Rationale and aims for long-term atrial fibrillation screening/search
Atrial fibrillation (AF) is the most common clinical arrhythmia with substantial health and socioeconomic impact on healthcare.1 The number of people affected by this condition was estimated to be 33.5 million in 2010, with an increasing prevalence and incidence over the coming years.2 In 2017, there were 37.6 million [95% confidence interval (CI): 32.5–42.6 million] individuals with AF/atrial flutter globally.3 The estimated number of subjects with AF in 2030 in Europe will be 14–17 million, and the number of new cases of AF per year at 120 000–215 000.4 Atrial fibrillation is independently associated with increased mortality and morbidity from complications such as ischaemic stroke, dementia, and cognitive dysfunction.5 Oral anticoagulation can significantly reduce the risk of stroke, dementia, and death.6 As a part of a holistic or integrated approach to AF care, it is associated with improved outcomes7–9 and is advocated in guidelines.10,11
In many patients, AF can be asymptomatic, and the diagnosis is established after the appearance of a complication typically associated with AF, such as ischaemic stroke/systemic embolic or heart failure. For example, the Event Monitoring Belt for Recording Atrial Fibrillation After a Cerebral Ischemic Event (EMBRACE) study showed that in patients after transient ischaemic attack (TIA) or cryptogenic stroke without known AF, 30 days of non-invasive event-triggered ambulatory electrocardiogram (ECG) monitoring allowed us to diagnose this arrhythmia in 16.1% of 280 individuals. At the same time, standard 24 h ECG revealed AF in only 3.2% of 277 patients.12 In individuals with stroke risk factors, implantable loop recorder (ILR) screening in the Implantable Loop recorder detection of atrial fibrillation to prevent stroke (LOOP) study resulted in a three-fold increase in AF detection and anticoagulation initiation.13 Hence, looking harder and longer with more sophisticated methods increases AF detection.
Early diagnosis of AF in some high-risk populations, together with appropriate antithrombotic therapy, could potentially prevent a substantial number of strokes and mortality.14,15 Additionally, it was shown by the results of the Early Rhythm-Control Therapy in Patients with Atrial Fibrillation (EAST) study that early AF detection and effective implementation of rhythm control strategies would reduce both deaths and hospitalizations. This rationalizes the need for active AF screening/search in at-risk populations. This need was reflected in the 2020 ESC guidelines that recommend opportunistic screening in individuals ≥65 years and suggests taking into consideration systematic screening in high-risk individuals and subjects aged ≥75 years to identify patients who could benefit from antithrombotic treatment. However, the recommended method(s) of screening/search that applies to the majority of the general population is not well established.10 The main objectives of the present consensus are to provide an overview of methods of preselection of participants for mass screening, establish the rationale for optimal monitoring time, and assess currently available AF detection methods in different clinical contexts.
Main issues in long-term atrial fibrillation screening/search
Atrial fibrillation definition: gaps and weaknesses of current atrial fibrillation definition
Diagnostic criteria for atrial fibrillation
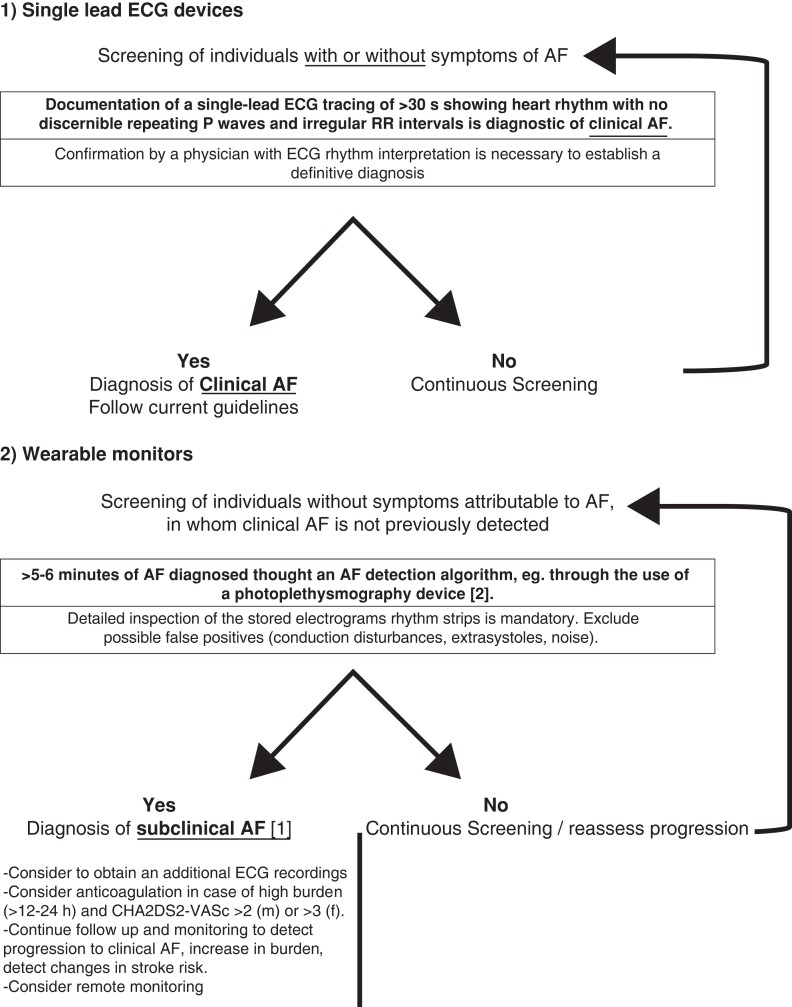
The diagnosis of AF requires rhythm documentation with an ECG tracing showing AF. A standard 12-lead ECG recording or a single-lead tracing of ≥30 s of heart rhythm with no discernable repeating P waves and irregular R-R intervals (when atrioventricular conduction is not impaired) is diagnostic for clinical AF with high specificity and sensitivity10,16
Implanted devices and wearable monitors allow for detecting atrial high-rate episode (AHRE) and subclinical AF (SCAF), respectively, which, by definition, cannot be regarded as identical to AF at conventional ECG tracings.17 Short monitoring using external devices is less likely to detect AHRE/SCAF. When AHRE/SCAF is detected by a device/wearable, inspection of stored electrograms/ECG rhythm strips is required.10,17
The primary definition and types of AF concerning the diagnostic criteria, the presence of symptoms, time of detection, and the kind of detecting devices or external monitors are presented in Table 1. As per the current ESC AF guidelines, the patient should be evaluated and characterized according to the 4S scheme: stroke, symptoms, severity of AF burden, and substrate.18
Table 1.
Definitions and types of atrial fibrillation
| AF pattern | Definition |
|---|---|
| Clinical AF | Symptomatic ‘overt’ or asymptomatic ‘silent’ AF that is documented by surface ECG. The minimum duration of an ECG tracing of AF required to establish the diagnosis of clinical AF is ≥30 s, or a 12-lead ECG.10,16 |
| First diagnosed AF | AF not diagnosed before, irrespective of its duration or presence/severity of AF-related symptoms.10 |
| AHRE | Events fulfilling programmed or specified criteria for AHRE that are detected by CIEDs with an atrial lead. Need to be visually inspected.10 |
| Subclinical AF | Includes AHRE confirmed to be AF, AFL or an AT, or AF episodes detected by insertable cardiac monitor or wearable monitor and visually confirmed.10 |
AHRE, atrial high-rate episode; AF, atrial fibrillation; ECG, electrocardiogram; AFL, atrial flutter; AT, atrial tachycardia; CIED, cardiac implantable electronic device; Based on 2020 ESC AFib Guidelines.10
Gaps and weaknesses of current atrial fibrillation definitions
A gap of evidence is to establish better the clinical significance of runs of atrial ectopics and atrial tachyarrhythmias lasting <30 s recorded by ECG Holter and other long-term ECG-monitoring devices, thus not fulfilling the criteria for diagnosing AF, and to assess the probability of progression to clinical AF, as well as their potential significance for thromboembolic events.
The current AF definition omits to allow a diagnosis of AF based on widely used screening tools based on photoplethysmography (PPG). The PPG signals registered by certified medical devices validated to the gold standard, as is ECG, could be considered comparable with the ECG due to their high sensitivity and specificity.8,9 The currently ongoing ‘Fitbit Heart Study’ is a significant clinical study designed to examine the validity of a novel PPG-based software algorithm for detecting AF.19 Nonetheless, we need to be sure of AF in an individual patient. Reported sensitivities/specificities are not enough to establish a definite diagnosis of AF without an ECG tracing, as suggested by guidelines.
The terms AHRE and SCAF are often used interchangeably. There are two ongoing randomized controlled trials (RCTs) dedicated to optimal management of AHRE/SCAF: ARTESiA20 and NOAH–AFNET 621 studies. Upcoming results should fill gaps in our knowledge in this area. Details of these studies are included in Supplementary material online, Table S1.
Atrial fibrillation screening and atrial fibrillation searching in atrial fibrillation diagnosis
According to current diagnostic criteria, a correct and definite AF diagnosis require electrocardiographic documentation of at least one episode, lasting 30 s or more. This recommendation is consistent with different guidelines.10,22 Due to the frequently asymptomatic nature of AF, current guidelines recommend systematic and opportunistic screening in populations at risk. Other screening methods and tools include pulse self-palpation,23 automated blood pressure monitors,24,25 watches,26,27 smartphone applications,28 single-lead ECG recorders, continuous ECG patches,29 long-term classical Holter, wearable belts30 for ECG recording, and ILRs31 have been postulated to identify individuals with AF effectively.
Nonetheless, only some of these methods allow for definitive AF diagnosis. Most methods require further ECG confirmation in individuals suspected to present AF. In this meaning, the main goal of screening is the identification of asymptomatic subjects with AF. If any screening method is insufficient to document AF with an ECG recording, a further search of AF using ECG-monitoring/recording devices must be performed. In patients with typical symptoms suggesting AF to whom classical ECG does not provide diagnosis, an active AF search using ECG-monitoring devices or patient’s self-activated ECG recorders is necessary to establish a final diagnosis of AF. The first requires devices with automatic identification of AF episodes based on internal or external algorithms. Patient-activated ECG recorders can also be effective in asymptomatic individuals if regular ECG recording is performed at the predefined time (e.g. twice daily as was done in the StrokeStop study).32,33 In contrast, in the population of cryptogenic stroke patients monitored with self-activated ECG recorders, symptoms triggered only 22% of all AF recordings.34
Assessment of silent atrial fibrillation risk and populations at risk of atrial fibrillation
Current ESC guidelines recommend opportunistic screening in individuals aged ≥65 years and emphasize the need for systematic screening in individuals ≥75 years to identify individuals who require anticoagulation.10 Nonetheless, effective, extensive routine screening in all eligible subjects seems unrealistic and not cost-effective. Based on the results of current studies, the number of individuals needed to screen in the general population to detect one AF case is estimated at almost 110.35 Additionally, because detection of AF remains challenging due to the unpredictable, short, self-terminating, or frequently asymptomatic nature of its episodes, the correct evaluation of a particular patient often requires long-time continuous monitoring. This necessitates large volumes of data to be processed. Therefore, for practical reasons, clinical risk stratification tools that better characterize the target population, decrease sample size, and identify subpopulations at risk are needed to increase the cost-effectiveness of future large populational screening programmes based on continuous long-term monitoring.
Some electrocardiographic36,37 (number of premature atrial beats) or echocardiographic38 (left atrial dysfunction) criteria (see Supplementary material online, Table S2) have been proposed to better select subjects with a high risk of AF. However, their practical accessibility is limited only to special populations of patients as patients after stroke or patients to whom Holter or echocardiographic assessments were performed due to other reasons. The use of these criteria for selecting participants of large populational screening programmes seems to be unpractical.
The need for better identifying of myocardial substrates of AF (especially atrial) with the use of large-scale longitudinal studies (combining: ECG, echocardiography, cardiovascular magnetic resonance, computed tomography, and traditional or genetic/genomic biomarkers) to demonstrate whether the estimated risk of AF and AF-related complications can be refined, was recently postulated.39 Practical implantation of results of such studies together with the use of artificial intelligence (AI) models should substantially improve the identification of high-risk populations and individuals.
Several predictive scores for risk stratification of AF have been proposed,10 but most are limited in defining high-risk populations suitable for large-scale screening projects. The major disadvantage of scores such as the Framingham Heart Study score,40 Cohorts for Heart and Aging Research in Genomic Epidemiology model for AF (CHARGE-AF) score,41 Atherosclerosis Risk in Communities score, Women's Health Study score,42 Maccabi Healthcare Services score,43 and Japan Medica Check-up score44 is that they have been developed to predict the long-term risk (7–10 years) of AF onset. Also, some of them require biomarkers or imaging data not readily available in the general population.
In the light of several suboptimal AF risk scores, the expectations for an improved risk score (i.e. easy to use, applicable to the general population, based on readily available information such as medical history/comorbidities) must be emphasized. Such AF risk score may also include/weigh risk factors for stroke in patients with AF. Such a score would facilitate identifying patients in whom AF detection and therapeutic intervention would yield a morbidity/mortality benefit.
In contrast to those mentioned thus far, the C2HEST score45 was a simple score based on clinical factors created to assess 1-year AF risk. Therefore, this scale appears to be more suitable for identifying the high-risk population of incident AF. Its usefulness as a possible opportunistic screening tool for incidents of AF has been confirmed in a healthy population in a Nationwide Danish Cohort Study46 and post-stroke patients in a French Nationwide Study.47 In the Huawei Heart Study, using the C2HEST score associated with symptoms (palpitations) was associated with a good prediction of newly diagnosed AF.27 In patients with cardiac implanted electrical devices (CIEDs), the C2HEST score was also predictive of AHREs (Table 2).48
Table 2.
Atrial fibrillation risk scores are potentially useful in defining of the population at risk of atrial fibrillation
| Score | Cohort | Subjects | C-idex | Risk factors |
|---|---|---|---|---|
| C2HEST45 | 100% Asian without structural heart diseases, 47% females, 47 years | 471 446/921 AF cases | 0.75 (0.73–0.77) | CAD/COPD, hypertension, age, systolic HF, thyroid diseases |
| MR-DASH49 | Stratified Polish nationwide cohort ≥65 years old, 50.9% females, 77.5 years | 3014/680 AF cases/279 SAF cases | 0.73 (0.68–0.78) | Male gender, renal failure, diabetes, age, stroke, HF |
AF, atrial fibrillation; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HF, hear failure.
| Position statement | Class | References |
|---|---|---|
| Recently a new clinical risk score (MR-DASH) was developed based on the population of NOMED-AF cross-sectional study to predict the risk of silent fibrillation (SAF).49 It was designed and validated in a cohort of 3014 individuals continuously monitored using a long-term vest-based ECG recording system. Brief characteristics of C2HEST and MR-DASH scores are included in Table 2. Better identification of populations at risk of AF using appropriate risk scales/scores is advised to increase the effectiveness of large systematic device-based AF screening projects. |

|
35,45 |
| The C2HEST score may be applied to identify populations at risk of incident AF. |

|
45–47 |
Optimal duration of long-term atrial fibrillation search
The appearance and duration of episodes of AF in paroxysmal AF are unpredictable. Even in symptomatic patients, some episodes of AF remain asymptomatic. For example, Israel et al.50 reported a cohort of 110 patients with previously diagnosed AF and implanted with dual-chamber pacemakers, that 52% had asymptomatic episodes; also, in 44% of patients with symptoms, no evidence of AF was present in recorded ECG or implanted device memory. In this study, 61% of patients were free of AF for ≥3 months despite earlier established diagnosis; and in 16% initially free of AF, symptomatic or asymptomatic AF recurrences lasting >48 h were observed during follow-up time lasting 19 ± 11 months.50
This work illustrates the central dilemma in evaluating optimal monitoring duration in AF screening/search. Even exceptionally long monitoring may be insufficient to detect all cases. Nonetheless, establishing a predefined standard monitoring time for particular situations seems to be necessary for either clinical practice or clinical and epidemiological studies. The time of monitoring is affected by the technical capability offered by the specifically selected method. For implanted devices such as pacemakers or implantable cardioverter defibrillators (ICDs), it is practically unlimited. For ILRs, it is constrained by battery longevity and usually lasts a few years. In external devices such as wearables (belts and vests), it is mainly limited by the patient physical and psychological ability. Additionally, some of these devices decrease recorded signal quality in more prolonged use. For practical reasons, all monitoring capabilities that allow monitoring beyond standard Holter devices (7 days) should be classified as long-term monitoring devices.
Searching for AF in the healthy population using invasive methods is difficult to justify and is not cost-effective. Nonetheless, for highly motivated, high-risk patients as patients after cryptogenic stroke or TIA, the use of ILRs seems to be acceptable. In such populations, prolongation of monitoring from 12 to 36 months yielded an increase in the incidence of diagnosed AF from 12.4 to 30.0%.31,51 Atrial fibrillation detection by continuous monitoring increased progressively throughout the study and was eight-fold higher at 36 months (30%) compared with the first month (3.7%).51 In another study evaluating the effectiveness of ILR in post-stroke patients, the detection rate of new AF after 630 days of monitoring was 17% (95% CI 7–26%); however, 90% of events were detected up to 118th day.52 This suggests that at least a few months of monitoring is necessary to identify the nearly complete number of patients with AF after stroke/TIA and supports using methods such as ILR. Nonetheless, prolongation of monitoring over 1 year after the index event seems to be unjustified due to the lack of temporal relationship between stroke and detected AF.
Different results were obtained using an external, belt-based, automatic ECG event recorder in a similar patient population. The detection rate of AF in 4 weeks was 14.8%, which was higher than in the control group using 24 h Holter (2.2%).12 Regardless of this discrepancy, either the use of ILR or up to 30 days of external ECG monitoring resulted in a three- to six-fold higher incidence of newly diagnosed AF in the population of patients after cryptogenic stroke or TIA than was reported with the use of 24 h or 3–7 days Holters.53,54
In populational screening aimed to detect AF, devices different from ILR devices should be applied, with shorter monitoring times. In two recently published studies of ECG population screening, the mSToPS trial29 and the STROKESTOP II32 study, a monitoring time was set up for two times of 14 days. Despite using different monitoring devices in both studies, the AF detection rates were four-fold higher in the monitoring cohort than in the control cohort, with 3.9 and 3% for the mSToPS29 and STROKESTOP32 studies, respectively. Further target group stratification based on NT-proBNP level, as was performed in STROKESOP II study, increased yield of AF detection from 2.6% (95% CI: 2.2–3.0%) in low (<125 ng/mL) NT-proBNP group to 4.4% (95% CI: 3.7–5.1%) in high (≥125 ng/mL) NT-proBNP group.55
The monitoring time in the Apple Heart Study was extensively longer (interquartile range 113–186 days). Still, the irregular pulse notification rate in the whole study population was 0.52%, and in participants aged ≥65 years, it was 3.14%.26 In the Huawei Heart Study, the median duration to first detected AF was 4 days (interquartile range 1–24) in a population with a ‘high-risk’ C2HEST score category, with a detection rate of 4.77%.27 Hence, prolonging monitoring over 30 days would not significantly increase the AF detection rate in populational studies.
The rationale for optimal monitoring duration should be limited by technical capability and assessing the clinical significance of detecting an episode of AF (especially short lasting) in a very long monitoring time. As shown in a newly published LOOP study that utilized ILR for AF searching in 70–90 years old, individuals with at least one additional stroke risk factor (i.e. hypertension, diabetes, previous stroke, or heart failure), elongation of monitoring time to a median of 64.5 months yielded a significant increase in AF detection from 12.2% in the control group (conventional care) to 31.8% in the ILR group. Nonetheless, initiation of anticoagulation in individuals with detected AF did not reduce stroke or systemic arterial embolism in the monitored vs. control group (4.5 vs. 5.6%; P = 0.11).13
| Position statement | Class | References |
|---|---|---|
| In systematic device-based, large-scale AF screening approaches for populations with unknown morbidity status, a monitoring duration of a minimum of 2 weeks of continuous monitoring is required to maximize AF detection. |

|
27,29,32 |
| In high-risk, highly motivated patients (e.g. after cryptogenic stroke), 1–12 months of monitoring with ILRs may be applied. |

|
13,31,51 |
Current capabilities of long-term atrial fibrillation screening/search based on devices
Devices for screening and search of atrial fibrillation
Due to the vast number of devices and methods capable of detecting AF, we propose to categorize these tools into two screening and diagnostic types:
Type 1:
Screening devices: simple methods, wildly accessible, detection of AF is a preliminary signal, and further diagnostic of AF is needed for the final AF confirmation (both commercial, uncertified devices, and medical, certified devices)
Diagnostic devices: tools and systems that establish AF diagnosis directly/immediately after AF detection by the device or system (medical, certified devices)
Type 2:
Screening devices: pulse palpitation, auscultation, oscillometric blood pressure measurements, wearable devices, patient-initiated PPG on a smartphone, semi-continuous PPG on a smartwatch or wearable, intermittent smartwatch ECG started by semi-continuous PPG with prompt notification of irregular rhythm or symptoms
Diagnostic devices: long-term non-invasive ECG recording (with the preferences of continuous): vests, belts, pocket ECG, long-term Holter monitoring, patch Holters, event monitors, ILRs, and implantable pacemakers or defibrillators with atrial pacing/sensing lead
According to the current rules of AF diagnosis, when AF is detected by a screening tool, registered ECG (single ECG tracing ≥30 s or 12-lead ECG) should be confirmed as AF by a physician with expertise in ECG interpretation. When AF detection is not based on an ECG recording (devices using plethysmography) or in case of uncertainty in the interpretation of registered ECG tracing, a final diagnosis of AF should be obtained using additional ECG recording (e.g. 12-lead ECG, Holter monitoring).56 In the opinion of the authors of this document, advanced, validated plethysmography tools utilizing mobile, and telemedicine technologies could be accepted equal to the confirmation of AF by ECG recording.56,57
Mobile health technology and telemedicine, including advanced systems, are currently used for screening and diagnosis. In the case of AF notification by the tool from screening type, there is an indication to confirm the final diagnosis of AF using the diagnostic type. The practical tools for screening and diagnostic classification are listed in Table 3.
Table 3.
The list of devices and methods for practical atrial fibrillation evaluation
| AF detection and diagnosis | |
|---|---|
| Tools for AF screening (detection) | Tools for AF search (detection and diagnosis) |
| Pulse palpitation, auscultation | Wearable vest, belts for continuous ECG recording |
| Patient or medical professional initiated oscillomerric blood pressure device | Long-term Holter (72 h to 7 days), pocket ECG |
| Patient-initiated photoplethysmogram on smartphone | Patch Holter Event Holter |
| Semi-continuous photoplethysmogram on a smartwatch | Implantable cardiac monitors and pacing devices with atrial electrode |
| Mobile or telemedic devices/systems detecting photoplethysmogram or ECG signala | |
AF, atrial fibrillation; ECG, electrocardiogram.
A or B category depending on the technology.
Wearables and other devices for atrial fibrillation screening based on pulse assessment
Smartphones and other wearable devices such as smartwatches are increasingly used in general. These devices’ wide availability and constant technological improvement provide options for the widespread use of medical-graded smartphone apps. These apps can provide data from remote and non-invasive screening on many health-related parameters. However, not all of the currently downloadable apps have been scientifically validated.
Most devices can readily provide PPG data by recording the pulse wave using the smartphone’s camera or specific sensors at the rear of the watch face. Particular algorithms can then detect the pulse irregularity that healthcare providers can use to diagnose AF early without additional hardware. Furthermore, smartwatches may also provide the option for long-term AF screening, possibly improving diagnostic yield.
The performance of PPG-based methods to detect AF is widely studied. Compared with a single-lead ECG recorded by a handheld device, the first prospective study of PPG-based AF detection based on a smartphone camera reported a sensitivity of 91.5% and a specificity of 99.6%.58 Other small studies also report excellent diagnostic accuracy of PPG-based screening, with sensitivity varying from 87 to 100% and specificity from 90 to 97% compared with ECG.59–63
Many studies have implemented smartphone-based strategies for AF screening. One of the most extensive mass screening programmes included 12 328 participants, who were offered by the lay press to download a detection app from the web and instructed to perform measurements twice daily for 7 days using the smartphone PPG technology via a specialized app.64 Tracings were classified as regular rhythm, some irregularities, possible AF, or insufficient quality. Possible AF was detected in 1.1% of the individuals, further referred to diagnose AF.
Two recent studies have evaluated the role of current technologies in screening for AF in a large series of smartwatch users. The Apple Heart Study included 419 297 subjects without a known history of AF who were monitored for an irregular pulse by PPG sensors incorporated in the Apple Watch®.26 One-minute tachograms were recorded and processed by the downloaded study app, which was also used to notify the study subjects and guide them through the necessary study procedures. Of the studied subjects, 0.52% (ranging from 3.1% in the age group >65 years to 0.16% age group 22–39 years) received irregular pulse notifications. Some of the notified subjects were monitored by an ECG patch for 6.3 days, which resulted in AF diagnosis in 34%, with most episodes exceeding 1 h in duration. The positive predictive value of irregular pulse for recording AF on the simultaneous ECG was 0.84 (95% CI: 0.76–0.92).
The Huawei Heart Study included 187 912 individuals screened for AF based on PPG smartwatch sensors incorporated in android-based wearable devices.56 With at least 14 days of monitoring by the specially designed PPG algorithm, 0.23% of the individuals received a ‘suspected AF’ notification. They were followed up, and AF was confirmed in 87% of them. The majority of confirmed cases (95.1%) entered a mobile app-based program for integrated AF management resulting in the initiation of anticoagulation therapy in almost 80% of the patients. The positive predictive value of PPG-derived data was 91.6% (95% CI: 91.5–91.8%).
However, limited data exist to date to advise on anticoagulation in asymptomatic patients diagnosed through these new technologies. These patients should thus be referred for further risk stratification on an individual basis. More specifically, the AF burden required to warrant anticoagulation might be different than in patients with overt clinical AF.65 Any screening programme should incorporate an integrated AF management care pathway to advice patients according to the diagnosed arrhythmia. With further developments in smartphones, smartwatches, and apps, also allowing single- or multiple-lead recordings, new data are expected in the coming years, hopefully also including clinical outcome data.
| Position statement | Class | References |
|---|---|---|
| Wearable devices (smartphones and smartwatches) with PPG sensors may be used for AF screening providing appropriate and approved software applications are installed and utilized. |

|
59–63,66 |
| When a screening test suggests a diagnosis of AF, ECG confirmation of at least 30 s duration is required. |

|
10 |
| Diagnosis via wearables without the capability of ECG recording should not guide the therapy/anticoagulation management. |

|
10 |
Atrial fibrillation search based on devices for non-invasive long-term electrocardiogram recording (vests, belts, and pocket electrocardiogram)
The use of classical ECG Holter systems for long-term (over 7 days) ECG monitoring is hampered by numerous factors, including skin reaction to wet adhesive Holter electrodes, deteriorating signal quality, problems with long-term power supply, and poor patient compliance. Due to hygiene reasons system must be taken off for short breaks necessary for bath or showering. Correct, repetitive self-placement of adhesive wet electrodes is unavailable for the typical patient even after instruction and training. Therefore, the simple construct as a vest or belt containing recording electrodes and would be easy to wear and take off seemed to be a reasonable solution allowing long-term ECG recording aimed to detect AF. Indeed, the EMBRACE first large study that proved the usefulness of long-term AF searching was based on the belt approach of recording.12,36
Currently, two strategies utilizing the vest/belt approach to ECG recording are available: External loop recorders and mobile cardiac telemetry (MCT). External loop recorders record ECG tracings lasting from a few seconds to several minutes surrounding trigger events and can detect both symptomatic and asymptomatic arrhythmias using an automatic algorithm identifying arrhythmia. Mobile cardiac telemetries are capable of real-time or intermittent transmission of electrogram directly to the reading centre via a wireless link using WiFi or/end GSM access. The MCT data are processed in a reading centre on the back end of the monitoring system using advanced signal processing algorithms. Trained technicians or cardiologists can confirm identified events. Both strategies allow for long-term continuous ECG monitoring for up to 30 days with minimal subject involvement. The monitoring must be interrupted only for a short time required for recovery of the skin, bathing or showering, and device charging or recorder exchange.67 External loop recorders potentially suffer from overlooking arrhythmic episodes that are not directly identified by intrinsic device algorithms or are out of range of predefined parameters.
The usefulness of belt/vest-based direct long-term ECG recording technology in AF detection was proved in several recently published studies characterized in Table 4. Most were performed on patients after cryptogenic stroke, with the monitoring time lasting up to 30 days. The diagnostic yield of AF detection was always superior to classical 24–48 h Holter recordings (in direct or indirect comparison) and was comparable with those obtained in implantable devices (ILR).
Table 4.
Studies that utilized belt/vest-based long-term electrocardiogram recording in atrial fibrillation search
| Study | Device | Study population | Time of monitoring | Diagnostic yield | References |
|---|---|---|---|---|---|
| DAF-ESUS registry | Nuubo®, Textile Wearable Holter vest | 100 patients after cryptogenic stroke 50 early/50 late after stroke | 21 days both groups | 14% (22% early, 6% late) | Rubio Campal et al.68 |
| Crypto-AF registry | Nuubo®, Textile Wearable Holter vest | 174 patients after cryptogenic stroke | Up to 28 days | 21.9% | Pagola et al.69 |
| EMBRACE | ER910AF Cardiac Event Monitor/Braemar Cardiac Bio-Systems belt | 280 patients intervention group/277 patients control group after cryptogenic stroke | Up to 30 days intervention group/24 h control group | 16.1 vs. 3.2% | Gladstone et al.12 |
| Beat2Phone | Beat2Phone belt | 15 patients after cryptogenic stroke | 14 days | 6.7% | Lumikari et al.30 |
Atrial fibrillation search based on classical and novel Holter electrocardiogram-based methods
Holter ECG is considered the gold standard for short-term heart rhythm diagnostics for up to 10 days. It is usually applied with three to five electrodes and allows an analysis of at least two different ECG channels. In contrast to automatically triggered devices, ECG Holter-based methods continuously document heart rhythm and will enable an analysis of the onset and end of suspicious episodes. The main drawback of Holter ECG is compliance over time. In a recent series of 200 patients with recent strokes, 75% of patients wore a 10-day Holter ECG for at least 8 days, but one-third of patients refused to have a second 10-day Holter ECG after 3 months.70
Extending the time of ECG registration will increase the diagnostic yield of Holter monitoring, especially for those rhythm disturbances that are infrequent but recurrent. The optimal duration of monitoring for AF, particularly after a stroke, is yet to be determined, but current evidence suggests monitoring for at least 72 h in all patients with stroke.71,72 Current ESC guidelines also recommend a preference for 72 h of monitoring.10
Event monitors do not record ECG continuously. Electrocardiogram is recorded only if the device identifies a suspected episode or the patient activates recording. Mobile cardiac telemetry is an evolution of event Holter, which analyses ECG continuously and transmits every beat in real-time to an attending cardiac unit.
Patch Holter uses a technology similar to a Holter ECG, using an adhesive patch attached to the patient chest instead of electrodes. This provides a single-channel ECG. The patch allows activities like showering without removing the device. The patches usually record heart rhythm continuously for 14 days. After this period, the patches are detached and mailed for remote analysis to a central core lab. The analysis of the ECG data is done by trained technicians and is included in the fee for the device.
Data on direct comparisons between ECG-monitoring modalities are scarce and often biased by different monitoring times. For example, Barrett et al.73 compared a 14-day patch Holter with 24 h of Holter monitoring. The patch detected significantly more arrhythmias than the Holter, mainly due to the different monitoring times. In the 24 h that both devices were worn in parallel, the yield of the Holter was significantly higher than the patch. Patients judged the patch to be more comfortable and were less likely affected by daily living activities.
Two large, randomized trials have used patch Holters for screening of AF. For example, in the mSToPS trial, 2659 individuals were randomized to either 2 weeks of rhythm monitoring with a patch Holter starting immediately after allocation or delayed 4 months.29 Atrial fibrillation was identified by 4 months in 3.9% of the immediate group vs. 0.9% in the delayed group. In the SCREEN-AF study, 856 patients with hypertension aged 75 years and above were randomized to 2 times 14 days of patch Holter monitoring or usual care.74 After 6 months, AF was detected in 5.3% in the patch Holter group and 0.5% in the standard care group. About 75% of the AF incidents detected by the patch were found with the first patch.
Another issue is the clinical significance of these asymptomatic AF episodes detected using extended ECG-monitoring devices.75 These should be carefully interpreted and individualized according to the clinical characteristics of each patient; for example, episodes of a few minutes of AF may not have the same significance in healthy middle-aged athletes as in hypertensive and diabetic patients after a cerebrovascular accident.
| Position statement | Class | References |
|---|---|---|
| 72 h of Holter ECG monitoring is required in high-risk patients (e.g. stroke patients) ahead of other long-term ECG-monitoring methods. |

|
10,71,72 |
| Patch Holter-based long-term non-invasive ECG recording in AF search can be used in populational screening studies. |

|
74,75 |
Implantable loop recorders and atrial fibrillation search
Implantable loop recorders are employed for AF search after cryptogenic stroke, AF monitoring after catheter and surgical ablation of AF, and AF detection in patients with unspecific palpitations. Modern ILR systems are small injectable recorders weighing between 2.5 and 4 g and have 2 and 4 years of battery capacity. All available devices allow telemonitoring and are MRI conditional. Device-related adverse events, including pain and wound infections, are rare throughout all published collectives. In one of the largest series of patients from the CRYSTAL-AF study, at 36 months, 5 (2.4%) of the 208 ILRs that were initially inserted had been removed owing to infection at the insertion site or pocket erosion. The most common adverse events associated with the ILR were infection [3 patients (1.4%)], pain [3 patients (1.4%)], and irritation or inflammation [4 patients (1.9%)] at the insertion site.31,51 In cryptogenic stroke, which represents about 25% of all stroke cases, early detection of clinically unapparent paroxysmal AF is essential for preventing recurrent stroke. The initiation of oral anticoagulation leads to a notable absolute risk reduction.76 The risk of recurrent stroke in patients with cryptogenic stroke is reported with 3–6% per year.77 Based on a RCT data, asymptomatic paroxysmal AF was diagnosed in a relevant proportion of patients with cryptogenic stroke after ILR implantation.31 A clear superiority was reported for the ILR compared with regular or irregular Holter ECG, with detection rates of 8.9% in ILR vs. 1.4% for conventional Holter ECG over only 6 months.31 These figures increased after 12 months and reached 30% for ILR vs. 3% for traditional Holter ECG after 3 years. These results are consistent with the findings of another randomized trial employing an external 30-day event-triggered recorder, which also displayed superiority compared with conventional Holter ECG.12 However, this method is limited to a specific time window and may be regarded as inferior to ILR with a potential monitoring period of up to 4 years.
The feasibility of ILR for the detection of SCAF is further underlined by a recent study in individuals without a history of AF who presented at least one accepted risk factor for systemic thromboembolism, including ≥70 years of age, hypertension, diabetes, and history of stroke or presence of heart failure. In this cohort, subclinical episodes of AF with at least 6 min were diagnosed by ILR implantation in 35% of all included patients. In all patients where AF was identified, oral anticoagulation was initiated according to current ESC guidelines.10 Similar results were obtained in the LOOP study. In 1501 individuals with at least one additional stroke risk factor (i.e. hypertension, diabetes, previous stroke, or heart failure) monitored with ILR, AF was detected in 477 (31.8%). In contrast, in 4503 individuals in the control group (usual care), only 550 (12.2%) were diagnosed with AF. Surprisingly, initiation of the anticoagulation due to newly detected AF resulted in no significant differences in the incidence of stroke or systemic arterial embolism between the ILR group and control groups (HR 0.80, 95% CI: 0.61–1.05; P = 0.11).13 The results of the LOOP study suggest that anticoagulation was not always initiated after any AF detection due to the occurrence of short episodes, which may be an explanation for these findings.
In patients who underwent AF ablation, ILR implantation correlated with a reduced prescription of antiarrhythmic and negative-dromotropic medication compared with patients in conventional follow-up. This can be explained by the higher confidence of the clinical team in the absence of an arrhythmia.78
Atrial fibrillation search in patients with implantable devices
Cardiac implanted electrical devices with an atrial lead or with the capability of rhythm discrimination (i.e. ILRs) allow to continuously monitor the cardiac rhythm and appropriately detect atrial tachyarrhythmias, including AF, as AHREs and recordings can be stored in device memories for review and specific diagnosis to be discriminated from oversensing due to double counting, repetitive non-reentrant ventriculoatrial synchrony, or artefacts caused by myopotentials or electrical interferences.17 Atrial high-rate episodes, currently defined as episodes of at least 5 min of atrial tachyarrhythmias/AF with an atrial rate >175 b.p.m.,10 are asymptomatic events discovered during routine device follow-up and classified in terms of duration of the single episode or time spent in atrial tachyarrhythmias during a day (AF burden, expressed as minutes to hours). The requirement for using the term AHRE, or SCAF, is that the patient has no previous history of AF and that AF was never documented with a standard 12-lead ECG or an ECG strip >30 s, the latter criteria qualifying for clinical AF.10
The prevalence of AHRE, often reported as AF burden among patients implanted with CIEDs, varies depending on underlying heart disease and observation periods. In general, AHRE can be detected in 10–15% of pacemaker patients and up to 20–40% in ICD and CRT patients. Patients with sinus node disease seem to be more at risk than patients with AV block.65,79–81
Although episodes of SCAF/AHREs are associated with a 2.0- to 2.5-fold increase in stroke risk compared with patients without these arrhythmias,65,79,82 the absolute risk of stroke among these patients is lower than the risk of patients with clinical AF.83 In a recent meta-analysis, AHRE was significantly associated with increased thromboembolic risk and increased incidence of clinical AF.84 Moreover, CIED-detected AHREs may occur with temporal dissociation regarding stroke events, thus suggesting that they may represent a marker rather than a risk factor for stroke75,82,85 or may develop clinical AF, as shown by the ASSERT trial where, during a follow-up period of 2.5 years, around 16% of the patients who had subclinical atrial tachyarrhythmias developed asymptomatic clinical AF.65 Careful monitoring of these patients is thus advised also considering remote monitoring, especially in patients with longer AHREs and a higher stroke risk profile.86
The threshold at which it is appropriate to initiate anticoagulation for AHRE below 24 h is not yet defined. Still, the risk of stroke is markedly increased when the duration of SCAF/AHRE is longer than 24 h, as shown by the ASSERT data.65 In patients with atrial arrhythmias lasting <5 min, no increase in stroke risk has been documented, and no anticoagulation is required. In patients with AHREs shorter than 24 h, the net clinical benefit of oral anticoagulation is currently studied in two randomized trials (details in Supplementary material online, Table S1).20,21
Patients with SCAF/AHREs show a substantial dynamicity with transitions from lower to higher AF burden categories depending on the AF burden at first detection and CHADS2 score.86 The longer the AF burden at first detection, the higher the probability of a faster transition to an AF. The threshold of >23–24 h has been reported in the literature to be associated with an essential increase in the risk of associated stroke.65 At present, decision-making on anticoagulation has to consider individualization of monitoring and clinical surveillance on top of clinical stroke risk stratification based on CHA2DS2-VASc.87
| Position statement | Class | References |
|---|---|---|
| A careful review of device memories is required at each follow-up of patients with CIEDs. |

|
17 |
| Risk stratification to identify individuals that require further investigation aimed at establishing AF diagnosis should be performed using the CHA2DS2-VASc score in patients with SCAF/AHREs (>5 min) |

|
86 |
| Intensified follow-up, eventually using remote monitoring, is justifiable in patients with AHREs <24 h in individuals at risk of thromboembolic episodes. |

|
87 |
| Based on their risk stratification, initiation of oral anticoagulation may be an option in patients with SCARF/AHREs lasting longer than 24 h. |

|
65 |
| No anticoagulation is needed in patients when asymptomatic atrial tachyarrhythmias lasting <5 min are discovered in device memories of CIEDs. |

|
10,75,82,85 |
E-medicine and artificial intelligence in current strategies of atrial fibrillation screening/search
If indicated, identification of AF before symptoms could promote the initiation of appropriate therapy, including risk-factor modification or oral anticoagulation.
Machine learning (ML) applications have advanced rapidly in the last years. A new novel ML model for implementing an AF prediction model was created using the data routinely collected from 3 million patients without a history of AF registered in the UK Clinical Practice Research Datalink.88 Using these data, a convolutional neural network, which considered 100 different baseline predictors, was identified as the optimal model with an area under the curve of 0.83 for AF prediction vs. 0.73 with the CHARGE-AF score. It should be noted that the complexity and variability of coding systems (variability over time, differences in coding between physicians) allow for possible divergences in coding, decreasing the validity in a given population.
Machine learning also allows to factor in the dynamic nature of risk factors and multimorbidity for incident AF and AF-related complications, such as stroke.89,90
An AI algorithm designed to detect nearly concomitant unrecognized AF based on sinus rhythm ECGs has been described.91 The algorithm was developed using almost half a million digitally stored ECGs recorded from more than 100 000 individuals. The model applied convolutions on a temporal axis to extract morphological and temporal characteristics during the training and validation process. Posteriorly, it was validated and tested in two different sets. Individuals with at least one ECG with AF were categorized as cases within 1 month after the sinus rhythm ECG. The algorithm showed a C statistic of 0.87 (95% CI: 0.86–0.88), a specificity of 79.5%, a sensitivity of 79.0%, and an accuracy of 79.4% in detecting individuals with documentation of AF within 1 month after the sinus rhythm ECG using only information from the sinus rhythm ECG.
Machine learning methods have also been applied to signals obtained from the single-lead ECG or photoplethysmography. For instance, a deep neural network has been developed to detect AF from photoplethysmography signals received from the Apple Watch (Apple Heart study) or the Huawei (Huawei Heart Study).26,56
Although these results are promising, the actual clinical utility of these solutions will be determined by the predictive values of each algorithm when applied to a population and by the cost related to follow-up diagnostic testing and therapies, mainly in the false-positive group. Indeed, these algorithms may facilitate targeted AF surveillance in subgroups of high-risk patients. A proposition of practical approaches based on individual risk factors is presented in Figure 1.
Figure 1.
Proposition of practical approaches of atrial fibrillation screening based on individual risk factors and devices.
Some important questions need to be addressed before these tools can be widely used in clinical practice, like the interpretation of deep-learning algorithms. Also, these black-box models may not allow stakeholders (patients and healthcare providers) to engage in the serious shared decision because of the opacity of the models.
New techniques for ECG analysis based on ML and AI and new technologies like wearables have opened up potentially significant opportunities for detecting and diagnosing AF. These innovations may help us personalize therapy and risk stratification in the near future.
Patient’s benefits of atrial fibrillation screening
In the event of identifying a case, the benefits of AF screening are multiple. From the clinical point of view, there is the prevention of stroke and heart failure with a reduction in cardiovascular mortality. From a biological point of view, there is the reversal of atrial remodelling and disease progression (Table 5). On the other hand, there can also be some possible negative aspects of screening, mainly related to anxiety, ECG misinterpretation, unnecessary procedures performed due to a false-positive test, and the associated costs.
Table 5.
Benefits and risk of screening for atrial fibrillation (modified from Guidelines 202010)
| Benefits | Risk | Exploring convergence points |
|---|---|---|
|
Clinical
Prevention of: Stroke using OAC if indicated
|
Stress and anxiety before and after the test. Possible divergence of patient interest vs. healthcare provider. |
Evaluate patient’s interest, values, goals, and preferences before proposing a screening test. Clarification that patient decision will be respected. Clarify possible misperceptions regarding to the procedure. |
|
Biological
Reversal/prevention:
|
False positives and ECG misinterpretation may promote overdiagnosis and overtreatment and also complications secondary to a positive test. Risk of harm and increase of cost. Bleeding secondary to OAC treatment. |
Establish an open discussion regarding the nature of AF and potential negative and positive aspects of the procedure. |
Regardless of obvious clinical and biological benefits resulting from participation in screening, some individuals can refuse to be enrolled. In the StrokeStop study, half of the subjects invited declined to participate, actually those with the highest risk.33
Reporting shared decision-making research has shown different perceptions between patients and healthcare professionals. In some cases, a misperception that patients might prefer not to be involved in decision-making but rather defer it to their physician has been identified.
Informing the patient about the disease, its possible consequences, and management should be done initially. Although a formal written informed consent is probably not mandatory, each patient should be able to accept or refuse to participate in a screening programme, being fully aware of the potential benefits or hurdles of the screening, the limitations of the chosen screening tool, and the consequences of a positive or negative test. This information could also be extended to the general population to increase awareness about AF, allowing everyone to decide whether or not he/she would be interested in being tested and how frequently the testing should be repeated.
Apart from ‘hard’ clinical outcomes (death, stroke, major bleeding, etc.), evaluating outcomes relevant to patients is essential during a screening process. As the first step of shared decision-making, identifying the patient’s values, goals, and preferences should be mandatory. In this context, an international consortium has identified the following patient-related outcomes as essential to measure for AF: health-related quality of life, physical and emotional functioning, cognitive function, symptom severity, exercise tolerance, and ability to work.
| Position statement | Class | References |
|---|---|---|
| Understanding each patient’s values and preferences should allow a tailored screening approach for each patient. |

|
33 |
| Comprehensive information about atrial fibrillation is necessary to allow every subject to decide whether or not to participate in a screening programme. |

|
66 |
Summary
The current AF definition requires recording in classical ECG or Holter ECG at least a 30-s episode of AF. According to the current definition, the presence of frequent shorter episodes of fast atrial arrhythmia or episodes of arrhythmia identified with widely used screening tools requires subsequent steps to establish a definite diagnosis of AF. The use of different clinical risk scores can help to refine target populations better. Due to the unpredictable and highly variable nature of AF episodes, a monitoring time lasting 2 weeks or longer is preferable to maximize the possibility of identifying subjects with AF.
Several capabilities are currently available for AF search/screening, including devices based on plethysmographic pulse assessment, belts and vests for long-term ECG monitoring, modern Holter capabilities, and ILRs. Decision-making regarding using particular of them should depend on proof of efficacy based on published data, patient characteristics, and purpose of monitoring (screening/search). Additionally, all subjects with CIED with the possibility of atrial sensing should be carefully evaluated to identify AHREs. In large-scale screening projects, ML and AI could provide the appropriate interpretation of large databases containing the results of a giant number of participants. From the patient perspective, participation in screening has positive but also negative aspects. Therefore, each patient should be able to accept or refuse to participate in a screening programme, being fully aware of the potential benefits or hurdles of the screening. As the first step of shared decision-making, identifying a patient’s values, goals, and preferences is mandatory.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgement
The authors thank the EHRA Scientific Document Committee: Dr Nikolaos Dagres, Prof. Thomas Deneke, Prof. Arthur Wilde, Prof. Frank R. Heinzel, Prof. Christian Meyer, Prof. Lucas Boersma, Prof. Radoslaw Lenarczyk, Prof. Luigi di Biase, Dr Elena Arbelo, Dr Avi Sabbag, Prof. Pierre Jais, Prof. Milos Taborsky, Asso. Prof. Markus Stühlinger.
Contributor Information
Zbigniew Kalarus, Department of Cardiology, DMS in Zabrze, Medical University of Silesia, Katowice, Poland; Department of Cardiology, Silesian Center for Heart Diseases, Zabrze, Poland.
Georges H Mairesse, Department of Cardiology and Electrophysiology, Cliniques du Sud Luxembourg—Vivalia, Arlon, Belgium.
Adam Sokal, Department of Cardiology, Silesian Center for Heart Diseases, Zabrze, Poland.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, Modena, Italy.
Beata Średniawa, Department of Cardiology, DMS in Zabrze, Medical University of Silesia, Katowice, Poland; Department of Cardiology, Silesian Center for Heart Diseases, Zabrze, Poland.
Ruben Casado-Arroyo, Department of Cardiology, Erasme Hospital, Brussels, Belgium.
Rolf Wachter, Clinic and Policlinic for Cardiology, University Hospital Leipzig, Leipzig, Germany.
Gerrit Frommeyer, Department of Cardiology II (Electrophysiology), University Hospital Münster, Münster, Germany.
Vassil Traykov, Department of Invasive Electrophysiology and Cardiac Pacing, Acibadem City Clinic Tokuda Hospital, Sofia, Bulgaria.
Nikolaos Dagres, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Heart and Chest Hospital, Liverpool, UK; Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Lucas Boersma, Department of Cardiology, St Antonius Hospital,, Utrecht, The Netherlands; Amsterdam University Medical Centers, Amsterdam, The Netherlands.
Petr Peichl, Klinika Kardiologie, IKEM, Prague, Czech Republic.
Dobromir Dobrev, Faculty of Medicine, Institute of Pharmacology, University Duisburg-Essen, Essen, Germany.
Alan Bulava, Faculty of Health and Social Sciences, University of South Bohemia in Ceske Budejovice, Czech Republic and Faculty of Medicine and Dentistry, Palacky University in Olomouc, Czech Republic.
Carina Blomström-Lundqvist, Department of Medical Science and Cardiology, Uppsala University Hospital, S-751 85 Uppsala, Sweden.
Natasja M S de Groot, Department of Cardiology-Electrophysiology, Erasmus Medical Center, Rotterdam, The Netherlands.
Renate Schnabel, Department of Cardiology, University Heart and Vascular Center, Hamburg, Germany.
Frank Heinzel, Department of Cardiology, Charité University Medicine, Campus Virchow-Klinikum, 13353 Berlin, Germany.
Isabelle C Van Gelder, Department Of Cardiology, University Medical Center Groningen, Groningen, The Netherlands.
Corrado Carbuccichio, Department of Arrhythmology, Centro Cardiologico Monzino IRCCS, Milan, Italy.
Dipen Shah, Department of Cardiology, Cantonal Hospital, CH-1211 Geneva, Switzerland.
Lars Eckardt, University Clinic of Munster (Ukm), Munster, Germany.
References
- 1. Burdett P, Lip GYH. Atrial fibrillation in the United Kingdom: predicting costs of an emerging epidemic recognising and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes 2020;8:187–94. [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang L, Ze F, Li J, Mi L, Han B, Niu H et al. Trends of global burden of atrial fibrillation/flutter from Global Burden of Disease Study 2017. Heart 2021;107:881–7. [DOI] [PubMed] [Google Scholar]
- 4. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. [DOI] [PubMed] [Google Scholar]
- 6. Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost 2017;117:1230–9. [DOI] [PubMed] [Google Scholar]
- 7. Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS et al. Improved population-based clinical outcomes of patients with atrial fibrillation by compliance with the simple ABC (atrial fibrillation better care) pathway for integrated care management: a Nationwide Cohort Study. Thromb Haemost 2019;119:1695–703. [DOI] [PubMed] [Google Scholar]
- 8. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D et al. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost 2022;122:406–14. [DOI] [PubMed] [Google Scholar]
- 9. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017;14:627–8. [DOI] [PubMed] [Google Scholar]
- 10. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 11. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH et al. 2021 Focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation: executive summary. Thromb Haemost 2022;122:20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. [DOI] [PubMed] [Google Scholar]
- 13. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP study): a randomised controlled trial. Lancet 2021;398:1507–16. [DOI] [PubMed] [Google Scholar]
- 14. Wallenhorst C, Martinez C, Freedman B. Risk of ischemic stroke in asymptomatic atrial fibrillation incidentally detected in primary care compared with other clinical presentations. Thromb Haemost 2022;122:277–85. [DOI] [PubMed] [Google Scholar]
- 15. Proietti M, Boriani G. Screening for atrial fibrillation in relation to stroke and mortality risk. Thromb Haemost 2021. doi: 10.1055/s-0041-1735189. [DOI] [PubMed] [Google Scholar]
- 16. Steinberg JS, O’Connell H, Li S, Ziegler PD. Thirty-second gold standard definition of atrial fibrillation and its relationship with subsequent arrhythmia patterns: analysis of a large prospective device database. Circ Arrhythm Electrophysiol 2018;11:e006274. [DOI] [PubMed] [Google Scholar]
- 17. Kaufman ES, Israel CW, Nair GM, Armaganijan L, Divakaramenon S, Mairesse GH et al. Positive predictive value of device-detected atrial high-rate episodes at different rates and durations: an analysis from ASSERT. Heart Rhythm 2012;9:1241–6. [DOI] [PubMed] [Google Scholar]
- 18. Potpara TS, Lip GYH, Blomstrom-Lundqvist C, Boriani G, Van Gelder IC, Heidbuchel H et al. The 4S-AF scheme (stroke risk; symptoms; severity of burden; substrate): a novel approach to In-depth characterization (rather than classification) of atrial fibrillation. Thromb Haemost 2021;121:270–8. [DOI] [PubMed] [Google Scholar]
- 19. Lubitz SA, Faranesh AZ, Atlas SJ, McManus DD, Singer DE, Pagoto S et al. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the Fitbit heart study. Am Heart J 2021;238:16–26. [DOI] [PubMed] [Google Scholar]
- 20. Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J 2017;189:137–45. [DOI] [PubMed] [Google Scholar]
- 21. Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener HC et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the non-vitamin K antagonist oral anticoagulants in patients with atrial high rate episodes (NOAH-AFNET 6) trial. Am Heart J 2017;190:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghazal F, Theobald H, Rosenqvist M, Al-Khalili F. Validity of daily self-pulse palpation for atrial fibrillation screening in patients 65 years and older: a cross-sectional study. PLoS Med 2020;17:e1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark CE, McDonagh STJ, McManus RJ. Accuracy of automated blood pressure measurements in the presence of atrial fibrillation: systematic review and meta-analysis. J Hum Hypertens 2019;33:352–64. [DOI] [PubMed] [Google Scholar]
- 25. Kyriakoulis KG, Kollias A, Anagnostopoulos I, Gravvani A, Kalogeropoulos P, Destounis A et al. Diagnostic accuracy of a novel cuffless self-blood pressure monitor for atrial fibrillation screening in the elderly. J Clin Hypertens (Greenwich) 2019;21:1797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo Y, Wang H, Zhang H, Chen Y, Lip GYH. Population-based screening or targeted screening based on initial clinical risk assessment for atrial fibrillation: a report from the Huawei heart study. J Clin Med 2020;9:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajakariar K, Koshy AN, Sajeev JK, Nair S, Roberts L, Teh AW. Accuracy of a smartwatch based single-lead electrocardiogram device in detection of atrial fibrillation. Heart 2020;106:665–70. [DOI] [PubMed] [Google Scholar]
- 29. Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018;320:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lumikari TJ, Pirinen J, Putaala J, Sibolt G, Kerola A, Pakarinen S et al. Prolonged ECG with a novel recorder utilizing electrode belt and mobile device in patients with recent embolic stroke of undetermined source: a pilot study. Ann Noninvasive Electrocardiol 2020;25:e12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- 32. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–84. [DOI] [PubMed] [Google Scholar]
- 33. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–506. [DOI] [PubMed] [Google Scholar]
- 34. Doliwa Sobocinski P, Anggårdh Rooth E, Frykman Kull V, von Arbin M, Wallén H, Rosenqvist M. Improved screening for silent atrial fibrillation after ischaemic stroke. Europace 2012;14:1112–6. [DOI] [PubMed] [Google Scholar]
- 35. Miyazawa K, Mairesse GH, Lip GYH. Screening for atrial fibrillation: look harder, look longer, and improve stroke outcomes with oral anticoagulation. Europace 2018;20:f278–9. [DOI] [PubMed] [Google Scholar]
- 36. Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke 2015;46:936–41. [DOI] [PubMed] [Google Scholar]
- 37. Chong BH, Pong V, Lam KF, Liu S, Zuo ML, Lau YF et al. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace 2012;14:942–7. [DOI] [PubMed] [Google Scholar]
- 38. Olsen FJ, Møgelvang R, Jensen GB, Jensen JS, Biering-Sørensen T. Relationship between left atrial functional measures and incident atrial fibrillation in the general population: the Copenhagen city heart study. JACC Cardiovasc Imaging 2019;12:981–9. [DOI] [PubMed] [Google Scholar]
- 39. Benjamin EJ, Go AS, Desvigne-Nickens P, Anderson CD, Casadei B, Chen LY et al. Research priorities in atrial fibrillation screening: a report from a national heart, lung, and blood institute virtual workshop. Circulation 2021;143:372–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB Sr, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet 2009;373:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J 2013;34:2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aronson D, Shalev V, Katz R, Chodick G, Mutlak D. Risk score for prediction of 10-year atrial fibrillation: a community-based study. Thromb Haemost 2018;118:1556–63. [DOI] [PubMed] [Google Scholar]
- 44. Hamada R, Muto S. Simple risk model and score for predicting of incident atrial fibrillation in Japanese. J Cardiol 2019;73:65–72. [DOI] [PubMed] [Google Scholar]
- 45. Li YG, Pastori D, Farcomeni A, Yang PS, Jang E, Joung B et al. A simple clinical risk score (C(2)HEST) for predicting incident atrial fibrillation in Asian subjects: derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest 2019;155:510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lip GYH, Skjøth F, Nielsen PB, Larsen TB. Evaluation of the C(2)HEST risk score as a possible opportunistic screening tool for incident atrial fibrillation in a healthy population (from a Nationwide Danish Cohort Study). Am J Cardiol 2020;125:48–54. [DOI] [PubMed] [Google Scholar]
- 47. Li YG, Bisson A, Bodin A, Herbert J, Grammatico-Guillon L, Joung B et al. C(2) HEST score and prediction of incident atrial fibrillation in poststroke patients: a French nationwide study. J Am Heart Assoc 2019;8:e012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li YG, Pastori D, Miyazawa K, Shahid F, Lip GYH. Identifying at-risk patients for sustained atrial high-rate episodes using the C(2)HEST score: the west Birmingham atrial fibrillation project. J Am Heart Assoc 2021;10:e017519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mitrega K, Lip GYH, Sredniawa B, Sokal A, Streb W, Przyludzki K et al. Predicting silent atrial fibrillation in the elderly: a report from the NOMED-AF cross-sectional study. J Clin Med 2021;10:2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Israel CW, Grönefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol 2004;43:47–52. [DOI] [PubMed] [Google Scholar]
- 51. Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RA et al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol 2016;9:e003333. [DOI] [PubMed] [Google Scholar]
- 52. Ritter MA, Kochhäuser S, Duning T, Reinke F, Pott C, Dechering DG et al. Occult atrial fibrillation in cryptogenic stroke: detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke 2013;44:1449–52. [DOI] [PubMed] [Google Scholar]
- 53. Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 2014;45:520–6. [DOI] [PubMed] [Google Scholar]
- 54. Shafqat S, Kelly PJ, Furie KL. Holter monitoring in the diagnosis of stroke mechanism. Intern Med J 2004;34:305–9. [DOI] [PubMed] [Google Scholar]
- 55. Kemp Gudmundsdottir K, Fredriksson T, Svennberg E, Al-Khalili F, Friberg L, Frykman V et al. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: the STROKESTOP II study. Europace 2020;22:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–75. [DOI] [PubMed] [Google Scholar]
- 57. Turakhia MP, Desai M, Hedlin H, Rajmane A, Talati N, Ferris T et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the Apple heart study. Am Heart J 2019;207:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brasier N, Raichle CJ, Dörr M, Becke A, Nohturfft V, Weber S et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 2019;21:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yan BP, Lai WHS, Chan CKY, Chan SC, Chan LH, Lam KM et al. Contact-free screening of atrial fibrillation by a smartphone using facial pulsatile photoplethysmographic signals. J Am Heart Assoc 2018;7:e008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lewis M, Parker D, Weston C, Bowes M. Screening for atrial fibrillation: sensitivity and specificity of a new methodology. Br J Gen Pract 2011;61:38–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chan PH, Wong CK, Poh YC, Pun L, Leung WW, Wong YF et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016;5:e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McManus MD, Chong JW, Soni A, Saczynski JS, Esa N, Napolitano C et al. PULSE-SMART: pulse-based arrhythmia discrimination using a novel smartphone application. J Cardiovasc Electrophysiol 2016;27:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eerikäinen LM, Bonomi AG, Schipper F, Dekker LRC, Vullings R, de Morree HM et al. Comparison between electrocardiogram- and photoplethysmogram-derived features for atrial fibrillation detection in free-living conditions. Physiol Meas 2018;39:084001. [DOI] [PubMed] [Google Scholar]
- 64. Verbrugge FH, Proesmans T, Vijgen J, Mullens W, Rivero-Ayerza M, Van Herendael H et al. Atrial fibrillation screening with photo-plethysmography through a smartphone camera. Europace 2019;21:1167–75. [DOI] [PubMed] [Google Scholar]
- 65. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 66. Pluymaekers N, Hermans ANL, van der Velden RMJ, Gawałko M, den Uijl DW, Buskes S et al. Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: TeleCheck-AF. Europace 2021;23:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017;14:e55–96. [DOI] [PubMed] [Google Scholar]
- 68. Rubio Campal JM, García Torres MA, Sánchez Borque P, Navas Vinagre I, Zamarbide Capdepón I, Miracle Blanco Á et al. Detecting atrial fibrillation in patients with an embolic stroke of undetermined source (from the DAF-ESUS registry). Am J Cardiol 2020;125:409–14. [DOI] [PubMed] [Google Scholar]
- 69. Pagola J, Juega J, Francisco-Pascual J, Moya A, Sanchis M, Bustamante A et al. Yield of atrial fibrillation detection with Textile Wearable Holter from the acute phase of stroke: pilot study of Crypto-AF registry. Int J Cardiol 2018;251:45–50. [DOI] [PubMed] [Google Scholar]
- 70. Wachter R, Gröschel K, Gelbrich G, Hamann GF, Kermer P, Liman J et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AF(RANDOMISED)): an open-label randomised controlled trial. Lancet Neurol 2017;16:282–90. [DOI] [PubMed] [Google Scholar]
- 71. Hariri E, Hachem A, Sarkis G, Nasr S. Optimal duration of monitoring for atrial fibrillation in cryptogenic stroke: a nonsystematic review. Biomed Res Int 2016;2016:5704963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J et al. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN International Collaboration. Circulation 2019;140:1834–50. [DOI] [PubMed] [Google Scholar]
- 73. Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014;127:95.e11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N et al. Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol 2021;6:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. [DOI] [PubMed] [Google Scholar]
- 76. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012;125:2298–307. [DOI] [PubMed] [Google Scholar]
- 77. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–38. [DOI] [PubMed] [Google Scholar]
- 78. Kapa S, Epstein AE, Callans DJ, Garcia FC, Lin D, Bala R et al. Assessing arrhythmia burden after catheter ablation of atrial fibrillation using an implantable loop recorder: the ABACUS study. J Cardiovasc Electrophysiol 2013;24:875–81. [DOI] [PubMed] [Google Scholar]
- 79. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014;35:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mairesse GH, Moran P, Van Gelder IC, Elsner C, Rosenqvist M, Mant J et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace 2017;19:1589–623. [DOI] [PubMed] [Google Scholar]
- 81. Gorenek BC, Bax J, Boriani G, Chen SA, Dagres N, Glotzer TV et al. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2017;19:1556–78. [DOI] [PubMed] [Google Scholar]
- 82. Freedman B, Boriani G, Glotzer TV, Healey JS, Kirchhof P, Potpara TS. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol 2017;14:701–14. [DOI] [PubMed] [Google Scholar]
- 83. Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J 2018;39:1407–15. [DOI] [PubMed] [Google Scholar]
- 84. Vitolo M, Imberti JF, Maisano A, Albini A, Bonini N, Valenti AC et al. Device-detected atrial high rate episodes and the risk of stroke/thrombo-embolism and atrial fibrillation incidence: a systematic review and meta-analysis. Eur J Intern Med 2021;92:100–6. [DOI] [PubMed] [Google Scholar]
- 85. Boriani G, Pettorelli D. Atrial fibrillation burden and atrial fibrillation type: clinical significance and impact on the risk of stroke and decision making for long-term anticoagulation. Vascul Pharmacol 2016;83:26–35. [DOI] [PubMed] [Google Scholar]
- 86. Boriani G, Glotzer TV, Ziegler PD, De Melis M, Mangoni di SSL, Sepsi M et al. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm 2018;15:376–83. [DOI] [PubMed] [Google Scholar]
- 87. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018;154:1121–201. [DOI] [PubMed] [Google Scholar]
- 88. Hill NR, Ayoubkhani D, McEwan P, Sugrue DM, Farooqui U, Lister S et al. Predicting atrial fibrillation in primary care using machine learning. PLoS One 2019;14:e0224582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lip GYH, Genaidy A, Tran G, Marroquin P, Estes C, Sloop S. Improving stroke risk prediction in the general population: a comparative assessment of common clinical rules, a new multimorbid index, and machine-learning-based algorithms. Thromb Haemost 2022;122:142–50. [DOI] [PubMed] [Google Scholar]
- 90. Lip GYH, Tran G, Genaidy A, Marroquin P, Estes C. Revisiting the dynamic risk profile of cardiovascular/non-cardiovascular multimorbidity in incident atrial fibrillation patients and five cardiovascular/non-cardiovascular outcomes: a machine-learning approach. J Arrhythm 2021;37:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.