-W, Li J. Adjunctive therapy with interferon-gamma for the treatment of pulmonary tuberculosis: a systematic review. Int J Infect Dis. 2011;15:e594–e600. doi: 10.1016/j.ijid.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Li F, Piattini F, Pohlmeier L, et al. Monocyte-derived alveolar macrophages autonomously determine severe outcome of respiratory viral infection. Sci Immunol. 2022;7:eabj5761. doi: 10.1126/sciimmunol.abj5761. [DOI] [PubMed] [Google Scholar]
- 45.Maquet C, Baiwir J, Loos P, et al. Ly6Chi monocytes balance regulatory and cytotoxic CD4 T cell responses to control virus-induced immunopathology. Sci Immunol. 2022;7:eabn3240. doi: 10.1126/sciimmunol.abn3240. [DOI] [PubMed] [Google Scholar]
- 46.Timsit J-F, de Kraker MEA, Sommer H, et al. Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: a perspective from COMBACTE’s STAT-Net. Intensive Care Med. 2017;43:1002–1012. doi: 10.1007/s00134-017-4802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meisel C, Schwab JM, Prass K, et al. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient-level data and full dataset and statistical code will be available upon reasonable request to the corresponding author. Consent was not obtained, but the presented data are anonymized, and the risk of identification is low. The potential benefits of sharing these data outweigh the potential harm.